Abstract
During meiotic prophase, the meiosis-specific telomere-binding protein TERB1 regulates chromosome movement required for homologous pairing and recombination by interacting with the telomeric shelterin subunit TRF1. Here, we report the crystal structure of the TRF1-binding motif of human TERB1 in complex with the TRFH domain of TRF1. Notably, specific disruption of the TERB1-TRF1 interaction by a point mutation in the mouse Terb1 gene results in infertility only in males. We find that this mutation causes an arrest in the zygotene-early pachytene stage and mild telomere abnormalities of autosomes but unpaired X and Y chromosomes in pachytene, leading to massive spermatocyte apoptosis. We propose that the loss of telomere structure mediated by the TERB1-TRF1 interaction significantly affects homologous pairing of the telomere-adjacent pseudoautosomal region (PAR) of the X and Y chromosomes in mouse spermatocytes. Our findings uncover a specific mechanism of telomeres that surmounts the unique challenges of mammalian X-Y pairing in meiosis.
Meiosis is a specialized cell division for gametogenesis that contributes to sexual reproduction by reducing the diploid chromosome number to the haploid1. During meiotic prophase I, homologous chromosomes undergo homolog pairing, synapsis, and reciprocal recombination, which are crucial for the chromosome segregation during metaphase I (refs.1-3). In most organisms studied to date, including mammals, repair of the programmed DNA double-stranded breaks (DSBs) introduced by SPO11 in leptotene initiates a genomewide search for homology4–7. This search drives the homolog pairing and alignment, leading to assembly of a proteinaceous structure called the synaptonemal complex along the length of the paired homologs8,9. The DSBs can be repaired either as a crossover or as a noncrossover7,8, the former resulting in chiasmata that are essential for the correct alignment and segregation of homologous chromosomes during metaphase I (refs.10-12). Despite the importance of SPO11-mediated DSBs in mammalian meiotic synapsis, a significant level of homolog pairing at chromosomal ends was detected in mouse spermatocytes before programmed DSBs13. This is consistent with the observation that synapsis appears to initiate in subtelomeric regions in human spermatocytes14, suggesting a positive role of telomeres and subtelomeres in the initiation of meiotic homolog pairing.
In recent years, mounting evidence has indicated that progression of mammalian meiotic prophase I depends on the telomere-led rapid prophase movements of chromosomes along the nuclear envelope, which has been observed in diverse eukaryote species15–17. A prerequisite for this rapid chromosome movement is the attachment of telomeres to the nuclear envelope, where the transmembrane LINC (linker of nucleoskeleton and cytoskeleton) complex serves as a structural bridge to connect telomeres to the cytoskeleton and transduce forces generated in the cytoplasm to the end of the chromosomes17,18. In addition to the LINC-complex protein SUN 1 (ref.19), several meiosis-related molecules also play important roles in mediating telomere-nuclear envelope attachment in mice, including TERB1 and TERB2 (telomere-repeat-binding bouquet-formation proteins 1 and 2)20–22, MAJIN (membrane-anchored junction protein)22, CDK2 (cyclin-dependent kinases 2)23 and Speedy/RINGO A (rapid inducer of G2/M progression in oocytes)24.
The meiosis-specific telomere regulator TERB1 is a molecular scaffold that simultaneously interacts with SUN1, TERB2, meiotic cohesin subunit SA3, and telomeric shelterin subunit TRF1, establishing telomere attachment to the inner nuclear membrane (INM) and driving the chromosome movement required for homologous pairing and recombination21,22. Knockout of the Terb1 gene in mice disrupts the entire interaction network and impairs homolog pairing, synapsis, and recombination, leading to early abolishment of both spermatogenesis and oogenesis21. However, the significance of each of the TERB1-mediated interactions and their molecular mechanisms in meiosis remain unclear.
In the present study, we characterized the TERB1-TRF1 interaction and determined the crystal structure of a short TRF1-binding motif of human TERB1 in complex with the TRF-homology (TRFH) domain of human TRF1. Our structural and biochemical characterization reveals that TRF1 recognizes a unique IxLxP motif on TERB1 via the peptide-binding site in its TRFH domain. We generated knock-in mice with the TRFl-binding-deficient mutation in the Terb1 gene and studied the functional roles ofthe TERB1-TRF1 interaction in mouse meiosis. Strikingly, specific disruption ofthe TERB1-TRF1 interaction led to infertility only in male mice. We found that the Terbl mutation caused an arrest in the zygotene-early pachytene stage of spermatogenesis and impaired X-Y chromosome pairing, resulting in massive spermatocyte apoptosis. The sex chromosomes possess short PARs25, and X-Y pairing is more challenging than autosomal pairing, owing to the deficiency of DNA interaction along the length of the chromosomes26‘27. Our studies suggest a mechanism of telomeres that helps surmount the unique challenges of X-Y pairing in meiosis.
RESULTS
Characterization of the interaction between TERB1 and TRF1
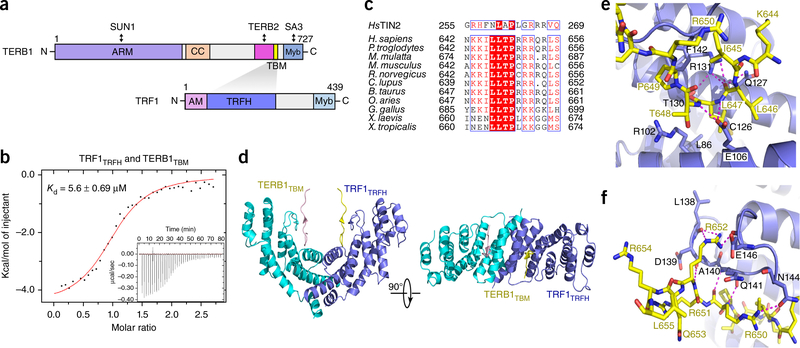
The TRFH domain of TRF1 is known to interact with TERB1, which functions together with TRF1 and SUN1 to regulate telomere-nuclear envelope attachment and telomere movement in meiosis20,21. TRFlTRFH was reported to directly interact with a C-terminal fragment of TERB1 (residues 522–662)21 (Fig. 1a). We confirmed this interaction using the yeast two-hybrid (Y2H) assay (Supplementary Fig. 1). To further map the TRFl-binding region in TERB1, we generated various TERB1 fragments of different lengths within TERB1522-662 to evaluate their ability to interact with TRFlTRFH by Y2H analysis. Our data revealed that a short fragment of TERB1 consisting of residues 642–656 was sufficient for binding to TRF1 (Supplementary Fig. 1). TERB1642-656 binds TRFlTRFH with an equilibrium dissociation constant (Kd) of 5.6 μΜ, measured by isothermal titration calorimetry (ITC) assay (Fig. 1b). Hereafter, we will refer to TERB1642–656 as TERBlTBM (TRFl-bind-ing motif) (Fig. 1a). Our previous studies have demonstrated that TRF1trfh specifically recognizes a short peptide motif with a signature sequence FxLxP (x, any amino acid) on its interacting proteins, including TIN2 (ref. 28). TERBlTBM contains a modified sequence FxLxP that highly resembles the TRFl-binding motif of TIN2 (ΤΙΝ2TΒΜ) (Fig. 1c), suggesting that TERB1Tbm might bind to TRF1trfh in the same manner as ΤΙΝ2TΒΜ.
Figure 1.
Structural studies of the interaction between TRF1trfh and TERBlTBM (a) Domain organization of TERB1 and TRF1. TRFH domain of TRF1 and TBM of TERB1 are colored in dark blue and yellow, respectively. Shading indicates the interaction between TRF1 and TERBl. ARM, armadillo repeats; CC, coiled coil; Myb, myeloblastosis domain; AM, acidic motif; TRFH, TRF homology domain, (b) ITC measurement ofthe interaction between TRF1trfh (residues 65–267) and TERB1tbm (residues 642–656), with Kd and error of the fit indicated, (c) Sequence alignment of the TBM of TIN2 and TERBl. Homo sapiens, NP_001129977.1; Pan troglodytes, XP_001159574.2; Macaca mulatta, XP_014981824.1; Mus musculus, NP_851289.2; Rattus norvegicus, XP_006255118.1; Canis lupus, XP_005620927.1; Bos taurus, XP_005195308.1; Ovis aries, XP_014955952.1; Gallus gallus, XP_001232198.3; Xenopus laevis, XP_018096997.1; Xenopus tropicalis, XP_002933719.2; human TIN2, NP_001092744.1. (d) Ribbon diagram of two orthogonal views of theTRFlTRFH-TERBlTBMcomplex. TRF1trfh is colored in dark blue and cyan, and TERB1tbm is in yellow and pink. (e,f) The TRF TRFlTRFH-TERBlTBM interface. The color scheme is the same as in d. Residues important for the interaction are shown as stick models. Salt bridges and hydrogen-bonding interactions are shown as magenta dashed lines.
Crystal structure of the TERBlTBM-TRFlTRFH complex
To reveal the structural basis for the specific recognition of TERBl by TRF1, we crystallized TRFlTRFH in complex with the TERBlTBM peptide and determined the structure at a resolution of 2.1 A by molecular replacement, using the previously determined structure of TRFlTRFH as a search model28 (Table 1, Fig. 1d and Supplementary Fig. 2a). Except for three residues at the N and Ctermini, the TERBlTBM peptide is well ordered, as evidenced by good electron density in the final atomic model (Supplementary Fig. 2a).
Table 1.
Data collection and refinement statistics
| TRF1trfh-TERB1tbm (PDB 5XUP)a | |
|---|---|
| Data collection | |
| Space group | P64 |
| Cell dimensions | |
| a, b, c(Å) | 161.58, 161.58, 45.93 |
| α, β, γ(°) | 90.00, 90.00, 120.00 |
| Resolution (Â) | 50.00–2.10 (2.18–2.10)b |
| Rmerge (%) | 12.1 (75.5) |
| I/σ(I) | 14.50 (2.00) |
| CC1/2 | 0.99 (0.83) |
| Completeness (%) | 100.0 (100.0) |
| Redundancy | 10.0 (9.8) |
| Refinement | |
| Resolution (Â) | 46.69–2.10 (2.16–2.10) |
| No. reflections | 40,329 |
| Rwork / Rfree (%) | 17.68/22.07 |
| No. atoms | |
| Protein | 3,357 |
| Water | 152 |
| B factors (À2) | |
| Protein | 44.82 |
| Water | 46.33 |
| R.m.s. deviations | |
| Bond lengths (Ä) | 0.006 |
| Bond angles (°) | 0.985 |
A single data set was scaled and merged for the crystal structure.
Values in parentheses are for highest-resolution shell.
The TRFH domain of TRF1 forms a homodimer, and each subunit contains ten meandering α-helices (Fig. 1d). The TERB1TBM peptide binds in an extended conformation across the concaved surface of each TRFlTRFH subunit, burying −700 A2 of total surface area at the intermolecular interface (Fig. 1d). Each of the TERBlTBM peptides contacts only one TRFlTRFH subunit and does not interfere with the dimerization of TRFlTRFH (Fig. 1d and Supplementary Fig. 2a). The N terminus (645ILLTP649) of the TERBlTBM peptide adopts a sharp-turn conformation and binds to TRF1trfh through both van der Waals and electrostatic interactions (Fig. 1e). The side chain of Leu647TERB1 is inserted deeply into a hydrophobic pocket formed by a panel of residues of TRF1trfh with hydrophobic and aliphatic side chains (Leu86, Argl02, Alal05, Glul06, Ilel09, Ilel23, Cysl26, Glnl27, and Thrl30) (Fig. 1e). This van der Waals contact is buttressed by an elaborate network of hydrogen bonds between the backbone of TERBItbm surrounding Leu647TERB1 and the side chains of TRF1 Glul06, Glnl27, Thrl30, and Argl31 (Fig. 1e). In addition to Leu647TERB1, Pro649TERB1 also contributes to complex formation via a stacking interaction between its pyrrolidine ring and the phenyl ring of Phel42TRE1 (Fig. 1e). The interaction between TRF1trfh and the C-terminal positively charged residues of TERBItbm (650RRR652) is dominated by electrostatic contacts (Fig. 1f and Supplementary Fig. 2b). This segment of TERBItbm adopts a short ß-strand conformation that runs antiparallel to the one formed by the conserved residues (139DAQ141) of TRF1 (Fig. 1d,f). This intermolecular β-sheet conformation is stabilized by electrostatic interactions; the guanidinium groups of Arg652TERB1 and Arg650TERB1 make four hydrogen-bonding interactions with Leul38, Asnl44, and Glul46 of TRF1 (Fig. 1f).
The structure of the TERB1tbm_TRF1trfh complex reveals a striking similarity to that of the TIN2tbm_TRF1trfh complex (Supplementary Fig. 3a-c). First, the central L-X-P motifs in both TRFH-binding motifs are identical in overall conformation, and the two key hydrophobic residues Leu647 and Pro649 of TERB1 interact with TRF1 in exactly the same way as their counterparts in TIN2tbm do (Supplementary Fig. 3a-c). Second, in both complex structures, the side chain of one arginine residue (Arg652 in TERB1 and Arg266 in TIN2) at the C terminus of both TBM motifs points to a concaved acidic surface on TRF1trfh (Supplementary Fig. 3a-c), suggesting that these electrostatic contacts make important contributions to the interaction between TRF1 and its binding partners. The major difference between the two structures is at the N termini before the central L-X-P motifs of the TBM peptides: 644KIL646 of TERB1 folds back onto the aliphatic side chain of Arg650TERB1 to form a closed configuration, whereas 257HFN259 of TIN2 adopts a rather extended conformation with the phenyl ring of Phe258TIN2 sitting on a flat hydrophobic surface of TRF1trfh (Supplementary Fig. 3a-c).
Mutational analysis of theTERB1-TRF1 interface
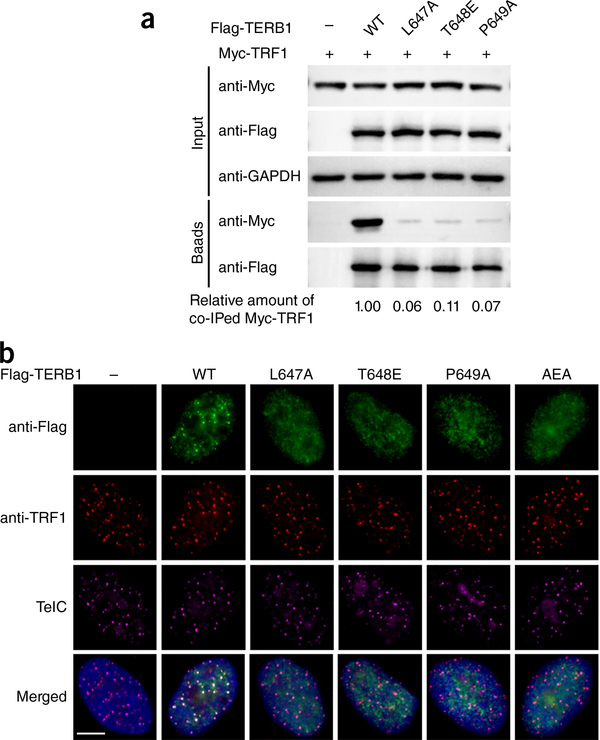
To test the validity of the TERB1-TRF1 interaction observed in the crystal structure, we mutated residues of the TERB1tbm Peptide that were predicted to be important for interacting with TRF1 and then examined binding. We first focused on two highly conserved hydrophobic residues in TERB1, Leu647 and Pro649, whose equivalents in other TRFH-binding proteins are critical for their respective interactions28–30. Consistent with the structural data, ITC analysis with purified TRF1trfh and TERB1tbm peptides showed that mutations of these key interacting residues either completely abolished (TERB1L647A) or substantially impaired (TERB1P649A) the TERB1-TRF1 interaction (Supplementary Fig. 4) A previous study showed that TERB1T648 can be phosphorylated by cyclin-dependent kinase and that this modification is not compatible with the interaction between TERB1 and TRF1 (ref. 22). Indeed, a TERB1tbm peptide with a phosphorylated Thr648 or a phosphomimetic glutamate residue at the position of Thr648 completely disrupted the interaction between TERB1tBM and TRF1TRFH (Supplementary Fig. 4). This finding is consistent with the crystal structure in which the hydroxyl group of TERBlT648 is surrounded by a negative electrostatic surface of TRF1, which would generate unfavorable electrostatic conflict with TERB1 if a phosphorylated threonine or a glutamate residue were at the position of TERBlT648(Supplementary Fig. 2b).
We then tested the interactions of mutant proteins transiently expressed in human embryonic kidney 293T cells, and the co-immunoprecipitation (co-IP) results were consistent with the in vitro ITC measurements (Fig. 2a). We therefore conclude that the observed TERB1-TRF1 interface is specific and necessary for both in vitro and in vivo binding ofTERBl to TRF1. Next, we tested the relevance of the TERB1-TRF1 interaction to the telomeric localization of TERBl in HeLa cells by transiently expressing Flag-tagged WT or mutant TERB1. Indirect immunofluorescence (IF) analysis revealed a nuclear punctate staining pattern for WT TERB1 that almost completely colocalized with TRF1 and telomeres (Fig. 2b). In contrast, TERB1 mutants containing single or multiple point mutations that disrupt the TRF1-TERB1 interaction were distributed throughout the nucleoplasm with no obvious accumulation at telomeres (Fig. 2b). This result confirms the structural information and indicates that the binding of TERB1tBM to the TRFH domain of TRF1 is required for the telomeric localization ofTERBl.
Figure 2.
Mutational analysis of the TERB1-TRF1 interaction. (a) Co-IP of TRF1 with WT or mutant Flag-tagged TERB 1 in 293T cells. The levels of each protein in the input and IP samples were analyzed by immunoblotting with the indicated antibodies. “Input” contains 5% of the input whole-cell lysate used for IPs. The numbers indicated below the blot images represent the relative signals of co-IPed Myc-TRFl normalized by the signals of anti-Flag. Band intensity of blots was quantified by the ImageJ software. The signal for WT sample was set as 1. Uncropped blot images are in Supplementary Data Set 1. (b) Telomere colocalization analysis of TRF1 with WT and mutant TERB1 in HeLa cells. Cells were immunostained with anti-Flag (green) and anti-TRFl (red), hybridized with the Cy3-TelC probe (purple) to detect telomeres, and stained with DAPI (blue) for nuclei. Scale bar, 5 μm.
Disruption of theTERB1-TRF1 interaction results in decreased fertility in male mice
To address the functional significance of the TERB1-TRF1 interaction in mammalian meiosis, we generated knock-in mice with the TRF 1-binding-deficient mutation 647LTP649 to 647AEA649 in the Terb1 gene using the CRISPR-Cas9 method31 (Supplementary Fig. 5a,b). The heterozygous TerblLTP/AEA mice were healthy and fertile and were intercrossed to produce homozygous Terb1AEA/AEA mice. Pups were born close to the expected Mendelian ratio, and homozygous Terb1AEA/AEA mice exhibited similar body weight compared to their WT and heterozygous littermates (data not shown), suggesting that the Terb1AEA/AEA mutation does not impair embryonic development. Quantitative PCR (qPCR) and western blotting with mouse testis revealed that the Terb1AEA/AEA mutation did not alter the in vivo mRNA and protein expression level of TERBl (Supplementary Fig. 5c,d). Strikingly, although some Terb1AEA/AEA males produced offspring, most were infertile. In sharp contrast, Terb1AEA/AEA females were fully fertile with normal litter sizes, strongly suggesting that the 647AEA649 mutation of TERBl only affects male fertility.
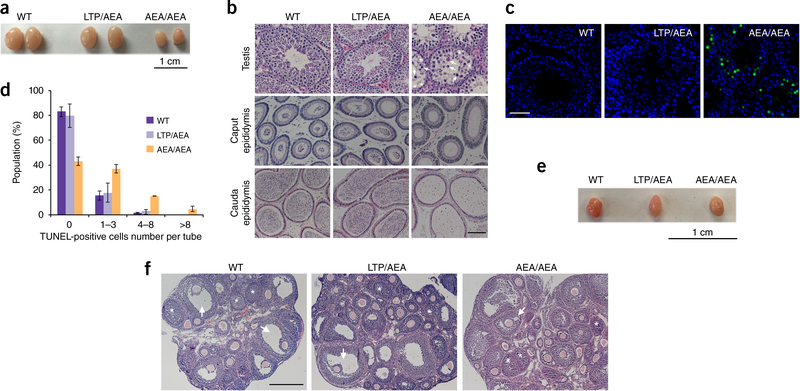
Six-week-old testes of Terb1AEA/AEA male mice were hypoplastic and about 35% in size compared with those of WT and heterozygous mice (Fig. 3a). Histopathological analysis showed that the seminiferous tubules were narrower and lacked postmeiotic cells (Fig. 3b). Furthermore, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) analysis of testis sections showed numerous apoptotic signals in Terb1AEA/AEA seminiferous tubules, indicating that spermatocytes were massively eliminated in Terb1AEA/AEA testes (Fig. 3c,d). Consequently, there were no or very few mature sperms in either the caput or cauda epididymal lumen of Terb1AEA/AEA testes (Fig. 3b). In sharp contrast, both the size of ovaries and the number of primordial follicles were indistinguishable between Terb1AEA/AEA and control females (Fig. 3e,f), in accordance with the observation that Terb1AEA/AEA females were fully fertile.
Figure 3.
Disruption of the TRF1-TERB1 interaction results in failure of spermatogenesis. (a) Testes from 6-week-old WT, heterozygous (LTP/AEA) and Terb1AEAIAEA (AEA/AEA) mice, (b) Hematoxylin-and-eosin-stained histological cross-sections of testes, caput epididymis, and cauda epididymis from adult mice. White arrows indicate abnormal spermatocyte-like cells in Terb1AEAIAEA mice. Asterisks show vacuolated seminiferous tubules in which both spermatozoa and spermatids are absent. Scale bar, 50 μm. (c) TUN EL assay using testis sections from 6–8-week-old mice. DNA was stained with DAPI (blue), and apoptosis was detected by immunofluorescence TUNEL (green). Many TUNEL-positive cells are observed in Terb1AEAJAEA testes. Scale bar, 50 μm. (d) Number of TUNEL-positive cells per tube, shown as mean and s.d., n = 3 mice of each genotype. Testis section samples were subjected to TUNEL assay, and more than 300 seminiferous tubules were assessed for each mouse, (e) Ovaries from 12-week-old WT, heterogynous and AEA/AEA female mice. (f) Hematoxylin-and-eosin-stained ovary sections from WT, heterozygous and Terb1AEAJAEA mice. Arrows indicate follicles and asterisks show corpora lutea. Scale bar, 200 μm.
Disruption of theTERB1-TRF1 interaction causes progressive loss of advanced spermatogenic cells
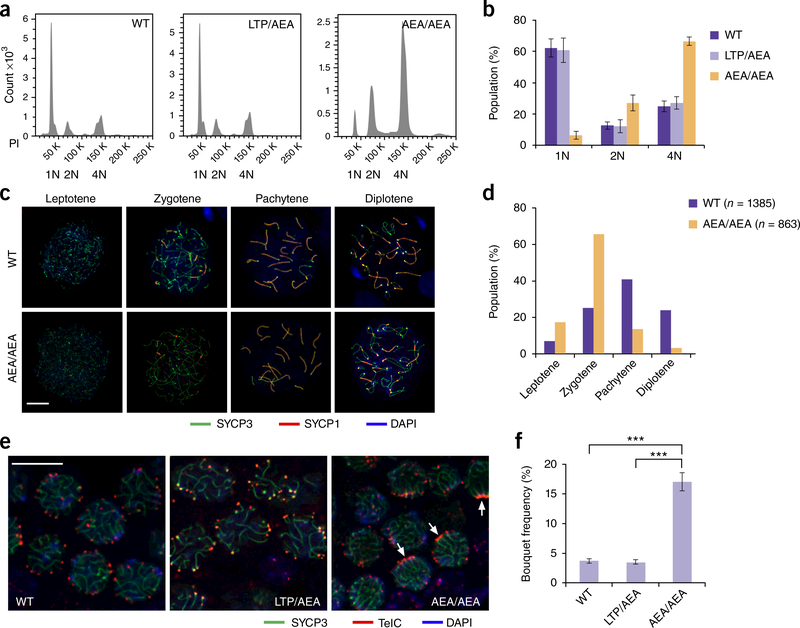
To reveal the reason for the absence of spermatozoa in Terb1AEA/AEA mice, we first analyzed meiotic progression using fluorescence-activated cell sorting (FACS) analysis. We found that although mature haploid sperms were generated in WT and heterozygous mice, the spermatogenesis process in Terb1AEA/AEA mice was disrupted at the tetraploid stage of primary spermatocytes (Fig. 4a,b). We further examined the progression of meiotic prophase I by immunostaining the axial element component SYCP3 and the synaptonemal complex protein SYCP1 on spermatocyte spreads32,33. In WT mice at 21 d postpartum, more than 60% of the spermatocytes that expressed SYCP3 reached the pachytene or later stages (Fig. 4c,d). In contrast, in Terb1AEA/AEA mice, most of the spermatocytes were arrested in the zygotene (~60%) or pachytene-like (~15%) stages, and very few spermatocytes reached the diplotene stage (Fig. 4c,d). Consistent with the immunostaining analysis, the Hoechst 33342 staining profile of Terb1AEA/AEA spermatocytes showed an arrest at the zygotene-early pachytene stage (Supplementary Fig. 6a-c). Next, we used fluorescence in situ hybridization (FISH) analysis of testis sections to further investigate the meiotic defect of Terb1AEA/AEA mice. There were very few spermatocytes exhibiting telomere bouquets in WT and heterozygous testis sections, consistent with the fact that telomere clustering in the bouquet configuration occurs transiently during the zygotene stage34–36 (Fig. 4e,f). In contrast, there was an ~4-fold increase of the frequency of nuclei with telomere bouquets in Terb1AEA/AEA testis sections (Fig. 4e,f). Collectively, these results suggest that spermatogenesis in Terb1AEA/AEA mice is delayed or arrested predominantly at the zygotene-early pachytene stage.
Figure 4.
Spermatogenesis process is arrested in Terb1AEAIAEA spermatocytes. (a) Propidium iodide (PI) fluorescence flow analysis of testicular cells from 6-week-old WT, heterozygous and Terb1AEAIAEA littermates. Results shown are from one out of three independent experiments showing similar results. 1N, haploid; 2N, diploid; 4N, tetraploid. (b) Quantification of subpopulations of a. Data are mean and s.d. for n = 3 mice of each genotype. One male mouse of each genotype from the same litter was used in each experiment. (c) Heterozygous and Terb1AEAIAEA spermatocytes stained for SYCP1 (red), SYCP3 (green) and DAPI (blue). Scale bar, 10 μm. (d) Quantification of the frequencies of meiotic stages shown in c. Numbers of spreads used for quantification are shown. (e) Representative images for immunofluorescence FISH assay using testis sections from 14-day-old mice stained for SYCP3 (green) and telomeres (telomeric PNAteIC, red). Arrows indicate clustered telomere signals. Scale bar, 10 μm. (f) Quantification of the frequencies of cells with telomere bouquet signals. Data show mean and s.d. of n = 3 independent experiments with different sets of mouse littermates. One male mouse of each genotype from the same litter was used in each experiment. Statistical significances (***P < 0.01) were assessed by two-tailed t tests.
Given that TERB1 plays an important role in homologous pairing, synapsis, and recombination during meiosis21, we next examined whether Terb1AEA/AEA spermatocytes exhibit defects in these processes. IF staining of SYCP3, SYCP1, and MLH1 showed that autosomal homologous pairing, synapsis, and MLH1 focus formation (a crossover marker) appeared normal in Terb1AEA/AEA spermatocytes (Fig. 4c and Supplementary Fig. 7a,b). To analyze whether the repair of meiotic programmed DSBs was impaired in Terb1AEA/AEA testes, we analyzed spermatocyte spreads using γ-Η2ΑΧ as a marker of DSBs37. WT and Terb1AEA/AEA spermatocytes exhibited a similar γ-Η2ΑΧ staining pattern with γ-Η2ΑΧ distributed throughout entire nuclei in leptotene and early zygotene, solely restricted to the sex body in midpachytene to late diplotene, and eliminated in dia-kinesis (Supplementary Fig. 7c). Taken together, our data suggest that, although the spermatogenesis is delayed in the zygotene-early pachytene stage, autosomal homologous crossing over, pairing, and synapsis are largely not affected in Terb1AEA/AEA mice.
Disruption of theTERB1-TRF1 interaction impairs telomeric localization ofTERBI and SUN1
We demonstrated that point mutations of any residue in 647LTP649 ofTERBI disrupted the interaction between TERB1 andTRFl when both proteins were tagged and coexpressed in HeLa cells (Fig. 2a and Supplementary Fig. 4). We next examined the effect of the 647AEA649 mutation of TERBI in the interaction between endogenous TERB1 and TRFl in Terb1AEA/AEA spermatocytes. Co-IP experiments showed that the 647AEA649 mutation greatly weakened the interaction (Supplementary Fig. 8a). This result confirmed that the TBM motif of TERBI is essential for TERB1-TRF1 interaction in spermatocytes. We then investigated whether this mutation causes any defect in telomere localization of TERBI in Terb1AEA/AEA spermatocytes. IF analysis of WT spermatocyte spreads showed discrete TERB1 foci completely colocalized with telomeres (Fig. 5a). In contrast, most telomeres exhibit substantially reduced TERB1 staining, and some of the telomeres completely lack TERB1 signal in Terb1AEA/AEA spreads (Fig. 5a,b). IF staining of SUN1 showed a similar defect in telomere localization in Terb1AEA/AEA spreads (Supplementary Fig. 8b,c), consistent with previous data that TERB1 recruits telomeres to INM through simultaneous interactions with both TRF1 and SUN1 in mouse spermatocytes21. Therefore, we conclude that disruption of the TERB1-TRF1 interaction impairs telomeric localization ofTERBI and SUNl in spermatocytes.
Figure 5.
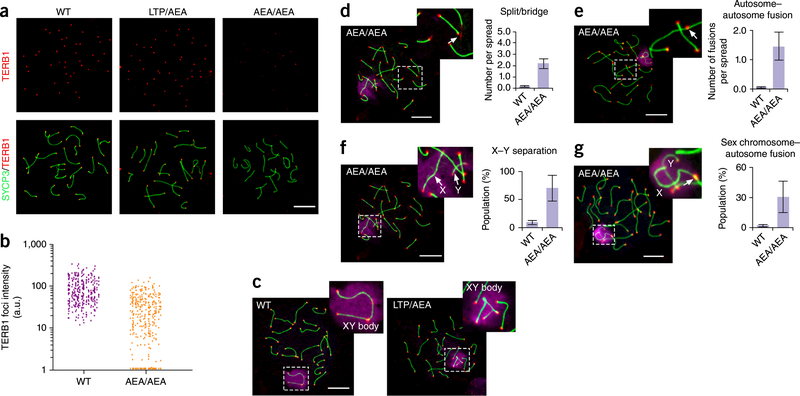
The 647aea649 mutation of TERB1 results in telomere localization defect of TERB1, autosomal telomere aberrance and unpaired X and Y chromosomes, (a) Localization of TERB1 (red) and SYCP3 (green) on pachytene spermatocyte spreads from WT and Terb1AEAJAEA testes. Scale bar, 10 μm. (b) Quantification of the intensity of TERB1 foci at chromosome termini. More than ten spermatocyte spreads were used for quantification for each mouse, a.u., arbitrary units. Results are representative of three independent experiments with 6-week-old littermates. One male mouse of each genotype from the same litter was used in each experiment. (c) Representative mid-pachytene spermatocyte spreads from WT and heterozygous mice stained for SYCP3 (green), telomere (teIC, red) and γ-Η2ΑΧ (purple). Scale bar, 10 μm. (d-g) Representative images showing chromosome structural abnormalities in Terb1AEAJAEA mid-pachytene spermatocyte spreads: telomere splitting and bridging on autosomes (d), autosome-autosome telomere fusions (e), X-Y separation (f), and sex chromosome-autosome telomere fusions (g). Three independent experiments with different sets of mouse littermates were performed with similar results. One male mouse of each genotype from the same litter was used in each experiment. More than 50 spreads were counted for each mouse. Scale bars, 10 μm. Graphs show quantification of structural abnormalities as mean and s.d. of n = 3 independent experiments.
Disruption of theTERB1-TRF1 interaction causes severe defect in sex-chromosome pairing
The telomere localization defect of the TERB1 647AEA649 mutant protein prompted us to investigate whether this mutation could cause structural abnormalities at telomeres such as splitting, bridging, and fusion, which are shown in Terb1spermatocytes21. Indeed, we observed on average two telomere splits or bridges and 1.5 telomere fusions between autosomal chromosomes per Terb1AEA/AEA spread (Fig. 5c-e). These aberrant telomeres affected ~10% of autosomal chromosomes, which is > 10-fold more frequent than in WT spermatocytes (Fig. 5c-e). Nevertheless, this phenotype is relatively mild and much less pronounced than the telomere aberrations in cells lacking TRF1 or TRF2 (refs. 38,39).
The most notable phenotype in Terb1AEA/AEA spermatocytes was a very high incidence (~70%) of unpaired sex chromosomes (X and Y chromosomes) (Fig. 5f), similar to the frequency of unpaired sex chromosomes observed in Spo11β-only spermatocytes27. In sharp contrast, no asynapsed autosomal chromosomes were found in Terb1AEA/AEA spermatocytes (Fig. 5d,g). In addition, Terb1AEA/AEA spermatocyte spreads also showed telomere fusions between sex and autosomal chromosomes with a frequency much higher than that of fusions between autosomal telomeres (Fig. 5g). Taken together, these data reveal that, although the 647AEA649 mutation of TERB1 causes telomere abnormalities in both autosomal and sex chromosomes, the defects of sex chromosomes are much more severe than those of autosomes.
DISCUSSION
In this report, we used both X-ray crystallography and mutational analyses to demonstrate that the TRFH domain of TRF1 specifically recognizes a short TBM sequence of TERB1, providing the first structural basis for how the telomere-INM connection is formed in meiosis. We have previously shown that the TRFH domain of TRF1 functions as a docking platform and recognizes a conserved TBM with a signature sequence of FxLxP in telomeric and telomere- associated proteins28. Notably, our data reveal that TERB1tBM contains a modified TBM sequence, FxLxP, and that this motif of TERB1 and proteins that contain TBM sequences, including TIN2, all bind to the same molecular-recognition surface of the TRF1Trph domain in a manner that could be mutually exclusive (Supplementary Fig. 3a-c). It is well established that TIN2 occupies a central position in the shelterin complex and simultaneously interacts with TRF1, TRF2, and TPP1, bridging three major DNA-binding modules in a highly organized manner40–42. The mutually exclusive TRF1 association between TERB1 and TIN2 suggests that there exist two separate TRF1-containing complexes in meiosis: the shelterin complex for telomere capping and the complex containing TERB1, TERB2, and MAJIN for specific telomere functions in meiosis.
TERB1, TERB2, and MAJIN form a meiotic telomere regulatory complex that anchors telomeres at the INM with the LINC complex and meiotic cohesin complex21‘22. This meiotic-specific interaction network is dynamically regulated and crucial for chromosome movement and homologous pairing, synapsis, and recombination21‘22. Disruption of the TERB1-TRF1 interaction partially abolishes this interaction network and leads to telomere aberrance and spermatogenesis arrest or delay in Terb1AEA/AEA mice (Figs. 4and 5). This telomere aberrance is relatively mild, and a substantial amount of Terb1AEA/AEA spermatocytes reach the pachytene stage with successful homolog pairing, synapsis, and recombination (Fig. 4c,d and Supplementary Fig. 7a,b). Unlike autosomes, most of the X and Y chromosomes are unpaired in Terb1AEA/AEA pachytene spermatocytes (Fig. 5f). X-Y pairing is intrinsically more challenging than autosomal pairing, because X and Y chromosomes have only a very short subtelomeric PAR (<1 Mb) and cannot mediate efficient multiple-DNA interactions along the entire length of the chromosomes. We propose that meiotic-specific telomere structure that is mediated by the TERB1-TRF1 interaction and immediately adjacent to the PAR may play an important role in promoting X-Y pairing. Disruption of the TERB1-TRF1 interaction results in unpaired X and Y chromosomes at pachytene, consequently triggering an apoptosis response and leading to male infertility. In contrast, homologous pairing and synapsis in females can take place along the nearly 170-Mb length of the X chromosome and is therefore unlikely to require special help from telomeres, so that the Terb1AEA/AEA female mice are fully fertile. Because the TERB1-TRF1 interface is conserved in humans, we speculate that TRF1-binding-deficient variants of TERB1 may be a human X-Y nondisjunction-susceptibility trait. We propose that aside from the critical capping function, telomeres play a vital role in meiosis via assisting X-Y pairing.
METHODS
Methods, including statements of data availability and any associated accession codes and references, are available in the online version of the paper.
ONLINE METHODS
Protein expression and purification.
Human TRF1trph (residues 65–267) and TERB1TBM (residues 642–656) were cloned into modified pET28a vector with a SUMO protein fused at the N terminus after the 6 x His tag and expressed in E. coli BL2-CodonPlus (DE3) cells (Stratagene). After induction for 18 h with 0.1 mM IPTG at 18 °C, the cells were harvested by centrifugation and the pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0,500 mM NaCl, 10% Glycerol, 1mM PMSF, 5 mM benzamidine, 1 μg/mL leupeptin and 1 μg/mL pep- statin). The cells were then lysed by sonication and the cell debris was removed by ultracentrifugation. The supernatant was mixed with Ni-NTA agarose beads (Qiagen) and rocked for 1 h at 4 °C before elution with 250 mM imidazole. Then Ulpl protease was added to remove the His-SUMO tag. TRF1trfh protein was further purified by gel-filtration chromatography on HiLoad Superdex75 column (GE Healthcare) equilibrated with 25 mM Tris-HCl, pH 8.0,150 mM NaCl and 5 mM DTT. The purified TRF1trfh protein was concentrated to 20 mg/mL and stored at −80 °C. The TERB1TBM peptide was further purified by gel-filtration chromatography on HiLoad Superdex75 column (GE Healthcare) equilibrated with 100 mM ammonium bicarbonate. The purified TERBItbm was concentrated by Speed Vac system and then lyophilized. The lyophilization products were then resuspended in water at a concentration of 50 mg/mL and stored at −80 °C. WT and mutant TERB1TBM peptides used for ITC measurements were expressed and purified similarly.
Crystallization, data collection, and structure determination.
TRF 1trfh and TERB1TBM were mixed at a molar ratio of 1:3 and the mixture was used for crystallization. Crystals of the TRF1trfh_TERB1tbm complex were grown using the sitting drop vapor diffusion method at 16 °C. The precipitant-well solution consisted of 10% 2-propanol, 0.1M Tris-HCl, pH 8.5. Crystals were gradually transferred into a harvesting solution containing 20% 2-propanol, 0.1M Tris-HCl, pH 8.5, and 25% glycerol before being flash frozen in liquid nitrogen for storage. Data sets were collected under cryogenic conditions (100 K) at the Shanghai Synchrotron Radiation Facility (SSRF) beamlines BL18U1 and BL19U1 (wavelength 0.97853 A) and were processed by HKL3000 (ref. 43). TRF1trfh-TERB1TBm complex structure was solved by molecular replacement by using the previously published TRF1TRFH-TIN2Tbm complex structure (PDB 3BQO) as a searching model. The model was then refined using Refmac44, together with manual building in Coot45. In the final Ramachandran plot, the favored and allowed residues are 96.8% and 100.0%, respectively. All of the crystal structural figures were generated using PyMOL46.
Isothermal titration calorimetry (ITC).
The equilibrium dissociation constants of the WT and mutant TERB1TBm interactions with TRF1trfh were determined using a MicroCal iTC200 calorimeter (Malvern). The enthalpies of binding between TRF1trfh (50–100 μΜ) and TERB1TBm (500–1,000 μΜ) were measured at 16 °C in 25 mM Tris-HCl, pH 8.0, 150 mM NaCl. Three independent experiments were performed for every interaction described here. ITC data were subsequently analyzed and fit using Origin 7 software (OriginLab) with blank injections of peptides into buffer subtracted from the experimental titrations before data analysis.
Yeast two-hybrid assay.
The yeast two-hybrid assays were performed as described previously47. Briefly, the L40 strain was transformed with pBTMl16 and PACT2 (Clontech) fusion plasmids, and colonies harboring the both plasmids were selected on -leucine -tryptophan plates. The β-galactosidase activities were measured with a liquid assay.
Animals.
Mice were housed under controlled environmental conditions with free access to water and food. Experimental protocols were approved by the regional ethical committee of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. We made every effort to minimize and refine our experiments to avoid animal suffering.
Generation of Terb1AEA/AEA mice.
The Terb1LTP/LTP to Terb1AEA/AEA mutation mice were generated using the CRISPR-Cas9 and single strand oligos as a donor31. Briefly, a single guide RNA (sgRNA: ACAAAGTTGTCTTCTTCTAC) was cloned into a sgRNA vector using OriGenes gRNA Cloning Services (Rockville, Maryland) and was then used as a template to synthesize sgRNAs using the MEGAshortscript T7 Kit (Life Technologies). Cas9 mRNA was in vitro transcribed from plasmid MLM3613 (Addgene #42251) using the mMESSAGE mMACHINE T7 Ultra Kit (Life Technologies). For microinjection, Cas9 mRNA (100 ng/μL) was mixed with sgRNA (50 ng/μL) and 100 ng/μL donor oligos ( AAC TAC TTA AAT TAC TGT TTT AAG TAT ACT AAA TGT TTT TTA TAT TTT TCA GAA ATT TTG GCG GAA GCA TGC AGA AGA AGA CAA CTT TGT AAA GAA TCT ACT GCC TCT GAA GAA CTA AGT AAG TAT ATT) and then microinjected into the cytoplasm of fertilized eggs collected from B6CBAF1/J FI hybrid mice (JAX). The injected zygotes were cultured overnight in M16 medium at 37 °C in 5% CO2. In next morning, the embryos that had reached two-cell stage of development were implanted into the oviducts of pseudo-pregnant foster mothers (Swiss Webster, Taconic Farm). The mice born to the foster mothers were genotyped by tail biopsy. Briefly, purified tail DNA samples were amplified by PCR using a pair of forward (TCT ACA GCC ACC AGG CAC) and reverse (AGG GAG GAA GAC GGT GTC) primers. The PCR bands were then sequenced using primers GTT CAC TA A GGA TTC CTA CTA G (forward orientation) and TCA TGA AAG CCA AGG GTT ATC (reverse orientation). Mice with overlapping sequencing peaks at the intended mutation sites were bred with C57BL/6 mice for passing the desired mutations through the germline.
Histological analysis.
The testes and ovaries were dissected and fixed in 4% paraformaldehyde in PBS for 4–12 h at 4 °C. Dehydration of tissues was done by passage through graded ethanol, and then tissues were cleared twice in xylene and embedded in paraffin. Tissue sections (5 μm) were prepared with a Leica RM2235 rotary microtome. HE staining was performed according to the standard protocol (Beyotime, CO 105). TUNEL assay was carried out with TUNEL Apo- Green Detection Kit (Biotool, B31112).
Quantitative real-time PCR.
Total RNA was purified from mouse testes using Trizol reagent (Invitrogen, 15596026) and total cDNA was prepared from total RNA using the PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, 6210A). Quantitative real-time PCR was performed with the Power SYBR Green PCR Master Mix (ABI, 4367659) using the ViiA 7 System (Thermo Fisher Scientific). The primers used were as follows: mTerbl-forward: 5’-TTCGTCACTGCACAGAGGATTG-3’; mTerbl-reverse: 5’-CACGTAAGTCGCAGCTTTGC-3’; mGAPDH-forward: 5’-GGTGAAGGTCGGTGTGAACG-3’; mGAPDH-reverse: 5’-CTCGCTCCTGGAAGATGGTG-3’
Antibody production.
cDNA fragment encoding mTERBl (residues 384–565) was inserted into pET28a vector with a His-SUMO tag and was expressed in E. coli BL2-CodonPlus (DE3) cells (Stratagene). Recombinant proteins were purified and His-SUMO tag was removed before immunizing rabbits. Polyclonal antibodies were affinity purified on antigen-coupled Sepharose beads (GE Healthcare).
Antibodies.
The following antibodies were used: rabbit polyclonal antibody against TERB1 (this study), SUNT (ref. 19), H2AX (p-S139) (Novus, NB100– 384), SYCP1 (Abcam, abl5087), SYCP3 (Abeam, abl5093), TRF1 (ref. 28), c-myc (Santa Cruz, sc-789); mouse monoclonal antibodies against MLH1 (Abcam, ab14206), SYCP3 (Abcam, ab97672), TRF1 (Abcam, ab66223), c-myc (Santa Cruz, sc-40), FLAG (Sigma, F3165), GAPDH (Cmctag, AT0002), actin (Sigma, A2228). Secondary antibody for western blot: Goat anti-Mouse IgG/AP (Beyotime, A0216), Donkey anti-Rabbit IgG/ΑΡ (BBI, D110057), Goat anti- Mouse IgG/HRP (ABclonal, AS003), Goat anti-Rabbit IgG/HRP (Proteintech, SA00001). Goat anti-mouse/rabbit secondary antibody for immunofluorescence: DyLight 488 (thermo, 35502, 35553), DyLight 550 (thermo, 84540, 84541), DyLight 633 (thermo, 35512,35562).
Cell culture.
293T and HeLa cells were obtained from the cell bank of type culture collection of Chinese Academy of Sciences and were cultured at 37 °C under 5% CO2 in DMEM supplemented with 10% FBS or cosmic calf serum (Hyclone). No mycoplasma contamination was detected in these cells.
HeLa stable cell lines.
Full-length hTERBl WT and mutants were constructed into pLVX-IRES-Puro (Clontech) and transfected into 293T cell with X-tremeGENE HP DNA Transfection Reagent (Roche) according to the manual. Lentiviruses are harvested 40 h later and filtered by 0.22 μm filter (Millipore, SLGP033R) before infecting plain HeLa cells. 48 h after infection,1 μg/mL puromycin was added to establish the stable-expressing populations. After 5 d of selection, cells were ready for immunofluorescence staining.
Co-IP and western blot.
cDNA fragments were constructed into pEGFP-N1 (Clontech) and transfected into 293T cells. After 36 h, cells were collected and lysed and sonicated in lysis buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% Glycerol, 0.5% TritonX-100,1 mM EDTA) supplemented with EDTA-free complete protease inhibitor (Roche), sonicated on ice, and centrifuged (13,000 r.p.m., 15 min, 4 °C). The resulting supernatants were then precleared and immu-noprecipitated with anti-FLAG M2 Affinity Gel (Sigma) overnight at 4 °C with constant rotation. Precipitates were then washed with lysis buffer, resuspended in Laemmli buffer, boiled for 5 min and used immediately on SDS-PAGE for immunoblotting. To detect WT and mutant TERB1 proteins in spermatocytes, testis samples from mouse littermates were homogenize in PBS buffer. Cells were pelleted and then lysed and sonicated in lysis buffer. Lysates were centrifuged and supernatants were prepared for SDS-PAGE. To detect the interaction between endogenous TERB1 and TRF1 in spermatocytes, mouse testis cell lysates were immunoprecipitated with anti-TERBl, and immunoprecipitated samples were subjected to western blot analysis. After SDS-PAGE, proteins were blotted onto PVDF membranes (Millipore). The blots were incubated in blocking buffer (5% fat-free milk in PBS buffer supplemented with 0.05% TWEEN-20) for 1 h at RT and were incubated in primary antibodies overnight at 4 °C. Blots were then washed and incubated in the alkaline-phosphatase-labeled or HRP-labeled secondary antibody for 1 h at RT. After being washed, blots were developed with BCIP/NBT substrate kit (Invitrogen, 002209) or ECL Prime western Blotting System (GE Healthcare, RPN2232).
Hoechst 33342 and PI staining for flow cytometry.
Testes tissues were prepared for FACS based on previous studies48,49 with minor modifications. Briefly, decapsulated testes were incubated in DMEM medium supplemented with 1 mg/ml Collagenase type IV (Sigma) and 10 μg/mL DNase I (Roche) in 50-ml tubes at 33 °C for 20 min and shaken vigorously by hand. 1 mg/mL trypsin (Gibco) was then added to the tissue incubation medium and samples were incubated at 33 °C for another 20 min, with the tube being inverted several times every 5 min at 33 °C. The dissociated testis samples were pipetted with a plastic disposable Pasteur pipet ten times, and 10% FBS was added to inactivate trypsin. For Hoechst 33342 staining, samples were incubated with 100 μg/mL Hoechst 33342 and 10 μg/ml DNase I at 33 °C for 1 h and then passed through 40-μm cell strainers (BD Falcon). 10 μg/mL PI was added before FACS processing performed using a BD Influx cell sorter. For PI staining of fixed whole cells, dissociated testis samples were centrifuged (2,000 r.p.m., 15 min, 4 °C) and cell pellets were resuspended in 100 μΕ PBS and then 900 μΕ 75% ethanol was added. Cells were fixed overnight at 4 °C and washed once with PBS. Cells were then resuspended in PI staining solution (PBS supplemented with 0.1% TritonX-100, 200 μg/mL DNase-free RNase and 20 μg/mL PI) and incubated at 37 °C for 30min and then used for FACS analysis with a BD FACSCalibur Flow Cytometer.
Spermatocytes chromosome spreading.
Chromosome spreading samples were prepared based on a previous study50. Briefly, testes were dissected and placed in PBS. Seminiferous tubules were separated and placed in a hypotonic extraction buffer (30 mM Tris-HCl, pH 8.2,50 mM sucrose, 17 mM sodium citrate, 5 mM EDTA, 0.5 mM DTT and protease inhibitor cocktail (Roche)) for 30–60 min. Tubules were then moved to 100 mM sucrose solution (pH 8.2) and spermatocytes were detached by pipetting. Drops of cell suspension were then place on the upper right corner of cover slips (Thermo, 12–545-84) that were soaked in a 1% paraformaldehyde solution (Sigma, P6148), pH 9.2, supplemented with 0.15% Triton X-100. Nuclei were dried overnight at RT and stored at −80 °C.
Immunofluorescence staining, telomere FISH, microscopy, and fluorescence- signal quantification.
For immunofluorescence staining of cultured cells, cells were grown on cover slips (Thermo, T_7011254584) and fixed in 4% paraformaldehyde, permeabilized with 0.15% Triton X-100, blocked with 3% BSA at RT for 1 h and incubated with primary antibodies. For immunofluorescence staining of spermatocyte spreads, spreads were washed with PBS buffer supplemented with 0.15% Triton X-100, blocked with 3% BSA at RT for 1 h and incubated with primary antibodies at 4 °C overnight. For immunofluorescence staining of paraffin-embedded tissue sections, sections on cover slips were dewaxed twice in fresh xylene for 15 min each, rehydrated in 100%, 95%, 75%, 50% ethanol and PBS for 10 min each. Sections were then incubated in retrieval solution (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) and boiled for 1 min for antigen retrieval. After washed with PBS at RT for 10 min and blocked with 3% BSA at RT for 1 h, cover slips with sections were incubated with primary antibodies at 4 °C overnight. Cover slips with cells, sections or spreads were then washed and incubated with fluorescence- conjugated secondary antibodies at RT for 1 h. Cover slips were washed and ready for microscopy imaging. Combinatorial immunofluorescence staining and telomere FISH were carried out following the protocol described previously with minor modifications51. Briefly, after being incubated with secondary antibodies, cover slips were washed and refixed with 4% paraformaldehyde for 10 min, dehydrated in 70%, 85%, and 100% ethanol, air dried, and hybridized to PNA telomere probe (Panagene) in hybridization buffer (20 mM Tris- HCl, pH 7.4, 70% formamide, 0.5% blocking reagent (Roche 11096176001)) using ThermoBrite system (Leica) (denatured at 85 °C for 5 min then kept at RT overnight for hybridization). Cover slips were washed once with hybridization buffer and then washed twice with 2x SSC buffer. Cover slips were then dehydrated in ethanol series and DNA was counterstained with DAPI. Microscopy imaging was then performed by LSM 710 (Zeiss) using 63×ΝΑ /1.40 oil. Fluorescence intensity quantification was carried out by using Imaris Image Analysis Software.
Data availability.
The atomic coordinates and structure factors for the TRF1trfh-TERB1TBm complex has been deposited to the Protein Data Bank (PDB) under the accession code PDB 5XUP. Source data for biochemical experiments that support the findings of this study are available from the corresponding author upon reasonable request. A Life Science Reporting Summary for this article is available.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Shi, X. Huang, Q. Shi, M. Tong, and J. Li for discussion. We thank M. Han (Fudan University) for the SUN1 antibody. We thank L. Wu, D. Yao, and R. Zhang from BL 18U1 and BL 19U1 beamlines at NCPSS and Shanghai Synchrotron Radiation Facility (SSRF) for help with crystal data collection and processing. We thank Y. Yu, S. He, Y. Wang and S. Li from NCPSS for help with confocal microscopy and FACS. This work was supported by grants from the Ministry of Science and Technology of China (2013CB910402 to M.L.), the National Natural Science Foundation of China (31330040 and 31525007 to M.L., 31500625 to J.W.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08010201 to M.L.), the Outstanding Academic Leader Program of Science and Technology Commission of Shanghai Municipality (16XD1405000 to M.L. and C.H.), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (to J.W.). Y.L. acknowledges support from the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
References
- 1.Handel MA & Schimenti JC Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat. Rev. Genet 11, 124–136 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Petronczki M, Siomos MF & Nasmyth K Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell 112, 423–440 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Kleckner N Meiosis: how could it work? Proc. Natl. Acad. Sci. USA 93, 8167–8174 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeney S, Giroux CN & Kleckner N Meiosis-specific DNA double-strand breaks are catalyzed by Spoil, a member of a widely conserved protein family. Cell 88, 375–384 (1997). [DOI] [PubMed] [Google Scholar]
- 5.San Filippo J, Sung P & Klein H Mechanism of eukaryotic homologous, recombination. Annu. Rev Biochem 77, 229–257 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Neale MJ & Keeney S Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442, 153–158 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Massy B Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu. Rev. Genet 47, 563–599 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Baudat F, Imai Y & de Massy B Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 14, 794–806 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Syrjänen JL, Pellegrini L & Davies OR A molecular model for the role of SYCP3 in meiotic chromosome organization. elite 3, e02963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolcun-Filas E & Schimenti JC Genetics of meiosis and recombination in mice. Int. Rev Cell Mol. Biol 298, 179–227 (2012). [DOI] [PubMed] [Google Scholar]
- 11. Hirose Y et al. Chiasmata promote monopolar attachment of sister chromatids and their co-segregation toward the proper pole during meiosis i. PLoS Genet. 7, e1001329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bascom-Slack CA, Ross LO & Dawson DS Chiasmata, crossovers, and meiotic chromosome segregation. Adv Genet. 35, 253–284 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Boateng KA, Bellani MA, Gregoretti IV, Pratto F & Camerini-Otero RD Homologous pairing preceding SP011-mediated double-strand breaks in mice. Dev. Cell 24, 196–205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown PW et al. Meiotic synapsis proceeds from a limited number of subtelomeric sites in the human male. Am. J. Hum. Genet 77, 556–566 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koszul R, Kim KP, Prentiss M, Kleckner N &> Kameoka S Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 133, 1188–1201 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koszul R &> Kleckner N Dynamic chromosome movements during meiosis: a way to eliminate unwanted connections? Trends Cell Biol. 19, 716–724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CY et al. Mechanism and regulation of rapid telomere prophase movements in mouse meiotic chromosomes. Cell Rep. 11, 551–563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraoka Y &> Dernburg AF The SUN rises on meiotic chromosome dynamics. Dev. Cell 17, 598–605 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Ding X et al. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12, 863–872 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Daniel K et al. Mouse CCDC79 (TERB1) is a meiosis-specific telomere associated protein. BMC Cell Biol. 15, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibuya H, Ishiguro K &> Watanabe Y The TRF1 binding protein TERB1, promotes chromosome movement and telomere rigidity in meiosis. Nat. Cell Bioi 16, 145–156 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Shibuya H et al. MAJIN links telomeric DNA to the nuclear membrane by exchanging telomere cap. Cell 163, 1252–1266 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Viera A et al. CDK2 regulates nuclear envelope protein dynamics and telomere attachment in mouse meiotic prophase. J. Cell Sci 128, 88–99 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Tu Z et al. Speedy A-Cdk2 binding mediates initial telomere-nuclear envelope attachment during meiotic prophase I independent of Cdk2 activation. Proc. Nati. Acad. Sci. USA 114, 592–597 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry J, Palmer S, Gabriel A &> Ashworth A A short pseudoautosomal region in laboratory mice. Genome Res. 11, 1826–1832 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauppi L, Jasin M &> Keeney S The tricky path to recombining X and Y, chromosomes in meiosis. Ann. NY Acad. Sci 1267, 18–23 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauppi L et al. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331, 916–920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y et al. A shared docking motif in TRF1 and TRF2 used for differential, recruitment of telomeric proteins. Science 319, 1092–1096 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Wan B et al. SLX4 assembles a telomere maintenance toolkit by bridging multiple endonucleases with telomeres. Cell Rep. 4, 861–869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rai R et al. NBS1 phosphorylation status dictates repair choice of dysfunctional telomeres. Mol. Cell 65, 801–817 e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Fuente R et al. Meiotic pairing and segregation of achiasmate sex chromosomes in eutherian mammals: the role of SYCP3 protein. PLoS Genet. 3, e198 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries FAT et al. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 19, 1376–1389 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klutstein M &> Cooper JP The chromosomal courtship dance-homolog pairing in early meiosis. Curr. Opin. Cell Biol 26, 123–131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherthan H Analysis of telomere dynamics in mouse spermatogenesis. Methods Moi. Bioi 558, 383–399 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Scherthan H et al. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J. Cell Biol 134, 1109–1125 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter N, Börner GV, Lichten M &> Kleckner N Gamma-H2AX illuminates meiosis. Nat. Genet 27, 236–238 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Celli GB &> de Lange T DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol 7, 712–718 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Sfeir A et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138, 90–103 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SH, Kaminker P &> Campisi J TIN2, a new regulator of telomere length in human cells. Nat. Genet 23, 405–412 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye JZ et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frescas D &> de Lange T TRF2-tethered TIN2 can mediate telomere protection by TPP1/POT1. Mol. Cell. Bioi 34, 1349–1362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 43.Minor W, Cymborowski M, Otwinowski Z & Chruszcz M HKL-3000: the integration of data reduction and structure solution-from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr 62, 859–866 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Murshudov GN, Vagin AA &> Dodson EJ Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr 53, 240–255 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Emsley P, Lohkamp B, Scott WG & Cowtan K Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrodinger L The PyMOL Molecular Graphics System, Version 1.8. (2015). [Google Scholar]
- 47.Moretti P, Freeman K, Coodly F & Shore D Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 8, 2257–2269 (1994). [DOI] [PubMed] [Google Scholar]
- 48.Bastos PI. et al. Flow cytometric characterization of viable meiotic and postmeiotic cells by Ploechst 33342 in mouse spermatogenesis. Cytometry A 65, 40–49 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Getun IV, Torres B &> Bois PR Flow cytometry purification of mouse meiotic cells. J. Vis. Exp 50, 2602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters AH, Plug AW, van Vugt MJ & de Boer PA drying-down technique for the spreading of mammalian meiocytes from the male and female germ line. Chromosome Res. 5, 66–68 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Scherthan PI. et al. Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores. Mol. Biol. Cell 11, 4189–4203 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates and structure factors for the TRF1trfh-TERB1TBm complex has been deposited to the Protein Data Bank (PDB) under the accession code PDB 5XUP. Source data for biochemical experiments that support the findings of this study are available from the corresponding author upon reasonable request. A Life Science Reporting Summary for this article is available.