Extracellular ATP signaling, mediated by the P2K1 receptor, directly changes the stability of JAZ1 protein in JA signaling to boost plant defense response.
Abstract
Damaged cells send various signals to stimulate defense responses. Recent identification and genetic studies of the plant purinoceptor, P2K1 (also known as DORN1), have demonstrated that extracellular ATP is a signal involved in plant stress responses, including wounding, perhaps to evoke plant defense. However, it remains largely unknown how extracellular ATP induces plant defense responses. Here, we demonstrate that extracellular ATP induces plant defense mediated through activation of the intracellular signaling of jasmonate (JA), a well-characterized defense hormone. In Arabidopsis (Arabidopsis thaliana) leaves, ATP pretreatment induced resistance against the necrotrophic fungus, Botrytis cinerea. The induced resistance was enhanced in the P2K1 receptor overexpression line, but reduced in the receptor mutant, dorn1-3. Mining the transcriptome data revealed that ATP induces a set of JA-induced genes. In addition, the P2K1-associated coexpression network contains defense-related genes, including those encoding jasmonate ZIM-domain (JAZ) proteins, which play key roles as repressors of JA signaling. We examined whether extracellular ATP impacts the stability of JAZ1 in Arabidopsis. The results showed that the JAZ1 stability decreased in response to ATP addition in a proteasome-dependent manner. This reduction required intracellular signaling via second messengers—cytosolic calcium, reactive oxygen species, and nitric oxide. Interestingly, the ATP-induced JAZ1 degradation was attenuated in the JA receptor mutant, coi1, but not in the JA biosynthesis mutant, aos, or upon addition of JA biosynthesis inhibitors. Immunoprecipitation analysis demonstrated that ATP increases the interaction between COI1 and JAZ1, suggesting direct cross talk between extracellular ATP and JA in intracellular signaling events. Taken together, these results suggest that extracellular ATP signaling directly impacts the JA signaling pathway to maximize plant defense responses.
Defense responses are initiated after the detection of a life-threatening event through the recognition of exogenous signals from various environmental changes and endogenous signals from damaged cells as danger signals. Endogenous danger signals are referred to as “damage-associated molecular patterns” (DAMPs). Many DAMPs in animals and plants are nuclear or cytosolic proteins and nonproteinaceous molecules with defined intracellular functions (Rubartelli and Lotze, 2007; Tanaka et al., 2014).
ATP—which typically serves as a universal intracellular energy currency, a genetic building block, and the phosphodonor substrate for various signal transduction pathways—becomes a DAMP signal after release into the extracellular milieu upon cellular damage. The role of extracellular ATP as a signal was first reported in the 1970s in plants and animals (Burnstock, 1972; Jaffe, 1973). Extracellular ATP is recognized at the cell surface by purinoceptors, evoking immune responses and damage healing. In animals, the two types of purinoceptors, ligand-gated channel P2X and G-protein coupled receptor P2Y, recognize extracellular ATP to activate intracellular signaling cascades, which have been well studied to develop therapeutic agents to dampen pathological inflammation and to control stress responses (Khakh and Burnstock, 2009). In plants, early studies identified the essential extracellular roles for ATP in plant growth, development, and stress responses (Foresi et al., 2007; Tanaka et al., 2010a; Sueldo et al., 2010; Chivasa and Slabas, 2012; Clark et al., 2014). The ATP-insensitive mutant, does not respond to nucleotide1 (dorn1), was recently identified. The dorn1 locus encodes the first plant purinoceptor, referred to as P2K1 (K is for kinase), which, unlike the purinoceptors in animal systems, is a lectin-receptor Ser/Thr kinase (Choi et al., 2014). Detailed analysis of the ATP-induced transcriptome in the Arabidopsis roots suggested that P2K1-mediated ATP signaling is involved in a range of plant defense responses (Cao et al., 2014). However, the downstream signaling pathway, after the reception of extracellular ATP through the P2K1 receptor, remains unknown.
A dozen studies showed that extracellular ATP signaling is associated with second messengers—cytosolic calcium (Ca2+), reactive oxygen species (ROS), and nitric oxide (NO; Demidchik et al., 2003; Jeter et al., 2004; Song et al., 2006; Foresi et al., 2007; Wu and Wu, 2008; Reichler et al., 2009; Tanaka et al., 2010b; Tonón et al., 2010; Clark et al., 2011; Sun et al., 2012; Lim et al., 2014). Some of them are suggested to be mediated by cellular components in the plasma membrane, including Ca2+ channels, heterotrimeric G Proteins, and NADPH oxidase (Song et al., 2006; Demidchik et al., 2009; Weerasinghe et al., 2009; Tanaka et al., 2010b; Hao et al., 2012; Wang et al., 2014). Recent studies also showed that phosphatidic acid formation and extracellular alkalinization are provoked during extracellular ATP signaling (Sueldo et al., 2010; Moroz et al., 2017). These signal transductions play essential roles in extracellular ATP-induced physiological responses including plant defense.
Plant defense is mediated through the coordinated activity of several stress hormones, generally through jasmonates (JAs), salicylates (SAs), and ethylene. The mode of action of these stress hormones has been well studied in plant biotic stress responses. JAs are primarily induced during necrotrophic pathogen attacks and chewing insect herbivores (Reymond and Farmer, 1998). The induced JAs activate specific intracellular signaling and eventually induce a set of defense-related genes against pathogens and chewing insects. Jasmonate ZIM-domain (JAZ) proteins, key negative regulators of JA signaling, block the activity of JA-specific master transcription factors, e.g. MYC2 (Chini et al., 2007). Once the JA receptor, coronatine-insensitive1 (COI1), binds to JA, JAZ proteins are degraded through an ubiquitination system (Thines et al., 2007); this permits transcription factors to activate downstream target genes in JA signaling.
Wounding and mechanical pressure, which elevate endogenous JA levels (Creelman et al., 1992, Koo and Howe, 2009; Chehab et al., 2012), also trigger the release of ATP (Weerasinghe et al., 2009; Jeter et al., 2004; Dark et al., 2011), which likely provokes a defense response against pathogens (Tanaka et al., 2014). It was reported that ATP application induces the expression of JA and ethylene biosynthesis genes (Jeter et al., 2004; Song et al., 2006). Moreover, ATP treatment of wounded tissue mimics a JA-dependent defense response, resulting in the secretion of extrafloral nectar in the lima bean (Phaseolus lunatus) to attract predators (Heil et al., 2012). Notably, approximately 60% of ATP-induced genes were also up-regulated through wounding (Choi et al., 2014), indicating that extracellular ATP induces the up-regulation of an array of genes involved in damage-related responses. Taken together, these results suggested that the release of ATP after cellular damage induces plant defense responses, some of which are activated via other stress hormones.
We hypothesized that ATP induces plant defenses via other stress hormone-mediated responses, e.g. JA. In this study, we investigated the role for ATP in plant defense responses mediated through JA signaling. ATP treatment enhanced resistance against infection by the necrotrophic fungus, Botrytis cinerea, which causes major pre- and postharvest diseases in numerous agronomic and horticultural crops, inflicting hundreds of millions of dollars in lost revenue. This resistance required the functional P2K1 receptor. A search of the public database revealed a set of genes induced through both ATP and JA, reflecting the observed ATP-induced pathogen resistance. We also show that ATP plays a role in the JA-induced defense response through the direct activation of JA signaling via second messengers, Ca2+, ROS, and NO. These data indicate that extracellular ATP enhances plant defense against pathogens through the direct activation of JA signaling.
RESULTS
A Set of ATP Up-Regulated Genes Are Induced by JA
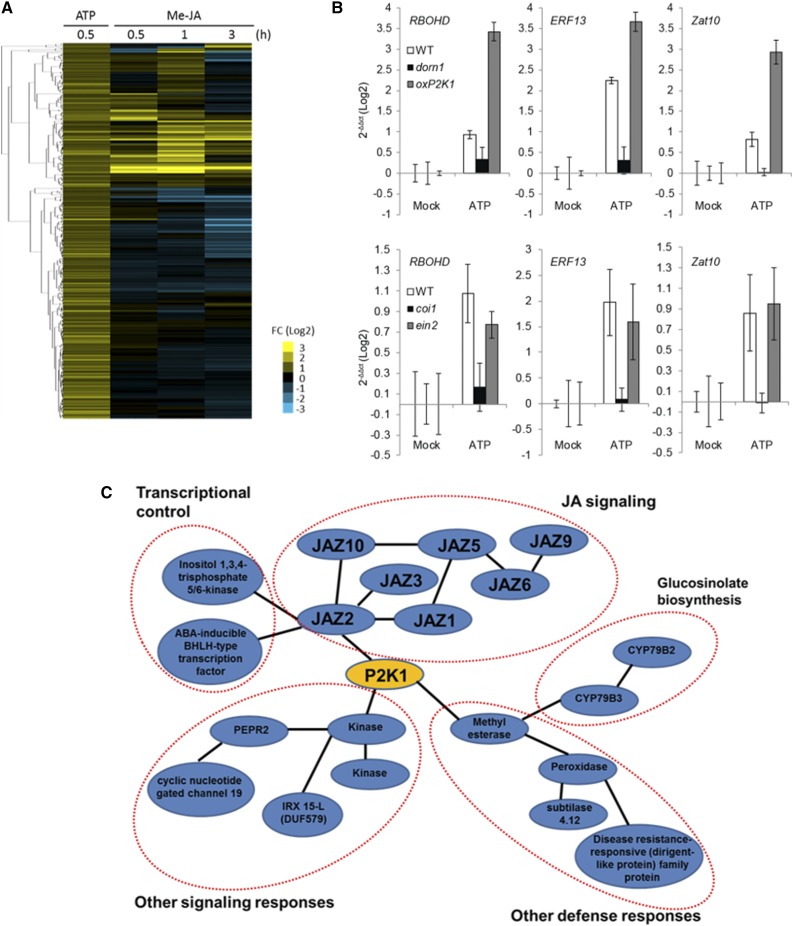
Detailed analysis of previous microarray data suggested that a number of extracellular ATP-induced genes are involved in plant responses to a variety of stresses, including defense-related genes (Cao et al., 2014; Supplemental Fig. S1). Hierarchical clustering analysis of ATP-induced genes (Choi et al., 2014) with methyl jasmonic acid (MeJA)-responsive genes (Kilian et al., 2007) showed that approximately 30% of ATP-induced genes respond to MeJA, suggesting that extracellular ATP activates a portion of the JA-responsive pathway (Fig. 1A). Among these genes (Supplemental Table S1), we selected RBOHD, ERF13, and ZAT10 as representative JA-responsive genes (Goda et al., 2008; Schweizer et al., 2013) and tested those responses upon ATP treatment in wild type, dorn1-3, oxP2K1, JA receptor mutant (coi1), and ethylene signaling mutant (ein2). The results showed that induction of JA-responsive genes by ATP required a functional JA-COI1-signaling pathway (Fig. 1B). In addition, transcriptome coexpression analysis using ATTED-II (Obayashi et al., 2011) indicated that the P2K1-associated coexpression network includes several defense-related genes, including JAZ genes (Fig. 1C). Based on these results, we hypothesized that ATP modulates the JA-dependent signaling pathway and more specifically with JAZ proteins to induce defense responses.
Figure 1.
ATP and JA induce a common set of genes. A, Hierarchical clustering of ATP up-regulated genes (log2-fold change > 0.58; Choi et al., 2014) in comparison to the profile of gene expression after MeJA treatment (log2-fold change > 1.0; Kilian et al., 2007). Note that the heatmap shows that approximately 30% of ATP up-regulated genes are induced by MeJA. Heatmap colors represent relative gene expression as indicated in the color bar of Log2-fold change. B, Gene expression of genes coregulated by ATP and JA in wild-type, dorn1-3, oxP2K1, coi1, and ein2 mutant seedlings. The seedlings were treated with 100 μM ATP for 30 min followed by real-time PCR analysis. Histograms show means with se as Log2 values relative to those of untreated controls. C, Transcriptome coexpression analysis for P2K1 (At5g60300) were performed using ATTED-II. Note that the P2K1-associated network shows a significant coexpression relationship with JAZ proteins that are involved in JA signaling. FC, fold change; Mock, untreated controls.
We also performed another hierarchical clustering analysis of ATP-induced genes in comparison to ethylene- and SA-inducible genes in addition to JA-inducible genes (Supplemental Fig. S2). The result suggested that a number of ATP-induced genes was up-regulated by SA and ethylene, in addition to JA, although some were down-regulated and vice versa. Interestingly, for example, SA-inducible genes (e.g. WRKY46, SARD1, and PAD4) were included as ATP up-regulated genes. The results demonstrate a potential role for extracellular ATP in mediating the plant responses not only via the JA pathway but also via the SA pathway. Collectively, the data suggest a central role for extracellular ATP in mediating the plant defense responses via typical stress hormones; i.e. JA, SA, and ethylene.
Extracellular ATP Confers Resistance to a Necrotrophic Fungus, B. cinerea
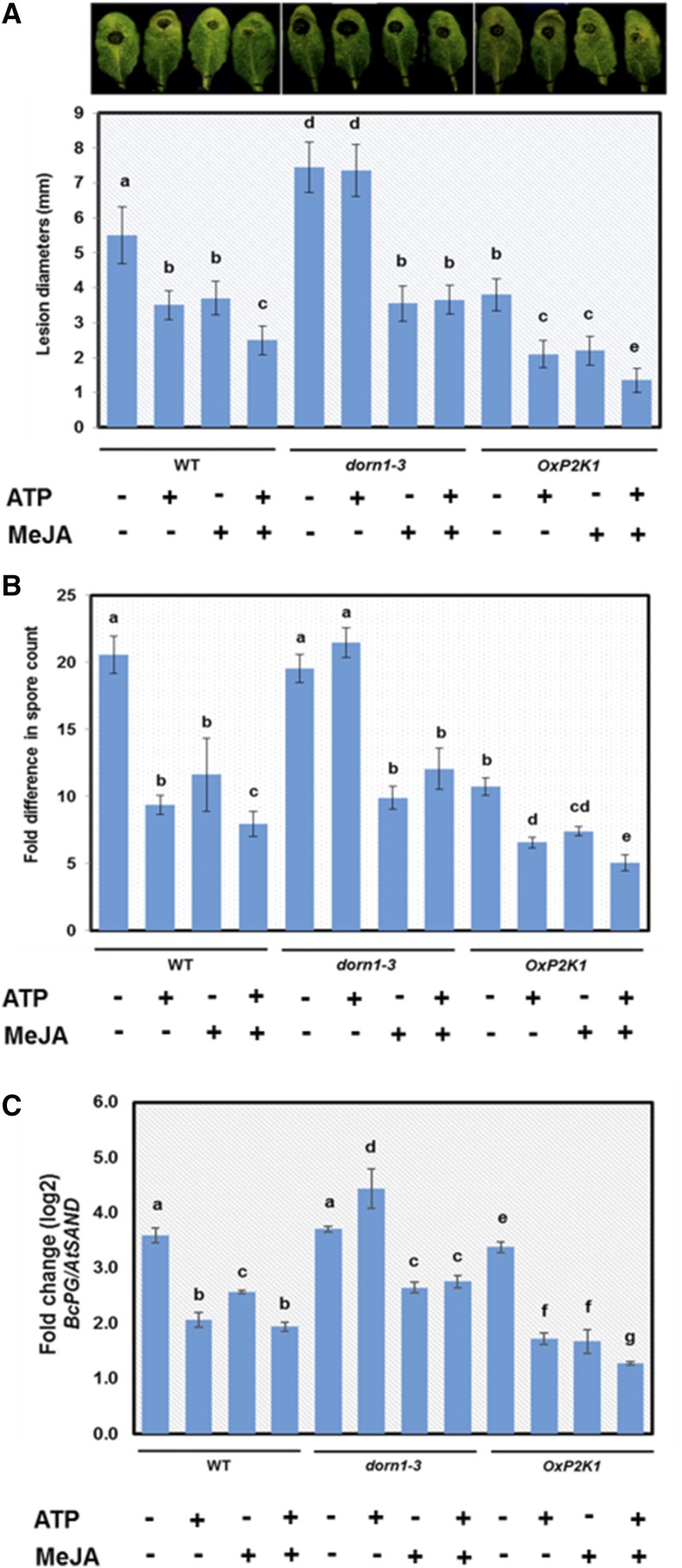
To examine whether extracellular ATP induces plant defense against biotic stresses, we used B. cinerea as this necrotrophic fungus has a broad host range and is a well-studied pathogen that induces jasmonate-dependent defense responses (Thomma et al., 1998). The leaves from 4-week-old Arabidopsis (Arabidopsis thaliana) plants of wild-type, dorn1-3, and OxP2K1 were pretreated with ATP and/or MeJA through needle-free syringe infiltration, and subsequently challenged with the pathogen (see “Materials and Methods”). Necrotic lesions were visible at 2 d after inoculation. The lesion diameters and spore numbers were measured to determine the extent of infection. Leaves treated with ATP showed reduced pathogen infection compared with mock treatment in wild-type and OxP2K1 plants, whereas this ATP-induced resistance was not observed in dorn1-3 mutant plants (Fig. 2A). The level of infection in OxP2K1 plants was markedly reduced compared with that in wild type. These data suggested that ATP-induced defense against B. cinerea is mediated through P2K1. Most notably, a coapplication of MeJA and ATP further enhanced the observed resistance compared with the single application of either ATP or JA (Fig. 2, A–D). Taken together, these results suggested that ATP synergistically induces plant defense with JA. Interestingly, JA induced resistance in dorn1-3 mutant plants in the same manner as that in wild-type plants, suggesting that the JA-induced defense response is not dependent on ATP signaling.
Figure 2.
Extracellular ATP-induced resistance against the necrotrophic fungus B. cinerea. Wild-type, dorn1-3, and OxP2K1 plants were infiltrated with ATP (500 μM) and/or MeJA (10 μM) followed by inoculation with B. cinerea (5 × 105 spores/mL). Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05). A, Pictures show infected leaves of wild type, dorn1-3, and OxP2K1 at 2-d postinoculation. Graph shows the lesion diameters induced by B. cinerea infection. Data are shown as means ± sd of three biological replicates (n = 10 leaves per treatment). B, Graph shows the fold change in the numbers of in planta-formed spores of B. cinerea 3-d postinoculation. Data are shown as means ± sd of three biological replicates (n = 15 leaves per treatment). C, Quantification of B. cinerea by real-time PCR was performed with DNA extracted from infected leaves of wild type (WT), dorn1-3, and OxP2K1. The data show expression values of B. cinerea gene BcPG relative to Arabidopsis reference gene AtSAND as means ± sd of three biological replicates (n = 6 leaves per treatment).
Extracellular ATP Reduces JAZ1 Protein Stability through the SCFCOI1-Proteasome Pathway
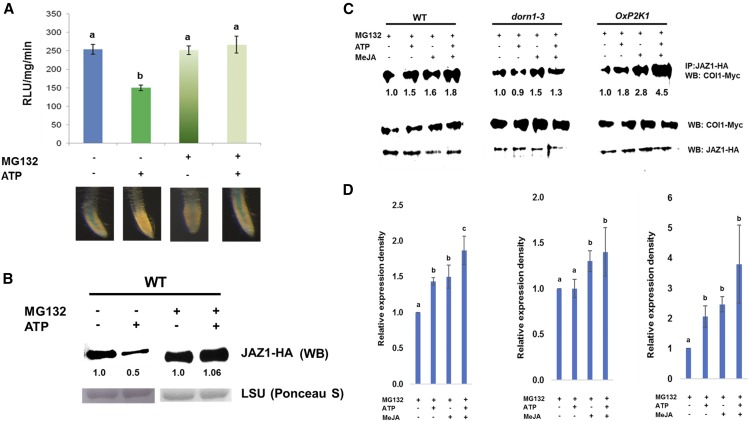
The results described above prompted us to examine whether extracellular ATP signaling via P2K1 influences JA signaling. JAZ proteins play a key role in jasmonate signaling, acting as signaling repressors, and these proteins are degraded through the SCFCOI1-proteasome pathway when JA signaling is activated (Chini et al., 2007; Thines et al., 2007). Therefore, we measured the stability of JAZ proteins to evaluate the effect of extracellular ATP on JA signaling. To monitor the protein stability of JAZ proteins, we used Arabidopsis plants expressing JAZ1 translationally fused to the GUS reporter (35S::JAZ1-GUS; Thines et al., 2007). First, we tested the effect of various concentrations of ATP on the stability of the JAZ1 protein. ATP significantly reduced JAZ1 stability from 100 µM of ATP (Supplemental Fig. S3). The reduction was saturated after 500 µM of ATP (Supplemental Fig. S3). The results were comparable to those in the previous reports, i.e. the dose-dependent kinetics of ATP-induced calcium response and ATP-induced reduction of PR gene expressions (Chivasa et al., 2009; Tanaka et al., 2010b). We decided to use 500 µM of ATP for further studies as it induced maximum response in reducing the stability of the JAZ1 protein. The application of ATP reduced the stability of JAZ1, which was attenuated by pretreatment with MG132, a 26S proteasome inhibitor (Fig. 3, A and B; Supplemental Fig. S4). This result suggests that the observed negative effects of extracellular ATP on B. cinerea pathogenesis could be explained by ATP-induced JA signaling. Consistent with the result in Figure 2, coapplication of MeJA and ATP further induced JAZ1 protein degradation in comparison with addition of ATP or JA alone (Supplemental Fig. S5).
Figure 3.
Effect of extracellular ATP on the stability of the JAZ1 protein. A, Seedlings expressing 35S::JAZ1-GUS were treated with ATP (500 μM) for 30 min in the presence or absence of pretreatment of MG132 (10 μM), a proteasome inhibitor. Top panel shows the data with means ± sd (n = 6) of fluorescence-based GUS biochemical assay to measure the JAZ1 protein level upon ATP addition. Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05). Lower panel shows GUS histochemical assay in roots. B, The protoplasts expressing JAZ1-HA were treated with 500 μM of ATP for 30 min in the presence or absence of 10 μM of MG132. Western blot (WB) was performed using anti-HA antibody as shown as JAZ1-HA. Rubisco large subunit (LSU) detected by Ponceau S staining represents as a loading control. Numbers below the blot show the expression of proteins relative to mock (i.e. non-ATP-treatment). C, Coimmunoprecipitation shows the interaction between COI1 and JAZ1 in wild type (WT), dorn1-3, and OxP2K1. The protoplasts coexpressing COI1-Myc and JAZ1-HA proteins were treated with ATP (500 μM) and/or MeJA (10 μM) for 30 min. To prevent proteasomal degradation upon COI1-JAZ1 interaction, protoplasts were preincubated with 10 μM of MG132 for 30 min. Immunoprecipitation (IP) was performed with anti-HA antibodies and western blots (WB) were probed with anti-Myc or anti-HA antibodies. Numbers below the blot show the expression of proteins relative to mock (i.e. non-ATP-treatment). D, Graph shows quantitative results of all three replications of coimmunoprecipitation (Fig. 3C; Supplemental Fig. S6). Data are shown as means ± sd of three biological replicates. Each graph from left to right shows the data in WT, dorn1-3, or OxP2K1. Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05).
To further understand how ATP influences JA signaling, we evaluated the protein interaction between COI1 and JAZ1 using coimmunoprecipitation in wild-type, dorn1-3, and OxP2K1 protoplasts. Because JAZ1 is degraded upon protein interaction, MG132 was used to prevent proteasomal degradation during the experiment. COI1-myc and JAZ1-HA were transiently cotransformed into protoplasts. The interaction between COI1 and JAZ1 was enhanced by ATP addition in wild-type and OxP2K1 plants, but not in dorn1-3 (Fig. 3, C and D; Supplemental Fig. S6). Interestingly, the COI1-JAZ1 interaction was further enhanced when ATP was added with JA in wild type and OxP2K1, whereas there was no significant increase observed in dorn1-3 lines (Fig. 3D). In addition, we found similar results of enhanced interaction of COI1 and JAZ1 in our bimolecular fluorescence complementation (BiFC) assays (Supplemental Fig. S7). The results suggest that ATP-JA synergism is mediated by the COI1-JAZ interactions, which require the functional P2K1 receptor.
Extracellular ATP Signaling Directly Activates JA Signaling, But Not via JA Biosynthesis
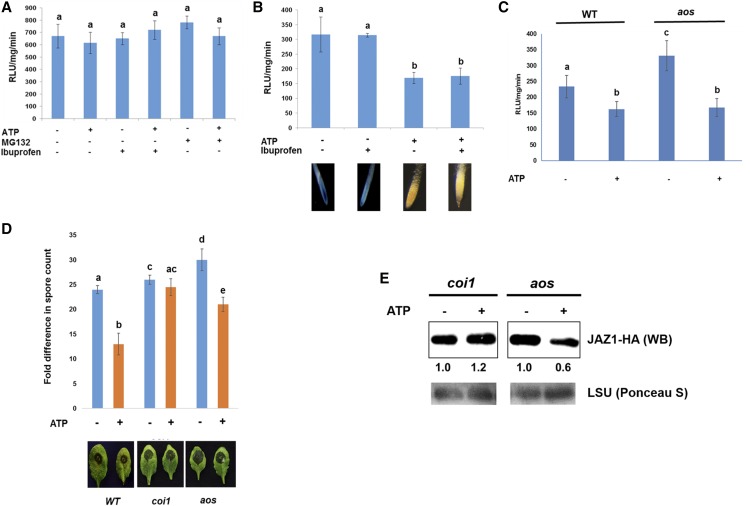
To confirm the ATP-mediated activation of JA signaling, we examined whether the stability of JAZ1 protein changes after ATP treatment in the JA receptor mutant, coi1-1 (Xie at al., 1998). ATP did not reduce the stability of the JAZ1 protein in the coi1-1 mutant, indicating that the active JA receptor COI1 is required for the ATP-dependent JAZ1 degradation (Fig. 4A; Supplemental Fig. S8). In addition, ATP-induced defense against B. cinerea was not observed in coi1-1 mutant plants (Fig. 4D). These results suggested that COI1 is required for ATP-induced destabilization of JAZ1 protein and ATP-induced defense against B. cinerea.
Figure 4.
ATP-induced activation of JA signaling requires the JA receptor function, but not JA biosynthesis. A, The coi1-1 mutant expressing 35S::JAZ1-GUS was treated with ATP (500 μM) for 30 min in the presence or absence of pretreatment of Ibuprofen (20 μM) or MG132 (10 μM). Graph shows the result of fluorescence-based GUS assay to measure the stability of the JAZ1 protein upon ATP addition. Data are shown with the means ± sd (n = 6). Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05). B, Wild-type seedlings expressing 35S::JAZ1-GUS were treated with ATP (500 μM) for 30 min in the presence or absence of Ibuprofen (20 μM), a JA biosynthesis inhibitor. Graph shows the result of fluorescence-based GUS assay to measure the JAZ1 stability in 30 min after ATP addition. Data are shown with the means ± sd (n = 6). Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05). Pictures represent the result of GUS histochemical assay in roots. C, Wild-type and aos mutant seedlings expressing 35S::JAZ1-GUS were treated with ATP (500 μM) for 30 min. Graph shows the result of fluorescence-based GUS assay to measure the JAZ1 stability in 30 min after ATP addition. Data are shown with the means ± sd (n = 6). Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05). D, Pictures show the infected leaves of the coi1-1 and aos mutants by B. cinerea (2 d after inoculation). Plants were infiltrated with ATP (500 μM) followed by inoculation of B. cinerea (5 × 105 spores/mL). Graph shows the fold change in number of in planta-formed spores of B. cinerea at 3-d postinoculation. Data are means ± sd (n = 15) of three biological replicates. Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05). E, Western blot showing the expression of JAZ1-HA in protoplasts of coi1 and aos mutant plants upon ATP treatment for 30 min. Western blot (WB) was performed using anti-HA antibody as shown as JAZ1-HA. Rubisco large subunit (LSU) detected by Ponceau S staining represents a loading control. Numbers below the blot show the expression of proteins relative to mock (i.e. non-ATP-treatment).
The destabilization of the JAZ1 protein by ATP treatment could be explained through the induction of JA biosynthesis. To test this hypothesis, we treated JAZ1-GUS seedlings with Ibuprofen or SHAM—lipoxygenase inhibitors that block a rate-limiting step of early JA biosynthesis (Creelman and Mullet, 1997; Nojiri et al., 1996)—or DIECA, which oxidizes the precursor pool leading to allene oxide (Farmer et al., 1994). Subsequently, we evaluated the changes in JAZ1 protein stability in response to ATP. The results showed that none of the inhibitors disrupted ATP-induced JAZ1 destabilization (Fig. 4B; Supplemental Fig. S9). We also used a knockout mutant, aos, defective in allene oxide synthase (AOS), which converts 13-hydroperoxylinolenate to allene oxide in JA biosynthesis (Park et al., 2002). A stable transgenic line of 35S:JAZ1-GUS in the aos mutant was subjected to ATP treatment. The results showed that application of ATP reduced JAZ1 protein stability, even in the aos mutant (Fig. 4, C and E). Similar results were obtained in transient expression of JAZ1-GFP in protoplasts of the aos mutant (Supplemental Fig. S10). Consistent with those results, ATP still induced resistance against a necrotrophic fungus, B. cinerea in the aos mutant (Fig. 4D). Those results were corroborated by JA measurement data, in which ATP did not elevate basal JA levels in seedlings (Supplemental Fig. S11).
Extracellular ATP-Activated JA Signaling via Ca2+, ROS, and NO
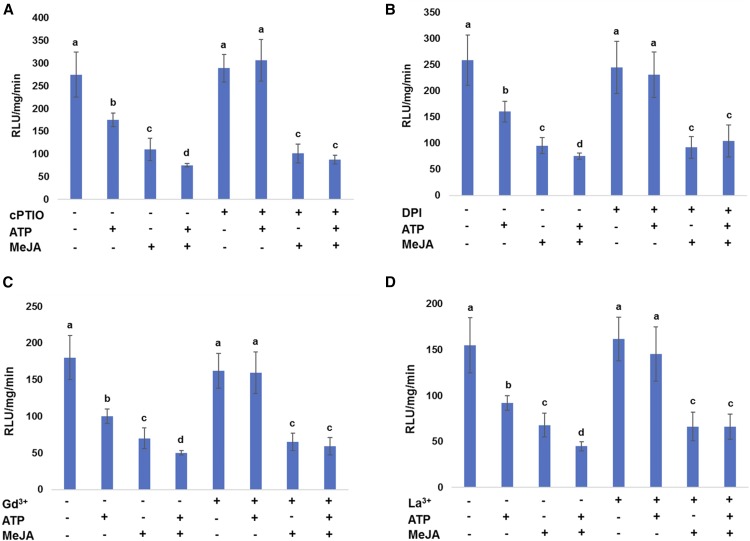
Extracellular ATP signaling is mediated by second messengers, Ca2+, ROS, and NO (Tanaka et al., 2010a). To test whether the ATP-mediated activation of JA signaling requires these second messengers, we evaluated the protein stability of JAZ1 upon ATP addition in the presence of the following inhibitors: the NO scavenger, cPTIO; the RBOH inhibitor, DPI; and the Ca2+ channel blockers Gd3+ and La3+. The JAZ1 protein stability was measured by GUS biochemical assay using seedlings expressing 35S::JAZ1-GUS. All inhibitors used attenuated ATP-induced JAZ1 degradation, but not degradation induced by JA (Fig. 5). The results suggest that Ca2+, ROS, and NO are important secondary signals mediating the ATP-activated JA signaling.
Figure 5.
ATP-induced JAZ1 degradation requires second messenger trio. Seedlings expressing 35S::JAZ1-GUS were treated with ATP (500 μM) and/or MeJA for 30 min in the presence of cPTIO (A), DPI (B), Gd3+ (C), and La3+ (D). Graphs shows the data with means ≥ sd (n = 6) of fluorescence-based GUS biochemical assay to measure the JAZ1 protein level upon ATP addition. Different letters denote statistically significant differences based on analysis performed using the mixed model and Tukey honest test (P < 0.05).
DISCUSSION
Plants experience multiple environmental factors in nature, where more than two signals are employed to activate defense responses (Ramegowda and Senthil-Kumar, 2015). Plant defense response is a coordination of activities among stress-related hormones and other signaling molecules (Thomma et al., 1998, Glazebrook, 2005; Duran-Flores and Heil, 2016). Extracellular ATP is a DAMP signal, and a key missing element in many stress responses previously identified as an effect of the stress stimulus, as ATP is released in response to many stresses (summarized in Tanaka et al., 2014). The results of this study highlighted how extracellular ATP serves as a danger signal in plant defense systems that interact with the signaling pathways of other stress hormones, e.g. JA signaling.
Several studies suggested a role for extracellular ATP in plant defense responses. For example, ATP induces the expression of JA and ethylene-induced genes (Jeter et al., 2004; Song et al., 2006) and mimics JA-dependent responses, i.e. secretion of extrafloral nectar to attract predators (Heil et al., 2012). In addition, the elevation of extracellular ATP levels through the suppression of extracellular ATP-hydrolyzing enzymes, ecto-apyrases, also induces the expression of genes involved in a number of immune responses (Lim et al., 2014). In contrast, when purified caterpillar apyrase was applied at the wound site, glandular trichome production as an antiherbivore reaction and the expression of JA- and ethylene-induced, defense-related genes was suppressed (Wu et al., 2012). Moreover, a reduced transcriptional response and an elevated response to both ATP treatment and wounding were observed in the dorn1 mutant and the P2K1 overexpression line, respectively (Choi et al., 2014). Notably, Balagué et al. (2017), using two knockout mutants and an overexpression line of P2K1, demonstrated that P2K1 is required for resistance to a bacteria pathogen Pseudomonas syringae through JA signaling. In the light of these reports mentioned above, extracellular ATP can be proposed to act as a central signal in many plant defense responses (Cao et al., 2014). In this study, the results support the function of extracellular ATP in plant defense responses as ATP addition induces resistance against B. cinerea, which required intact P2K1, the receptor for extracellular ATP (Fig. 2). Detailed analysis of the ATP-induced transcriptome indicates that extracellular ATP signaling via P2K1 is involved in plant responses to a variety of stresses (Cao et al., 2014; Supplemental Fig. S1). Moreover, in silico studies suggested that extracellular ATP-induced genes are induced through MeJA, and the P2K1 receptor is highly coexpressed with genes encoding JAZ family proteins, which is a key repressor on JA signaling (Fig. 1). These results suggest that the release of ATP after cellular damage via pathogen infection activates downstream signaling, e.g. via JA signaling, which eventually activates plant defense systems.
Our data suggest that extracellular ATP acts on JA signaling through the direct enhancement of the COI1-JAZ1 interaction followed by JAZ1 protein degradation (Fig. 3; Supplemental Fig. S6), without the requirement for enhanced JA biosynthesis (Fig. 4). Recent studies suggested that JAZ proteins are pivotal interfaces to mediate synergism and antagonism between JA and other plant hormones, and that JA signaling can be modulated directly in the absence of effects on JA biosynthesis (Song et al., 2014; Huot et al., 2014). For example, DELLA repressors, a key player in gibberellin signaling, directly interact with JAZ proteins to regulate JA signaling and vice versa (Hou et al., 2010; Yang et al., 2012). This cross talk has been implicated in the antagonistic regulation of growth and immunity based on growth-defense tradeoffs in plants. In addition, a recent study shows that the HopX1 effector from Pseudomonas syringae pv. tabaci 11528 targets JAZ proteins to modulate host defense responses (Gimenez-Ibanez et al., 2014). Therefore, based on our observations, together with previous studies, we speculate that downstream signaling factors, after the recognition of extracellular ATP by the P2K1 receptor, interact directly with (or modify) JAZ proteins to promote the formation of the CO1-JAZs complex, which coordinates ATP-JA synergism.
Interestingly, our data suggest that these molecular components are exclusively regulated by second messengers Ca2+, ROS, and NO (Fig. 5). Previous studies demonstrated that the second messengers Ca2+, ROS, and NO were all interconnected during extracellular ATP signaling (Foresi et al., 2007; Song et al., 2006; Tanaka et al., 2010b; Sueldo et al., 2010). Notably, Casalongué et al. (2015) pointed out that extracellular ATP and NO are coplayers during the wound response, and ATP-induced NO likely plays a key role for S-nitrosylation of proteins. Indeed, Terrile et al. (2012) reported that NO impacts on S-nitrosylation of TIR1 auxin receptor, which enhances TIR1-Aux/IAA interaction to facilitate Aux/IAA degradation for activation of auxin signaling. Given the mechanistical similarities between TIR1 and COI1, ATP-induced NO likely influences COI1-JAZ1 interaction through yet-unidentified modification of the COI1 protein. Alternatively, ATP-induced Ca2+ may directly stimulate JA signaling. This speculation is supported by the finding by Lu et al. (2016) in which Ca2+ elevation through CNGC2 is required for activation of JA signaling.
How does ATP enhance the COI1-JAZ1 interaction and induce JAZ protein degradation without exogenous JA addition? A similar observation was reported by Zhou et al. (2015), in which protein degradation of JAZs was promoted by RIN4 or AHA1 in transient coexpression assays in tobacco without addition of COR or JA-Ile. It is conceivable that this event is mediated by endogenous JA, given that there is a certain level of JAs, e.g. approximately 20 pmol/g of JA and approximately 3.5 pmol/g of JA-Ile, without any stimuli (Supplemental Fig. S11). Inositol polyphosphates are reported as a cofactor for COI1 receptor function and crucial for enhancing COI1-JAZ interactions (Laha et al., 2015; Mosblech et al., 2011; Sheard et al., 2010). A previous report suggested that ATP induces the formation of phosphatidic acid via phospholipase C (Sueldo et al., 2010). Given that the phospholipase C-mediated pathway produces multiple inositol polyphosphates, it is tempting to speculate that extracellular ATP signaling regulates COI1 function by changing a cofactor level, and thereby contributes to enhancement of the COI1-JAZ1 interaction. It is not entirely inconceivable that local concentration of JA-Ile can be changed without bulk changes in cellular JA-Ile content if the uptake of JA-Ile into the nucleus is preferentially enhanced by the recently identified transporter AtJAT1/AtABCG16 (Li et al., 2017). Whether extracellular ATP can stimulate the activity of AtJAT1/AtABCG16 remains to be determined.
Liu et al. (2016) demonstrated a noncanonical pathway at the early stage of ETI in which the activation of JA signaling is mediated through direct degradation of JAZs by SA receptors, NPR3 and NPR4, in a COI1-independent manner. As it was attenuated in a sid2 mutant, SA is a necessary signal for the activation of JA signaling in this noncanonical pathway. The authors speculated that SA-mediated JAZ1 degradation, i.e. SA-mediated activation of JA signaling, during ETI provides the resistance against necrotrophs during defense response to biotrophs. It is possible that extracellular ATP may activate JA signaling via this noncanonical pathway. However, ATP does not accumulate SA at an early time point (Supplemental Fig. S11), and ATP reduces endogenous SA level at a late time point (Chivasa et al., 2009). Furthermore, ATP is still able to induce JA-inducible genes in the sid2 mutant (Supplemental Fig. S12), which disagreed with the fact that the noncanonical pathway requires intact SID2. Finally, ATP-mediated JAZ1 degradation is COI1-dependent (Fig. 4). Therefore, ATP-induced resistance against a necrotrophic pathogen is not likely mediated through the noncanonical pathway, although ATP induced some SA-responsive genes (Supplemental Fig. S2). Extracellular ATP may independently enhance JA and SA signaling.
We conclude that extracellular ATP signaling induces plant defense responses, which in part reflects the direct enhancement of COI1-JAZ1 complex formation in JA signaling (Fig. 3) without inducing JA biosynthesis. This signaling cross talk proceeds downstream of extracellular ATP recognition by the P2K1 receptor. Considering the synergistic relationship between ATP and JA in plant pathogen resistance (Fig. 2), and that JA biosynthesis is not required for stimulation of JA signaling by ATP addition (Fig. 4), this ATP-activated JA signaling likely expedites plant defense responses against pathogen attacks. Because JA still induced resistance in the P2K1 receptor mutant, dorn1 (Fig. 2), damage-induced JAs can cause defense responses independent of ATP signaling. This notion supports the idea that ATP-activated JA signaling is an early event for the synergistic enhancement of JA-mediated plant responses. The event requires signaling via the second messenger trio, Ca2+, ROS, and NO (Fig. 5). Several questions still need to be addressed for a better understanding of the interaction between extracellular ATP and JA. For example, what is the key factor for ATP-induced COI1-JAZ1 complex formation? Further studies concerning how COI1 or JAZ proteins are modified upon ATP treatment might provide some insight into the cross-talk mechanism between extracellular ATP signaling and JA signaling.
It is worth discussing how extracellular ATP is involved in SA signaling because our data showed that ATP induced expression of some SA-inducible genes. Given that ATP treatment does not change SA content at an early time point (Supplemental Fig. S11), and also reduces SA levels at a late time point (Chivasa et al., 2009), it is possible that ATP directly enhances SA signaling in a similar manner to that seen in ATP-mediated enhancement of JA signaling as described above. It is conceivable that ATP-induces dynamic and transient changes in the intracellular concentration of the second messengers directly impacting SA signaling. For example, Ca2+-mediated signaling evokes the expression of pathogen-related genes, some of which are regulated by SA signaling (Yuan et al., 2017). It was proposed by Casalongué et al. (2015) that, during wounding, ATP-mediated NO might be involved in thioredoxin-mediated S-nitrosylation modifications of NPR1 (a key transcriptional regulator of the SA signaling pathway). The modification induces the redox-based conformation changes of the protein to regulate defense responses (Tada et al., 2008). Collectively, these experimental results further support a possible mechanism of direct enhancement of SA signaling by ATP-induced second messengers. However, future studies focused on exploring the role for extracellular ATP on SA signaling will provide a better understanding.
MATERIALS AND METHODS
Chemicals
For the stock solution, ATP (Sigma-Aldrich) was dissolved in water at a concentration of 50 mM, and the pH was adjusted to 5.7 with NaOH. The proteasome inhibitor, MG132 (Enzo Life Sciences) was dissolved in DMSO and used at a final concentration of 10 μM. MeJA (Bedoukian Research) was dissolved in ethanol and used at a final concentration of 10 μM. JA biosynthesis inhibitors, SHAM (Alfa Aesar), Ibuprofen, and DIECA (Sigma-Aldrich) were dissolved in DMSO and used at a final concentration of 20 μM. All stock solutions were stored at −20°C until further use. The final concentration of the solvent under assay conditions did not exceed 0.1% (v/v). Calcium channel blockers, LanthanumIII chloride heptahydrate (Sigma-Aldrich), GadoliniumIII chloride hexahydrate (Sigma-Aldrich), NADPH oxidase inhibitor, Diphenyleneiodonium chloride (Sigma-Aldrich), and nitric oxide inhibitor, Carboxy-PTIO (Enzo Life Sciences) were used at a final concentration of 500 μM.
Plant Materials
All Arabidopsis (Arabidopsis thaliana) lines had a Col-0 background. The OxP2K1 (35S::P2K1) and JAZ1-GUS (35S::JAZ1-GUS) lines and the dorn1-3, aos, coi1-1 mutants have been previously described (Choi et al., 2014; Park et al., 2002; Thines et al., 2007; Xie et al., 1998). Sterilized Arabidopsis seeds were first sown onto half-strength Murashige & Skoog (MS) medium containing 2.2 g/L of MS salt with vitamins (Caisson Labs), 1% (w/v) of Suc, 1% (w/v) of agar, and 0.05% (w/v) of MES (pH 5.7). After incubation at 4°C for 3 d, the seedlings were germinated and vertically grown in a growth chamber (Conviron) at 22°C under a 16-h light/8-h dark cycle (100 μmol m−2 s−1 light intensity). Ten-day-old seedlings were used for most of the experiments. For pathogen assays, 10-d-old seedlings were transferred to soil (Sunshine mix) and further grown for 4 to 5 weeks in a growth chamber at 22°C with 70% humidity under an 8 h light/16 h dark light cycle (150 μmol m−2 s−1 light intensity). JAZ1-GUS in the aos mutant background was made by crossing, and homozygous plants were selected by sterile phenotype (which is rescued by JA treatment on the flower) and histochemical GUS staining of seedlings.
β-Glucuronidase Reporter Assays
GUS (β-glucuronidase) reporter assays were performed according to the method of Jefferson et al. (1987) with certain modification. The GUS biochemical assay was performed using 10-d-old seedlings. After chemical treatments, the seedlings were ground, and the protein was extracted in extraction buffer, including 50 mm of sodium P buffer (pH 7.2), 0.5 mm of EDTA, 0.2% (v/v) of Triton X-100, 5% (w/v) of Sarcosyl, and 5 mm of 2-mercaptoethanol. Ten-microgram aliquots of the protein lysates were incubated with 4-methylumbelliferyl-β-d-glucuronide substrate and relative light units were recorded using a plate reader (EnSpire; PerkinElmer) after incubating at 37°C for 2 h. GUS histochemical assay was performed on the 7- to 10 d-old seedlings. After ATP treatment for 30 min, the seedlings were immersed in 90% (v/v) acetone and incubated in a vacuum for at least 5 min. Acetone was replaced with the staining buffer, including 50 mm sodium P (pH 7.0), 1 mm 5-bromo-4-chloro-3-indolyl-b-d-glucuronide, 10 mm EDTA, 1% (w/v) Triton X-100, 0.5 mm potassium ferricyanide, and 0.5 mm potassium ferrocyanide, and the seedlings were incubated for 6 h at 37°C. The histochemically stained seedlings were subsequently cleared using a series of ethanol washes (20%, 35%, and 50% [v/v]) for 30 min each. The seedlings were stored in 70% (v/v) ethanol. The stained tissue was observed under a stereoscope (Carl Zeiss) after several washes with 50%, 25%, and 10% (v/v) ethanol and a final wash with water.
Transient Gene Expression in Protoplasts and Immunoprecipitation
Transient expression in leaf mesophyll protoplasts was performed according to Yoo et al. (2007). Briefly, 5 × 105 protoplasts were cotransformed with 50 μg of pUC18 harboring the 35S::COI1-Myc cassette and 50 μg of pUC18 harboring the 35S::JAZ1-HA cassette. Protoplasts were resuspended in W1 solution (4 mm MES, pH 5.7, 0.5 m mannitol, and 20 mm KCl) and incubated at room temperature in the dark for 16 h to 20 h. After ATP or JA treatments, the protoplasts were harvested and lysed with lysis buffer (50 mm Tris-HCl, pH 7.8, 100 mm NaCl, 10% [w/v] glycerol, and 0.1% [w/v] Tween 20) in the presence of protease inhibitor cocktail (Thermo Fisher Scientific). Immunoprecipitation was performed with anti-HA or anti-Myc antibodies (Proteintech). Approximately 1 μg of the antibodies was added to 10 μL of protein A beads (Thermo Fisher Scientific) for 1 h at 4°C, and subsequently, the antibody-protein A beads complex was cross linked after incubation with 5 mm of disuccinimidyl suberate (DSS) for 30 min at room temperature as described by the manufacturer (Thermo Fisher Scientific). After removing the residual DSS, 50 μg of protein lysate was mixed with the antibody complex. The protein complex was washed three times with 10 mm of Tris buffer (pH 8.0) and immunoprecipitated proteins were eluted in 50 μL of double-strength sample buffer (0.2 m Tris-Cl, pH 8.0, 10% [w/v] SDS, 10 mm DTT, and 20% [w/v] glycerol).
Western Blotting
Proteins were separated by 12% (w/v) SDA-PAGE, and electrophoretically transferred to PVDF membranes (Bio-Rad). Transblots were blocked and then incubated with primary antibody, anti-HA, or anti-myc antibodies (Proteintech) at 1:5000 dilution. Immunoreactive bands were visualized using ECL substrate (Bio-Rad) with HRP-conjugated secondary antibodies (Proteintech) at 1:10,000 dilution. Ponceau S (Advansta) staining was used as the loading control. The band intensity was measured by the software ImageJ (National Institutes of Health) and densitometric data were normalized by the loading control values. The relative intensity compared to the mock control is shown in the figures.
Fluorescence Measurement
Transient expression of the 35S::JAZ1-GFP cassette was performed in the protoplast of wild-type and aos mutant plants as described earlier. Protoplasts were resuspended in W5 solution (2 mm MES, pH 5.7, 154 mm NaCl, 125 mm CaCl2, 20 mm KCl) and incubated at room temperature for 16 h to 20 h. An equal number of protoplasts were distributed for each treatment. Fluorescence was measured using a plate reader (EnSpire; PerkinElmer). For BiFC assays, COI1 and JAZ1 were cloned into pUC-pSPYNE and pUC-pSPYCE expression vectors (Walter et al., 2004) to fuse with split-YFP, N-terminal part (YFPn), and C-terminal part (YFPc), respectively. Transient coexpression of COI1-YFPn and JAZ1-YFPc was performed in an equal number of protoplasts of WT, dorn1-3, and OxP2K1. After 16-h incubation in light, fluorescence from reconstitute YFP was measured using the plate reader described above.
Botrytis cinerea Infection Assay
Four-week-old plants were infiltrated with water (mock), ATP (500 μM), or MeJA (10 μM). After 24 h, the leaves were placed in plastic petri dishes containing 1% (w/v) phytoagar. Approximately 5 × 105 spores of grape (B. cinerea) were suspended in 1 mL of potato dextrose broth, and 5 μL of spore suspension was spotted onto the leaves as described by van Wees et al. (2013). Each petri dish contained 10 to 15 leaves of each genotype. Plates were sealed to prevent the desiccation of the inoculation droplets. The plates were maintained at 22°C. The lesion diameters were recorded at 2-d postinoculation using a digital caliper. After incubation with B. cinerea spore suspension for 3 d, leaves were washed to extract spores and the numbers of planta-formed spores were counted from the infected leaves using a hemocytometer under a light microscope (Leica Microsystems) as described by van Wees et al. (2013). For additional quantification, the amount of the pathogen-specific gene, endopolygalacturonase (BcPG) in the infected leaves, was measured through real-time PCR (Kasza et al., 2004) using CFX96 (Bio-Rad). Arabidopsis SAND gene (At2g28390) was used as a reference gene for normalization of BcPG gene.
Quantification of JAs and SA
Measurement of JA and JA-Ile and SA using ultra performance liquid chromatography-electrospray ionization-tandem mass spectrometry was performed as described in Koo et al. (2009), Smith et al. (2014), and Poudel et al. (2016). Dihydro-JA, [13C6]JA-Ile, and deuterated SA were used as internal standards.
Statistical Analysis
All experiments were independently performed at least three times with similar results. Results are shown as means ± sd. Statistically significant differences between the possible combinations of genotype and treatment was analyzed by the Student’s t test and/or by the software SAS (v. 9.0; SAS Institute) using the mixed model and Tukey honest significant difference test (P < 0.05).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. List of JA up-regulated genes (Kilian et al., 2007) in an ATP up-regulated gene pool (Choi et al., 2014).
Supplemental Figure S1. Gene-ontology term enrichment analysis of ATP up-regulated genes.
Supplemental Figure S2. ATP-responsive genes are regulated by other stress hormones.
Supplemental Figure S3. ATP-induced dose-dependent response in reducing the JAZ1 protein stability.
Supplemental Figure S4. Western blot showing the JAZ-HA protein level (a repeated trial for Fig. 3B).
Supplemental Figure S5. Stability of the JAZ1 protein was reduced by ATP and JA additions.
Supplemental Figure S6. Replicated experiments of coimmunoprecipitation for the COI1-JAZ1 interaction.
Supplemental Figure S7. BiFC assay to show the interaction between COI1 and JAZ1 upon ATP and/or JA treatment.
Supplemental Figure S8. Western blot showing the JAZ-HA protein level (a repeated trial for Fig. 4E).
Supplemental Figure S9. JA biosynthesis inhibitors did not attenuate ATP-induced reduction of the JAZ1 protein stability.
Supplemental Figure S10. JA biosynthesis mutant did not attenuate ATP-induced reduction of the JAZ1 protein stability.
Supplemental Figure S11. ATP does not induce JAs and SA in the Arabidopsis seedlings.
Supplemental Figure S12. Gene expression of ATP/JA coregulated genes in wild-type and sid2 mutant seedlings.
Acknowledgments
Special thanks to Drs. John Browse, Cynthia Gleason, and Scot Hulbert at Washington State University for manuscript review. We are also grateful to Dr. Jörg Kudla at Universität Münster for providing BiFC vectors, Dr. John Browse at Washington State University for providing B. cinerea and Arabidopsis lines (35S::JAZ1-GUS, aos, and coi1-1), David Wheeler and Sierra Windsor at Washington State University for technical supports for the pathogen assay, and Dr. Jeremy Jewell for propagating the 35S::JAZ-GUS transgenic line in the aos mutant background.
Footnotes
This project was supported by the National Science Foundation (NSF grant no. IOS-1557813 to K.T. and IOS-1557439 to A.J.K) and also by the Next-Generation BioGreen 21 Program Systems and Synthetic Agrobiotech Center, Rural Development Administration, Republic of Korea (grant no. PJ01116604 to G.S.). This work was also supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIGMS, NIH grant no. R01GM121445 to G.S.), and PPNS No. 0719, Department of Plant Pathology, College of Agriculture, Human and Natural Resource Sciences, Agricultural Research Center, Hatch Project No. WNP00833, Washington State University, Pullman, WA, 99164-6430.
Articles can be viewed without a subscription.
References
- Balagué C, Gouget A, Bouchez O, Souriac C, Haget N, Boutet-Mercey S, Govers F, Roby D, Canut H (2017) The Arabidopsis thaliana lectin receptor kinase LecRK-I.9 is required for full resistance to Pseudomonas syringae and affects jasmonate signalling. Mol Plant Pathol 18: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. (1972) Purinergic nerves. Pharmacol Rev 24: 509–581 [PubMed] [Google Scholar]
- Cao Y, Tanaka K, Nguyen CT, Stacey G (2014) Extracellular ATP is a central signaling molecule in plant stress responses. Curr Opin Plant Biol 20: 82–87 [DOI] [PubMed] [Google Scholar]
- Casalongué CA, Fiol DF, D’Ippólito S, Tonón C, París R (2015) Insights into the participation of nitric oxide and extra cellular ATP in wounding. In Khan M, Mobin M, Mohammad F, Corpas F. (eds), Nitric Oxide Action in Abiotic Stress Responses in Plants. Springer, Cham, Switzerland. [Google Scholar]
- Chehab EW, Yao C, Henderson Z, Kim S, Braam J (2012) Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr Biol 22: 701–706 [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP, Slabas AR (2009) Extracellular ATP is a regulator of pathogen defence in plants. Plant J 60: 436–448 [DOI] [PubMed] [Google Scholar]
- Chivasa S, Slabas AR (2012) Plant extracellular ATP signalling: new insight from proteomics. Mol Biosyst 8: 445–452 [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Liang Y, Cao Y, Lee SY, Stacey G (2014) Extracellular ATP, a danger signal, is recognized by DORN1 in Arabidopsis. Biochem J 463: 429–437 [DOI] [PubMed] [Google Scholar]
- Clark G, Fraley D, Steinebrunner I, Cervantes A, Onyirimba J, Liu A, Torres J, Tang W, Kim J, Roux SJ (2011) Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis. Plant Physiol 156: 1740–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GB, Morgan RO, Fernandez M-P, Salmi ML, Roux SJ (2014) Breakthroughs spotlighting roles for extracellular nucleotides and apyrases in stress responses and growth and development. Plant Sci 225: 107–116 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48: 355–381 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE (1992) Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA 89: 4938–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark A, Demidchik V, Richards SL, Shabala S, Davies JM (2011) Release of extracellular purines from plant roots and effect on ion fluxes. Plant Signal Behav 6: 1855–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM (2003) Is ATP a signaling agent in plants? Plant Physiol 133: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, Schachtman DP, Shabala SN, et al. (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58: 903–913 [DOI] [PubMed] [Google Scholar]
- Duran-Flores D, Heil M (2016) Sources of specificity in plant damaged-self recognition. Curr Opin Plant Biol 32: 77–87 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Caldelari D, Pearce G, Walker-Simmons MK, Ryan CA (1994) Diethyldithiocarbamic acid inhibits the octadecanoid signaling pathway for the wound induction of proteinase inhibitors in tomato leaves. Plant Physiol 106: 337–342 [Google Scholar]
- Foresi NP, Laxalt AM, Tonón CV, Casalongué CA, Lamattina L (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Boter M, Fernández-Barbero G, Chini A, Rathjen JP, Solano R (2014) The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol 12: e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Goda H, Sasaki E, Akiyama K, Maruyama-Nakashita A, Nakabayashi K, Li W, Ogawa M, Yamauchi Y, Preston J, Aoki K, Kiba T, Takatsuto S, et al. (2008) The AtGenExpress hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. Plant J 55: 526–542 [DOI] [PubMed] [Google Scholar]
- Hao LH, Wang WX, Chen C, Wang YF, Liu T, Li X, Shang ZL (2012) Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol Plant 5: 852–864 [DOI] [PubMed] [Google Scholar]
- Heil M, Ibarra-Laclette E, Adame-Álvarez RM, Martínez O, Ramirez-Chávez E, Molina-Torres J, Herrera-Estrella L (2012) How plants sense wounds: damaged-self recognition is based on plant-derived elicitors and induces octadecanoid signaling. PLoS One 7: e30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Lee LY, Xia K, Yan Y, Yu H (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19: 884–894 [DOI] [PubMed] [Google Scholar]
- Huot B, Yao J, Montgomery BL, He SY (2014) Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7: 1267–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe MJ. (1973) The role of ATP in mechanically stimulated rapid closure of the Venus’s flytrap. Plant Physiol 51: 17–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16: 2652–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza Z, Vagvölgyi C, Févre M, Cotton P (2004) Molecular characterization and in planta detection of Sclerotinia sclerotiorum endopolygalacturonase genes. Curr Microbiol 48: 208–213 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G (2009) The double life of ATP. Sci Am 301: 84–90, 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Koo AJ, Gao X, Jones AD, Howe GA (2009) A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 59: 974–986 [DOI] [PubMed] [Google Scholar]
- Koo AJK, Howe GA (2009) The wound hormone jasmonate. Phytochemistry 70: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha D, Johnen P, Azevedo C, Dynowski M, Weiß M, Capolicchio S, Mao H, Iven T, Steenbergen M, Freyer M, Gaugler P, de Campos MK, et al. (2015) VIH2 regulates the synthesis of inositol pyrophosphate InsP8 and jasmonate-dependent defenses in Arabidopsis. Plant Cell 27: 1082–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zheng J, Li S, Huang G, Skilling SJ, Wang L, Li L, Li M, Yuan L, Liu P (2017) Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol Plant 10: 695–708 [DOI] [PubMed] [Google Scholar]
- Lim MH, Wu J, Yao J, Gallardo IF, Dugger JW, Webb LJ, Huang J, Salmi ML, Song J, Clark G, Roux SJ (2014) Apyrase suppression raises extracellular ATP levels and induces gene expression and cell wall changes characteristic of stress responses. Plant Physiol 164: 2054–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Sonbol FM, Huot B, Gu Y, Withers J, Mwimba M, Yao J, He SY, Dong X (2016) Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat Commun 7: 13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Zhang Y, Tang S, Pan J, Yu Y, Han J, Li Y, Du X, Nan Z, Sun Q (2016) AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. J Exp Bot 67: 809–819 [DOI] [PubMed] [Google Scholar]
- Moroz N, Huffaker A, Tanaka K (2017) Extracellular alkalinization assay for detection of early defense response. Curr Protoc Plant Biol 5: 210–220 [DOI] [PubMed] [Google Scholar]
- Mosblech A, Thurow C, Gatz C, Feussner I, Heilmann I (2011) Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J 65: 949–957 [DOI] [PubMed] [Google Scholar]
- Nojiri H, Sugimori M, Yamane H, Nishimura Y, Yamada A, Shibuya N, Kodama O, Murofushi N, Omori T (1996) Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol 110: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T, Nishida K, Kasahara K, Kinoshita K (2011) ATTED-II updates: condition-specific gene coexpression to extend coexpression analyses and applications to a broad range of flowering plants. Plant Cell Physiol 52: 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12. [DOI] [PubMed] [Google Scholar]
- Poudel AN, Zhang T, Kwasniewski M, Nakabayashi R, Saito K, Koo AJ (2016) Mutations in jasmonoyl-L-isoleucine-12-hydroxylases suppress multiple JA-dependent wound responses in Arabidopsis thaliana. Biochim Biophys Acta 1861(9 Pt B): 1396–1408 [DOI] [PubMed] [Google Scholar]
- Ramegowda V, Senthil-Kumar M (2015) The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol 176: 47–54 [DOI] [PubMed] [Google Scholar]
- Reichler SA, Torres J, Rivera AL, Cintolesi VA, Clark G, Roux SJ (2009) Intersection of two signalling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J Exp Bot 60: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404–411 [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Lotze MT (2007) Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 28: 429–436 [DOI] [PubMed] [Google Scholar]
- Schweizer F, Bodenhausen N, Lassueur S, Masclaux FG, Reymond P (2013) Differential contribution of transcription factors to Arabidopsis thaliana defense against Spodoptera littoralis. Front Plant Sci 4: 13.10.3389/fpls.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, He SY, Rizo J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Leslie ME, Robinson SJ, Korasick DA, Zhang T, Backues SK, Cornish PV, Koo AJ, Bednarek SY, Heese A (2014) Loss of Arabidopsis thaliana Dynamin-Related Protein 2B reveals separation of innate immune signaling pathways. PLoS Pathog 10: e1004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol 140: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Huang H, Gao H, Wang J, Wu D, Liu X, Yang S, Zhai Q, Li C, Qi T, Xie D (2014) Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26: 263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueldo DJ, Foresi NP, Casalongué CA, Lamattina L, Laxalt AM (2010) Phosphatidic acid formation is required for extracellular ATP-mediated nitric oxide production in suspension-cultured tomato cells. New Phytol 185: 909–916 [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang X, Deng S, Zhang C, Wang M, Ding M, Zhao R, Shen X, Zhou X, Lu C, Chen S (2012) Extracellular ATP signaling is mediated by H2O2 and cytosolic Ca2+ in the salt response of Populus euphratica cells. PLoS One 7: e53136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Choi J, Cao Y, Stacey G (2014) Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front Plant Sci 5: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Gilroy S, Jones AM, Stacey G (2010a) Extracellular ATP signaling in plants. Trends Cell Biol 20: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Swanson SJ, Gilroy S, Stacey G (2010b) Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol 154: 705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrile MC, París R, Calderón-Villalobos LI, Iglesias MJ, Lamattina L, Estelle M, Casalongué CA (2012) Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J 70: 492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonón C, Cecilia Terrile M, José Iglesias M, Lamattina L, Casalongué C (2010) Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J Plant Physiol 167: 540–546 [DOI] [PubMed] [Google Scholar]
- van Wees SC, van Pelt JA, Bakker PA, Pieterse CM (2013) Bioassays for assessing jasmonate-dependent defenses triggered by pathogens, herbivorous insects, or beneficial rhizobacteria. Methods Mol Biol 1011: 35–49 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, Harter K, Kudla J (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang F, Jia J, Wang Y, Wang W, Chen Y, Liu T, Shang Z (2014) Hyperpolarization-activated Ca2+ channels in guard cell plasma membrane are involved in extracellular ATP-promoted stomatal opening in Vicia faba. J Plant Physiol 171: 1241–1247 [DOI] [PubMed] [Google Scholar]
- Weerasinghe RR, Swanson SJ, Okada SF, Garrett MB, Kim SY, Stacey G, Boucher RC, Gilroy S, Jones AM (2009) Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett 583: 2521–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Peiffer M, Luthe DS, Felton GW (2012) ATP hydrolyzing salivary enzymes of caterpillars suppress plant defenses. PLoS One 7: e41947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Wu JY (2008) Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J Exp Bot 59: 4007–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, Xiao LT, Sun TP, Li J, Deng XW, Lee CM, Thomashow MF, et al. (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci USA 109: E1192–E1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y-H, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yuan P, Jauregui E, Du L, Tanaka K, Poovaiah BW (2017) Calcium signatures and signaling events orchestrate plant-microbe interactions. Curr Opin Plant Biol 38: 173–183 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wu Y, Yang Y, Du M, Zhang X, Guo Y, Li C, Zhou JM (2015) An Arabidopsis plasma membrane proton ATPase modulates JA signaling and Is exploited by the Pseudomonas syringae effector protein AvrB for stomatal invasion. Plant Cell 27: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]