Abstract
Importance:
Antagonist antibodies to programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have shown remarkable activity in multiple tumor types. Recent US Food and Drug Administration approval of such agents for advanced melanoma, non–small cell lung cancer, and renal cell carcinoma has hastened the need to better characterize their unique toxicity profiles.
Objective:
To provide a clinical and pathologic description of the lichenoid mucocutaneous adverse effects seen in patients receiving anti–PD-1/PD-L1 treatment.
Design, Setting, and Participants:
Patients with advanced cancer who were referred to dermatology at Yale–New Haven Hospital, a tertiary care hospital, after developing cutaneous adverse effects while receiving an anti–PD-1 or PD-L1 antibody therapy either as monotherapy or in combination with another agent were identified. Medical records from 2010 to 2015 and available skin biopsy specimens were retrospectively reviewed.
Main Outcomes and Measures:
Patient demographic characteristics, concurrent medications, therapeutic regimen, type of disease, previous oncologic therapies, clinical morphology of cutaneous lesions, treatment of rash, peripheral blood eosinophil count, tumor response, and skin histologic characteristics if biopsies were available.
Results:
Patients were 13 men and 7 women, with a mean (range) age of 64 (46-86) years. The majority of cases (16 [80%]) had a clinical morphology consisting of erythematous papules with scale in a variety of distributions. Biopsies were available from 17 patients; 16 (94%) showed features of lichenoid interface dermatitis. Eighteen patients were treated with topical corticosteroids, and only 1 patient required discontinuation of anti–PD-1/PD-L1 therapy. Only 4 of 20 patients (20%) developed peripheral eosinophilia. Sixteen patients (80%) were concurrently taking medications that have been previously reported to cause lichenoid drug eruptions.
Conclusions and Relevance:
Papular and nodular eruptions with scale, as well as mucosal erosions, with lichenoid features on histologic analysis were a distinct finding seen with anti–PD-1/PD-L1 therapies and were generally manageable with topical steroids. Concurrent medications may play a role in the development of this cutaneous adverse effect.
Introduction
Immunotherapy represents the next generation of anticancer therapy. Within the last several years, numerous immuno-oncology agents have emerged as effective treatment options for patients with cancer. One immune target of particular interest is programmed cell death 1 (PD-1), an inhibitory molecule found on the surface of T cells that maintains immune tolerance to self-antigens.1 Numerous malignant tumors express programmed cell death ligand 1 (PD-L1), which acts to inhibit antitumor T-cell function,2 allowing cancers to evade the host immune response. Blockade of PD-L1 has been shown to improve immune function of tumor-specific T cells and increase tumor lysis.3
Nivolumab and pembrolizumab are IgG4 antagonist antibodies to PD-1, which can relieve inhibition of tumor-specific T cells, restoring effective antitumor immunity. Both have been approved by the US Food and Drug Administration (FDA) for the treatment of advanced melanoma and non–small-cell lung cancer (NSCLC). Nivolumab was also recently FDA approved for the treatment of metastatic renal cell carcinoma and relapsed or refractory classical Flodgkin lymphoma. Toxicity of anti–PD-1 therapies is primarily related to autoimmunity unmasked by releasing self-protective PD-1 inhibition. Compared with ipilimumab, an antagonist antibody to another immune inhibitory molecule, cytotoxic T lymphocyte–associated protein 4, anti–PD-1 therapy is better tolerated, with less severe autoimmune adverse effects.4 Two of the most common immune-related adverse events (irAEs) with anti–PD-1 therapy are the mucocutaneous adverse effects of rash and pruritus. Antibodies targeting the ligand PD-L1 (eg, atezolizumab and durvalumab) are still under active investigation in clinical trials and show similar dermatologic adverse effects.
Given that these immunotherapeutic agents have only emerged recently, their toxicity profiles are still being fully characterized. In this study, we aim to characterize the clinical and histopathologic features of cutaneous eruptions that developed in a series of patients receiving anti–PD-1 or anti–PD-L1 therapy.
Methods
With the approval of the Yale University Institutional Review Board, cases were collected based on a consecutive list of patients from Yale–New Haven Hospital who were sent to the Yale Oncodermatology Clinic for a dermatology consultation. Data for the cases were collected retrospectively, and informed consent was waived due to the retrospective nature of the study. Patients were included if they were receiving treatment with either an anti–PD-1 or anti–PD-L1 agent alone, or if they were receiving an anti–PD-1 or anti–PD-L1 agent in combination with other therapy, and if they were referred for dermatologic evaluation of rash. Data for patients evaluated between 2010 and 2015 were collected, and included demographic characteristics, concurrent medications, therapeutic regimen, type of disease, previous oncologic therapies, clinical morphology and distribution of cutaneous lesions, treatment of rash, peripheral blood eosinophil count, and tumor response. Concurrent medications at the time of presentation were recorded. The peripheral blood eosinophil count was recorded at the time of biopsy, and for those patients without biopsy, eosinophil count was recorded at the time of presentation of cutaneous toxic effect. Tumor response was determined from documentation from the patients’ treating oncologists and was characterized on the basis of RECIST (Response Evaluation Criteria in Solid Tumors) criteria. Time to disease progression was calculated from the first dose of anti–PD-1/PD-L1 treatment to progression, which was determined by imaging. Any other irAEs that were documented were recorded. The histopathologic features of available biopsy specimens were reviewed by 2 dermatopathologists (N.R., M.B.) and tabulated. For each available case, light microscopic examination of tissue sections prepared with hematoxylin-eosin staining was performed. In addition, for 3 of the cases (numbers 2, 5, and 9), a panel of immunoperoxidase stains, including stains for CD3, CD4, CD8, and CD20, was performed.
Results
A total of 20 patients were included in this study (13 men and 7 women). Ten patients were treated with nivolumab alone, while 4 were treated with nivolumab in combination with ipilimumab. One patient was treated with nivolumab in combination with bevacizumab, and 1 patient was initially treated with nivolumab in addition to erlotinib and then continued taking nivolumab alone. Two patients were treated with pembrolizumab alone, 1 patient was treated with the anti–PD-L1 agent atezolizumab alone, and 1 patient received atezolizumab in combination with carboplatin and paclitaxel. Twelve patients (60%) had received prior systemic therapy for their cancer, with 3 of 20 patients having received prior immune checkpoint inhibitors. One of these patients had already had a previous course of nivolumab and ipilimumab combination therapy, while 2 patients had therapy with ipilimumab. Table 1 summarizes the characteristics of the included patients.
Table 1.
Clinical and Histologic Profile of 20 Patients With Cutaneous Adverse Effects While Receiving Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Treatment
| No./Sex | Cancer Type |
Oncologic Agent |
Prior Therapy |
Time to Rash, mo |
Morphology | Anatomic Distribution |
Pruritus | Treatment Held for Rash |
Treatment of Rash |
Tumor Response |
PFS, mo | Other irAE |
Histologic Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M | Lung | Nivolumab | None | 12.8 | Grover disease | Trunk | Yes | No | Triamcinolone | CR | 33.7a | None | Lichenoid, spongiotic |
| 2/F | Lung | Nivolumab | Carboplatin + gemcitabine, pemetrexed | 1.8 | Papular | Extremities | Yes | No | Clobetasol | PR | 38.0 | None | Lichenoid (CD3+, CD4+ CD8+ CD20−) |
| 3/M | Lung | Nivolumab | Carboplatin + pemetrexed | 1.2 | Papulopustular | Trunk | No | No | Clobetasol, minocycline | PD | 1.7 | None | Lichenoid, spongiotic |
| 4/F | Lung | Nivolumab | None | 4.6 | Papular | Trunk, extremities | Yes | No | Topical steroidsb | PR | 8.9 | Autoimmune diabetes | NA |
| 5/M | RCC | Nivolumab | HD IL-2, bevacizumab | 2.0 | Papular | Generalized | No | No | Topical steroidsb | PR | 9.5 | None | Lichenoid (CD3+, CD4+, CD8+, CD20−) |
| 6/F | RCC | Nivolumab | None | 1.6 | Papular, palmoplantar | Palms, soles, mouth | Yes | Yes | Clobetasol, PUVA | SD | 10.4 | Hypothyroidism, colitis | Lichenoid |
| 7/M | MM | Nivolumab | HD IL-2 | 13.0 | Papular | Chest (shawl-like) | No | No | None | PR | 75.0b | None | Lichenoid, spongiotic |
| 8/M | Lung | Nivolumab | Carboplatin + gemcitabine | 0.8 | Papular | Lower back, left upper arm | Yes | No | Triamcinolone | CR | 32.3 | Possible pneumonitis | Lichenoid, spongiotic |
| 9/F | Lung | Nivolumab | Vinorelbine and cisplatin + cetuximab, cetuximab, gemcitabine, erlotinib, docetaxel + retaspimycin hydrochloride | 10.2 | Mucositis | Mouth | No | Yes | Clobetasol, Valacyclovir | PR | 55.6b | None | Lichenoid, spongiotic (CD3+, CD4+, CD8+, CD20−) |
| 10/M | MM | Nivolumab | None | 0.5 | Erosive lichen planus | Penis, mouth | Yes | No | Clobetasol | SD | 4.2b | None | Lichenoid |
| 11/M | MM | Pembrolizumab | High-dose interferon, ipilimumab | c | Papular | Extremities, trunk | Yes | No | Clobetasol | PD | 3.9 | LFT elevation | NA |
| 12/M | MM | Pembrolizumab | Ipilimumab | 2.1 | Hypertrophic plaques | Lower extremities | Yes | Yes | Clobetasol, Prednisone | PR | 3.5b | LFT elevation | Lichenoid |
| 13/M | MM | Nivolumab + ipilimumab | None | 0.1 (3 d) | Papular | Generalized | Yes | No | Topical steroidsb | PR | 39.5b | None | Lichenoid |
| 14/M | MM | Nivolumab + ipilimumab | Interferon, previous course of nivolumab + ipilimumab | 2.8 | Papular, annular, inflammation of seborrheic keratoses | Extremities, trunk | Yes | No | Triamcinolone | PD | 2.8 | None | Lichenoid, spongiotic |
| 15/M | Lung | Nivolumab + ipilimumab | None | 2.5, 6.0 | Papular, lichenoid keratosis | Left forearm, left upper thigh | Yes | No | Triamcinolone | PR | 10.5b | None | Lichenoid, spongiotic |
| 16/M | Lung | Nivolumab + ipilimumab | None | 4.5 | Papular | Back, extensor arms, upper chest | Yes | No | Triamcinolone | PR | 10.7 | Adrenal insufficiency, acute interstitial nephritis | NA |
| 17/F | Lung | Nivolumab + bevacizumab | Carboplatin + pemetrexed + bevacizumab | 1.5 | Papular | Face, neck, left arm | No | No | Triamcinolone | PD | 2.0 | None | Lichenoid, spongiotic |
| 18/F | Lung | Nivolumab + erlotinib, then nivolumab alone | Erlotinib | 1.9, 2.3d | Papular | Extremities, trunk | Yes | Yes | Prednisone | PR | 35.9 | LFT elevation, low thyrotropin | Vacuolar interface dermatitis |
| 19/F | RCC | Atezolizumab | Sunitinib | 8.3 | Papular, palmar | Palms, arms, mouth | Yes | No | Clobetasol, NBUVB | PR | 16.7 | None | Lichenoid |
| 20/M | Lung | Atezolizumab + paditaxel and carboplatin | None | 3.1 | Papular | Generalized | Yes | Yes | Clobetasol | PR | 6.6b | None | Lichenoid |
Abbreviations: CR, complete response; HD IL-2, high dose interleukin 2; irAE, immune-related adverse effect; LFT, liver function test; MM, metastatic melanoma; NA, not applicable; NBUVB, narrow-band UV-B therapy; PD, progression of disease; PFS, progression-free survival: PR, partial response; PUVA, psoralen and UV-A therapy; RCC, renal cell carcinoma; SD, stable disease.
Response ongoing at time of data collection.
Specific strength of topical steroids unknown.
Exacerbation within 5 d of an existing rash patient had developed while taking ipilimumab.
This patient had 2 acute cutaneous eruptions that appeared to be temporally related to erlotinib administration.
The time of onset to cutaneous eruption was variable, with a mean (range) time of 4 months (3 days to 12.8 months). The majority of cases (16 [80%]) had a clinical morphology consisting of erythematous papules with scale, in either a focal distribution such as localized lesions on an extremity, neck, or chest (11 [55%]) (patient number 4) (Figure 1A), or in a more generalized distribution of coalescing larger plaques on the trunk and extremities (9 [45%]). Other clinical morphologies were variable, ranging from keratotic plaques resembling hypertrophic lichen planus (patient 12) (Figure 1B) to discrete papules on the trunk that looked typical of Grover disease, or transient acantholytic dermatosis (patient number 1). Of note, 2 patients (numbers 6 and 19) (Figure 1D and E) had papules and plaques limited to a striking palmoplantar distribution with additional oral mucosal lesions. Four patients (numbers 6, 9, 10, 19) developed oral lesions that varied in appearance involving the tongue, buccal mucosa, lips, and/or gingivae. One patient (number 6) developed 1- to 2-mm whitish flat-topped papules with apparent Wickham striae on the bilateral buccal mucosae extending onto the lateral commissures, whereas the other patients developed erosions resembling oral lichen planus. Other unique presentations included inflammation of existing seborrheic keratoses (patient 14) (Figure 1C) and erosive lesions on the penis, clinically resembling erosive genital lichen planus (patient 10) (Figure 1F).
Figure 1. Cutaneous Eruptions Consisting of Erythematous Papules With Scale Due to Anti–Programmed Cell Death 1 and Anti–Programmed Cell Death Ligand 1 Therapy.
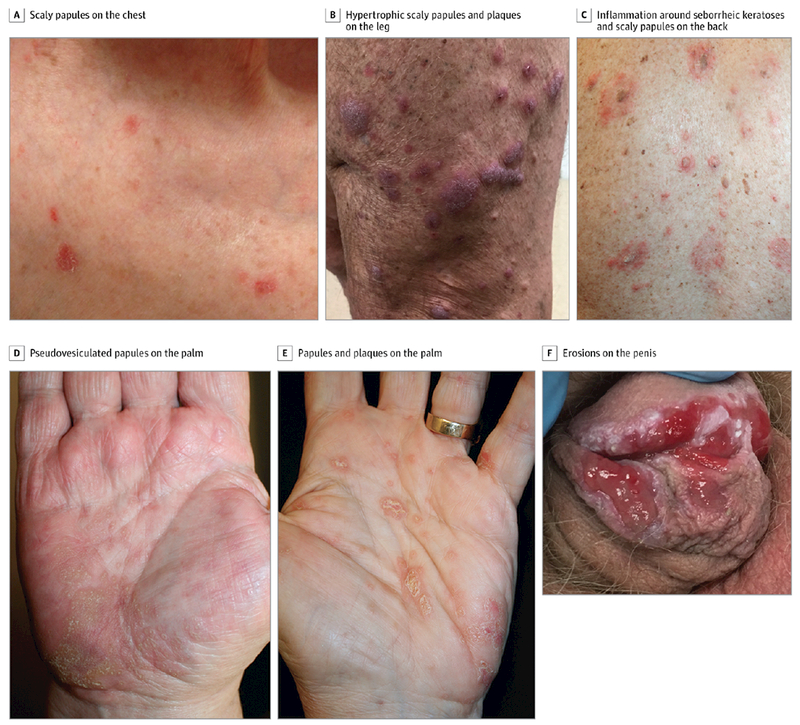
A, Small number of discrete scaly papules on the chest (patient number 4). B, Hypertrophic scaly papules and plaques on the lower extremity (patient number 12). C, Inflammation of and around existing seborrheic keratoses, in addition to new-onset scaly papules, on the back (patient number 14). D, Coalescent pseudovesiculated papules on the palm (patient number 6). E, Scaly, discrete papules and plaques on the palm (patient number 19). F, Numerous erosions on the penis, resembling erosive lichen planus (patient number 10).
Most patients (15 [75%]) were noted to experience pruritus with the lesions. The most common treatment was topical corticosteroids. One patient (number 18) who developed 2 acute eruptions that appeared temporally related to erlotinib administration required oral prednisone treatment. The 2 patients who developed palmoplantar lesions (numbers 6 and 19) were treated with phototherapy, 1 with psoralen and UV-A, and the other with narrow-band UV-B, with improvement. Five patients (25%) required dose delay of the oncologic agent due to cutaneous adverse effects. Eosinophil counts were substantially elevated in only 4 patients (20%) at the time of cutaneous eruptions. The majority of patients (16 [80%]) were taking concurrent medications that have been previously reported to cause lichenoid drug eruptions. Table 2 lists the concurrent medications at the time of presentation and the absolute eosinophil counts in patients at time of biopsy or at time of presentation of cutaneous eruption if biopsy was not performed.
Table 2.
Concurrent Medications and Peripheral Eosinophil Counts
| Patient No. | Concurrent Medications | Serum Eosinophils (Absolute Count, Cells/μL) | |
|---|---|---|---|
| Not Reported to Cause Lichenoid Drug Eruptions | Reported to Cause Lichenoid Drug Eruptions5,6(p616) | ||
| 1 | Brimonidine, cholecalciferol, coenzyme Q10, iron, loperamide, tetrahydrozoline, nitroglycerin | Clopidogrel, metformin, metoprolol, simvastatin, aspirin | 1050a |
| 2 | Coumadin, amiodarone | Aspirin, metformin | 104 |
| 3 | Rosuvastatin, zolpidem | … | 504a |
| 4 | Insulin | … | 0 |
| 5 | Chlorthalidine | Lorazepam, amlodipine, atenolol | 212 |
| 6 | Montelukast, diphenhydramine | Tiotropium, metoprolol, hydrochlorothiazide | 252 |
| 7 | Levothyroxine, tamsulosin | Hydrochlorothiazide | 747a |
| 8 | Ipratropium-albuterol, oxycontin, oxycodone-acetaminophen, fluticasone/salmeterol, rosuvastatin, fenofibrate | Tiotropium, alprazolam, aspirin | 138 |
| 9 | … | Ibuprofen | 84 |
| 10 | Prochlorperazine, sertraline, mirtazapine | Omeprazole, allopurinol, atorvastatin, naproxen | 72 |
| 11 | Nitroglycerin | Aspirin, atorvastatin, glipizide, lisinopril, metformin, metoprolol | 930a |
| 12 | Celecoxib, levetiracetam, phenobarbital, vitamin B12 | … | 135 |
| 13 | Vitamin D | … | 310 |
| 14 | Zolpidem | Aspirin, ibuprofen, omeprazole | 126 |
| 15 | Cholecalciferol, rivaroxaban, famotidine, moxifloxacin | Atorvastatin, colchicine | 304 |
| 16 | Albuterol, famotidine, hydrocortisone, hydroxyzine, zolpidem, levetiracetam | Aspirin, lorazepam, omeprazole | 150 |
| 17 | Mirtazapine, morphine | Lorazepam | 66 |
| 18 | Eszopiclone | Sertraline | 208 |
| 19 | Levothyroxine, bupropion | Omeprazole, sertraline | 159 |
| 20 | Acetaminophen, bupropion, tadalafil, digoxin, fluticasone-salmeterol, morphine, ondansetron, prochlorperazine, rivaroxaban | Atorvastatin, metoprolol, omeprazole, tiotropium | 0 |
SI conversion factor: To convert eosinophils to billions per liter, multiply by 0.001.
Peripheral eosinophilia, defined as greater than 500 cells/μL.
Tumor response, time to progression, and development of any other irAEs were also assessed (Table 1). Of 6 patients with melanoma, 3 had a partial response, 1 had stable disease, and 2 had progression of disease. Of 11 patients with NSCLC, 2 patients had complete response, 7 had a partial response, and 2 had progression of disease. Of 3 patients with renal cell carcinoma, 2 patients had a partial response, and 1 patient had stable disease. The mean progression-free survival (PFS) was 20.1 months, with a wide range between 1.7 and 75.0 months. This large range was due to prolonged PFS (mean, 26.9 [range, 3.5-75.0] months) in those patients who experienced tumor response, compared with a much shorter PFS (4.2 [range, 1.7-10.4] months) in patients who had either stable disease or progression.
Histologic analysis was available from 17 of the 20 patients. Nearly all cases (16 of 17 [94%]) showed features of lichenoid interface dermatitis (Figure 2A-C). In addition, many of the cases also showed features of spongiotic dermatitis (8 of 17 [47%]). One case, the patient (number 18) who developed an acute eruption in temporal association with erlotinib administration, showed evidence of vacuolar interface changes. Of the 3 biopsies for which ancillary immunostaining was performed, all showed intradermal and intraepithelial lymphocytes that were CD3 positive (Figure 2D). Intradermal lymphocytes were CD4 positive, while intraepithelial lymphocytes were CD8 positive; CD20 stains had negative results (Figure 2E-G). Table 1 summarizes the histopathological features of each skin biopsy.
Figure 2. Photomicrographs Showing Lichenoid Interface Dermatitis.
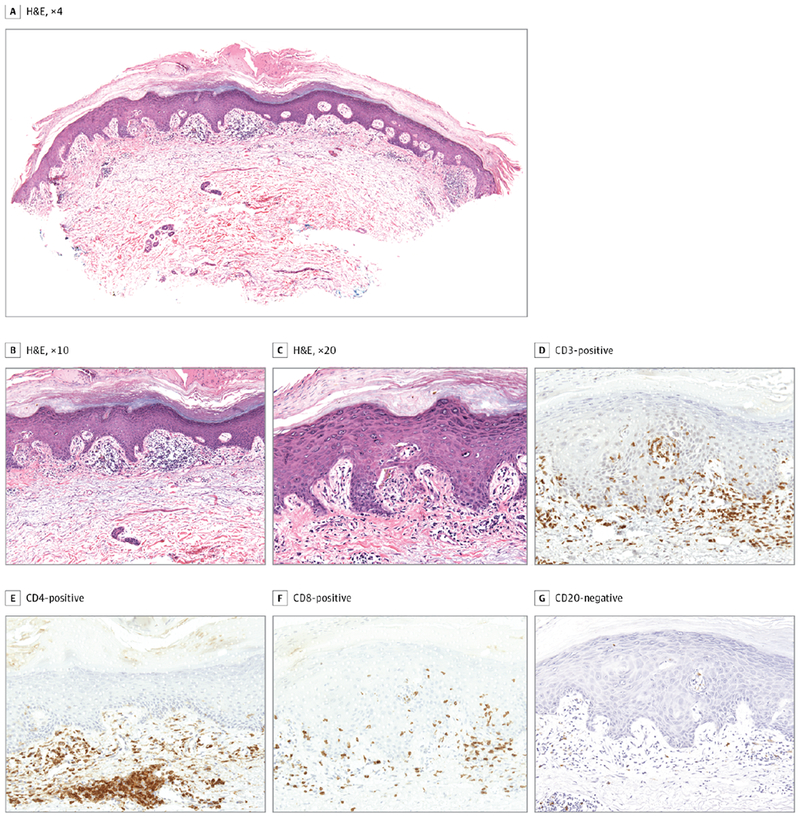
A-C, Hematoxylin-eosin (H&E) staining, original magnification ×4, ×10, and ×20, respectively. Staining of lymphocytic infiltrate revealed the following immunoprofile: D, CD3-positive (both intradermal and intraepithelial lymphocytes); E, CD4-positive (intradermal lymphocytes); F, CD8-positive (intraepithelial lymphocytes); and G, CD20-negative.
Discussion
Cutaneous adverse effects associated with treatment with anti–PD-1 antibodies most commonly include rash (4%-27% of patients), pruritus (2%-23%), and less frequently vitiligo (5%-11 %),7–11 with comparable incidences seen with pembrolizumab and nivolumab use. Similar adverse effects are seen with anti–PD-L1 antibody therapy, including pruritus (25%) and rash (16%).12 These adverse effects are usually manageable and do not generally require discontinuation of therapy.
Whereas “rash” has been commonly reported as an adverse effect in many oncologic trials evaluating treatment with anti–PD-1/PD-L1 antibodies, further details about the specific nature of these cutaneous eruptions are often not completely described. Our study aimed to characterize both the clinical and histological features of cutaneous adverse effects associated with anti–PD-1/PD-L1 therapy. Clinically, the eruption seen with use of these agents consisted of erythematous scaly papules or plaques that were usually pruritic. The distribution of lesions varied, with either a small number of discrete papules or plaques on a limited area of the body or a generalized distribution of larger plaques with a predilection for the trunk. There was also a wide range in time to cutaneous presentation after initiation of anti–PD-1/PD-L1 therapy, from 3 days to 13 months. For those patients with delayed eruptions up to 1 year into therapy, no other identifiable triggers were noted. In a recent publication, cutaneous adverse effects with onset up to 60 weeks after treatment initiation with anti–PD-1 therapy have been described.13
Although the clinical morphology varied, a striking finding was that the histologic features were remarkably consistent among the patients. Nearly all of the cases for which biopsies were performed in our study (16 [94%]) showed lichenoid interface changes. Three biopsies for which immunohistochemical staining was available showed that the lichenoid infiltrate was composed of predominantly CD4-positive T cells within the dermis, with a few CD8-positive intraepithelial lymphocytes. In addition, many showed concurrent features of spongiotic dermatitis, an atypical finding when lichenoid interface changes are appreciated. A previous case series reported similar findings of lichenoid dermatitis on histologic analysis in 3 patients receiving pembrolizumab as treatment for melanoma.14 Clinically, the patients presented with papular lesions as well, primarily on the trunk and extremities, between 4 and 9 weeks after starting treatment with pembrolizumab. Two of these patients had previously received immunotherapy with ipilimumab. All 3 cases showed a CD3-positive lymphocytic infiltrate, with a more prominent CD4 component than CD8; 10% of the T cells expressed PD-1. Tumor response was noted in 2 of the 3 patients, and consisted of 1 partial and 1 complete response. All 3 patients had relatively mild adverse effects, and oncologic treatment was not discontinued. In another recent case series of 5 patients treated with anti–PD-1/PD-L1 agents, histologic examination revealed lichenoid dermatitis with greater histiocytic infiltrates, increased spongiosis, and increased epidermal necrosis, compared with biopsies of non–drug-related lichen planus and lichen planus–like keratoses.15 Our results are consistent with these, showing a cutaneous lichenoid eruption that is unique to anti–PD-1/PD-L1 therapy.
Another noteworthy finding was that most cutaneous eruptions were mild and were managed adequately with topical corticosteroids. Only 1 patient (number 12) developed hypertrophic plaques on the extremities that did not substantially improve with administration of topical steroids or oral prednisone, and required complete discontinuation of anti–PD-1 therapy due to the severity of his cutaneous lesions. Only 4 other patients required doses to be held, including 2 who developed oral lesions, but these patients were able to restart oncologic treatment, with eventual resolution of their cutaneous lesions. Most patients did not need to discontinue or interrupt oncologic therapy, even when presenting with mucosal lesions.
Several patients in this study were being treated with anti–PD-1 or anti–PD-L1 therapy with other concurrent medications. While ipilimumab also causes a cutaneous eruption consisting of erythematous papules coalescing into thin plaques, it is usually associated with a concurrent increase in peripheral blood eosinophil levels.16 Eosinophilia was not seen in the majority of patients in our series or in the 4 patients who specifically received ipilimumab. Furthermore, the lichenoid changes on histologic analysis of the patients in our series are distinct from the superficial, perivascular CD4-predominant infiltrate with eosinophils that has been previously described in ipilimumab-related eruptions. Lichenoid eruptions have not previously been reported with use of ipilimumab, bevacizumab, epidermal growth factor receptor inhibitors such as erlotinib, or traditional cytotoxic chemotherapies such as carboplatin or paclitaxel. Thus, it seems likely that these lichenoid eruptions are associated with anti–PD-1 therapy. In addition, the clinical appearance and lichenoid changes on histologic analysis are consistently seen among both anti–PD-1 agents, nivolumab and pembrolizumab, in addition to anti–PD-L1 agents, supporting the idea that this cutaneous reaction may be a direct, on-target effect on the PD-1/PD-L1 pathway rather than a nonspecific hypersensitivity reaction.
The mechanism through which anti–PD-1/PD-L1–induced drug eruptions occur remains to be elucidated. The PD-1 pathway has been implicated to play an important role in the induction and/or maintenance of tolerance. Subsequent work has examined the mechanisms by which PD-1 and its ligands can control self-reactive T-cell responses.17 Perhaps the focal distribution seen in some of our patients suggests an underlying “unmasking” of an immune response to a preexisting antigen that is localized to a specific site in the body. Only once there is blockade of the PD-1 pathway does the body now produce an inflammatory response to this antigen. These findings may have implications for the pathogenesis of lichen planus, a T-cell–mediated disease that bears a clinical resemblance to the lesions seen in our patients. Lichen planus can also affect the oral mucosa, and blockade of the PD-1/PD-L1 pathway significantly increases the proliferation of peripheral blood T cells in oral lichen planus, suggesting an inhibitory role of PD-1.18 Histologically, lichen planus also shows a similar lichenoid interface dermatitis, with a dense, bandlike lymphohistiocytic infiltrate at the dermal-epidermal junction. Interestingly, the majority (16 of 20 [80%]) of patients in this series were also receiving concurrent medications that have been reported in the literature to cause lichenoid drug reactions (Table 2). These patients had all previously tolerated these medications, and the fact that anti–PD-1/PD-L1 therapy was the only new medication for these patients suggests that it may be the drug culprit. An alternative explanation may be that the administration of an anti–PD-1 or PD-L1 therapeutic agent may have unmasked an immune response to a medication that was previously tolerated, resulting in these lichenoid eruptions. Interestingly, 1 patient (number 18) seemed to develop acute rashes that were temporally related to erlotinib administration, even though she had previously tolerated a course of erlotinib without such dermatologic adverse effects 2 years prior, possibly representing an activation of the immune system by anti–PD-1 therapy to mount a more exuberant inflammatory response.
There is evidence that development of cutaneous adverse effects during anti–PD-1 therapy is associated with longer PFS,19 tumor response,20 and overall survival.21 In our group, 5 of 6 patients (83%) with NSCLC treated with anti–PD-1 or PD-L1 monotherapy showed a response, compared with the typical response rates of 14% to 20% with nivolumab7,22 and 19% with pembrolizumab.23 Six of 20 patients (30%) developed other definitive irAEs that were associated with therapy. Four of these 6 patients showed a response to therapy, which may suggest a possible association between irAE development and clinical response. Given the small number of patients, definitive conclusions about the association of cutaneous adverse effects with tumor response in this group cannot be drawn, but further research into this area is intriguing.
Conclusions
There appears to be a range of clinical presentations and distributions of the cutaneous adverse effects seen with anti–PD-1/PD-L1 agents, but the eruption is typically papular in morphology with associated scale. The lichenoid pattern on histologic analysis is a remarkably consistent finding and appears to be a distinct feature compared with cutaneous reactions seen with other immunotherapies. Notably, the eruptions are usually relatively mild and can be typically adequately managed with topical corticosteroids. Future investigation is needed to determine whether there is an association between cutaneous adverse effects or other irAEs and tumor response. This series of patients adds further characterization to the emerging toxicity profiles of anti–PD-1/PD-L1 therapies.
Key Points.
Question:
What are the features of the cutaneous adverse effects associated with anti–programmed cell death 1 and anti–programmed cell death ligand 1 therapy?
Findings:
In this case series of 20 patients, the clinical morphology of cutaneous eruptions consisted of erythematous papules with scale, with skin histologic analysis predominantly showing lichenoid interface changes.
Meaning:
There is a distinct cutaneous lichenoid eruption associated with anti–programmed cell death 1 and anti–programmed cell death ligand 1 therapy.
Footnotes
Conflict of Interest Disclosures: Drs Gettinger and Choi have served as advisory board members for Bristol-Meyers Squibb. No other disclosures are reported.
References
- 1.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192(7): 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003; 170(3): 1257–1266. [DOI] [PubMed] [Google Scholar]
- 3.Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006; 119(2):317–327. [DOI] [PubMed] [Google Scholar]
- 4.Weide B, Di Giacomo AM, Fonsatti E, Zitvogel L. Immunologic correlates in the course of treatment with immunomodulating antibodies. Semin Oncol 2015;42(3):448–458. [DOI] [PubMed] [Google Scholar]
- 5.Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Scientific World Journal. 2014;2014:742826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litt JZ. Litt’s Drug Eruption Reference Manual. 14th ed. London, England: Informa Healthcare; 2008. [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33(18):2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. [DOI] [PubMed] [Google Scholar]
- 10.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16(8):908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 12.Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol 2013;31 (suppl):abstr 9010. [Google Scholar]
- 13.Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy [published online March 8, 2016]. Clin Cancer Res doi: 10.1158/1078-0432.CCR-15-2872. [DOI] [PubMed] [Google Scholar]
- 14.Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res 2015;3(1):18–22. [DOI] [PubMed] [Google Scholar]
- 15.Schaberg KB, Novoa RA, Wakelee HA, et al. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J Cutan Pathol 2016;43(4):339–346. [DOI] [PubMed] [Google Scholar]
- 16.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol 2006;142(2):166–172. [DOI] [PubMed] [Google Scholar]
- 17.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou G, Zhang J, Ren XW, Hu JY, Du GF, Xu XY. Increased B7-H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J Clin Immunol 2012;32(4):794–801. [DOI] [PubMed] [Google Scholar]
- 19.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151(11):1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152(1):45–51. [DOI] [PubMed] [Google Scholar]
- 21.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016;22(4):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]


