Abstract
Introduction:
Neutrophil CD64 (nCD64) has been found to identify sepsis from nonseptic patients. It is also reported to be a predictor of survival and severity of sepsis. The goal of this study was to correlate serial nCD64 with Intensive Care Unit (ICU) outcome and severity of sepsis.
Materials and Methods:
A prospective observational study was conducted in 12-bedded critical care unit of a tertiary care center. Adult patients with sepsis were included in this study. Demographics, illness severity scores, clinical parameters, laboratory data, and 28-day outcome were recorded. Serial nCD64 analysis was done (on days 0, 4, and 8) in consecutive patients.
Results:
Fifty-one consecutive patients were included in the study. Median Acute Physiology and Chronic Health Evaluation II was 16 (12–20) and mean Sequential Organ Failure Assessment was 9 (8–10). Compared to survivors, nonsurvivors had higher nCD64 on day 8 (P = 0.001). nCD64 was higher in the septic shock group compared to sepsis group on days 0 and 8 (P < 0.05). Survivors showed improving trend of nCD64 over time while nonsurvivors did not. This trend was similar in the presence or absence of septic shock. nCD64 count was a good predictor of the septic shock on day 0 (area under the curve [AUC] = 0.747, P = 0.010) and moderate predictor at day 8 (AUC = 0.679, P = 0.028).
Conclusion:
Monitoring serial nCD64 during ICU stay may be helpful in determining the clinical course of septic patients.
Keywords: Antibiotics, biomarkers, Intensive Care Unit, neutrophil CD64, prognosis, sepsis
INTRODUCTION
Sepsis is a major cause of mortality in Intensive Care Unit (ICU).[1] Early identification and timely interventions hold a key role to improve morbidity and mortality in sepsis.[2] Despite improvements in better understanding of pathophysiology and research over the years, early diagnosis of sepsis still remains a challenge for clinicians. Conventional investigations such as total leukocyte count (TLC)[3] and C-reactive protein (CRP) have produced variable results. Microbiological culture is still the gold standard for identifying sepsis,[4] but it takes time with chances of false negativity. Many factors, including the use of empiric antibiotic therapy started before taking the samples, may influence the results.[5] These issues encouraged research on biomarkers for aggressive identification of sepsis. Recently, the new generation and improved diagnostics for infection workup have been directed toward soluble biomarkers in the serum or plasma. Tumor necrosis factor, interleukins, procalcitonin (PCT),[6] and neutrophil CD64 (nCD64) expressions are being evaluated extensively. nCD64 seems to be a clinically useful diagnostic cell-based parameter of a systemic acute inflammatory response[7,8,9,10,11,12,13,14] or sepsis.
nCD64 is a high-affinity receptor for Fcy part of the IgG heavy chain.[15] Phagocytosis of bacteria and other microorganisms is mediated by this FcyR1 receptor. It is constitutively expressed on macrophages, monocytes, and eosinophils and to a very low extent on resting neutrophils (approximately 1000 molecules per cell). However, CD64 expression on neutrophils increases once they become activated by pro-inflammatory cytokines produced in response to infections. Their level starts to increase within 4–6 h to up to more than 10 times than the resting levels, allowing discrimination between resting and activated neutrophils.[16,17] When these stimulation factors subside, CD64 expression substantially decreases within 48 h and restores to baseline values after 7 days.
Some recent studies suggested that nCD64 had better diagnostic ability in differentiating sepsis from nonsepsis, compared with PCT.[10,13] It was also found to be more appropriate than CRP in guiding de-escalation of antibiotics.[18] Some investigators described its role as a highly sensitive and specific marker for sepsis or bacterial infection in adults, neonates, and children.[7,8,9,10,11,12,13,14,19,20,21] nCD64 has been utilized to predict outcome and severity in critically ill patients.[9] High nCD64 was found to be associated with poor prognosis.[9,13,17] However, till now, very few studies have tried to highlight its role in defining the clinical course in ICU.
In the present study, we have assessed serial nCD64 in patients with sepsis and septic shock over the first 8 days of ICU stay and evaluated its correlation with the patient's characteristics and outcome.
MATERIALS AND METHODS
This was a prospective observational study conducted in a tertiary care 12-bedded medical-surgical ICU over a period of 12 months from February 2016 to January 2017. The study was approved by institutes' ethical committee. We included adult patients (age >18 years) who presented with features of sepsis (sepsis was defined by the survival sepsis guideline 2012[2]). Informed consent was taken from the patients or next to kin.
Data collection
The baseline clinical and laboratory parameters were noted on admission to ICU. The data included age, sex, comorbidities, source and type of admission, and possible causes for sepsis. Admission Sequential Organ Failure Assessment (SOFA) score and Acute Physiology and Chronic Health Evaluation II (APACHE II) were also noted. Other laboratory data were collected which included complete blood count and lactate for the study purpose.
Blood cultures along with culture from possible source of infection (as decided by the treating clinicians) were also sent for all patients at admission. The samples were collected and processed according to standard microbiological procedures in our institute.
Neutrophil CD64 measurement
For nCD64 measurement, 14 ml of venous blood sample was collected in ethylenediaminetetraacetic acid (EDTA) vial for each patient and sent to laboratory within 15 min. nCD64was measured by flow cytometry (FCM) (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA, Food and Drug Administration approved) in the department of immunology in our institute. The polymorphonuclear cells were separated from other blood cells by their characteristic side scatter profile. The neutrophils were then gated according to CD64 activity. The results were expressed as percentage (%) of neutrophil expressing CD64 positivity. It is a kit-based assay, and at a time, 200 samples can be processed.
Serial measurements of nCD64 were done on days 0, 4, and 8 of ICU stay (day 0 being the day of admission).
The patients were followed up till death or discharge up to the 28th day. nCD64 values did not guide patient management at any point of time.
We further observed mortality and severity of sepsis (the presence or absence of septic shock) and observed their correlation with nCD64.
Sample size estimation
Assuming ratio of survivor and nonsurvivor patients in ICU as 2:1, calculated sample size came out to be 38 (survivor = 25 and nonsurvivor = 13) at minimum 80% power of the study and 0.05 significance level. The sample size was calculated to detect a mean difference in CD64 count (mean ± standard deviation) between survivor and nonsurvivor (40 ± 26 vs. 65.5 ± 24.5) on day 8 (input was taken from a pilot study, conducted on 10 patients in ICU). Finally, in this study, the number of survivor and nonsurvivor patients was 34 and 17, respectively. This achieved 99.7% power of the study in the observed mean difference in CD64 count on day 8. The sample size was calculated using Power Analysis and Sample Size version 8 (PASS-8, NCSS, Utah, USA) was used to calculate sample size.
Statistical analysis
Variables were reported as median and interquartile range (IQR). Normality of continuous variables was tested using Kolmogorov–Smirnov test. For normally distributed data, parametric test was used, while for nonnormal data, nonparametric methods were used. We compared the variables between the two groups using the independent t-test or Mann-Whitney U-test, while for three or more groups, Kruskal–Wallis H-test was used. Friedman test was used to evaluate the changes in nCD64 count over the time intervals. Multiple comparisons were followed when P value was found significant. Spearman's rank correlation coefficient was used to test the correlation between CD64 and other variables. SPSS version 23 (IBM, Chicago, USA) was used for statistical analysis. P < 0.05 was considered to be statistically significant.
RESULTS
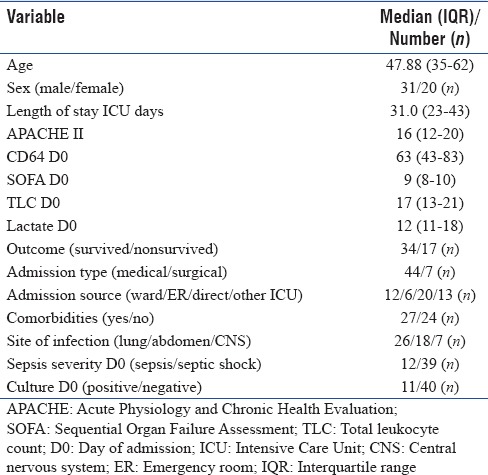
We enrolled 65 patients in the study out of a total 125 admission during the study period. Data were analyzed for the patients who survived for at least 8 days in our ICU and who could be followed up to day 28. Fifty-one patients were included in this study as per the inclusion criteria with measurement of a total of 153 samples over the study period. Table 1 shows demographic and clinical characteristics of our patients. Median (IQR) age of the patients was 47.88 (35–62) years with range of 18–83 years. Median (IQR) length of ICU stay was 31.0 (23–43) days with range of 8–90 days. Median (IQR) APACHE II score was 16 (12–20) while SOFA was 9 (8–10) [Table 1]. Most common source of sepsis was lung (26/51, 50%). Medical reasons were the primary cause for their sepsis. Almost 50% of patients had one or more comorbidities at admission. The initial cultures were positive in 11 patients. Thirty-nine patients survived at day 28. At admission, median (IQR) of nCD64 value was 63 (43–83) [Table 1].
Table 1.
Demographic and clinical characteristics of the study patients at admission
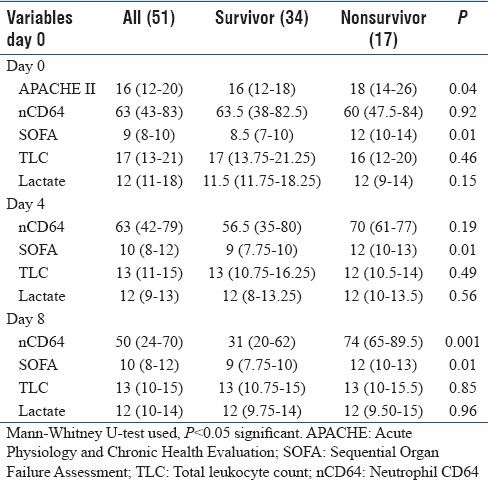
In Table 2, median values of the nCD64, APACHE II, SOFA, TLC, and lactate were compared between survival and nonsurvival patients at days 0, 4, and 8, respectively. On days 0 and 4, SOFA was statistically significant between the groups; however, nCD64 did not show any difference. On day 8, both SOFA and nCD64 were significantly higher in the nonsurvivor group (P < 0.05).
Table 2.
Neutrophil CD64 and correlation with outcome
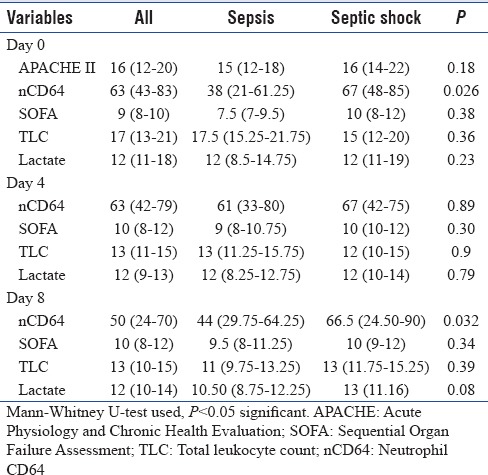
Neutrophil CD64 kinetics based on severity of sepsis
In Table 3, median score of the nCD64, APACHE II, SOFA, and TLC was compared between sepsis and septic shock patients at days 0, 4, and 8, respectively. nCD64 was significantly higher in the septic shock group as compared to sepsis patients on days 0 and 8. SOFA, APACHE II, TLC, and lactate were comparable between the groups.
Table 3.
Neutrophil CD64 and correlation with severity of sepsis
In Table 4 and Figure 1, the changes in nCD64 over the time intervals for survivor and nonsurvivor patients were represented. The results showed that there was a significant decrease in nCD64 counts in survivor patients over the time (P < 0.001). Multiple comparison revealed that nCD64 count on day 8 was significantly different with days 0 and 4 (P < 0.05) while day 4 value was not significantly different from day 0 (P > 0.05). In nonsurvivor patients, there was nonsignificant change over the time intervals (P > 0.05). The decrease in nCD64 among the survivors was significant in sepsis as well as septic shock patients [Figure 2]. Among the septic shock patients, nCD64 showed an increasing trend in the nonsurvivors (P < 0.05).
Table 4.
Change in neutrophil CD64 value over the time intervals between survivor and nonsurvivor patients
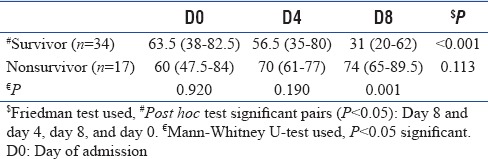
Figure 1.
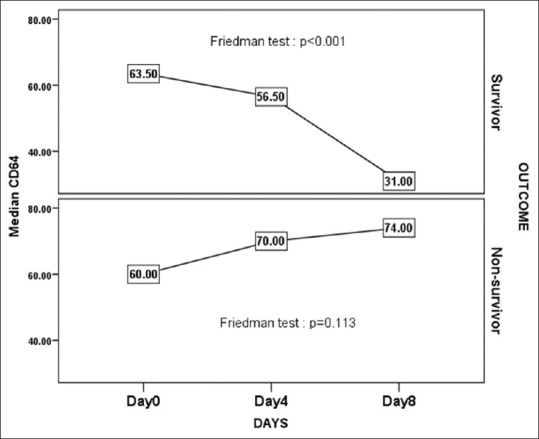
Change in neutrophil CD64 value over the time intervals between survivor and nonsurvivor patients
Figure 2.
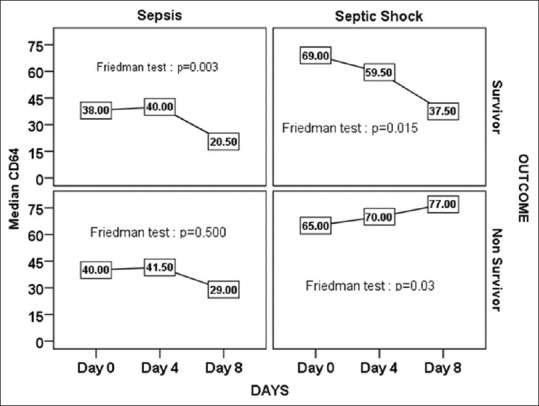
Change in neutrophil CD64 value over the time intervals between survivor and nonsurvivor in both the sepsis and septic shock patients
nCD64 count was compared between survivor and nonsurvivor patients [Table 4] at days 0, 4, and 8. Mann–Whitney U-test indicated that there was no significant difference at days 0 and 4 (P > 0.05) but was significant at day 8 (P < 0.05).
nCD64 count was significantly different between sepsis and septic shock [Table 5] patients at days 0 and 8 but not significant at day 4. Receiver operating characteristic curve was plotted for nCD64 count to discriminate the septic shock with sepsis at days 0 and 8 (not plotted for day 4: not significant). Result revealed that nCD64 count is good predictor of the septic shock on day 0 (area under the curve [AUC] = 0.747, P = 0.010) [Figure 3a] and moderate predictor at day 8 (AUC = 0.679, P = 0.028) [Figure 3b].
Table 5.
Neutrophil CD64 sepsis versus septic shock
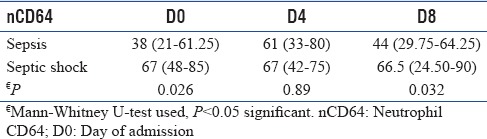
Figure 3.
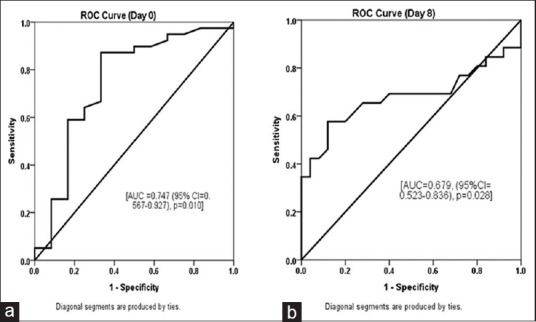
(a and b) Accuracy of the neutrophil CD64 to discriminate the septic shock with sepsis using receiver operating characteristic curve
DISCUSSION
In spite of years of extensive research, managing sepsis and preventing mortality remain a constant challenge. Much is debated regarding initial management, but appropriate initial antibiotic therapy remains the cornerstone of sepsis therapy.[22] Identifying sepsis is important to avoid unnecessary antibiotic use in nonsepsis conditions. Many recent studies have observed good sensitivity and specificity of nCD64 for identifying sepsis compared with other global markers of inflammation such as CRP and PCT.[10,13,18] Some studies also showed that nCD64 distinguishes the stages of sepsis.[9] However, these studies have limitations of small sample size with heterogeneous patient populations. The clinical status of sepsis patients may change significantly in the initial few days of ICU stay as a result of resuscitation and antibiotic therapy. Clinical course in the 1st week, results of microbiological cultures and laboratory values play a major role in the outcome. There are limited studies[11,23,24] on serial estimation of nCD64 in critically ill patients. We followed nCD64 through the 1st week of ICU stay and observed its correlation with the patient's characteristics and outcome.
In our study, nCD64 was higher in the nonsurvivor group on days 4 and 8. Many studies observed similar findings. Livaditi et al.[9] showed that increased nCD64 was favoring mortality in patients with sepsis. Muller et al.[25] also suggested the same finding in their study. Increased nCD64 could predict 28-day mortality in disseminated intravascular coagulation (DIC) patients. nCD64 value was significantly correlated with DIC severity as well.[17] Authors could find only two studies[11,26] which suggested an opposite correlation between nCD64 level and mortality. Cid et al.[26] showed that patients who survived had higher nCD64 expression compared to nonsurvivors. The possible explanation may be due to “exhaustion” of cells due to continuous stimulation by systemic cytokines in nonsurvivors.[27]
In our study, nCD64 was higher in septic shock patients compared to sepsis group on days 0 and 8 (P < 0.05). This finding corroborated with studies by several other authors.[9,13,27] In our study, survivors had a decrease in trend of nCD64 over time compared to nonsurvivors. This trend was also significant when we observed sepsis and septic shock. The nonsurvivors among the septic shock patients rather showed an increasing trend of nCD64 (P < 0.05). Icardi et al.[28] reported that patients treated with appropriate antibiotic therapy had a quick decrease of nCD64 along with improvement of their clinical condition as well.
Many recent studies have already defined the role of nCD64 in differentiating sepsis from nonsepsis. The biochemical property of nCD64 is self-explanatory for its higher value in septic shock compared to sepsis. However, if improving trend of nCD64 can be correlated with survival, it may be used as a marker of appropriate therapy during the initial days of treatment.
Regarding analytical aspects, FCM is the method of choice for nCD64 determinations.[29] The test can be done with small volume of EDTA anticoagulated blood, and this is stable for 36–72 h at room temperature.[16,30] The determination of nCD64 lacks standardization, and various methods have been used in expressing nCD64. We used percentage of nCD64 being activated by antigenic stimulus, and this approach is fairly reported in literature.[31] Even the cutoff values to differentiate sepsis and nonsepsis vary between studies.[32] Studies on nCD64 evaluation in sepsis were mostly with small sample size, inclusion criteria are different, and cutoff values and units are not universal. Moreover, there is a scarcity of literature on longitudinal follow-up of nCD64.[11]
Our study is limited by a small sample size. Besides, we received patients who were already on antibiotic therapy. We did not take account of severe sepsis group, rather evaluated sepsis patients based on the presence and absence of shock.
CONCLUSION
Monitoring serial nCD64 during ICU stay may be helpful in determining the clinical course of septic patients. However, further randomized trial with larger sample size is needed to establish its diagnostic and prognostic efficacy of such trend.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: Secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10:R42. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Müller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirn TJ, Weinstein MP. Update on blood cultures: How to obtain, process, report, and interpret. Clin Microbiol Infect. 2013;19:513–20. doi: 10.1111/1469-0691.12180. [DOI] [PubMed] [Google Scholar]
- 5.Pazin GJ, Saul S, Thompson ME. Blood culture positivity: Suppression by outpatient antibiotic therapy in patients with bacterial endocarditis. Arch Intern Med. 1982;142:263–8. [PubMed] [Google Scholar]
- 6.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609–34. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis BH, Bigelow NC. Comparison of neutrophil CD64 expression, manual myeloid immaturity counts, and automated hematology analyzer flags as indicators of infection or sepsis. Lab Hematol. 2005;11:137–47. doi: 10.1532/LH96.04077. [DOI] [PubMed] [Google Scholar]
- 8.Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med. 2006;130:654–61. doi: 10.5858/2006-130-654-NCIAII. [DOI] [PubMed] [Google Scholar]
- 9.Livaditi O, Kotanidou A, Psarra A, Dimopoulou I, Sotiropoulou C, Augustatou K, et al. Neutrophil CD64 expression and serum IL-8: Sensitive early markers of severity and outcome in sepsis. Cytokine. 2006;36:283–90. doi: 10.1016/j.cyto.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Cardelli P, Ferraironi M, Amodeo R, Tabacco F, De Blasi RA, Nicoletti M, et al. Evaluation of neutrophil CD64 expression and procalcitonin as useful markers in early diagnosis of sepsis. Int J Immunopathol Pharmacol. 2008;21:43–9. doi: 10.1177/039463200802100106. [DOI] [PubMed] [Google Scholar]
- 11.Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol. 2008;154:87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobreglio GB, d'Aversa P, Leo L, Scolozzi S, Fiore G. Quantitative expression of CD64 on neutrophil granulocytes as early marker of sepsis or severe infection. Haematologica. 2008;93:21. [Google Scholar]
- 13.Hsu KH, Chan MC, Wang JM, Lin LY, Wu CL. Comparison of fcγ receptor expression on neutrophils with procalcitonin for the diagnosis of sepsis in critically ill patients. Respirology. 2011;16:152–60. doi: 10.1111/j.1440-1843.2010.01876.x. [DOI] [PubMed] [Google Scholar]
- 14.Gámez-Díaz LY, Enriquez LE, Matute JD, Velásquez S, Gómez ID, Toro F, et al. Diagnostic accuracy of HMGB-1, sTREM-1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Acad Emerg Med. 2011;18:807–15. doi: 10.1111/j.1553-2712.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 15.Schiff DE, Rae J, Martin TR, Davis BH, Curnutte JT. Increased phagocyte fc gammaRI expression and improved fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects. Blood. 1997;90:3187–94. [PubMed] [Google Scholar]
- 16.Davis BH. Improved diagnostic approaches to infection/sepsis detection. Expert Rev Mol Diagn. 2005;5:193–207. doi: 10.1586/14737159.5.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Song SH, Kim HK, Park MH, Cho HI. Neutrophil CD64 expression is associated with severity and prognosis of disseminated intravascular coagulation. Thromb Res. 2008;121:499–507. doi: 10.1016/j.thromres.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann JJ. Neutrophil CD64: A diagnostic marker for infection and sepsis. Clin Chem Lab Med. 2009;47:903–16. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- 19.Ng PC, Li G, Chui KM, Chu WC, Li K, Wong RP, et al. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res. 2004;56:796–803. doi: 10.1203/01.PDR.0000142586.47798.5E. [DOI] [PubMed] [Google Scholar]
- 20.Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M, et al. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med. 2009;35:1950–8. doi: 10.1007/s00134-009-1637-7. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: Neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–34. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 23.Fischer G, Schneider EM, Moldawer LL, Karcher C, Barth E, Suger-Wiedeck H, et al. CD64 surface expression on neutrophils is transiently upregulated in patients with septic shock. Intensive Care Med. 2001;27:1848–52. doi: 10.1007/s00134-001-1135-z. [DOI] [PubMed] [Google Scholar]
- 24.de Jong E, de Lange DW, Beishuizen A, van de Ven PM, Girbes AR, Huisman A, et al. Neutrophil CD64 expression as a longitudinal biomarker for severe disease and acute infection in critically ill patients. Int J Lab Hematol. 2016;38:576–84. doi: 10.1111/ijlh.12545. [DOI] [PubMed] [Google Scholar]
- 25.Muller Kobold AC, Tulleken JE, Zijlstra JG, Sluiter W, Hermans J, Kallenberg CG, et al. Leukocyte activation in sepsis; correlations with disease state and mortality. Intensive Care Med. 2000;26:883–92. doi: 10.1007/s001340051277. [DOI] [PubMed] [Google Scholar]
- 26.Cid J, Garcia-Pardo G, Aguinaco R, Sanchez R. Neutrophil CD 64: Diagnostic accuracy and prognostic value in patients presenting to the emergency department. Eur J Clin Microbiol Inf Dis. 2011;30:845–52. doi: 10.1007/s10096-011-1164-7. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh M, Mahamid E, Bashenko Y, Hirsh I, Krausz MM. Overexpression of the high affinity Fc-g receptor (CD64) is associated with leucocyte dysfunction in sepsis. Shock. 2001;16:102–8. doi: 10.1097/00024382-200116020-00003. [DOI] [PubMed] [Google Scholar]
- 28.Icardi M, Erickson Y, Kilborn S, Stewart B, Grief B, Scharnweber G, et al. CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol. 2009;47:3914–9. doi: 10.1128/JCM.00628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann JJ. Neutrophil CD64 as a sepsis biomarker. Biochem Med (Zagreb) 2011;21:282–90. doi: 10.11613/bm.2011.038. [DOI] [PubMed] [Google Scholar]
- 30.Tillinger W, Jilch R, Jilma B, Brunner H, Koeller U, Lichtenberger C, et al. Expression of the high-affinity IgG receptor fcRI (CD64) in patients with inflammatory bowel disease: A new biomarker for gastroenterologic diagnostics. Am J Gastroenterol. 2009;104:102–9. doi: 10.1038/ajg.2008.6. [DOI] [PubMed] [Google Scholar]
- 31.Bauer PR, Kashyap R, League SC, Park JG, Block DR, Baumann NA, et al. Diagnostic accuracy and clinical relevance of an inflammatory biomarker panel for sepsis in adult critically ill patients. Diagn Microbiol Infect Dis. 2016;84:175–80. doi: 10.1016/j.diagmicrobio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Li ZY, Zeng L, Zhang AQ, Pan W, Gu W, et al. Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients: A meta-analysis. Crit Care. 2015;19:245. doi: 10.1186/s13054-015-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


