Abstract
Stroke is a leading cause of death and disability and new therapies are desperately needed. Given the complex nature of ischemic brain injury, it has been postulated that cell-based therapies may be useful. However, cell resources, invasive extraction procedures, immunological rejection, tumorigenesis and ethical challenges make it unlikely that many stem cell types could serve as a practical source for therapy. By contrast, these issues do not pertain to human amnion epithelial cells (hAECs), which are placenta-derived stem cells. We recently assessed the effects of systemically delivered hAECs on stroke outcome using four animal models of stroke. We demonstrated that when injected intravenously after ischemia onset, hAECs migrate preferentially to the spleen and injured brain to limit apoptosis and inflammation, and attenuate early brain infiltration of immune cells, progression of infarction and systemic immunosuppression and to ultimately ameliorate functional deficits. When administration of hAECs is delayed by 1-3 days post-stroke, long-term functional recovery can still be enhanced in young and aged mice of either sex. Moreover, our proof-of-principle findings suggest that hAECs are effective at limiting post-stroke infarct development in non-human primates. Overall, the results suggest that hAECs could be a viable clinical stroke therapy.
Keywords: ischemic stroke, cerebral infarction, stem cells, human amnion epithelial cells, inflammation, immunosuppression, brain repair, mouse, non-human primate
Introduction
Stroke is the world's second leading cause of death after ischemic heart disease, accounting for 6.7 million deaths annually (Mozaffarian et al., 2016). It is also the greatest cause of long-term disability in adults, with up to half of all survivors failing to regain independence (Mozaffarian et al., 2016). Currently, there is only one approved pharmacological agent available to treat stroke, recombinant tissue plasminogen activator (rtPA), which must be administered within a 4.5 hours window of stroke onset and only after computed tomography (CT) or magnetic resonance imaging (MRI) has diagnosed a thrombotic cause. Due to these strict timing limitations, only approximately 15% of stroke patients are eligible to receive rtPA (Phan et al., 2018). An additional therapy involving endovascular clot retrieval (ECR), for large clot removal, has been recently approved for use up to 8 hours from the onset of stroke in patients who have received rtPA or who are already taking anti-coagulant medication (Phan et al., 2018). However, even with this extended time window, less than 5% of additional patients are eligible for ECR (Phan et al., 2018). Consequently, there is a desperate need to identify additional effective therapies to improve outcome for a larger proportion of stroke patients.
Cell-based therapies, which have the potential to target multiple injury mechanisms in a more coordinated manner, have been gaining interest as a treatment option for stroke. While a number of different cell types have been evaluated for stroke therapy, the most commonly used type of cells is stem cells. Stem cells are defined as undifferentiated cells capable of self-renewal, and can be broadly classified as being of embryonic, fetal or adult origin (Broughton et al., 2013). Administration of various stem cell lineages has been reported to reduce brain injury and facilitate recovery after experimental stroke. Notably, it has been documented that stem cells can be administered up to several days after stroke and still promote improvement or recovery (Broughton et al., 2013). Further, there have been a number of clinical trials assessing the safety and/or efficacy of stem cell therapy for stroke, which have reported varying levels of success (Nagpal et al., 2017). The most frequently used stem cell types in stroke research include embryonic, mesenchymal (usually derived from bone marrow or umbilical cord), neural and induced pluripotent stem cells (Broughton et al., 2013). Whilst all of these cell types have been demonstrated to have efficacy at improving experimental stroke outcome, they possess a number of limitations, which may restrict their use as a routine treatment option. These limitations include immune rejection, tumor formation, limited sources and availability, invasive extraction, unsuitability for systemic delivery and/or ethical concerns (Broughton et al., 2013). However, none of these limitations appear to pertain to the human amnion epithelial cell (hAEC) (Broughton et al., 2013; Evans et al., 2018).
hAECs
hAECs are derived from the epithelial layer of the amnion, which is the sac that encloses the developing fetus and is attached to the placenta. hAECs are fetal in origin and thus display a high degree of pluripotency. However, as these cells are obtained from discarded term placentae, they do not possess ethical constraints to their use as do embryonic stem cells, for example (Broughton et al., 2013). Also unlike some of the other stem cell lineages, hAECs are readily available and do not require invasive extraction procedures for harvesting. Likely reflecting their role in maintaining maternal immune tolerance of the fetus during pregnancy, hAECs are immunologically inert and have low surface expression of human leukocyte antigen (HLA)-A, HLA-B, HLA-C and HLA-DR, which are key antigens involved in transplant rejection (Broughton et al., 2013; Phan et al., 2018). On the other hand, hAECs express and release the non-polymorphic, non-classical antigen, HLA-G, which is able to directly suppress immune responses. Consistent with these properties, it has been known for over 35 years that hAECs can be safely transplanted into humans without evidence of immune rejection (Broughton et al., 2013). Amnion cells or strips of amnion membrane have long been used to reduce scarring and promote healing in burn victims or following corneal surgery, also suggesting hAECs are well tolerated by a recipient's immune system. Furthermore, hAECs do not form tumors in vivo as they lack telomerase enzyme, nor do they differentiate into fibroblasts, as can mesenchymal stem cells, for example (Broughton et al., 2013; Phan et al., 2018). Overall this profile of characteristics suggests that hAECs are an inherently safer cell for transplantation than many other stem cell types.
hAECs as A Potential Therapy for Stroke
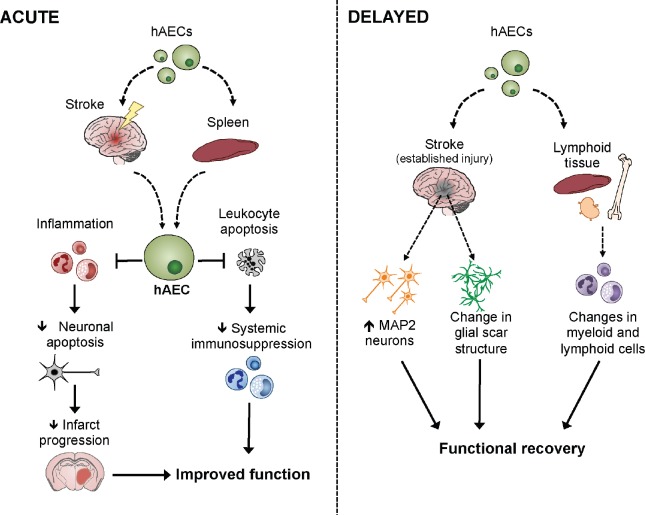
To exploit the above-mentioned beneficial properties, we recently tested the efficacy of systemically delivered hAECs to improve a number of outcome measures in four animal models of ischemic stroke (Evans et al., 2018). The cells were harvested from term placentae of healthy women following uncomplicated pregnancies, and were stored frozen under good manufacturing practice conditions until use. Importantly, the cells were administered, without further manipulation, to immune competent animals with no evidence of immune rejection. Our data indicate that hAECs provided substantial benefit even when administered up to 3 days following a stroke. Specifically we showed that when administered to mice acutely after onset of cerebral ischemia, hAECs migrate to the injured brain in a C-X-C chemokine receptor (CXCR)4-dependent manner to limit apoptosis and inflammation, attenuate early brain infiltration of immune cells, reduce progression of infarction and ultimately ameliorate functional deficits (Figure 1). We also showed that a considerable number of hAECs migrate to the spleen, and that an intact spleen is required for hAECs to fully elicit their neuroprotective effects. Additionally, we found that hAECs are effective at blunting systemic post-stroke immunosuppression, a phenomenon that leaves stroke patients particularly vulnerable to infectious complications (Anrather and Iadecola, 2016) (Figure 1). Moreover, when administration of hAECs was delayed by 1–3 days post-stroke, long-term functional recovery was still observed to be enhanced in young and aged mice of either sex, regardless of infarct size. The data also suggest that hAECs may enhance endogenous brain repair processes, as we observed changes to the structure of the glial scar, and more microtubule-associated protein 2 (MAP-2)-positive cells present in the peri-infarct region in animals treated with hAECs (Figure 1). To further assess and extend the potential translatability of our findings we also obtained proof-of-principle evidence that intravenous (i.v.) administration of hAECs reduces infarct development in non-human primates (i.e., marmosets). Overall, these findings strongly indicate that hAECs could be a viable and effective clinical stroke therapy.
Figure 1.
A schematic of the likely mechanisms by which human amnion epithelial cells (hAECs) improve outcome after stroke.
When administered acutely after ischemia onset (1.5 hours post-stroke), hAECs preferentially migrate to the spleen and injured brain to limit apoptosis and inflammation, and attenuate early brain infiltration of immune cells, progression of infarction and systemic immunosuppression to ultimately ameliorate functional deficits. When administration of hAECs is delayed by 1–3 days post-stroke, long-term functional recovery can still be enhanced, likely by increasing numbers of microtubule-associated protein 2 (MAP-2) positive neurons as well as altering the structure of the glial scar and numbers of immune cells in lymphoid tissue. Diagram adapted from Evans et al., 2018.
An important feature of our pre-clinical study was that hAECs were administered systemically post-stroke (Evans et al., 2018). While one previous study reported that hAECs could reduce infarct volume and behavioral deficits following ischemic stroke when administered directly into the injured brain tissue (Liu et al., 2008), intracerebral administration of hAECs is unlikely to be practicable clinically for several reasons. First, intracerebral administration will likely require expensive imaging equipment and expertise not readily available in many stroke centres. Intracerebral administration may itself cause further brain injury, and it is an approach which does not likely target the systemic mechanisms that contribute to impaired outcomes after stroke, particularly the systemic immune response (Anrather and Iadecola, 2016).
Studies indicate that hAECs possess potent immunomodulatory properties and can reduce inflammation in several experimental disease settings (Broughton et al., 2013; Tan et al., 2014, 2015). It is therefore not surprising that a common finding across our mouse models of cerebral ischemia was that hAECs were able to modify the post-stroke immune response. More specifically, when administered acutely following transient cerebral ischemia, hAECs therapy limited the numbers of immune cells (neutrophils, monocytes/macrophages, B cells and T cells) and M1 polarized macrophages present in the brain. Following permanent cerebral ischemia, hAECs also reduced numbers of brain-infiltrating T cells. Further, when hAECs were administered in a delayed manner (after 24 hours) following photothrombotic stroke, they were still able to modify the stroke-induced changes occurring to peripheral myeloid and lymphoid cell populations. It is likely that early administration of hAECs is necessary to sequester inflammation, which contributes to secondary brain damage following acute stroke. However, we predict that modulating the immune response once stroke-induced brain injury is established would still be beneficial for overall recovery. For instance, it is known that hAECs can promote T regulatory cell expansion and M2 macrophage polarization (Tan et al., 2014, 2015), which may assist in brain repair processes (Anrather and Iadecola, 2016).
Lung infections are a major cause of post-stroke morbidity and mortality and can delay recovery processes (Anrather and Iadecola, 2016; Stanley et al., 2016). As mentioned, these infections are thought be a consequence of a profound systemic immunosuppression, which develops over the hours to days following a stroke (Anrather and Iadecola, 2016). Importantly, we found that hAECs were also effective at preventing post-stroke systemic immunosuppression, particularly the reduction in splenic and circulating leukocytes (by apoptosis) and the associated splenic atrophy (Evans et al., 2018). These changes occurred in association with improved functional outcome, which may suggest that such mice would develop fewer infections. While we did assess levels of bacteria in the lungs at 72 hours, this only included aerobic (not anaerobic) bacteria. It is currently thought that following a stroke, the gut becomes leaky and as the host's immune system is compromised, this allows for the translocation of bacteria from the gut to the lungs, resulting in infection (Stanley et al., 2016). As the majority of bacteria in the gut are anaerobic, future studies should also examine the effect of hAECs on anaerobic lung infections after stroke to fully understand the efficacy of hAECs to prevent post-stroke infections.
Translational Perspectives
In 1999, a series of conferences was held called the ‘Stroke Treatment Academic Industry Roundtable’ or ‘STAIR’ (Stroke Therapy Academic Industry Roundtable (STAIR), 1999). The intention of these conferences was to develop guidelines on how to best proceed in the development of stroke therapies in order to overcome the translational roadblock from laboratory to clinic. Similarly, the Stem cell Therapies as an Emerging Paradigm in Stroke or ‘STEPS’ meeting was organized to create a framework to guide investigations in stem cell therapy for stroke (Stem Cell Therapies as an Emerging Paradigm in Stroke Participants, 2009). Importantly, our studies were performed in support of STAIRs and STEPs recommendations, as we utilized models of permanent and transient cerebral ischemia, models that include cortical and subcortical infarcts, animals of both sexes, as well as aged animals. We assessed different time-points at which hAECs could be delivered post-stroke, and outcomes during acute (1–3 days) and delayed (7–56 days) phases. Furthermore, our data from six marmosets were in accordance with the guidelines for therapeutic development, which recommend that once efficacy is established in rodents, studies be carried out in gyrencephalic species, such as non-human primates, before taking the therapeutic through to studies in humans. Hence, the next stage of this research will be to assess the safety and efficacy of hAECs in humans following a stroke. Indeed we have now registered a Phase I clinical trial to assess the maximum tolerable dose of hAECs in human ischemic stroke patients (Australian New Zealand Clinical Trials Registry: ACTRN12618000076279p; Phan et al., 2018). From a safety perspective, it is known that hAECs can be injected into healthy humans without evidence of immune rejection or tumor formation and it is therefore predicted that hAECs can also be safely administered to stroke patients (Broughton et al., 2013; Phan et al., 2018). Further, with nearly 4 million births per year in the USA alone, and an average yield of 150–200 million cells per placenta, hAECs are a readily available and relatively cheap source of cells for subsequent clinical application.
Conclusions
Our pre-clinical study (Evans et al., 2018) provides the first evidence that systemically delivered hAECs can improve outcomes following experimental ischemic stroke. Overall, we suggest that hAECs represent a genuinely new opportunity for the treatment of ischemic stroke with a therapeutic window of efficacy that extends substantially beyond the current 4.5 hours for rtPA.
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Lim R, Arumugam TV, Drummond GR, Wallace EM, Sobey CG. Post-stroke inflammation and the potential efficacy of novel stem cell therapies: focus on amnion epithelial cells. Front Cell Neurosci. 2013;6:66. doi: 10.3389/fncel.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MA, Lim R, Kim HA, Chu HX, Gardiner-Mann CV, Taylor KWE, Chan CT, Brait VH, Lee S, Dinh QN, Vinh A, Phan TG, Srikanth VK, Ma H, Arumugam TV, Fann DY, Poh L, Hunt CPJ, Pouton CW, Haynes JM, et al. Acute or delayed systemic administration of human amnion epithelial cells improves outcomes in experimental stroke. Stroke. 2018;49:700–709. doi: 10.1161/STROKEAHA.117.019136. [DOI] [PubMed] [Google Scholar]
- Liu T, Wu J, Huang Q, Hou Y, Jiang Z, Zang S, Guo L. Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock. 2008;29:603–611. doi: 10.1097/SHK.0b013e318157e845. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, et al. (2016) Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation. 133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nagpal A, Choy FC, Howell S, Hillier S, Chan F, Hamilton-Bruce MA, Koblar SA. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: a systematic review and meta-analysis. Stem Cell Res Ther. 2017;8:191. doi: 10.1186/s13287-017-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Ma H, Lim R, Sobey CG, Wallace EM. Phase I trial of amnion cell therapy for ischaemic stroke (I-ACT) Front Neurol. 2018 doi: 10.3389/fneur.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, Wong CH. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Tan JL, Chan ST, Wallace EM, Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 2014;23:319–328. doi: 10.3727/096368912X661409. [DOI] [PubMed] [Google Scholar]
- Tan JL, Chan ST, Lo CY, Deane JA, McDonald CA, Bernard CC, Wallace EM, Lim R. Amnion cell-mediated immune modulation following bleomycin challenge: controlling the regulatory T cell response. Stem Cell Res Ther. 2015;6:8. doi: 10.1186/scrt542. [DOI] [PMC free article] [PubMed] [Google Scholar]