Abstract
Taste and flavor (retronasal olfaction) interact in the brain. The rules of that interaction are not well understood. This study uses 2 taste modifiers that alter sweet to examine the effects on flavors. Subjects used the Global Sensory Intensity Scale to assess the aroma, sweetness, sourness, and flavor of 10 foods. As previous work had shown, miracle fruit added sweetness to acids, which secondarily reduced sourness (mixture suppression) and Gymnema sylvestre reduced sweetness in sweet foods as well as the sweetness induced by miracle fruit. In this study, multiple regression showed that both sweet and sour contribute to flavor. Gymnema sylvestre reduced the perceived sweet of predominantly sweet foods (chocolate and maple syrup) as expected; reducing the sweet, reduced the flavor. The effects of miracle fruit were complicated by its dual action: intensification of sweet and reduction of sour. Predominantly sour foods (vinegar, lemon, mustard, pickle) were sweetened by miracle fruit but any flavor enhancement associated with the added sweet appears to have been countered by the flavor reduction associated with reduced sourness. Moderately sour foods that are also sweet (tomatoes, strawberries) were sweetened by miracle fruit and thus flavor was enhanced; flavor loss through sour reduction was apparently not sufficient to counter the flavor enhancement due to increased sweet so the net result was that tomato and strawberry flavors were enhanced. The flavors of control foods (not predominantly sweet or sour [sausage, peanuts]) showed only small changes.
Keywords: Global Sensory Intensity Scale, Gymnema sylvestre, miracle fruit, retronasal olfaction, sour, sweet
Introduction
Eating foods stimulates complex sensations. The anatomy is clear. Taste stimuli (nonvolatiles) excite cranial nerves (CNs) VII, IX, and X. Olfactory stimuli (volatiles) excite CN I. Touch, irritation/pain, and temperature excite CNs V and IX. Chemosensory terminology is less clear. In everyday language, the terms “taste” and “flavor” are used as synonyms to refer to the complex of sensations resulting from chewing and swallowing food. However, these terms are also used to refer to specific sensations within this complex. Perhaps the greatest confusion arises because olfactory stimuli can be perceived in 2 ways. Sniffing brings volatiles from the environment through the nostrils and up into the nose to binding sites on the olfactory receptors in the olfactory mucosa; this is called “orthonasal olfaction” (commonly called “smell”). When foods that emit volatiles are chewed and swallowed, the volatiles are forced up behind the palate and into the nose from the rear where they ultimately contact the binding sites; this is called “retronasal olfaction.”
Historically, we find commentary about the chemical senses among the works of the Greek philosophers. Olfactory sensations were seen as resulting from inhalation and taste from placing substances in the mouth (Beare 1906). Because retronasal olfaction is produced when foods are in the mouth even though the sensations arise from the olfactory nerve, retronasal olfactory sensations were confused with taste sensations. The orthonasal/retronasal distinction was not made correctly until 1812 (Prout 1812; Brock 1967).
Pathology made important contributions to analysis of the complex of sensations evoked by foods. Ogle (1870) described patients with damage to the olfactory nerve. They could not smell but retained the ability to perceive sweet, salty, sour, and bitter. Patrick (1897) administered an extensive set of chemosensory stimuli to anosmic subjects and concluded that they could perceive sweet, salty, sour, and bitter, but lacked “gustatory smelling,” Patrick’s name for retronasal olfaction. “Gustatory smelling” was presumably the translation of Zwaardemaker’s “gustatorische Reichen” (Zwaardemaker 1895). The term “retronasal olfaction” came along almost a century later (Burdach et al. 1984).
Modern use of “taste” and “flavor”
The word “taste” can be used as a verb: “I taste food.” The word “flavor” can also be used as a verb, “I flavor food.” However, when flavor is used as a verb it refers to adding a flavoring ingredient to food, not perceiving the flavor of the food. English lacks a verb that describes perceiving retronasal olfaction. Thus the sentence “I taste food” can have dual meaning. “Taste” can refer to a sensation arising from taste buds (e.g., I taste sweet) or it can refer to retronasal olfaction (e.g., I taste cinnamon). There is a wonderful example of this in the Merriam-Webster Dictionary (https://www.merriam-webster.com/): “taste: to ascertain the flavor of by taking a little into the mouth.”
However, if an individual is asked to name the sensations attributed to food, there is no confusion between “taste” and “flavor.” For example, cinnamon toast can be described as having a sweet taste, feeling warm and crisp, and having a cinnamon flavor; subjects can rate the intensity of each of these sensory attributes (sweet, warm, crisp, flavor) without confusing them.
Some modern experts use the word “flavor” to encompass all of the possible attributes of the experience of eating foods. For example, consider Moncrieff (1967): “Flavour is a complex sensation. It comprises taste, odour, roughness or smoothness, hotness or coldness, and pungency or blandness. The factor which has the greatest influence is odour. If odour is lacking then the food loses its flavor and becomes chiefly bitter, sweet, sour or saline.” Note that Moncrieff acknowledges that when retronasal olfaction is lacking we say that “food loses its flavor”; the sensation lost is that imparted by retronasal olfaction. Consider another quote from Moncrieff: “Synura in water [a type of algae] will give a cucumber flavor to it.” When describing the sensory attributes of water contaminated by algae, Moncrieff uses the word “flavor” to refer to the sensation associated with retronasal olfaction. Moncrieff found it easy to switch from the definition of “flavor” as complex to its more targeted usage to describe a specific attribute: the cucumber-like sensation evoked by retronasal olfaction.
In this article, when the word “flavor” is used, it is intended to refer to the sensations evoked by volatiles perceived retronasally. Of special interest for this study, retronasal olfaction and taste can alter one another, presumably in the brain. The rules governing these interactions are still not well understood; some examples are discussed in the following sections.
Sweet or sour taste can alter retronasal olfaction
As early as 1955, the food industry made use of sugar to enhance the flavors of a variety of foods (Sjöström and Cairncross 1955). Beginning in the 1970s, work by chemosensory investigators confirmed that adding sweet taste could enhance some retronasal olfactory stimuli (Murphy et al. 1977; Murphy and Cain 1980; Burdach et al. 1984; Frank and Byram 1988; Frank et al. 1989; Green et al. 2012). Although most studies showing taste alteration of retronasal olfaction have been focused on the effects of sweet, some studies have observed effects with sour (Noble 1996). In particular, the addition of sour taste has been reported to enhance lemon flavor (McBride and Johnson 1987; Kuo et al. 1993), although not all studies support this conclusion (Green et al. 2012). Interactions between taste and retronasal olfaction have also been observed in studies focused on the sensory properties of tomatoes. The addition of either sugars or acids to tomato puree intensified some tomato flavor attributes (Baldwin et al. 2008).
Removing taste as well as adding it can demonstrate taste/retronasal olfaction interactions. Anesthesia of taste was shown to reduce the retronasal olfactory sensations evoked by some foods leaving orthonasal olfaction unchanged (Snyder et al. 2001, 2007; Snyder 2010). This study includes results we interpret as reduction of flavor through reduction of sourness.
Retronasal olfaction can alter sweet taste
In the 1970s, a few volatiles were identified that intensified sweet (see Bartoshuk and Klee (2013) for a brief review). For example, subjects rated a solution of sucrose with strawberry volatiles added as sweeter than the sucrose solution alone (Frank et al. 1989). In the tomato research mentioned earlier, the addition of a few volatiles intensified sweetness (Baldwin et al. 2008).
However, studies conducted at our university that were designed to increase tomato and strawberry palatability (Tieman et al. 2012; Schwieterman et al. 2013) led to the discovery that fruits contain many more such volatiles. These studies were conducted using multiple regression. Multiple regression quantifies associations between several independent (predictor) variables and a dependent (criterion) variable. An independent variable is said to “contribute” to a criterion variable when there is a significant association between them. In our studies, we measured chemical constituents (e.g., sugar content as well as the content of various volatiles) and asked subjects to rate sensory attributes of the fruit including sweet, and fruit flavor. Multiple regression analyses were performed initially with perceived sweet as the dependent variable with sugar content and perceived fruit flavor as the independent variables. These analyses showed significant contributions of perceived flavor to the perceived sweet that were independent of the contributions of sugar. Multiple regression analyses with perceived sweet as the dependent variable but with sugar content and a given volatile as independent variables allowed us to determine which of the volatiles that made up the flavor of the fruit were responsible for the contribution to perceived sweet. We mixed those volatiles (at concentrations approximately equal to those in the fruit) with a 2% sucrose solution; subjects rated the mixture as nearly twice as sweet as the 2% sucrose solution alone. Thus, the perceived sweetness of tomatoes and of strawberries is actually the sum of sweetness from sugars and sweetness provided by volatile-enhanced sweetness (Colquhoun et al. 2015).
In this study, we used 2 taste modifiers to increase and decrease sweet taste. Synsepalum dulcificum, more commonly known as “miracle fruit,” is a plant native to tropical West Africa (Daniell 1852). Its berries contain a glycoprotein called miraculin. Although an early theory suggested that miracle fruit worked by suppressing sour (Dzendolet 1969), other work showed that miracle fruit added a sweet taste in the presence of acid (Bartoshuk et al. 1969; Kurihara and Beidler 1969; Kurihara et al. 1969; Bartoshuk et al. 1974; Koizumi et al. 2011). The reduction of sourness associated with miracle fruit appears to be the result of mixture suppression; miracle fruit and the simple addition of sucrose suppress sourness to a similar degree (Bartoshuk et al. 1974).
A contrasting effect is provided by an herb from southern and central India known as Gymnema sylvestre. The leaves contain gymnemic acids, which are known to suppress the sweetness of a variety of sweet tastes (see Bartoshuk et al. (1969) for a brief review). Applied after miracle fruit, G. sylvestre suppresses the sweet taste produced by miracle fruit and the sour taste returns to close to its original value (Bartoshuk et al. 1969). This supports the explanation of sour suppression by miracle fruit as an example of mixture suppression. The effects of both miracle fruit and G. sylvestre are temporary—only lasting between 30 min and 1 h.
The emerging information about the interactions between taste and flavor (noted earlier) suggests that taste modifiers should affect flavors as well as taste. Anecdotal observations led to the experiments conducted at the 2010 meeting of the Association for Psychological Science and a Distinctive Voices lecture presented under the auspices of the National Academy of Sciences (Bartoshuk et al. 2011). Attendees (N = 822) used the general Labeled Magnitude Scale (gLMS) to rate the aroma, sweetness, sourness, and flavor of strawberry and lemon before and after miracle fruit. The strawberry flavor was enhanced by miracle fruit; the lemon flavor was not enhanced. One purpose of this study was to examine the effects of miracle fruit and G. sylvestre on flavor and explain why miracle fruit enhanced strawberry flavor but not lemon flavor.
In the present research, subjects were asked to quantify both flavor and taste sensations to examine interactions between the two. Rozin (1982) noted: “Adults usually know (learn) what sensory system is being stimulated when they experience specific sensations.” However, a problem can arise if subjects are not presented with the categories necessary to describe those sensations. Lawless and Heymann (2010) reviewed the history of the phenomenon of “dumping,” inflation of some sensations resulting when subjects are not provided with appropriate categories for sensations. Mindful of this, we specifically named the taste sensations historically affected (sweet and sour for miracle fruit; sweet for G. sylvestre) and provided visual identification of the foods for aroma and flavor.
Recreational use of miracle fruit (i.e., miracle fruit parties (Farrell and Bracken 2008; Mayhew 2009; D’Onfro 2014)) and clinical use for cancer patients experiencing taste alterations (Soares et al. 2010; Wilken and Satiroff 2012; Swamy et al. 2014) have been cited recently. The result of this study may help interpret this usage because the enhanced palatability of food may depend as much on intensification of flavor as on sweetening per se.
Materials and methods
Panelists
This study was conducted according to the principles of the 1975 Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the University of Florida Institutional Review Board. Written and verbal informed consent was obtained from each participant. One hundred panelists (18–55 years) were recruited from students and staff of the University of Florida. Subjects were excluded if they were vegetarians (the foods included chicken sausage), had allergies to any of the foods to be tested, or disliked any of the foods (and so were reluctant to taste them).
All panelists participated in a training session before the tasting experiments to familiarize themselves with the Global Sensory Intensity Scale (GSIS). The training and tasting sessions took place in the University of Florida Food Science and Human Nutrition Department sensory laboratory. This facility contains 10 individual booths equipped with computers running Compusense.
Verbal instructions were given by the panel leader and also repeated on the computer screen. Subjects provided demographic information including age, gender, height, weight, and ethnic background.
Three independent experiments were conducted on separate days. Not all subjects were available for each of the 3 experiments; thus, the numbers of subjects varied across the 3 experiments. In each experiment, subjects first tasted the 10 foods and then tasted them again after exposure to modifier(s). The order of the foods was randomized each time they were tasted.
In the first experiment (N = 97; 49 men, 48 women), subjects tasted the 10 foods, then tongues were exposed to miracle fruit and subjects re-tasted the foods. Finally, tongues were exposed to G. sylvestre and subjects again re-tasted the foods. The primary purpose of this experiment was to induce sweetness with miracle fruit to see the effects of G. sylvestre on that sweetness. In the second experiment (N = 84; 40 men, 44 women), subjects tasted the foods, were exposed to G. sylvestre, and re-tasted the foods. The third experiment (N = 80; 38 men, 42 women) was similar to the second experiment but the modifier used was miracle fruit. At the end of the study, subjects were compensated with supermarket gift cards for their participation.
Psychophysical method
Panelists used the GSIS to rate the intensities of 10 food samples (Kalva et al. 2014). Panelists sniffed the food samples to rate aroma and placed the samples in the mouth, chewed, and swallowed to rate sweetness, sourness, and the flavor of each food.
The GSIS is the result of methodological research aimed at providing valid comparisons between groups (see Bartoshuk et al. 2005). The technique underlying valid comparisons was first used to compare groups that differed with regard to genetic sensitivities to PTC (phenylthiocarbamide) and its chemical relative PROP (6-n-propylthiouracil) (Hall et al. 1975; Bartoshuk 1979) and was subsequently named “magnitude matching” (Marks and Stevens 1980; Stevens and Marks 1980; Marks et al. 1988). In brief, subjects were asked to rate stimuli relative to a standard that is assumed to show no systematic variation across the groups to be compared. Labeled scales can be devised that permit magnitude matching; the first of these was the gLMS. That scale ran from 0 (no sensation) to 100 (strongest sensation of any kind imaginable) with intermediate intensity descriptors (see Bartoshuk et al. 2004). Subsequent research suggested removing “imaginable” and the intermediate descriptors (Snyder et al. 2008). The GSIS runs from 0 (no sensation) to 100 (strongest sensation of any kind ever experienced) with no intermediate intensity descriptors. To provide valid comparisons of taste intensities, the top of the scale (strongest sensation of any kind ever experienced) must be independent of taste. To insure this, panelists were asked to note the experience that was the strongest to them. Only 1 panelist selected a taste experience as the strongest ever experienced (a bitter medicine). Panelists then rated the following experiences from memory: loudest sound ever heard, loudness of a conversation, loudness of a whisper, brightest light ever seen (usually the sun), brightness of a well-lit room, and brightness of a dimly lit restaurant. These ratings both provided practice in using the scale and also served as a test for comprehension of the scaling instructions.
Panelists rated the intensities of aroma, sweetness, sourness, and flavor of the 10 food samples before and after exposure of the tongue to the taste modifiers under investigation.
Stimuli
Ten food samples were selected to represent a range of foods containing varying levels of sweet and/or sour tastes. Foods were predominantly sour (apple cider vinegar, lemons, French’s yellow mustard, Mt. Olive dill pickle chips), predominantly sweet (Hershey’s dark chocolate Kisses, Aunt Jemima original maple syrup), or both sour and sweet (tomatoes and strawberries). Control foods had very little sour or sweet taste (Armour chicken Vienna sausages and Planters unsalted peanuts).
Unsalted crackers and water were provided at all times; subjects were instructed to cleanse their palate before and after each sample. Samples were served at room temperature (23 °C) in covered 116 mL soufflé cups. Each cup contained a representative amount of each sample (e.g., 1 piece of each fruit sample and approximately 29 mL of a liquid product).
Miracle fruit
Commercially available freeze-dried miracle fruit tablets, “mberry,” were obtained from My M Fruit, LLC. Each tablet was stated to be equivalent to approximately 1 miracle fruit berry. Panelists were instructed to let the tablet dissolve in the mouth, moving it around without chewing. In approximately 5 min, panelists began the food sampling and rating the 10 foods.
Gymnema sylvestre tea
Gymnema sylvestre leaves (Penn Herb Company, Ltd) were brewed as a tea beverage shown to reduce perceived sweetness according to the recipe originally developed by Meiselman: 1500 mL hot water and 100 g tea leaves stirred for 1 h at 95 °C (Meiselman and Halpern 1970). The tea was stored in a refrigerator and warmed to room temperature before using. Subjects held 10 mL in the mouth for 30 s and then expectorated. Panelists rated the foods after exposure of the tongue to the G. sylvestre tea.
Results
Table 1 shows results for all 3 experiments. Note that the experiments were performed on separate days in the order indicated under “Panelists”; however, in Table 1 the results are presented ordering the modifiers conceptually: miracle fruit, G. sylvestre, and miracle fruit followed by G. sylvestre. As noted earlier, the foods were selected based on the magnitudes of the sour and sweet sensations (before modification) and they were organized into those groups shown in Table 1. The left-most columns show the results for miracle fruit. Ratings before and after miracle fruit were compared using paired t-tests with Bonferroni corrections. That is, given 40 observations (10 foods, 4 qualities) the individual P value for a t-test had to be less than 0.00125 to be considered significant at P < 0.05 (“–” for a decrease or “+” for an increase). The center columns of Table 1 show the results for G. sylvestre with similar Bonferroni corrections. The right-most columns show the results for the experiment that tested first miracle fruit and then G. sylvestre. The “aft MF” column compares ratings before to those after miracle fruit. The “aft MF and GS” column compares ratings after miracle fruit to those after both modifiers. t-Tests comparing ratings before either modifier to ratings after both modifiers are described in the text. Bonferroni corrections: given 10 foods, 4 qualities, and 3 comparisons, the individual P values for t-tests had to be less than 0.00042 to be considered significant at P < 0.05.
Table 1.
Effects of miracle fruit (MF) and Gymnema sylvestre (GS) on sour, sweet, flavor and aroma
| bef | aft MF | bef | aft GS | bef | aft MF | aft MF and GS | ||
|---|---|---|---|---|---|---|---|---|
| Predominantly sour foods | ||||||||
| Sour | Vinegar | 48 | 20 – | 51 | 47 | 54 | 28 – | 47 + |
| Lemon | 47 | 13 – | 46 | 45 | 53 | 17 – | 42 + | |
| Mustard | 26 | 11 – | 27 | 28 | 29 | 16 – | 27 + | |
| Pickle | 25 | 10 – | 25 | 22 | 30 | 13 – | 22 + | |
| Sweet | Vinegar | 6 | 32 + | 6 | 3 – | 5 | 27 + | 4 – |
| Lemon | 5 | 38 + | 5 | 2 – | 5 | 41 + | 9 – | |
| Mustard | 6 | 26 + | 6 | 3 – | 6 | 25 + | 5 – | |
| Pickle | 6 | 19 + | 7 | 4 – | 7 | 18 + | 4 – | |
| Flavor | Vinegar | 52 | 45 – | 56 | 51 | 61 | 48 – | 52 |
| Lemon | 48 | 41 – | 47 | 45 | 53 | 44 – | 44 | |
| Mustard | 33 | 31 | 35 | 55 | 38 | 34 | 33 | |
| Pickle | 28 | 25 | 30 | 26 | 34 | 28 – | 27 | |
| Aroma | Vinegar | 50 | 47 | 52 | 46 – | 54 | 52 | 50 |
| Lemon | 29 | 26 | 28 | 25 | 33 | 33 | 29 | |
| Mustard | 33 | 31 | 31 | 29 | 34 | 35 | 31 | |
| Pickle | 28 | 26 | 30 | 27 | 33 | 32 | 29 | |
| Sour and sweet foods | ||||||||
| Sour | Strawberry | 10 | 2 – | 15 | 15 | 15 | 3 – | 15 + |
| Tomato | 10 | 2 – | 9 | 10 | 14 | 4 – | 14 + | |
| Sweet | Strawberry | 24 | 45 + | 22 | 8 – | 30 | 49 + | 12 – |
| Tomato | 10 | 31 + | 13 | 4 – | 11 | 32 + | 6 – | |
| Flavor | Strawberry | 27 | 42 + | 28 | 21 – | 33 | 45 + | 24 – |
| Tomato | 17 | 30 + | 19 | 14 – | 24 | 32 + | 20 – | |
| Aroma | Strawberry | 20 | 19 | 17 | 17 | 24 | 23 | 22 |
| Tomato | 7 | 9 | 7 | 6 | 9 | 11 | 12 | |
| Predominantly sweet foods | ||||||||
| Sour | Syrup | 1 | 1 | 1 | 2 | 1 | 1 | 2 |
| Chocolate | 2 | 1 | 2 | 3 | 3 | 2 | 4 | |
| Sweet | Syrup | 38 | 40 | 39 | 10 – | 45 | 42 | 11 – |
| Chocolate | 32 | 36 | 33 | 9 – | 36 | 38 | 10 – | |
| Flavor | Syrup | 36 | 38 | 37 | 15 – | 38 | 39 | 17 – |
| Chocolate | 34 | 36 | 37 | 17 – | 38 | 37 | 19 – | |
| Aroma | Syrup | 25 | 24 | 24 | 23 | 27 | 26 | 24 |
| Chocolate | 22 | 23 | 23 | 22 | 27 | 26 | 24 | |
| Control foods: little sour or sweet | ||||||||
| Sour | Sausage | 5 | 4 | 6 | 6 | 6 | 5 | 6 |
| Peanut | 1 | 1 | 2 | 2 | 3 | 2 | 3 | |
| Sweet | Sausage | 8 | 13 + | 9 | 8 | 9 | 13 + | 7 – |
| Peanut | 6 | 9 | 6 | 3 – | 7 | 10 | 3 – | |
| Flavor | Sausage | 23 | 25 | 26 | 24 | 27 | 25 | 23 |
| Peanut | 17 | 16 | 17 | 11 – | 21 | 19 | 15 – | |
| Aroma | Sausage | 26 | 28 | 27 | 24 | 29 | 29 | 26 |
| Peanut | 18 | 18 | 18 | 18 | 23 | 19 | 20 | |
Significant at P < 0.05 after Bonferroni correction: increase marked with +, decrease marked with –.
Analysis by food groupings
Results are presented by food group moving from left to right through Table 1.
Predominantly sour foods (vinegar, lemon, mustard, pickle)
After miracle fruit these foods tasted sweeter and less sour. Flavor was not intensified and, in fact, was significantly reduced for vinegar and lemon.
Gymnema sylvestre had relatively little effect on these foods because they produce little sweetness; however, after G. sylvestre the small amount of sweetness present was reduced. Aroma was essentially unchanged except that the aroma of vinegar was slightly reduced. This was the only effect on aroma in all 3 experiments so aroma will not be further described.
In the experiment with both modifiers, after the first modifier, miracle fruit, the effects of miracle fruit alone were essentially duplicated; the only difference was that the pickle flavor was significantly reduced (along with the flavors of vinegar and lemon). After G. sylvestre the increased sweetness and decreased sourness associated with miracle fruit went back toward premodifier ratings.
Comparisons between premodifier ratings and those after both modifiers showed that sourness returned to the premodifier values for vinegar and mustard, but remained significantly lower than the premodifier values for lemon and pickle.
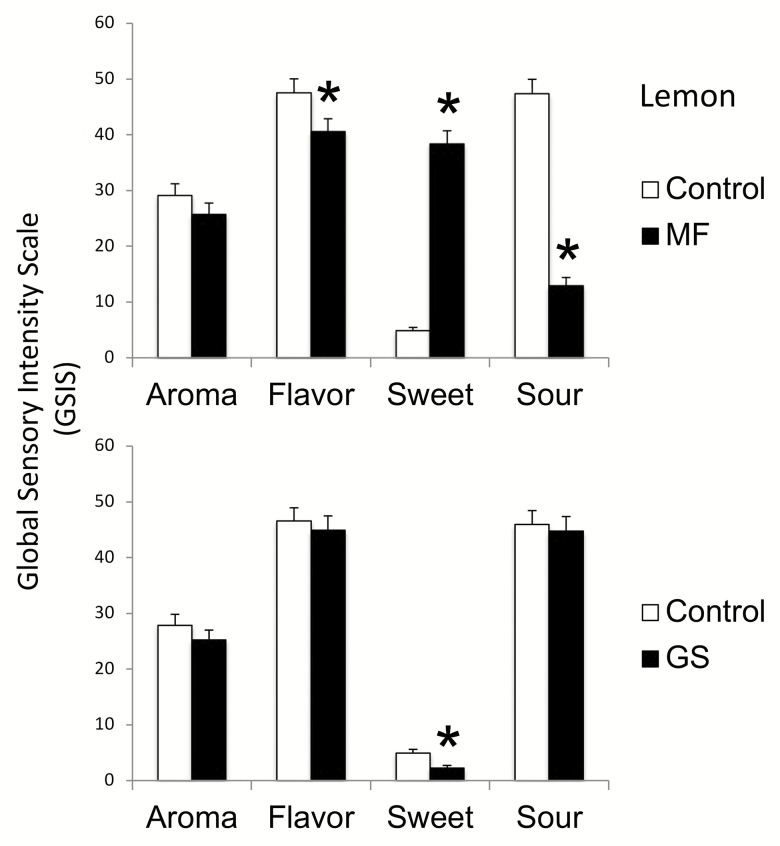
Figure 1 illustrates the effects of miracle fruit and G. sylvestre on predominantly sour foods, using lemon as an example.
Figure 1.
Aroma, flavor, sweet and sour tastes of lemon (± SE) after miracle fruit (MF) and Gymnema sylvestre (GS). Significant differences (shown in Table 1) are indicated by stars.
Sour and sweet foods (strawberry and tomato)
After miracle fruit, these foods tasted sweeter and less sour just as for predominantly sour foods. However, for these sour and sweet foods, flavor was intensified after miracle fruit.
After G. sylvestre, sweetness and flavor were reduced.
In the experiment with both modifiers, the effects after miracle fruit were the same as when miracle fruit was the only modifier presented, as expected; foods were sweeter and less sour; flavor was intensified.
After G. sylvestre and miracle fruit, sweetness and flavor were reduced and sourness was intensified.
Comparisons of ratings before either modifier with those after both modifiers showed that sweetness ratings were reduced to values below those before either modifier. Flavor ratings were also reduced to values below those before either modifier, but the reduction was only significant for strawberry. Sourness returned to the premodifier values.
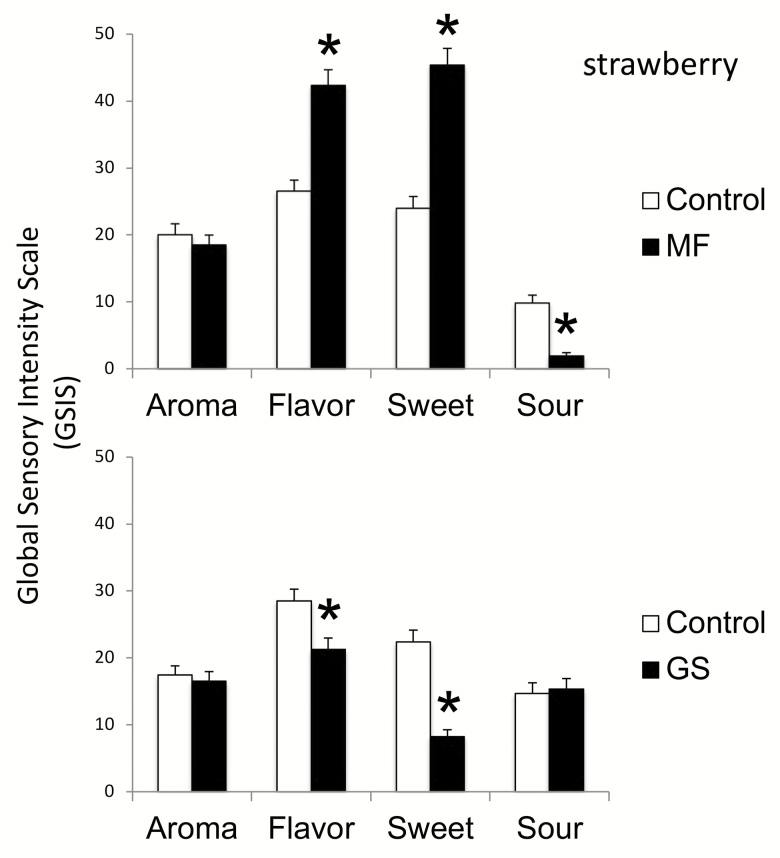
Figure 2 illustrates the effects of miracle fruit and G. sylvestre on the sour–sweet foods using strawberry.
Figure 2.
Aroma, flavor, sweet and sour tastes of strawberry (± SE) after miracle fruit (MF) and Gymnema sylvestre (GS). Significant differences (shown in Table 1) are indicated by stars.
Predominantly sweet foods (syrup and chocolate)
Miracle fruit had no effects on these foods.
After G. sylvestre, sweetness and flavor were reduced.
In the experiment with both modifiers, the effects after miracle fruit were the same as when miracle fruit was the only modifier presented, as expected; miracle fruit had no effect.
After G. sylvestre and miracle fruit, the effects were also the same as for G. sylvestre alone; sweetness and flavor were reduced.
Comparisons of ratings before either modifier with those after both modifiers showed sweetness and flavor ratings were significantly below the premodifier ratings.
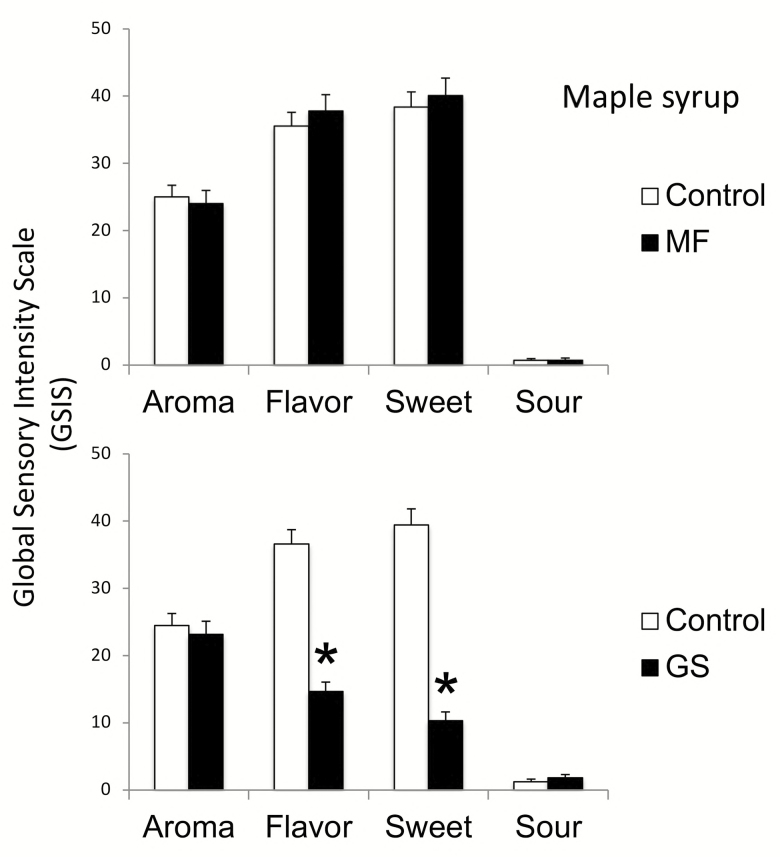
Figure 3 illustrates the effects of miracle fruit and G. sylvestre on the predominantly sweet foods using maple syrup.
Figure 3.
Aroma, flavor, sweet and sour tastes of maple syrup (± SE) after miracle fruit (MF) and Gymnema sylvestre (GS). Significant differences (shown in Table 1) are indicated by stars.
Control foods: little sour or sweet (chicken sausage and peanuts)
For the most part, these control foods showed only small changes with either modifier; however, some of them achieved statistical significance. In general, these small changes are similar to the changes noted earlier: sweetness was intensified after miracle fruit; sweetness and flavor were reduced after G. sylvestre.
Analysis of contributions of sourness and sweetness to flavor
In this study, we used multiple regression (SPSS; SPSS Inc.) to study associations between sourness, sweetness, and food flavor (Brace et al. 2013); data used came from the first occasion on which subjects tasted the foods (“before” condition for the experiment using both modifiers). Each of the foods that were either predominantly sour or both sour and sweet (vinegar, lemons, mustard, pickles, strawberries, and tomatoes) was analyzed individually with the perceived food flavor as the dependent variable and the perceived sweetness and perceived sourness as the independent variables. Bonferroni corrections (6 foods with 2 taste qualities for each) showed that for all 6 foods, sourness was a significant contributor to flavor at P < 0.01. Sweetness was also a significant contributor to flavor for strawberry (P < 0.01) and tomato (P = 0.01).
Discussion
Effects of miracle fruit and G. sylvestre on the tastes of the foods
After miracle fruit, sweetness was intensified in predominantly sour foods as well as sour and sweet foods. The chicken sausage was the only additional food to show a significant increase in sweetness (albeit a small increase); this was presumably due to a small amount of acid in the sausage. This corroborates (using foods) the results of previous studies using pure acids.
After G. sylvestre, all of the sweet tastes (with the exception of a small amount of sweetness reported for the chicken sausage) were reduced. This corroborates (using foods) the results of previous studies using a variety of sweeteners.
In the experiment using both modifiers, not surprisingly for the sour and sweet foods (strawberry and tomato), after G. sylvestre, the original sweetness and the sweetness intensification following miracle fruit were reduced.
We previously showed that after miracle fruit, pure acids tasted sweet as well as less sour. After subsequent G. sylvestre, that sweetness was abolished and sourness returned to near original values. The results of this study show similar results when sweet and sour foods were the stimuli.
Effects of miracle fruit and G. sylvestre on the flavors of the foods
This study confirms previous studies showing that sweet and sour tastes can intensify flavor. Thus, we predicted that the changes in sweetness and sourness induced by modifiers should alter flavors in foods. Elevations in sweet should intensify flavor; reductions in sweet or sour should reduce flavor.
Sweet was reduced by G. sylvestre in the sweet foods and flavor was reduced in all of the cases where sweet reduction was substantial, as predicted. The only failures of flavor reduction occurred for the small amount of sweetness reduction in the predominantly sour foods.
Sweet was enhanced by miracle fruit in the sour foods. However, flavor was only enhanced in foods that were sour and sweet; flavor was not enhanced in predominantly sour foods. We suggest that the failure of miracle fruit to intensify flavor in the predominantly sour foods is due to the dual action of miracle fruit: Miracle fruit intensifies sweetness but also reduces sourness. Because both sweet and sour contribute to flavor, in predominantly sour foods (vinegar, lemon, mustard, pickle) miracle fruit induces opposing effects: Increased sweetness intensifies flavor, whereas reduced sourness reduces it. The sum total appears to be little or no change in the overall flavor.
For the sour and sweet foods (strawberry and tomato), enough acid was present to activate miracle fruit, but the sweetness added by miracle fruit was apparently enough to outweigh the loss of flavor due to the reduction of sourness.
Recently, popular accounts of miracle fruit parties have added anecdotal descriptions of the effects of miracle fruit (Farrell and Bracken 2008; Mayhew 2009; D’Onfro 2014). One particularly insightful observation noted that when miracle fruit is used with acidic foods and drinks, “underlying layers of flavor become more perceptible” (Mayhew 2009). We suggest that this may be due to intensification of flavor produced by the intensification of sweetness associated with miracle fruit.
A few studies have shown that miracle fruit makes food more palatable to patients having cancer (Soares et al. 2010; Wilken and Satiroff 2012; Swamy et al. 2014). One of the comments made by a patient having cancer was that food had “more flavor than usual.” Note that these comments suggest that part of the success of miracle fruit in enhancing eating is an intensification of flavor rather than simply the addition of a sweet taste. The results of this study support such anecdotes and may help select the foods where enhancement of flavor will be most likely to occur.
Funding
This work was supported by the National Institutes of Health [R21 DC013751].
Conflict of interest
Lorenzo Puentes is employed by Merieux Nutrisciences Company and Shawn C. Dotson is employed by Coca-Cola Company. There have been no involvements that might raise the questions of bias in the work reported or in the conclusions, implications, or opinions stated.
References
- Baldwin EA, Goodner K, Plotto A. 2008. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J Food Sci. 73:S294–S307. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. 1979. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 205:934–935. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Bohannon J, Cahill D, Cogan E, Heiphetz L, Hobbs K, Hudson S, Lincoln SH, Lorig T, Marton R et al. 2011. Miracle fruit adds a sweet taste to acidic foods but also intensifies flavor. Annual Meeting of the Association for Psychological Science; Boston, MA. [Google Scholar]
- Bartoshuk LM, Dateo GP, Vandenbelt DJ, Buttrick RD, Long L. 1969. Effects of Gymnema sylvestre and Synsepalum dulcificum on taste in man. In: Pfaffmann C, editor. Olfaction and taste III. New York: Rockefeller University Press; p. 436–444. [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. 2004. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82:109–114. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Fast K, Snyder D. 2005. Differences in our sensory worlds: invalid comparisons with labeled scales. Curr Dir Psychol Sci. 14:122–125. [Google Scholar]
- Bartoshuk LM, Gentile RL, Molkowitz HR, Meiselman HL. 1974. Sweet taste induced by miracle fruit (Synsepalum dulcificum). Physiol Behav. 12:449–456. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Klee HJ. 2013. Better fruits and vegetables through sensory analysis. Curr Biol. 23:R374–R378. [DOI] [PubMed] [Google Scholar]
- Beare JL. 1906. Greek theories of elementary cognition from Alcmaeon to Aristotle. Oxford: Clarendon Press. [Google Scholar]
- Brace N, Kemp R, Snelgar R. 2013. SPSS for psychologists. New York: Routledge. [Google Scholar]
- Brock WH. 1967. William Prout on taste, smell, and flavor. J Hist Med Allied Sci. 22:184–187. [Google Scholar]
- Burdach KJ, Kroeze JH, Köster EP. 1984. Nasal, retronasal, and gustatory perception: an experimental comparison. Percept Psychophys. 36:205–208. [DOI] [PubMed] [Google Scholar]
- Colquhoun TA, Schwieterman ML, Snyder DJ, Stamps JJ, Sims CA, Odabasi AZ, Klee HJ, Tieman DM, Olmstead JW, Clark DG et al. 2015. Laboratory demonstration of volatile-enhanced-sweetness. Chem Senses. 40:622–623. [Google Scholar]
- Daniell WF. 1852. On the Synsepalum dulcificum, De Cand.; or miraculous berry of Western Africa. Pharm J. 11:445–448. [Google Scholar]
- D’Onfro J. 2014. We were blown away by the miracle berry that let us down shots of vinegar. Business Insider; https://www.businessinsider.com.au/miracle-berry-flavor-tripping-party-2014-6 [Google Scholar]
- Dzendolet E. 1969. Theory for the mechanism of action of “miracle fruit”. Percept Psychophys. 6:187–188. [Google Scholar]
- Farrell P, Bracken K. 2008. A tiny fruit that tricks the tongue. New York: New York Times. [Google Scholar]
- Frank RA, Byram J. 1988. Taste–smell interactions are tastant and odorant dependent. Chem Senses. 13:445–455. [Google Scholar]
- Frank RA, Ducheny K, Mize SJS. 1989. Strawberry odor, but not red color, enhances the sweetness of sucrose solutions. Chem Senses. 14:371–377. [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J. 2012. Enhancement of retronasal odors by taste. Chem Senses. 37:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Bartoshuk LM, Cain WS, Stevens JC. 1975. PTC taste blindness and the taste of caffeine. Nature. 253:442–443. [DOI] [PubMed] [Google Scholar]
- Kalva JJ, Sims CA, Puentes LA, Snyder DJ, Bartoshuk LM. 2014. Comparison of the hedonic general labeled magnitude scale with the hedonic 9-point scale. J Food Sci. 79:S238–S245. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Tsuchiya A, Nakajima K, Ito K, Terada T, Shimizu-Ibuka A, Briand L, Asakura T, Misaka T, Abe K. 2011. Human sweet taste receptor mediates acid-induced sweetness of miraculin. Proc Natl Acad Sci USA. 108:16819–16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-L, Pangborn RM, Noble AC. 1993. Temporal patterns of nasal, oral, and retronasal perception of citral and vanillan and interaction of these odourants with selected tastants. Int J Food Sci Technol. 28:127–137. [Google Scholar]
- Kurihara K, Beidler LM. 1969. Mechanism of the action of taste-modifying protein. Nature. 222:1176–1179. [DOI] [PubMed] [Google Scholar]
- Kurihara K, Kurihara Y, Beidler LM. 1969. Isolation and mechanism of taste modifiers: taste modifying protein and gymnemic acids. In: Pfaffmann C, editor. Olfaction and taste. New York: Rockefeller Press; p. 450–469. [Google Scholar]
- Lawless HT, Heymann H. 2010. Sensory evaluation of food. New York: Springer. [Google Scholar]
- Marks LE, Stevens JC. 1980. Measuring sensation in the aged. In: Poon LW, editor. Aging in the 1980’s: psychological issues. Washington: American Psychological Association; p. 592–598. [Google Scholar]
- Marks LE, Stevens JC, Bartoshuk LM, Gent JG, Rifkin B, Stone VK. 1988. Magnitude matching: the measurement of taste and smell. Chem Senses. 13:63–87. [Google Scholar]
- Mayhew LJ. 2009. Miracle fruit. Imbibe Magazine; http://imbibemagazine.com/miracle-fruit/ [Google Scholar]
- McBride RL, Johnson RL. 1987. Perception of sugar-acid mixtures in lemon juice drink. Int J Food Sci Technol. 22:399–408. [Google Scholar]
- Meiselman HL, Halpern BP. 1970. Effects of Gymnema sylvestre on complex tastes elicited by amino acids and sucrose. Physiol Behav. 5:1379–1384. [DOI] [PubMed] [Google Scholar]
- Moncrieff RW. 1967. The chemical senses. London: Leonard Hill. [Google Scholar]
- Murphy C, Cain WS. 1980. Taste and olfaction: independence vs interaction. Physiol Behav. 24:601–605. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM. 1977. Mutual action of taste and olfaction. Sens Processes. 1:204–211. [PubMed] [Google Scholar]
- Noble AC. 1996. Taste–aroma interactions. Trends Food Sci Technol. 7:439–444. [Google Scholar]
- Ogle W. 1870. Anosmia, or cases illustrating the physiology and pathology of the sense of smell. Med Chir Trans. 53:263–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GTW. 1899. On the analysis of perception of taste. Univ Iowa Stud Psychol. 2:85–127. [Google Scholar]
- Prout W. 1812. Observations upon the sensations of taste and smell. Med Phys J. 22:457–461. [PMC free article] [PubMed] [Google Scholar]
- Rozin P. 1982. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 31:397–401. [DOI] [PubMed] [Google Scholar]
- Schwieterman ML, Colquhoun TA, Jaworski EA, Bartoshuk LM, Gilbert JL, Tieman DM, Odabasi AZ, Moskowitz HK, Folta KM, Klee HJ et al. 2013. Strawberry flavor: diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS One. 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström LB, Cairncross SE. 1955. Role of sweeteners in food flavor. In: Use of sugars and other carbohydrates in the food industry. Washington, D.C: American Chemical Society; p. 108–113. [Google Scholar]
- Snyder DJ. 2010. Multimodal interactions supporting oral sensory capture and referral. In: Neuroscience. New Haven, CT: Yale University, PhD thesis [Google Scholar]
- Snyder DJ, Clark CJ, Catalanotto FA, Mayo V, Bartoshuk LM. 2007. Oral anesthesia specifically impairs retronasal olfaction. Chem Senses. 32:A15. [Google Scholar]
- Snyder DJ, Dwivedi N, Mramor A, Bartoshuk LM, Duffy VA. 2001. Taste and touch may contribute to the localization of retronasal olfaction: unilateral and bilateral anesthesia of cranial nerves V/VII. In: Society of neuroscience abstract, Vol. 27 San Diego (CA): Society for Neuroscience; p. AA-2, Program No. 727.711. [Google Scholar]
- Snyder DJ, Puentes LA, Sims CA, Bartoshuk LM. 2008. Building a better intensity scale: which labels are essential?Chem Senses. 33:S142. [Google Scholar]
- Soares HP, Cusnir M, Schwartz MA, Pizzolato J, Lutsky RJ, Campbell JL, Beaumont JL, Eton D, Stonick S, Lilenbaum R. 2010. Treatment of taste alterations in chemotherapy patients using the “miracle fruit”. J Clin Oncol. 28:e19523. [Google Scholar]
- Stevens JC, Marks LE. 1980. Cross-modality matching functions generated by magnitude estimation. Percept Psychophys. 27:379–389. [DOI] [PubMed] [Google Scholar]
- Swamy KB, Hadi SA, Sekaran M, Pichika MR. 2014. The clinical effects of Synsepalum dulcificum: a review. J Med Food. 17:1165–1169. [DOI] [PubMed] [Google Scholar]
- Tieman D, Bliss P, McIntyre LM, Blandon-Ubeda A, Bies D, Odabasi AZ, Rodríguez GR, van der Knaap E, Taylor MG, Goulet C et al. 2012. The chemical interactions underlying tomato flavor preferences. Curr Biol. 22:1035–1039. [DOI] [PubMed] [Google Scholar]
- Wilken MK, Satiroff BA. 2012. Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin J Oncol Nurs. 16:E173–E177. [DOI] [PubMed] [Google Scholar]
- Zwaardemaker H. 1895. Die physiologie des geruchs. Leipzig: Verlag von Wilhelm Engelmann. [Google Scholar]