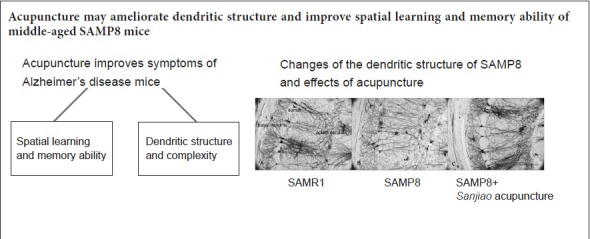
Keywords: nerve regeneration, Alzheimer's disease, senescence-accelerated prone mouse 8, acupuncture, cognition, dendrite, hippocampal CA1 region, Morris water maze, Golgi staining, neural regeneration
Abstract
Acupuncture can improve the cognitive state of Alzheimer's disease, but its mechanism is not clear. Dendritic atrophy and synaptic loss in Alzheimer's disease brain are positively correlated with cognitive damage. Therefore, we speculated that the effect of acupuncture on improving cognitive function may be associated with reduced dendritic damage in the brain. Acupuncture at Qihai (CV6), Zhongwan (CV12), Danzhong (CV17), bilateral Zusanli (ST36), and bilateral Xuehai (SP10) acupoints was performed once a day (1-day rest after 6-day treatment) for 14 consecutive days. Senescence-accelerated mouse prone 8 (SAMP8) mice without acupuncture and senescence-accelerated mouse resistant 1 (SAMR1) mice were used as normal controls. After 14 days of treatment, spatial learning and memory ability of mice was assessed in each group using the Morris water maze. Dendritic changes of pyramidal cells in the hippocampal CA1 region were analyzed by quantitative Golgi staining. Our results showed that acupuncture shortened escape latency and lengthened retention time of the former platform quadrant in SAMP8 mice. Further, SAMP8 mice exhibited a significant increase in the number of apical and basal dendritic branches and total length of apical and basal dendrites after acupuncture. These results suggest that acupuncture improves spatial learning and memory ability of middle-aged SAMP8 mice by ameliorating dendritic structure.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease and the major cause of senile dementia. High incidence and mortality rates of AD have caused a heavy burden on society and the family. According to an Alzheimer's Association's report in 2016, one new case of Alzheimer's dementia developed every 66 seconds, and an estimated 700,000 Americans aged ≥ 65 years will die with AD (Alzheimer's Association, 2016). There are no effective treatments to stop and reverse progression of AD. Acupuncture as a complementary and alternative therapy has a long history in treating patients with nervous system diseases (No authors listed, 1998), and can achieve desired results (Kim et al., 2012). A systematic review and meta-analysis of randomized controlled trials found that acupuncture treatment is safe and may be more effective than drugs in improving the daily lives of patients with AD (Zhou et al., 2015). Sanjiao acupuncture was put forward based on traditional Chinese medicine and its effect on improving cognitive status had been proven in long-term clinical practice (Han, 2007). Acupuncture might improve cognitive function by reducing oxidative stress, amyloid beta (Aβ) protein deposition, and apoptosis, as well as regulating glucose metabolism and enhancing neurotransmission and autophagy (Leung et al., 2014; Cao et al., 2016; Lai et al., 2016). However, there are few reports on the effects of acupuncture on changes in dendritic structure.
Cognitive damage positively correlates with dendritic atrophy and synapse loss in AD (Falke et al., 2003; Zhang et al., 2016). Some studies have suggested that AD is a disease of synaptic disorder (Selkoe, 2002; Marcello et al., 2012). Indeed, Cochran et al., (2014) first proposed the “dendritic hypothesis” of AD, with dystrophic neurites, reduced dendritic complexity, and loss of dendritic spines being dendritic defects found in AD. Soluble Aβ causes impaired dendritic structure, spine density, and synaptic ultrastructure of neurons in the hippocampal CA1 region, which leads to cognitive disorder in early AD (Pozueta et al., 2013; Price et al., 2014). Senescence-accelerated mouse prone 8 (SAMP8) is an ideal animal model of AD. Spatial learning and memory ability of SAMP8 mice declines along with typical pathological hallmarks of AD including senile plaques, tau-like neurofibrillary tangles, and neuropathological changes (Cheng et al., 2014). Spine density starts to decrease in SAMP8 mice at 6 months and worsens at 9 months (del Valle et al., 2012). In this study, we examined the relationship of acupuncture with cognitive function and dendrites in AD.
Materials and Methods
Animals
Twenty male 7.5-month-old SAMP8 and 10 male age-matched senescence-accelerated mouse resistant 1 (SAMR1) mice were used in this study. Their phenotypes have been described previously (Takeda et al., 1997). SAMR1 mice were used as normal controls. All mice were obtained from our breeding colony, maintained as an inbred strain, and originally obtained from Professor Takeda of Kyoto University, Japan. The study protocol was approved by the Animal Ethics and Welfare Board of Tianjin Medical University of China (license No. TCM-LAEC20170025). Mice were housed at 23 ± 1°C and humidity of 55 ± 10%, and allowed free access to food and water in a 12-hour light/dark cycle.
Acupuncture treatment
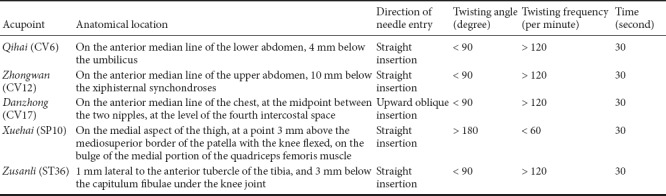
SAMP8 mice were randomly divided into two groups (n = 10 per group): SAMP8 + SA group (acupuncture) and SAMP8 group (model control). The SAMP8 + SA group received Sanjiao acupuncture once daily with a 0.25 mm × 25 mm acupuncture needle (Changchun AIK Medical Devices Co., Ltd., Changchun, China) (Luo et al., 2017). Qihai (CV6), Zhongwan (CV12), Danzhong (CV17), bilateral Zusanli (ST36), and bilateral Xuehai (SP10) acupoints were selected, according to the ‘‘animal acupuncture points map’’ reported by the Experimental Acupuncture–Moxibustion Research Association of China (Academy of Acupuncture–Moxibustion) (Table 1). Acupuncture was administered once a day (1-day rest after 6-day treatment) for 14 consecutive days. Mice in the SAMP8 group and SAMR1 group (10 SAMR1 mice) were handled using the same degree of grasp. The whole treatment lasted for 14 days with a rest on day 7.
Table 1.
Acupuncture points, anatomical position, and innervation
Morris water maze (MWM)
A pilot study using a cueing procedure demonstrated no significant differences in sensorimotor or motivational function in each group (data not shown). Thus, to test hippocampus-dependent spatial cognition, SAMR1 and SAMP8 mice were trained in the standard MWM with a hidden platform after acupuncture. A 10-day testing protocol was used, as previously described (Li et al., 2013). Briefly, mice were gently placed facing the wall of the water pool in different locations for each trial (two trials per day), and allowed 90 seconds to locate a submerged platform. Mice were then allowed to stay on the platform for an additional 10 seconds. If mice failed to find the platform within 90 seconds, they were guided gently onto the platform and remained for 10 seconds. On day 6, the swimming paths of mice were recorded over 60 seconds after being placed in the same point of the pool without the hidden platform. Retention time of the former platform quadrant was analyzed. From days 7 to 9, the hidden platform was moved to the opposite quadrant and the following steps operated according to the hidden platform. On day 10, the platform was 2 cm above the water and marked by yellow tape, with the same operation process as for the hidden platform trial. Data were recorded using image tracking software (China Daheng Group, Inc., Beijing Image Vision Technology Branch, Beijing, China).
Golgi staining
Hito Golgi-Cox OptimStain™ Kit (HTKNS1125, Hitobiotec, Wilmington, DE, USA) was used in accordance with the manufacturer's protocol. Briefly, mice were euthanized at 10 days after acupuncture. The brains were rapidly removed and handled with care to avoid damage. Brain tissue was rinsed briefly in double distilled water for 2–3 seconds to remove blood from the surface, and then transferred into impregnation solution that was at least five-times the tissue volume. Brain tissue was stored at room temperature in the dark for 12–24 hours. To avoid non-specific staining, impregnation time could not be extended. Brain tissue was then transferred into at least five-times the tissue volume of solution 3, and incubated at 4°C in the dark for 12 hours. Solution was replaced with fresh solution. Afterwards, brain tissue was further stored at 4°C in the dark for 24–72 hours. Next, blocks were rapidly frozen in Tissue-Ted solution, wrapped with aluminum foil, and stored at −80°C. Sections (100-μm thickness) were cut using a cryostat chamber at −19°C. The following procedure was performed according to a user manual. Images of pyramidal neurons in the hippocampal CA1 region were captured by randomly selecting well-stained neurons at 200× magnification using stereology software (MBF Bioscience, Williston, VT, USA). Selection criteria for image analysis of neurons were: (1) pyramidal neurons had to reside in the hippocampal CA1 region, as defined by cytoarchitectural characteristics within 100 μm section depth; (2) cell position and overall profile were clear, while the colors of cell bodies and branches were uniform and consistent; and (3) intact tertiary branches, except for branches that extended beyond 50 μm from the cell soma in a radial distance. The number of dendrites was automatedly quantified using the Neuron J analysis plugin of Image J software (National Institutes of Health, Bethesda, MD, USA). Dendritic complexity was assessed by Sholl analysis (Morris et al., 1982). The number of dendritic intersections was counted with concentric spheres positioned at radial intervals of 40 μm. Dendritic structure was assessed by a blinded analyzer.
Statistical analysis
All data are presented as the mean ± SD and were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Escape latencies in MWM were analyzed by repeated measure two-way analysis of variance (ANOVA). Comparison between groups was performed using one-way ANOVA followed by post hoc Bonferroni testing (multiple comparisons). A value of P < 0.05 was considered statistically significant.
Results
Acupuncture improved spatial learning and memory ability in SAMP8 mice
After five-days of training, escape latency of mice was significantly different among groups (P < 0.05). There was a significant interaction between training days and groups (P < 0.05). Dynamic change and tendency of mice in each group are shown in Figure 1A. Escape latency progressively shortened with increasing training days in the SAMR1 group and SAMP8 + SA group. Escape latency was significantly longer in the SAMP8 group compared with the SAMR1 group. Acupuncture significantly shortened the escape latency of SAMP8 mice (P < 0.05). There was no significant difference in escape latency between the SAMR1 group and SAMP8 + SA group (P > 0.05). In the probe trial, acupuncture significantly increased retention time of SAMP8 mice in the original platform quadrant (P < 0.001). There was no significant difference in retention time between the SAMR1 group and SAMP8 + SA group (P > 0.05; Figure 1B). During the reversal trial, there was a significant difference among groups (P < 0.05), but no interaction between groups and training days (P > 0.05). Acupuncture significantly shortened the escape latency of SAMP8 mice (P < 0.05). There was no significant difference in escape latency between the SAMP8 + SA group and SAMR1 group (P > 0.05; Figure 1C).
Figure 1.
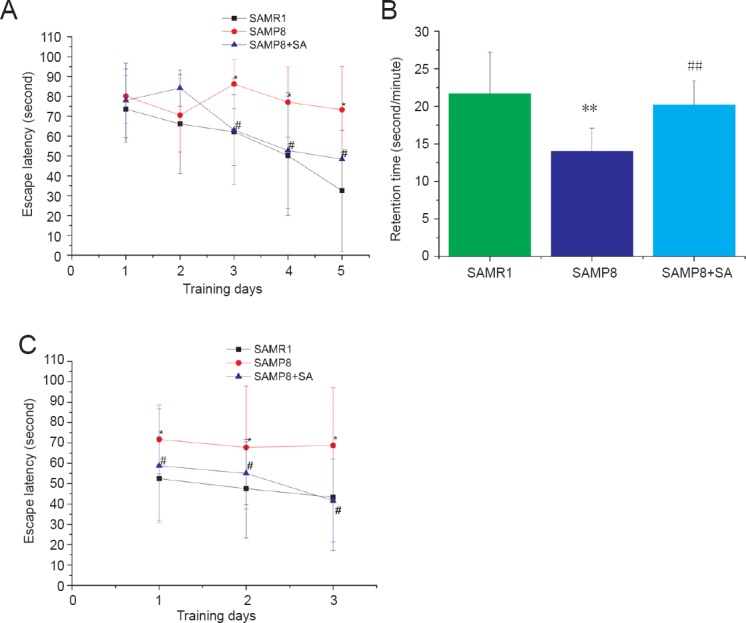
Acupuncture improved spatial learning and memory ability of SAMP8 mice in the Morris water maze.
(A) Escape latency of mice in each group in the hidden platform trial. (B) Retention time of mice in each group in the probe trial. (C) Escape latency of mice in each group in the reversal trial. Data are expressed as the mean ± SD (n = 10). Escape latency in the Morris water maze was analyzed by repeated measures two-way analysis of variance. Comparison between groups was performed using one-way analysis of variance followed by post hoc Bonferroni testing (multiple comparisons). *P < 0.05, **P < 0.001, vs. SAMR1 group; #P < 0.05, ##P < 0.001, vs. SAMP8 group. SAMP8: Senescence-accelerated mouse prone 8; SAMR1: senescence-accelerated mouse resistant 1.
Effect of acupuncture on dendritic structure in SAMP8 mice
Dendritic complexity was assessed by Golgi staining (Figure 2). SAMP8 mice exhibited a significant decrease in number of apical dendritic branches (P < 0.001, vs. SAMR1 group) and total length of apical dendrites (P < 0.001, vs. SAMR1 group). With acupuncture treatment, both the number of apical dendritic branches and total length of apical dendrites increased in the SAMP8 + SA group compared with the SAMP8 group (P < 0.05; Figure 3B, D).
Figure 2.
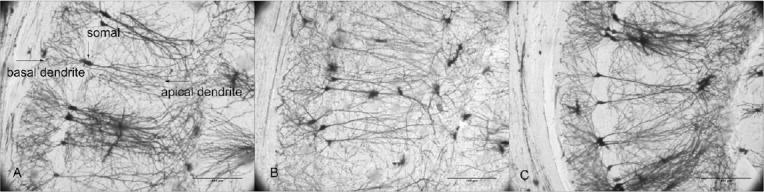
Morphology of pyramidal neurons in hippocampal CA1 region of SAMP8 and SAMR1 mice at 10 days after acupuncture (Golgi staining)
(A) Morphology of pyramidal neurons was good in the hippocampal CA1 region of SAMR1 mice. (B) Morphology of pyramidal cells was damaged in the hippocampal CA1 region of SAMP8 mice. (C) Morphology of pyramidal neurons in the hippocampal CA1 region of SAMP8 mice was improved by acupuncture treatment. Scale bars: 100 μm. SAMP8: Senescence-accelerated mouse prone 8; SAMR1: senescence-accelerated mouse resistant 1; SA: Sanjiao acupuncture.
Figure 3.
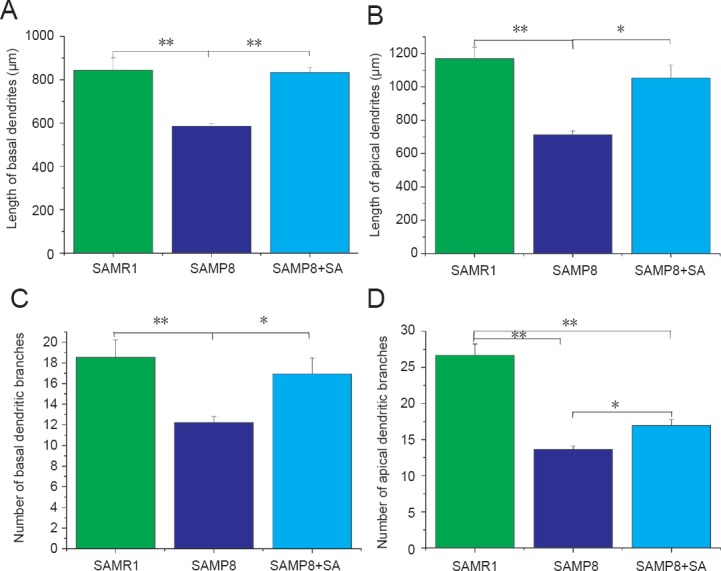
Neuron J analysis of dendritic morphology in hippocampal CA1 region of SAMP8 mice at 10 days after acupuncture.
Dystrophic neurites and reduced dendritic complexity are common dendritic abnormalities in AD. (A) Acupuncture had an effect on the length of basal dendrites in the hippocampal CA1 region of SAMP8 mice. (B) Acupuncture had an effect on the length of apical dendrites in the hippocampal CA1 region of SAMP8 mice. (C) Acupuncture had an effect on the number of basal dendritic branches in the hippocampal CA1 region of SAMP8 mice. (D) Acupuncture had an effect on the number of apical dendritic branches in the hippocampal CA1 region of SAMP8 mice. *P < 0.05, **P < 0.001 (mean ± SD; n = 10; analysis of variance followed by post hoc Bonferroni testing). SAMP8: Senescence-accelerated mouse prone 8; SAMR1: senescence-accelerated mouse resistant 1; AD: Alzheimer's disease; SA: Sanjiao acupuncture.
Regarding basal dendrites, the SAMP8 group also showed decreased branch number and total length compared with the SAMR1 group (P < 0.001). In the SAMP8 + SA group, both branch number and total length were significantly increased compared with the SAMP8 group (P < 0.05), and were similar to the SAMR1 group (P > 0.05; Figure 3A, C). Sholl analysis was also performed to examine the branching characteristics of neurons. Sholl analysis showed that the intersections of dendritic branches were greatest at a distance of 40 μm from the soma, before decreasing gradually. In the SAMP8 group, the number of intersections at a distance of 40–160 μm from the soma was significantly lower compared with the SAMR1 group (P < 0.05). Acupuncture had a therapeutic effect on nodes at a distance of 80–160 μm from the soma, with the distance being significantly increased in the SAMP8 + SA group compared with the SAMP8 group (P < 0.05; Figure 4).
Figure 4.
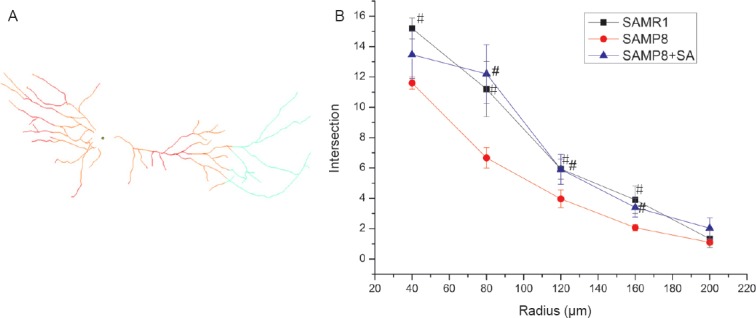
Sholl analysis of dendritic morphology in hippocampal CA1 region of SAMP8 mice at 10 days after acupuncture.
(A) Schematic diagram of Sholl analysis. (B) Sholl analysis curve of dendrites. #P < 0.05, vs. SAMP8 group (mean ± SD, n = 10, analysis of variance followed by post hoc Bonferroni testing). SAMP8: Senescence-accelerated mouse prone 8; SAMR1: senescence-accelerated mouse resistant 1; SA: Sanjiao acupuncture.
Discussion
The MWM is used to assess spatial memory and learning ability (Morris et al., 1982). The hidden platform trial evaluates spatial memory acquisition, while the probe trial is used to test animal spatial memory retention capacity, and the reverse trial reflects animal relearning ability. Using other experimental methods, Yanai and Endo (2016) found age-related behavioral alterations in SAMP8 mice as early as 4-month-old. In this study, 8-month-old SAMP8 mice were examined, with our results showing visibly impaired cognitive function in the SAMP8 group compared with the SAMR1 group, which is consistent with a previous study (Jin et al., 2016).
Dendritic structure is essential for brain function and is the structural basis of cognition (Kulkarni and Firestein, 2012). Overall shape of the dendritic arbor determines neuronal input and how it is processed, thereby affecting synaptic output (Elston and Fujita, 2014). Vulnerability of pyramidal cells in the hippocampal CA1 region plays an important role in occurrence of cognitive impairment (Counts et al., 2014). Our Golgi staining results show that dendritic length and dendritic branches are both altered in the hippocampal CA1 region of SAMP8 mice. This is consistent with studies by Siskova et al., (Siskova et al., 2014), on amyloid precursor protein/presenilin 1 (APP/PS1) mice, and Wang et al., (Wang et al., 2016), on Aβ1–42-induced AD mice. Further, this is partly consistent with the study by Price et al., on a mouse model of AD with soluble Aβ oligomers, in which dendritic structure changes only occurred in apical dendrites (Price et al., 2014). Differences in results may be due to model differences. Changes in dendritic complexity of SAMP8 are first reported here.
Acupuncture is different from drug therapy and achieves a better effect with minimal side effects during treatment of multiple chronic conditions (Luo and Du, 2014). Sanjiao acupuncture was proposed by Professor Han Jingxian, who emphasized that AD was not a disease of an individual organ, and that Sanjiao pneumatolysis disorder caused AD (Cheng et al., 2008). Sanjiao pneumatolysis links to the five internal organs (heart, liver, spleen, lung, and kidney), with only correct gasification function necessary to ensure humans are disease-free. Gasification arrhythmia is the fundamental mechanism of aging. Sanjiao acupuncture focuses on regulating visceral functions covered by Sanjiao, which reflects the Chinese perspective and achieves good clinical effects (Yu et al., 2006). Acupuncture at Qihai (CV6), Zhongwan (CV12), and Tanzhong (CV17) can regulate the lower energizer, middle energizer, and upper energizer, respectively. Acupuncture at Zusanli (ST36) can regulate Sanjiao, while at Xuehai (SP10) can regulate and harmonize the blood. Our previous studies have revealed the mechanism of acupuncture in terms of neurons (Cheng et al., 2008; Li et al., 2012) and oxidation-reduction (Liu et al., 2006). This study determined whether acupuncture can prevent dendritic degeneration and improve behavioral outcome in SAMP8 mice. Our results show that acupuncture dramatically reduces dendritic damage and preserves dendritic complexity. Branch number and total length of both apical and basal dendrites in the SAMP8 + SA group were remarkably increased compared with the SAMP8 group.
In conclusion, dendrites are the therapeutic target of acupuncture for AD, and acupuncture may ameliorate dendritic structure and improve spatial learning and memory ability of middle-aged SAMP8 mice. This study provides scientific evidence for acupuncture treatment of AD. In further studies, dendritic changes in SAMP8 mice at different ages, and sustained changes in mice after acupuncture will be studied to further reveal molecular mechanisms of acupuncture effects on dendrites.
Additional file: Open peer review report 1 (8.2KB, pdf) .
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81603686, 81603684; the High School Science and Technology Fund Planning Project of Tianjin of China, No. 20120211; the Natural Science Foundation of Tianjin of China (Key Program), No. 15JCZDJC36700, 16JCZDJC37500; the Natural Science Foundation of Tianjin of China, No. 17JCYBJC26200. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: The study protocol was approved by the Animal Ethics and Welfare Board of Tianjin University of Traditional Chinese Medicine of China (license No. TCM-LAEC20170025). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Maria Solesio, New York University, USA.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81603686, 81603684; the High School Science and Technology Fund Planning Project of Tianjin of China, No. 20120211; the Natural Science Foundation of Tianjin of China (Key Program), No. 15JCZDJC36700, 16JCZDJC37500; the Natural Science Foundation of Tianjin of China, No. 17JCYBJC26200.
(Copyedited by James R, Frenchman B, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhang LW, Wang J, Du SQ, Xiao LY, Tu JF, Liu CZ. Mechanisms of acupuncture effect on Alzheimer's disease in animal- based researches. Curr Top Med Chem. 2016;16:574–578. doi: 10.2174/1568026615666150813144942. [DOI] [PubMed] [Google Scholar]
- Cheng H, Yu J, Jiang Z, Zhang X, Liu C, Peng Y, Chen F, Qu Y, Jia Y, Tian Q, Xiao C, Chu Q, Nie K, Kan B, Hu X, Han J. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett. 2008;432:111–116. doi: 10.1016/j.neulet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Cheng XR, Zhou WX, Zhang YX. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer's disease animal model. Ageing Res Rev. 2014;13:13–37. doi: 10.1016/j.arr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Cochran JN, Hall AM, Roberson ED. The dendritic hypothesis for Alzheimer's disease pathophysiology. Brain Res Bull. 2014;103:18–28. doi: 10.1016/j.brainresbull.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ. Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology. 2014;79:172–179. doi: 10.1016/j.neuropharm.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle J, Bayod S, Camins A, Beas-Zárate C, Velázquez-Zamora DA, González-Burgos I, Pallàs M. Dendritic spine abnormalities in hippocampal CA1 pyramidal neurons underlying memory deficits in the SAMP8 mouse model of Alzheimer's disease. J Alzheimers Dis. 2012;32:233–240. doi: 10.3233/JAD-2012-120718. [DOI] [PubMed] [Google Scholar]
- Elston GN, Fujita I. Pyramidal cell development: postnatal spinogenesis, dendritic growth, axon growth, and electrophysiology. Front Neuroanat. 2014;8:78. doi: 10.3389/fnana.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke E, Nissanov J, Mitchell TW, Bennett DA, Trojanowski JQ, Arnold SE. Subicular dendritic arborization in Alzheimer's disease correlates with neurofibrillary tangle density. Am J Pathol. 2003;163:1615–1621. doi: 10.1016/S0002-9440(10)63518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JX. Acupuncture principle of tonifying qi and regulating blood, supporting the root and fostering the source on aging and senile diseases. Chin J Integr Med. 2007;13:166–167. doi: 10.1007/s11655-007-0166-x. [DOI] [PubMed] [Google Scholar]
- Jin G, Bai D, Yin S, Yang Z, Zou D, Zhang Z, Li X, Sun Y, Zhu Q. Silibinin rescues learning and memory deficits by attenuating microglia activation and preventing neuroinflammatory reactions in SAMP8 mice. Neurosci Lett. 2016;629:256–261. doi: 10.1016/j.neulet.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee H, Chae Y, Park HJ, Lee H. A systematic review of cost-effectiveness analyses alongside randomised controlled trials of acupuncture. Acupunct Med. 2012;30:273–285. doi: 10.1136/acupmed-2012-010178. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50:10–20. doi: 10.1016/j.mcn.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Lai X, Ren J, Lu Y, Cui S, Chen J, Huang Y, Tang C, Shan B, Nie B. Effects of acupuncture at HT7 on glucose metabolism in a rat model of Alzheimer's disease: an 18F-FDG-PET study. Acupunct Med. 2016;34:215–222. doi: 10.1136/acupmed-2015-010865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MC, Yip KK, Ho YS, Siu FK, Li WC, Garner B. Mechanisms underlying the effect of acupuncture on cognitive improvement: a systematic review of animal studies. J Neuroimmune Pharmacol. 2014;9:492–507. doi: 10.1007/s11481-014-9550-4. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang X, Cheng H, Shang X, Xie H, Zhang X, Yu J, Han J. Acupuncture improves cognitive deficits and increases neuron density of the hippocampus in middle-aged SAMP8 mice. Acupunct Med. 2012;30:339–345. doi: 10.1136/acupmed-2012-010180. [DOI] [PubMed] [Google Scholar]
- Li G, Cheng H, Zhang X, Shang X, Xie H, Zhang X, Yu J, Han J. Hippocampal neuron loss is correlated with cognitive deficits in SAMP8 mice. Neurol Sci. 2013;34:963–969. doi: 10.1007/s10072-012-1173-z. [DOI] [PubMed] [Google Scholar]
- Liu CZ, Yu JC, Zhang XZ, Fu WW, Wang T, Han JX. Acupuncture prevents cognitive deficits and oxidative stress in cerebral multi-infarction rats. Neurosci Lett. 2006;393:45–50. doi: 10.1016/j.neulet.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Luo B, Zhao L, Zhang X, Kan B, Liu Y, Jia Y, Han J, Yu J. Acupuncture upregulates G protein coupled activity in SAMP8 mice. Acupunct Med. 2017;35:289–296. doi: 10.1136/acupmed-2016-011139. [DOI] [PubMed] [Google Scholar]
- Luo QQ, Du YJ. Clinical research progress of acupuncture to treat Alzheimer's disease. Liaoning Zhongyi Zazhi. 2014;41:594–597. [Google Scholar]
- Marcello E, Epis R, Saraceno C, Di Luca M. Synaptic dysfunction in Alzheimer's disease. Adv Exp Med Biol. 2012;970:573–601. doi: 10.1007/978-3-7091-0932-8_25. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- No authors listed (1998) NIH Consensus Conference. Acupuncture. JAMA. 280:1518–1524. [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Ribe EM, Troy CM, Arancio O, Shelanski M. Caspase-2 is required for dendritic spine and behavioural alterations in J20 APP transgenic mice. Nat Commun. 2013;4:1939. doi: 10.1038/ncomms2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KA, Varghese M, Sowa A, Yuk F, Brautigam H, Ehrlich ME, Dickstein DL. Altered synaptic structure in the hippocampus in a mouse model of Alzheimer's disease with soluble amyloid-beta oligomers and no plaque pathology. Mol Neurodegener. 2014;9:41. doi: 10.1186/1750-1326-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Siskova Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T, Pitsch J, Schoch S, Becker A, von der Kammer H, Remy S. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer's disease. Neuron. 2014;84:1023–1033. doi: 10.1016/j.neuron.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp Gerontol. 1997;32:105–109. doi: 10.1016/s0531-5565(96)00036-8. [DOI] [PubMed] [Google Scholar]
- Wang S, Yu L, Yang H, Li C, Hui Z, Xu Y, Zhu X. Oridonin attenuates synaptic loss and cognitive deficits in an abeta1-42-induced mouse model of Alzheimer's disease. PLoS One. 2016;11:e0151397. doi: 10.1371/journal.pone.0151397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai S, Endo S. Early onset of behavioral alterations in senescence-accelerated mouse prone 8 (SAMP8) Behav Brain Res. 2016;308:187–195. doi: 10.1016/j.bbr.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang X, Liu C, Meng Y, Han J. Effect of acupuncture treatment on vascular dementia. Neurol Res. 2006;28:97–103. doi: 10.1179/016164106X91951. [DOI] [PubMed] [Google Scholar]
- Zhang S, Huang XY, Liu S, Li YJ, Zhao JC. Effects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons Effects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:224–229. [Google Scholar]
- Zhou J, Peng W, Xu M, Li W, Liu Z. The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e933. doi: 10.1097/MD.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


