Multiple sclerosis (MS) is a chronic, inflammatory and neurodegenerative disease of the central nervous system (CNS) affecting at least 2.5 million people worldwide. While the relapsing subtypes of MS are well treatable, the disease per se remains incurable and results in progressive disability. Its etiology is complex and far from being understood. However, it is well-established that its central histopathological hallmark is demyelination - the autoimmune destruction of myelin sheaths. These elaborate structures wrap around axons electrically isolating them and provide accelerated electrical transmission as well as physical and trophic support in the brain and spinal cord (Lassmann, 2018). Demyelination impairs axonal integrity which leads to permanent disability (Lassmann, 2018). Whereas relapsing MS (RMS) which is most common at disease onset is characterized by episodes of autoimmune attacks (relapse) followed by spontaneous partial functional recovery (remission), most patients eventually develop a progressive disease course. Progressive MS stages, however, are mainly characterized by reduced or absent immune cell infiltration but ongoing neurodegeneration. Neuroregeneration in MS, on the other hand, basically refers to myelin repair - a process that can repair some of the existing lesions via recruitment of resident oligodendroglial precursor cells (OPCs) and neural stem cells (NSCs) which can differentiate and produce new axonal myelin sheaths restoring axonal integrity (Franklin and Ffrench-Constant, 2017). However, the repair capacity of precursor- and stem cells declines with age and disease progression. Moreover, differences in the extent of myelin regeneration can be observed between lesions and patients, potentially indicating heterogeneous underlying mechanisms which interfere with myelin restoration (Franklin and Ffrench-Constant, 2017). In this regard, several oligodendroglial differentiation inhibitors have been identified which are supposed to prevent successful cell maturation in an inflammatory environment (Kremer et al., 2011).
Of note, whereas a number of RMS treatments exist that successfully reduce relapse rate, none of the currently available disease-modifying therapies (DMT) has been shown to effectively enhance lesion repair. Hence, there is an unmet clinical need to develop new disability-reversing therapies aiming at the preservation of both axons and oligodendroglial cells. Different strategies for enhancing remyelination are conceivable including cell-based therapies relying on exogenous cell supply (Scolding et al., 2017), the neutralization of differentiation inhibitors (Kremer et al., 2011) or direct stimulatory approaches for improved adult oligodendrogenesis (Kremer et al., 2016). As cell-based therapies are restricted in their practical feasibility, stimulation and promotion of endogenous cell-based repair represents a more promising avenue. To this end, the development of new drugs, acting on inhibitory or stimulatory glial pathways, and the repurposing of existing drugs represent possible approaches. The strategy of drug repurposing offers the advantage of identifying new targets for known drugs already clinically approved for other indications minimizing risks and costs. Hence, high-throughput drug-screenings (Mei et al., 2014) as well as computational drug-repurposing approaches (Azim et al., 2017) are being used. In this context, recent screenings identified the ability of the histamine H1 receptor blocker clemastine, the muscarinic receptor antagonist benztropine and the atypical neuroleptic quetiapine, to promote myelin repair (Mei et al., 2014). On the other hand, ocrelizumab, a monoclonal antibody directed against CD20 on B cells and initially designed for treatment of rheumatoid arthritis (RA), demonstrated efficacy on disability progression in primary progressive MS patients (Montalban et al., 2017; Kremer et al., 2018). Moreover, the phosphodiesterase inhibitor ibudilast, an approved asthma treatment, was found to reduce brain atrophy in progressive MS patients (Kremer et al., 2018). Investigating its effects in secondary progressive MS, a clinical study found that siponimod, a novel sphingosine-1-phosphate (S1P)-receptor modulator, reduced confirmed disability progression by 21% over three months of treatment (Kappos et al., 2018). Finally, in our recent own contribution to the field, we closer investigated the effect of teriflunomide, currently used as an immunomodulatory DMT for RMS. In our preclinical study we focused on oligodendroglial cells, their differentiation capacity and their ability to differentiate and wrap around central nervous system (CNS) axons. We could demonstrate that teriflunomide - beyond its role as an immune modulator acting via inhibition of pyrimidine biosynthesis in activated lymphocytes - can also significantly promote OPC differentiation and internode formation (Figure 1), particularly when applied early and in pulses (Göttle et al., 2018). Future in vivo studies will reveal to what degree teriflunomide also holds promise for remyelination in hostile in vivo settings and whether such repair processes could explain the reduced disability progression as observed in the teriflunomide multiple sclerosis trials (TEMSO, ClinicalTrials.gov number NCT00134563; TOWER, ClinicalTrials.gov number NCT00751881) (Freedman et al., 2018). At the current time-point it can therefore be concluded that, given the heterogeneity of clinical MS subtypes and the dynamics of lesion development in time and space, it will be challenging to find the appropriate window of opportunity for therapy – no matter if using newly designed drugs or repurposed ones.
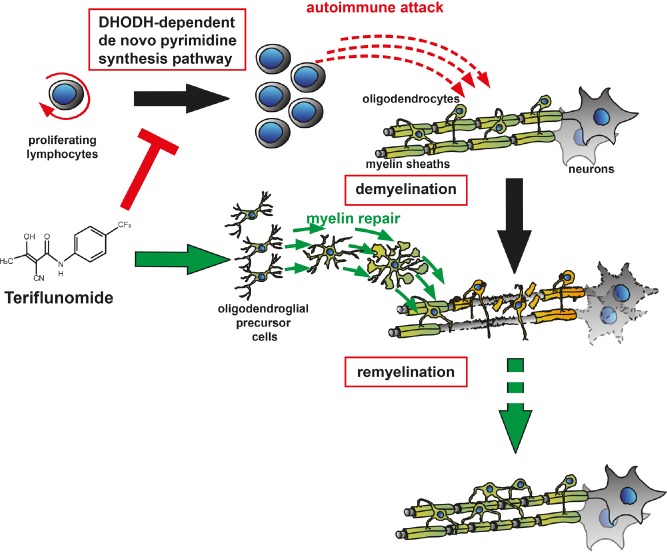
Figure 1.
Teriflunomide's mode of action.
It has been well accepted that teriflunomide reduces the number of activated peripheral T and B lymphocytes, which could potentially infiltrate the central nervous system (CNS). Our new study provides evidence that local parenchymal teriflunomide concentrations could also positively affect oligodendroglial precursor cells by means of promoting cell differentiation, maturation and subsequent generation of myelin sheaths around previously demyelinated axons.
Cited research work in the laboratory of the authors was supported by the Jürgen Manchot Foundation Düsseldorf, by the research commission of the medical faculty of Heinrich-Heine-University of Düsseldorf and by a grant of Sanofi Genzyme. The MS Center at the Department of Neurology is supported in part by the Walter and Ilse Rose Foundation and the James and Elisabeth Cloppenburg, Peek & Cloppenburg Düsseldorf Stiftung.
Additional file: Open peer review reports 1 (126.9KB, pdf) , 2 (99.8KB, pdf) .
Footnotes
Copyright transfer agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Tetsuya Akaishi, Tohoku University, Japan; Anna Maria Colangelo, University of Milano-Bicocca, Italy.
References
- Azim K, Angonin D, Marcy G, Pieropan F, Rivera A, Donega V, Cantu C, Williams G, Berninger B, Butt AM, Raineteau O. Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol. 2017;15:e2000698. doi: 10.1371/journal.pbio.2000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM, Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18:753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Wolinsky JS, Comi G, Kappos L, Olsson TP, Miller AE, Thangavelu K, Benamor M, Truffinet P, O’Connor PW TEMSO and TOWER Study Groups. The efficacy of teriflunomide in patients who received prior disease-modifying treatments: Subgroup analyses of the teriflunomide phase 3 TEMSO and TOWER studies. Mult Scler. 2018;24:535–539. doi: 10.1177/1352458517695468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttle P, Manousi A, Kremer D, Reiche L, Hartung HP, Küry P. Teriflunomide promotes oligodendroglial differentiation and myelination. J Neuroinflammation. 2018;15:76. doi: 10.1186/s12974-018-1110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, Arnold DL, Arnould S, Scherz T, Wolf C, Wallström E, Dahlke F EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- Kremer D, Küry P, Hartung HP. ECTRIMS/ACTRIMS 2017: Closing in on neurorepair in progressive multiple sclerosis. Mult Scler. 2018;24:696–700. doi: 10.1177/1352458518768770. [DOI] [PubMed] [Google Scholar]
- Kremer D, Aktas O, Hartung HP, Küry P. The complex world of oligodendroglial differentiation inhibitors. Ann Neurol. 2011;69:602–618. doi: 10.1002/ana.22415. [DOI] [PubMed] [Google Scholar]
- Kremer D, Göttle P, Hartung HP, Küry P. Pushing forward: remyelination as the new frontier in CNS diseases. Trends Neurosci. 2016;39:246–263. doi: 10.1016/j.tins.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med. 2018;8:a028936. doi: 10.1101/cshperspect.a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SPJ, Shen YA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, Etxeberria A, Xiao L, Franklin RJM, Green A, Hauser SL, Chan JR. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med. 2014;20:954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X, Belachew S, Wolinsky JS. Ocrelizumab in primary progressive and relapsing multiple sclerosis. N Engl J Med. 2017;376:1694. doi: 10.1056/NEJMc1702076. [DOI] [PubMed] [Google Scholar]
- Scolding NJ, Pasquini M, Reingold SC, Cohen JA International Conference on Cell-Based Therapies for Multiple Sclerosis, International Conference on Cell-Based Therapies for Multiple Sclerosis, International Conference on Cell-Based Therapies for Multiple Sclerosis. Cell-based therapeutic strategies for multiple sclerosis. Brain. 2017;140:2776–2796. doi: 10.1093/brain/awx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.