Abstract
Introduction:
The risk for development of a second primary lung cancer (SPLC) after treatment of an initial primary lung cancer (IPLC) is around 1% to 2% per patient per year. The present screening and surveillance guidelines do not adequately address this particular patient population.
Methods:
We retrospectively reviewed patients in the Surveillance, Epidemiology, and End Results database from 1992 to 2007 to assess the frequency of occurrence of SPLC with regard to multiple patient demographics and calculated standardized incidence ratios (SIRs).
Results:
The SIRs for SPLCs were high for both men and women at any age but highest if the IPLC occurred at a younger age. Women had the highest SIR values irrespective of age and race, with the highest SIR reported for the youngest age group (20–49 years) (SIR = 15.26, 95% confidence interval: 12.81–18.04). The rate of SPLC development was 1.10% per patient per year, with median time intervals between the IPLC and SPLC diagnoses of 59 and 62 months, respectively, for men and women. The cumulative risk for development of SPLC increased over time and did not plateau.
Conclusions:
These findings suggest that there is a continued risk for development of SPLC. Surveillance strategies for this population must be addressed.
Keywords: Lung cancer, Second primary, SEER, Surveillance
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States.1 For patients who have been treated for an initial primary lung cancer (IPLC), there is a subsequent risk for development of a second primary lung cancer (SPLC). SPLC is defined as a new primary lung cancer that develops after curative intent therapy for the IPLC. The incidence of SPLC has been estimated at approximately 1% to 2% per patient per year.2 The development of a SPLC has been associated with an overall worse survival even after treatment.3 The screening guidelines for lung cancer were restricted, in that individuals with a history of lung cancer were excluded from participating in the National Lung Screening Trial.4 The current recommendations for screening also do not address any surveillance strategy for monitoring development of a SPLC.5 Most of the data on SPLC have been restricted to analyses of subgroups of IPLCs according to stage or intervention, and the overall SPLC occurrence rate is unknown.6,7 Considering the trend toward better survival among treated patients with earlier-stage lung cancer, it is important to have a clear understanding of the incidence of SPLC.
The aim of this study was to evaluate the incidence of SPLC by utilizing the Surveillance, Epidemiology, and End Results (SEER) database. For lung cancer, SEER defines the occurrence of multiple primary tumors on the basis of (1) topography, (2) histology code, (3) a single tumor occurring in each lung, (4) tumors diagnosed more than 3 years apart, or (5) an invasive tumor diagnosed more than 60 days after diagnosis of an in situ tumor.8,9
The intention was also to establish the frequency of occurrence on the basis of patient demographics, including sex, age, race, the respective histologic types and stages at presentation of the IPLC and SPLC, the interval between diagnoses, and cumulative risk for development of a SPLC in this patient population.
Materials and Methods
Data Sources
Patients were identified from the Surveillance, Epidemiology, and End Results (SEER) public use database. The SEER Program of the National Cancer Institute is responsible for the collection and reporting of cancer incidence and survival data from several population-based central cancer registries that cover approximately 30% of the U.S. population. SEER data include patient demographic information, as well as primary tumor site, tumor morphology, stage at diagnosis, first course of cancer treatment, and follow-up for vital status. This study was limited to the SEER 13 registries (consisting of Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, rural Georgia, and the Alaska Native Tumor Registry) with complete longitudinal data necessary for the analyses.
Patient Cohorts and Study Parameters
Our initial query of the SEER database included all patients 20 years of age or older in whom a first primary lung cancer was diagnosed between 1992 and 2007. Follow-up for the diagnoses of a SPLC was through 2012. Lung cancer diagnoses were defined by using the SEER site recode International Classification of Diseases for Oncology third edition site codes C34.0 through C34.9 and excluding histology codes 9050 through 9055, 9140, and 9590 through 9992.
A 6-month latency exclusion period was set to further distinguish SPLCs from late metastatic disease of the IPLC. Information on age at diagnosis, date of diagnosis, sex, race, histologic type, and stage at presentation of IPLCs and SPLCs, as well as the intervals between diagnoses, were obtained from SEER. Age was evaluated in four categories (20–49 years, 50–59 years, 60–69 years, and 70 years or older), and year of IPLC diagnosis was evaluated in three categories (1992–1996, 1997–2001, and 2002–2007). Race was assessed by using the standard SEER race recode (black, white, and other). Histologic type was categorized into four groups by the following International Classification of Diseases for Oncology histology codes: squamous cell carcinoma (8070–8073 and 8083), adenocarcinoma (8140, 8250–8253, 8255, 8260, 8323, 8480–8481, 8550, 8560, 8570, and 8574), and other NSCLC and SCLC (8041and 8045). Tumor staging was characterized as localized, regional, distant, and unknown, as defined by the SEER Program Coding and Staging Manual.8
Statistical Analysis
The distribution of IPLC characteristics (sex, race, age group, histologic type, and stage) were summarized by using counts and percentages, and differences by the development of a SPLC were determined using chisquare tests. The incidence of SPLCs was compared with the expected incidence in the general population by calculating standardized incidence ratios (SIRs) and 95% confidence intervals. SIRs were calculated overall and stratified by sex and race, age group, sex and age group, histologic type, SEER summary stage, year of IPLC diagnosis, and sex and year of IPLC diagnosis group. The concordance between each patient’s IPLC and SPLC in terms of histologic type and stage was evaluated by using counts and percentages. The cumulative risk for development of SPLC was calculated by the Kaplan-Meier method. SIR analyses were completed using the SEER*Stat program,10 and all other analyses were done using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
An IPLC was diagnosed in 156,494 patients in the SEER 13 database between 1992 and 2007. The characteristics of these patients are reported in Table 1. Of those patients, 54% (83,971) were male, 46% (72,523) were female, and 80% were white. NSCLC was more prevalent than SCLC (88% versus 12% of cases, respectively). The distribution of the presenting stages of the IPLCs was as follows: distant, 38%; regional, 32%; local, 23%; and unknown, 7%. A SPLC developed in 3% percent of patients (4622) (Table 1).
Table 1.
Demographic and Disease Characteristics of Patients Age 20 Years and Older with a Diagnosis of Malignant First Primary Lung Cancer in SEER-13 from 1992 to 2007
| All Patients |
Development of a Second Lung Primary |
||||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| Characteristic | n | % | n | % | n | % | p Value |
| Total | 156,494 | 151,872 | 4622 | ||||
| Sex | <0.0001 | ||||||
| Male | 83,971 | 53.7% | 81,653 | 53.8% | 2318 | 50.2% | |
| Female | 72,523 | 46.3% | 70,219 | 46.2% | 2304 | 49.8% | |
| Race | <0.0001 | ||||||
| White | 125,762 | 80.4% | 121,921 | 80.3% | 3841 | 83.1% | |
| Black | 17,392 | 11.1% | 16,911 | 11.1% | 481 | 10.4% | |
| Other | 13,340 | 8.5% | 13,040 | 8.6% | 300 | 6.5% | |
| Age at diagnosis, y | <0.0001 | ||||||
| 20-49 | 11,890 | 7.6% | 11,645 | 7.7% | 245 | 5.3% | |
| 50-59 | 28,866 | 18.4% | 27,908 | 18.4% | 958 | 20.7% | |
| 60-69 | 48,762 | 31.2% | 46,842 | 30.8% | 1920 | 41.5% | |
| ≥70 | 66,976 | 42.8% | 65,477 | 43.1% | 1499 | 32.4% | |
| Histologic type | <0.0001 | ||||||
| Squamous cell | 32,326 | 20.7% | 31,061 | 20.5% | 1265 | 27.4% | |
| SCLC | 18,585 | 11.9% | 18,356 | 12.1% | 229 | 5.0% | |
| Adenocarcinoma | 58,031 | 37.1% | 55,696 | 36.7% | 2335 | 50.5% | |
| Other NSCLC | 47,552 | 30.4% | 46,759 | 30.8% | 793 | 17.2% | |
| SEER stage | <0.0001 | ||||||
| Local | 36,414 | 23.3% | 33,853 | 22.3% | 2561 | 55.4% | |
| Regional | 49,568 | 31.7% | 47,965 | 31.6% | 1603 | 34.7% | |
| Distant | 59,239 | 37.9% | 58,904 | 38.8% | 335 | 7.2% | |
| Unstaged | 11,273 | 7.2% | 11,150 | 7.3% | 123 | 2.7% | |
SEER, Surveillance, Epidemiology, and End Results.
SIR Analysis
SIRs were used to evaluate the incidence of SPLCs in a cohort of patients with IPLC in relation to the expected incidence of lung malignancies in the general population. Women had the highest SIR values across all ages and races (Table 2), with the highest SIR reported for the youngest (age 20–49 years) female cohort (SIR = 15.26, 95% confidence interval: 12.81–18.04). For both men and women, SIR values increased with the year of IPLC diagnosis. For all analyses, each stratum had statistically significant SIR values greater than 2.00.
Table 2.
SIR Analysis of SPLCs in Patients with a History of an IPLC by Sex, Race, Age, and Year of Diagnosis, 1992-2007 with Follow-up through 2012, SEER-13
| Characteristic | Observed | Expected | Observed/Expected | 95% CI | |
|---|---|---|---|---|---|
| Total | 4622 | 1161.6 | 3.98 | 3.87 | 4.1 |
| Sex and race | |||||
| All men | 2318 | 686.7 | 3.38 | 3.24 | 3.52 |
| White men | 1903 | 548.4 | 3.47 | 3.32 | 3.63 |
| Black men | 247 | 86 | 2.87 | 2.53 | 3.25 |
| Men of other race | 168 | 50.8 | 3.31 | 2.83 | 3.85 |
| All women | 2304 | 474.9 | 4.85 | 4.66 | 5.05 |
| White women | 1938 | 413.7 | 4.68 | 4.48 | 4.9 |
| Black women | 234 | 39.7 | 5.9 | 5.17 | 6.7 |
| Women of other race | 132 | 20.5 | 6.43 | 5.38 | 7.62 |
| Age at diagnosis, y | |||||
| 20–49 | 245 | 19.2 | 12.74 | 11.19 | 14.44 |
| 50–59 | 958 | 136.5 | 7.02 | 6.58 | 7.48 |
| 60–69 | 1920 | 429.1 | 4.47 | 4.28 | 4.68 |
| >70 | 1499 | 576.7 | 2.6 | 2.47 | 2.73 |
| Age (y) and sex | |||||
| Men | |||||
| 20–49 | 108 | 10.3 | 10.53 | 8.64 | 12.71 |
| 50–59 | 467 | 79.4 | 5.88 | 5.36 | 6.44 |
| 60–69 | 987 | 256.6 | 3.85 | 3.61 | 4.09 |
| >70 | 756 | 340.5 | 2.22 | 2.07 | 2.38 |
| Women | |||||
| 20–49 | 137 | 9 | 15.26 | 12.81 | 18.04 |
| 50–59 | 491 | 57.1 | 8.6 | 7.85 | 9.39 |
| 60–69 | 933 | 172.6 | 5.41 | 5.07 | 5.77 |
| >70 | 743 | 236.3 | 3.14 | 2.92 | 3.38 |
| Histologic type | |||||
| Squamous cell | 1265 | 293.83 | 4.31 | 4.07 | 4.55 |
| SCLC | 229 | 66.63 | 3.44 | 3.01 | 3.91 |
| Adenocarcinoma | 2335 | 512.08 | 4.56 | 4.38 | 4.75 |
| Other NSCLC | 793 | 289.06 | 2.74 | 2.56 | 2.94 |
| SEER stage | |||||
| Local | 2561 | 539.98 | 4.74 | 4.56 | 4.93 |
| Regional | 1603 | 392.77 | 4.08 | 3.88 | 4.29 |
| Distant | 335 | 163.17 | 2.05 | 1.84 | 2.29 |
| Unstaged | 123 | 65.68 | 1.87 | 1.56 | 2.23 |
| Year of diagnosis | |||||
| 1992–1996 | 1505 | 427.3 | 3.52 | 3.35 | 3.7 |
| 1997–2001 | 1483 | 378.1 | 3.92 | 3.73 | 4.13 |
| 2002–2007 | 1634 | 356.2 | 4.59 | 4.37 | 4.82 |
| Year and sex | |||||
| Men | |||||
| 1992–1996 | 816 | 268.4 | 3.04 | 2.84 | 3.26 |
| 1997–2001 | 729 | 221 | 3.3 | 3.06 | 3.55 |
| 2002–2007 | 773 | 197.3 | 3.92 | 3.65 | 4.2 |
| Women | |||||
| 1992–1996 | 689 | 158.9 | 4.34 | 4.02 | 4.67 |
| 1997–2001 | 754 | 157.1 | 4.8 | 4.46 | 5.16 |
| 2002–2007 | 861 | 158.9 | 5.42 | 5.06 | 5.79 |
SIR, standardized incidence ratio; SPLC, second primary lung cancer; IPLC, initial primary lung cancer; SEER, Surveillance, Epidemiology, and End Results; CI, confidence interval.
Histologic Type and Staging
The predominant histologic types of the IPLCs were adenocarcinoma and squamous cell carcinoma (58% of all IPLCs). This proportion was higher for the SPLCs with adenocarcinoma and squamous cell carcinomas, which comprised 78% of all cases. IPLC adenocarcinomas most frequently had SPLC adenocarcinomas (62%). IPLC squamous cell carcinomas and SCLCs most often presented with SPLC squamous cell carcinomas (45 and 41%, respectively). Only 8% of SPLCs were SCLCs (Tables 3 and 4), which was higher in incidence than the 5% of IPLCs that were SCLCs. Most patients in whom a second primary developed (55%) initially had a localized IPLC, but a small fraction of patients (7%) had distant stage IPLCs (Table 1). Most SPLCs (56%) presented at advanced stages (regional or distant) or were of an unknown stage, whereas only 44% of SPLCs had localized disease.
Table 3.
Distribution of SPLC Histologic Type by IPLC Histologic Type in Patients with a History of an IPLC
| IPLC histologic type | SPLC Histologic Type |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Squamous cell |
SCLC |
Adenocarcinoma |
Other NSCLC |
Total | |||||
| N | Row % | N | Row % | N | Row % | N | Row % | ||
| Squamous cell | 575 | 45% | 156 | 12% | 242 | 19% | 292 | 23% | 1265 |
| SCLC | 94 | 41% | 26 | 11% | 47 | 21% | 62 | 27% | 229 |
| Adenocarcinoma | 313 | 13% | 120 | 5% | 1446 | 62% | 456 | 20% | 2335 |
| Other NSCLC | 197 | 25% | 80 | 10% | 284 | 36% | 232 | 29% | 793 |
| Total | 1179 | 26% | 382 | 8% | 2019 | 44% | 1042 | 23% | 4622 |
SPLC, second primary lung cancer; IPLC, initial primary lung cancer.
Table 4.
Distribution of SPLC SEER Stage by IPLC SEER Stage in Patients with a History of an IPLC
| IPLC SEER stage | SPLC SEER Stage |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Localized |
Regional |
Distant |
Unstaged |
Total | |||||
| N | Row % | N | Row % | N | Row % | N | Row % | ||
| Localized | 1184 | 46% | 657 | 26% | 570 | 22% | 150 | 6% | 2561 |
| Regional | 694 | 43% | 402 | 25% | 378 | 24% | 129 | 8% | 1603 |
| Distant | 132 | 39% | 86 | 26% | 90 | 27% | 27 | 8% | 335 |
| Unstaged | 44 | 35% | 38 | 31% | 33 | 27% | 8 | 6% | 123 |
| Total | 2055 | 44% | 1184 | 26% | 1070 | 23% | 313 | 7% | 4622 |
SPLC, second primary lung cancer; SEER, Surveillance, Epidemiology, and End Results; IPLC, initial primary lung cancer.
Cumulative Risk for Development of a SPLC
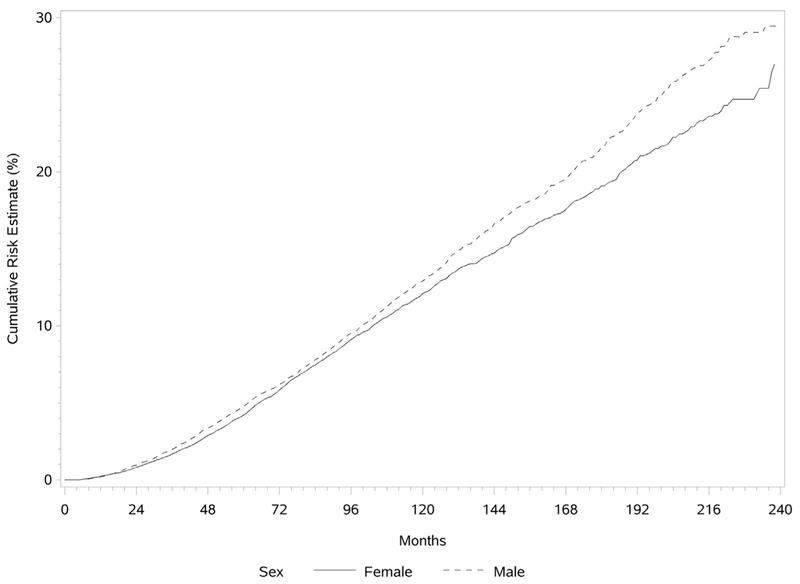
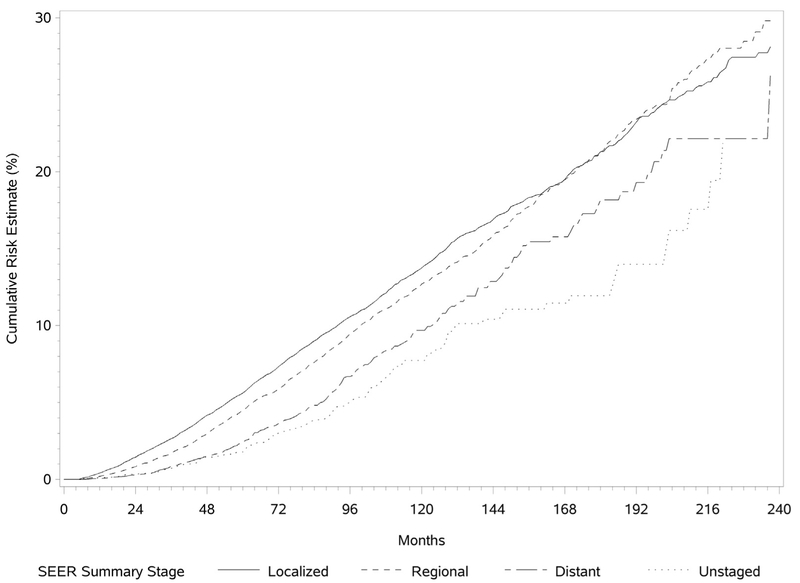
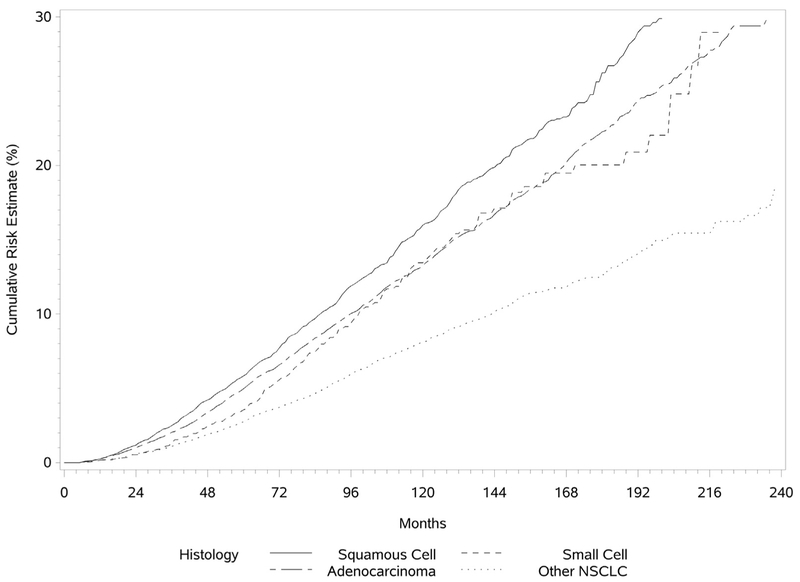
The cumulative risk for development of a SPLC was determined by the Kaplan-Meier method and is illustrated in Figure 1. The risk for development of a SPLC was found to be continuously increasing as a function of time, with the risk slightly higher for men. The median intervals between the diagnoses of IPLC and SPLC were 59 and 62 months for men and women, respectively. Overall, in patients with an IPLC, the incidence rate for development of a SPLC was 1.10% per patient per year. The risk did not plateau over time. The cumulative risk for development of SPLC was higher if the IPLC was a squamous cell carcinoma and for those with initial localized lung cancer (Figs. 2 and 3).
Figure 1.
Cumulative risk for development of a second primary lung cancer stratified by sex.
Figure 2.
Cumulative risk for development of a second primary lung cancer stratified by Surveillance, Epidemiology, and End Results (SEER) summary stage.
Figure 3.
Cumulative risk for development of a second primary lung cancer stratified by histologic type.
Discussion
This SEER-based study indicates that development of a new primary lung malignancy is uniformly more likely in patients with a history of lung cancer than in the general population. Overall, SPLCs developed in 3% of patients with a prior lung cancer. To properly classify these risks, SIRs were evaluated for different patient groups with respect to age, sex, race, and year of diagnosis. The SIR values for all cohorts were greater than 2.2. In general, younger men and women had the highest SIR values. The SIRs in women were found to be higher than those in their male counterparts even though women are at lower risk for development of lung cancer. It has been postulated that lung cancer has a different natural history in women than in men. This has been shown in global analyses in which women had a lower risk for development of lung cancer as well as lower mortality when compared with men.11 Another SEER analysis showed that although the rates of lung cancer in women have increased significantly since the 1970s, they are still lower than the rates in men.12 This potentially explains the higher SIRs for women even though their overall risk for development of lung cancer is lower than that of men. Although specific reasons for the lower risk in women are unknown, it has been postulated that exposure to hormonal therapy and the influence of reproductive factors such as menarche, menopause, and parity may play a role. A prospective study by Weiss et al. demonstrated that factors such as late menopause, multiparity, use of intrauterine devices, and longer reproductive periods were associated with decreased lung cancer risk in nonsmoking women.13 It has been suggested that the use of hormone replacement therapy, especially estrogen, may decrease lung cancer risk as well as mortality in women, even though the data on association with smoking patterns have been conflicting.14–17 Considering their better outcomes when compared with those of men, women with lung cancer may be more likely to survive their IPLC and go on to experience development of a SPLC. Similarly, younger patients had higher SIRs than older patients did—likely for the same reason.18,19 An additional explanation relates to the fact that overall cancer incidence increases with age, so the difference between the observed and expected risk for development of lung cancer in the elderly group will be lower. In the SEER data, the survival rate at 5 years for patients with lung cancer who are age 80 years or older is 7.4% compared with a 15.5% survival rate in patients younger than 70 years.19 Elderly patients with lung cancer are more likely to succumb to their disease, preventing development of SPLCs.
In this study we found that the cumulative risk for development of SPLC was progressive over time and was higher in men than in women. This suggests that the risk for development of lung cancer persists for a long time and never plateaus. Thus, the combined effect of the increased risk for lung cancer among patients who have had lung cancer previously compared with that among the general population and the increasing risk with age may explain the progressive increase in risk for development of a second lung cancer over time.
Most IPLCs in this series were either adenocarcinoma or squamous cell carcinoma, which mirrors the general current trend with regard to histologic type.20 These two histologic types were also the most common SPLC presentations, which is consistent with published reports.6,21,22 The occurrence of a SPLC with the identical histologic type as the IPLC may make it difficult to distinguish a SPLC from recurrence of the original lung cancer. This is of particular importance in cases of synchronous tumors or those that have a close temporal presentation, and if the distinction of separate primaries affects treatment decisions. Molecular adjuncts such as multigene next-generation sequencing could prove invaluable in this situation.23 The incidence of development of SCLC as a SPLC was uniform at around 10% to 12% for all histologic types except when adenocarcinoma was the primary histologic type (5%). This is close to the overall incidence of SCLC as an IPLC, which has shown a downward trend over the past several decades.24 Given this observation, a SPLC will not necessarily be of the same histologic type as the IPLC.
Our data show that for all patients with lung cancer, there was a 1.1% risk for development of a SPLC per patient per year. This value is consistent with the 1% to 2% risk previously reported in the literature.2 The risk for development of a SPLC was found to be cumulative and thus continuously increasing over time. This could be explained by the fact that the intervals examined for this report were longer than those previously studied (between 16.5 and 51 months), and additionally, the quoted numbers from published data are derived from studies restricted to only early-stage NSCLC.7,25 It is possible that the increasing risk over time may be related to smoking trends. Not only does smoking contribute to a higher risk for IPLC, but the risk does not return to baseline even after 35 years of smoking cessation.26 It is well established that cigarette smoking is the predominant risk factor for development of lung cancer, but the SEER data unfortunately do not capture this information.27 Although this may in part explain the continued risk for development of SPLCs in individuals who continue to smoke, we were unable to study the effects of cigarette smoking (both before and after diagnosis of IPLCs) on SPLCs, which is a limitation of this study. We acknowledge that further study of SPLCs may be enhanced by knowledge of smoking habits.
In conclusion, this study demonstrates that patients in whom a lung malignancy has been diagnosed are at high risk for development of SPLCs and that this risk increases over time, with median times to development longer than 5 years. In addition, most SPLCs do not present at an early stage. This information could have major implications regarding surveillance after treatment of an initial lung primary, considering that current guidelines are geared toward concern for the recurrence of the IPLC rather than the development of a SPLC. Given the propensity for SPLCs to present late and the general practice trends toward relaxation of surveillance over time, these data suggest that clinical follow-up, possibly including imaging, should be maintained indefinitely. It would be beneficial to establish a risk profile for the development of SPLCs. Potential culprits with regard to risk could be identified by studying patients in whom SPLCs have developed. A prospective multi-institutional trial could be planned to study not only patient demographics but also molecular and genomic correlates that may contribute to the risk.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345. [DOI] [PubMed] [Google Scholar]
- 3.Ha D, Choi H, Chevalier C, Zell K, Wang XF, Mazzone PJ. Survival in patients with metachronous second primary lung cancer. Ann Am Thorac Soc. 2015;12:79–84. [DOI] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN guidelines in oncology: lung cancer screening. Version 2.2018. https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed March 20, 2017. [Google Scholar]
- 6.Kawaguchi T, Matsumura A, Iuchi K, et al. Second primary cancers in patients with stage III non-small cell lung cancer successfully treated with chemo-radiotherapy. Jpn J Clin Oncol. 2006;36:7–11. [DOI] [PubMed] [Google Scholar]
- 7.Rice D, Kim HW, Sabichi A, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. Ann Thorac Surg. 2003;76:1001–1007 [discussion 1007–1008]. [DOI] [PubMed] [Google Scholar]
- 8. Adamo M, Dickie L, Ruhl J. SEER Program Coding and Staging Manual. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 9.National Cancer Institute. The multiple primary and histology coding rules, SEER program. https://seer.cancer. gov/tools/mphrules/. Accessed September 11, 2017. [Google Scholar]
- 10.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–1093. [DOI] [PubMed] [Google Scholar]
- 11.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jamal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 12.Egleston BL, Meireles SI, Flieder DB, Clapper ML. Population-based trends in lung cancer incidence in women. Semin Oncol. 2009;36:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss JM, Lacey JV Jr, Shu XO, et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol. 2008;168:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Gu X, Zhu J, Yuan D, Song Y. Hormone replacement therapy in females can decrease the risk of lung cancer: a meta-analysis. PLoS One. 2013;8:e71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10:113–123. [DOI] [PubMed] [Google Scholar]
- 16.Clague J, Reynolds P, Henderson KD, et al. Menopausal hormone therapy and lung cancer-specific mortality following diagnosis: the California Teachers Study. PLoS One. 2014;9:e103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chlebowski RT, Anderson GL, Manson JE, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women’s health initiative randomized trial. J Natl Cancer Inst. 2010;102:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. http://seer.cancer.gov/csr/1975_2012/. Accessed March 20, 2017. [Google Scholar]
- 19.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–5577. [DOI] [PubMed] [Google Scholar]
- 20.Maggiore C, Mule A, Fadda G, et al. Histological classification of lung cancer. Rays. 2004;29:353–355. [PubMed] [Google Scholar]
- 21.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–81 [discussion: 81–82]. [DOI] [PubMed] [Google Scholar]
- 22.Reinmuth N, Stumpf A, Stumpf P, et al. Characteristics and outcome of patients with second primary lung cancer. Eur Respir J. 2012;42:1668–1676. [DOI] [PubMed] [Google Scholar]
- 23.Klempner SJ, Ou S-HI, Costa DB, et al. The clinical use of genomic profiling to distinguish intrapulmonary metastases from synchronous primaries in non-small-cell lung cancer: a mini-review. Clin Lung Cancer. 2015;16:334–339. [DOI] [PubMed] [Google Scholar]
- 24.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. [DOI] [PubMed] [Google Scholar]
- 25.Spratt DE, Wu AJ, Adeseye V, et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non-small-cell lung cancer: implications for surveillance. Clin Lung Cancer. 2015;17(3):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer—relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonato L, Agudo A, Ahrens W, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer. 2001;91:876–887. [DOI] [PubMed] [Google Scholar]