Abstract
Purpose
This study aimed to measure the serum levels of interleukin (IL)-17, IL-10, and IL-35 in patients with stable chronic obstructive pulmonary disease (COPD) and disclose the correlations between their expression levels and clinical factors of patients.
Methods
A total of 75 patients with stable COPD (47 males and 28 females) and 30 healthy controls (15 males and 15 females) were included in this study. The serum levels of IL-17, IL-10, and IL-35 were determined by enzyme-linked immunosorbent assay. The correlations between their expression levels and clinical factors of patients were determined using linear regression methods.
Results
The serum level of IL-17 was upregulated in stable COPD, and increased IL-17 expression was positively correlated with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) grading, modified Medical Research Council (mMRC) score, and long clinical history (P<0.05), but negatively correlated with the pulmonary function (P<0.05) of patients. The serum levels of IL-10 and IL-35 were downregulated in stable COPD, and decreased IL-10 and IL-35 levels negatively correlated with the smoking status, GOLD grading, mMRC score, and long clinical history (P<0.05), but positively correlated with the pulmonary function (P<0.05) of patients. Moreover, the level of IL-17 negatively correlated with IL-10 and IL-35, but IL-10 positively correlated with IL-35.
Conclusion
The serum levels of IL-17, IL-10, and IL-35 correlated with the clinical factors of COPD, indicating that they can serve as indicators to estimate the progression of COPD.
Keywords: chronic obstructive pulmonary disease, interleukin-17, interleukin-10, interleukin-35, serum
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease characterized by persistent airflow restriction, which is associated with increased chronic inflammatory response of the airway and lung to toxic particles or gases.1–3 Risk factors for COPD include exposure to tobacco smoke, people with asthma who smoke, occupational exposure to dusts and chemicals, exposure to fumes from burning fuel, age, and genetics.1,3–5 COPD is characterized by chronic inflammation of the airway, lung parenchyma, and pulmonary vessels.1,2,5 In this process, activated inflammatory cells release a variety of cytokines and inflammatory mediators, including leukotriene B4, interleukin (IL)-8, tumor necrosis factor alpha, and other mediators, which further destroy the structure of the lungs and/or promote the inflammatory responses of COPD.1,4,6,7 In addition, the protease and antiprotease imbalance, oxidation and antioxidant imbalance, and autonomic nervous system dysfunction of the lungs play important roles in the incidence and development of COPD.1,8
Some scholars believe that IL-17 and its related factors IL-35 and IL-10 play important roles in COPD pathogenesis.9–13 IL-17 is mainly secreted by T helper (Th)17 cells, which can be produced through the induction of chemokines, thereby inducing the activation and accumulation of neutrophils. IL-17 promotes inflammation and participates in the pathogenesis of various autoimmune diseases.9,10,14 However, unlike IL-17 proinflammatory factor, IL-10 and IL-35 are secreted by activating lymphocytes, macrophages, and other important inflammatory factors, which can restrain the response of Th1 cells, the antigen presentation of macrophages, and the aggregation of neutrophils.11,13 IL-10 can inhibit the function of IL-17 indirectly by amplifying the inhibition of regulatory T cells (Tregs) to Th cells.15 Moreover, IL-35 can suppress the differentiation of Th17 cells and the synthesis of IL-17.
However, the expression status and possible biological functions of IL-17, IL-10, and IL-35 in COPD have yet to be elucidated clearly. In the present study, we tested the expression levels of IL-17, IL-10, and IL-35 in the serum of patients with COPD and analyzed the correlations between their expression levels and the clinical parameters of COPD.
Methods
Ethics statement
The research was approved by the research ethics committees of the Affiliated Hospital of Qingdao University, Qingdao, China, and the Affiliated Hospital of Jining Medical University, Jining, China. All patients were approached in accordance with approved ethical guidelines. They agreed to participate in this study and signed informed consent forms. We state that all methods in the study were performed in accordance with the relevant guidelines and regulations developed by the aforementioned ethics committees.
Included objects in the study
A total of 75 stable patients with COPD randomly selected from June 2016 to June 2017 were recruited as the observation group (Affiliated Hospital of Qingdao University, Qingdao, China; Affiliated Hospital of Jining Medical University, Jining, China). A healthy control group of 30 cases was recruited from the hospital’s health checkup center mentioned above. No significant differences were observed in terms of age, gender, and smoking status of patients between the observation and control individuals (P>0.05). Clinical data of all individuals are given in detail in Table 1.
Table 1.
Clinical data of included patients (75 cases)
| Items | Groups | COPD group (n, %) | Control group (n, %) |
|---|---|---|---|
| Gender | Male | 47 (62.7) | 15 (50) |
| Female | 28 (37.3) | 15 (50) | |
| Age (years) | <70 | 33 (44) | 16 (53.3) |
| ≧70 | 42 (56) | 14 (46.7) | |
| Smoking status | Yes | 39 (52) | 11 (36.7) |
| No | 36 (48) | 19 (63.3) | |
| GOLD classification | 1 | 7 (9.3) | |
| 2 | 13 (17.3) | ||
| 3 | 29 (38.7) | ||
| 4 | 26 (34.7) | ||
| Scores of mMRC | 2 | 12 (16) | |
| 3 | 31 (41.3) | ||
| 4 | 32 (42.7) | ||
| Clinical history (years) | <10 | 25 (33.3) | |
| ≧10<20 | 21 (28) | ||
| ≧20 | 29 (38.7) |
Notes: GOLD-1, the predicted FEV1 percentage is ≥80%; GOLD-2, the predicted FEV1 percentage is ≥50%, but <80%; GOLD-3, the predicted FEV1 percentage is ≥30%, but <50%; and GOLD-4, the predicted FEV1 percentage is <30%.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global initiative for chronic Obstructive Lung Disease; mMRC, modified British Medical Research Council for dyspnea scale for symptom classification of COPD.
Diagnosis of COPD
Diagnosis of COPD was confirmed in accordance with the diagnostic criteria of the Global initiative for chronic Obstructive Lung Disease (GOLD).16,17 Patients with the following characteristics were diagnosed with COPD: 1) chronic cough, phlegm, progressive dyspnea, and history of exposure to COPD risk factors, even if no symptoms of respiratory distress were observed and 2) the ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) <0.7, which can be considered as permanent airflow limitation. Stable COPD is defined as follows: the cough, expectoration, and shortness of breath are in stable condition in the patient; showing only mild symptoms; or the condition is basically restored to a stable state before acute exacerbation.6
Inclusion criteria for the study
The inclusion criteria were as follows: 1) patients confirmed as having COPD and completed lung function testing;6 2) patients with stable COPD; 3) patients who received no antibiotics, oxygen therapy, glucocorticoids, and theophylline medications within 2 weeks before entering the study; 4) patients showing nonacute exacerbation that is not associated with purulent sputum, fever, chest pain, sign of lung consolidation, and lung moist rale; 5) patients whose imaging studies showed no evidence of lung infection; 6) patients who completed lung function examination; and 7) patients who signed informed consent forms for voluntary participation in the study.
Exclusion criteria for the study
The exclusion criteria were as follows: 1) patients with acute exacerbations (they may be accompanied by infection, multiple complications, and complex treatment measures, which may affect the natural process of IL-17, IL-10, and IL-35 in COPD and influence the reliability of conclusion); 2) patients with COPD accompanied by pulmonary interstitial fibrosis, bronchial asthma, tuberculosis, pneumonia, bronchial pneumonia, and lung cancer; 3) white blood cell count of >10×109 or <4×109/L, with or without the presence of left nucleus; 4) patients with major diseases of nonrespiratory system, such as diabetes, severe cardiovascular and cerebrovascular diseases, and neurological diseases or liver and kidney dysfunction; and 5) patients with mental illness and cognitive impairment.
Severity grading of patients with COPD
COPD grading was performed in accordance with the GOLD criteria, as follows:2,6 GOLD-1, the predicted FEV1 percentage is ≥80%; GOLD-2, the predicted FEV1 percentage is ≥50%, but <80%; GOLD-3, the predicted FEV1 percentage is ≥30%, but <50%; and GOLD-4, the predicted FEV1 percentage is <30%. To study the relationship between clinical history and test values, we divided the clinical history of COPD into three sections, namely, <10, ≥10<20, and ≥20 years.
Collection and treatment of blood samples
The peripheral venous blood of the patient was drawn on an empty stomach in the early morning, and the blood samples were numbered according to the order of the patients. The blood collection volume of each patient was 5 mL and it was packed into anticoagulant test tubes. The collected blood samples were centrifuged at 3,000 rpm, and the serum was cryopreserved by placing in a −80°C refrigerator after separation. The frozen serum was defrosted at room temperature when testing.
Implementation of enzyme-linked immunosorbent assay (ELISA)
The serum levels of IL-17, IL-10, and IL-35 were measured with the double-antibody sandwich ABC-ELISA in accordance with the manufacturer’s instructions. Human IL-17 and IL-10 ELISA test kits were purchased from Shanghai Heng Yuan Biotechnology Co., Ltd. (Shanghai, China). Human IL-35 kit was provided by Shanghai Xitang Biotechnology Co., Ltd. (Shanghai, China). In brief, the antibody was diluted to a protein content of 3–6 μg/mL with a buffer, and then 0.1 mL was added into the enzyme label plate hole at 4°C overnight. The following day, the solution in the wells was discarded, and the plate was washed thrice for 3 minutes each with a wash buffer. A 0.1 mL aliquot of the sample to be tested (blank control, negative control, and positive control at the same time) was added to the reaction well. Afterward, the reaction well was placed in a humid chamber at 37°C for 1 hour, and the plate was washed thrice with a wash buffer for 3 minutes each. Then, 0.1 mL of freshly diluted enzyme-labeled antibody was added in each reaction well at 37°C for 1 hour and was washed thrice with a wash buffer for 3 minutes each. A 0.1 mL aliquot of the 3,3′,5,5′-tetramethyl-benzidine substrate solution was added to each well at 37°C for 10–30 minutes and 0.05 mL of stop solution was added to each reaction well. The color intensity was measured at a wavelength of 450 nm by using a photometer. The standard curve was plotted based on the concentration of the standard sample and the OD of each well.
Key observation indicators
Modified Medical Research Council (mMRC) score
Degree of breathing difficulty of patients was evaluated by mMRC score, which consists of five statements as follows:18 grade 0: “I only get breathless with strenuous exercise”; grade 1: “I get short of breath when hurrying on level ground or walking up a slight hill”; grade 2: “On level ground, I walk slower than people of the same age because of breathlessness, or I have to stop for breath when walking at my own pace on the level”; grade 3: “I stop for breath after walking about 100 yards or after a few minutes on level ground”; and grade 4: “I am too breathless to leave the house or I am breathless when dressing”.18
Lung function test
All patients enrolled in this study had been asked to undergo pulmonary function examination. Each patient was tested thrice and the average value of three measurements was considered as the final result. The FEV1/FVC values and the predicted FEV1 percentage were recorded in detail for subsequent statistical analysis because both these values are directly related to the diagnosis and classification of COPD.
Statistical analysis
The data in this study were analyzed by SPSS 21.0 statistical software (IBM Corporation, Armonk, NY, USA). The count data were expressed in terms of constituent ratio (%), and the statistical results of the measurement data were expressed as mean±SD (X±SD). All data were tested using the Kolmogorov–Smirnov test to determine whether the data were normally distributed. The normal distribution of data was tested using Student’s t-test and one-way analysis of variance, and the non-normal distribution was compared with the results of the Wilcoxmann–Whitney test. The correlation between two variables was tested using linear correlation analysis. Statistical significance was set at P<0.05.
Results
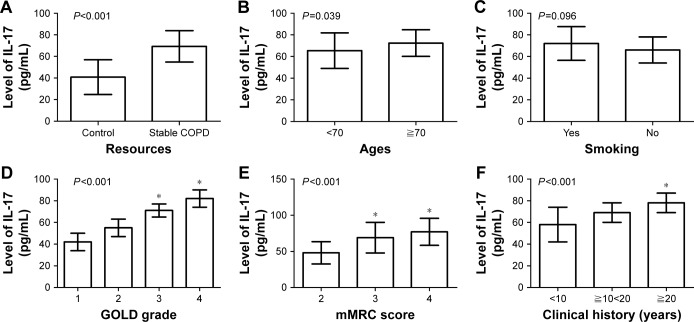
Increased serum IL-17 level positively correlated with GOLD grade, mMRC score, and clinical history of patients with stable COPD.
As shown in Table 2, the expression of serum IL-17 was upregulated in patients with stable COPD (69.31±14.57 pg/mL) compared with those in the control group (40.77±16.07 pg/mL; P<0.001; Figure 1A). Moreover, increased serum IL-17 level positively correlated with the age of patients (72.38±12.31 pg/mL for patients aged ≥70 years or older vs 65.41±16.40 pg/mL for patients aged <70 years or older; P=0.039; Figure 1B), but not with smoking (P=0.096; Figure 1C). The serum level of IL-17 in patients with GOLD-3 (71.61±6.34 pg/mL) and GOLD-4 (81.09±8.35 pg/mL) was higher than that in patients with GOLD-1 (42.07±8.36 pg/mL) and GOLD-2 (55.28±8.74 pg/mL), with P<0.001 (Figure 1D). Patients with mMRC scores of 3 (69.13±10.93 pg/mL) and 4 (77.37±10.59 pg/mL) showed higher serum levels of IL-17 compared with those with mMRC score of 2 (48.28±10.74 pg/mL; P<0.001; Figure 1E). In addition, patients with a long clinical history (78.22±9.18 pg/mL for patients with a clinical history of ≧20 years) displayed a higher expression of serum IL-17 (P<0.001; Figure 1F) than those with a short history (58.89±16.35 pg/mL for patients with a clinical history of <10 years).
Table 2.
Correlations between clinical features and the serum levels of IL-17, IL-10, and IL-35 in patients with stable COPD (N=75)
| Parameter | Group | n | Level of IL-17
|
Level of IL-10
|
Level of IL-35
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (M±SD, pg/mL) | Statistical value | P-value | (M±SD, pg/mL) | Statistical value | P-value | (M±SD, pg/mL) | Statistical value | P-value | |||
| Resources | Healthy control | 30 | 40.77±16.07 | −8.80 | <0.001 | 320.63±33.46 | 14.81 | <0.001 | 353.61±57.12 | 17.30 | <0.001 |
| Stable COPD | 75 | 69.31±14.57 | 188.34±44.05 | 169.28±45.83 | |||||||
| Gender | Male | 47 | 70.71±15.89 | 1.077 | 0.285 | 187.97±45.29 | −2.75 | 0.071 | 164.08±46.09 | −1.27 | 0.207 |
| Female | 28 | 66.97±11.95 | 205.74±36.37 | 177.99±45.03 | |||||||
| Age (years) | <70 | 33 | 65.41±16.40 | −2.10 | 0.039 | 206.14±46.96 | 3.30 | 0.001 | 191.84±46.51 | 4.17 | <0.001 |
| ≧70 | 42 | 72.38±12.31 | 174.35±36.41 | 151.55±31.76 | |||||||
| Smoking | Yes | 39 | 72.00±15.58 | 1.68 | 0.096 | 172.51±45.68 | −3.47 | 0.001 | 158.12±48.8 | −2.25 | 0.027 |
| No | 36 | 66.39±12.99 | 205.48±35.43 | 181.36±39.72 | |||||||
| GOLD grade | 1 | 7 | 42.07±8.36 | 64.84 | <0.001 | 267.86±25.76 | 74.61 | <0.001 | 252.41±22.13 | 136.33 | <0.001 |
| 2 | 13 | 55.28±8.74 | 220.39±17.38 | 212.69±17.19 | |||||||
| 3 | 29 | 71.61±6.34* | 194.24±15.80* | 172.67±11.82* | |||||||
| 4 | 26 | 81.09±8.35* | 144.32±28.27* | 121.40±23.51* | |||||||
| mMRC score | 2 | 12 | 48.28±10.74 | 31.95 | <0.001 | 247.05±32.76 | 24.31 | <0.001 | 236.48±27.38 | 38.66 | <0.001 |
| 3 | 31 | 69.13±10.93** | 189.05±30.17 | 172.76±32.42** | |||||||
| 4 | 32 | 77.37±10.59** | 165.63±38.77** | 169.27±45.89** | |||||||
| Clinical history (years) | <10 | 25 | 58.89±16.35 | 16.86 | <0.001 | 221.75±39.79 | 20.31 | <0.001 | 203.32±37.21 | 27.63 | <0.001 |
| ≧10<20 | 21 | 69.41±9.81 | 188.18±33.32 | 178.08±30.07 | |||||||
| ≧20 | 29 | 78.22±9.18*** | 159.64±33.60*** | 133.39±46.89*** | |||||||
Notes: For IL-17: *, GOLD grades 3 and 4 compared with GOLD grades 1 and 2; **, mMRC scores of 3 and 4 compared with mMRC score of 2; ***, a clinical history of ≥20 years compared with a history of <10 years. For IL-10: *, GOLD grades 3 and 4 compared with GOLD grades 1 and 2; **, mMRC score of 4 compared with mMRC scores of 3 and 2; ***, a clinical history of ≥20 years compared with a history of <10 years. For IL-35: *, GOLD grades 3 and 4 compared with GOLD grades 1 and 2; **, mMRC scores of 4 and 3 compared with mMRC score of 2; ***, a clinical history of ≥20 years compared with a history of <20 years.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; IL, interleukin; mMRC, modified British Medical Research Council.
Figure 1.
Correlation between clinical factors and the serum level of IL-17 in patients with stable COPD.
Notes: (A) The expression of serum IL-17 was upregulated in patients with stable COPD compared with those in the control group (P<0.001). (B) Increased serum IL-17 level positively correlated with the age of patients (P=0.039). (C) Serum IL-17 expression did not correlate with the smoking status of patients (P=0.096). (D) The serum level of IL-17 in patients with GOLD-3 and -4 was higher than that in patients with GOLD-1 and -2 (*P<0.001). (E) Patients with mMRC scores of 3 and 4 showed higher serum levels of IL-17 compared with those with mMRC score of 2 (*P<0.001). (F) Patients with a long clinical history displayed a higher expression of serum IL-17 than those with a short history (*P<0.001).
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic obstructive Lung Disease; IL, interleukin; mMRC, modified British Medical Research Council.
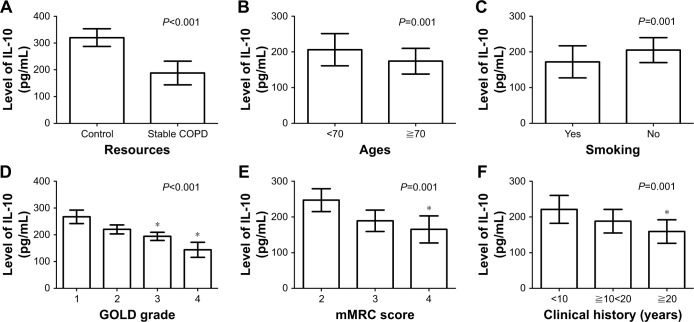
Decreased serum IL-10 expression negatively correlated with GOLD grade, mMRC score, and clinical history of patients with stable COPD
As shown in Table 2, patients with stable COPD (188.34±44.05 pg/mL) had lower levels of serum IL-10 than those in the control group (320.63±33.46 pg/mL; P<0.001; Figure 2A). Moreover, decreased IL-10 negatively correlated with the age of patients (174.35±36.41 pg/mL for patients aged ≧70 years or older vs 206.14±46.96 pg/mL for patients aged <70 years or older; P=0.001; Figure 2B) and smoking status (172.51±45.68 pg/mL for smoking patients vs 205.48±35.43 pg/mL for patients who did not smoke; P=0.001; Figure 2C). Patients with GOLD-3 (194.24±15.80 pg/mL) and GOLD-4 (144.32±28.27 pg/mL) showed lower levels of serum IL-10 than those with GOLD-1 (267.86±25.76 pg/mL) and GOLD-2 (220.39±17.38 pg/mL), with P<0.001 (Figure 2D). Patients with an mMRC score of 4 (165.63±38.77 pg/mL) showed lower levels of serum IL-10 than those with mMRC scores of 3 (189.05±30.17 pg/mL) and 2 (247.05±32.76 pg/mL), with P<0.001 (Figure 2E). In addition, patients with longer clinical history (159.64±33.60 pg/mL for patients with a clinical history of ≧20 years) displayed a lower expression of serum IL-10 (P<0.001; Figure 2F) than those with a short history (221.75±39.79 pg/mL for patients with a clinical history of <10 years).
Figure 2.
Correlation between clinical factors and the serum level of IL-10 in patients with stable COPD.
Notes: (A) Patients with stable COPD showed lower levels of serum IL-10 than those in the control group (P<0.001). (B) Decreased IL-10 negatively correlated with the age of patients (P=0.001). (C) Decreased IL-10 negatively correlated with the smoking status of patients (P=0.001). (D) Patients with GOLD-3 and -4 showed lower levels of serum IL-10 than those with GOLD-1 and -2 (*P<0.001). (E) Serum level of IL-10 in patients with mMRC score of 4 was lower than in those with mMRC scores of 2 and 3 (*P<0.001). (F) Patients with longer clinical history displayed a lower expression of serum IL-10 than those with a short history (*P<0.001).
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; IL, interleukin; mMRC, modified British Medical Research Council.
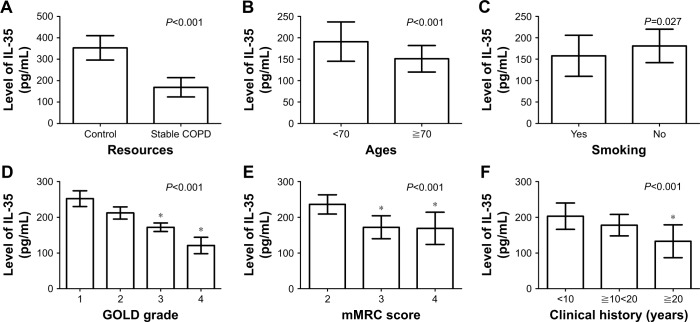
Decreased serum IL-35 expression negatively correlated with GOLD grade, mMRC score, and clinical history of stable patients
As shown in Table 2, a lower level of serum IL-35 was observed in patients with stable COPD (169.28±45.83 pg/mL) compared with those in the control group (353.61±57.12 pg/mL; P<0.001; Figure 3A). Moreover, the upregulation of serum IL-35 negatively correlated with the age of patients (151.55±31.76 pg/mL for patients aged ≧70 years or older vs 191.84±46.51 pg/mL for patients aged <70 years or older; P<0.001; Figure 3B) and smoking status (158.12±48.8 pg/mL for patients who smoked vs 181.36±39.72 pg/mL for patients who did not smoke; P=0.027; Figure 3C). Patients with GOLD-3 (172.67±11.82 pg/mL) and GOLD-4 (121.40±23.51 pg/mL) showed lower levels of serum IL-35 than those with GOLD-1 (252.41±22.13 pg/mL) and GOLD-2 (212.69±17.19 pg/mL), with P<0.001 (Figure 3D). Patients with mMRC scores of 3 (172.76±32.42 pg/mL) and 4 (169.27±45.89 pg/mL) showed lower levels of serum IL-35 than those with mMRC score of 2 (236.48±27.38 pg/mL; P<0.001; Figure 3E). In addition, patients with a long clinical history (133.39±46.89 pg/mL for patients with a clinical history of ≥20 years) displayed a lower expression of serum IL-35 (P<0.001; Figure 3F) than those with a short history (203.32±37.21 pg/mL for patients with a clinical history of <10 years).
Figure 3.
Correlation between clinical factors and the serum level of IL-35 in patients with stable COPD.
Notes: (A) A lower level of serum IL-35 was observed in patients with stable COPD compared with those in the control group (P<0.001). (B) The upregulation of serum IL-35 negatively correlated with the age of patients (P<0.001). (C) The upregulation of serum IL-35 negatively correlated with the smoking status of patients (P=0.027). (D) Patients with GOLD-3 and -4 showed lower levels of serum IL-35 than those with GOLD-1 and -2 (*P<0.001). (E) Patients with mMRC scores of 3 and 4 showed lower levels of serum IL-35 than those with mMRC score of 2 (*P<0.001). (F) Patients with a long clinical history displayed a lower expression of serum IL-35 than those with a short history (*P<0.001).
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; IL, interleukin; mMRC, modified British Medical Research Council.
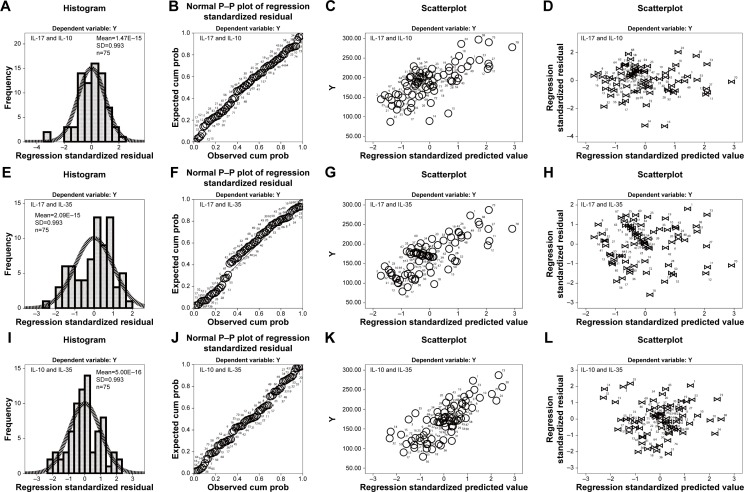
Expression of IL-17 negatively correlated with the serum expression levels of IL-10 and IL-35 in stable COPD
As shown in Table 3, statistical results showed that the serum expression levels of IL-17 and IL-10 had a negative linear correlation. The correlation coefficient of Pearson’s correlation test was −0.717 (P<0.001). Meanwhile, the F-value of variance analysis was 77.25 (P<0.001). The T-value of the correlation coefficient test was −19.39, whereas the T-value of the regression coefficient was −2.17 (P<0.001, 95% CI=−2.658 to −1.676) in stable patients with COPD. Furthermore, the final regression equation was ^Y=338–2.17X (Figure 4A–D). Moreover, the serum expression levels of IL-17 and IL-35 had a negative linear correlation. The correlation coefficient of Pearson’s correlation test was −0.765 (P<0.001), and the F-value of variance analysis was 103.27 (P<0.001). Moreover, the T-value of the correlation coefficient test was −20.03, whereas the T-value of the regression coefficient was −2.41 (P<0.001, 95% CI=−2.880 to −1.937). The final regression equation was ^Y=336–2.41X (Figure 4E–H).
Table 3.
Linear correlation analysis of important indicators (N=75)
| Items | Linear correlation analysis of continuous variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Correlations | Variance analysis | Coefficients | ||||||||
| Pearson’s correlation | P-value | Adjusted square | F-value | P-value | Regression coefficient | T-value | P-value | 95% CI | Equation | |
| IL-17 and IL-10 | −0.717 | <0.001 | 0.51 | 77.25 | <0.001 | −2.17 | −19.39 | <0.001 | −2.658 to −1.676 | ^Y=338−2.17X |
| IL-17 and IL-35 | −0.765 | <0.001 | 0.58 | 103.27 | <0.001 | −2.41 | −20.03 | <0.001 | −2.880 to −1.937 | ^Y=336−2.41X |
| IL-10 and IL-35 | 0.775 | <0.001 | 0.59 | 109.53 | <0.001 | 0.81 | 10.46 | <0.001 | 0.653–0.961 | ^Y=17+0.807X |
| IL-17 and FEV1/FVC | −0.789 | <0.001 | 0.62 | 120.46 | <0.001 | −0.008 | −10.976 | <0.001 | −0.009 to −0.006 | ^Y=−0.969−0.008X |
| IL-17 and FEV1% predicted | −0.847 | <0.001 | 0.71 | 185.35 | <0.001 | −0.011 | −13.61 | <0.001 | −0.013 to −0.010 | ^Y=−1.217−0.011X |
| IL-10 and FEV1/FVC | 0.778 | <0.001 | 0.601 | 112.29 | <0.001 | 0.003 | 10.597 | <0.001 | 0.002–0.003 | ^Y=−0.035+0.003X |
| IL-10 and FEV1% predicted | 0.815 | <0.001 | 0.659 | 143.98 | <0.001 | 0.004 | 12.000 | <0.001 | 0.003–0.004 | ^Y=−0.244+0.004X |
| IL-35 and FEV1/FVC | 0.834 | <0.001 | 0.692 | 166.68 | <0.001 | 0.003 | 12.922 | <0.001 | 0.002–0.003 | ^Y=−0.001+0.003X |
| IL-35 and FEV1% predicted | 0.869 | <0.001 | 0.751 | 224.21 | <0.001 | 0.004 | 14.976 | <0.001 | 0.003–0.004 | ^Y=−0.19+0.004X |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IL, interleukin.
Figure 4.
Correlation between the serum levels of IL-17, IL-10, and IL-35 in patients with stable COPD.
Notes: (A) Histogram of serum IL-17 and IL-10 shows that the normalized residuals belonged to normal distribution (P>0.05). (B) The P–P plot suggests that scatter points derived from IL-17 and IL-10 had normal distribution (P<0.05). (C) The dependent variable is approximately linear with the standardized predictive value, which indicates that IL-17 and IL-10 had a negative linear correlation (P<0.001). (D) The vast majority of normalized residuals did not exceed 3, suggesting no abnormal values of IL-17 and IL-10 were found. (E) Histogram of IL-17 and IL-35 shows that the normalized residuals belonged to normal distribution (P>0.05). (F) The P–P plot suggests that scatter points derived from IL-17 and IL-35 showed a normal distribution (P<0.05). (G) The dependent variable is approximately linear with the standardized predictive value, which indicates that IL-17 and IL-35 had a negative linear correlation (P<0.001). (H) The vast majority of normalized residuals did not exceed 3, suggesting no abnormal values of IL-17 and IL-35 were found. (I) Histogram of IL-10 and IL-35 shows that the normalized residuals had normal distribution (P>0.05). (J) The P–P plot suggests that scatter points derived from IL-10 and IL-35 had normal distribution (P<0.05). (K) The dependent variable is approximately linear with the standardized predictive value, which indicates that IL-10 and IL-35 had a positive linear correlation (P<0.001). (L) The vast majority of normalized residuals did not exceed 3, suggesting no abnormal values of IL-10 and IL-35 were found.
Abbreviations: COPD, chronic obstructive pulmonary disease; IL, interleukin.
Expression of serum IL-10 positively correlated with the serum IL-35 in stable COPD
As shown in Table 3, the serum expression levels of IL-17 and IL-35 displayed a positive linear correlation. The correlation coefficient of Pearson’s correlation test was 0.775 (P<0.001), and the F-value of variance analysis was 109.53 (P<0.001). Furthermore, the T-value of correlation coefficient test was 10.46, whereas the T-value of the regression coefficient was 0.81 (P<0.001, 95% CI=0.653–0.961) in stable patients with COPD. The final regression equation was ^Y=17+0.807X (Figure 4I–L).
Correlation between the expression levels of IL-17, IL-10, and IL-35 and lung function of stable patients with COPD
As shown in Table 3, the serum expression of IL-17 was significantly negatively correlated with FEV1/FVC (P<0.001; regression equation: ^Y=−0.969–0.008X; Figure 5A and B) and the predicted FEV1 percentage (P<0.001; regression equation: ^Y=−1.217–0.011X; Figure 5C and D) in stable patients with COPD. However, the expression of serum IL-10 positively correlated with FEV1/FVC (P<0.001; regression equation: ^Y=−0.035+0.003X; Figure 5E and F) and the predicted FEV1 percentage (P<0.001; regression equation: ^Y=−0.244+0.004X; Figure 5G and H) in patients with stable COPD. Furthermore, the IL-35 serum level positively correlated with FEV1/FVC (P<0.001; regression equation: ^Y=−0.001+0.003X; Figure 5I and J) and the predicted FEV1 percentage (P<0.001; regression equation: ^Y=−0.19+0.004X; Figure 5K and L) in patients with stable COPD.
Figure 5.
Correlation between the serum levels of IL-17, IL-10, and IL-35 and pulmonary function in patients with stable COPD.
Notes: (A) Histogram of serum IL-17 and FEV1/FVC suggests that these variables had a characteristic of normal distribution (P>0.05). (B) The dependent variable is approximately linear with the standardized predictive value, which indicates that serum IL-17 and FEV1/FVC of patients had a negative linear correlation (P<0.001). (C) Histogram of serum IL-17 and FEV1% predicted suggests that these variables had a characteristic of normal distribution (P>0.05). (D) The dependent variable is approximately linear with the standardized predictive value, which indicates that serum IL-17 and FEV1% predicted of patients had a negative linear correlation (P<0.001). (E) Histogram of serum IL-10 and FEV1/FVC suggests that these variables had a characteristic of normal distribution (P>0.05). (F) The dependent variable is approximately linear with the standardized predictive value, which indicates that serum IL-10 and FEV1/FVC of patients had a negative linear correlation (P<0.001). (G) Histogram of serum IL-10 and FEV1% predicted suggests that these variables had a characteristic of normal distribution (P>0.05). (H) The dependent variable is approximately linear with the standardized predictive value, which indicates that serum IL-10 and FEV1% predicted of patients had a negative linear correlation (P<0.001). (I) Histogram of serum IL-35 and FEV1/FVC suggests that these variables had a characteristic of normal distribution (P>0.05). (J) The dependent variable is approximately linear with the standardized predictive value, which indicates that serum IL-35 and FEV1/FVC of patients had a negative linear correlation (P<0.001). (K) Histogram of serum IL-35 and FEV1% predicted suggests that these variables had a characteristic of normal distribution (P>0.05). (L) The dependent variable is approximately linear with the standardized predictive value, which indicates that serum IL-35 and FEV1% predicted of patients had a negative linear correlation (P<0.001).
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IL, interleukin.
Discussion
The etiology and pathogenesis of COPD have not yet been fully explained. The root cause of its development is believed to be systemic or local chronic inflammation.19,20 IL-17 is a proinflammatory cytokine produced by memory CD4+ T cells, and its overexpression may lead to the upregulation of chemokines.21 IL-10 is a cytokine that is closely related to the Th2 immune response,22 and IL-35 plays an immunomodula-tory role in the human body.23 In the present study, we tested the serum levels of IL-35, IL-10, and IL-35 in patients with stable COPD and attempted to elucidate the roles of these three cytokines in the pathogenesis of COPD.
The expression of serum IL-17 showed a significant increase as compared with that in the control group. Moreover, increased serum level of IL-17 positively correlated with GOLD grading, mMRC score, and clinical history of patients with stable COPD, which indicated that IL-17 was positively associated with the progression of COPD. This result suggested that IL-17 can be used as an indicator of the degree COPD progression. IL-17 is an early promoter of T-cell-induced inflammatory response; it also promotes the release of proinflammatory cytokines.9,24 IL-17 can promote the secretion of IL-6 and tumor necrosis factor alpha, thereby affecting the construction of airway fiber connective tissue and hyperplasia of smooth muscles. Thus, it may be involved in the adjustment of airway remodeling of COPD.9,13,21 In addition, the levels of IL-10 and IL-35 in patients with stable COPD significantly decreased compared with those in the control group. We also found that decreased IL-10 and IL-35 negatively correlated with the smoking status, GOLD grading, mMRC score, and clinical history of patients with stable COPD. This result indicates that IL-10 and IL-35 are protective factors for COPD. IL-10 inhibits the response of Th1 cells, the antigen presentation of macrophages, and the aggregation of neutrophils; it also promotes neutrophil apoptosis, thereby inhibiting the body’s inflammatory response.11,25 The anti-inflammatory effect of IL-35 is achieved by promoting the proliferation and differentiation of induced Tregs and promoting the secretion of anti-inflammatory factor IL-10 and inhibiting the proliferation and differentiation of effector T cells.14,26–28 On the basis of our results, we believe that IL-10 and IL-35 both have inhibitory effects on the inflammatory development of COPD. An in-depth study on IL-10 and IL-35 may aid in finding new drugs for the treatment of COPD.
After conducting a linear correlation regression analysis, we found that the serum IL-17 level negatively correlated with the serum levels of IL-10 and IL-35 in stable COPD. A previous study showed that IL-35 can inhibit Th17 cell differentiation and reduce IL-17 synthesis.12 Moreover, IL-10 can enhance the function of Tregs to inhibit the effects of Th cells, which indirectly inhibit the effect of IL-17 on the inflammatory reactions.13,15 We believe that the more severe the chronic inflammatory response is, the higher the level of IL-17 (proinflammatory cytokine) and the lower the levels of IL-10 and IL-35 (anti-inflammatory cytokines) will be. This imbalance of inflammatory factors in the lungs promotes the accumulation of inflammatory cells and inflammatory mediators and cytokines, further leading to chronic inflammation in the airways of COPD. However, we found that the serum expression levels of IL-10 and IL-35 had a positive linear correlation. A previous study showed that IL-35 can promote the secretion of IL-10.14 Whether IL-10 can also promote the secretion of IL-35 has not been reported yet. However, as anti-inflammatory cytokines, both IL-35 and IL-10 may play roles in the development of COPD. Thus, the study of the anti-inflammatory mechanisms of IL-10 and IL-35 in COPD should be strengthened further in the future.
We found that the serum expression of IL-17 negatively correlated with FEV1/FVC and the predicted FEV1 percentage in patients with stable COPD. IL-17 plays a positive role in airway inflammation and airway remodeling in both chronic and acute respiratory diseases. Given the progress of the course of disease, the synthesis and secretion of IL-17 will increase, resulting in the aggravation of airway fibrosis and reduction of pulmonary function.9,13,21 IL-17 is a major cytokine for neutrophilic inflammation and has been linked to COPD pathogenesis.29 A previous study showed that IL-17 is increased in COPD and is correlated with decreased pulmonary function. Moreover, IL-17 may contribute to disease progression by activating C-X-C motif chemokine 12 in the peripheral lungs of patients with severe-to-very severe COPD.30 IL-17 may play a complex role in the cigarette smoke-induced loss of pulmonary function and development of emphysema; it also cannot be characterized as a central mediator of cigarette smoke-induced lung damage.31 A recent study has evaluated the efficacy and safety of the anti-IL-17A monoclonal antibody CNTO 6,785 in patients with symptomatic moderate-to-severe COPD. This study showed that IL-17 is unlikely to be a dominant driver in the pathology as a viable therapeutic target to maintain COPD treatment. The pathological process of COPD may involve IL-17 to some extent through its interaction with other cytokines, which may require further investigation.32
We observed that the expression levels of serum IL-10 and IL-35 positively correlated with FEV1/FVC and the predicted FEV1 percentage in patients with stable COPD. This result suggests that high levels of IL-10 and IL-35 predict a better lung function of patients. An increase in the serum level of IL-10 can reduce the effects of various inflammatory factors, such as IL-6 and IL-8, and further inhibit the airway inflammatory response and airway secretions, thereby improving the ventilation function.33,34 IL-35 exerts a certain inhibitory effect on inflammatory response, and it also negatively regulates the activity of immune factors, thereby improving the lung function of patients.35 Another study suggested that IL-35 plays a role in retarding the pathogenesis of COPD by regulating the inflammatory response of Th2- and Th17-mediated COPD.36
However, this study has some drawbacks. First, patients with acute exacerbations may be accompanied by infection and multiple complications, which may affect the natural processes of IL-17, IL-10, and IL-35, thereby disturbing the reliability of conclusion. Thus, patients with acute exacerbation of COPD were not enrolled in the study. Second, the size of the study was limited; selected patients were from two hospitals only. Thus, patient’s selective bias may be present. Third, this study did not involve the signal mechanisms of IL-17, IL-10, and IL-35 in COPD. Future research should pay attention to the aforementioned topic to elucidate the regulation mechanisms of IL-17, IL-10, and IL-35 in COPD. Moreover, confirming the clinical reliability of the results requires multicenter studies with large sample sizes.
Conclusion
The serum level of IL-17 increased in patients with stable COPD. In addition, the increased level of IL-17 positively correlated with GOLD grade, mMRC score, and clinical history of patients, but negativity correlated with the pulmonary ventilation function of patients. The serum levels of IL-10 and IL-35 decreased in patients with stable COPD. In addition, decreased serum levels of IL-10 and IL-35 negatively correlated with smoking, GOLD grade, mMRC score, and clinical history of patients, but positively correlated with the pulmonary ventilation function of patients. In addition, the expression of IL-17 negatively correlated with the expression levels of IL-10 and IL-35. Also, the expression levels of IL-10 and IL-35 showed a positive linear correlation. These results suggest that these cytokines may be used as dynamic indicators to understand the disease progression of COPD.
Acknowledgments
This study was supported by the Shandong Provincial Medical and Health Science and Technology Development Plan Project (grant number 2016WS0176).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:995–1013. doi: 10.2147/COPD.S82518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger CR, Peters SP. Outpatient chronic obstructive pulmonary disease management: going for the GOLD. J Allergy Clin Immunol Pract. 2015;3(4):471–478. doi: 10.1016/j.jaip.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Zhou A, Zhou Z, Zhao Y, Chen P. The recent advances of phenotypes in acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1009–1018. doi: 10.2147/COPD.S128604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machin M, Amaral AF, Wielscher M, et al. Systematic review of lung function and COPD with peripheral blood DNA methylation in population based studies. BMC Pulm Med. 2017;17(1):54. doi: 10.1186/s12890-017-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadhra S, Kurmi OP, Sadhra SS, Lam KB, Ayres JG. Occupational COPD and job exposure matrices: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:725–734. doi: 10.2147/COPD.S125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. 2017;22(3):575–601. doi: 10.1111/resp.13012. [DOI] [PubMed] [Google Scholar]
- 7.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. doi: 10.2147/COPD.S42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adcock IM, Marwick J, Casolari P, et al. Mechanisms of corticosteroid resistance in severe asthma and chronic obstructive pulmonary disease (COPD) Curr Pharm Des. 2010;16(32):3554–3573. doi: 10.2174/138161210793797889. [DOI] [PubMed] [Google Scholar]
- 9.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17(5):435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 10.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 11.Mckinstry KK, Strutt TM, Buck A, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182(12):7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37(11):3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Cheng Z, Liu W, Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD. 2013;10(4):459–465. doi: 10.3109/15412555.2013.770456. [DOI] [PubMed] [Google Scholar]
- 14.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182(10):6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhry A, Samstein RM, Treuting P, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montes de Oca M, López Varela MV, Laucho-Contreras ME, et al. Classification of patients with chronic obstructive pulmonary disease according to the Latin American Thoracic Association (ALAT) staging systems and the global initiative for chronic obstructive pulmonary disease (GOLD) Arch Bronconeumol. 2017;53(3):98–106. doi: 10.1016/j.arbres.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 18.Launois C, Barbe C, Bertin E, et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012;12:61. doi: 10.1186/1471-2466-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. doi: 10.2147/COPD.S152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223(4–5):383–396. doi: 10.1016/j.imbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Q, Liu GQ, Zeng YY, et al. Role of IL-17 in LPS-induced acute lung injury: an in vivo study. Oncotarget. 2017;8(55):93704–93711. doi: 10.18632/oncotarget.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia JM, Stillings SA, Leclerc JL, et al. Role of interleukin-10 in acute brain injuries. Front Neurol. 2017;8:244. doi: 10.3389/fneur.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang A, Cheng L, He M, Nie J, Wang J, Jiang K. Interleukin-35 on B cell and T cell induction and regulation. J Inflamm. 2017;14:16. doi: 10.1186/s12950-017-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 25.Glynn P, Coakley R, Kilgallen I, Murphy N, O’Neill S. Circulating interleukin 6 and interleukin 10 in community acquired pneumonia. Thorax. 1999;54(1):51–55. doi: 10.1136/thx.54.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Mai J, Virtue A, et al. IL-35 is a novel responsive anti-inflammatory cytokine – a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7(3):e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirtz S, Billmeier U, Mchedlidze T, Blumberg RS, Neurath MF. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141(5):1875–1886. doi: 10.1053/j.gastro.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collison LW, Chaturvedi V, Henderson AL, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11(12):1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos AB, Sethi S, Nikota J, et al. IL-17A and the promotion of neutrophilia in acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):428–437. doi: 10.1164/rccm.201409-1689OC. [DOI] [PubMed] [Google Scholar]
- 30.Roos AB, Sandén C, Mori M, Bjermer L, Stampfli MR, Erjefält JS. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke-induced lymphoid neogenesis. Am J Respir Crit Care Med. 2015;191(11):1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 31.Voss M, Wolf L, Kamyschnikow A, et al. Il-17A contributes to maintenance of pulmonary homeostasis in a murine model of cigarette smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2015;309(2):L188–L195. doi: 10.1152/ajplung.00388.2014. [DOI] [PubMed] [Google Scholar]
- 32.Eich A, Urban V, Jutel M, et al. A randomized, placebo-controlled Phase 2 trial of CNTO 6785 in chronic obstructive pulmonary disease. COPD. 2017;14(5):476–483. doi: 10.1080/15412555.2017.1335697. [DOI] [PubMed] [Google Scholar]
- 33.Silva BSA, Lira FS, Ramos D, et al. Severity of COPD and its relationship with IL-10. Cytokine. 2018;106:95–100. doi: 10.1016/j.cyto.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Huang AX, Lu LW, Liu WJ, Huang M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-α levels correlate with pulmonary function in patients with asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome. Med Sci Monit. 2016;22:2800–2808. doi: 10.12659/MSM.896458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Herminé A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181(10):6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 36.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]