Summary
Background
Intraventricular haemorrhage is a subtype of intracerebral haemorrhage, with 50% mortality and serious disability for survivors. We aimed to test whether attempting to remove intraventricular haemorrhage with alteplase versus saline irrigation improved functional outcome.
Methods
In this randomised, double-blinded, placebo-controlled, multiregional trial (CLEAR III), participants with a routinely placed extraventricular drain, in the intensive care unit with stable, non-traumatic intracerebral haemorrhage volume less than 30 mL, intraventricular haemorrhage obstructing the 3rd or 4th ventricles, and no underlying pathology were adaptively randomly assigned (1:1), via a web-based system to receive up to 12 doses, 8 h apart of 1 mg of alteplase or 0·9% saline via the extraventricular drain. The treating physician, clinical research staff, and participants were masked to treatment assignment. CT scans were obtained every 24 h throughout dosing. The primary efficacy outcome was good functional outcome, defined as a modified Rankin Scale score (mRS) of 3 or less at 180 days per central adjudication by blinded evaluators. This study is registered with ClinicalTrials.gov, NCT00784134.
Findings
Between Sept 18, 2009, and Jan 13, 2015, 500 patients were randomised: 249 to the alteplase group and 251 to the saline group. 180-day follow-up data were available for analysis from 246 of 249 participants in the alteplase group and 245 of 251 participants in the placebo group. The primary efficacy outcome was similar in each group (good outcome in alteplase group 48% vs saline 45%; risk ratio [RR] 1·06 [95% CI 0·88–1·28; p=0–554]). A difference of 3·5% (RR 1·08 [95% CI 0·90–1·29], p=0–420) was found after adjustment for intraventricular haemorrhage size and thalamic intracerebral haemorrhage. At 180 days, the treatment group had lower case fatality (46 [18%] vs saline 73 [29%], hazard ratio 0·60 [95% CI 0·41–0·86], p=0–006), but a greater proportion with mRS 5 (42 [17%] vs 21 [9%]; RR 1·99 [95% CI 1·22–3·26], p=0–007). Ventriculitis (17 [7%] alteplase vs 31 [12%] saline; RR 0·55 [95% CI 0·31–0·97], p=0–048) and serious adverse events (114 [46%] alteplase vs 151 [60%] saline; RR 0·76 [95% CI 0·64–0·90], p=0–002) were less frequent with alteplase treatment. Symptomatic bleeding (six [2%] in the alteplase group vs five [2%] in the saline group; RR 1·21 [95% CI 0·37–3·91], p=0–771) was similar.
Interpretation
In patients with intraventricular haemorrhage and a routine extraventricular drain, irrigation with alteplase did not substantially improve functional outcomes at the mRS 3 cutoff compared with irrigation with saline. Protocol-based use of alteplase with extraventricular drain seems safe. Future investigation is needed to determine whether a greater frequency of complete intraventricular haemorrhage removal via alteplase produces gains in functional status.
Introduction
In patients with spontaneous intracerebral haemorrhage, intraventricular haemorrhage is associated with devastating consequences.1–4 Mortality is reported to be greater than 50%, with fewer than 20% of survivors having good functional outcomes. Mortality and function seem to be altered if thrombolysis is used.5,6 Systematic review and meta-analysis suggest removal of intraventricular haemorrhage improves survival and long-term functional outcome by relieving acute obstructive hydrocephalus and reducing neurotoxicity.5–9 We hypothesised that small intracerebral haemorrhage with large intraventricular haemorrhage describes a subgroup of patients whose severe prognosis is reversible.1,6 Thus, we organised the phase 3 Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Haemorrhage (CLEAR III) trial.
Preliminary data showed that alteplase (Genentech Inc, San Francisco, CA, USA) can safely remove clot from the ventricle in patients with an extraventricular drain, if precautions are taken to avoid reactivating brain bleeding.10–13 Therefore, in patients with intraventricular haemorrhage and an extraventricular drain, we tested the hypothesis that irrigating the ventricle with alteplase would improve functional outcome as measured by a modified Rankin Scale (mRS) score 0–3 (mRS ≤3, so-called good outcome) compared with normal saline (0·9%).
Methods
Study design and participants
In this multicentre, randomised, prospective phase 3 trial (CLEAR III)13 done at 73 sites in Brazil, Canada, Germany, Hungary, Israel, Spain, the UK, and the USA, following local and country ethics approval, we tested a strategy of ventricular clearance with alteplase via extraventricular drains. Although extraventricular drain placement is not standard practice for all cases of intraventricular haemorrhage, it is used to manage hydrocephalus and intracranial pressure. All patients had a clinical diagnosis of obstructive hydrocephalus, and placement of an extraventricular drain pre-trial was a routine clinical care decision. Participants were aged 18–80 years with known symptom onset within 24 h of the initial CT scan confirming intraventricular haemorrhage and 3rd or 4th ventricle obstruction. Eligibility criteria included supratentorial intracerebral haemorrhage volume 30 mL or less, measured by the ABC/2 method,18,19 and clot stability (no measured expansion >5 mL) on repeat CT scan at least 6 h after extraventricular drain placement.13 Additional eligibility criteria included a historical mRS of 1 or less, no limitations to care, and no ongoing coagulopathy, suspicion of aneurysm, arteriovenous malformation, or other vascular anomaly (see appendix p 5: methodological details of the treatment protocol and inclusion and exclusion criteria for additional details).13 All data were captured electronically and pertinent source documentation uploaded by local site personnel using an internet-based electronic data capture (EDC) system (VISION, Prelude Dynamics, LLC, Austin, TX, USA). The EDC provided field-level and form-to-form range and value edit checks during data entry. Independent quality assurance monitors (Emissary International, LLC, Austin, TX, USA) used the uploaded source documentation to perform risk-based, remote monitoring of key data variables. Monitored data were then exported by the data management centre where additional data edit checks were applied before form/participant finalisation. Site personnel were notified of and responded to data discrepancies identified during these review processes with the EDC system query tool, with resulting data corrections captured by the electronic audit trail.
Randomisation and masking
All participants and trial personnel, except for the local and central pharmacists and the unblinded statistician, were masked to treatment assignments. After the local principal investigator determined eligibility and written, informed consent was obtained, site personnel randomly assigned patients (1:1) within 72 h of ictus, using a web-based enrolment system (VISION, Prelude Dynamics, LLC, Austin, TX, USA) that generated a treatment allocation and emailed the treatment assignment code directly to the local, trained pharmacist. All other site and coordinating centre personnel remained masked to allocation. After 100 participants were assigned by simple randomisation, a Pocock-Simon14,15 covariate adaptive algorithm was implemented to balance study groups by baseline intraventricular haemorrhage size (≤20 mL, 20–50 mL, and >50 mL, measured on the diagnostic CT) and intracerebral haemorrhage location (thalamus or other, determined by centralised CT reading). Imbalances in these factors were determined at each enrolment, and patients were randomised with a weighted coin (80/20) favouring assignment to the treatment group, which improved balance in intracerebral haemorrhage location and intracerebral haemorrhage size.6,13,14 To ensure treatment balance at the site, patients were adaptively randomised only after a given site had recruited two saline and two alteplase patients. All participants remained masked during data collection and interim analyses. Masking was assessed by an external monitor.
Procedures
Participants received up to 12 doses, 8 h apart, of 1 mg alteplase or 0·9% saline via the extraventricular drain. CT scans were obtained every 24 h throughout dosing. All participants were managed using the American Heart Association recommendations for the treatment of spontaneous intracerebral haemorrhage as the basis for a standard approach to airway, ventilation, intracranial pressure monitoring, sedation, and pharmacological treatment of mass effect.16,17 Investigators were asked to remove as much clot as possible, until a stopping point was obtained: 3rd and 4th ventricles open; intraventricular haemorrhage mass effect relieved; 80% of clot removed; or 12 doses given.
To optimise accuracy and minimise investigator bias, clot volumes were analysed by a core laboratory using semi-automated segmentation and Hounsfield thresholds.18 This was done with OsiriX software (version 4.1, Pixmeo; Geneva, Switzerland) on DICOM images of each participant’s stability and daily scans. This approach has been validated for accuracy and inter-rater reliability.18,19 Core laboratory values were used in all analyses. Core lab defined location as either thalamus or other (lobar, putamen, caudate).
Participants had an NIHSS assessment at day 7, clinic visits on days 30, 180, and 365, and phone contacts at days 90 and 270. A site-identified, certified examiner assessed the mRS (a score of 0 indicates no symptoms, 5 indicates severe disability, and 6 indicates death), extended Glasgow Outcome Scale (eGOS; eight-level disability scale from 8 [upper good recovery] to 1 [death]), Barthel Index (0 to 100 daily activities scale with 0 indicating no activities performed and 100 all activities performed), Stroke Impact Scale (SIS; self-reported scale of 16 activity domains from 1 [most impaired] to 5 [not impaired]), and National Institutes of Health Stroke Scale (NIHSS, clinic visits only; scores range from 0 to 42, with higher scores indicating a more severe neurological deficit). CT was repeated at 30 and 365 days. The mRS assessment, the primary outcome, was video-recorded and sent to a core laboratory (Robertson Centre for Biostatistics, University of Glasgow, Glasgow, UK) for blinded assessment by an independent panel of experts.13 All other assessments were secondary. Full details of mRS, eGOS, and NIHSS are given in the appendix (pp 7–10).
Outcomes
The primary aim was to assess clinical efficacy of extraventricular drain plus alteplase by estimating the difference in the proportion of centrally adjudicated mRS scores, dichotomised as 3 or less versus more than 3, at 180 days. We estimated the average benefit comparing treatment versus control, using the intention to treat (ITT) principle. Specifically, we estimated the difference between the probability of an mRS score of 3 or less at 180 days, referred to as a good outcome, comparing alteplase versus saline. In accordance with the literature on covariate-adaptive randomised designs, the estimate of the adjusted treatment effect was based on a weighted average of the difference in proportions for each of the six strata defined by the possible baseline combinations for covariates used in randomisation: intraventricular haemorrhage volume and intracerebral haemorrhage location. Weights were set proportional to the number in each stratum (pooled across groups). The 95% CI was computed by the non-parametric bias corrected and accelerated (BCa) bootstrap method,20 such that the covariate-adaptive design is adhered to in each resampled (ie, bootstrap replicated) data set. Multivariable logistic regression models were used to estimate the conditional effect of treatment on good mRS outcome for baseline variables.
For the planned secondary efficacy analyses, we used Kaplan-Meier time-to-event analysis to estimate the survival functions and the log-rank test to compare survival by treatment; and the logistic model relating clot removed to mRS 0-3 proportion was used (appendix p 24). These models were informed by univariate regression and well established clinical and epidemiological considerations of the important prognostic factors of age, initial Glasgow Coma Scale (GCS) score (scores range from 3 to 15, with lower scores indicating reduced levels of consciousness), intraventricular haemorrhage size as a continuous variable, and the results of the planned intervention, clot removal. Intensity of ICU management by treatment type was compared via χ2 or Fisher’s exact test, as appropriate. Results of planned analyses of key demographic subgroups for heterogeneity of treatment effect are presented: intra-ventricular haemorrhage and intracerebral haemorrhage size, location, GCS, age, sex, and ethnic origin.
Post-hoc analyses were done for two unexpected but clinically important findings: 1) when the number of participants in the mRS 5 category at 180 days was inspected for disproportion, a treatment comparison was made; and 2) when inspection of protocol-associated clot removal showed that alteplase achieved the hypothesised differential removal for participants with intraventricular haemorrhage volume >20 mL but not in the group with <20 mL, subgroup by treatment group was undertaken. Interaction terms for treatment by baseline intraventricular haemorrhage stability volume at 20 mL were considered. No correction for multiplicity was applied, because secondary analyses were considered hypothesis generating.
The safety aim of the trial was to achieve near total clot dissolution without procedure-related safety events endangering subjects beyond the risks associated with intensive medical treatment.21 Analyses tested the null hypothesis that use of alteplase is safe for the treatment of intraventricular haemorrhage, relative to standard care of extraventricular drain alone under prespecified thresholds for 30-day case fatality (40%), symptomatic rebleeding (25%), and bacterial brain infection (20%). We tested these three thresholds and all safety event rates as interim analyses by the unblinded statistician (ES) after 100, 175, 250, and 350 participants were enrolled. A final analysis was then done for the full 500 participants over 180 days with Fisher’s exact test and reviewed with the DSMB. The overall occurrence rate of serious adverse events was tested between the two treatment groups.
End of treatment (EOT) was defined as 24 h after the last dose.18 Additionally, area under the curve (AUC) of the intraventricular haemorrhage time course from stability to EOT was calculated with the trapezoidal rule to quantify clot removal over time. AUC values were normalised by the time elapsed from stability to EOT, to account for variability in treatment times. Logistic and Cox regression analyses were done to evaluate the relation between intraventricular haemorrhage removal, represented by the normalised AUC of intraventricular haemorrhage clot on mRS ≤3, and 180-day case fatality, respectively, after adjustment for intracerebral haemorrhage clot location, age, intracerebral haemorrhage volume at stability, and randomisation GCS.
Statistical analysis
The trial planning was informed by data from the previously completed phase 2 CLEAR studies, which recorded 30-day outcomes.6 Sample size planning assumed an average removal difference attributable to total alteplase exposure of 12 mL. The sample size of CLEAR III was planned to be 250 patients per group to provide at least 82% power to detect a risk difference of 13% in the proportion of good outcome from extraventricular drain plus alteplase treatment compared with control, assuming a 22% proportion of good outcome among controls.
The conjectured risk difference and good outcome rate under control were based on extrapolating from the previously completed phase 2 CLEAR studies, which recorded baseline intraventricular haemorrhage clot volume and location (for intracerebral haemorrhage) and 30-day mRS outcomes.6 To do this, models were first fit using the data from these studies, and then used for simulating hypothetical trials at sample size 500 as described in the appendix (p 13).
We present data in three groups: the planned ITT primary efficacy analyses of functional outcome; additional mRS efficacy analyses including other mRS analyses of various cut points and secondary analyses of case fatality, clot removal, and ICU care; and safety. Adjusted analyses are indicated in the appendix (p 19). The primary and key secondary analyses were designated in 2008, at the start of the trial.
All data analyses were done with two-sided tests with a type I error rate of 0·05, done with the statistical packages STATA 13.0 or higher (STATA Corp, College Station, TX, USA) and R version 3.2 (R Foundation for Statistical Computing, Vienna, Austria). All data are presented as median (IQR), unless otherwise specified.
Role of the funding source
The principal investigator (DFH) conceived, organised, and executed this trial. He had full access to all study data and had final responsibility for the decision to submit for publication. The sponsor NIH/NINDS provided input regarding the study design during the grant review process and the NIH/NINDS-appointed DSMB provided the same during active recruitment. The NIH/NINDS-appointed DSMB and Genentech, Inc. approved the decision to submit the paper for publication.
Results
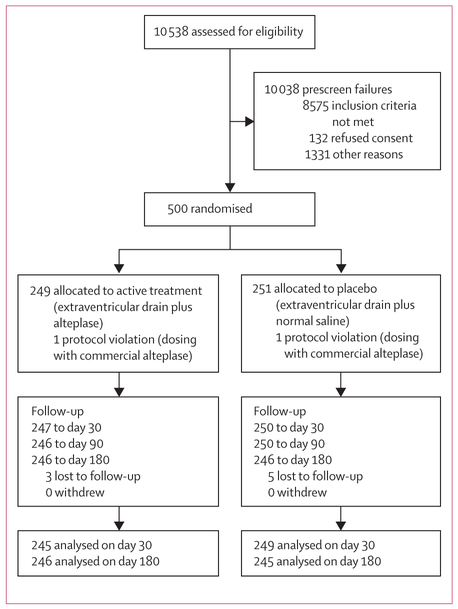
Between Sept 18, 2009, and Jan 13, 2015, 500 patients were randomised: 249 to the alteplase group and 251 to the saline group, with the last participant completing follow-up on Jan 13, 2016 (figure 1). The trial completed with 180-day follow-up data available for analysis from 246 of 249 participants in the alteplase group and 245 of 251 participants in the placebo group. Admission demographics and clinical severity factors are shown in table 1 and were similar between groups. Overall, participants arrived at hospital within mean 1·5 h (IQR 0·8–3·5) of ictus and underwent a CT by 2·3 h (1·4—4·9). Intracerebral haemorrhage clot location was in the thalamus in 293 (59%), other in 161 (32%), and primary intraventricular haemorrhage (no identifiable intracerebral haemorrhage) in 46 (9%). The median baseline intra-ventricular haemorrhage size was 21·8 mL (IQR 12·7–36·8) and intracerebral haemorrhage size was 7·9 mL (2·5–15·0). Baseline mean arterial pressure and intracranial pressure were similar by group. Participants received the first extraventricular drain at 7·5 h (IQR 5·0–12·0) and bleeding was stable by 43·5 h (26·9–57·9) post ictus.
Figure 1: Trial profile.
In the alteplase group, one participant who was initially thought to be lost to follow-up at day 30 was located and assessed at day 180.
Table 1:
Demographic and baseline participant characteristics by group
| Alteplase (n=249) | Saline (n=251) | |
|---|---|---|
| Demographic variables | ||
| Age (years) | 59 (51–66) | 59 (51–67) |
| Women | 105 (42%) | 117 (47%) |
| Ethnic origin | ||
| White | 144 (58%) | 161 (64%) |
| African American | 92 (37%) | 78 (31%) |
| American Indian or Alaskan Native | 0 | 1 (<1%) |
| Other | 13 (5%) | 11 (4%) |
| Ethnicity: Hispanic/Latino | 28 (11%) | 32 (13%) |
| Baseline variables | ||
| Tobacco use | 73 (29%) | 59 (24%) |
| Cocaine use | 12 (5%) | 18 (7%) |
| Anticoagulated at registration | 20 (8%) | 29 (12%) |
| Antihypertensive medication compliant (self-report) | 168 (67%) | 202 (80%) |
| Hyperlipidaemia medication compliant (self-report) | 240 (96%) | 245 (98%) |
| On antiplatelet at registration | 56 (22%) | 72 (29%) |
| Randomisation MAP | 96 (86–106) | 94 (86–104) |
| Randomisation GCS* total | 10 (7–13) | 9 (7–12) |
| Randomisation NIHSS | 19 (11–32, n=231) | 20 (11–35, n=232) |
| Stability CT (last CT before enrolment) | ||
| IVH volume (mL) | 21·2 (12·7–34·2) | 22·4 (12·7–39·1) |
| ICH volume (mL) | 8·3 (2·9–15·2) | 7·2 (2·3–14·7) |
| Index clot location | ||
| Thalamus | 149 (60%) | 144 (57%) |
| Primary IVH | 19 (8%) | 27 (11%) |
| Ictus to hospital arrival (h) | 1·5 (0·8–3·3) | 1·5 (0·8–3·6) |
| Ictus to first CT (h) | 2·3 (1·3–4·6) | 2·3 (14–5·2) |
| Ictus to first EVD (h) | 7·1 (4·5–11·9) | 7·9 (5–1–12–0) |
| Ictus to stability CT (h) | 43·1 (25·4– 58·8) | 44–0 (28–2–57–0) |
| Ictus to randomisation (h) | 51·8 (36·4– 65·8) | 52–2 (41–2-66–8) |
Data are median (IQR) or n (%).
Scores on the Glasgow Coma Scale (GCS) range from 15 (fully conscious) to 3 (deep coma). MAP=mean arterial pressure. GCS=Glasgow Coma Scale. NIHSS=NIH Stroke Scale. IVH=ventricular haemorrhage. ICH= intracerebral haemorrhage. EVD=extraventricular drain.
Randomisation occurred at 52·1 h (IQR 39·1–66·5), with first treatment given 3·0 h (1·7—5·5) later. Five (IQR 3–8) doses of alteplase and 12 (9–12) doses of saline were given. EOT occurred at 2·5 days (1·8–3·7) post randomisation for alteplase and 4·7 days (4·0–5·1) for saline. In the alteplase group, the 3rd and 4th ventricles opened more rapidly than in the saline group (p<0–0001). 106 (21%) of all participants achieved 80% removal of intraventricular haemorrhage. Overall, 137 (27%) participants received two extraventricular drains (dual catheters), one in each lateral ventricle. During treatment, most participants (78%) had at least one intracranial pressure reading ≤20 mm Hg. Cerebral perfusion pressure less than 70 mm Hg occurred in 310 (62%) participants despite continuous extraventricular drain drainage; the proportion of cerebral perfusion pressure compromise was 2% less in the alteplase group (table 2). Permanent ventriculo-peritoneal shunts were placed in 90 participants (18%).
Table 2:
Treatment variables by group
| Alteplase (N=249) | Saline (N=251) | p value | |
|---|---|---|---|
| Randomisation to first dose (h) | 3·0 (1·7–5·3) | 3·1 (1·7–5·7) | 0·62 |
| Total number of doses | 5 (3–8) | 12 (9–12) | <0·0001 |
| Duration of dosing (days) | 1 (1–2) | 4 (3–4] | <0·0001 |
| Randomisation to EOT (days) | 2·5 (1·8–3·7) | 4·7 (4·0–5·1) | <0·0001 |
| EOT IVH volume (mL) | 5·9 (1·9–13·0) | 11·5 (5·8– 23·1) | <0·0001 |
| Time to open ventricles (days) | 2 (2–3) | 5 (3–7) | <0·0001 |
| ICP ≥20 mm Hg: mean proportion of events mean of patient-specific proportions) |
9·8 | 10·2 | 0·45 |
| CPP <70 mm Hg | 644 (7%) | 867 (9%) | <0·0001 |
| One or more ICP therapies | 67 (27%) | 77 (31%) | 0·35 |
| Dual EVD placed | 66 (27%) | 71 (28%) | 0·66 |
| Day 0-180 bacterial ventriculitis | 17 (7%) | 31 (12%) | 0·05 |
| Symptomatic bleeding ≤72 h post last dose | 6 (2%) | 5 (2%) | 0·77 |
| Day 0-180 serious adverse events | 114 (46%) | 151 (60%) | 0·002 |
| Days in ICU | 14 (11–21) | 15 (12–22) | 0·10 |
| Withdrawal of care | 27 (11%) | 30 (12%) | 0·70 |
| Ventilator support | 184 (74%) | 192 (76%) | 0·50 |
| Pressor or inotrope use | 60 (24%) | 63 (25%) | 0·80 |
| Ventriculoperitoneal shunt | 46 (18%) | 44 (18%) | 0·78 |
Additional outcome variable data are included in the appendix. EOT=end of treatment. IVH=intraventricular haemorrhage. ICP=intracranial pressure. CPP=cerebral perfusion pressure. EVD=extraventricular drain. ICU=intensive care unit. Day 0=day of ictus.
Retention to day 180 was 98%. The primary ITT analysis comparing groups achieving mRS 3 or less was achieved by 117 (48%) in the alteplase group and 110 (45%) in the saline group (RR 1·06 [95% CI 0· 88–1· 28], p=0–554; figure 2; table 3). The difference in good outcome (alteplase–saline) adjusted for intraventricular haemorrhage size and thalamic intracerebral haemorrhage was 3· 5% (RR 1·08 [95% CI 0·90–1·29], p=0–420), not significantly different from zero. One participant (<1%) received alteplase after completion of 12 doses of saline (crossover). A subsequent sensitivity analysis was done with this participant moved to the active treatment group. The primary results did not change.
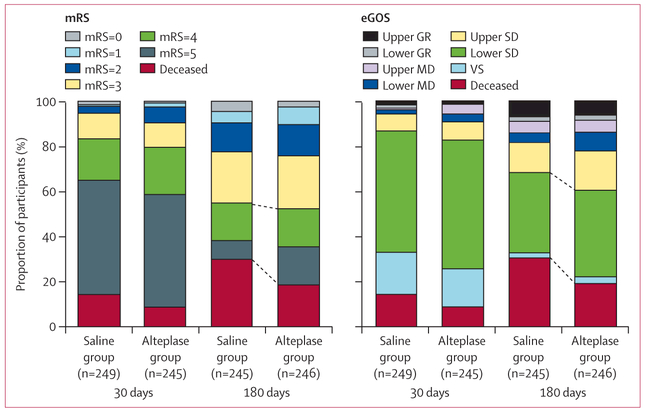
Figure 2: Outcome dichotomies of mRS (left panel; scores range from 0 [no disability] to 6 [death]) and eGOS (right panel; scores range from 8 [upper good recovery] to 1 [death]) scores at 30 days and 180 days by treatment.
The left panel blue lines indicate the differences in proportion of 180 day mRS ≤3 (112 [45%] in the saline group vs 118 [48%] in the alteplase group; p=0·477) and deceased participants (73 [30%] in the saline group vs 46 [19%] in the alteplase group; p=0O04). mRS=modified Rankin Scale. eGOS=extended Glasgow Outcome Scale. GR=good recovery. MD=moderate disability. SD=severe disability. VS=vegetative state.
Table 3:
mRS score frequencies for the 30-day and 180-day timepoints
| 30 days |
180 days |
|||
|---|---|---|---|---|
| Saline (n=249) | Alteplase (n=245) | Saline (n=245) | Alteplase (n=246) | |
| 0 | 4 (2%) | 2 (1%) | 11 (4%) | 6 (2%) |
| 1 | 2 (1%) | 4 (2%) | 13 (5%) | 19 (8%) |
| 2 | 7 (3%) | 17 (7%) | 31 (13%) | 34 (14%) |
| 3 | 28 (11%) | 27 (11%) | 55 (22%) | 58 (24%) |
| 4 | 46 (18%) | 51 (21%) | 41 (17%) | 41 (17%) |
| 5 | 126 (51%) | 122 (50%) | 21 (9%) | 42 (17%) |
| 6 | 36 (14%) | 22 (9%) | 73 (30%) | 46 (19%) |
The 180-day data represent the primary, unadjusted outcome. Corresponding eGOS data for the same timepoints can be seen in the appendix (p 24). In the alteplase group, one participant who was initially thought to be lost to follow-up at day 30 was located and assessed at day 180. mRS=modified Rankin Scale.
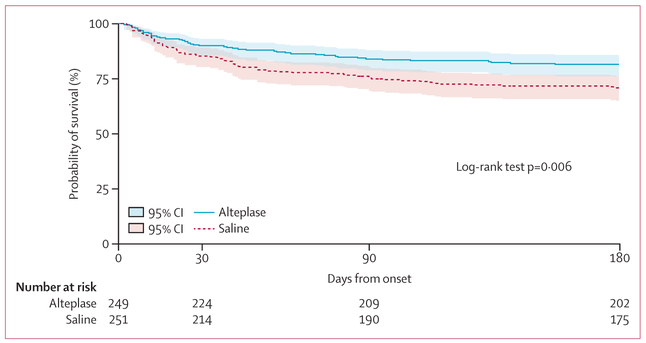
There was a 50% decrease in the odds of being dead (mRS 6) for alteplase versus saline (adjusted OR 0·50 [95% CI 0·31–0·80], p=0–004). No effect of hospital site was shown for the primary mRS 0–3 outcome (appendix p 19). Estimated Kaplan-Meier survival probabilities were greater throughout 180 days of follow-up for the alteplase group than for the saline group (cumulative case fatality: 46 [18%] vs 73 [29%]; p=0–006; figure 3). Safety parameters favoured alteplase: bacterial ventriculitis (17 [7%] vs 31 [12%]; RR 0·55 [95% CI 0·31–0·97], p=0–048) and serious adverse events (114 [46%] vs 151 [60%]; RR 0·76 [95% CI 0· 64–0· 90]; p=0–002). The frequency of symptomatic bleeding was similar between groups (six in the alteplase group [2%] and five [2%] in the saline group; RR 1·21 [95% CI 0·37–3·91], p=0–771). Appendix (p 19) shows the primary, secondary and safety outcomes. Table 4 shows the safety profile by group and by the Medical Dictionary for Regulatory Activities (MedDRA) body system classifications. Fewer neurological, respiratory, and sudden deaths (found in the MedDRA general disorders classification) were noted in the alteplase group than in the saline group. This is consistent with the hypothesis that early removal of blood corrects a severe life threatening cerebral anatomic defect and possibly limits the structural brain injury as well as limits the effects of immobility on cardiorespiratory risks inherent with structural brain injury.
Figure 3: Kaplan-Meier survival estimates from day of randomisation to observed day of death with truncation at 193 days for late and missed 180-day visits (n=36), which corresponds to the longest in-window 180-day visit.
Estimated survival probabilities were higher throughout 180 days of follow-up with alteplase compared with saline (p=0·006). Shading shows 95% CI.
Table 4:
Serious adverse events by treatment group
| Alteplase (N=249) | Saline (N=251) | |
|---|---|---|
| Blood and lymphatic disorders | 1 (<1%) | 0 |
| Cardiac disorders | 6 (2%) | 14 (6%) |
| Gastrointestinal disorders | 4 (2%) | 2 (1%) |
| General disorders and administration site conditions | 17 (7%) | 33 (13%) |
| Hepatobiliary disorders | 1 (<1%) | 0 |
| Infections, non-neurological | 9 (4%) | 7 (3%) |
| Injury, poisoning, and procedural complication | 2 (1%) | 0 |
| Investigations (laboratory) | 0 | 1 (<1%) |
| Metabolism and nutrition disorders | 0 | 1 (<1%) |
| Musculoskeletal and connective tissue disorders | 0 | 2 (1%) |
| Nervous system disorders | 39 (16%) | 50 (20%) |
| Psychiatric disorders | 1 (<1%) | 1 (<1%) |
| Renal and urinary disorders | 3 (1%) | 2 (1%) |
| Respiratory, thoracic, and mediastinal disorders | 26 (10%) | 34 (14%) |
| Surgical and medical procedures | 1 (<1%) | 0 |
| Vascular disorders | 4 (2%) | 4 (2%) |
A post-hoc analysis showed a greater proportion of patients with mRS 5 (ie, bedbound) in the alteplase group than in the saline group (42 [17%] vs 21 [9%]; RR 1·99 (95% CI 1· 22–3· 26), p=0–007). Other post-hoc analyses showed no difference in the proportion of participants in a vegetative state, measured by the eGOS scale (eight [3%] in the alteplase group and six [3%] in the saline group; RR 1–33 [95% CI 0·47–3·78]; p=0–787), nor for participants surviving in long-term care facilities (34 [14%] in the alteplase group vs 29 [12%] in the saline group; RR 1·18 [95% CI 0·74–1·88], p=0A79; figure 2; appendix p 24). Neither the Barthel index nor the EuroQol Visual Analog Scale (EQ-VAS; self-reported, quality of life scale with scores ranging from 0 [worst] to 100 [best] imaginable health state) differed between groups (appendix p 19).
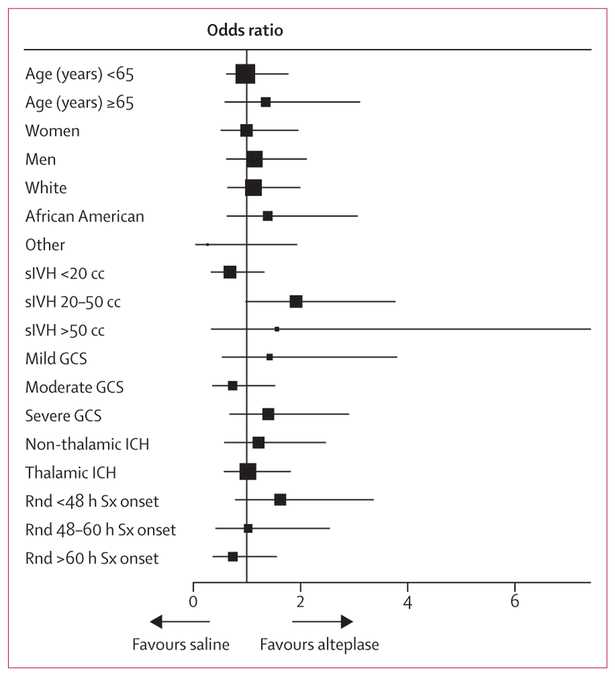
Removal of clot varied widely, dependent on number and location of extraventricular drains and number of alteplase doses. 82 participants (33%) in the alteplase group and 24 participants (10%) in the saline group achieved the 80% removal endpoint. The planned secondary analysis relating mRS score to the amount and timing of clot removal in all participants showed a significant relation between clot removal (per clot remaining [mL] as measured by normalised AUC) and both mRS ≤3 (adjusted OR 0·96 [95% CI 0·94–0·97]; p<0–0001) and case fatality (adjusted HR of death per mL of time-weighted clot volume remaining 1·03 [95% CI 1·02–1·04]; p<0–0001), adjusted for age, thalamic intracerebral haemorrhage location, stability intracerebral haemorrhage volume, and randomised GCS (appendix p 24). The results of the subgroup analyses prespecified in the protocol are shown in figure 4. No p values for interaction were significant.
Figure 4: Subgroup analysis.
Forest plot of interaction terms, adjusted for age, sex, thalamic intracerebral haemorrhage location, stability intracerebral haemorrhage, intraventricular haemorrhage volume, and GCS (mild, 13–15; moderate, 9–12; severe, 3–8) at admission. The size of points indicates the relative sizes of the subgroups. Scores on the Glasgow Coma Scale (GCS) range from 15 (fully conscious) to 3 (deep coma). sIVH=stability intraventricular haemorrhage volume. ICH=intracerebral haemorrhage. Rnd Sx=randomised after symptom onset.
Discussion
In the CLEAR III trial, irrigation of the ventricles with alteplase via a routine extraventricular drain did not improve functional outcomes in patients with intraventricular haemorrhage. Analyses of our secondary outcome measures, 180 day case fatality, was significantly lower in the alteplase group; however, most of these survivors ended up with severe disability (ie, mRS 4 or 5 or eGOS lower and upper significant disability). Clot removal analyses showed an association between amount of removal and improved odds of mRS ≤3. Alteplase seemed safe when compared with saline. These findings suggest possible value to the concept of removing greater amounts of intraventricular haemorrhage volume. On the face of the current evidence, however, alteplase at the dose of 1 mg every 8 h cannot be recommended as an intervention to improve functional outcome in patients with intraventricular haemorrhage.
There are limitations to this trial. This was the first phase 3 trial of intraventricular haemorrhage thrombolysis and evidence-based standards for participant selection and treatment endpoint did not exist. Current guidelines do not mandate the use, number, or location of extraventricular drain catheters, which are important factors affecting the amount of intraventricular haemorrhage removed. Routine practice produced good adherence to extraventricular drain safety, and opening of the midline ventricles occurred but poor adherence to removal of more than 80% intraventricular haemorrhage; this lack of adherence could have limited the stringency of the test of our hypothesis. Not all severity factors are known, so imbalances in severity could have existed. For example, non-specific factors (eg, type and extent of intensive care unit care) could have differed between the treatment groups and affected outcomes. This does not seem likely, because participant severity, “withdrawal of care”, and intensive care unit care were similar between groups. CLEAR III was a small sample of current clinical practice taken from the most aggressive end of the treatment spectrum: those participants whose physicians used extraventricular drains and the control intervention represented an aggressive level of care not offered to every participant with intraventricular haemorrhage. The survival and good functional outcomes observed in the CLEAR III controls was greater than in our previous study6 or in the expected levels from the general population (where low frequencies of extraventricular drain use are coupled with very high reliance on medical care as the sole supportive intervention for intraventricular haemorrhage).2,3,6,7,9 Only convenience sample data exists for outcomes of medically managed patients; thus, our knowledge about risk and benefit for the intervention in the general population is restricted. Another possible limitation is that the CLEAR III sample might not represent a true general intraventricular haemorrhage population, rather a milder or more severe population. Assessments of the general population of intraventricular haemorrhage, concurrently performed, have demographics and severity factors matching CLEAR III. They show full population estimates of mortality (40–60%) and low good functional outcome (10–30%) suggesting less intense therapies might not produce as many benefits.2,5 Finally, the main outcome measure: mRS 0–3 versus mRS 4–6 proportion is only one measure of disability. Further research will be needed to clarify the divergent picture within the more severe disability segments of mRS and eGOS.22 If survival comes at the cost of living with unacceptable impairment, this or any treatment could be seen as limited in value.
Potential to improve practice is evident with the findings that cerebral perfusion pressure and intracranial pressure are not always controlled by a single extraventricular drain routinely placed into the anatomically largest pool of cerebrospinal fluid or least bloody site. This is a starting point to investigate multiple catheters where the clot is large, bilateral, trapping the ventricle, or creating a local mass effect. Placing a second catheter near or into the largest portion of the clot leads to greater and more rapid removal23 and possibly greater clinical benefit. The precise clinical definitions for the at-risk population will need to be tested in a surgically standardised trial setting.24 The signal of benefit from greater clot removal and the low percentage of participants achieving 80% removal raise the possibility that benefit of alteplase might be possible if greater clot removal could be achieved and, if it is achieved, more rapidly. As CLEAR III did not show improved rates of good functional recovery with alteplase rather than saline, future investigation will need improved surgical placement of catheters to achieve effective clot reduction more frequently and more rapidly. A possible solution is an adaptively designed, efficacy-to-effectiveness trial25 that shows a better clot removal protocol can be integrated into routine stroke care and tests for influence on function, disability, and case fatality.
Supplementary Material
Research in context
Evidence before this study
We searched the literature from Jan 1, 1950, until Nov 1, 2015, on PubMed with the terms: IVH, IVH AND ICH, IVH AND TPA, IVH AND thrombolytic, IVH AND cross-sectional, and intraventricular haemorrhage AND treatment. Search filters for “adult” and “human subjects” publications were applied and were restricted to English language publications. Meta-analysis of case series, one small multisite trial, and single-site convenience samples suggest mortality and perhaps functional impairment can be mitigated via enhanced clearance of the intraventricular haemorrhage through thrombolysis. Before and after the CLEAR III trial, when caring for patients with a small intracerebral haemorrhage and large intraventricular haemorrhage, clinicians have no class 1 evidence regarding the effectiveness of intraventricular haemorrhage thrombolysis with alteplase.
Added value of this study
CLEAR III is a randomised, double-blinded study designed to provide a test of the combination of extraventricular drainage and low dose alteplase as a method to remove intraventricular haemorrhage and improve functional outcomes. This multisite study is the first, to our knowledge, to prospectively collect several objective functional performance (modified Rankin Scale [mRS] and extended Glasgow Outcome Scale [eGOS]) as well as patient-based (Euro-QoL [EQ], Stroke Impact Scale [SIS]) measures of satisfaction. Medical care in the intensive care unit was standardised and assessed rigorously. Data were prospectively defined and collected in a uniform manner and monitored thoroughly providing evidence of type, intensity, and duration of intensive care unit care required. Precise measurement of intraventricular haemorrhage size occurred in this trial possibly improving estimates of severity and treatment performance. The results of CLEAR III provide a robust estimate of the proportion of patients with mRS 0–3 (about 45–48%) that occurs, if participants are supported until the extraventricular drain is no longer needed. This led to an adjusted (intraventricular haemorrhage size and thalamic location) estimate of treatment effect of 3·5% (95% CI −4 to 12). This effect size did not differ between alteplase and saline treatment plans, although case fatality did. The absolute proportion of patients with mRS 0–3 found in all CLEAR III participants compares favourably to the untreated participants in the literature. The study provides detailed evidence that a protocoled approach to remove intraventricular haemorrhage with alteplase is safe and that the 3rd and 4th ventricles open sooner if alteplase is used. A legitimate concern could be raised about greater infection rates due to frequent injections of alteplase or saline in the extraventricular drain; however, infection rates in CLEAR were in the same range as that reported in a meta-analysis of infections from published extraventricular drain series where injections were not given. The data shown characterise substantial variations in current extraventricular drain-related neurosurgical practice. They suggest usefulness of the measures of initial severity (Glasgow Coma Scale [GCS], location, and intracerebral haemorrhage/intraventricular haemorrhage size) and the elements of treatment needed for precise characterisation of prognosis in aggressively treated intraventricular haemorrhage patients. The subgroup analysis suggests increased focus on larger intraventricular haemorrhage and earlier treatment times is appropriate.
Implications of all the available evidence
The trial was neutral on primary outcome of functional improvement. Therefore, we do not think practice should change. Other post-hoc results are consistent with the hypothesis that clot removal saves lives and possibly improves function. The issue of survival with disability is now well defined by our primary results and other measures of disability. The secondary results leave open a possible role of intraventricular haemorrhage volume reduction as a biomarker for treatment, with better outcomes more likely achieved with enhanced intraventricular haemorrhage clearance, particularly in patients with larger initial intraventricular haemorrhage volume. The results are consistent with previous convenience reports and meta-analyses showing that good functional outcome can occur in up to 50% of treated patients. The data provide a sound basis to critically redefine short-term neurosurgical and intensive care unit management around the task of volume removal. A trial testing these more objective goals is needed, if we are to be assured that aggressive removal of intraventricular haemorrhage is safe and can predictably produce increased independent function and decreased case fatality. Current information suggests as many as 25% of intracerebral haemorrhages have large intraventricular haemorrhage. How many of these patients would be eligible for treatment is not known. An estimate of the full benefit of intervention will require a combined epidemiological and RCT intervention approach that randomises available participants and collects information about usual care controls. A novel trial would provide a standardised surgical task, treat subjects more rapidly, and require greater care team adherence to the removal of large amounts of the intraventricular haemorrhage, not just removal of enough blood clot to open the 3rd and 4th ventricles. Sharing the full results of CLEAR III is likely to stimulate further investigations of a worldwide problem that is serious, growing, and could be treatable.
Acknowledgments
We thank the CLEAR III patients and their families and the CLEAR III investigators and coordinators who provided and cared for them. We thank the Johns Hopkins University Brain Injury Outcomes (BIOS) Coordinating Center staff and site managers Bing Cao, Amanda Bistran-Hall, Tracey Hartmann, and Noeleen Ostapkovich, the Reading Center readers, Timothy Morgan, Saman Nekoovaght-Tak, Hasan Ali, Vikram Madan, Alexandra Baker, and Krissia Rivera Perla, and Coordinating Center Pharmacists, Janet Mighty and Esther Jeon; the University of Chicago Surgical Center managers, Agnieszka Stadnik, Jennifer Jaffe, and Michael Jesselson; Emissary International’s Sarah Lenington, Nicki Karlen, and Carolyn Koenig for monitoring management; Prelude Dynamics for developing the data management and randomisation systems; the Newcastle Neurosurgical Foundation and Mawdsley-Brooks & Co (Salford, UK) for supporting the trial in the European Union; the University of Glasgow outcomes assessors and translators and Robertson Centre for Biostatistics programmers; the Johns Hopkins Bloomberg School of Public Health Biostatistics Department data managers, Rachel Dlugash, Malathi Ram, Gwendolyn Clemens, Andre Hackman, and Jennifer Houser, Ryan Majkowski and Maningbe Kieta for their project management and technical editing. We thank the NIH-appointed DSMB Bob S Carter (chairman), Kyra J Becker, James C Torner, and Alex B Valadka for their guidance and oversight of the safety of the trial participants. The DSMB approved changes to analysis plan and this publication. We also thank the US FDA Orphan Products Development program for supporting a high-risk idea that became a programme of investigation. CLEAR III was supported by the grant 5U01 NS062851-05, awarded to DFH from the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS). DFH was awarded research support of grants number R01NS046309, 5U01 NS062851-05, and 5U01 NS080824-02.
Declaration of interests
After funding of the trial by the US National Institutes of Health, Genentech provided drug at no cost. This is not a regulatory study sponsored by Genentech. There has been no other scientific input from other companies or entities. HA reports grants from Johns Hopkins University, during the conduct of the study. SA reports grants from National Institute of Neurological Disorders and Stroke, during the conduct of the study; personal fees from BARD MEDICAL, outside of the submitted work. IA reports grants from NIH/NINDS, during the conduct of the study. CT reports grants from National Institute of Neurological Disorders and Stroke, during the conduct of the study. BG reports grants from Johns Hopkins University (CLEAR III NIH grant), during the conduct of the study; grants from National Institute of Health Research (UK) HTA, outside the submitted work. DFH reports grants from NIH/NINDS, during the conduct of the study; grants and personal fees from EKOS Corp, grants and personal fees from Brainscope, personal fees from Rand Corp, personal fees from medico-legal, outside the submitted work. MH reports grants from NIH, during the conduct of the study. KLa reports grants from NINDS, during the conduct of the study. KLe reports personal fees from Boehringer Ingelheim, outside the submitted work. SM reports grants from NIH/NINDS, during the conduct of the study; personal fees from Johns Hopkins University, outside the submitted work. NM reports grants from National Institute of Neurological Disorders and Stroke, during the conduct of the study. ADM reports grants from NIH, during the conduct of the study; non-financial support from Newcastle Neurosurgery Foundation Ltd, personal fees from Stryker and Draeger, outside the submitted work. WAM reports grants from NINDS, during the conduct of the study. JM reports grants from T32AG000247 NIA Training Grant, during the conduct of the study. ES reports grants from NIH/NINDS, during the conduct of the study. GY reports grants from National Institute of Neurological Disorders and Stroke, during the conduct of the study; personal fees from The RAND Corporation, Health Advisory Services, outside the submitted work. WZ reports personal fees from HeadSense Medical Inc, outside the submitted work. OA, EFA, JB, KB, JC, JLC, JD, MD-W, DG, SH, JJ, SJa, SJo, CK, PK, DL, GLMR, RT, ST, NU, DW, and MZ declare no competing interests.
Funding National Institute of Neurological Disorders and Stroke.
Footnotes
Contributors
DFH and IAA organised the trial hypotheses, designed the trial, and provided guidance about the data analysis and interpretation and presentation of the data. DFH drafted most of the sections of the manuscript. KL, NMcB, WZ, ST, ADM, BG, KB, PV, DWW, PMK, MDW, SJo, SH, GL, EFA, MRH, SA, JJ, JLC, DL, OA, MZ, and HPA were involved in the design of the study and provided contributions to the writing and revising of the manuscript. KL, NMcB, and SWM organised and managed the trial including trial start-up, data collection, quality assurance, and trial close-out. KRL and JD provided the independent review and central adjudication of the modified Rankin Scale scores. DG, NU, and WAM provided the region of interest calculations for all volumetric measurement results. WZ, CSK, and JRC provided independent review and adjudication of all safety events. MR and RET generated the random allocation method. RET, JM, JFB, MR, CBT, EAS, and GY were involved in the statistical analysis and data interpretation, and contributed to the development and revisions to the manuscript. SJa provided critical review of the manuscript. The CLEAR III investigators contributed equally to the identification and, when eligible, randomisation of trial participants.
See Online for appendix
Contributor Information
Daniel F Hanley, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Karen Lane, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Nichol McBee, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Wendy Ziai, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Stanley Tuhrim, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kennedy R Lees, University of Glasgow, Glasgow, UK.
Jesse Dawson, University of Glasgow, Glasgow, UK.
Dheeraj Gandhi, University of Maryland, Baltimore, MD, USA.
Natalie Ullman, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
W Andrew Mould, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Steven W Mayo, Emissary International LLC, Austin, TX, USA.
A David Mendelow, Newcastle University, Newcastle upon Tyne, UK.
Barbara Gregson, Newcastle University, Newcastle upon Tyne, UK.
Kenneth Butcher, University of Alberta, Edmonton, AB, Canada.
Paul Vespa, University of California, Los Angeles, CA, USA.
David W Wright, Emory University, Atlanta, GA, USA.
Carlos S Kase, Boston University, Boston, MA, USA.
J Ricardo Carhuapoma, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Penelope M Keyl, Johns Hopkins University, School of Medicine, Brain Injury Outcomes Division, Baltimore, MD, USA.
Marie Diener-West, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
John Muschelli, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Joshua F Betz, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Carol B Thompson, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Elizabeth A Sugar, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Gayane Yenokyan, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Scott Janis, National Institutes of Health, National institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Sayona John, Rush University, Chicago, IL, USA.
Sagi Harnof, Chaim Sheba Medical Center, Ramat Gan, Israel.
George A Lopez, University of Texas, Houston, Houston, TX, USA.
E Francois Aldrich, University of Maryland, Baltimore, MD, USA.
Mark R Harrigan, University of Alabama at Birmingham, Birmingham, AL, USA.
Safdar Ansari, University of Utah, Salt Lake City, UT, USA.
Jack Jallo, Thomas Jefferson University Hospital, Philadelphia, PA, USA.
Jean-Louis Caron, University of Texas, San Antonio, San Antonio, TX, USA.
David LeDoux, North Shore Long Island Jewish Medical Center, Manhasset, NY, USA.
Opeolu Adeoye, University of Cincinnati, Cincinnati, OH, USA.
Mario Zuccarello, University of Cincinnati, Cincinnati, OH, USA.
Harold P Adams, Jr, University of Iowa, Iowa City, IA, USA.
Michael Rosenblum, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Richard E Thompson, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Issam A Awad, University of Chicago, Chicago, IL, USA.
References
- 1.Hanley DF. Intraventricular hemorrhage severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 2009; 40: 1533–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen BM, Morgan TC, Betz J, et al. Intraventricular extension of supratentorial intracerebral hemorrhage: the modified graeb scale improves outcome prediction in lund stroke register. Neuroepidemiology 2015; 46: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical Care Med 1999; 27: 617–21. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. New Engl J Med 2001; 344: 1450–60. [DOI] [PubMed] [Google Scholar]
- 5.Moradiya Y, Murthy SB, Newman-Toker DE, Hanley DF, Ziai WC. Intraventricular thrombolysis in intracerebral hemorrhage requiring ventriculostomy a decade-long real-world experience. Stroke 2014; 45: 2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naff N, Williams MA, Keyl PM, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage the intraventricular hemorrhage thrombolysis trial. Stroke 2011; 42: 3009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaberel T, Magheru C, Parienti J-J, Huttner HB, Vivien D, Emery E. Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage a meta-analysis. Stroke 2011; 42: 2776–81. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurology 2000; 247: 117–21. [DOI] [PubMed] [Google Scholar]
- 9.Staykov D, Bardutzky J, Huttner HB, Schwab S. Intraventricular fibrinolysis for intracerebral hemorrhage with severe ventricular involvement. Neurocritical Care 2011; 15: 194–209. [DOI] [PubMed] [Google Scholar]
- 10.Ziai WC, Muschelli J, Thompson CB, et al. Factors affecting clot lysis rates in patients with spontaneous intraventricular hemorrhage. Stroke 2012; 43: 1234–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley DF, Thompson RE, Muschelli J, et al. , for the MISTIE Investigators, Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol 2016; 15: 1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephson CB, White PM, Krishan A, Al-Shahi Salman R. Computed tomography angiography or magnetic resonance angiography for detection of intracranial vascular malformations in patients with intracerebral haemorrhage. Cochrane Database Syst Rev 2014; 9: CD009372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziai WC, Tuhrim S, Lane K, et al. A multicenter, randomized, double-binded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int J Stroke 2014; 9: 536–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31: 103–15. [PubMed] [Google Scholar]
- 15.Weir CJ, Lees KR. Comparison of stratification and adaptive methods for treatment allocation in an acute stroke clinical trial. Stats Med 2003; 22: 705–26. [DOI] [PubMed] [Google Scholar]
- 16.Morgenstern L, Hemphill J 3rd, Anderson C, et al. American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010; 41: 2108–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broderick J, Connolly S, Feldmann E, et al. American Stroke Association Stroke Council; American Heart Association/American Stroke Association High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American heart association/American stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Circulation 2007; 116: e391–13. [DOI] [PubMed] [Google Scholar]
- 18.Mould WA, Carhuapoma JR, Muschelli J, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 2013; 44: 627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan TC, Dawson J, Spengler D, et al. The modified Graeb score an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke 2013; 44: 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efron B Better bootstrap confidence intervals. J Am Stat Assoc 1987; 82: 171–85. [Google Scholar]
- 21.Mattle HP, Raabe A. CLEAR Intraventricular Hemorrhage More Than a Glimmer of Hope. Stroke 2011; 42: 2999–3000. [DOI] [PubMed] [Google Scholar]
- 22.Teasdale GM, Pettigrew Le, Wilson Jl, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma 1998; 15: 587–97 [DOI] [PubMed] [Google Scholar]
- 23.Hinson HE, Melnychuk E, Muschelli J, Hanley DF, Awad IA, Ziai WC. Drainage efficiency with dual versus single catheters in severe intraventricular hemorrhage. Neurocritical Care 2012; 16: 399–405. [DOI] [PubMed] [Google Scholar]
- 24.Vespa PM, Martin N, Zuccarello M, Awad I, Hanley DF. Surgical trials in intracerebral hemorrhage. Stroke 2013; 44: S79–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selker HP, Oye KA, Eichler HG, et al. A proposal for integrated efficacy-to-effectiveness (E2E) clinical trials. Clinical Pharmacology Therapeutics 2014; 95: 147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.