Abstract
Degeneration of the locus coeruleus noradrenergic system is thought to play a key role in the pathogenesis of Parkinson's disease (PD), whereas pharmacological approaches to increase noradrenaline bioavailability may provide neuroprotection. Noradrenaline inhibits microglial activation and suppresses pro-inflammatory mediator production (e.g., tumor necrosis factor-α, interleukin-1β & inducible nitric oxide synthase activity), thus limiting the cytotoxicity of midbrain dopaminergic neurons in response to an inflammatory stimulus. Neighbouring astrocyte populations promote a neurotrophic environment in response to β2-adrenoceptor (β2-AR) stimulation via the production of growth factors (e.g., brain derived neurotrophic factor, cerebral dopamine neurotrophic factor & glial cell derived neurotrophic factor which have shown promising neuroprotective and neuro-restorative effects in the nigrostriatal dopaminergic system. More recent findings have demonstrated a role for the β2-AR in down-regulating expression levels of the human α-synuclein gene SNCA and relative α-synuclein protein abundance. Given that α-synuclein is a major protein constituent of Lewy body pathology, a hallmark neuropathological feature in Parkinson's disease, these findings could open up new avenues for pharmacological intervention strategies aimed at alleviating the burden of α-synucleinopathies in the Parkinsonian brain. In essence, the literature reviewed herein supports our hypothesis of a tripartite neuroprotective role for noradrenaline in combating PD-related neuropathology and motor dysfunction via (1) inhibiting nigral microglial activation & pro-inflammatory mediator production, (2) promoting the synthesis of neurotrophic factors from midbrain astrocytes and (3) downregulating α-synuclein gene expression and protein abundance in a β2-AR-dependent manner. Thus, taken together, either pharmacologically enhancing extra-synaptic noradrenaline bioavailability or targeting glial β2-ARs directly makes itself as a promising treatment option aimed at slowing/halting PD progression.
Keywords: noradrenaline, microglia, astrocytes, inflammation, Parkinson's disease, neuroprotection, animal model, dopamie
Chronic microglial activation, pro-inflammatory mediator production & oxidative stress can precipitate nigrostriatal neurodegeneration and ensuing motor dysfunction, as occurs in Parkinson's disease (PD) (Jenner, 2003). Toll-like receptor 4 (TLR4) is expressed on brain-resident microglia and TLR4-mediated signalling can induce potent inflammatory responses and is thought to be implicated in the pathogenesis of PD (Panaro et al., 2008). Lipopolysaccharide (LPS) is an endotoxin and TLR4 agonist derived from Gram-negative bacteria that has been previously used in rodents to study how inflammatory processes contribute to the pathogenesis of PD (Liu and Bing, 2011). Thus, promoting TLR4 signalling in nigral microglia with LPS is a useful method to recapitulate glial-derived pro-inflammatory mediator production and dopaminergic neuropathology in experimental PD.
The locus coeruleus (LC), situated in the outermost layer of the pontine tegmentum is the main source of noradrenergic (NA) cell bodies in the central nervous system (CNS) and these cells are reduced at autopsy by approximately 60% in PD patients relative to age-matched healthy controls (Marien et al., 2004). Consequently, NA inputs to the substantia nigra (SN) and striatum are decreased and it has been suggested that this loss of NA tone is a significant contributor to the progression of PD-related neuropathology and symptomology, including both motor and non-motor aspects of the disease (Gesi et al., 2000; Vazey and Aston-Jones, 2012). Indeed, studies by (Remy et al., 2005) using [11C]RTI-32 PET, an in vivo marker for dopamine & noradrenaline transporter binding on 8 and 12 PD patients with and without a history of depression respectively, have shown that the depressed cohort had lower [11C]RTI-32 binding in the LC and several limbic areas such as the amygdala, thalamus, anterior cingulate cortex and in the ventral striatum, indicating NA loss in these brain regions. The severity of anxiety in depressed PD patients was further shown to be inversely correlated with [11C]RTI-32 binding in most of these brain regions and the degree of apathy in these patients was inversely correlated with [11C]RTI-32 binding in the ventral striatum, thus highlighting a link between degeneration of the LC-NA system and loss of NA innervation to limbic brain regions with a higher frequency of depression & anxiety in PD patients.
In an experimental setting, selective lesioning of the LC-NA system and subsequent NA depletion is chiefly performed through the use of N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP4) (Jonsson et al., 1981). Treatment with DSP4 (50 mg/kg; intraperitoneal (i.p.) injection every 2 weeks) commencing 6 months post systemic LPS challenge (5 mg/kg; i.p.) exacerbates the loss of dopaminergic neurons in the rodent substantia nigra pars compacta (SNpc) in vivo whereas pre-treatment of primary mesencephalic neuron/glia cultures with submicromolar concentrations of noradrenaline suppresses LPS-induced microglial activation and proinflammatory mediator production in vitro (Jiang et al., 2015). Interestingly, the same authors demonstrate that pre-treatment of LPS-challenged mesencephalic neuron/glia cultures derived from β2-adrenergic receptor (AR)-deficient mice with NA does not totally abolish the neuroprotection observed, indicating that at a submicromolar level (10- to 100-fold lower than its lower than its binding affinity to AR binding affinity to AR receptors) the neuroprotection afforded by NA occurs at least partially, in a β2-AR-independent fashion. Indeed, data derived from the same studies show that noradrenaline, at submicromolar concentrations, exerts an anti-inflammatory effect on activated microglia by attenuating LPS-induced pro-inflammatory mediator production (such as tumor necrosis factor-α [TNFα] & nitric oxide) and inhibiting microglial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated superoxide production. Hence, degeneration of LC-NA neurons (up to 80%), as occurs in PD even before the onset of dopaminergic neuronal loss (Zarow et al., 2003; Baloyannis et al., 2006), is likely to enhance neuroinflammation & PD-related neuropathology whereas NA augmentation strategies could curtail these inflammatory processes & slow/halt disease progression.
Studies by Rommelfanger et al. (2007) have also shown that lesioning the LC-NA system with N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP4), or dopamine β-hydroxylase knockout (Dbh–/–) mice (which lack NA altogether) display greater motor abnormalities than 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-injected mice with 80% dopaminergic (DA) nerve terminal loss. Data from the same study showed that acute pharmacological restoration of central noradrenaline levels with L-threo-DOPS however, improved motor function in Dbh–/– mice. Moreover, MPTP-induced degeneration of the nigrostriatal DA system and ensuing dopamine loss is attenuated in noradrenaline transporter knockout (NAT–/–) mice, a protective effect similarly conferred upon treatment with the specific NAT inhibitor nisoxetine (Rommelfanger et al., 2004). Thus, given that the LC-NA system functionally modulates the survival rate of target DA neurons within the SN (Jiang et al., 2015), we hypothesized that the ensuing loss of LC noradrenergic inputs to the midbrain & striatum may be a major contributor to PD progression, whereas pharmacological enhancement of central noradrenergic tone may provide anti-inflammatory effects & provide neuroprotection against LPS-induced PD-related neuropathology and motor dysfunction.
Atomoxetine (marketed under brand name: Strattera by Eli Lilly and Company) is a selective noradrenaline reuptake inhibitor (NRI) approved by the US Food and Drug Administration for the treatment of attention deficit hyperactivity disorder (ADHD). Atomoxetine blocks presynaptic noradrenaline transporters (NAT) and thus, enhances extracellular noradrenaline concentration which further promotes NA signalling. Idazoxan is a selective α2-adrenoceptor antagonist that blocks presynaptic α2-AR's (which normally act as auto-receptors to regulate noradrenaline release) and thus potentiates extra-synaptic noradrenaline bioavailability. Previous data from our laboratory has demonstrated that treatment with atomoxetine (10 mg/kg; i.p.) attenuates LPS-induced increases in TNFα, interleukin-1β (IL-1β) & inducible nitric oxide synthase (iNOS) gene expression levels and suppresses nuclear factor-κB (NfκB) activation, findings which were accompanied by reductions in the microglial activation markers CD40 & CD11b in the rat cortex (O’Sullivan et al., 2009). Noradrenaline can affect a wide array of microglial functions through adrenergic signalling. Cultured rat microglia express mRNA encoding α1a-, α2a-, β1- and β2-ARs (Mori et al., 2002). Nevertheless, it is likely that NA also influences other cell populations. Astrocytes in particular have been identified as a possible, primary extra-synaptic target of noradrenaline and similar to microglia, rat astrocytes express functional α1-, α2-, β1- and β2-ARs (Hertz et al., 2004). We assessed the efficacy of treatment with atomoxetine alone or in combination with idazoxan in the inflammatory-based intra-nigral LPS rat model of PD. Treatment with atomoxetine (3 mg/kg; i.p.) alone or in combination with idazoxan (1 mg/kg; i.p.) commenced 4 hours post unilateral lesioning of the substantia nigra with bacterial LPS (10 μg/2 μL) and continued twice daily (b.i.d.) for 7 days. A previous in vivo microdialysis study by (Swanson et al., 2006) investigating the effect of atomoxetine (3 mg/kg; i.p.) and idazoxan (1 mg/kg; i.p.) on extracellular noradrenaline concentrations in freely moving rats has demonstrated a 7-fold increase in cortical noradrenaline efflux greater than that of either compound alone, indicating a synergistic effect when these agents are administered in combination, and thus we deemed these treatment dosages appropriate for elevating extracellular noradrenaline concentrations in the rat brain. Overall, our data demonstrated that enhancing central extra-synaptic noradrenergic bioavailability inhibits LPS-induced microglial activation within the SN, abrogates DA neurodegeneration, attenuates the ensuing nigrostriatal dopamine loss and provides partial protection against the associated motor dysfunction in vivo (Yssel et al., 2018).
Motor deficits are a salient feature of PD and become apparent in patients with ongoing DA neurodegeneration after an approximate 80% loss of striatal dopamine content (Cheng et al., 2010). Thus, we used a battery of motor function tests to assess the impact of pharmacologically enhancing NA tone on different aspects of PD-related motor dysfunction relative to the human condition, namely forelimb akinesia (stepping test), asymmetric limb use (cylinder test & D-amphetamine challenge) and skilled motor function (staircase test). Our data showed that in the stepping test of forelimb akinesia, intra-nigral LPS injection reduced the number of contralateral adjusting steps made in the forehand direction. Treatment with atomoxetine alone and in combination with idazoxan rescued these animals from LPS-induced forelimb akinesia in the forehand direction as indexed by increases in the number of adjusting steps made at 7 and 13 days post-lesioning relative to LPS-injected rats treated with saline, thus indicating a protective role for NA-augmentation against akinetic behaviour. Moreover, treatment with atomoxetine in combination with idazoxan partially protected against LPS-induced reductions in contralateral forelimb wall placements in the cylinder test and also reduced the number of amphetamine-induced ipsilateral rotations in LPS-lesioned rats, thus indicating a protective effect of enhancing NA tone against LPS-induced asymmetric limb use. Intra-nigral LPS reduced the contralateral forelimb success rate in the staircase test at 7, but not 13 days post-lesioning. In contrast to the stepping test and cylinder test, these deficits in skilled motor function were not restored by treatment with atomoxetine and/or idazoxan. Hence, it seems likely that with respect to the varying levels of sensitivity across the behavioural tests, the detection of different aspects of motor dysfunction are therefore dependent on the extent of LPS-induced degeneration of the nigrostriatal tract, and conversely the varying level of protection against motor dysfunction afforded by pharmacologically enhancing NA bioavailability likely depends on the degree of restored functionality of the neural circuitry responsible for performing these different motor tasks as discussed in the original research article.
Moreover, given that idazoxan only conferred a therapeutic benefit when co-administered with atomoxetine, highlights that the therapeutic benefits in terms of motor improvements across the board are more attributable to blockade of NAT due to treatment with atomoxetine as opposed to antagonism of α2-ARs post idazoxan administration. Although the influence of NA on subthalamic nuclei (STN) electrical activity remains unclear, it has been hypothesized that loss of NAergic input to this brain region increases the firing pattern of STN neurons resulting in hypokinesia. Conversely, improvements in motor function are observed following modulation of STN activity, through electrical LC stimulation or pharmacological manipulations. Indeed, α2-AR antagonism in the STN reduced motor abnormalities in 6-hydroxydopamine (6-OHDA) lesioned rats due to its heavy innervation by NAergic afferents expressing both α1- and α2-AR (Belujon et al., 2007), lending support to the role of idazoxan and modulation of this brain region in mediating improvements in asymmetric limb use observed in the LPS PD model. Moreover, blockade of α2-adrenoceptors exerts an ameliorative effect on levodopa (L-DOPA)-induced dyskinesia's in MPTP parkinsonian mice and nonhuman primates (Grondin et al., 2000; Archer and Fredriksson, 2003). Similarly, the neuroprotective effect of 28-day treatment with the α2-adrenoceptor antagonist dexefaroxan (0.63 mg/kg; i.p.) on devascularisation-induced degeneration of cholinergic neurons in the nucleus basalis was coincident with persistent NGF production in areas surrounding the cortical infarct (Debeir et al., 2004). It is possible that dexefaroxan-induced α2-AR blockade enhances NA tone, leading to the activation of astrocytic β2-ARs culminating towards amplified NGF production (Lu et al., 1991; Semkova et al., 1996) and the protection of basal forebrain cholinergic neurons.
These behavioural improvements observed following atomoxetine treatment in the intra-nigral LPS inflammatory model of PD in rats were fortified by our post mortem analysis demonstrating a protective effect of atomoxetine treatment against LPS-mediated neurotoxicity of the nigrostriatal DA system. On average, intra-nigral injection of LPS induced an approximate 66% loss of tyrosine hydroxylase (TH)+ dopamine neurons within the SNpc and an associated 64% loss of TH+ nerve terminals in the ipsilateral striatum. Treatment with atomoxetine alone or in combination with idazoxan abrogated the LPS-induced DA neurodegeneration within the SN and suppressed the affiliated DA nerve terminal loss in the striatum. Consistent with this immunohistochemical data, intra-nigral LPS induced an approximate 55% loss of dopamine content in the midbrain and an approximate 86% loss in the ipsilateral striatum. Treatment with atomoxetine alone almost completely attenuated the LPS-induced loss of nigrostriatal dopamine content to levels comparable to that of vehicle-injected control animals (Yssel et al., 2018).
Given that the midbrain contains the highest density of microglial cells in the entire rodent brain (Kim et al., 2000), the root, and indeed progression of this inflammatory-derived neuropathology and ensuing motor dysfunction is likely to be congruent on the activation state of nigral microglia. In essence, nigral microgliosis & subsequent pro-inflammatory mediator production is a cellular prerequisite for LPS-mediated neurotoxicity of dopamine neurons (Gayle et al., 2002). Treatment with atomoxetine alone or in combination with idazoxan however, inhibited nigral microglial activation in response to lesioning with LPS. Intra-nigral LPS injection lead to a robust increase in the number of Iba1+ microglial cells within the SN with a morphologically distinct phenotype (retracted processes & enlarged cell soma) from quiescent microglia (extended processes & smaller cell soma). Treatment with atomoxetine alone or in combination with idazoxan inhibited the LPS-induced Iba1+ nigral microgliosis and rendered the nigral microglia morphologically indicative of a quiescent phenotype. In support of the well documented anti-inflammatory effect of noradrenaline on microglial activation and pro-inflammatory cytokine secretion (Dello Russo et al., 2004; McNamee et al., 2010a), in the present study, treatment with atomoxetine also attenuated the LPS-induced increases in TNFα and IL-1β pro-inflammatory gene expression within the midbrain, a finding which is likely to have underpinned the attenuated loss of nigrostriatal dopamine neurons and dopamine concentrations. Previous data has shown that stimulation of CNS β2-adrenoceptors with clenbuterol (0.5 mg/kg; i.p.) suppresses NfκB activity and ameliorates expression of the NfκB-inducible genes TNFα and intercellular cell adhesion molecule-1 (ICAM-1) in response to central injection of bacterial LPS (1 μg/5μL; intracerebroventricular (icv)), whilst concurrently elevating the temporal expression of the NfκB-inhibitory protein nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α (IκBα) (Ryan et al., 2013). Moreover, noradrenaline negatively regulates the IL-1 system in glial cells via upregulating IL-1Ra and the IL-1RІІ decoy receptor in vitro (McNamee et al., 2010b) and in vivo (McNamee et al., 2010c) and raises CNS expression levels of the broad spectrum anti-inflammatory cytokine IL-10 and its downstream signalling molecule suppressor of cytokine signaling 3 (SOCS-3) in a β-adrenoceptor-dependent manner (McNamee et al., 2010b). Similarly, enhancing NA tone ameliorates LPS (250 μg/kg; i.p.) induced increases in cortical IL-1β, TNFα, iNOS, CD11b and CD40 gene expression (O’Sullivan et al., 2009), and decreases the elevated expression of the chemokines RANTES and interferon-inducible protein-10 (IP-10), as well as the cell adhesion molecules ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) in the CNS following systemic inflammatory insult (O’Sullivan et al., 2010). Thus, both noradrenaline augmentation strategies and β2-AR stimulation drive an anti-inflammatory phenotype in the CNS and may be of great therapeutic value in conditions where inflammation contributes to neuropathology (Figure 1).
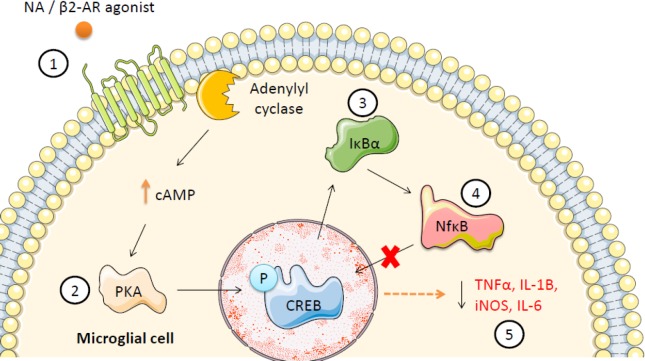
Figure 1.
Proposed anti-inflammatory mechanism of action of noradrenaline/β2-adrenoceptor (β2-AR) agonists on nigral microglia in the inflamed substantia nigra; a molecular signalling pathway towards neuroprotection.
Stimulation of Toll-like receptor 4 (TLR4) receptors on microglia with lipopolysaccharide (LPS) triggers a signalling cascade which leads to the phosphorylation and subsequent degradation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α (IκBα), nuclear factor-κB (NfκB) nuclear translocation and ensuing increases in pro-inflammatory gene expression. In the current schematic, stimulation of the β2-AR with endogenous noradrenergic (NA) or with a β2-AR agonist (e.g., clenbuterol/formoterol) may suppress NfκB transcriptional activity via the following pathway: (1) Stimulation of glial β2-ARs activates adenylyl cyclase which raises intracellular cyclic adenosine monophosphate (cAMP) leading to (2) activation of protein kinase A (PKA) which in turn phosphorylates cAMP-response element binding protein (CREB) and (3) induces de novo synthesis of the NfκB inhibitory protein IκBα (and possibly preventing its phosphorylation), thus stabilizing cytosolic levels of IκBα which (4) inhibits transcriptional activity of NfκB by preventing its translocation into the nucleus, ultimately (5) decreasing pro-inflammatory gene expression. TNFα: Tumor necrosis factor-α; IL: interleukin; iNOS: inducible nitric oxide synthase.
Enhancing NA availability also induced a marked increase in growth factor production within the midbrain. Treatment with atomoxetine significantly increased glial cell derived neurotrophic factor (GDNF) and brain derived neurotrophic factor (BDNF) mRNA expression within the midbrain of LPS-lesioned rats. Cerebral dopamine neurotrophic factor (CDNF) mRNA expression was also observably increased in these animals, albeit to a lesser extent to that of GDNF & BDNF. Here, we propose that treatment with atomoxetine is exerting a glial-derived neurotrophic response to raised extra-synaptic NA tone on foot of blockade of the NAT. The increases in GDNF are particularly promising from a neuro-protective perspective, as delayed intra-striatal delivery of this growth factor has previously been shown to prevent DA neurodegeneration within the SNpc, increase striatal dopamine concentration and promote functional recovery in 6-OHDA-lesioned rats (Wang et al., 2002). Moreover, direct intra-putamenal infusion of GDNF in 5 PD patients increases dopamine storage in the putamen, improves UPDRS motor scores and reduces medication-induced dyskinesias (Gill et al., 2003). Thus, in conjunction with the anti-inflammatory effect of atomoxetine treatment on activated microglia, we now show that atomoxetine also induces growth factor expression within the midbrain, most likely from astrocytes in an AR-dependent manner. Thus, atomoxetine has a dual effect on midbrain glial cells, inducing a tonic inhibition on microglial activation & pro-inflammatory gene expression, whilst concurrently promoting the synthesis of neurotrophic factors from astrocytes. Both aspects are likely to contribute to the neuroprotection afforded by treatment with atomoxetine.
Noradrenaline is hypothesised to play a bi-modal neuroprotective role in the brain in a variety of neurodegenerative disease states via its interactions with glial cells, particularly by down-regulating microglial pro-inflammatory gene expression (Dello Russo et al., 2004; Jiang et al., 2015), and also by promoting a neurotrophic effect in the brain via astrocytic growth factor production in a β-AR dependent manner (Culmsee et al., 1999a, b). Pre-treatment with NA protects primary rat hippocampal cells against Aβ1–42 and Aβ25–35 mediated toxicity via the production of NGF and BDNF downstream of β-AR signalling (Counts and Mufson, 2010). Moreover, in the multiple sclerosis field, CNS noradrenaline deficiency exacerbates experimental autoimmune encephalitis (EAE), whereas dual treatment with the NRI atomoxetine (20 mg/kg; i.p.) and L-threo-DOPS (400 mg/kg; subcutaneous (s.c.)) administered three times weekly improves EAE clinical scores in NA-depleted mice (Simonini et al., 2010). Similarly, the serotonin noradrenaline reuptake inhibitor venlafaxine suppresses CD3, CD8, IL-12 p40, TNFα, IFN-γ, CCL2 and RANTES gene transcripts in the CNS lesions of an experimental adoptive myelin-specific T-cell model of EAE, whilst concomitantly upregulating BDNF expression in the inflamed spinal cord of these animals (Vollmar et al., 2009).
In addition to our findings, studies by Mittal et al. (2017) on the pharmaceutical history of over 4 million Norwegians who were taking one of the β2-AR agonists for other medical problems over an 11 year period has shown that usage of the β2-AR agonist salbutamol (a brain-penetrant asthma medication) was associated with a decreased risk of developing PD, and conversely, that blockade of the β2-AR with the antagonist propranolol was associated with an increased risk of developing PD. Interestingly, out of 1126 Food and Drug Administration (FDA)-approved drugs & compounds screened, 4 significantly reduced alpha synuclein gene (SNCA) & protein expression (a major constituent of pathological Lewy Body inclusions in PD brains); three of them being the selective β2-AR agonists metaproterenol, clenbuterol & salbutamol. Furthermore, treatment with clenbuterol (20 μM) reduced SNCA mRNA expression and α-synuclein (α-Syn) protein levels in SNCA-triplication patient iPSC-derived neuronal precursor cells and attenuated the loss of TH+ dopaminergic neurons in the SNpc of MPTP-lesioned mice in vivo. Moreover, the authors demonstrate that the β2-AR regulates the transcription of the human α-synuclein gene SNCA through H3K27 acetylation (H3K27ac) of promoters and enhancers in the human SNCA locus and that treatment with clenbuterol is correlated with a decrease in H3K27ac levels and relative expression of SNCA mRNA levels. Data derived from the same study also shows that knockout of the β2-AR gene (Adrb2) in murine primary neurons, RNA interference-induced silencing of the β2-AR in human SK-N-MC cells or chemical antagonism of the β2-AR with the β-blocker propranolol in SK-N-MC cells consistently increases SNCA mRNA expression and α-Syn protein abundance. Conversely, transfection of SK-N-MC cells with ADRB2 constructs reduces α-Syn SNCA mRNA levels and genetic silencing of the β2-AR with siRNA's or its blockade with propranolol abrogates the SNCA expression-lowering effects induced by treatment with the β2-AR agonist clenbuterol. Thus, these results are strong evidence indicating that the β2-AR is linked to transcription of α-syn and risk of developing PD, and that pharmacological stimulation of glial β2-ARs may provide neuroprotection.
In conclusion, we propose that noradrenaline is exerting a tripartite neuroprotective role in the pathologic CNS by (a) inhibiting microglial activation within the substantia nigra and attenuating the ensuing increases in pro-inflammatory gene expression, (b) stimulating the production of neurotrophic factors from neighbouring astrocytes and (c) regulating SNCA gene expression and α-syn protein levels. Our results demonstrate that pharmacological enhancement of extra-synaptic NA bioavailability via blockade of the noradrenaline transporter may be a pertinent candidate for consideration as a future PD pharmacotherapy and in related illnesses where inflammation contributes to neuropathology. Future studies will aim to determine if pharmacologically targeting glial β2-ARs directly will elicit anti-inflammatory and neuroprotective effects in the intra-nigral LPS and α-syn transgenic rat models of PD.
Additional file: Open peer review report 1 (104.4KB, pdf) .
Acknowledgments
The authors acknowledge the support of Trinity Foundation, Trinity College Dublin.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: Eoin O’Neill was supported by a Trinity College postgraduate award.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Yi Pang, University of Mississippi Medical Center, USA.
Funding: Eoin O’Neill was supported by a Trinity College postgraduate award.
References
- Archer T, Fredriksson A. An antihypokinesic action of α2-adrenoceptors upon MPTP-induced behaviour deficits in mice. J Neural Transm. 2003;110:183–200. doi: 10.1007/s00702-002-0777-5. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Costa V, Baloyannis IS. Morphological alterations of the synapses in the locus coeruleus in Parkinson's disease. J Neurol Sci. 2006;248:35–41. doi: 10.1016/j.jns.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Belujon P, Bezard E, Taupignon A, Bioulac B, Benazzouz A. Noradrenergic modulation of subthalamic nucleus activity: behavioral and electrophysiological evidence in intact and 6-hydroxydopamine-lesioned rats. J Neurosci. 2007;27:9595–9606. doi: 10.1523/JNEUROSCI.2583-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson's disease and the neurobiology of axons. Ann Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem. 2010;113:649–660. doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Semkova I, Krieglstein J. NGF mediates the neuroprotective effect of the β2-adrenoceptor agonist clenbuterol in vitro and in vivo: evidence from an NGF-antisense study. Neurochem Int. 1999a;35:47–57. doi: 10.1016/s0197-0186(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Stumm RK, Schäfer MKH, Weihe E, Krieglstein J. Clenbuterol induces growth factor mRNA, activates astrocytes, and protects rat brain tissue against ischemic damage. Eur J Pharmacol. 1999b;379:33–45. doi: 10.1016/s0014-2999(99)00452-5. [DOI] [PubMed] [Google Scholar]
- Debeir T, Marien M, Ferrario J, Rizk P, Prigent A, Colpaert F, Raisman-Vozari R. In vivo upregulation of endogenous NGF in the rat brain by the alpha2-adrenoreceptor antagonist dexefaroxan: potential role in the protection of the basalocortical cholinergic system during neurodegeneration. Exp Neurol. 2004;190:384–395. doi: 10.1016/j.expneurol.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1β production. J Neuroinflammation. 2004;1:1–15. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle DA, Ling Z, Tong C, Landers T, Lipton JW, Carvey PM. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-α, interleukin-1β, and nitric oxide. Brain Res Dev Brain Res. 2002;133:27–35. doi: 10.1016/s0165-3806(01)00315-7. [DOI] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Grondin R, Hadj Tahar A, Doan DV, Ladure P, Bédard PJ. Noradrenoceptor antagonism with idazoxan improves l-dopa-induced dyskinesias in MPTP monkeys. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:181–186. doi: 10.1007/s002109900167. [DOI] [PubMed] [Google Scholar]
- Hertz L, Chen Y, Gibbs M, Zang P, Peng L. Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:239–267. doi: 10.2174/1568007043337535. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jiang L, Chen SH, Chu CH, Wang SJ, Oyarzabal E, Wilson B, Sanders V, Xie K, Wang Q, Hong JS. A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia. 2015;63:1057–1072. doi: 10.1002/glia.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson G, Hallman H, Ponzio F, Ross S. DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine)--a useful denervation tool for central and peripheral noradrenaline neurons. Eur J Pharmacol. 1981;72:173–188. doi: 10.1016/0014-2999(81)90272-7. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Bing G. Lipopolysaccharide animal models for Parkinson's disease. Parkinsons Dis. 2011;2011:327089. doi: 10.4061/2011/327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Yokoyama M, Dreyfus C, Black I. NGF gene expression in actively growing brain glia. J Neurosci. 1991;11:318–326. doi: 10.1523/JNEUROSCI.11-02-00318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- McNamee EN, Ryan KM, Kilroy D, Connor TJ. Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1β-induced neurotoxicity. Eur J Pharmacol. 2010a;626:219–228. doi: 10.1016/j.ejphar.2009.09.054. [DOI] [PubMed] [Google Scholar]
- McNamee EN, Ryan KM, Griffin EW, Gonzalez-Reyes RE, Ryan KJ, Harkin A, Connor TJ. Noradrenaline acting at central α-adrenoceptors induces interleukin-10 and suppressor of cytokine signaling-3 expression in rat brain: Implications for neurodegeneration. Brain Behav Immun. 2010b;24:660–671. doi: 10.1016/j.bbi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- McNamee EN, Griffin ÉW, Ryan KM, Ryan KJ, Heffernan S, Harkin A, Connor TJ. Noradrenaline acting at β-adrenoceptors induces expression of IL-1β and its negative regulators IL-1ra and IL-1RII, and drives an overall anti-inflammatory phenotype in rat cortex. Neuropharmacology. 2010c;59:37–48. doi: 10.1016/j.neuropharm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, Abo KM, Long E, Jin M, Xu B, Xiang YK, Rochet JC, Engeland A, Rizzu P, Heutink P, Bartels T, Selkoe DJ, Caldarone BJ, Glicksman MA, Khurana V, et al. (2017) β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson's disease. Science. 357:891–898. doi: 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express α1, α2, β1 and β2 adrenergic receptors. Neuropharmacology. 2002;43:1026–1034. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JB, Ryan KM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J Neuroimmunol. 2010;220:34–42. doi: 10.1016/j.jneuroim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol. 2009;12:687–699. doi: 10.1017/S146114570800967X. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Lofrumento DD, Saponaro C, De Nuccio F, Cianciulli A, Mitolo V, Nicolardi G. Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson’s-like disease. Immunopharmacol Immunotoxicol. 2008;30:729–740. doi: 10.1080/08923970802278557. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D, Miller GW. Reduced MPTP toxicity in noradrenaline transporter knockout mice. J Neurochem. 2004;91:1116–1124. doi: 10.1111/j.1471-4159.2004.02785.x. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KJ, Griffin E, Yssel JD, Ryan KM, McNamee EN, Harkin A, Connor TJ. Stimulation of central α2-adrenoceptors suppresses NFκB activity in rat brain: A role for IκB. Neurochem Int. 2013;63:368–378. doi: 10.1016/j.neuint.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Semkova I, Schilling M, Henrich-Noack P, Rami A, Krieglstein J. Clenbuterol protects mouse cerebral cortex and rat hippocampus from ischemic damage and attenuates glutamate neurotoxicity in cultured hippocampal neurons by induction of NGF. Brain Res. 1996;717:44–54. doi: 10.1016/0006-8993(95)01567-1. [DOI] [PubMed] [Google Scholar]
- Simonini MV, Polak PE, Sharp A, McGuire S, Galea E, Feinstein DL. Increasing CNS noradrenaline reduces EAE severity. J Neuroimmune Pharmacol. 2010;5:252–259. doi: 10.1007/s11481-009-9182-2. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology. 2006;50:755–760. doi: 10.1016/j.neuropharm.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Vazey E, Aston-Jones G. The emerging role of norepinephrine in cognitive dysfunctions of Parkinson's disease. Front Behav Neurosci. 2012;6:48. doi: 10.3389/fnbeh.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar P, Nessler S, Kalluri SR, Hartung HP, Hemmer B. The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int J Neuropsychopharmacol. 2009;12:525–536. doi: 10.1017/S1461145708009425. [DOI] [PubMed] [Google Scholar]
- Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Hanazono Y, Kume A, Urano F. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson's disease. Gene Ther. 2002;9:381. doi: 10.1038/sj.gt.3301682. [DOI] [PubMed] [Google Scholar]
- Yssel JD, O’Neill E, Nolan YM, Connor TJ, Harkin A. Treatment with the noradrenaline re-uptake inhibitor atomoxetine alone and in combination with the α2-adrenoceptor antagonist idazoxan attenuates loss of dopamine and associated motor deficits in the LPS inflammatory rat model of Parkinson's disease. Brain Behav Immun. 2018;69:456–469. doi: 10.1016/j.bbi.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.