Abstract
Background
Neuroendocrine cell carcinoma is a rare variant of esophageal carcinoma. The characteristic clinical features and diagnosis of superficial neuroendocrine cell carcinoma remain to be established. We report a rare case of superficial coexistence of neuroendocrine cell carcinoma with squamous cell carcinoma treated by endoscopic submucosal dissection, and regional lymph node metastasis was detected after additional surgical treatment.
Case presentation
A 77-year-old Japanese man with esophageal squamous cell carcinoma received endoscopic submucosal dissection in en-bloc resection. Histopathological findings showed that lymphovascular invasion by the neuroendocrine cell carcinoma component occurred in the deep part of the muscularis mucosa. Regional lymph node metastasis was identified after additional surgical treatment. After surgical treatment, our patient received chemotherapy consisting of etoposide and carboplatin for 3 months. He is alive and shows no sign of disease recurrence 12 months after surgery.
Conclusions
This case report highlights the fact that even if neuroendocrine cell carcinoma is small and limited to superficial, the tumor has the potential for metastasis if lymphovascular invasion by the neuroendocrine cell carcinoma component occurs. In addition, this case indicates the necessity of close follow-up of small neuroendocrine cell carcinoma after treatment.
Keywords: Neuroendocrine cell carcinoma, Esophagus, Squamous cell carcinoma, Endoscopic submucosal dissection, Metastasis
Background
Neuroendocrine cell carcinoma (NEC) is rare variant of esophageal carcinoma. NEC is less common than squamous cell carcinoma (SCC) and adenocarcinoma, and it is a relatively rare disease with a reported incidence between 0.4% and 2% [1–3]. The prognosis of NEC of the esophagus is poor because the tumor is often at an advanced stage of disease at diagnosis. Superficial NEC of the esophagus is rare. To the best of our knowledge, only a few superficial NECs of the esophagus have been reported in the English literature [4]. Accordingly, the characteristic endoscopic features and diagnosis of superficial NEC remain to be established.
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) offer non-invasive treatment for esophageal cancer limited to the mucosa and without lymph node metastasis [5]. ESD has a significantly higher curative resection rate and lower local recurrence rate than EMR, particularly in lesions less than 2 cm [6]. However, whether endoscopic treatment is sufficient for disease control for superficial NEC is unclear.
We report a rare case of superficial coexistence of NEC with SCC treated by ESD, and regional lymph node metastasis was detected after additional surgical treatment.
Case presentation
A 77-year-old Japanese man presented to our hospital with esophageal mucosal abnormality in the middle thoracic esophagus. This abnormality was discovered in a barium study for a health checkup. His medical history was significant for primary hypertension. He reported a 50-year history of smoking 8–10 cigarettes per day. There was no family history. Physical and neurological examinations were unremarkable. Esophagogastroduodenoscopy showed 20-mm, reddish, elevated, and flat lesions in the middle thoracic esophagus (Fig. 1a). Narrow band imaging (NBI) endoscopy showed dot-like microvessels in elevated lesions (Fig. 1b, c). However, the microvascular pattern showed irregular, fine, reticular blood vessels, of Japan Esophageal Society (JES) classification type R, near the center of the lesions (Fig. 1d). Magnifying endoscopy with NBI revealed type B1 in elevated area, and type R near the center of the lesion in the JES classification. Endoscopic ultrasound showed that the lesion was localized in the mucosa (Fig. 1e). Therefore, this area of invasion was: cancer limited to the epithelium (EP)/cancer invading into the lamina propria (LPM) to cancer invading into the muscularis mucosa (MM). Biopsies showed SCC of the esophagus. Computed tomography (CT) showed no evidence of lymph node and distant metastases. En bloc resection of the tumor was performed successfully by esophageal ESD without any complications (Fig. 1f).
Fig. 1.
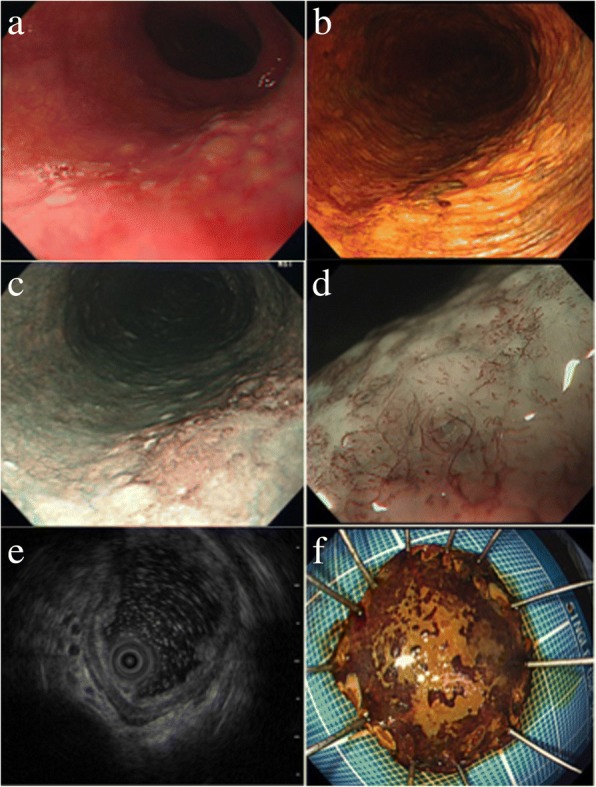
a Esophagogastroduodenoscopy shows an irregular, reddish, flat lesion in the posterior wall of the mid-esophagus. b Lugol’s iodine chromoendoscopy shows an unstained lesion that is located in the posterior wall of the mid-esophagus. c Narrow band imaging endoscopy shows a brownish area. d Magnifying endoscopy with narrow band imaging shows a microvascular pattern with irregular, fine, reticular blood vessels near the center of the lesion. e Endoscopic ultrasound image of the lesion limited to the mucosa. f En bloc resection by endoscopic submucosal dissection
Histopathological findings showed an admixture of endocrine cell tumor with SCC with an invasion depth into the muscularis mucosa (Fig. 2a). No complications were related to the procedure. Immunohistochemical analysis showed positivity for CD56, chromogranin, and synaptophysin in the NEC component (Fig. 2b–f). Small cell type NEC was arranged in a sheet fashion existing in the center of the tumor, and these were partially surrounded by well-differentiated SCC. Lymphovascular invasion of the NEC component occurred in the deep part of the muscularis mucosa. Our patient underwent additional surgical treatment consisting of video-assisted thoracoscopic esophagectomy, three-field lymph node dissection from the cervix, mediastinum, and abdomen, and gastric conduit construction. Regional lymph node metastasis was identified in 1 of 76 nodes (number 108 lymph node), and the node metastasis stage was pN1. This lymph node contained NEC and no metastasis of SCC (Fig. 3a–d). After surgical treatment, he received chemotherapy consisting of etoposide and carboplatin for 3 months. He is alive and shows no sign of disease recurrence 12 months after surgery.
Fig. 2.
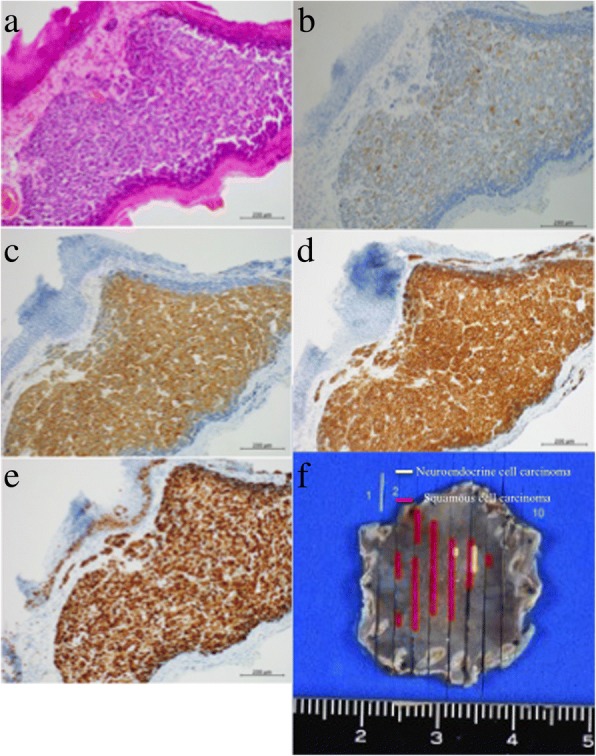
Resected specimen by endoscopic submucosal dissection. This specimen shows neuroendocrine cell carcinoma arranged in a sheet fashion with mixed squamous cell carcinoma. Neuroendocrine cell carcinoma formed a duct and it is surrounded by squamous cell carcinoma. a Hematoxylin and eosin staining. Immunohistochemical staining showing: b chromogranin A, c synaptophysin, d CD56, and e Ki-67. f The fixed resected specimen is mapped by yellow and red lines. Red lines squamous cell carcinoma, yellow lines neuroendocrine cell carcinoma
Fig. 3.
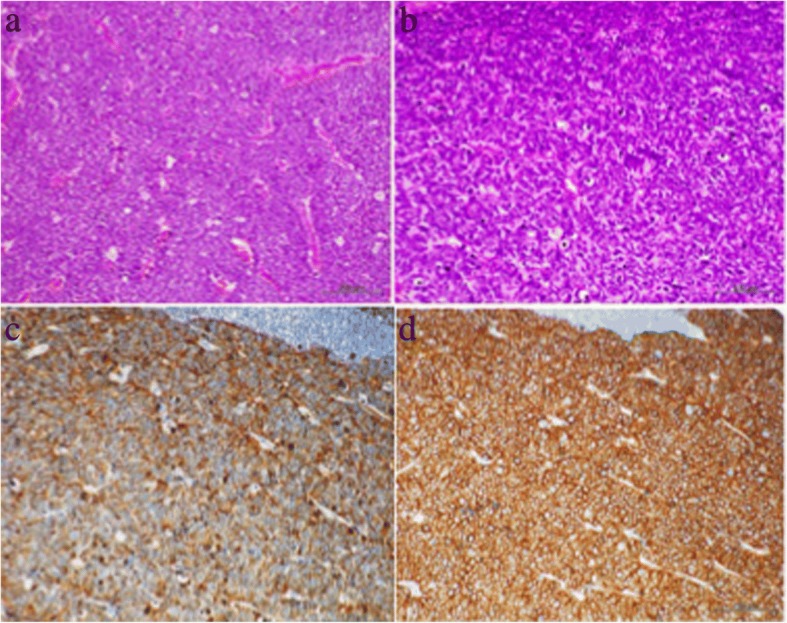
Histological examination of a resected lymph node. a Low-power view and b high-power view (hematoxylin and eosin staining). Immunohistochemical staining shows: b chromogranin A, c synaptophysin, and d CD56
Discussion
NEC was formerly called small cell carcinoma. The first description of small cell carcinoma of the esophagus was reported in 1952 by McKeown [7]. The World Health Organization (WHO) definition for NEC includes positive endocrine markers, such as CD56, chromogranin A, and synaptophysin. A Ki-67 or mitotic index of 20% or higher is also necessary for diagnosing NEC. Tumors with less than 20% Ki-67 positivity are diagnosed as a neuroendocrine tumor [8].
NEC is categorized into two morphological types of small cell type and large cell type, and coexistence of SCC and/or adenocarcinoma is also often observed [9]. The small cell type is more aggressive than the large cell type, and it is frequently found in the advanced stage with lymph node and distant metastases [10].
We ascertained two clinically important issues based on the findings in our case. With regard to the first clinical issue, a small neuroendocrine tumor may occur with lymph node metastasis at an early stage, and attention should be paid to metastasis. Neuroendocrine tumors of the esophagus more frequently present in the middle of the esophagus and stain positive for CD56, synaptophysin, and chromogranin A [1, 11]. These tumors are slow growing, but high-grade neuroendocrine tumors of the esophagus have a poor prognosis and a 5-year survival of approximately 25% [1, 11]. The therapeutic strategy for NEC of the esophagus has not been well defined because of the small number of cases [10]. To date, there has only been one case of collision of SCC and NEC of the esophagus treated by ESD [4].
Saddoughi et al. performed a retrospective analysis of malignant esophageal cancers including NEC [12]. They found that 1-year survival after surgical resection for rare types of malignant esophageal cancers was 69%, 5-year survival was 43%, and 10-year survival was 37%. Surgical resection for rare types of esophageal malignancies should be considered part of effective treatment. In our case, a resected lymph node contained NEC without an SCC component, and regional lymph node metastasis was identified at the early stage of the NEC. Which patients will benefit from additional therapy at the early stage of NEC has not been well established. Therefore, additional therapy, such as esophagectomy and chemoradiotherapy, is required if histology from endoscopic resection shows a tumor invasion depth into the muscularis mucosa with lymphovascular invasion by NEC components.
Concerning the second clinical issue, the diagnosis of NEC before treatment is difficult. Table 1 summarizes cases of esophageal endocrine cell carcinoma treated by endoscopic resection. Characteristic endoscopic features of NEC include submucosal growth, which is usually covered by normal epithelium with or without an ulcerous lesion in the center [8]. Biopsy sometimes fails to reach any diagnosis of NEC before treatment [4]. In our case, the microvascular pattern showed irregular, fine, reticular blood vessels near the center of the lesion. Vessels with a reticular pattern are defined as plexiform microvessels [13]. This vascular pattern is often found in invasive SCC or a non-SCC type of malignant epithelial neoplasm (for example, basaloid carcinoma, adenosquamous carcinoma, and endocrine tumor) with an infiltrative growth pattern [13, 14]. Therefore, vessels with a reticular pattern are not a specific sign for diagnosis of an early stage of NEC, but physicians have to consider the possibility of non-SCC types of malignancies.
Table 1.
Cases of superficial esophageal endocrine cell carcinoma treated by endoscopic resection
| Case | Author and reference number | Year | Age | Sex | Resection method | Size, mm | Morphology | Depth | Lymphovascular invasion | Treatment after ER | Recurrence after ER, duration after ER (months) | Prognosis, period (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Takeshita et al. [15] | 2000 | 73 | F | EMR | 5 | 0–IIa | Muscularis mucosa (M3) | None | Carboplatin + etoposide | None | Alive, no recurrence, 28 |
| 2 | Ozawa and Wachi [16] | 2009 | 78 | M | ESD | 7 | 0–IIa | Shallow submucosa (SM1) | None | CPT-11 + CDDP | Abdominal lymph node metastasis, 9 | Alive, 9 |
| 3 | Kobayashi et al. [17] | 2011 | 61 | M | ESD | 22 | 0–IIc | Shallow submucosa (SM1) | Yes | 5-FU + CDDP, additional surgery, FP + docetaxel, and radiation therapy | Lymph node metastasis around thoracic aorta, 14 | Dead, 25 |
| 4 | Watanabe et al. [4] | 2014 | 55 | M | ESD | 30 | 0–IIa + IIc | Deep submucosa | Yes | CPT-11 + CDDP | None | Alive, 55 |
| 5 | Present case | 2017 | 77 | M | ESD | 20 | 0–IIa | Muscularis mucosa (M3) | Yes | Carboplatin + etoposide | Regional lymph node metastasis, 1 | Alive, 12 |
5-FU 5-fluorouracil, CDDP cisplatin, CPT-11 irinotecan, EMR endoscopic mucosal resection, ER endoscopic resection, ESD endoscopic submucosal dissection, F female, FP 5-Fluorouracil plus cisplatin, M male, M3 muscularis mucosae, SM1 submucosa
Conclusions
In summary, we report a rare case of small superficial NEC showing regional lymph node metastasis. This case report highlights the fact that even if a NEC is small and limited to superficial, the tumor has the potential for metastasis if lymphovascular invasion by the NEC component occurs. In addition, this case indicates the necessity of close follow-up of small NEC after treatment.
Acknowledgements
We thank Nobuya Ohara for skillful technical assistance.
Availability of data and materials
Data sharing is not applicable to this article, because no datasets were generated or analyzed during the present study.
Abbreviations
- CT
Computed tomography
- EMR
Endoscopic mucosal resection
- EP
Cancer limited to the epithelium
- ESD
Endoscopic submucosal dissection
- JES
Japan Esophageal Society
- LPM
Cancer invading into the lamina propria
- MM
Cancer invading into the muscularis mucosa
- NBI
Narrow band imaging
- NEC
Neuroendocrine cell carcinoma
- SCC
Squamous cell carcinoma
- WHO
World Health Organization
Authors’ contributions
SF was the major contributor to writing the manuscript. Endoscopic treatment was performed by MN and TY. The surgical treatment was performed by MK and AM. AY, AD, and HK supported the entire scope of work. TM reviewed and approved the final version of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shintaro Fujihara, Phone: +81-87-723-3111, Email: joshin@med.kagawa-u.ac.jp.
Masahiko Kobayashi, Email: koba.mm@soleil.ocn.ne.jp.
Masako Nishi, Email: kwmkwm@kagawah.johas.go.jp.
Tatsuo Yachida, Email: tyachida@med.kagawa-u.ac.jp.
Akira Yoshitake, Email: sannai@kms.ac.jp.
Akihiro Deguchi, Email: akihiro41@me.com.
Atsushi Muraoka, Email: marugame@kagawah.johas.go.jp.
Hideki Kobara, Email: kobara@med.kagawa-u.ac.jp.
Tsutomu Masaki, Email: tmasaki@med.kagawa-u.ac.jp.
References
- 1.Huang Q, Wu H, Nie L, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467–483. doi: 10.1097/PAS.0b013e31826d2639. [DOI] [PubMed] [Google Scholar]
- 2.Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013;20:4239–44. doi: 10.1245/s10434-013-3167-3. [DOI] [PubMed] [Google Scholar]
- 3.Yun JP, Zhang MF, Hou JH, et al. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer. 2007;7:38. doi: 10.1186/1471-2407-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe K, Hikichi T, Sato M, et al. A case of endocrine cell carcinoma combined with squamous cell carcinoma of the esophagus resected by endoscopic submucosal dissection. Fukushima J Med Sci. 2014;60:187–191. doi: 10.5387/fms.2014-2. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Sato Y, Sugaya S, et al. Endoscopic mucosal resection for early-stage gastrointestinal cancers. Best Pract Res Clin Gastroenterol. 2005;19:871–887. doi: 10.1016/j.bpg.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20:5540–5547. doi: 10.3748/wjg.v20.i18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeown F. Oat-cell carcinoma of the oesophagus. J Pathol Bacteriol. 1952;64:889–891. doi: 10.1002/path.1700640420. [DOI] [PubMed] [Google Scholar]
- 8.Rindi G, Arnold R, Bosman FT, Capella C, Kilmstra DS, Klöppel G, Komminoth P, Solcia E. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 9.Egashira A, Morita M, Kumagai R, et al. Neuroendocrine carcinoma of the esophagus: clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12:e0173501. doi: 10.1371/journal.pone.0173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730–2739. doi: 10.1200/JCO.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 11.Lee CG, Lim YJ, Park SJ, et al. The clinical features and treatment modality of esophageal neuroendocrine tumors: a multicenter study in Korea. BMC Cancer. 2014;14:569. doi: 10.1186/1471-2407-14-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saddoughi SA, Taswell J, Harmsen WS, et al. Surgical resection of rare esophageal cancers. Ann Thorac Surg. 2016;101:311–315. doi: 10.1016/j.athoracsur.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Arima M, Tada M, Arima H. Evaluation of microvascular patterns of superficial esophageal cancers by magnifying endoscopy. Esophagus. 2005;2:191–197. doi: 10.1007/s10388-005-0060-6. [DOI] [Google Scholar]
- 14.Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–112. doi: 10.1007/s10388-016-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeshita S, Akahosi R, Sagara K, Oda T, Kiyozumi T, Kurano R, Mera K. A case of small cell carcinoma of the esophagus treated by endoscopic mucosal resection followed by chemotherapy (in Japanese with English abstract) Gastroenterol Endosc. 2000;42:258–261. [Google Scholar]
- 16.Ozawa T, Wachi E. Endocrine cell carcinoma of the esophagus resected by ESD after magnifying endoscopy with narrow band imaging (in Japanese with English abstract) Gastroenterol Endosc. 2009;51:2437–2446. [Google Scholar]
- 17.Kobayashi K, Aoki T, Nishioka K, Takachi K, Ko-mori T, Hatano H, Yoshida K, Hayashi E. A case of esophageal endocrine cell carcinoma that recurred the lymph nodes around the thoracic aorta after ESD (in Japanese with English abstract) Gastroenterol Endosc. 2011;53:28–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, because no datasets were generated or analyzed during the present study.


