Keywords: nerve regeneration, neural tube defects, advanced glycation end products, diabetic embryopathy, oxidative stress, N-(carboxymethyl)lysine, malondiadehyde, N(ε)-(carboxyethyl) lysine, embryo, H2O2, bovine serum albumin, neural regeneration
Abstract
Our previous study showed an association between advanced glycation end products (AGEs) and neural tube defects (NTDs). To understand the molecular mechanisms underlying the effect of AGEs on neural tube development, C57BL/6 female mice were fed for 4 weeks with commercial food containing 3% advanced glycation end product bovine serum albumin (AGE-BSA) or 3% bovine serum albumin (BSA) as a control. After mating mice, oxidative stress markers including malondialdehyde and H2O2 were measured at embryonic day 7.5 (E7.5) of gestation, and the level of intracellular reactive oxygen species (ROS) in embryonic cells was determined at E8.5. In addition to evaluating NTDs, an enzyme-linked immunosorbent assay was used to determine the effect of embryonic protein administration on the N-(carboxymethyl) lysine reactivity of acid and carboxyethyl lysine antibodies at E10.5. The results showed a remarkable increase in the incidence of NTDs at E10.5 in embryos of mice fed with AGE-BSA (no hyperglycemia) compared with control mice. Moreover, embryonic protein administration resulted in a noticeable increase in the reactivity of N-(carboxymethyl) lysine and N(ε)-(carboxyethyl) lysine antibodies. Malondialdehyde and H2O2 levels in embryonic cells were increased at E7.5, followed by increased intracellular ROS levels at E8.5. Vitamin E supplementation could partially recover these phenomena. Collectively, these results suggest that AGE-BSA could induce NTDs in the absence of hyperglycemia by an underlying mechanism that is at least partially associated with its capacity to increase embryonic oxidative stress levels.
Introduction
Neural tube defects (NTDs) are severe birth defects of the central nervous system. The prevalence of NTDs varies widely between 1 and 10 per 1000 births, depending on geographic region and ethnic grouping (Au et al., 2010; Machín et al., 2015). Both genetic and non-genetic factors are involved in the etiology of NTDs (Copp and Greene, 2010; Hall and Solehdin, 2015). Although interventions such as folic acid supplementation and glucose level control have been recommended to prevent NTDs, a high incidence of NTDs still occurs in rats (Fleming and Copp, 1998; Suhonen, 2000; Loffredo et al., 2001; Liu et al., 2017).
Glycation is the nonenzymatic reaction of glucose or other reducing sugars with primary amino groups of proteins. Major products of glycation and oxidation of proteins and lipids include advanced glycation end products (AGEs) such as fluorescent pentosidine (Bhat et al., 2014; Siddiqui et al., 2015), nonfluorescent Nε-(carboxymethyl) lysine (CML), and other species (Ramasamy et al., 2005). AGEs are generated in vivo as a normal consequence of metabolism, but their formation is accelerated under conditions of hyperglycemia. Chronic accumulation of AGEs is associated with diabetes, rheumatoid arthritis, systemic lupus crythematosus, renal insufficiency, inflammation, and aging (Cerami, 1997; Nicholl and Bucala, 1998; Rodriguez-Garcia, 1998; Mohamed et al., 1999; Schmidt et al., 2000; Singh, 2001). However, the effects of AGEs on embryonic development remain unclear.
The results of a study suggest that AGEs could play an important role in diabetic embryopathy (Li and Chen, 2014). As AGEs can cause single-strand breaks in genomic DNA, they can have serious teratogenic effects. While the incidence of congenital abnormalities is not increased in short-term, pregnancy-induced hyperglycemia, it is increased if long-term poor glycemic control predates conception, further implicating a role for AGEs in diabetic embryopathy (Millis, 1988; Eriksson and Wentzel, 2016). Our recent study indicated serum AGEs level to be an important risk factor for NTD occurrence (Li et al., 2014; Daglar et al., 2016). These findings support the notion that AGEs could be an important factor in the mechanism underlying increased incidence of NTDs in diabetic or non-diabetic mothers.
An abundance of evidence supports the involvement of AGEs in a vicious cycle of inflammation, oxidative stress, amplified AGEs production, followed by more inflammation and more oxidative stress production (Yan, 1997; Obrosova, 2002; Jiang, 2004). Increased generation of reactive oxygen species (ROS) in response to AGEs could occur through multiple mechanisms, for example the ligation of receptor for AGEs (RAGE) and catalase (Ramasamy et al., 2005; Lin et al., 2016). As RAGE is expressed in early embryos (Yan, 1997; Wang et al., 2008), AGEs might exert their effects on embryonic development through RAGE or other mechanisms to induce oxidative stress.
The present study sought to determine if exogenous AGE-modified proteins could induce NTDs in mice and, if so, the molecular mechanism underlying this phenomenon.
Materials and Methods
Animals
One hundred and ninety-two 4–6-week-old (40–60 g) specific pathogen-free C57BL/6 mice (48 males, 144 females) were obtained from the Animal Center of Xi’an Jiaotong University of China [SCXK (Shaan) 2017-003]. All experimental procedures were approved by the Animal Ethics Committee of Northwest University of China (Approval No. NWU-AWC-20170605M).
Preparation of AGEs
AGE bovine serum albumin (AGE-BSA) was produced by incubating BSA (Sigma, St. Louis, MO) at a concentration of 50 g/L with 1 M D-glucose (Sangon Bio, SHH, China) in 0.1 M phosphate-buffered saline (PBS, pH 7.4) at 37°C for up to 8 weeks. Unbound glucose and low molecular weight reactants were removed through dialysis against PBS. AGE-BSA was lyophilized and resuspended in PBS. The products were quantified by measuring optical density (OD) at 405 nm.
Induction of diabetes
Diabetes was induced in 4–6-week-old female C57BL/6 mice with 100 mg/kg streptozotocin (Sigma) dissolved in 10 mM sodium citrate (pH 4.5). Normal blood glucose levels were maintained in streptozotocin-diabetic mice by subcutaneously implanting these animals with insulin pellets (Sigma) before pregnancy, until development of hyperglycemia was induced at E4.5 (D group), thereby exposing the embryo to hyperglycemia during the entire post-implantation period (Li et al., 2005). Mice in the control group were only administered PBS. Afterwards, female and male mice were placed in the same cage. On the day of copulation, hyperglycemic mice were fed with commercial food containing 0.125% (w/w) vitamin E (VitE) (D/VitE group).
Animal intervention
A total of 192 C57BL/6 mice (48 males, 144 females) were randomly assigned to three groups: an AGE-BSA group (n = 72) consisting of 54 females and 18 males fed with commercial food containing 3% AGE-BSA, a BSA group (n = 48) consisting of 36 females and 12 males fed with commercial food containing 3% BSA, and a control group (n = 72) consisting of 54 females and 18 males fed with common commercial food.
Four weeks after feeding, the AGE-BSA group was subdivided into three subgroups: a D + AGEs group consisting of six females and two males with induced diabetes fed with commercial food after embryonic day 15 (E0.5); an AGE-BSA/VitE group of six females and two males fed with commercial food containing 0.125% (w/w) VitE; and an AGE-BSA group with six females and two males fed with commercial food after E0.5.
The BSA group was subdivided into two groups: a BSA/VitE group of six female and two male mice fed with commercial food containing 0.125% (w/w) VitE, and group of six female mice and two male mice fed with commercial food after E0.5.
The control group was subdivided into three groups: a D/VitE group of six females and two males in which diabetes was induced, and whom were fed with commercial food containing 0.125% (w/w) VitE after E0.5; a D group of six females and two males in which diabetes was induced, and whom were fed with commercial food after at E0.5; and a control group of six females and two males fed with commercial food after E0.5.
Male mice in the experiment were used only for breeding and were not ultimately included in the final analysis.
Detection of serum AGEs levels in pregnant mice
Blood samples were collected from the tails of pregnant mice after 4 weeks of feeding with 3% AGE-BSA commercial food or 3% BSA commercial food. Blood was centrifuged at 1500 × g for 10 minutes. Serum was collected in 1.5-mL tubes for AGEs assay. Determination of AGEs was based on spectrofluorimetric detection modified from a previously published method (Kalousova et al., 2002). Briefly, serum was diluted at 1:50 with PBS (pH 7.4) and fluorescence intensity was measured at the emission maximum (440 nm) upon excitation at 365 nm with a microplate reader (Tecan, Shanghai, China).
Preparation of CML-BSA and N(ε)-(carboxyethyl) lysine (CEL)-BSA
CML-BSA and CEL-BSA were prepared as previously described (Koito et al., 2004). Briefly, for CML-BSA, 50 g/L BSA was incubated at 37°C for 24 hours with 45 mM glyoxylic acid (Sigma) and 150 mM sodium cyanoborohydride (NaCNBH3) (Sigma) in 2 mL of 0.2 M phosphate buffer (pH 7.4), followed by dialysis against 0.01 M PBS (pH 7.4). For CEL-BSA, 50 g/L BSA was incubated at 37°C for 24 hours with 45 mM pyruvic acid (Sigma) and 150 mM NaCNBH3 in 2 mL of 0.2 M phosphate buffer (pH 7.4), followed by dialysis against 0.01 M PBS (pH 7.4).
Preparation of polyclonal anti-CML-BSA and anti-CEL-BSA antibodies
One mg of each immunogen was emulsified in 50% Freund's complete adjuvant (Boao, Beijing, China) and intradermally injected into rabbits. This was followed by four booster injections of 0.5 mg immunogen in 50% Freund's incomplete adjuvant (Boao) at 14, 28, and 42 days. Obtained serum was subjected to further affinity purification. Antibodies to CML-BSA and CEL-BSA were purified by affinity chromatography.
Preparation and immunochemical reactivity of embryonic protein
Embryos were collected from mice in each of the five groups (AGE-BSA, BSA, AGE-BSA/VitE, BSA/VitE, and control). The tissue was homogenized using a pestle for 20 strokes in lysis buffer and incubated on ice for 20 minutes. Samples were then centrifuged at 880,000 × g for 10 minutes. After centrifugation, the supernatant was transferred into a new tube. Protein concentration was measured using a Bradford assay. Each well was coated with mouse embryonic proteins (10 μg/mL) and then blocked with 1% non-fat milk. Following three washes with PBS containing Tween-20, each well was incubated with purified mouse polyclonal anti-CML and -CEL antibodies (1:4000) and washed three times. Horseradish peroxidase-conjugated anti-rabbit IgG (1:3000; Sangon, Shanghai, China) was added and incubated for 1 hour at 37°C. After washing three times, TMP solution was added and OD values were measured at 490 nm with a microplate reader (Tecan, Shanghai, China).
Preparation of embryonic cell suspensions and assessment of intracellular ROS production
E8.5 embryos were dissected from uteri in PBS, and extraembryonic structures were placed in Dulbecco's Modified Eagle's Medium (DMEM). Whole embryos were minced and passed through a cell strainer with 40-μM nylon mesh (BD FalconTM, Shanghai, China), to generate single-cell suspensions. The suspension was prepared at a density of 2 × 107 cells/mL. Embryonic cells were cultured in DMEM under a range of AGE-BSA concentrations for 24 hours: 0, 200, 400, and 800 μg/mL in DMEM. Cells were subsequently washed twice with PBS. Intracellular ROS production was measured with 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) (Molecular Probes, Shanghai, China). Embryonic cells were incubated with DCF-DA (5 μM) in serum-free medium at 37°C for 30 minutes and then washed with PBS. DCF fluorescence was excited at 488 nm, and emission at 530 nm was measured on a Gemini EM Reader (Molecular Devices, Shanghai, China). Fluorescence intensity values are presented as the percentage of control values after subtraction of background fluorescence.
Measurement of malondialdehyde (MDA) and H2O2
E7.5 embryos were collected for to assay MDA and H2O2. MDA was assayed using an ultraviolet-visible spectrophotometer and photometer (Agilent, Beijing China) using the thiobarbituric acid test (Ohya et al., 1993). H2O2 was assayed using a kit from Cayman Chemicals (Shanghai, China) according to the manufacturer's instructions. Results were normalized to protein concentrations in cell extracts.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed by SPSS software (SPSS 19.0, IBM, Armonk, NY). One-way analysis of variance followed by a least significant difference test was used to examine differences between treatment groups. P values less than 0.05 were considered statistically significant.
Results
Verification of AGE-BSA
The OD at 405 nm of AGE-BSA gradually increased from 0 to 2.4, while that of control BSA prepared under the same conditions without glucose was lower than 0.15 (Figure 1).
Figure 1.
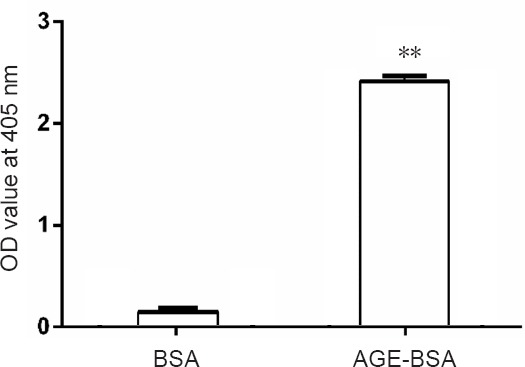
Optical density (OD) value at 405 nm of advanced glycation end product bovine serum albumin (AGE-BSA) and bovine serum albumin (BSA) control at the end of the 8-week incubation.
**P < 0.01, vs. BSA group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference test).
Serum AGEs level in AGE-BSA feeding pregnant mice
Serum levels of AGEs increased significantly (P < 0.01) in AGE-BSA pregnant mice compared with BSA control pregnant mice. VitE supplementation did not affect levels of AGEs in AGE-BSA or BSA control pregnant mice (P > 0.05; Figure 2).
Figure 2.

Serum levels of AGEs in mice fed with AGE-BSA or BSA.
**P < 0.01, vs. BSA group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by least significant difference test). Control group: Mice were fed with normal commercial food. AGE-BSA group: Female mice were fed with commercial food containing 3% AGE-BSA. AGE-BSA/VitE group: Female mice were fed with 3% AGE-BSA for 4 weeks and then fed with 0.125% (w/w) VitE at day 0.5 of gestation. BSA group: Female mice were fed with commercial food containing 3% BSA. BSA/VitE group: Female mice were fed with 3% BSA for 4 weeks and then fed with 0.125% (w/w) VitE at day 0.5 of gestation. AGEs: Advanced glycation end products; BSA: bovine serum albumin; AGE-BSA: advanced glycation end product bovine serum albumin; VitE: vitamin E; AU: arbitrary units.
Immunochemical reactivity of embryonic proteins
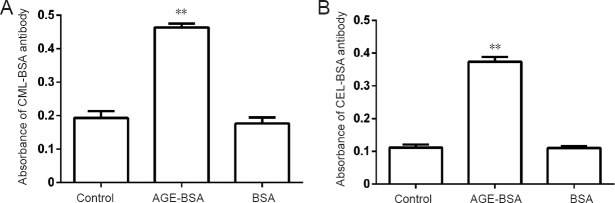
Reactivity of CML-BSA and CEL-BSA antibodies, which recognize embryonic proteins, increased significantly in embryos of AGE-BSA mice compared with control mice and BSA mice, indicating that mice fed with AGE-BSA food could increase embryonic CEL and CML contents (P < 0.01; Figure 3). VitE supplementation did not change embryo protein reactivity to CML and CEL (data not shown).
Figure 3.
Immunochemical reactivity of antibody against CML-BSA (A) and CEL-BSA (B) with embryo proteins.
**P < 0.01, vs. BSA group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference test). Control group: Mice were fed with normal commercial food. AGE-BSA group: Female mice were fed with commercial food containing 3% AGE-BSA. BSA group: Female mice were fed with commercial food containing 3% BSA. AGEs: Advanced glycation end products; BSA: bovine serum albumin; AGE-BSA: advanced glycation end product bovine serum albumin; CML-BSA: Nε-(carboxymethyl) lysine-bovine serum albumin; CEL-BSA: N(epsilon)-(carboxyethyl) lysine-bovine serum albumin.
AGE-BSA induces NTDs in mice in vivo
As shown in Figure 4, the incidence of NTDs was significantly increased in AGE-BSA mice at E10.5, similar to the effect of hyperglycemia (Figure 5). Incidence of NTDs was also significantly increased in diabetic AGE-BSA mice compared with diabetic mice or AGE-BSA mice (P < 0.05). In contrast, there was no effect of BSA or saline control administration on embryonic developmental malformation. The increased incidence of NTDs induced by AGE-BSA administration in mice could be partially blocked by VitE supplementation (Figure 4 & Table 1).
Figure 4.
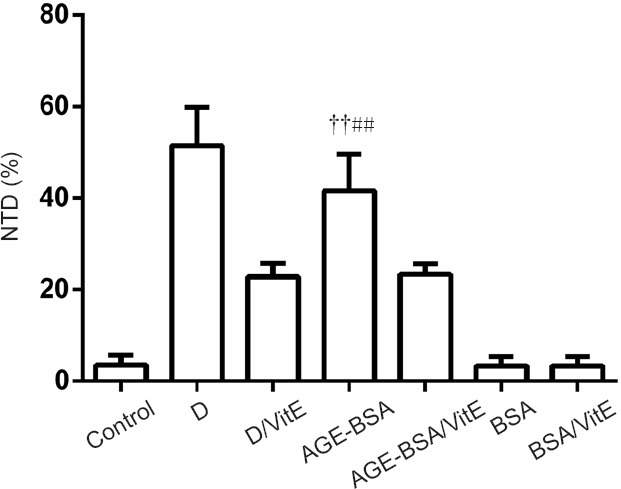
Effects of AGE-BSA on incidence of NTDs in C57BL/6 mice.
After feeding for 4 weeks with food containing AGE-BSA, C57BL/6 mice displayed significantly higher incidence of NTDs compared with control (fed with normal commercial food) mice (††P < 0.01, vs. control). VitE could decrease, but not recover all, NTDs in diabetic and AGE-BSA mice (##P < 0.01, vs. AGE-BSA/VitE). Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by least significant difference test). Control group: Female mice were fed with normal commercial food; D group: Diabetes was induced in female mice. D/VitE group: Diabetic female mice were fed with 0.125% (w/w) VitE at day 0.5 of gestation. AGE-BSA group: Female mice were fed with commercial food containing 3% AGE-BSA. AGE-BSA/VitE group: Female mice were fed with 3% AGE-BSA for 4 weeks and then with 0.125% (w w) VitE at day 0.5 of gestation. BSA group: Female mice were fed with commercial food containing 3% BSA. BSA/VitE group: Female mice were fed with 3% BSA for 4 weeks and then with 0.125% (w/w) VitE at day 0.5 of gestation. AGEs: Advanced glycation end products; BSA: bovine serum albumin; AGE-BSA: advanced glycation end product bovine serum albumin; VitE: vitamin E; NTDs: neural tube defects.
Figure 5.
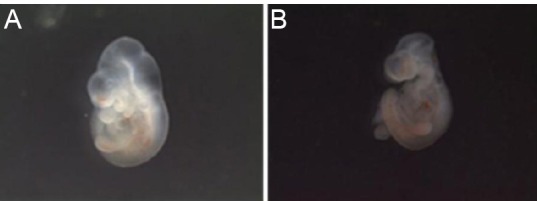
Embryos with normal neural tube formation and neural tube defects.
(A)Normal embryo at embryonic day 10.5 (E10.5) from mother fed with 3% bovine serum albumin. (B) E10.5 embryo exhibiting neural tube defects from mother fed with 3% advanced glycation end product bovine serum albumin.
Table 1.
Morphology of embryonic day 10.5 mouse embryos from different groups

Serum levels of MDA and H2O2 in embryos of mice fed with AGE-BSA
To further test whether increased incidence of NTDs is the result of oxidative stress induced by AGE-BSA, oxidative stress markers MDA and H2O2 were assayed in embryos. Significant increases in MDA levels (P < 0.01; Figure 6) and H2O2 (P < 0.01) were observed in embryos of AGE-BSA mice compared with BSA control mice. Moreover, MDA and H2O2 levels were significantly higher in diabetic AGE-BSA mice compared with diabetic mice or AGE-BSA mice. VitE significantly decreased MDA and H2O2 levels (P < 0.01) in embryos of AGE-BSA mice compared with BSA control mice (Figure 6).
Figure 6.
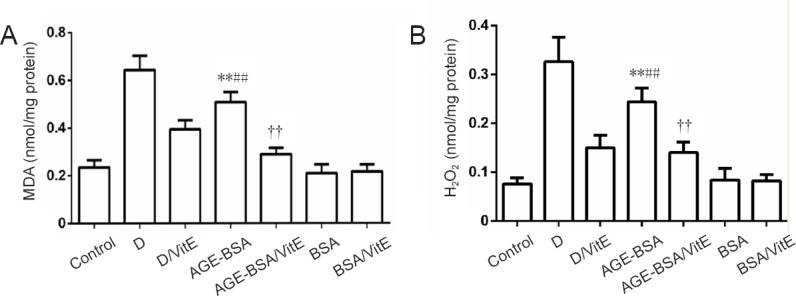
MDA and H2O2 levels in AGE-BSA and control mice with different treatments.
For MDA (A) and H2O2 (B) assays, **P < 0.01, vs. BSA group; ##P < 0.01, vs. AGE-BSA/VitE group; ††P < 0.01, vs. control group. Data are expressed as mean ± SD (n = 6; one-way analysis of variance followed by least significant difference test). Control group: Female mice were fed with normal commercial food. D: Diabetes was induced in female mice. D/VitE group: Diabetic female mice were fed with 0.125% (w/w) VitE at day 0.5 of gestation. AGE-BSA: Female mice were fed with commercial food containing 3% AGE-BSA. AGE-BSA/VitE group: Female mice were fed with 3% AGE-BSA for 4 weeks and then with 0.125% (w/w) VitE at day 0.5 of gestation. BSA group: Female mice were fed with commercial food containing 3% BSA. BSA/VitE group: Female mice were fed with 3% BSA for 4 weeks and then with 0.125% (w/w) VitE at day 0.5 of gestation. AGEs: Advanced glycation end products; BSA: bovine serum albumin; AGE-BSA: advanced glycation end product bovine serum albumin; VitE: vitamin E; MDA: malondialdehyde.
Intracellular ROS in embryonic cells
In situ ROS production by embryonic cells at E8.5, as measured by DCF fluorescence, indicated significantly increased ROS levels after 24-hour incubation with 800 μg/mL AGE-BSA. Intracellular ROS production increased by 150.40 ± 29.17% in embryonic cells cultured in 800 μg/mL AGE-BSA medium compared with control cells cultured in 800 μg/mL BSA medium (100%; P < 0.01; Figure 7).
Figure 7.
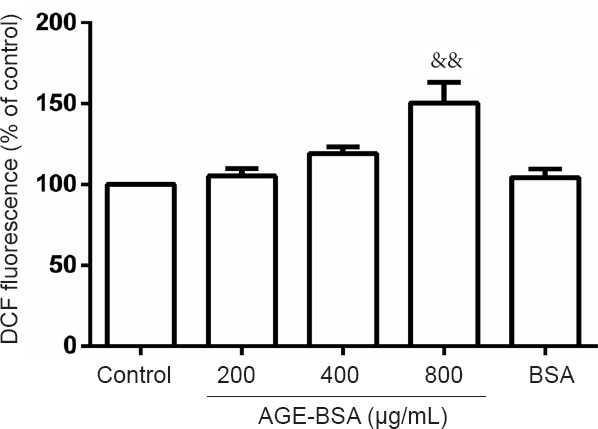
Effects of AGE-BSA on reactive oxygen species production in embryonic cells on embryonic day 8.5 (E8.5).
After culturing embryonic cells harvested at E8.5 in DMEM containing 800 μg/mL AGE-BSA for 24 hours, increased reactive oxygen species production was observed (&&P < 0.01, vs. other 4 groups). Data are expressed as mean ± SD (n = 6; one-way analysis of variance followed by least significant difference test). Control group: E8.5 cells were cultured in DMEM. 200 μg/mL AGE-BSA group: E8.5 cells were cultured in DMEM containing 200 μg/mL AGE-BSA. 400 μg/mL AGE-BSA group: E8.5 cells were cultured in DMEM containing 400 μg/mL AGE-BSA. 800 μg/mL AGE-BSA group: E8.5 cells were cultured in DMEM containing 800 μg/mL AGE-BSA. BSA group: E8.5 cells were cultured in DMEM containing BSA. AGEs: Advanced glycation end products; BSA: bovine serum albumin; AGE-BSA: advanced glycation end product bovine serum albumin; DMEM: Dulbecco's Modified Eagle's Medium.
Discussion
Contributions and limitations of previous studies
AGEs, a heterogenous group of molecules formed by the nonenzymatic reaction of glucose with protein, are critical pathogenic mediators of diabetes complications and other associated diseases such as aging, atherosclerosis, and Alzheimer's disease. The formation of AGEs occurs at a constant and slow rate in normal conditions, but is markedly accelerated in hyperglycemia because of increased availability of glucose, and significantly increased by eating habits or lifestyle choices such as smoking. However, the role of AGEs in embryonic development has remained in question.
Preparation of AGE-BSA by incubation of BSA and glucose in vitro has been widely used to mimic the effects of AGEs (Schmidt et al., 1995). By incubating BSA and glucose in vitro for 8 weeks, an AGE-BSA mixture including intermediate and late AGEs is produced (Schmidt et al., 1995; Alam et al., 2016; Koch et al., 2017; Pietsch et al., 2017). AGE-specific fluorescence, which has been described in several publications (Monnier, 1981; Lo, 1994; Leclere, 2001), is widely used to detect AGEs in vivo and in vitro (Nagai, 2000; Valencia et al., 2004). One study showed that fluorescence detected at an excitation/emission of 365/440 nm is a good choice for glucose-modified human serum albumin (Schmitt et al., 2005). Indeed, 365/440 nm has been proven to be a very effective wavelength to detect specific AGEs in blood and tissue samples (Kalousova, 2002; Jing et al., 2009; Bohlooli et al., 2013). As such, 365/440 nm was used in this study to detect AGEs levels in mouse blood.
Apart from endogenous AGE formation, AGEs are also absorbed by the body from exogenous sources including cigarette smoke and highly heated processed foods, which contain high amounts of AGEs (Stirban and Tschöpe, 2015). Kinetic studies indicated that 10–30% of dietary AGEs consumed are intestinally absorbed. Thus, plasma concentrations of AGEs appear to be directly influenced by dietary AGE intake and the body's capacity for AGE elimination.
Distinguishing characteristics of the current study
In the present study, AGE-BSA was fed to mice to examine the effect of AGEs (Peppa et al., 2003; Chen et al., 2013). The results showed that serum levels of AGEs significantly increased in AGE-BSA mice compared with BSA control mice. Moreover, CML-BSA and CEL-BSA reactivity were remarkably increased in the embryos of AGE-BSA mice compared with control mice, indicating increased CEL and CML contents in embryos of mice fed with AGE-BSA food. Thus, feeding is a suitable method to administer AGEs to animals.
The results of this study demonstrated a significantly higher incidence of NTDs in AGE-BSA mice compared with control mice. In addition, a higher incidence of NTDs was observed in diabetic AGE-BSA mice compared with diabetic mice. This result is consistent with previous results indicating that AGEs is a risk factor for induction of NTDs in the absence of hyperglycemia (Carmichael, 2003; Suarez et al., 2012). As food is the major source of exogenous AGEs, it is reasonable to conclude that lifestyle, especially eating behaviors, could be an important factor for pregnant women. Notably, VitE supplementation could inhibit the increased incidence of NTDs caused by AGE-BSA compared with the non-VitE group, similar to its effect on diabetic mice. Therefore, the mechanism underlying the teratogenic effect of AGE-BSA on embryonic development may be similar to the mechanism induced by diabetes, at least with regard to increased oxidative stress.
While overproduction of oxidative stress is associated with embryonic dysmorphology in diabetic pregnancy, administration of antioxidants could attenuate this teratogenic effect in embryos subjected to a hyperglycemic environment both in vivo and in vitro (Loeken, 2005). For non-diabetic pregnant women, oxidative stress may also be a risk factor for inducing embryonic malformation. First, a high level of free radicals can directly damage DNA or inhibit Pax3 expression, which is crucial for neural tube development. Second, oxidative stress caused by AGE-RAGE interaction is the major mechanism underlying the biological effect of AGE. Although we did not observe significantly different serum MDA levels between NTDs-affected and unaffected control pregnant women in the presence of different AGEs levels (Li et al., 2014), we found significantly different MDA levels in embryos between AGE-BSA and control mice. One explanation for this is that the materials used to assay MDA were different: one is for human serum, while the animal experiment assayed MDA levels of whole embryos. Second, as the background, diet, and other lifestyle habits of mice are identical, it is easy to observe the effect of AGEs on MDA levels to the exclusion of other factors. In contrast, many factors vary between individual humans, including different eating habits. Furthermore, we observed that the antioxidant VitE could decrease the incidence of NTDs caused by AGE-BSA feeding but could not totally block the teratogenic effect. Thus, increased oxidative stress is unlikely to be the only mechanism by which AGEs induce NTDs.
Limitations
The results of this study clearly indicated that AGE-BSA could induce NTDs in embryonic mice, at least in part by elevating oxidative stress levels. However, this mechanism is not necessarily the only cause of NTDs. Further studies will be important to demonstrate the detailed localization of CML and CEL in pathological embryonic tissues with specific antibodies, as well as changes in signaling pathways downstream of the oxidative stress caused by AGEs.
Significance
In conclusion, exogenous application of AGE-BSA to C57BL/6 mice resulted in an increased incidence of NTDs in the absence of hyperglycemia, thus replicating the effects of maternal diabetes on embryonic development. These findings both confirm and provide a new mechanistic basis for the role of AGEs in NTDs occurring in nondiabetic pregnancies and NTDs caused by maternal diabetes.
Footnotes
Conflicts of interest: The authors declare that there is no duality of interest associated with this manuscript.
Financial support: This work was supported by the grant from Shaanxi Technology Committee of China, No. 2013JM4001, and the China Scholarship Council for Ru-Lin Li. Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the papaer for publication.
Institutional review board statement: The procedures were in accordance with ethical standards of the Animal Ethics Committee of Northwest University of China (approval No. NWU-AWC-20170605M).
Reporting statement: This study follows the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Laboratory for Development, College of Life Sciences, Northwest University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the grant from Shaanxi Technology Committee of China, No. 2013JM4001; and the China Scholarship Council (CSC).
(Copyedited by Van Deusen A, Haase R, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Alam MS, Pathania S, Sharma A. Optimization of the extrusion process for development of high fibre soybean-rice ready-to-eat snacks using carrot pomace and cauliflower trimmings. Lebenson Wiss Technol. 2016;74:135–144. [Google Scholar]
- Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16:6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat SA, Sohail A, Siddiqui AA, Bano B. Effect of non-enzymatic glycation on cystatin: a spectroscopic study. J Fluoresc. 2014;24:1107–1117. doi: 10.1007/s10895-014-1391-2. [DOI] [PubMed] [Google Scholar]
- Bohlooli M, Moosavi-Movahedi AA, Taghavi F, Maghami P, Saboury AA, Moosavi-Movahedi Z, Farhadi M, Hong J, Sheibani N, Habibi-Rezaei M. Investigation of thermal reversibility and stability of glycated human serum albumin. Int J Biol Macromol. 2013;62:358–364. doi: 10.1016/j.ijbiomac.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Bucala R, Model P, Cerami A. Modification of DNA by reducing sugars: a possible mechanism for nucleic acid aging and age-related dysfunction in gene expression. Proc Natl Acad Sci U S A. 1984;81:105–109. doi: 10.1073/pnas.81.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Schaffer, DM, Laurent C, Selvin S. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127–1131. doi: 10.1093/aje/kwg286. [DOI] [PubMed] [Google Scholar]
- Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed, Y, Vlassara H, Bucala R, Cerami A. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94:13915–13920. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Abell AM, Moon YS, Kim K. An advanced glycation end product (AGE)-receptor for AGEs (RAGE) axis restores adipogenic potential of senescent preadipocytes through modulation of p53 protein function. J Biol Chem. 2013;288:10949. doi: 10.1074/jbc.M112.399790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010;220:217. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglar K, Tokmak A, Kirbas A, Guzel AI, Erkenekli K, Yucel A, Uygur D. Maternal serum vitamin D levels in pregnancies complicated by neural tube defects. J Matern Fetal Neonatal Med. 2016;29:298–302. doi: 10.3109/14767058.2014.999037. [DOI] [PubMed] [Google Scholar]
- Eriksson UJ, Wentzel P. The status of diabetic embryopathy. Ups J Med Sci. 2016;121:96–112. doi: 10.3109/03009734.2016.1165317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Hall JG, Solehdin F. Genetics of neural tube defects. developmental disabilities research reviews 4:269-281. Biomed Environ Sci. 2015;17:79–86. [Google Scholar]
- Jiang JM, Wang Z, Li DD. Effects of AGEs on oxidation stress and antioxidation abilities in cultured astrocytes. Biomed Environ Sci. 2004;17:79–86. [PubMed] [Google Scholar]
- Jing H, Yap M, Wong PYY, Kitts DD. Comparison of physicochemical and antioxidant properties of egg-white proteins and fructose and inulin maillard reaction products. Food Bioproc Tech. 2009;4:1489–1496. [Google Scholar]
- Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51:597–604. [PubMed] [Google Scholar]
- Koito W, Araki T, Horiuchi S, Nagai R. Conventional antibody against Nepsilon-(carboxymethyl)lysine (CML) shows cross-reaction to Nepsilon-(carboxyethyl)lysine (CEL): immunochemical quantification of CML with a specific antibody. J Biochem. 2004;136:831–837. doi: 10.1093/jb/mvh193. [DOI] [PubMed] [Google Scholar]
- Koch LM, Emin A, Shchuchmann HP. Influence of processing conditions on the formation of whey protein-citrus pectin conjugates in extrusion. J Food Eng. 2017;193:1–9. [Google Scholar]
- Leclere J, Birlouez-Aragon I. The fluorescence of advanced maillard products is a good indicator of lysine damage during the maillard reaction. J Agric Food Chem. 2001;49:4682–4687. doi: 10.1021/jf001433o. [DOI] [PubMed] [Google Scholar]
- Lee AT, Cerami A. Elevated glucose 6-phosphate levels are associated with plasmid mutations in vivo. Proc Natl Acad Sci U S A. 1987;84:8311–834. doi: 10.1073/pnas.84.23.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Chase M, Jung SK, Smith PJ, Loeken MR. Hypoxic stress in diabetic pregnancy contributes to impaired embryo gene expression and defective development by inducing oxidative stress. Am J Physiol Endocrinol Metab. 2005;289:E591–E599. doi: 10.1152/ajpendo.00441.2004. [DOI] [PubMed] [Google Scholar]
- Li R, Chen X. Erythrocyte osmotic fragility increases with serum advanced glycated end products in cigarette smokers. Clin Hemorheol Microcirc. 2014;57:85–92. doi: 10.3233/CH-131801. [DOI] [PubMed] [Google Scholar]
- Li R, Yang P, Chen X, Wang L. Maternal serum AGEs levels in pregnancies associated with neural tube defects. Int J Dev Neurosci. 2014;33:57–61. doi: 10.1016/j.ijdevneu.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Lin JA, Wu CH, Lu CC, Hsia SM, Yen GC. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: An emerging biological factor in cancer onset and progression. Mol Nutr Food Res. 2016;60:1850. doi: 10.1002/mnfr.201500759. [DOI] [PubMed] [Google Scholar]
- Liu J, Li Z, Greene NDE, Li H, Ren A. The recurrence risk of neural tube defects (NTDs) in a population with high prevalence of NTDs in northern China. Oncotarget. 2017;8:72577–72583. doi: 10.18632/oncotarget.19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo TW, Westood ME, Mclellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. J Biol Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- Loeken MR. Current perspectives on the causes of neural tube defects resulting from diabetic pregnancy. Am J Med Genet C Semin Med Genet. 2005;135C:77–87. doi: 10.1002/ajmg.c.30056. [DOI] [PubMed] [Google Scholar]
- Loffredo CA, Wilson PD, Ferencz C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64:98. doi: 10.1002/tera.1051. [DOI] [PubMed] [Google Scholar]
- Millis JL, Knopp RH, Simpson JL, Jovanovic-Peterson L, Metzqer BE, Holmes LB, Aarons JH, Brown Z, Reed GF, Bieber FR. Lack of relation of increased malformation rates in infants of diabetic mothers to glycemic control during organgenesis. J Wildl Dis. 1988;18:389–391. doi: 10.1056/NEJM198803173181104. [DOI] [PubMed] [Google Scholar]
- Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler R, Nawroth PP. The role of oxidative stress and NF-kappa B activation in late diabetic complications. Biofactors. 1999;10:157–167. doi: 10.1002/biof.5520100211. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Cerami A. Nonenzymatic browing in vivo possible process for aging of long-lived proteins. Science. 1981;211:491. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Nagai R, Matsumoto K, Ling X, Suzuki H, Araki T, Horiuchi S. Glycolaldehyde, a reactive intermediate for advanced glycation end products, platys an important role in the generation of an active ligand for the macrophage scavenger receptor. Diabetes. 2000;49:1714. doi: 10.2337/diabetes.49.10.1714. [DOI] [PubMed] [Google Scholar]
- Nicholl ID, Bucala R. Advanced glycation endproducts and cigarette smoking. Cell Mol Biol (Noisy-le-grand) 1998;44:1025–1033. [PubMed] [Google Scholar]
- Obrosova IG. How does glucose generate oxidative stress in peripheral nerve. Int Rev Neurobiol. 2002;50:33–35. doi: 10.1016/s0074-7742(02)50071-4. [DOI] [PubMed] [Google Scholar]
- Ohya T. Reactivity of alkanals towards malondialdehyde (MDA) and the effect of alkanals on MDA determination with a thiobarbituric acid test. Biol Pharm Bull. 1993;16:1078–1082. doi: 10.1248/bpb.16.1078. [DOI] [PubMed] [Google Scholar]
- Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, Croitoru A, Thung S, Vlassara H. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52:2805. doi: 10.2337/diabetes.52.11.2805. [DOI] [PubMed] [Google Scholar]
- Pietsch VL, Emin MA, Schuchmann HP. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J Food Eng. 2017;198:28–35. [Google Scholar]
- Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Rodríguezgarcía J, Requena JR, Rodríguezsegade S. Increased concentrations of serum pentosidine in rheumatoid arthritis. Clin Chem. 1998;44:250–255. [PubMed] [Google Scholar]
- Rodriguez-Garcia J, Requena JR, Rodriguez-Segade S. A new antibody in rheumatoid arthritis targeting glycated IgG. Br J Rheumatol. 1998;37:1307–1314. doi: 10.1093/rheumatology/37.12.1307. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochimica Et Biophysica Acta. 2000;1498:99. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1): a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Schmitt J, Munch G, Gasic-Milencovic J. Characterization of advanced glycation end products for biochemical studies: side chain modifications and fluorescence characteristics. Anal Biochem. 2005;338:201–215. doi: 10.1016/j.ab.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation End-products (AGEs): an emerging concern for processed food industries. J Food Sci Tech. 2015;52:7561–7576. doi: 10.1007/s13197-015-1851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AA, Sohail A, Bhat SA, Rehman MT, Bano B. Non-enzymatic glycation of almond cystatin leads to conformational changes and altered activity. Protein Pept Lett. 2015;22:449–459. doi: 10.2174/0929866522666150326105704. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products_ a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Suarez L, Felkner M, Brender JD, Canfield MA. Dieting to lose weight and occurrence of neural tube defects in offspring of mexican–american women. Matern Child Health J. 2012;16:844–849. doi: 10.1007/s10995-011-0806-9. [DOI] [PubMed] [Google Scholar]
- Stirban A, Tschöpe D. Vascular effects of dietary advanced glycation end products. Int J Endocrinol. 2015;2015:836498. doi: 10.1155/2015/836498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen L, Hiilesmaa V, Teramo K. Glycemic control during early pregnancy and fetal malformations in women with type 1 diabetes mellitus. Diabetologia. 2000;43:79–82. doi: 10.1007/s001250050010. [DOI] [PubMed] [Google Scholar]
- Valencia JV, Weldon SC, Quinn D, Kiers GH, DeGroot J, TeKoppele JM, Hughes TE. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem. 2004;324:68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Wang L, Li S, Jungalwala FB. Receptor for advanced glycation end products (RAGE) mediates neuronal differentiation and neurite outgrowth. J Neurosci Res. 2008;86:1254–1266. doi: 10.1002/jnr.21578. [DOI] [PubMed] [Google Scholar]
- Westwood ME, Thornalley PJ. Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J Protein Chem. 1995;14:359–372. doi: 10.1007/BF01886793. [DOI] [PubMed] [Google Scholar]
- Yan H, Harding JJ. Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem J. 1997;328:599–605. doi: 10.1042/bj3280599. [DOI] [PMC free article] [PubMed] [Google Scholar]