Abstract
Background
Cytomegalovirus (CMV) colitis typically presents in immunocompromised and inflammatory bowel disease (IBD) patients. Several studies have been conducted on the endoscopic characteristics of CMV colitis in IBD patients.
Objectives
The endoscopic findings of CMV colitis in non-IBD patients and their relationship with inhospital mortality are unclear. We aimed to describe the endoscopic presentation in these patients and to determine the endoscopic predictors of inhospital mortality.
Patients and methods
Patients with CMV colitis diagnosed using histology between April 2002 and December 2016 at the Linkou Chang Gung Memorial Hospital, Taiwan, were retrospectively enrolled. Patients diagnosed with IBD during follow-up were excluded. Patient data, including underlying diseases, endoscopic presentation, laboratory data, clinical course, complications, and clinical outcomes, were collected. The independent risk factors for inhospital mortality were analyzed with logistic regression. The difference of overall survival was compared using Kaplan–Meier survival curve and log rank test. All statistical calculations were performed using SPSS software, version 21.
Results
Sixty-nine patients were enrolled, and 8 IBD patients were excluded. Within the 61 non-IBD patients, 31 were diagnosed by colonoscopy and others by sigmoidoscopy. Ulceration (77%) was the most common endoscopic finding, followed by a cobblestone appearance (19.7%), colitis with/without erosions (9.8%), pseudomembrane (9.8%), and tumor/polyp-like lesions (8.2%). Among the patients who underwent full-length colonoscopy, 35.3% presented with right-sided colitis, 23.5% with left-sided colitis, and 32.4% with pancolitis. Pancolitis was identified as a negative predictor of inhospital mortality (odds ratio, 6.8; 95% confidence interval, 1.233–37.497; p=0.028) and overall survival (log rank p=0.018).
Conclusion
Colonoscopy is recommended for precise CMV colitis diagnosis and outcome prediction in non-IBD patients.
Keywords: cytomegalovirus colitis, CMV, inflammatory bowel disease, endoscopy, colonos-copy, mortality
Introduction
Cytomegalovirus (CMV) colitis is mostly diagnosed in immunocompromised and inflammatory bowel disease (IBD) patients and is associated with a high mortality.1–5 Several studies have demonstrated the endoscopic presentation of CMV colitis in IBD patients,6–9 particularly in those with ulcerative colitis, but few have done so in non-IBD patients.10 In a Korean study conducted in 2010, 43 patients were reviewed and 21 received full-length colonoscopy.10 The endoscopic findings varied, with 8 (38%) patients displaying grossly normal mucosa in the rectosigmoid colon. However, the relationship between endoscopic findings and patient mortality remained unknown. We aimed to examine the endoscopic presentation of CMV colitis in non-IBD patients and to assess its relationship with mortality. In this way, we hoped to predict and decrease patient mortality in CMV colitis.
Patients and methods
Compliance with ethical standards
This study was approved by Chang Gung Medical Foundation Institutional Review Board on February 20, 2017, No 201700193B0 “Clinical presentations and outcome of cytomegalovirus infection in gastrointestinal tract” during February 1, 2017 to February 1, 2018. Informed consent from individual patients to review the medical records covering patients’ data was not required for retrospective studies with the electronic medical record system, as stated by Chang Gung Medical Foundation Institutional Review Board. We confirmed patient data confidentiality. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution’s human research committee.
Patients
Under the approval of the Chang Gung Medical Foundation Institutional Review Board (No 201700193B0), we retrospectively retrieved clinical data of all patients with pathologically proven CMV colitis from the electronic pathology database who presented at the Linkou Chang Gung Memorial Hospital between April 2002 and December 2016. Histology specimens were obtained using sigmoidoscopy or colonoscopy, and diagnosis was confirmed by the presence of CMV inclusion bodies in the tissue and a positive immunohistochemistry (IHC) staining. IHC staining was performed using monoclonal antibodies directed against the CMV pp65 antigen (Novocastra™ lyophilized mouse monoclonal antibody, Leica Biosystems Newcastle Ltd, Newcastle upon Tyne, UK).
Patients with a history of solid organ or bone marrow transplantation, HIV infection, and immunosuppressive drug use, including corticosteroids (oral or intravenous prednisone or equivalent, ≥20 mg/d for >2 weeks), or chemotherapeutic agent use within 6 months were classified as immunocompromised.
Clinical and endoscopic data collection
Clinical data collected included age; gender; dates of admission; underlying diseases; diagnosis and death, follow-up data; general condition a week prior to diagnosis; medication history; and treatment duration and outcomes. Endoscopic data collected included the distribution and appearance of colonic lesions. Laboratory data included total white blood cell and platelet counts and levels of hemoglobin, alanine aminotransferase, bilirubin, albumin, C-reactive protein and CMV pp65 antigenaemia, DNA (226-bp segment on Glyco-protein B gene, LightMix® Kit human cytomegalovirus, TIB MOLBIOL, Berlin, Germany), and serology.
Statistical analysis
Numerical data with normal distribution are presented as mean ± standard deviation and those with nonnormal distribution are presented as medium and range. Categorical data are expressed as absolute number and percentage. We used logistic regression to identify independent risk factors for inhospital mortality, and the results are presented as odds ratio, with 95% confidence interval and p-value. We evaluated the overall survival using the Kaplan–Meier survival curve and log rank test. p<0.05 was considered statistically significant. All statistical calculations were performed using SPSS software, version 21 (IBM Corporation, Armonk, NY, USA).
Results
Baseline clinicopathological characteristics
Sixty-one non-IBD patients were enrolled. The average age was 61.2±20.0 years, and males (59%) were predominant. Among these, 35 (57.4%) patients were classified as immunocompetent. Overall, 45 (73.8%) patients received antiviral therapy, including 19 (73.1%) immunocompromised and 26 (74.3%) immunocompetent patients. In the patients with CMV antigenemia, medium of viral load was 12/500,000 PMN (range 1–731/500,000 PMN). There were 4 cases with evidence of Clostridium difficile coinfection and 1 with HIV infection among them. In view of complications and outcome evaluation, 2 (3.3%) patients developed bowel perforations and 6 (9.8%) underwent surgical resection. The inhospital mortality was 29.5% and overall survival was 60.7%. All of them died from poor general condition, especially septic shock instead of CMV colitis itself. Other data are shown in Table 1.
Table 1.
Baseline characteristics of CMV colitis patients without IBD
| Characteristics | Result |
|---|---|
| Case number | 61 |
| Age, years | 61.2±20.0 |
| Gender (male) | 36 (59%) |
| Hospital stay, day | 172.7±639.9 |
| Follow-up duration (day) | 116 (3–5,178) |
| General condition within 1 week | |
| Sepsis | 45 (73.8%) |
| Shock | 22 (36.1%) |
| Respiratory failure | 22 (36.1%) |
| Clinical symptom triggering colonoscopy | |
| Melena | 25 (41%) |
| Diarrhea | 27 (44.3%) |
| Abdominal pain | 6 (9.8%) |
| Positive stool occult blood test | 2 (3.3%) |
| Other (no definite record) | 1 (1.6%) |
| Immunocompromised status | 26 (42.6%) |
| Underlying diseases | |
| Liver cirrhosis | 4 (6.6%) |
| Coronary artery disease | 8 (13.1%) |
| End-stage renal disease | 8 (13.1%) |
| Diabetes mellitus | 20 (32.8%) |
| HIV infection | 8 (13.1%) |
| Immunosuppressive medication | |
| Immunosuppressant | 6 (9.8%) |
| Chemotherapy | 1 (1.6%) |
| Steroid | 17 (27.9%) |
| Laboratory data | |
| Total white blood cell count,/µL | 7,800 (800–27,300) |
| Hemoglobin level, g/dL | 10.4±2.1 |
| Platelet count, ×1,000/mm3 | 228.0±105.8 |
| ALT, IU/L | 25.1±17.3 |
| Bilirubin, mg/dL | 0.5 (0.2–7.8) |
| Albumin, g/dL | 2.6 (1.7–4.2) |
| C-reactive protein, mg/dL | 73.8±76.6 |
| Viral markers | |
| CMV pp65 antigenemia (n=31) | 21 (67.7%) |
| CMV PCR positive (n=7) | 4 (57.1%) |
| CMV IgG positive, IgM negative (n=34) | 26 (76.5%) |
| CMV IgG negative, IgM positive (n=34) | 0 (0.0%) |
| CMV IgG positive, IgM positive (n=34) | 6 (17.6%) |
| Ganciclovir or valganciclovir treatment | 45 (73.8%) |
| Treatment duration, day | 19 (1–151) |
| Outcome | |
| Perforation | 2 (3.3%) |
| Surgical resection | 6 (9.8%) |
| Inhospital mortality | 18 (29.5%) |
| Overall survival | 37 (60.7%) |
Notes: Numerical data with normal distribution are presented as mean ± standard deviation and those with non-normal distribution are presented as medium (range). Categorical data are expressed as absolute number (percentage).
Abbreviations: ALT, alanine transaminase; CMV, cytomegalovirus; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; PCR, polymerase reaction.
Endoscopic findings of CMV colitis in non-IBD patients
Thirty-one (50.8%) patients with CMV colitis underwent full-length colonoscopy. Most patients receiving incomplete colonoscopy or sigmoidoscopy were critical, with 36.1% of them having shock or respiratory failure. Endoscopy showed a diverse presentation and scattered distribution (Figures 1 and 2, Table 2). When including colitis with/without erosions (45.2%), varied ulcers were the most common presentation (77%). Among these ulcers, those coated with white membrane and the typical, deep ulcers (punched-out ulcers) appeared more characteristic of CMV colitis. Furthermore, 25 (40.98%) patients had more than 1 endoscopic finding simultaneously. It was difficult to accurately diagnose or completely exclude CMV colitis using endoscopic findings without pathological confirmation.
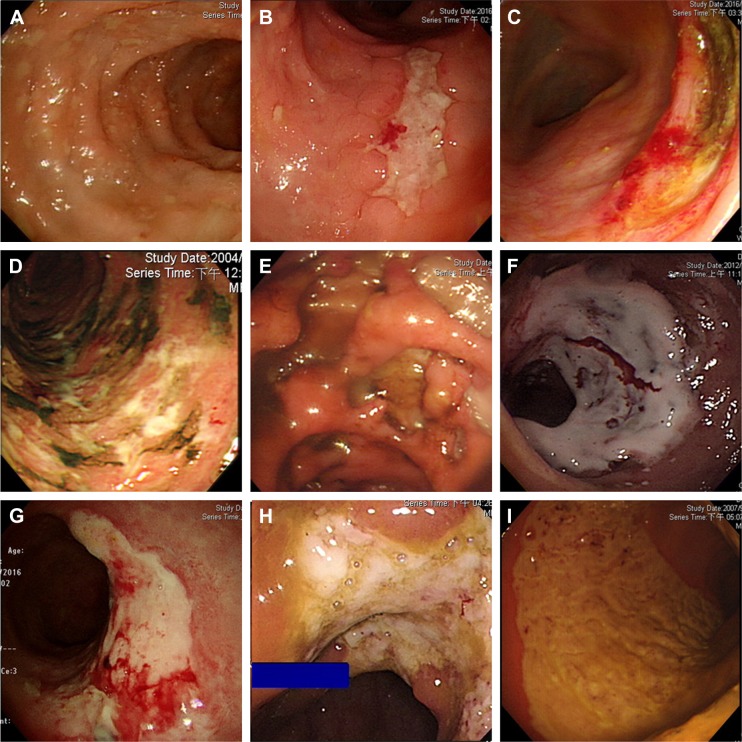
Figure 1.
The ulcers of CMV colitis in patients without IBD.
Note: (A) Well-demarcated small ulcer, (B) longitudinal ulcer, (C) semi-lunate ulcer, (D) irregular ulcer, (E) deep ulcer (including punch-out ulcer), (F–H) ulcer coated with white membrane, and (I) segmental mucosal defect.
Abbreviations: CMV, cytomegalovirus; IBD, inflammatory bowel disease.
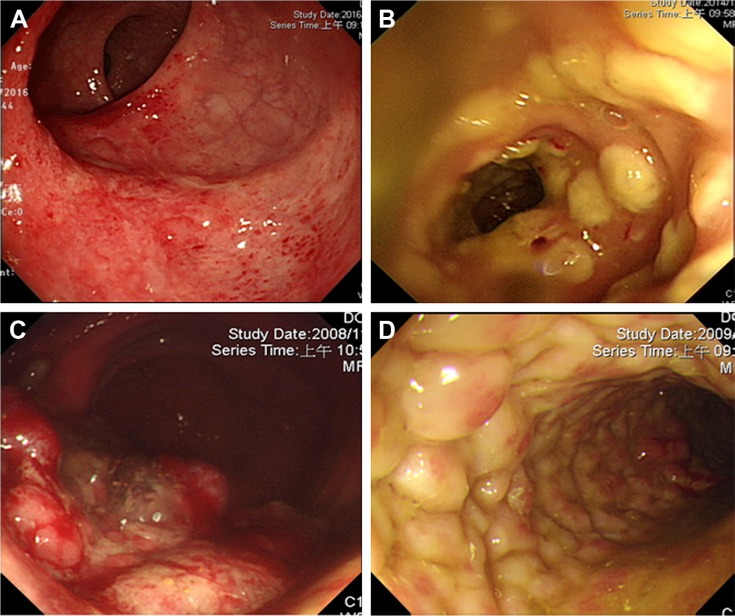
Figure 2.
Endoscopic findings of CMV colitis in patients without IBD, except ulcers.
Note: (A) Colitis with/without erosion, (B) pseudomembrane, (C) tumor/polyp-like, and (D) cobblestone appearance.
Abbreviations: CMV, cytomegalovirus; IBD, inflammatory bowel disease.
Table 2.
Endoscopic findings of CMV colitis patients without IBD
| Characteristics | Result, n (%) |
|---|---|
| Diagnostic tools | |
| Colonoscopy | 31 (50.8) |
| Sigmoidoscopy | 30 (49.2) |
| Involved area | |
| Rectum (n=61) | 35 (57.4) |
| Sigmoid colon (n=60) | 26 (43.3) |
| Descending colon (n=48) | 13 (27.1) |
| Transverse colon (n=39) | 19 (48.7) |
| Ascending colon (n=34) | 18 (52.9) |
| Cecum (n=31) | 13 (41.9) |
| Ileocecal valve (n=18) | 4 (22.2) |
| Endoscopic finding | |
| Colitis with/without erosion, only | 6 (9.8) |
| Ulcer | 47 (77) |
| Well-demarcated small ulcer | 27 (57.5) |
| Longitudinal ulcer | 5 (10.6) |
| Semi-lunate ulcer | 21 (44.7) |
| Irregular ulcer | 12 (25.5) |
| Deep ulcer (including punch-out ulcer) | 11 (23.4) |
| Ulcer coating with white membrane | 7 (14.9) |
| Segmental mucosal defect | 14 (29.8) |
| Pseudomembrane | 6 (9.8) |
| Tumor/polyp-like | 5 (8.2) |
| Cobblestone appearance | 12 (19.7) |
| Skip lesions | 18 (29.5) |
| Stricture during follow-up | 2 (3.3) |
Abbreviations: CMV, cytomegalovirus; IBD, inflammatory bowel disease.
To assess the distribution of CMV colitis, we focused on the 31 patients who underwent full-length colonoscopy. The incidence of right- and left-sided colitis and pancolitis were 35.3%, 23.5%, and 32.4%, respectively. If sigmoidoscopy was performed for CMV colitis diagnosis in non-IBD patients, 35.3% patients would be missed; therefore, colonoscopy is the preferred diagnostic tool in patients with suspected CMV colitis.
Pancolitis increases mortality rate of patients with CMV colitis
To accurately evaluate the role of endoscopic findings in inhospital mortality, we focused on 31 patients who underwent full-length colonoscopy. Among these, pancolitis was the only predictor of inhospital mortality (odds ratio, 6.8; 95% confidence interval, 1.233–37.497; p=0.028) (Table 3). Patients with pancolitis also displayed a higher overall mortality rate (log rank p=0.018) according to the Kaplan–Meier survival curve (Figure 3). Neither immunological status nor endoscopic presentation was related to mortality. However, pancolitis was noted related to antigenemia or the reasons for endoscope.
Table 3.
Clinical factors associated with inhospital mortality of CMV colitis patients without IBD (31 patients with full-length colonoscopy)
| Characteristics | Odd ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Age | 1.036 | 0.993–1.081 | 0.104 |
| Gender (male/female) | 0.188 | 0.035–1.000 | 0.050 |
| Immunological status | 0.286 | 0.056–1.466 | 0.133 |
| Underlying diseases | |||
| Liver cirrhosis | 0.000 | 0.000 | 1.000 |
| End-stage renal disease | 5.923×109 | 0–>1012 | 0.999 |
| Diabetes mellitus | 3.600 | 0.655–19.78 | 0.141 |
| HIV infection | 0.000 | 0.000 | 0.999 |
| Immunosuppressive medication | |||
| Immunosuppressant | 2.625 | 0.146–47.183 | 0.513 |
| Chemotherapy | 4.443 | 0–>1012 | 1.000 |
| Steroid | 0.500 | 0.083–3.011 | 0.449 |
| Laboratory data | |||
| Total white blood cell count,/µL | 1.000 | 1.000 | 0.934 |
| Hemoglobin level, g/dL | 0.690 | 0.436–1.091 | 0.112 |
| Platelet count, ×1,000/mm3 | 0.993 | 0.984–1.003 | 0.164 |
| ALT, IU/L | 0.957 | 0.886–1.034 | 0.267 |
| Bilirubin, mg/dL | 1.508 | 0.827–2.750 | 0.180 |
| Albumin, g/dL | 0.308 | 0.055–1.736 | 0.182 |
| C-reactive protein, mg/dL | 1.009 | 0.997–1.022 | 0.146 |
| Involved area | |||
| Right-sided colitis (n=12, 35.3%) | 0.343 | 0.058–2.036 | 0.239 |
| Left-sided colitis (n=8, 23.5%) | 0.268 | 0.028–2.578 | 0.254 |
| Pancolitis (n=11, 32.4%) | 6.800 | 1.233–37.497 | 0.028* |
| Endoscopic finding | |||
| Colitis with/without erosion, only | |||
| Ulcer | 2.353 | 0.235–23.601 | 0.467 |
| Well-demarcated small ulcer | 0.863 | 0.254–2.932 | 0.813 |
| Longitudinal ulcer | 1.667 | 0.254–10.931 | 0.594 |
| Semi-lunate ulcer | 2.051 | 0.647–6.501 | 0.222 |
| Irregular ulcer | 1.978 | 0.533–7.340 | 0.308 |
| Deep ulcer (including punch-out ulcer) | 1.469 | 0.372–5.811 | 0.583 |
| Ulcer coating with white membrane | 3.810 | 0.757–19.172 | 0.105 |
| Segmental mucosal defect | 0.943 | 0.253–3.520 | 0.930 |
| Pseudomembrane | 4.443×109 | 0–>1012 | 1.000 |
| Tumor/polyp-like | 1.250 | 0.099–15.796 | 0.863 |
| Cobblestone appearance | 0.563 | 0.054–5.864 | 0.630 |
| Skip lesions | 1.071 | 0.206–5.584 | 0.935 |
| Ganciclovir or valganciclovir treatment | 9.693×108 | 0–.1012 | 0.999 |
| Treatment duration | 0.937 | 0.830–1.057 | 0.287 |
Note:
p<0.05.
Abbreviations: ALT, alanine transaminase; CMV, cytomegalovirus; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease.
Figure 3.
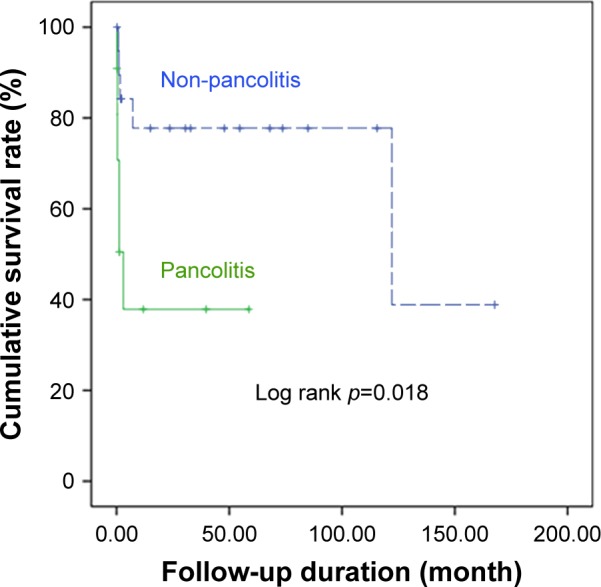
Survival curves of CMV colitis patients with/without pancolitis.
Note: The patients of CMV colitis with pancolitis (solid line) had worse overall survival than the ones without pancolitis (dashed line) with log rank p=0.018 according to Kaplan–Meier survival curve analysis.
Abbreviation: CMV, cytomegalovirus.
Discussion
CMV can involve the entire gastrointestinal tract, but the colon is the most common site.11 CMV colitis is mostly reported in immunocompromised and IBD patients. While pathological diagnosis is the gold standard for CMV colitis, the location and number of biopsies are also important.9 Diverse endoscopic presentations also make the endoscopic study of CMV colitis in non-IBD patients challenging.
The endoscopic findings were commonly divided into well-demarcated ulceration (50%), ulceroinfiltrative (25%), and pseudomembrane (25%) types according to a study conducted on 12 patients in 2012.12 However, other case reports have shown different presentations, such as pseudotumor, longitudinal ulcers, and pseudomembranes.13–16 Although several studies have discussed the endoscopic findings of CMV colitis in IBD patients, only 1 Korean study was conducted in non-IBD patients,10 in which among 43 enrolled patients, 21 received full-length colonoscopy and presented 4 types of endoscopic findings, namely normal, colitis, ulceration, and colitis with ulceration.
In our study, we collected data from 61 CMV colitis non-IBD patients, 31 of whom underwent full-length colonoscopy. Right-sided colitis (35.3%) was the most common in this group of patients; therefore, a full-length colonoscopy was of greater relevance in CMV colitis diagnosis. We reported 5 major types of endoscopic findings in CMV colitis, including colitis with/without erosions, ulceration and pseudomembrane, tumor/polyp-like or cobblestone appearance. Although varied ulcers were the most common finding, only 23.4% patients presented with a typical deep or punched-out ulcer. Forty-one percent patients had at least 2 types of endoscopic findings during the diagnosis. Because of diverse endoscopic presentations of CMV colitis, we should always keep its diagnosis in mind, even in immunocompetent patients.
The inhospital mortality of CMV colitis in non-IBD patients was 29.5%, and most of these patients died from poor general condition, especially septic shock rather than from CMV colitis itself. Pancolitis may reflect a worse clinical condition, and thus can be a negative predictor of inhospital mortality and overall survival. This is the first study to point out the relationship between endoscopic finding and mortality of CMV colitis in non-IBD patients. However, a cohort study and more cases are needed to clarify the endoscopic features.
In CMV colitis, right-sided colitis was most common, and full colonoscopy could also help us to predict the outcome. Besides, melena and diarrhea were most common presentations. Therefore, endoscopic exam was indicated in the patients with melena or diarrhea. However, critical patients had higher risks to receive full colonoscopy. In our opinion, we should advance to right side of the colon in order to completely rule out CMV, tuberculous, or other infectious disease when we could not find active lesion over the left side of the colon in the critical patients.
Conclusion
Right-sided colitis and ulceration were the most common presentations of CMV colitis in non-IBD patients. Pancolitis was a negative predictor of inhospital mortality and overall survival. Colonoscopy is preferred for the diagnosis and outcome prediction of CMV colitis in non-IBD patients. The limitation of this study was small number of included cases. We will integrate more data and collect more patients to validate the conclusion in a further study.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Prof Liang Kung-Hao, Liver Research Center, Linkou; Chang Gung of the Memorial Hospital, Taoyuan, Taiwan, provided statistical consultation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nakase H, Herfarth H. Cytomegalovirus colitis, cytomegalovirus hepatitis and systemic cytomegalovirus infection: common features and differences. Inflamm Intest Dis. 2016;1(1):15–23. doi: 10.1159/000443198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitley RJ, Jacobson MA, Friedberg DN, et al. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. International AIDS Society-USA. Arch Intern Med. 1998;158(9):957–969. doi: 10.1001/archinte.158.9.957. [DOI] [PubMed] [Google Scholar]
- 3.Matthes T, Kaiser L, Weber D, Kurt AM, Dietrich PY. Cytomegalovirus colitis – a severe complication after standard chemotherapy. Acta Oncol. 2002;41(7–8):704–706. doi: 10.1080/028418602321028346. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Kim YH, Kim JS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012;46(1):51–56. doi: 10.1097/MCG.0b013e3182160c9c. [DOI] [PubMed] [Google Scholar]
- 5.Weng MT, Tung CC, Lee YS, et al. Cytomegalovirus colitis in hospitalized inflammatory bowel disease patients in Taiwan: a referral center study. BMC Gastroenterol. 2017;17(1):28. doi: 10.1186/s12876-017-0586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirayama Y, Ando T, Hirooka Y, et al. Characteristic endoscopic findings and risk factors for cytomegalovirus-associated colitis in patients with active ulcerative colitis. World J Gastrointest Endosc. 2016;8(6):301–309. doi: 10.4253/wjge.v8.i6.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16(10):1245–1251. doi: 10.3748/wjg.v16.i10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Zhou W, Lv H, et al. The association between CMV viremia or endoscopic features and histopathological characteristics of CMV colitis in patients with underlying ulcerative colitis. Inflamm Bowel Dis. 2017;23(5):814–821. doi: 10.1097/MIB.0000000000001095. [DOI] [PubMed] [Google Scholar]
- 9.McCurdy JD, Enders FT, Jones A, et al. Detection of cytomegalovirus in patients with inflammatory bowel disease: where to biopsy and how many biopsies? Inflamm Bowel Dis. 2015;21(12):2833–2838. doi: 10.1097/MIB.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Bahng S, Kang KJ, et al. Cytomegalovirus colitis in patients without inflammatory bowel disease: a single center study. Scand J Gastroenterol. 2010;45(11):1295–1301. doi: 10.3109/00365521.2010.499962. [DOI] [PubMed] [Google Scholar]
- 11.Galiatsatos P, Shrier I, Lamoureux E, Szilagyi A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci. 2005;50(4):609–616. doi: 10.1007/s10620-005-2544-6. [DOI] [PubMed] [Google Scholar]
- 12.Seo TH, Kim JH, Ko SY, et al. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology. 2012;59(119):2137–2141. doi: 10.5754/hge10825. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Wakabayashi N, Fukumoto K, et al. Toxic megacolon associated with cytomegalovirus infection in a patient with steroid-naive ulcerative colitis. Intern Med. 2012;51(19):2739–2743. doi: 10.2169/internalmedicine.51.8145. [DOI] [PubMed] [Google Scholar]
- 14.Vegunta AS, Dasar SK, Joshi SK, Rao RV. Spontaneous partial vanishing cytomegalovirus pseudotumour of colon in an immunocompetent patient. J Clin Diagn Res. 2015;9(8):TD07–TD09. doi: 10.7860/JCDR/2015/13803.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falagas ME, Griffiths J, Prekezes J, Worthington M. Cytomegalovirus colitis mimicking colon carcinoma in an HIV-negative patient with chronic renal failure. Am J Gastroenterol. 1996;91(1):168–169. [PubMed] [Google Scholar]
- 16.Roskell DE, Hyde GM, Campbell AP, Jewell DP, Gray W. HIV associated cytomegalovirus colitis as a mimic of inflammatory bowel disease. Gut. 1995;37(1):148–150. doi: 10.1136/gut.37.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]