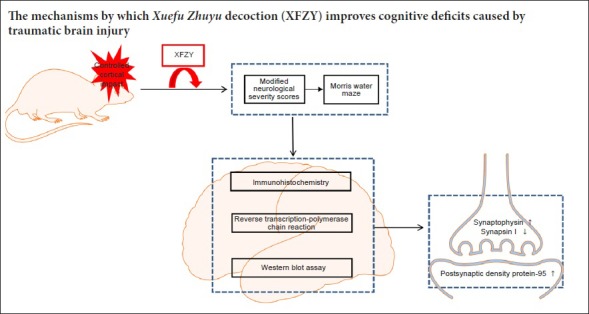
Keywords: nerve regeneration, traumatic brain injury, Xuefu Zhuyu decoction, neurological dysfunction, synapsin, synaptophysin, synapsin I, postsynaptic density protein-95, neuroprotection, neural regeneration
Abstract
Xuefu Zhuyu decoction has been used for treating traumatic brain injury and improving post-traumatic dysfunction, but its mechanism of action needs further investigation. This study established rat models of traumatic brain injury by controlled cortical impact. Rat models were intragastrically administered 9 and 18 g/kg Xuefu Zhuyu decoction once a day for 14 or 21 days. Changes in neurological function were assessed by modified neurological severity scores and the Morris water maze. Immunohistochemistry, western blot assay, and reverse-transcription polymerase chain reaction were used to analyze synapsin protein and mRNA expression at the injury site of rats. Our results showed that Xuefu Zhuyu decoction visibly improved neurological function of rats with traumatic brain injury. These changes were accompanied by increased expression of synaptophysin, synapsin I, and postsynaptic density protein-95 protein and mRNA in a dose-dependent manner. These findings indicate that Xuefu Zhuyu decoction increases synapsin expression and improves neurological deficits after traumatic brain injury.
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and long-term disability in the general population (Haring et al., 2015). Public epidemiological statistics indicate that around 250 per 100,000 persons undergo TBI each year (Popescu et al., 2015). In China, the incidence of TBI is dramatically increasing owing to increasing use of vehicles and high-rise buildings (Zhao et al., 2012). Approximately 15–30% of TBI patients incur cognitive impairments each year (Wada et al., 2012; Yorke et al., 2016). Cognitive impairments following TBI are considered the most disabling and distressing symptom for individuals, families, and society (Barman et al., 2016; Khan et al., 2017; Zhou et al., 2017).
As TBI is due to mechanical forces acting immediately on the brain, the primary injury cannot be treated. Hence, neuroscientists and doctors focus on therapeutic strategies during secondary injury post-TBI. Secondary injury is triggered progressively in minutes to days after the primary injury, and aggravates brain damage by oxidative stress, inflammation, and excitotoxicity (Ayton et al., 2014). TBI leads to neuronal loss and disruption of axonal microtubule networks, which are associated with neurological dysfunction (Kondo et al., 2015).
Current knowledge suggests that synaptic dysfunction mainly contributes to neurological dysfunction after TBI. Synaptogenesis, axonal sprouting, and neurogenesis of surviving neurons are crucial to spontaneous motor recovery (Oshima et al., 2009). In the central nervous system, synaptic proteins play a critical role in processing and transferring information. Synaptophysin (SYN) is a synaptic vesicle glycoprotein that is widely expressed on presynaptic membranes and strongly associated with synaptic plasticity (Liu and Wang, 2017). Elevated SYN levels have a positive effect on consolidation of learning and memory after injury (Ferrer, 1999). SYN serves as a marker for presynaptic axon terminals, and has been used to quantify synapse amount after injury (Bonanomi et al., 2007). Synapsin I is a nerve terminal-specific phosphoprotein involved in synaptic transmission (Cesca et al., 2010), neural development (Fornasiero et al., 2010), and neurite outgrowth (Wang et al., 2011). Downregulation of synapsin I in lesion models suggests reduced trafficking or decreased number of synapses (Gulino et al., 2007, 2010). Postsynaptic density protein-95 (PSD-95) functions as a core scaffolding protein, and is largely expressed within excitatory synapses. PSD-95 plays an essential role in synaptic plasticity (Gardoni et al., 2001), and facilitates maturation of pre-synaptic and post-synaptic components implicated in dendritic spine formation and neurotransmission. Previous studies have confirmed that loss of SYN, synapsin I, and PSD-95 in brain tissue underlies cognitive disorders following TBI (Pan et al., 2016; Wang and Peng, 2016). TBI triggers a series of pathological and biophysiological reactions, which result in long-term functional impairments. Unfortunately, although vast sums of money and efforts have been invested in TBI treatment, its therapeutic efficacy remains unsatisfactory (Hu et al., 2016). Accordingly, finding satisfactory effective neuroprotective and neurorestorative pharmacotherapies is a significant challenge (Villapol et al., 2015).
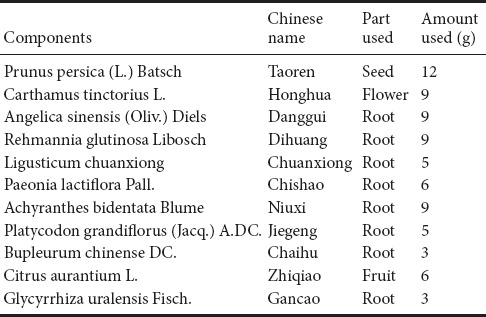
Traditional Chinese medicine has attracted increasing attention for its associated multi-potent properties (Sucher, 2013). Traditional Chinese medicine can interact with various pathways to improve TBI-associated pathological changes. Existing clinical research has shown the efficacy of traditional Chinese medicine by amelioration of dysfunction after TBI (Saito et al., 2010; Zhao et al., 2015). Xuefu Zhuyu decoction (XFZY), a famous classical herbal formula consisting of 11 herbs (Table 1), is described in Wang Qing-ren's “Yi Lin Gai Cuo”. XFZY is not only efficient in alleviating cardiovascular disease (Shi et al., 2013), but is also capable of reversing TBI-induced neurological dysfunction (Li et al., 2015) by activating blood circulation and preventing blood stasis (Fu et al., 2016). Thirty-four bioactive compounds from XFZY have been shown to exert anti-oxidative, anti-atherogenic, and anti-inflammatory effects (Sun et al., 2010; Paoletti et al., 2013; Fu et al., 2016). However, the detailed mechanisms involved in synaptic regulation of XFZY after TBI have not yet been determined.
Table 1.
Recipe of Xuefu Zhuyu decoction formulation
In this study, we aimed to investigate protective effects of XFZY on neurological dysfunction and variation of relevant synaptic proteins (including SYN, synapsin I, and PSD-95) in a rat model of TBI. Our research may provide a potential theoretical basis for pharmacological effects of XFZY on neurological function and synaptic plasticity following TBI.
Materials and Methods
Animals
Seventy-eight specific-pathogen-free male Sprague-Dawley rats weighing 200–250 g and aged 10 weeks were purchased from the Experimental Animal Research Center of Central South University of China (license number: SCXK 2014-0011). Experimental animals were housed in 12-hour light/dark cycles at 22°C, and allowed free access to food and water. All animal experimental procedures were strictly undertaken in accordance with the guidelines of the Animal Ethics Committee of Xiangya Hospital of Central South University and the Guidelines for the Care and Use of Laboratory Animals (approved number: 201603112). Best efforts were given to reduce animal mortality.
Rats were randomly assigned to four groups: (1) Sham group (n = 6), controlled cortical impact (CCI) without dura impact; (2) TBI group (n = 24), TBI + normal saline; (3) 9 g/kg XFZY group (n = 24), TBI + 9 g/kg XFZY; and (4) 18 g/kg XFZY (n = 24), TBI + 18 g/kg XFZY. Each injury group was randomly divided into two subgroups of 14 and 21 days post-injury (n = 12 each sub-group; with 6 brains used for immunohistochemistry and 6 for reverse-transcription polymerase chain reaction [RT-PCR] and western blot assay).
Model establishment of TBI
Throughout the surgical procedure, rats were deeply anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally) and their temperature maintained using an underlying heating pad. The rat was mounted in the stereotaxic frame of the CCI injury device, with the head in a horizontal plane. After shaving, a 2.0 cm sagittal incision was made in the scalp. The skull was exposed using hemostats. A 5.0 mm diameter bone window was made on the left parietal cortex: 3.0 mm anterior and lateral to bregma. The bone flap was removed, and the impounder tip of the device positioned to the exposed dura. Cortical injury was implemented with an electronic controlled pneumatic impact device (TBI 0310; Precision Systems & Instrumentation, Fairfax Station, VA, USA). The following parameters were used: striking speed, 6.0 m/s; blow depth, 5.0 mm; and dwell time, 500 ms. The incision was closed with sutures, and the rats then placed on a warm blanket with their body temperature maintained at 37.0 ± 0.5°C. Sham rats underwent the same anesthesia and craniotomy but without dura impact.
Drug preparation
The components and volume of XFZY are shown in Table 1. Herbs were obtained from the Pharmacy of Xiangya Hospital, Changsha, China. Voucher specimens (No. M20130042) were kept in Xiangya Hospital of China, and processed into lyophilized powder according to established standard procedures (Wang et al., 2016). After preparation, each 1 g of powder contained 5.2 g of unprocessed herbs. Finally, lyophilized powder was dissolved in distilled water to 1 g/mL for use. Each group received intragastric administration of XFZY or distilled water once a day after TBI for 14 or 21 days.
Neurobehavioral scoring
The neurological function of animals was assessed by modified neurological severity scores (mNSS) on days 14 and 21 post-injury. mNSS includes motor, sensory, balance, and reflex tests. Test was performed based on a previous study (Yang and Rosenberg, 2011). Rats that failed to accomplish the task were awarded 1 score. Scores ranged from 0 to 18 (normal score: 0; maximal damage: 18), and rats with higher scores were regarded as having more severe injury.
Morris water maze
The Morris water maze was used to assess spatial learning and memory function in rats’ post-injury. The test was strictly conducted as previously described (Zhu et al., 2014). Each rat was positioned in the tank and faced the wall at a randomly selected quadrant. The time taken to reach the hidden platform (escape latency) within 90 seconds was recorded. All experiments were tracked (ANY-maze, San Diego, CA, USA).
Immunohistochemistry
Rats were anesthetized completely with sodium pentobarbital (50 mg/kg) and perfused intracardially with saline followed by 4% paraformaldehyde. Ipsilateral brain tissue was removed and rapidly immerged in 4% paraformaldehyde. Sections (10 μm thickness) were immersed in 3% hydrogen peroxide to block endogenous peroxidase. After washing thoroughly three times with PBS, immunostaining was performed with the following primary antibodies: rabbit anti-SYN (1:1000; Proteintech, Chicago, IL, USA), rabbit anti-synapsin I (1:1000; Cell Signaling Technology, Boston, MA, USA), and rabbit anti-PSD 95 (1:1000; Cell Signaling Technology) at 4°C overnight. Sections were then washed followed by incubation with biotinylated-conjugated anti-rabbit secondary antibody (1:800; Proteintech) for 2 hours at room temperature. Last, sections were stained with 3,3′-diaminobenzidine and counterstained with hematoxylin. Images were measured using Image-Pro-Plus (Media Cybernetics, Rockville, MD, USA). Three random fields per section were selected from each animal, and the optical density ratio was calculated: this included the optical density of the region and background from each section.
Quantitative RT-PCR
Ipsilateral brain tissue was collected at given time points. Total RNA was isolated from tissue using Trizol isolation reagent (Invitrogen, Carlsbad, CA, USA), and the quality and concentration verified at optical density 260/280 nm. Subsequently, 3 μg of total RNA was used for RT-PCR. Amplification was performed using a SYBR PCR kit (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA). The PCR amplification procedure was: initial denaturation at 95°C for 10 minutes, then denaturation 95°C for 10 seconds with annealing at 57°C (for SYN) or 59°C (for synapsin I and PSD-95) for 50 seconds for a total of 40 cycles. PCR results were evaluated by the comparative threshold cycle method (Livak and Schmittgen, 2001). The gene sequences of primers are shown in Table 2.
Table 2.
Primers for genes

Western blot assay
Ipsilateral brain tissue samples were homogenized in radioimmunoprecipitation assay lysis buffer, and centrifuged at 12,000 r/min for 5 minutes at 4°C. Supernatants were collected. Protein concentration was determined by bicinchoninic acid assay. Equal amounts of protein (30 μg per lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to nitrocellulose membranes. Membranes were incubated with rabbit anti-synaptophysin (1:500; Proteintech), rabbit anti-PSD 95 (1:1000; Cell Signaling), and rabbit anti-synapsin I (1:1000; Cell Signaling) overnight at 4°C. After washing, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:5000; Proteintech) for 50 minutes at room temperature. Enhanced chemiluminescence solution (Thermo Fisher Scientific) was used for detecting immunoreactive bands. Last, membranes were developed using a Bio-Rad ChemiDoc XRS digital documentation system (Bio-Rad). Synapsin band densities were quantified and normalized using mouse anti-β-actin (1:4000). Results are presented by averaging the values (protein/β-actin).
Statistical analysis
All data are expressed as the mean ± SD. Data were analyzed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). The mNSS test was evaluated by repeated-measures analysis of variance. The Morris water maze task was analyzed by repeated-measures analysis of variance mixed model and two-way repeated-measures analysis of variance (group × time). Biochemical data were analyzed by analysis of variance. A value of P < 0.05 was considered statistically significant.
Results
XFZY improved behavioral functional recovery after CCI
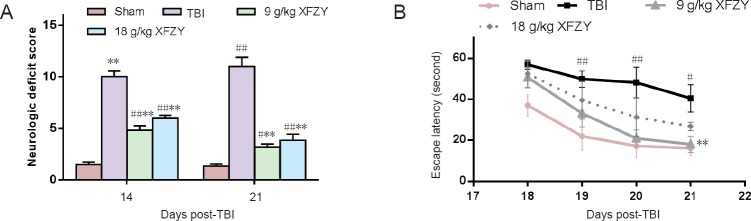
As shown in Figure 1A, neurological function was evaluated by mNSS at 14 and 21 days after CCI. Rats in groups that underwent CCI all exhibited markedly higher scores compared with the sham group. However, administration of two different doses of XFZY resulted in significantly lower scores compared with the TBI group at the evaluated time points (P < 0.01).
Figure 1.
Effect of XFZY on neurological and cognitive functions of rats with TBI.
(A) mNSS: Rats showed neurological deficits after TBI, while administration of XFZY (9 and 18 g/kg) significantly lowered mNSS on days 14 and 21 compared with the TBI group. (B) Escape latency in the Morris water maze: TBI group showed significantly longer escape latency compared with the sham group, while the 9 g/kg XFZY group showed a significantly reduced time on day 21 compared with the TBI group. Data are expressed as the mean ± SD (n = 6). Data were analyzed by repeated-measures analysis of variance (mNSS) or repeated-measures analysis of variance mixed model and two-way repeated-measures analysis of variance (Morris water maze task). #P < 0.05, ##P < 0.01, vs. sham group; **P < 0.01, vs. TBI group. XFZY: Xuefu Zhuyu decoction; TBI: traumatic brain injury; CCI: controlled cortical impact; mNSS: modified neurological severity score.
XFZY alleviated cognitive dysfunction after TBI
As shown in Figure 1B, rats undergoing CCI performed worse than sham injury rats in the Morris water maze. The TBI group displayed significantly longer escape latency to the hidden platform compared with the sham group from 19 to 21 post-injury days (P < 0.01 at 19 and 20 days, P < 0.05 at 21 days). This finding was reversed by XFZY administration, and XFZY treatment trended to reduce the gap between TBI rats and sham-operated rats. In particular, escape latency was significantly reduced in the 9 g/kg XFZY group compared with the TBI group on day 21 (P < 0.01).
XFZY increased SYN expression in brain tissue after TBI
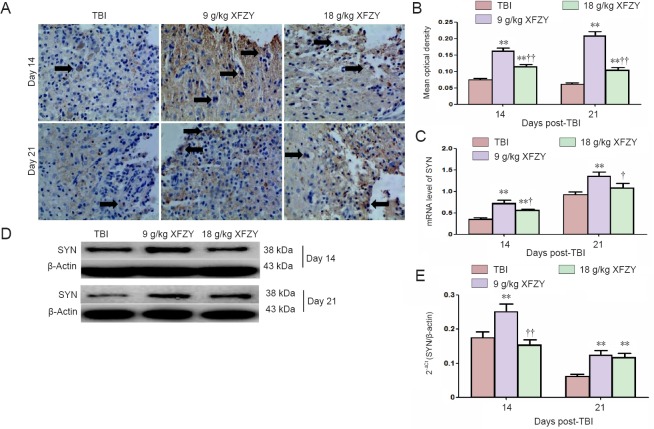
SYN immunohistochemistry results are shown in Figure 2A and B. Immunoreactive deposition of SYN in the 9 and 18 g/kg XFZY groups was visibly increased compared with the TBI group (P < 0.01). Interestingly, the 9 g/kg XFZY group was superior to the 18 g/kg XFZY group at 14 and 21 days post-injury (P < 0.01).
Figure 2.
Effect of XFZY treatment on SYN expression levels in the brain of TBI rats.
(A, D) Representative immunohistochemistry (× 400) and western blot images of SYN in different groups. Arrows: Immunoreactivity of SYN. (B–D) Quantification of SYN expression levels from immunohistochemistry and western blot assays. (E) SYN gene expression levels. Data are expressed as the mean ± SD (n = 6), and were analyzed by analysis of variance. *P < 0.05, **P < 0.01, vs. TBI group; †P < 0.05, ††P < 0.01, vs. 9 g/kg group. XFZY: Xuefu Zhuyu decoction; TBI: traumatic brain injury; SYN: synaptophysin.
As shown in Figure 2C, the same trend was observed with SYN mRNA expression after injury. SYN mRNA expression was markedly upregulated in the 9 g/kg XFZY group compared with the TBI group (P < 0.01) and 18 g/kg XFZY group (P < 0.05) at 21 days post-injury.
Further, SYN protein content was examined by western blot assay. SYN protein expression levels in brain tissue were higher in the 9 g/kg XFZY group compared with the TBI group and 18 g/kg XFZY group at 14 days post-injury (P < 0.01). While SYN protein expression levels were significantly increased in the 9 and 18 g/kg XFZY groups compared with the TBI group at 21 days post-injury (P < 0.01; Figure 2D, E).
XFZY increased synapsin I expression in brain tissue after TBI
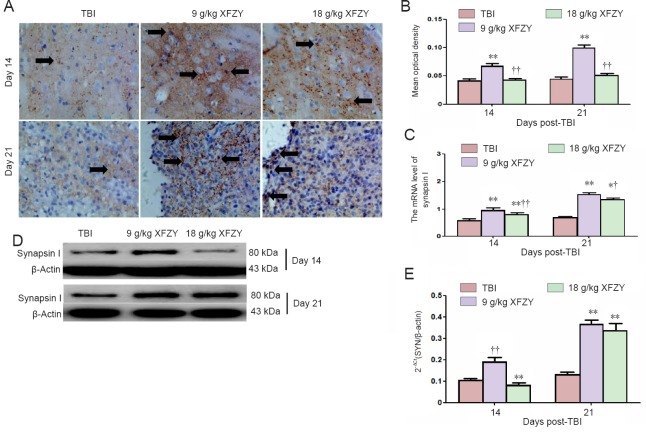
Synapsin I immunohistochemistry is shown in Figure 3A and B. Immunoreactive deposition of synapsin I was significantly increased in the 9 g/kg XFZY group compared with the TBI group and 18 g/kg XFZY group (P < 0.01; Figure 3B).
Figure 3.
Effect of XFZY treatment on synapsin I expression levels in the brain of TBI rats.
(A, D) Representative immunohistochemistry (400×) and western blot images of synapsin I in different groups. Arrows: Immunoreactivity of synapsin I. (B–D) Quantification of synapsin I expression levels from immunohistochemistry and western blot assays. (E) Synapsin I gene expression levels. Data are expressed as the mean ± SD (n = 6), and were analyzed by analysis of variance. **P < 0.01, vs. TBI group; †P < 0.05, ††P < 0.01, vs. 9 g/kg XFZY group. XFZY: Xuefu Zhuyu decoction; TBI: traumatic brain injury.
Synapsin I mRNA expression was significantly higher in the 9 and 18 g/kg XFZY groups than the TBI group (P < 0.01; Figure 3C). Additionally, the 9 g/kg XFZY group was superior to the 18 g/kg XFZY group at 14 and 21 days post-injury (P < 0.01 and P < 0.05).
Synapsin I protein content was examined by western blot assay. Synapsin I protein expression levels were higher in brain tissue of the 9 g/kg XFZY group than the TBI group (P < 0.01). Significantly higher synapsin I expression was found in the 9 g/kg XFZY group compared with the 18 g/kg XFZY group at 14 days after injury (P < 0.01; Figure 3D, E).
XFZY increased PSD-95 expression in brain tissue after TBI
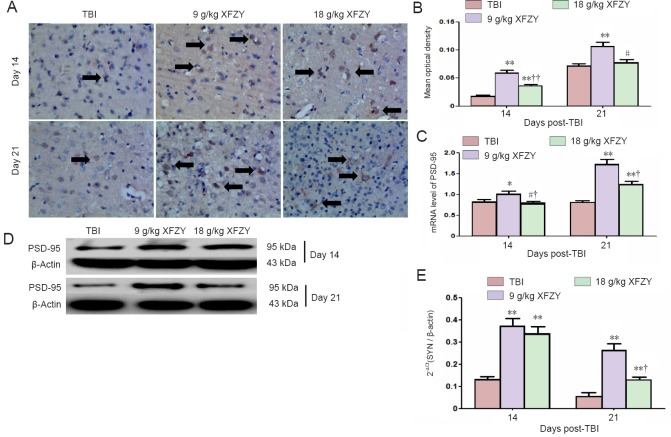
PSD-95 immunohistochemistry is shown in Figure 4A and B. Immunoreactive deposition of PSD-95 was higher in the 9 g/kg XFZY group compared with the TBI group and 18 g/kg XFZY group at 14 days (P < 0.05; Figure 4B).
Figure 4.
Effect of XFZY treatment on PSD-95 expression in the brain of TBI rats.
(A, D) Representative immunohistochemistry (× 400) and western blot images of PSD-95 in different groups. Arrows: Immunoreactivity of PSD-95. (B–D) Quantification of PSD-95 expression levels from immunohistochemistry and western blot assays. (E) PSD-95 gene expression levels. Data are expressed as the mean ± SD (n = 6), and were analyzed by analysis of variance. **P < 0.01, vs. TBI group; †P < 0.05, ††P < 0.01, vs. 9 g/kg XFZY group. XFZY: Xuefu Zhuyu decoction; TBI: traumatic brain injury; PSD-95: postsynaptic density protein-95.
The same trend was found with PSD-95 mRNA expression levels in brain tissue after injury. After administration of 9 g/kg XFZY, PSD-95 mRNA expression was visibly upregulated compared with the TBI group (21 days) and 18 g/kg XFZY group (14 days) (P < 0.01; Figure 4C).
PSD-95 protein content was examined by western blot assay. PSD-95 protein expression levels were higher in the 9 and 18 g/kg XFZY groups than the TBI group (P < 0.01). PSD-95 was markedly increased in the 9 g/kg XFZY group compared with the 18 g/kg XFZY group at 21 days after injury (P < 0.05; Figure 4D, E).
Discussion
In the present study, XFZY significantly improved performance outcomes on mNSS tests and the Morris water maze task. Furthermore, XFZY markedly reversed expression of SYN, synapsin I, and PSD-95 in ipsilateral brain tissue of CCI rats. Interestingly, the neuroprotective effect of XFZY at a dose of 9 g/kg was superior to 18 g/kg. Taken together, XFZY attenuated neurological dysfunction via upregulation of SYN, synapsin I, and PSD-95 in the ipsilateral brain of CCI rats (Figure 5).
Figure 5.
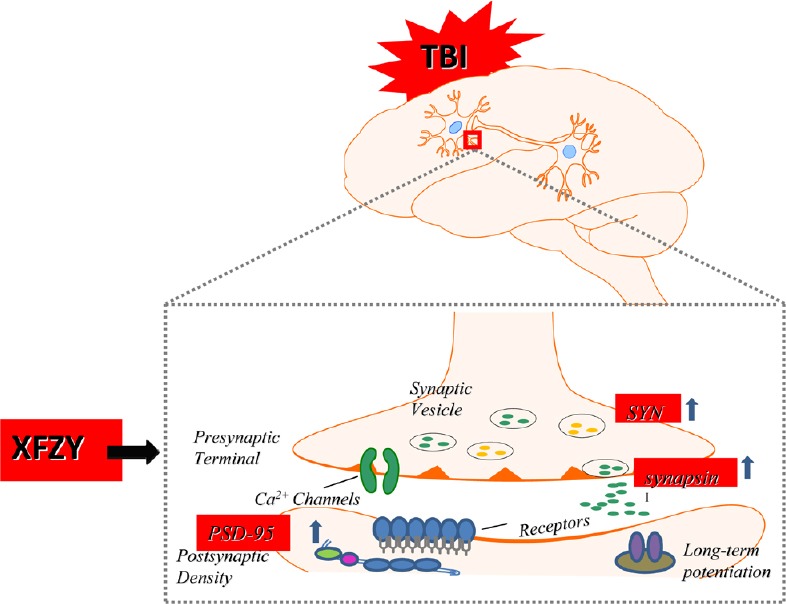
XFZY attenuated controlled cortical impact-induced cognitive deficits via upregulation of SYN, synapsin I, and PSD-95.
XFZY: Xuefu Zhuyu decoction; TBI: traumatic brain injury; PSD-95: postsynaptic density protein-95.
TBI is a leading cause of mortality and disability worldwide (Bruns and Hauser, 2003). It can trigger tissue alterations and result in long-term cognitive deficits and neurological disorders in patients. Therefore, TBI causes a series of social, financial, and personal problems (Lin et al., 2016; Lou et al., 2016). It is evident that synaptic function mainly contributes to cognitive deficits and neurological disorders post-TBI. Previous studies show that TBI leads to synaptic damage by triggering multiple pathological events through cellular and molecular mechanisms (Perez et al., 2016). Thus, synaptic damage causes a significant reduction of synapses in both the cortex and hippocampus (Ansari et al., 2008a, b). Moreover, secondary damage following TBI (including oxidative stress and inflammatory responses) aggravates the degree of synapse reduction, and ultimately contributes to neurological dysfunction (Wu et al., 2004, 2007). Beyond the site of direct cortical injury, dramatic changes distant from primary lesions also disturb hippocampal circuits and lead to behavioral dysfunction (Atkins, 2011). After TBI, decreasing expression of long-term potentiation changes N-methyl-D-aspartic acid (NMDA)-type glutamate receptor-mediated synaptic transmission. TBI induced disorders of synaptic transmission are strongly associated with hippocampal dysfunction, and result in cognitive and behavioral deficits (Aungst et al., 2014). Synaptic transmission is fundamentally important to neurological function. Motivation of synaptic expression will improve functional outcome (Thickbroom and Mastaglia, 2009). Enhancing synapse expression in brain tissue can accelerate neural restoration. Thus, synaptic regulation may be a promising therapeutic strategy for cognitive functional recovery.
Here, we assessed neuroprotective effects and cognitive improvements in CCI rats after administration of XFZY. As shown in Figure 1B, latency to reach a hidden platform was significantly longer in rats from the TBI group compared with the sham group, which suggests that TBI damaged learning and memory capacities. Interestingly, the 9 g/kg XFZY group showed significant improvements of spatial learning and memory impairments. Furthermore, upregulation of synaptic proteins (including SYN, synapsin I, and PSD-95) was observed after administration of XFZY.
SYN is a presynaptic membrane marker (Oliveira et al., 2010) that is a crucial synapsin for axonal reorganization and synaptogenesis. The amount of SYN serves as a reflection of the level of synaptic function and synaptic transmission. Moreover, synapsin I is a main downstream effector of brain-derived neurotrophic factor, and plays an extensive role in synaptic transmission and neurite outgrowth (Futai et al., 2007; Wu et al., 2007). In addition, PSD-95 interacts with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA glutamate receptors, and plays an essential role in maturation of glutamatergic synapses. Several studies show that PSD-95 increases the number and size of dendritic spines, and facilitates synaptic stabilization and plasticity (El-Husseini et al., 2000; Steiner et al., 2008). Consequently, reduction of PSD-95 may disturb neuronal networks and connectivity (Lang et al., 2007). SYN, synapsin I, and PSD-95 and their modifications play a dynamic role in learning and memory (Wu et al., 2006; Gorczyca et al., 2007). Upregulation of these three synaptic proteins can contribute to memory consolidation. Based on our western blot and RT-PCR analyses, XFZY significantly enhanced expression of SYN, synapsin I, and PSD-95 in brain tissue of CCI rats. These changes were accompanied by improvements in neurological function. Overall, these results demonstrate that XFZY attenuates CCI-induced cognitive deficits via upregulation of SYN, synapsin I, and PSD-95. In future studies, the absorbed bioactive compositions of XFZY that were effective in neural restoration should be determined.
Altogether, our present study shows significant improvements of XFZY on cognitive dysfunction in CCI rats. The underlying mechanism involves regulation at the level of synaptic proteins. XFZY may represent a novel therapeutic approach for TBI treatment.
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81673719, 81173175 and 81303074; a grant from China Postdoctoral Science Foundation, No. 2016M600639 and 2017T100614. Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement: The study protocol was approved by the Animal Ethics Committee of Xiangya Hospital of Central South University of China (approval number: 201603112).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Gabriole Siciliano, University of Pisa, Italy.
Comments to authors: This preclinical study investigates the effects of Xuefu Zhuyu decoction, at two different dosages, on rat model of traumatic brain injury. Both clinical neurological dysfunction and immunoistochemical assay of synaptic proteins were performed, with a detailed description of the methods. The nutraceutic is a very actual field in clinical research and this paper is very interesting.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81673719, 81173175 and 81303074; a grant from China Postdoctoral Science Foundation, No. 2016M600639 and 2017T100614.
(Copyedited by James R, Stow A, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008a;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008b;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- Atkins CM. Decoding hippocampal signaling deficits after traumatic brain injury. Transl Stroke Res. 2011;2:546–555. doi: 10.1007/s12975-011-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab. 2014;34:1223–1232. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, Bush AI, Frugier T, Crack PJ, Duce JA. Ceruloplasmin and beta-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic Biol Med. 2014;69:331–337. doi: 10.1016/j.freeradbiomed.2014.01.041. [DOI] [PubMed] [Google Scholar]
- Barman A, Chatterjee A, Bhide R. Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J Psychol Med. 2016;38:172–181. doi: 10.4103/0253-7176.183086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi D, Rusconi L, Colombo CA, Benfenati F, Valtorta F. Synaptophysin I selectively specifies the exocytic pathway of synaptobrevin 2/VAMP2. Biochem J. 2007;404:525–534. doi: 10.1042/BJ20061907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement Geriatr Cogn Disord 10 Suppl. 1999;1:55–60. doi: 10.1159/000051214. [DOI] [PubMed] [Google Scholar]
- Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F. The role of synapsins in neuronal development. Cell Mol Life Sci. 2010;67:1383–1396. doi: 10.1007/s00018-009-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Xia Z, Liu Y, Lu H, Zhang Z, Wang Y, Fan X. Qualitative analysis of major constituents from Xue Fu Zhu Yu Decoction using ultra high performance liquid chromatography with hybrid ion trap time-of-flight mass spectrometry. J Sep Sci. 2016;39:3457–3468. doi: 10.1002/jssc.201600083. [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M. Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J Neurosci. 2001;21:1501–1509. doi: 10.1523/JNEUROSCI.21-05-01501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca D, Ashley J, Speese S, Gherbesi N, Thomas U, Gundelfinger E, Gramates LS, Budnik V. Postsynaptic membrane addition depends on the Discs-Large-interacting t-SNARE Gtaxin. J Neurosci. 2007;27:1033–1044. doi: 10.1523/JNEUROSCI.3160-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino R, Perciavalle V, Gulisano M. Expression of cell fate determinants and plastic changes after neurotoxic lesion of adult mice spinal cord by cholera toxin-B saporin. Eur J Neurosci. 2010;31:1423–1434. doi: 10.1111/j.1460-9568.2010.07170.x. [DOI] [PubMed] [Google Scholar]
- Gulino R, Dimartino M, Casabona A, Lombardo SA, Perciavalle V. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neurosci Res. 2007;57:148–156. doi: 10.1016/j.neures.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Haring RS, Narang K, Canner JK, Asemota AO, George BP, Selvarajah S, Haider AH, Schneider EB. Traumatic brain injury in the elderly: morbidity and mortality trends and risk factors. J Surg Res. 2015;195:1–9. doi: 10.1016/j.jss.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Hu Q, Manaenko A, Xu T, Guo Z, Tang J, Zhang JH. Hyperbaric oxygen therapy for traumatic brain injury: bench-to-bedside. Med Gas Res. 2016;6:102–110. doi: 10.4103/2045-9912.184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Khan H, Singh I, Singh AK. Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury. Neural Regen Res. 2017;12:696–701. doi: 10.4103/1673-5374.206632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523:431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SB, Stein V, Bonhoeffer T, Lohmann C. Endogenous brain-derived neurotrophic factor triggers fast calcium transients at synapses in developing dendrites. J Neurosci. 2007;27:1097–1105. doi: 10.1523/JNEUROSCI.3590-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Wang JC, Cui J, Jia J, Qiao BL, Wang QH. Effect of Xuefu Zhuyu decoction on the neural functional recovery and living ability in patients with craniocerebral injury. Xiandai Zhongxiyi Jiehe Zazhi. 2015;24:350–352. [Google Scholar]
- Lin W, Yang LK, Cai S, Zhu J, Feng Y, Yang LX, Feng ZZ, Li PP, Chen JH, Wang YH. Cognitive function and biomarkers after traumatic brain injury: protocol for a prospective inception cohort study. Asia Pac J Clin Trials Nerv Syst Dis. 2016;1:170–176. [Google Scholar]
- Liu X, Wang H. Effect of Danshen injection with neural stem cells transplantation in rats with craniocerebral injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:4709–4715. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lou YL, Chen P, Jiang Y, Zhang H, Min YH. Neural stem cell transplantation for sequela of traumatic brain injury: the best timing for treatment. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:1474–1480. [Google Scholar]
- Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff KE, Wenk MR, Arancio O, Di Paolo G. Phospholipase d2 ablation ameliorates Alzheimer's disease-linked synaptic dysfunction and cognitive deficits. J Neurosci. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T. TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res. 2009;1290:102–110. doi: 10.1016/j.brainres.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Pan W, Han S, Kang L, Li S, Du J, Cui H. Effects of dihydrotestosterone on synaptic plasticity of the hippocampus in mild cognitive impairment male SAMP8 mice. Exp Ther Med. 2016;12:1455–1463. doi: 10.3892/etm.2016.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti I, De Gregorio V, Baroni A, Tufano MA, Donnarumma G, Perez JJ. Amygdalin analogues inhibit IFN-gamma signalling and reduce the inflammatory response in human epidermal keratinocytes. Inflammation. 2013;36:1316–1326. doi: 10.1007/s10753-013-9670-7. [DOI] [PubMed] [Google Scholar]
- Perez EJ, Cepero ML, Perez SU, Coyle JT, Sick TJ, Liebl DJ. EphB3 signaling propagates synaptic dysfunction in the traumatic injured brain. Neurobiol Dis. 2016;94:73–84. doi: 10.1016/j.nbd.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu C, Anghelescu A, Daia C, Onose G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J Med Life. 2015;8:272–277. [PMC free article] [PubMed] [Google Scholar]
- Saito S, Kobayashi T, Osawa T, Kato S. Effectiveness of Japanese herbal medicine yokukansan for alleviating psychiatric symptoms after traumatic brain injury. Psychogeriatrics. 2010;10:45–48. doi: 10.1111/j.1479-8301.2010.00313.x. [DOI] [PubMed] [Google Scholar]
- Shi WL, Zhang JS, Hu YQ. Prevention and treatment of blood vessels related diseases by xuefu zhuyu decoction (see text): its clinical application and progress in researches of mechanisms. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:712–716. [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ. The application of Chinese medicine to novel drug discovery. Expert opinion on drug discovery. 2013;8:21–34. doi: 10.1517/17460441.2013.739602. [DOI] [PubMed] [Google Scholar]
- Sun X, Wei X, Qu S, Zhao Y, Zhang X. Hydroxysafflor Yellow A suppresses thrombin generation and inflammatory responses following focal cerebral ischemia-reperfusion in rats. Bioorg Med Chem Lett. 2010;20:4120–4124. doi: 10.1016/j.bmcl.2010.05.076. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Mastaglia FL. Plasticity in neurological disorders and challenges for noninvasive brain stimulation (NBS) J Neuroeng Rehabil. 2009;6:4. doi: 10.1186/1743-0003-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Balarezo MG, Affram K, Saavedra JM, Symes AJ. Neurorestoration after traumatic brain injury through angiotensin II receptor blockage. Brain. 2015;138:3299–3315. doi: 10.1093/brain/awv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Asano Y, Shinoda J. Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR Am J Neuroradiol. 2012;33:2117–2122. doi: 10.3174/ajnr.A3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peng RY. Basic roles of key molecules connected with NMDAR signaling pathway on regulating learning and memory and synaptic plasticity. Mil Med Res. 2016;3:26. doi: 10.1186/s40779-016-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, Schachner M, Kleene R. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31:7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li H, Yu J, Hong M, Zhou J, Zhu L, Wang Y, Luo M, Xia Z, Yang ZJ, Tang T, Ren P, Huang X, Wang J. Protective effects of chinese herbal medicine rhizoma drynariae in rats after traumatic brain injury and identification of active compound. Mol Neurobiol. 2016;53:4809–4820. doi: 10.1007/s12035-015-9385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorke AM, Littleton S, Alsalaheen BA. Concussion attitudes and beliefs, knowledge, and clinical practice: survey of physical therapists. Phys Ther. 2016;96:1018–1028. doi: 10.2522/ptj.20140598. [DOI] [PubMed] [Google Scholar]
- Zhao J, Tu EJ, McMurray C, Sleigh A. Rising mortality from injury in urban China: demographic burden, underlying causes and policy implications. Bull World Health Organ. 2012;90:461–467. doi: 10.2471/BLT.11.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Wang C, Li Z, Chen L, Li J, Cui W, Ding S, Xi Q, Wang F, Jia F, Xiao S, Guo Y, Zhao Y. Efficacy and safety of transcutaneous electrical acupoint stimulation to treat muscle spasticity following brain injury: a double-blinded, multicenter, randomized controlled trial. PLoS One. 2015;10:e0116976. doi: 10.1371/journal.pone.0116976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZW, Li YD, Gao WW, Chen JL, Yue SY, Zhang JN. Cold water swimming pretreatment reduces cognitive deficits in a rat model of traumatic brain injury. Neural Regen Res. 2017;12:1322–1328. doi: 10.4103/1673-5374.213553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Park J, Golinski J, Qiu J, Khuman J, Lee CC, Lo EH, Degterev A, Whalen MJ. Role of Akt and mammalian target of rapamycin in functional outcome after concussive brain injury in mice. J Cereb Blood Flow Metab. 2014;34:1531–1539. doi: 10.1038/jcbfm.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]