Abstract
Prenatal opioid exposure has reached epidemic proportions. In the last 10 years, there has been a 242% increase in the number of babies born with the drug withdrawal syndrome known as Neonatal Opioid Withdrawal Syndrome (NOWS). Developmental outcome studies of infants with prenatal opioid exposure are limited by methodological issues including small sample sizes and lack of control for confounding variables such as exposure to poverty and maternal psychopathology. Thus, there is a critical gap in the literature that limits our ability to predict short-term effects of opioid exposure. Here we review direct neurotoxic, indirect, and stress-related pathophysiologies of prenatal opioid exposure. We describe the literature on short and long-term neurodevelopmental outcomes of children with prenatal opioid exposure, highlighting sex differences and the role of early life stress. We conclude by prioritizing avenues for future research for this group of underserved women and their children at risk for neurodevelopmental delays.
Over the last 10 years, there has been a 242% increase in the number of babies born with the drug withdrawal syndrome known as Neonatal Opioid Withdrawal Syndrome (NOWS), also referred to as neonatal abstinence syndrome (NAS; Desai et al., 2015; Reddy et al., 2017). NOWS is characterized by increased central nervous system excitability and hyperirritability, such as excessive crying, hypertonia, tremors, sleep disturbances, gastrointestinal problems, poor feeding, vomiting and diarrhea, respiratory distress, sweating, and sneezing (Finnegan and Kaltenbach, 1992; Kakko et al., 2008). Not all newborns exposed to opioids in utero develop NOWS. The incidence of NOWS is highly variable, with estimates ranging from 50 to 80% (Kakko et al., 2008; Reddy et al., 2017). This variability can likely be attributed to a variety of factors including the type of opioid to which the infant was exposed (e.g., heroin, buprenorphine, methadone), the number of newborns who require pharmacological treatment, and the diverse ways in which NOWS is assessed (Reddy et al., 2017). There is a critical gap in our understanding of the neurodevelopmental and psychiatric consequences of prenatal exposure to opioids. The existing literature is also plagued by low sample sizes, a lack of consideration of confounding stressors and/or effects of other substances over-and-above opioid exposure, and a lack of appropriate comparison groups. The aim of this review is to: (1) review the pathophysiology of prenatal opioid exposure; (2) outline additional stressors to which the fetus may be exposed; (3) briefly review the extant literature on prenatal opioid exposure and neurodevelopmental outcomes; (4) describe how outcomes could vary by infant sex; and (5) introduce an agenda for future research focused on developmental sequelae of prenatal opioid exposure.
1. Pathophysiology of prenatal opioid exposure
Opioids are defined as any class of drug that bind to G-protein coupled receptors, of which there are four subtypes: μ (mu), Κ (kappa) and δ (delta), and nociception-orphanin FQ (Reddy et al., 2017). These opioids include “street drugs” such as heroin, as well as prescription pain relievers such as oxycodone, hydrocodone, codeine, morphine, methadone, and buprenorphine. Methadone and buprenorphine are the two most common medications given to pregnant women addicted to opioids (Reddy et al., 2017). Methadone is a complete μ-opioid receptor agonist and results in heroin-like effects (Farid et al., 2008). However, because of its long half-life, it typically prevents withdrawal effects if patients receive a daily dose. Buprenorphine is a partial μ-opioid receptor agonist and complete Κ-receptor antagonist. It can therefore prevent withdrawal symptoms without resulting in strong euphoric effects (Farid et al., 2008; Reddy et al., 2017). Because it is a partial, as opposed to complete agonist, it has a ceiling effect beyond which a higher dose is not more effective (Reddy et al., 2017).
We first describe direct effects of opioids on the mother. Opioids are metabolized in the liver and also cross the blood-brain barrier (Zuckermann et al., 1995). Opioids act on the central nervous system and bind at endogenous opioid receptors (Behnke et al., 2013; Brown and Zuckerman, 1991). Direct behavioral effects of opioid use in adults include analgesia, lowered anxiety, euphoria, and drowsiness (Zuckermann et al., 1995). Physiologically, respiratory depression and intestinal peristalsis also occur (Zuckermann et al., 1995). Among pregnant women, opioids can alter maternal glucose regulation, and decrease arterial pressure and uterine blood flow (Szeto, 1995). These effects, in addition to depressed respiratory function, can affect the amount of oxygen the fetus receives in utero (Szeto, 1995). Opioid-induced maternal hypotension can also reduce uterine blood flow (Szeto, 1995).
Opioid receptor concentrations fluctuate across development (Farid et al., 2008). Opioid binding sites are present in the fetus before birth and direct exposure effects are likely mediated by the presence of these opioid receptors as well as overstimulation of μ opioid receptors (Little et al., 1996). In rat fetal neurons, methadone binding occurs at rates 2–14 times higher than maternal neurons (Pertschuk et al., 1977). Following exposure to opioids, there are direct effects on fetal cardiovascular, respiratory, neurobehavioral, metabolic and neuroendocrine systems (Szeto, 1995). For example, fetuses exposed to buprenorphine (n = 6) showed higher fetal heart rate variability, more accelerations in fetal heart rate, and greater coupling between heart rate and movement in the second trimester compared to methadone-exposed fetuses (n = 11), and in the third trimester fetuses exposed to buprenorphine exhibited more motor activity (Jansson et al., 2011, Jansson et al., 2017). After birth, newborns with prenatal opioid exposure also exhibited greater heart rate variability during nutritive and non-nutritive sucking compared to non-exposed controls (Hambleton et al., 2013).
These direct effects of opioids on the mother are mediated via placenta transfer of opioids from the woman to the fetal compartment. Indirect exposure therefore occurs via alterations in maternal-placental physiology (Szeto, 1995). The opioids meperidine, morphine and fentanyl can be detected in cord blood, neonatal plasma, and neonatal urine (Szeto, 1995). Methadone and, to a lesser extent, buprenorphine, crosses the human placenta, and this permeability is dependent on the number of tissue layers between fetal and maternal blood (Farid et al., 2008). There is wide variability across women in the degree to which methadone is metabolized by the placenta, which could contribute to the range of neurodevelopmental outcomes seen in newborns with prenatal exposure to methadone (Nanovskaya et al., 2008). These outcomes include intrauterine growth restriction, placental abruption, and preterm birth (Stover and Davis, 2015).
In rats, prenatal morphine exposure has been shown to affect the migration and survival of rat neurons (Walhovd et al., 2009). Morphine increases apoptosis in human fetal microglia and neurons and disrupts neural maturation (Slotkin, 1983; Tripathi et al., 2008). Prenatal exposure to opioids such as methadone and buprenorphine can also impact myelination (Sanchez et al., 2008; Vestal-Laborde et al., 2014). Opioid receptors are found on the surface of oligodendrocytes, which are involved in the production of myelin (Vestal-Laborde et al., 2014). Prenatal exposure to methadone in rodents sped up the timing of myelination in the corpus callosum and accelerated the maturation of preoligodendrocytes (Vestal-Laborde et al., 2014). Prenatal exposure to moderate levels of buprenorphine in rodents also resulted in precocious myelination as well as the thinning of the myelin sheath (Sanchez et al., 2008), and exposure to higher doses resulted in a reduction of myelinated axons in the rat corpus callosum (Sanchez et al., 2008). This accelerated myelination has the potential of dysregulating normal neural connectivity in the fetal brain (Vestal-Laborde et al., 2014).
Prolonged exposure to opioids in utero suppresses opioid G protein coupled receptors which in turn leads to increases in adenyl cyclase activity and subsequent dysregulation of multiple neuroendocrine pathways (Kocherlakota, 2014). Withdrawal from opioids in the newborn results in increased levels of norepinephrine, corticotrophin, noradrenaline, and acetylcholine. Increases in these stress neurotransmitters are related to tremors and hyperthermia. Decreases in dopamine and serotonin have also been observed in newborns exposed to opioids in utero, which likely plays a role in hyperirritability and sleep fragmentation, respectively (Kocherlakota, 2014).
We have described here direct effects of opioids on the mother and indirect (e.g., via placenta transfer) effects of prenatal opioid exposure on the fetus. A third, and largely unexplored pathophysiology of prenatal opioid exposure, may be indirect effects that occur due to exposure to early life stress which often covaries with prenatal opioid exposure.
2. Prenatal opioid exposure and the effects of early life stress
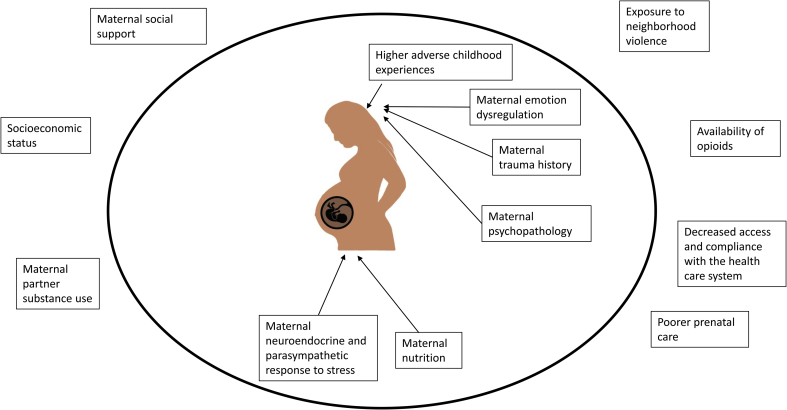
In Fig. 1 we highlight the important stressors that mothers with opioid use disorders are disproportionately more likely to experience compared to mothers who are not using opioids while pregnant. Children exposed to opioids in utero also often experience the effects of poverty, maternal psychopathology and emotion dysregulation, disruptions in maternal care, and problematic (e.g., insensitive) interactions with their primary caregiver (Hans and Jeremy, 2001). Women who use opioids while pregnant are also more likely to have poor nutrition, decreased access to and compliance with the health care system, and increased exposure to violence (Behnke et al., 2013). These women are also more likely to have a history of trauma (Saia et al., 2016). Approximately 50–80% of women with substance use disorders have also experienced physical, sexual, and/or emotional abuse (Saia et al., 2016) which is associated with neuroendocrine dysregulation (Carpenter et al., 2007).
Fig. 1.
Factors that may exert programming effects on the developing fetus exposed to opioids in utero. Adapted from Conradt et al. (2018).
Women who use opioids while pregnant are also more likely to use other substances, such as nicotine, alcohol, antidepressants, and benzodiazepines compared to pregnant women who aren't using opioids (Heberlein et al., 2012; Winklbaur et al., 2009). Furthermore, they are more likely to experience a range of psychiatric comorbidities in addition to their substance use disorder such as anxiety, depression, and bipolar disorder (Whiteman et al., 2014). Given the high rates of polysubstance use and mental health comorbidities among women with opioid use disorders it is necessary to examine the shared mechanisms underlying development of these disorders. In other words, a transdiagnostic approach to investigating processes that give rise to the constellation of psychiatric symptoms among women who use opioids while pregnant is needed (Garland and Howard, 2013).
Maternal emotion dysregulation. We highlight here the importance of assessing emotion dysregulation in pregnant women with an opioid use disorder. Emotion dyregulation is a transdiagnostic vulnerability for a variety of psychiatric disorders, including substance use disorders (Beauchaine, 2015). It is a multi-faceted construct that includes a lack of awareness and acceptance of emotions, an inability to control behaviors when experiencing distress, a lack of access to adaptive strategies for regulating emotion, and an unwillingness to experience emotional distress (Beauchaine, 2015; Leventhal and Zvolensky, 2015). Maternal emotion dysregulation is both a cause and consequence of stress and is often seen among those with anxiety, depression, borderline personality disorder, and bipolar disorder, discrete diagnoses that are commonly found among women with opioid use disorders (Whiteman et al., 2014).
Adults with substance use disorders experience greater overall levels of emotion dysregulation compared to adults who do not use substances (Barahmand et al., 2016; Weiss et al., 2013). They report difficulties accepting and regulating their emotions, engaging in goal-directed behavior, controlling impulsive behavior when distressed, and identifying appropriate strategies for regulating emotions (Barahmand et al., 2016). These symptoms are heightened if individuals with substance use disorders also engage in self-harm (Anestis et al., 2012). Furthermore, emotion dysregulation may mediate the association between childhood trauma and later substance use disorders (Weiss et al., 2013). Emotion dysregulation may therefore be a key construct that could explain the etiology of opioid use disorders in pregnant women, particularly those with an early trauma history. For researchers interested in examining maternal effects on child risk, including impaired developmental outcomes in children with prenatal opioid exposure, it may be worthwhile including an assessment of this construct.
Importantly, emotion dysregulation can be assessed at multiple levels of analysis, including self-reports, behavioral observations, laboratory tasks, and biomarkers. One key biomarker of risk for emotion dysregulation is respiratory sinus arrhythmia (RSA; also known as high frequency heart rate variability; HF-HRV), which is an index of parasympathetic nervous system functioning (Beauchaine, 2015). RSA is a measure of the natural beat-to-beat variability in heart rate that occurs with respiration. Specifically, heart rate increases during inhalation, and decreases with exhalation and RSA indexes the component of this variability that is due to parasympathetic influences (Berntson et al., 1993). Changes in RSA (increases or decreases) are thought to occur in response to stress (Beauchaine, 2001). RSA has been assessed in women in a methadone maintenance treatment program (Jansson et al., 2009). Jansson and colleagues found that any changes in RSA in response to methadone use in the mother were predictive of symptoms of NOWS. Mothers with no change in RSA in response to methadone administration were significantly less likely to have infants with symptoms of NOWS (Jansson et al., 2009). It may therefore be that changes in RSA in response to opioid administration was experienced as a stressor for these women, with consequences for the development of NOWS in the fetus.
Studies that have examined these factors, including maternal psychopathology, in addition to prenatal opioid exposure have found some support for the hypothesis that the effect of opioids no longer predicts offspring cognitive outcomes after controlling for associated stressors (Hans and Jeremy, 2001; Messinger et al., 2004). For example, Hans and Jeremy (2001) found that the effects of opioid exposure on poorer mental development outcomes, as assessed using the Bayley scales of mental and motor development in early childhood, were no longer significant after controlling for social-environmental risk factors like low maternal education, low family SES, and maternal-infant interactions. Messinger et al. (2004) likewise found that the effects of opioid exposure on psychomotor development using the Bayley were no longer significant when controlling for covariates such as SES and quality of the home environment. These findings highlight the need to consider “confounds,” such as early life stress exposure and sociodemographic risk and also suggest the need to consider potential programming effects. Indeed, prenatal programming of fetal stress response systems may be an additional pathway by which prenatal opioid exposure contributes to risk for impaired developmental outcomes.
3. Programming effects of prenatal opioid exposure
As reviewed above, prenatal opioid exposure does not occur in isolation. In addition to the direct and indirect pathophysiological effects of prenatal opioid exposure, the fetus is more likely to be exposed to high levels of prenatal stress, which can exert programming effects on stress response systems, including the neuroendocrine system. We argue here that exposure to opioids prenatally and associated stressors could disrupt in utero homeostasis and result in adjustments that confer immediate survival advantage, with potential costs to long-term health (Lester and Padbury, 2009).
The programming hypothesis as it relates to prenatal substance exposure was originally proposed by Lester and Padbury in reference to children with prenatal cocaine exposure and it can be extended to children with prenatal opioid exposure (Lester and Padbury, 2009). It has been well-documented that early life stress impacts functioning of the hypothalamic-pituitary-adrenal (HPA) axis in the fetus and in early childhood (for reviews, see Glover et al., 2010; Gunnar and Quevedo, 2007; Murgatroyd and Spengler, 2011). It is therefore plausible that early life stress in addition to prenatal opioid exposure could exert additive or interactive programming influences on fetal HPA axis functioning. Paraventricular corticotrophin releasing hormone neurons, which activate the hypothalamic-pituitary-adrenal (HPA) axis, express μ-opioid receptors and are modulated by β-endorphin neurons (Wand, 2002). Individuals expressing the μ-opioid receptor variant ASP40 have been shown to have disruptions in HPA axis functioning and altered responses to other physiological processes regulated through activation of the μ-opioid receptor (Wand, 2002). Furthermore, removing the endogenous inhibitory opioid tone using naloxone, an opioid receptor antagonist, induces a rise in ACTH and cortisol (Wand et al., 2011). In humans, those who are addicted to opioids show a blunted HPA axis response to stress (Sinha, 2008). The HPA axis tends to be more reactive during a period of withdrawal and shows the typical response to stress during treatment with a methadone maintenance program (Fatseas et al., 2011).
These effects on the HPA axis could also extend to the postnatal period via disruptions in maternal caregiving. Rodent models suggest that the infant neuroendocrine system can be programmed by maternal caregiving behaviors such as licking and grooming and arched back nursing (Weaver et al., 2004). Some studies (though not all; Jeremy and Bernstein, 1984) have found that women with substance use disorders are less sensitive when interacting with their infants (LaGasse, 2003). For example, LaGasse and colleagues found that mothers who used cocaine while pregnant were less flexible and engaged, and also exhibited more activity with their infants during a feeding interaction compared to mothers who had not used cocaine while pregnant (LaGasse, 2003). Children exposed to cocaine and opioids were also less likely to be securely attached compared to infants exposed to drugs other than cocaine and opioids (Seifer et al., 2004). In an independent sample, Eiden et al. (2011) found that mothers who used cocaine while pregnant showed more negative affect and less sensitivity when interacting with their 13-month-old infants. It is therefore possible that both prenatal and postnatal programming can alter the neuroendocrine and associated stress response systems among children with prenatal opioid exposure.
4. Prenatal opioid exposure effects on genetic, epigenetic, and neurodevelopmental outcomes
There is a dearth of information about the short and long-term neurodevelopmental effects of prenatal exposure to opioids in humans, and even less is known about effects on the developing infant brain. The vast majority of information comes from short-term follow-up studies, and includes clinical obstetric and pediatric outcomes such as birth weight, head circumference, and APGAR scores. It appears as though the effect of prenatal opioid exposure on the neurodevelopment of the neonate depends on a wide range of factors, including type of opioid (e.g., buprenorphine, methadone, or heroin) as well as associated early life stressors, such as maternal education and socioeconomic status. In one of the first studies on prenatal opioid exposure, Jeremy and Bernstein, 1984 found that methadone-exposed neonates (n = 29) were more jittery, had more tremors, and were more tense, active, and could put their hand to their mouth more easily compared to control infants (n = 37). Hans and Jeremy (2001) followed these infants to two years of age and found no differences in cognitive outcomes between methadone-exposed and unexposed infants, though the exposed infants had poorer motor outcomes.
Coyle and colleagues (2012) found similar newborn neurobehavioral effects when comparing methadone-expose to buprenorphine-exposed newborns, with infants exposed to buprenorphine showing better newborn neurobehavioral outcomes compared to infants exposed to methadone. In another study comparing methadone-exposed (n = 11) and buprenporphine-exposed (n = 10) newborns using the NICU Network Neurobehavioral Scale (NNNS), newborns exposed to buprenorphine had higher arousal and excitability levels on postnatal days 5 and 7 (but not postnatal day 3, 10, or 14), compared to methadone-exposed newborns (Jones et al., 2010). Furthermore, infants requiring treatment for NAS/NOWS showed poorer quality of movement, excitability, and lethargy scores compared to exposed newborns who did not need treatment, regardless of the type of opioid to which they were exposed (Jones et al., 2010).
Johnson et al. (2001) studied three buprenorphine-exposed infants and found that they had typical birth outcomes, mild symptoms of NOWS, and required no pharmacological treatment. In a study of only buprenorphine-exposed newborns, Velez and colleagues found that greater exposure to buprenorphine was related to poor quality of movement and self-regulation, and more central nervous systems signs of stress at day 3. They found no differences in newborn neurobehavioral outcomes as assessed by the NNNS between infants who required pharmacotherapy for NOWS and those who did not require treatment on day 3. We only found two studies that compared neurodevelopmental effects in infants who received treatment for NOWS compared with those who did not. Beckwith and Burke (2015) discovered that, at an average of 55 days old, infants treated for NOWS (n = 28) had lower language and cognition scores compared to a historical control. This study should be interpreted with caution, however, given that infants were assessed at a mean age of 55 days, when it is difficult to capture variability in language and cognition using the Bayley. Heller et al. (2017) examined differences in neurodevelopmental outcome using the NNNS among 6-week old infants with prenatal methadone exposure who did (n = 23) or did not (n = 16) require pharmacological treatment for NOWS compared to a demographically matched control group (n = 21; Heller et al., 2017). Infants who were exposed to methadone and who required treatment had more difficulty self-regulating and had poorer quality movement compared to an unexposed control and compared to infants who were exposed but did not require treatment (Heller et al., 2017).
There is a small, but growing literature on genetic and epigenetic predictors of NOWS. Epigenetic methods can be useful in uncovering the mechanisms by which prenatal opioid exposure could lead to impaired neurodevelopmental outcome. Most of the focus thus far has been on epigenetic variation in the mu-opioid receptor gene in infants with NOWS (Wachman et al., 2014). Greater methylation at specific CpG sites on OPRM1 was associated with greater need for medications to treat NOWS (Wachman et al., 2014) and see (Wachman et al., 2018a, Wachman et al., 2018b) for a partial replication of these findings in an independent sample. In an independent sample, McLaughlin et al. (2017) also found that increased DNA methylation in OPRM1 was found among infants exposed to opioids in utero and that variability in DNA methylation of ABCB1, and CYP2D6 related to prenatal opioid exposure. Wachman et al. (2015) identified single nucleotide polymorphisms associated with NOWS severity in PNOC and OPRK1, genes from the opioid-receptor family. In a partial replication study, infants with a specific allele of the PNOC gene and specific alleles of the maternal COMT gene were less likely to require treatment as infants (Wachman et al., 2017). These findings indicate that there may be genetic indicators of risk for NOWS.
There are very few longitudinal follow-up studies with opioid-exposed infants. In one such study with 72 exposed and 58 comparison (unexposed) infants, the difference in IQ between female infants with and without prenatal opioid exposure increased from 1 to 8.5 years, even when controlling for foster care placement and heroin exposure, while boys with prenatal opioid exposure exhibited consistently lower levels of IQ over time compared to unexposed control boys (Nygaard et al., 2015). In a study of only children with prenatal opioid exposure, Kaltenbach and colleagues found no differences in cognitive outcomes using the Bayley at three years of age between methadone-exposed and buprenorphine-exposed preschoolers (Kaltenbach et al., 2018). At two years of age, Levine and Woodward (2018) found that methadone-exposed infants showed poorer inhibitory control. At 4 years methadone-exposed infants showed poorer short-term memory and inhibition, though these effects were partially explained by maternal education (Konijnenberg and Melinder, 2015; Levine and Woodward, 2018), maternal benzodiazepine use (Levine and Woodward, 2018), and maternal employment (Konijnenberg and Melinder, 2015).
It is unclear what the effects of prenatal opioid exposure may be on neurodevelopment given that the sample sizes tend to be low and few studies control for confounding factors like maternal psychopathology, polysubstance use, maternal nutrition, or socioeconomic status. Those that have controlled for these factors tend to find that the effects of opioids are no longer significant. Even fewer studies have controlled for the effects of polysubstance exposure. In other words, it is unknown whether the neurodevelopmental outcomes are due to opioids, the effects of other stressors, or the effects of other drugs, like nicotine. For example, in a retrospective chart review, Patrick et al. (2015) found that prenatal nicotine exposure, in addition to opioid exposure, type of opioid used, and selective serotonin reuptake inhibitor exposure was related to increased likelihood of developing NOWS. Large sample sizes are thus needed to try to disentangle the effects of the opioid on neurodevelopment above and beyond additional substances and associated environmental stressors.
5. Sex differences in response to prenatal opioid exposure
There is very little known about how the effects of prenatal opioid exposure may vary by infant sex, and the vast majority of studies are underpowered to detect sex differences. In studies with human newborns, males showed more symptoms of NOWS, and the symptoms they did express were more severe. As a result they received more pharmacological treatment compared to females (Jansson et al., 2007). In a study of newborn neurobehavioral outcomes, males exposed to opioids prenatally had higher scores on habituation, a measure of the neonate's ability to tune out external stimuli, compared to females exposed prenatally to opioids (Jones et al., 2010). In a large cohort study that included a review of 102,695 medical records, Charles and colleagues (Charles et al., 2017) found that males were more likely than females to be diagnosed with NOWS. However, no sex differences in NOWS severity were identified.
The theory of a viability-vulnerability tradeoff proffered by Sandman and colleagues (Sandman et al., 2013) is the only theory to date describing how sex differences due to fetal programming may occur. According to this hypothesis, prenatal exposure to stress could affect males by decreasing their viability and making it more likely that they exhibit maturational delays. This hypothesis supports the above reviewed finding by Jansson et al. (2007). While females may be more likely to survive a stressful prenatal environment, the effects of this exposure on developmental outcomes may be subtle and they may be at increased likelihood of experiencing fear and anxiety as a direct consequence of this exposure. While the literature on sex differences in prenatal opioid exposure is scarce, it will be important to consider in future work whether males exposed to opioids are more likely to show developmental delays while females could be more likely to show increases in fear and anxiety, as predicted by the theory of viability-vulnerability tradeoff (Sandman et al., 2013).
5.1. Future research priorities
Substance use disorders are complex illnesses and there are a number of challenges associated with designing and conducting rigorous research on women who use substances while pregnant, and on the neurodevelopmental consequences of that use for the child (Kakko et al., 2008). First, very few studies examine maternal characteristics that predict newborn and infant neurodevelopmental outcomes. It will be important in future research to include comprehensive assessments of the mother, including her mental health and trauma history, as well as a detailed substance use history that can be verified using toxicology screens. Another novel approach is to examine whether maternal physiological stress signs are predictive of NOWS symptom severity (Jansson et al., 2009). For example, Jansson and colleagues found that any maternal response in the parasympathetic nervous system to administration of methadone was positively related to NOWS symptom severity and need for treatment. Individual differences in maternal parasympathetic response to methadone treatment may provide the field with more information about which children are at risk for developing NOWS.
In designing studies with humans, it will be nearly impossible to study women who are solely using opioids. Polysubstance use is the rule rather than the exception (Kakko et al., 2008) and studies can either covary for the effects of other substances, such as antidepressant exposure or exposure to nicotine, or create a cumulative substance exposure “risk” score (see for example (Conradt et al., 2014). Studies attempting to examine effects of prenatal opioid exposure will therefore benefit from maternal report of substance use in addition to toxicology screens at birth.
There is also no standardized assessment of NOWs that has widespread acceptance in the prenatal opioid literature. A number of studies use the Finnegan or an adaptation of the Finnegan (see for example Jansson et al., 2007), though there is wide variability in the way clinicians diagnose and treat NOWS (Reddy et al., 2017). In addition, the Finnegan has been shown to have poor psychometric properties (Jones et al., 2016). Short, standardized, reliable, and valid assessments for the diagnosis of NOWS, especially ones that can be easily used by pediatricians and neonatologists, are sorely needed.
The studies examining predictors of NOWS symptom severity tend to be retrospective. While work on the genetic and epigenetic predictors of NOWS is growing, there are very few studies examining prenatal or early neonatal predictors of NOWS (though for exceptions see Jansson et al., 2007; McLaughlin et al., 2017; Sharpe, 2004; Velez et al., 2009; Wachman et al., 2014, 2015). Those studies that have examined early life predictors tend to use newborn neurobehavioral assessments, such as the NNNS. For example, Velez et al. (2009) examined newborns exposed to methadone in utero and found that infants who were ultimately treated for NOWS showed higher habituation scores, they were more aroused, excitable, and hypertonic compared to newborns who did not require treatment. In another study Sharpe (2004) examined differences in NOWS outcomes between women using methadone for pain and those using methadone for drug treatment. They found that 11% of infants exposed to methadone because of maternal pain developed NOWS while 58% of infants exposed to methadone because of maternal substance use disorder developed NOWS.
NOWS symptom severity and even diagnosis could also be impacted by maternal sensitive caregiving. The percentage of newborns exposed to opioids prenatally who require pharmacological treatment to manage withdrawal symptoms ranges from 30 to 80% (Wachman et al., 2018b). This variability in treatment is likely due to several factors including non-pharmacologic care (e.g., swaddling, skin-to-skin, and rooming in with the mother), as well as exposure to other substances such as nicotine (Kakko et al., 2008; Wachman et al., 2018a, Wachman et al., 2018b). Several studies have recently published data demonstrating that rooming-in practices, breast feeding, and kangaroo care are related to a 7–60% decrease in the necessity for pharmacological treatment for NOWS and that these practices significantly reduce length of hospital stays (Holmes et al., 2016; Wachman et al., 2018a, Wachman et al., 2018b). However, few studies have tested the efficacy of these nonpharmacological interventions using methodologically rigorous randomized controlled trials (Wachman et al., 2018a, Wachman et al., 2018b), and no studies have examined mechanisms of effects (e.g., maternal sensitivity, changes in maternal HPA axis functioning), which are significant research gaps that are ripe for future research.
The long-term developmental consequences of prenatal exposure to opioids and related stressors are also unknown. Importantly, there are very few studies examining long-term neurodevelopmental consequences of infants exposed to opioids who then developed NOWS compared with exposed infants who did not develop NOWS. These studies are needed in order for clinicians to inform pregnant women about potential effects on infant development and to answer critical questions about whether opioids alter neurodevelopmental outcomes above and beyond associated stressors, such as exposure to poverty and parental psychopathology.
Finally, it will be important to consider whether the neurodevelopmental consequences of prenatal opioid exposure and correlated stressors differ between male and female fetuses. Current hypotheses suggest that males may be more at risk for experiencing developmental delays while females may experience more subtle effects that manifest later in life as mood disorders (Sandman et al., 2013). However, this literature is still quite limited.
In sum, developmental outcome studies of infants with prenatal opioid exposure are small and are plagued by methodological issues, including small sample sizes and lack of control for confounding variables such as exposure to poverty and maternal psychopathology. Thus, there is a critical gap in the literature that affects our ability to predict short-term effects of drug exposure. There is an even smaller literature on the long-term neurodevelopmental consequences of prenatal opioid exposure, particularly studies that examine differences between infants who develop NOWS compared to those that do not. Attention to these critical factors is important if we are to support the development of these underserved infants at risk for neurodevelopmental delays.
Financial disclosure
This study was supported a Career Development Award from the National Institute on Drug Abuse 7K08DA038959-02 (to E.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, or the National Institutes of Health.
Conflict of interest
The authors report no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.08.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Anestis M.D., Tull M.T., Bagge C.L., Gratz K.L. The moderating role of distress tolerance in the relationship between posttraumatic stress disorder symptom clusters and suicidal behavior among trauma exposed substance users in residential treatment. Arch. Suicide Res. 2012;16(3):198–211. doi: 10.1080/13811118.2012.695269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahmand U., Khazaee A., Hashjin G.S. Emotion dysregulation mediates between childhood emotional abuse and motives for substance use. Arch. Psychiatr. Nurs. 2016;30(6):653–659. doi: 10.1016/j.apnu.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Beauchaine Theodore. Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev. Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine T.P. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology. 2015;3:43–47. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith A.M., Burke S.A. Identification of early developmental deficits in infants with prenatal heroin, methadone, and other opioid exposure. Clin. Pediatr. 2015;54:328–335. doi: 10.1177/0009922814549545. [DOI] [PubMed] [Google Scholar]
- Behnke M., Smith V.C., Committee on substance abuse, Committee on fetus and newborn Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–e1024. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson G.G., Cacioppo J.T., Quigley K.S. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Brown E.R., Zuckerman B. The infant of the drug-abusing mother. Pediatr. Ann. 1991;20(10):555–563. doi: 10.3928/0090-4481-19911001-07. [DOI] [PubMed] [Google Scholar]
- Carpenter L.L., Carvalho J.P., Tyrka A.R., Wier L.M., Mello A.F., Mello M.F. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatr. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M.K., Cooper W.O., Jansson L.M., Dudley J., Slaughter J.C., Patrick S.W. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp. Pediatr. 2017;7(6):328–334. doi: 10.1542/hpeds.2016-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E., Abar B., Lester B.M., LaGasse L.L., Shankaran S., Bada H. Child Development; 2014. Cortisol Reactivity to Social Stress as a Mediator of Early Adversity on Risk and Adaptive Outcomes. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E., Adkins D.E., Crowell S.E., Monk C., Kobor M.S. An epigenetic pathway approach to investigating associations between prenatal exposure to maternal mood disorder and newborn neurobehavior. Dev. Psychopathol. 2018;30:881–890. doi: 10.1017/S0954579418000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R.J., Huybrechts K.F., Hernandez-Diaz S., Mogun H., Patorno E., Kaltenbach K. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ. 2015;350(may14 1) doi: 10.1136/bmj.h2102. h2102–h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden R.D., Schuetze P., Coles C.D. Maternal cocaine use and mother-infant interactions: Direct and moderated associations. Neurotoxicol. Teratol. 2011;33:120–128. doi: 10.1016/j.ntt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid W., Dunlop S., Tait R., Hulse G. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr. Neuropharmacol. 2008;6(2):125–150. doi: 10.2174/157015908784533842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatseas M., Denis C., Massida Z., Verger M., Franques-Rénéric P., Auriacombe M. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol. Psychiatr. 2011;70(8):720–727. doi: 10.1016/j.biopsych.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Finnegan Loretta P., Kaltenbach Karol. Primary Pediatric Care. second ed. Mosby; St. Louis: 1992. Neonatal abstinence syndrome; pp. 1367–1378. [Google Scholar]
- Garland E.L., Howard M.O. Mindfulness-oriented recovery enhancement reduces pain attentional bias in chronic pain patients. Psychother. Psychosom. 2013;82(5):311–318. doi: 10.1159/000348868. [DOI] [PubMed] [Google Scholar]
- Glover V., O'Connor T.G., O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 2010;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Quevedo K.M. vol. 167. Elsevier; 2007. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability; pp. 137–149. (Progress in Brain Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton M.T., Reynolds E.W., Sithisarn T., Traxel S.J., Patwardhan A.R., Crawford T.N. Autonomic nervous system function following prenatal opiate exposure. Frontiers in Pediatrics. 2013;1 doi: 10.3389/fped.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S.L., Jeremy R.J. Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Ment. Health J. 2001;22(3):300–315. doi: 10.1002/imhj.1003. [DOI] [Google Scholar]
- Heberlein A., Leggio L., Stichtenoth D., Hillemacher T. The treatment of alcohol and opioid dependence in pregnant women. Curr. Opin. Psychiatr. 2012;25(6):559–564. doi: 10.1097/YCO.0b013e328358ad36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller N.A., Logan B.A., Morrison D.G., Paul J.A., Brown M.S., Hayes M.J. Neonatal abstinence syndrome: neurobehavior at 6 weeks of age in infants with or without pharmacological treatment for withdrawal. Dev. Psychobiol. 2017;59(5):574–582. doi: 10.1002/dev.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.V., Atwood E.C., Whalen B., Beliveau J., Jarvis J.D., Matulis J.C., Ralston S.L. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6) doi: 10.1542/peds.2015-2929. e20152929–e20152929. [DOI] [PubMed] [Google Scholar]
- Jansson L.M., Dipietro J.A., Elko A., Velez M. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J. Matern. Fetal Neonatal Med. 2007;20(9):677–685. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- Jansson L.M., Dipietro J.A., Velez M., Elko A., Knauer H., Kivlighan K.T. Maternal methadone dosing schedule and fetal neurobehaviour. J. Matern. Fetal Neonatal Med. 2009;22(1):29–35. doi: 10.1080/14767050802452291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L.M., DiPietro J.A., Velez M., Elko A., Williams E., Milio L. Fetal neurobehavioral effects of exposure to methadone or buprenorphine. Neurotoxicol. Teratol. 2011;33(2):240–243. doi: 10.1016/j.ntt.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L.M., Velez M., McConnell K., Spencer N., Tuten M., Jones H.E., King V.L., Gandorta N., Milio L.A., Voegtline K., DiPietro J.A. Maternal buprenorphine treatment and fetal neurobehavioral development. Am. J. Obstet. Gynecol. 2017;216:529.e1–529.e8. doi: 10.1016/j.ajog.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremy R.J., Bernstein V.J. Dyads at risk: methadone-maintained women and their four-month-old infants. Child Dev. 1984;55(4):1141. doi: 10.2307/1129983. [DOI] [PubMed] [Google Scholar]
- Johnson R.E., Jones H.E., Jasinski D.R., Svikis D.S., Haug N.A., Jansson L.M., Kissin W.B., Alpan G., Lantz M.E., Cone E.J., Wilkins D.G., Golden A.S., Huggins G.R., Lester B.M. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Jones H.E., O'Grady K.E., Johnson R.E., Velez M., Jansson L.M. Infant neurobehavior following prenatal exposure to methadone or buprenorphine: results from the neonatal intensive care unit Network neurobehavioral scale. Subst. Use Misuse. 2010;45(13):2244–2257. doi: 10.3109/10826084.2010.484474. [DOI] [PubMed] [Google Scholar]
- Jones H.E., Seashore C., Johnson E., Horton E., O'Grady K.E., Andringa K. Psychometric assessment of the neonatal abstinence scoring system and the MOTHER NAS scale: NAS psychometrics. Am. J. Addict. 2016;25(5):370–373. doi: 10.1111/ajad.12388. [DOI] [PubMed] [Google Scholar]
- Kakko J., Heilig M., Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96(1–2):69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K., O'Grady K.E., Heil S.H., Salisbury A.L., Coyle M.G., Fischer G. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40–49. doi: 10.1016/j.drugalcdep.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547–e561. doi: 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
- Konijnenberg C., Melinder A. Visual selective attention is impaired in children prenatally exposed to opioid agonist medication. Eur. Addiction Res. 2015;21(2):63–70. doi: 10.1159/000366018. [DOI] [PubMed] [Google Scholar]
- LaGasse L.L. Prenatal drug exposure and maternal and infant feeding behaviour. Arch. Dis. Child. Fetal Neonatal Ed. 2003;88(5):391F–399F. doi: 10.1136/fn.88.5.F391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B.M., Padbury J.F. Third pathophysiology of prenatal cocaine exposure. Dev. Neurosci. 2009;31(1–2):23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Leventhal A.M., Zvolensky M.J. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion–smoking comorbidity. Psychol. Bull. 2015;141(1):176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T.A., Woodward L.J. Early inhibitory control and working memory abilities of children prenatally exposed to methadone. Early Hum. Dev. 2018;116:68–75. doi: 10.1016/j.earlhumdev.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Little P.J., Price R.R., Hinton R.K., Kuhn C.M. Role of noradrenergic hyperactivity in neonatal opiate abstinence. Drug Alcohol Depend. 1996;41(1):47–54. doi: 10.1016/0376-8716(96)01236-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin P., Mactier H., Gillis C., Hickish T., Parker A., Liang W.-J., Osselton M.D. Increased DNA methylation of ABCB1, CYP2D6, and OPRM1 genes in newborn infants of methadone-maintained opioid-dependent mothers. J. Pediatr. 2017;190:180–184. doi: 10.1016/j.jpeds.2017.07.026. e1. [DOI] [PubMed] [Google Scholar]
- Messinger D.S., Bauer C.R., Das A., Seifer R., Lester B.M., Lagasse L.L. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113(6):1677–1685. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C., Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress. 2011;14(6):581–589. doi: 10.3109/10253890.2011.602146. [DOI] [PubMed] [Google Scholar]
- Nanovskaya T.N., Nekhayeva I.A., Hankins G.D.V., Ahmed M.S. Transfer of methadone across the dually perfused preterm human placental lobule. Am. J. Obstet. Gynecol. 2008;198(1):126. doi: 10.1016/j.ajog.2007.06.073. e1-126.e4. [DOI] [PubMed] [Google Scholar]
- Nygaard E., Moe V., Slinning K., Walhovd K.B. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr. Res. 2015;78(3):330–335. doi: 10.1038/pr.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertschuk L.P., Ford D.H., Rainford E.A. Localization of methadone in fetal rat eye by the immunofluorescence technic. Exp. Eye Res. 1977;24(6):547–552. doi: 10.1016/0014-4835(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Patrick S.W., Dudley J., Martin P.R., Harrell F.E., Warren M.D., Hartmann K.E., Ely E.W., Grijalva C.G., Cooper W.D. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135:842–850. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy U.M., Davis J.M., Ren Z., Greene M.F. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the eunice kennedy shriver national Institute of child health and human development, american college of obstetricians and gynecologists, american academy of pediatrics, society for maternal-fetal medicine, centers for disease control and prevention, and the march of dimes foundation. Obstet. Gynecol. 2017;130(1):10–28. doi: 10.1097/AOG.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saia K.A., Schiff D., Wachman E.M., Mehta P., Vilkins A., Sia M. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Current Obstetrics and Gynecology Reports. 2016;5(3):257–263. doi: 10.1007/s13669-016-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E.S., Bigbee J.W., Fobbs W., Robinson S.E., Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56(9):1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman C.A., Glynn L.M., Davis E.P. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013;75(4):327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer R., LaGasse L.L., Lester B., Bauer C.R., Shankaran S., Bada H.S. Attachment status in children prenatally exposed to cocaine and other substances. Child Dev. 2004;75(3):850–868. doi: 10.1111/j.1467-8624.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Sharpe C. Outcomes of infants born to mothers receiving methadone for pain management in pregnancy. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89(1):33F–36F. doi: 10.1136/fn.89.1.F33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T.A. Effects of perinatal exposure to methadone on development of neurotransmission: biochemical bases for behavioral Alterations1. In: Schlumpf M., Lichtensteiger W., editors. vol. 9. S. Karger AG; 1983. pp. 153–158. (Frontiers of Neurology and Neuroscience). [DOI] [PubMed] [Google Scholar]
- Stover M.W., Davis J.M. Opioids in pregnancy and neonatal abstinence syndrome. Semin. Perinatol. 2015;39(7):561–565. doi: 10.1053/j.semperi.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto Hazel H. vol. 149. 1995. Opioid receptor approaches for the development of medications for pregnant women; pp. 100–115. (NIDA Research Monographs). [PubMed] [Google Scholar]
- Tripathi A., Khurshid N., Kumar P., Iyengar S. Expression of δ- and μ-opioid receptors in the ventricular and subventricular zones of the developing human neocortex. Neurosci. Res. 2008;61(3):257–270. doi: 10.1016/j.neures.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Velez M.L., Jansson L.M., Schroeder J., Williams E. Prenatal methadone exposure and neonatal neurobehavioral functioning. Pediatr. Res. 2009;66(6):704–709. doi: 10.1203/PDR.0b013e3181bc035d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal-Laborde A.A., Eschenroeder A.C., Bigbee J.W., Robinson S.E., Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev. Neurosci. 2014;36(5):409–421. doi: 10.1159/000365074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman E.M., Hayes M.J., Lester B.M., Terrin N., Brown M.S., Nielsen D.A., Davis J.M. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J. Pediatr. 2014;165(3):472–478. doi: 10.1016/j.jpeds.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman E.M., Hayes M.J., Sherva R., Brown M.S., Davis J.M., Farrer L.A., Nielsen D.A. Variations in opioid receptor genes in neonatal abstinence syndrome. Drug Alcohol Depend. 2015;155:253–259. doi: 10.1016/j.drugalcdep.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman E.M., Hayes M.J., Sherva R., Brown M.S., Shrestha H., Logan B.A. Association of maternal and infant variants in PNOC and COMT genes with neonatal abstinence syndrome severity: PNOC and COMT and NAS Severity. Am. J. Addict. 2017;26(1):42–49. doi: 10.1111/ajad.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman E.M., Hayes M.J., Shrestha H., Nikita F.N.U., Nolin A., Hoyo L. Brain and Behavior; Genes: 2018. Epigenetic Variation in OPRM1 Gene in Opioid-exposed Mother-infant Dyads. [DOI] [PubMed] [Google Scholar]
- Wachman E.M., Schiff D.M., Silverstein M. Neonatal abstinence syndrome: advances in diagnosis and treatment. J. Am. Med. Assoc. 2018;319(13):1362. doi: 10.1001/jama.2018.2640. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Moe V., Slinning K., Siqveland T., Fjell A.M., Bjørnebekk A., Smith L. Effects of prenatal opiate exposure on brain development – a call for attention. Nat. Rev. Neurosci. 2009;10(5) doi: 10.1038/nrn2598-c1. 390–390. [DOI] [PubMed] [Google Scholar]
- Wand G. The mu-opioid receptor gene polymorphism (A118G) alters HPA Axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26(1):106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wand G.S., Weerts E.M., Kuwabara H., Frost J.J., Xu X., McCaul M.E. Naloxone-induced cortisol predicts mu opioid receptor binding potential in specific brain regions of healthy subjects. Psychoneuroendocrinology. 2011;36(10):1453–1459. doi: 10.1016/j.psyneuen.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C.G., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weiss N.H., Tull M.T., Anestis M.D., Gratz K.L. The relative and unique contributions of emotion dysregulation and impulsivity to posttraumatic stress disorder among substance dependent inpatients. Drug Alcohol Depend. 2013;128(1–2):45–51. doi: 10.1016/j.drugalcdep.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman V.E., Salemi J.L., Mogos M.F., Cain M.A., Aliyu M.H., Salihu H.M. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. Journal of Pregnancy. 2014;2014:1–8. doi: 10.1155/2014/906723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B., Baewert A., Jagsch R., Rohrmeister K., Metz V., Aeschbach Jachmann C. Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Eur. Addiction Res. 2009;15(3):150–156. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]
- Zuckermann B., Frank D., Brown E. vol. 149. US Government Printing Office; Washington (DC): 1995. Overview of the effects of abuse and drugs on pregnancy and offspring. (Medications Development for the Treatment of Pregnant Addicts and Their Infants. In NIDA Research Monograph). NIH Publication No.: 95-3891. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.