Abstract
Background: Numerous studies have been conducted to evaluate the frequency of hypovitaminosis D in patients with fibromyalgia syndrome (FMS) and its association with FMS symptoms. This study aimed at assessing the effect of hypovitaminosis D on the symptoms and quality of life of patients with fibromyalgia.
Methods: A total of 74 FMS patients with hypovitaminosis D were randomly assigned into group A (Trazodone 25 mg at bedtime + vitamin D 50 000 IU weekly) and group B (Trazodone 25 mg at bedtime + placebo). Serum vitamin D level, Widespread Pain Index (WPI), Fibromyalgia Impact Questionnaire (FIQ), Pittsburgh Sleep Quality Index (PSQI), and Short Form Health Survey (SF-36) were used at the beginning of the treatment and 4 and 8 weeks post treatment.
Results: Significant improvements were observed in WPI, FIQ, and PSQI scores in both groups. Moreover, combination of vitamin D and Trazodone resulted in significant improvement of SF-36 scores compared to Trazodone therapy. Improvement in pain-related indices including the WPI and the physical component score (PCS) fraction of SF-36 was more noticeable in vitamin D/Trazodone combination therapy.
Conclusion: This study suggests that vitamin D supplementation has significant therapeutic benefits in the management of FMS, especially in pain reduction of patients with fibromyalgia. According to our results, a combination of vitamin D supplements and a conventional antidepressant, when given to vitamin D-deficient fibromyalgia patients, could significantly improve both physical and psychological symptoms
Keywords: Fibromyalgia syndrome, Vitamin D, Pain, Quality of life
↑ What is “already known” in this topic:
Considering that fibromyalgia is defined as a syndrome with both physical and mental aspects and bearing in mind the results of previous reports showing the positive effects of vitamin D on the physical symptoms of FMS, we hypothesized that adding vitamin D to trazodone could improve both physical and psychological symptoms of the patients.
→ What this article adds:
According to our results, in vitamin D-deficient fibromyalgia patients, a combination of vitamin D supplements and a conventional antidepressant, such as trazodone, could significantly improve both physical and psychological symptoms of the affected patients.
Introduction
Fibromyalgia syndrome (FMS) is a complex disorder with unknown etiology, characterized by a combination of physical and psychological phenotypes (1-3).
Several studies have reported a significant correlation between FMS and vitamin D deficiency (4-8). To the best of our knowledge, only one published randomized controlled trial (RCT) is available on the role of vitamin D in the pathogenesis of fibromyalgia, which indicates the efficacy of 25-hydroxy vitamin D optimization in reduction of pain and morning fatigue symptoms of the affected patients (5). Several other studies have also reported the role of vitamin D deficiency in musculoskeletal pain resulting from various diseases including fibromyalgia (9-13).
Low-dose trazodone, a traditional antidepressant, is recommended by the American Pain Society to improve sleep quality in patients with fibromyalgia (14). Its favourable price and insurance coverage in Iran makes its administration more practical than duloxetine and pregabalin, both FDA-approved medications of FMS.
During several years of practice in management of FMS, we noticed an acceptable psychological improvement following trazodone administration. However, because FMS is defined as a syndrome with both physical and mental aspects and the RCT performed by Wepner et al. confirmed the positive effect of vitamin D on the physical symptoms of FMS including pain reduction, we hypothesized that adding vitamin D to trazodone could improve both physical and psychological symptoms of FMS.
In this study, we designed an RCTto evaluate the effect of combination of vitamin D and trazodone in vitamin D-deficient fibromyalgia patients.
Methods
Study design
This was a parallel, randomized, double-blind, and placebo-controlled trial conducted at our academic medical center. Sample size planning included 37 individuals per group. According to similar studies on vitamin D treatment in fibromyalgia patients (5), standardized effect size of 0.8 was implemented. Patients were randomized in predesigned blocks. Block randomization was done by a computer-generated random number list prepared by an investigator with no clinical involvement in the study. Informed consent was obtained from all the patients; then, they were allocated into predesigned groups. Staff members, participants, and investigators were kept blind to group assignment during data collection.
Data
A total of 108 patients diagnosed with FMS were referred to a rheumatology center from October 2012 to March 2014. Diagnostic criteria adopted by the American College of Rheumatology Criteria (ACR 2010) were used to confirm the diagnosis of FMS for all patients participating in this study (15). Serum levels of 25-hydroxyvitamin D [25(OH) D] were measured with a commercial radioimmunoassay kit (immune diagnostic systems, Boldon, UK). The serum levels of 30 ng/mL were considered as normal, < 20 ng/mL as deficiency, and 20.1 to 29 ng/mL as vitamin D insufficiency. Females aged 20 to 70 years, who met the ACR criteria of FMS and had serum levels of 25 (OH) D under 30 ng/mL, were included in the study. Patients with confirmed diagnosis of the following disorders were all excluded from this study: metabolic disease; diabetes mellitus (DM); rheumatic disorders; psychological disorders, such as major depression; liver diseases; chronic kidney disease (CKD); cancer; cardiovascular diseases; malabsorption; pregnancy; and intolerance of trazodone. Patients taking steroids or vitamin D supplements and hospitalized patients were also excluded. Therefore, of the initial 108 patients, 74 with the mean age and standard deviation (± SD) of 41.6 ± 10.5 years met the inclusion criteria. Among them, 64 patients were identified with vitamin D deficiency and 10 with vitamin D insufficiency.
The process of including the patients in the study has been conclusively depicted in the flow diagram of the study (Fig. 1).
Fig. 1.
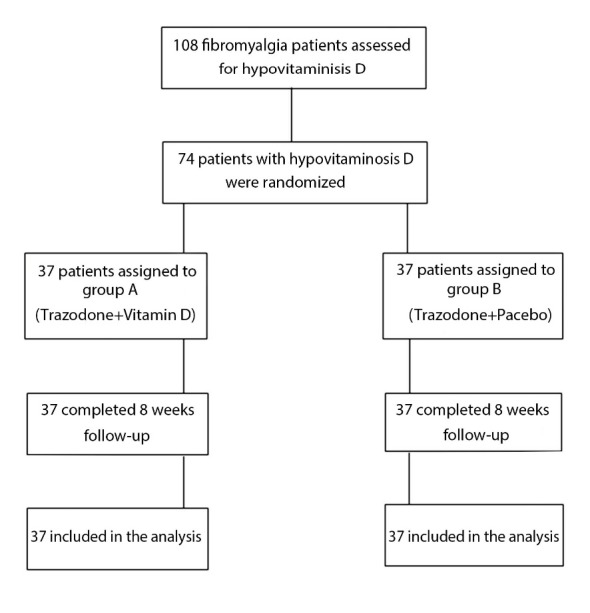
Flow diagram of patients’ selection, allocation, and follow-up
Patients who satisfied the inclusion criteria were randomly and equally assigned into 2 study groups: A and B. In group A, patients received 50 000 IU oral vitamin D (D-Vitin, Zahravi Pharmaceuticals, Iran) weekly along with 25 mg (half a tablet) dose of trazodone (Trazodone, Darupakhsh, 50 mg TAB) at bedtime daily. In group B, patients received the same dose of trazodone at bedtime along with a placebo.
Treatment started at the beginning of the study and continued in both groups till the end of the study period. All patients were individually visited at the beginning (baseline) and 4 and 8 weeks post treatment. The Short Form Health Survey (SF-36) in Persian (16) was used at baseline and after 8 weeks. Serum 25(OH) D levels, the Fibromyalgia Impact Questionnaire (FIQ) in Persian (16, 17), Pittsburgh Sleep Quality Index (PSQI) in Persian (18), and the Widespread Pain Index (WPI) (15) were used in each visit (baseline, Week 4, and Week 8). Patients were informed about the possible side effects of the medication and provided with a contact number to report any incidence of adverse events. During the study, none of the patients missed any of the scheduled follow-up visits.
This study was approved by the Ethics Committee of Iran University of Medical Sciences and was registered in the Iranian Registry of Clinical Trials (IRCT2012100610960N2). Moreover, this study was approved by the Ethics Committee of Tehran University of Medical Sciences (130/1605). Informed consent was obtained from all participants.
Statistical analysis
Statistical analysis was performed using IBM SPSS for Windows Version 21. Paired t test was used to analyse the change of scores within each group. To compare the variations of vitamin D, FIQ, PSQI, and WPI at different time points, two-way repeated measures ANOVA was used. The SF36 scores of the 2 groups was analyzed using ANCOVA test at Weeks 0 and 8. Significance level was set at 0.05.
Results
Table 1 summarizes the clinical and demographic data of the enrolled patients and the results of the baseline test scores administered during their primary evaluations in the initial visit. The effects of intervention in the 2 groups have been demonstrated in the following paragraphs.
Table 1. Baseline clinico-demographic data of the 2 groups .
| Variables |
Group A (Trazodone +Vitamin D) (n = 37) |
Group B (Trazodone + Placebo) (n = 37) |
| Age (year) | 42.1 ± 10.8 | 41 ± 10.3 |
|
25(OH)D (ng/ml) Deficiency (%) Sufficiency (%) |
11.4 ± 6.5 | 13.4 ± 7.3 |
| 34 (89.5) | 30 (83.3) | |
| 4 (10.5) | 6 (16.7) | |
| SF-36 PCS | 50.3 ± 9.6 | 49.6± 9.3 |
| SF-36 MCS | 49.8 ±11.3 | 50.2 ± 9.1 |
| WPI score | 12.2 ± 2.4 | 13.5 ± 3.4 |
| FIQ total score | 53.4 ± 16.6 | 50.7 ± 16 |
| PSQI total score | 10 ± 3.4 | 10.75± 4.42 |
Data are presented as mean ± SD.
Vitamin D levels
Among 74 patients, 10 (13.5%) were identified with vitamin D insufficiency and 64 (86.5%) with vitamin D deficiency. All patients in group A showed serum vitamin D levels ≥ 30 ng/mL at Week 8, indicating that the form of vitamin D included in the combination therapy effectively normalized the serum levels of 25(OH) D.
The results of two-way ANOVA with repeated measures revealed a significant difference at the mean vitamin D level between the 2 groups (p< 0.001). Moreover, a considerably higher mean vitamin D levels was observed in group A at Weeks 4 and 8 compared to Group B. Because vitamin D was administered to group A as a part of the combination therapy, such improvement in serum vitamin D levels was expected (Table 2).
Table 2. Comparison of the mean vitamin D, WPI, FIQ, and PSQI score of Weeks 0, 4, and 8 .
| Variable | Trazodone+Vitamin D | Trazodone+ Placebo | F (P) * |
| Vit D level (Week 0) | 11.4 (6.7) | 13.4 (7.3) |
104.97 (<0.001) |
| Vit D level (Week 4) | 23.9 (9.1) | 13.5 (7.3) | |
| Vit D level (Week 8) | 33.5 (12.2) | 13.3 (7.2) | |
| WPI (Week 0) | 12.2 (2.3) | 13.5 (3.4) |
7.77 (0.007) |
| WPI (Week 4) | 6.13 (3.2) | 8.86 (4.9) | |
| WPI (Week 8) | 4.47 (2.5) | 8 (4.8) | |
| FIQ (Week 0) | 53.4 (16.6) | 50.7 (16) |
3.54 (0.064) |
| FIQ (Week 4) | 37.7 (13.1) | 42.8 (16.7) | |
| FIQ(Week 8) | 29.7 (14) | 40.4 (15.3) | |
| PSQI (Week 0) | 10 (3.3) | 10.75 (4.4) |
10.35 (0.002) |
| PSQI (Week 4) | 7.5 (2.9) | 8.6 (3.8) | |
| PSQI (Week 8) | 6.2 (2.2) | 8.2 (3.7) |
Data are shown as mean (SD).
*Between groups’ comparison using two-way repeated measures ANOVA
Psychometric assessments
Comparison of the various components of the SF-36 test at the baseline versus Week 8 showed that group A had more significant improvements in all SF- 36 subscales, while group B only showed a significant improvement in 4 SF-36 subscales, namely, physical functioning, role limitation due to emotional problems, social functioning, and pain (Table 3).
Table 3. Change of SF-36 subscores from baseline to Week 8 .
| SF-36 Scores |
Week 8 minus week 0 Group A (Trazodone + Vit D) (n=37 |
p* |
Week 8 minus week 0 Group B (Trazodone + Placebo) (n=37) |
p* |
| Mental component score | 2.4 (10.8) | 0.11 | -2.5(9.2) | 0.14 |
| Physical component score | 3 (7.8) | 0.02 | -3.1(7.4) | 0.01 |
| Physical functioning | 17.63 (15.34) | 0.0001 | 8.74 (14.64) | 0.001 |
| Role limitation due to physical health | 36.18 (43.76) | 0.0001 | 8.33 (35.85) | 0.08 |
| Role limitation due to emotional problems | 39.38 (37.94) | 0.0001 | 13.88 (34.15) | 0.001 |
| Fatigue | 17.23 (21.76) | 0.0001 | 3.12 (20.38) | 0.21 |
| Emotional wellbeing | 16.42 (19.98) | 0.0001 | 5.05 (17.07) | 0.09 |
| Social functioning | 11.74 (20.68) | 0.001 | 8.29 (24.27) | 0.048 |
| Pain | 18.35 ( 21.35) | 0.0001 | 9.94 (21.98) | 0.01 |
| General health | 13.94 (21.31) | 0.0001 | 3 (19.13) | 0.353 |
Results are presented as mean (SD).
* Within group’s comparison by Paired T-test.
The observed change was more evident in the physical component score (PCS) of SF-36. In this regard, baseline PCS of group A revealed a considerable improvement at Week 8, while baseline PCS of group B considerably decreased at Week 8 (p= 0.001). Such pattern of change was also observed in mental component score (MCS) of group A compared to group B (p= 0.04) (Table 4).
Table 4. The SF-36 scores of the 2 study groups at baseline and Week 8 .
| Variable |
Trazodone +Vitamin D (Group A) |
Trazodone + Placebo (Group B) |
p * |
| SF36 PCS (Week 0) | 50.3 ± 11.24 | 49.6± 9.3 | 0.001 |
| SF36 PCS (Week 8) | 53.3 ± 7.03 | 46.5 ± 11.24 | |
| SF36 MCS (Week 0) | 49.8±11.3 | 50.19±9.1 | 0.04 |
| SF36 MCS (Week 8) | 52.3±9.2 | 47.69±10.8 |
Results are presented as Mean ± SD.
* Between groups’ comparison by ANCOVA test
PCS = Physical component score; MCS= Mental component score
The mean±SD baseline WPI decreased from 12.2±2.3 to 4.47+2.5 at Week 8 in group A and from 13.5±3.4 to 8±4.8 in group B. Despite a considerable decrease in the WPI of the both study groups, this decrease was more significant in group A (p= 0.007) (Table 2).
With respect to FIQ scores, patients treated with vitamin D revealed improvements in all subscores of FIQ compared to the patients of group B. A more considerable improvement was observed in the subscores of morning tiredness, stiffness, anxiety, and depression in patients in group A compared to those in group B (Table 5).
Table 5. The observed improvement in total and subscales of the FIQ score from baseline to Week 8 in each group .
|
FIQ Score |
Week 8 minus week 0 Group A (Trazodone + Vit D) (n = 37) |
p* |
Week 8 minus week 0 Group B (Trazodone + Placebo) (n = 37) |
p* |
| Total score | -23.68 ± 18.58 | 0.002 | -10.27 ± 17.13 | 0.002 |
| Work | -2.34 ± 4.24 | 0.004 | -1.61 ± 2.84 | 0.072 |
| Pain | -1.65 ± 3.37 | 0.0001 | -0.91 ± 0.96 | 0.023 |
| Fatigue | -2.81 ± 3.27 | 0.0001 | -1.36 ± 3.42 | 0.053 |
| Morning tiredness | -3.65 ± 3.40 | 0.0001 | -1.27 ± 3.91 | 0.026 |
| Stiffness | -3.18 ± 3.36 | 0.0001 | -1.22 ± 3.15 | 0.01 |
| Anxiety | -3.55 ± 3.10 | 0.0001 | -1.50 ± 3.29 | 0.238 |
| Depression | -3.21 ± 4.16 | 0.0001 | -0.75 ± 3.74 | 0.001 |
Results are presented as mean ± SD
*Within group’s comparison by paired t test
Moreover, a considerable improvement was observed in total FIQ score of group A compared to group B. The mean±SD total baseline FIQ score decreased from 53.4±16.6 to 29.7±14 at Week 8 in group A and from 50.7±16 to 40.4±15.3 in group B. However, this difference was not statistically significant (p = 0.064) (Table 2).
Considerable improvements were also observed in the PSQI score of the both study groups. The mean PSQI score reduced from 10 at baseline to 6.1 at Week 8 in group A and from 10.4 to 8.1 in group B (Fig. 2). This difference was statistically significant (p= 0.002) (Table 2).
Fig. 2.
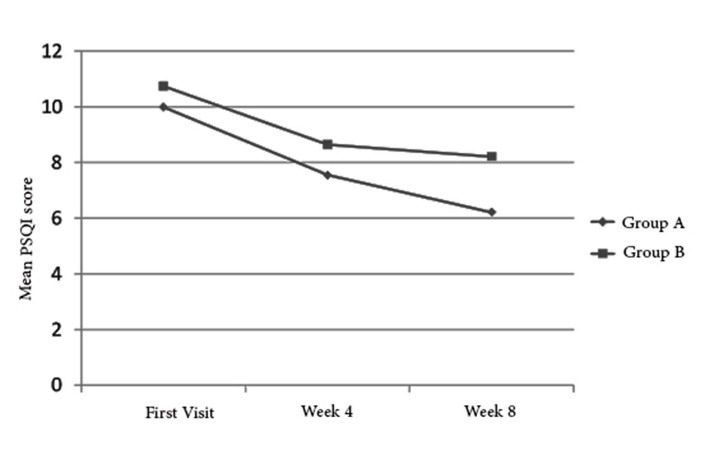
Mean PSQI score in the 2 study groups before and after intervention
Side effects
We have previously evaluated the safety and effectiveness of high doses of orally administered vitamin D for hypovitaminosis D patients; no adverse reaction was reported when vitamin D was administered as a single therapy (19). As a result, the observed side effects could be attributed to either trazodone or the combination of trazodone and vitamin D. The most frequently observed side effects included dry mouth (n= 6, 8.1%; 50% observed in group A and 50% in group B) and dizziness (n= 6, 8.1%; 83% observed in group A and 16% in group B). Less frequent side effects, all observed in group B, included tachycardia (n= 3, 4.1%). None of these side effects were severe enough to cause discontinuation of the medications.
Discussion
In this study, we aimed at assessing the effect of the treatment of hypovitaminosis D on the symptoms and quality of life of fibromyalgia patients. Our findings indicated that Vitamin D supplementation is a safe and economical treatment and can significantly improve the quality of life of FMS patients.
Earlier studies have reported a correlation between vitamin D deficiency and the severity of symptoms in patients suffering from fibromyalgia. Such correlations between vitamin D deficiency and chronic pain have been reported in other diseases as well (13, 20, 21). In addition, vitamin D has an important role in bone and muscle strength, central nervous system, and autoimmune and cardiovascular health (22, 23). Vitamin D deficiency is prevalent around the world. In particular, it has a high incidence in the Iranian population. In one cross-sectional study on 100 Iranian medical students, the authors found that 99% of the participants had 25(OH) D levels below 30 ng/mL (24). According to our results, hypovitaminosis D was seen in 68% of fibromyalgia patients, 64% of whom were considered to be vitamin D deficient. Although some studies have reported a higher rate of hypovitaminosis D among patients with fibromyalgia (21, 25), other studies found no differences in vitamin D levels between these patients and healthy individuals (7, 26). Nevertheless, based on the well-known role of vitamin D in both the central nervous system and muscles, hypovitaminosis D could be a risk factor for fibromyalgia (27). Therefore, the evaluation of the effects of vitamin D supplement in vitamin D-deficient fibromyalgia patients is the core purpose of some recent investigations. Matthana has studied serum 25 (OH) D levels in 100 women suffering from FMS. Patients with vitamin D deficiency were treated weekly with 50 000 IU of Ergocalciferol until their serum 25(OH) D levels exceeded 50 ng/mL. FIQ scores were investigated to evaluate the symptoms of fibromyalgia before and after vitamin D repletion. The results of this study demonstrate that the condition of 42 of the 61 vitamin D-deficient fibromyalgia patients significantly improved when their serum of 25(OH) D levels reached or exceeded 30 ng/mL. This improvement became more significant when their serum of 25(OH) D level exceeded 50 ng/mL (6). Abokrysha also found that treatment with high-dose vitamin D resulted in clinical improvement in the condition of fibromyalgia patients (28). To the best of our knowledge, Wepner et al. published the first and only RCT to evaluate the effect of vitamin D on 30 fibromyalgia patients randomly divided into treatment and control groups. Their goal was to increase the serum levels of 25(OH) D in the treatment group by oral supplementation of cholecalciferol and compare the effect of such intervention between the 2 study groups. Their analysis indicated that the optimization of 25 (OH)D levels in FMS had a positive effect on the perception of pain (5).
We randomized vitamin D-deficient fibromyalgia patients in 2 groups (trazodone/placebo and trazodone/vitamin D therapy) to evaluate the effect of vitamin D supplementation.
Trazodone, as an antidepressant agent, has been administered to manage fibromyalgia (14, 29). However, it is not an FDA-approved drug for fibromyalgia. One might ask why trazodone should be administered instead of FDA-approved drugs like pregabalin, duloxetine, and milnacipran. In response, it should be pointed out that while the cost of trazodone therapy is covered by Iranian insurance companies, they do not cover the cost of pregabalin and duloxetine, and thus they are not affordable for patients. In addition, milnacipran is not available in the Iranian drug market. Moreover, considerable adverse effects of pregabalin and duloxetine impose the need for new alternative medications (30, 31).
As a result, trazodone is mainly considered as the antidepressant drug of choice in Iran. The effects of trazodone on sleep quality, but not on pain reduction, have been shown in some earlier studies (32, 33). Morillas-Arques et al. used a flexible dose of trazodone and found significant improvements in different sleep parameters, particularly in the quality, duration, and efficiency of sleep in FM patients. However, they found no reduction in pain intensity at the conclusion of their trial (33).
Based on our results, the WPI score showed a significant improvement in patients receiving vitamin D, confirming the results of the previous studies including those of Wapner et al. (5, 34). Although Wapner et al. used the visual analogue scale scores (VAS score) to determine the severity of pain, our results, which applied the WPI score, are in agreement with Wapner’s results. Interestingly, in our study, the trazodone/vitamin D-treated group showed a significant improvement in both PCS and MCS of SF-36 compared to the control group receiving trazodone/placebo. However, Wapner’s study found no significant improvement in PCS or MCS. In addition, while a slight improvement in FIQ score was seen in the vitamin D-treated group in Wapner’s study, our study showed a considerable improvement in all subscales of FIQ in the group treated with trazodone/vitamin D. Significant reduction in the PSQI scores of both groups was observed in our study as well, which could be correlated to trazodone’s hypnotic effect.
The short-term follow-up of the enrolled patients could be considered as the limitation of our study, and no loss of follow-up could be regarded as the strength of our study, which could be attributed to the revisit reminder calls and the short follow-up period of our study. Further similar studies with longer follow-up periods in future investigations will further explore the impact of vitamin D optimization in the quality of life of fibromyalgia patients.
Conclusion
Our study provided preliminary evidence that Vitamin D supplementation up to 30 ng/mL is a safe and economical treatment that could lead to a significant improvement in the quality of life of FMS patients. Vitamin D supplementation adjunct to psychotropic drugs such as trazodone could be suggested for the improvement of both physical and psychological symptoms of FMS.
Acknowledgments
We gratefully acknowledge Dr. Effat Emamian for critically reviewing the manuscript.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Mirzaei A, Zabihiyeganeh M, Jahed SA, Khiabani E, Nojomi M, Ghaffari S. Effects of vitamin D optimization on quality of life of patients with fibromyalgia: A randomized controlled trial. Med J Islam Repub Iran. 2018(5 Apr);32:29. https://doi.org/10.14196/mjiri.32.29
References
- 1. Wolfe F. Fibromyalgia. Prognosis in the Rheumatic Diseases: Springer; 1991. p. 321-32.
- 2.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 3.Burckhardt CS, Clark S, Bennett RR. Fibromyalgia and quality of life. J Rheumtol. 1993;20(3):475–9. [PubMed] [Google Scholar]
- 4.Armstrong D, Meenagh G, Bickle I, Lee A, Curran E-S, Finch M. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26(4):551–4. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 5.Wepner F, Scheuer R, Schuetz-Wieser B, Machacek P, Pieler-Bruha E, Cross HS. et al. Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo-controlled trial. PAIN. 2014;155(2):261–8. doi: 10.1016/j.pain.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Matthana MH. The relation between vitamin D deficiency and fibromyalgia syndrome in women. Saudi Med J. 2011;32(9):925–9. [PubMed] [Google Scholar]
- 7.Tandeter H, Grynbaum M, Zuili I, Shany S, Shvartzman P. Serum 25-OH vitamin D levels in patients with fibromyalgia. Isr Med Assoc J. 2009;11(6):339–42. [PubMed] [Google Scholar]
- 8.Block SR editor. Vitamin D deficiency is not associated with nonspecific musculoskeletal pain syndromes including fibromyalgia. Mayo Clin Proc. 2004;79(12):1585–6. doi: 10.4065/79.12.1585. [DOI] [PubMed] [Google Scholar]
- 9.Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine. 2003;28(2):177–9. doi: 10.1097/00007632-200301150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Atherton K, Berry DJ, Parsons T, Macfarlane GJ, Power C, Hyppönen E. Vitamin D and chronic widespread pain in a white middle-aged British population: evidence from a cross-sectional population survey. Ann Rheum Dis. 2009;68(6):817–22. doi: 10.1136/ard.2008.090456. [DOI] [PubMed] [Google Scholar]
- 11.Erkal M, Wilde J, Bilgin Y, Akinci A, Demir E, Bödeker R. et al. High prevalence of vitamin D deficiency, secondary hyperparathyroidism and generalized bone pain in Turkish immigrants in Germany: identification of risk factors. Osteoporos Int. 2006;17(8):1133–40. doi: 10.1007/s00198-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 12.Straube S, Moore RA, Derry S, McQuay HJ. Vitamin D and chronic pain. PAIN. 2009;141(1):10–3. doi: 10.1016/j.pain.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Badsha H, Daher M, Kong KO. Myalgias or non-specific muscle pain in Arab or Indo-Pakistani patients may indicate vitamin D deficiency. Clin Rheumatol. 2009;28(8):971–3. doi: 10.1007/s10067-009-1146-7. [DOI] [PubMed] [Google Scholar]
- 14. Burckhardt CS, Goldenberg DL. Guideline for the management of fibromyalgia syndrome pain in adults and children: American Pain Society; 2005.
- 15.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P. et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 16.Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res. 2005;14(3):875–82. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 17.Bidari A, Hassanzadeh M, Mohabat M-F, Talachian E, Khoei EM. Validation of a Persian version of the Fibromyalgia Impact Questionnaire (FIQ-P) Rheumatol Int. 2014;34(2):181–9. doi: 10.1007/s00296-013-2883-0. [DOI] [PubMed] [Google Scholar]
- 18.Moghaddam JF, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P) Sleep Breath. 2012;16(1):79–82. doi: 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 19.Zabihiyeganeh M, Jahed A, Nojomi M. Treatment of hypovitaminosis D with pharmacologic doses of cholecalciferol, oral vs intramuscular; an open labeled RCT. Clin Endocrinol. 2013;78(2):210–6. doi: 10.1111/j.1365-2265.2012.04518.x. [DOI] [PubMed] [Google Scholar]
- 20.Turner MK, Hooten WM, Schmidt JE, Kerkvliet JL, Townsend CO, Bruce BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. 2008;9(8):979–84. doi: 10.1111/j.1526-4637.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 21.Plotnikoff GA, Quigley JM, editors editors. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 22.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Experimen Biol and Med. 2004;229(11):1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 23.Souberbielle J-C, Body J-J, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ. et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9(11):709–15. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Zabihiyeganeh M, Jahed SA, Sarami S, Nojomi M. Hypovitaminosis D: Are medical students at risk? Int J Prev Med. 2014;5(9):1161. [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatty SA, Shaikh NA, Irfan M, Kashif SM, Vaswani AS, Sumbhai A. et al. Vitamin D deficiency in fibromyalgia. J Pak Med Assoc. 2010;60(11):949. [PubMed] [Google Scholar]
- 26.Warner AE, Arnspiger SA. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. JCR: J Clin Rheumatol. 2008;14(1):12–6. doi: 10.1097/RHU.0b013e31816356a9. [DOI] [PubMed] [Google Scholar]
- 27.Jesus CA, Feder D, Peres MF. The role of vitamin D in pathophysiology and treatment of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):1–7. doi: 10.1007/s11916-013-0355-6. [DOI] [PubMed] [Google Scholar]
- 28.Abokrysha NT. Vitamin D deficiency in women with fibromyalgia in Saudi Arabia. Pain Med. 2012;13(3):452–8. doi: 10.1111/j.1526-4637.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 29.Morillas-Arques P, Rodriguez-Lopez CM, Molina-Barea R, Rico-Villademoros F, Calandre EP. Trazodone for the treatment of fibromyalgia: an open-label, 12-week study. BMC Musculoskelet Disord. 2010;11(1):1. doi: 10.1186/1471-2474-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: A systematic review and meta‐analysis of randomized controlled trials. Epilepsia. 2011;52(4):826–36. doi: 10.1111/j.1528-1167.2010.02966.x. [DOI] [PubMed] [Google Scholar]
- 31.Wernicke JF, Gahimer J, Yalcin I, Wulster-Radcliffe M, Viktrup L. Safety and adverse event profile of duloxetine. Expert Opin Drug Saf. 2005;4(6):987–93. doi: 10.1517/14740338.4.6.987. [DOI] [PubMed] [Google Scholar]
- 32.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994;21(6):1113–7. [PubMed] [Google Scholar]
- 33.Morillas-Arques P, Rodriguez-Lopez CM, Molina-Barea R, Rico-Villademoros F, Calandre EP. Trazodone for the treatment of fibromyalgia: an open-label, 12-week study. BMC Musculoskelet Disord. 2010;11(1):204. doi: 10.1186/1471-2474-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solmaz D, Avci O, Yalcin B, Kara S, Oran M. AB0944 Vitamin D Deficiency Might Contribute Fatigue and Disease Activity in Patients with Fibromyalgia. Ann Rheum Dis. 2015;74(Suppl 2):1215. [Google Scholar]


