Abstract
CD4+CD25+ regulatory T cells can prevent and resolve intestinal inflammation in the murine T cell transfer model of colitis. Using Foxp3 as a marker of regulatory T cell activity, we now provide a comprehensive analysis of the in vivo distribution of Foxp3+CD4+CD25+ cells in wild-type mice, and during cure of experimental colitis. In both cases, Foxp3+CD4+CD25+ cells were found to accumulate in the colon and secondary lymphoid organs. Importantly, Foxp3+ cells were present at increased density in colon samples from patients with ulcerative colitis or Crohn’s disease, suggesting similarities in the behaviour of murine and human regulatory cells under inflammatory conditions. Cure of murine colitis was dependent on the presence of IL- 10, and IL-10-producing CD4+CD25+ T cells were enriched within the colon during cure of colitis and also under steady state conditions. Our data indicate that although CD4+CD25+ T cells expressing Foxp3 are present within both lymphoid organs and the colon, subsets of IL- 10-producing CD4+CD25+ T cells are present mainly within the intestinal lamina propria suggesting compartmentalization of the regulatory T cell response at effector sites.
Keywords: T Cells, Mucosa, Tolerance/Suppression/Anergy
Introduction
Increasing evidence suggests that functionally specialized subsets of CD4+ T cells play a key role in the regulation of immune responses (1,2). In particular, naturally occurring CD4+CD25+ regulatory T cells (TR) have been shown to prevent both T cell-mediated and innate immune pathology in a number of disease models (3–5). CD4+CD25+ TR cells with similar properties to those described in mice are also present in humans and impaired function of these cells has been observed in patients with autoimmune disease (6–11). The transcription factor Foxp3 is differentially expressed by CD4+CD25+ TR cells and plays a key role in their development and function (12–14). Accordingly, mice lacking functional Foxp3 develop a multiorgan- inflammatory disease (12, 15, 16). Similarly, loss of function mutations in FOXP3 have been shown to be responsible for the human autoimmune and inflammatory disease, immune polyendocrine X-linked enteropathy, providing evidence that TR cells also contribute to immune homeostasis in humans (17–18).
The T cell transfer model of colitis provides a good system with which the mechanisms of TR function can be dissected. In this model, transfer of naive CD4+CD45RBhigh T cells into immunodeficient mice leads to a Th1-mediated colitis, while co-transfer of CD4+CD25+ TR cells can completely prevent disease (4). In addition, recent studies in models of type 1 diabetes and inflammatory bowel disease (IBD) have shown that CD4+CD25+ TR not only prevent development of disease but can also actually reverse established inflammation (19–22). Thus, using the T cell transfer model of colitis, we found that CD4+CD25+ T cells can reverse an established T cell-mediated inflammatory response in the intestinal mucosa by reducing the pathogenic T cell infiltrate, ultimately leading to restoration of normal intestinal architecture. It is likely that the mechanisms by which TR can prevent or cure colitis differ. In prevention of colitis, TR must control activation of a predominantly naive population of cells, while in cure of colitis, they must act on Ag-experienced cells and an aggressive inflammatory response.
The ability of CD4+CD25+ TR cells to resolve established inflammation in model systems raises the possibility that these cells may be useful as therapeutic agents for chronic inflammatory diseases in humans. With this in mind, it will be important to establish whether the properties of TR cells determined through study of prevention of colitis also apply to cure of colitis. Attempts to design effective therapeutic strategies will also be aided by knowledge of the location and behaviour of TR in the human IBDs.
We have previously shown that, during cure of experimental colitis, CD4+CD25+ T cells proliferate and accumulate in the mesenteric lymph nodes (MLN) and also in the colonic lamina propria (LP). At both sites, the progeny of CD4+CD25+ T cells are in direct contact with CD11c+ dendritic cells, as well as effector T cells (20). These findings suggest that regulation of an active immune response by CD4+CD25+ T cells occurs in the draining lymph node, as well as at the site of inflammation. However, these studies have been limited by the lack of specific markers for naturally arising TR cells. Although useful, CD25 expression does not uniquely identify TR cells, as the CD4+CD25+ pool can contain activated effector cells and not all TR cells express CD25. Identification of Foxp3 as a more specific marker for TR cells provides an opportunity to track the fate of TR cells in vivo (23–24), although studies to date have primarily focused on expression of Foxp3 mRNA.
Cure of colitis by CD4+CD25+ T cells has also been shown to be functionally dependent on IL- 10, however, it is not known whether TR cells themselves are the important source of IL-10 and where it is produced (19). In this study, we have used anti-Foxp3 Abs together with analysis of IL-10 secretion to further investigate the functional and phenotypic characteristics of CD4+CD25+ T cells under physiological conditions and during resolution of intestinal inflammation. Our results show that during cure of intestinal inflammation the majority of Foxp3+ cells and IL-10-secreting cells derive from CD4+CD25+ precursors. However, whereas Foxp3+ cells are present in similar frequencies in both the secondary lymphoid organs and LP of colitic animals, IL-10-producing CD4+CD25+ T cells selectively enrich within the colonic LP. In addition, we have extended these findings to analysis of human IBD and show an accumulation of Foxp3+CD4+CD25+ cells in the inflamed intestine of patients with IBD. This suggests that chronic intestinal inflammation in humans is not simply a consequence of a lack of TR cells at the inflammatory site.
Results
In situ distribution of Foxp3+CD4+CD25+ T cells in wild-type mice
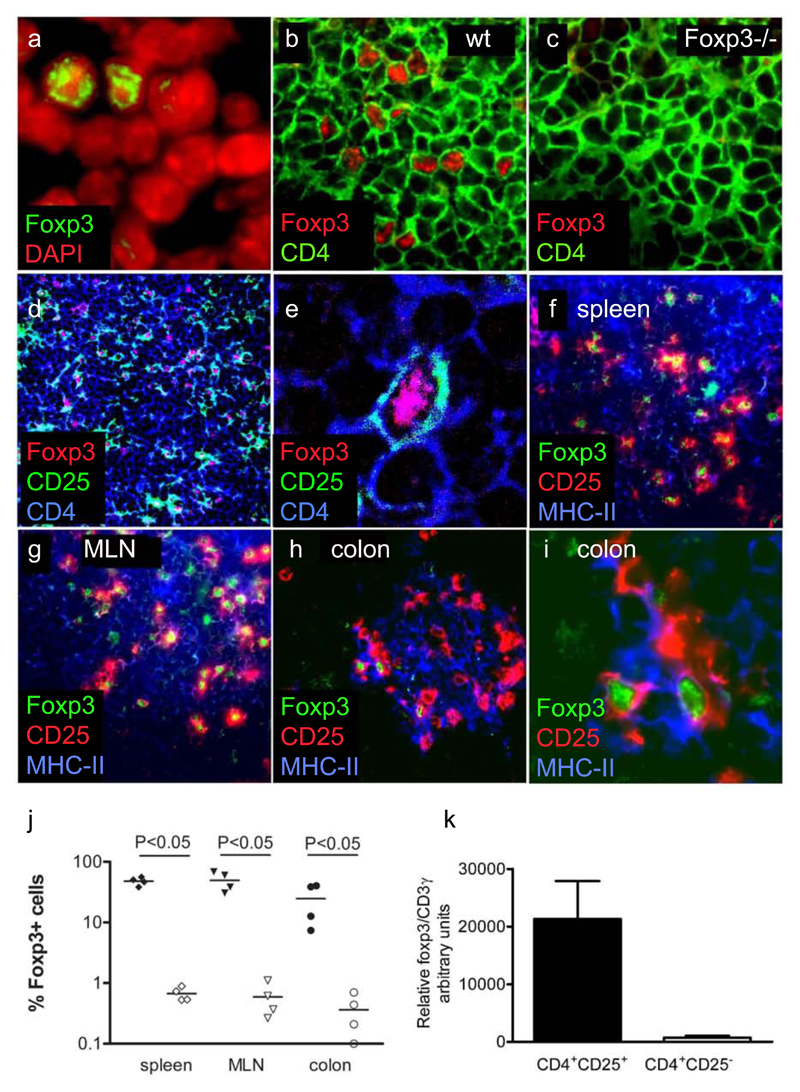
Numerous studies have demonstrated that TR activity enriches within the CD4+CD25+ subset, and this is consistent with its high level of expression of Foxp3. However, most studies have analysed expression of Foxp3 mRNA at the population level. The frequency of bona fide Foxp3 protein-expressing TR cells within the CD4+CD25+ T cell pool and their distribution in vivo remain to be established. To investigate this, expression of CD25 was analysed in combination with Foxp3 (Figure 1). In tissue sections from spleen, MLN, and colon of wild-type mice, a minority of DAPI-stained cell nuclei also stained in a dotted pattern with the polyclonal rabbit anti-Foxp3 serum (Figure 1). Control experiments using normal rabbit serum did not lead to any nuclear staining, whereas the rabbit anti-histone H2B Ab stained all cell nuclei (data not shown). The specificity of the staining was further confirmed by the absence of nuclear staining in secondary lymphoid organs of Foxp3−/− mice (Figure 1 b and c). Foxp3 staining was found within a subpopulation of CD4+ cells homogenously distributed in the T cells areas of secondary lymphoid organs. Furthermore, Foxp3 expression was largely associated with the expression of CD25 (Figure 1 d–j). Thus, 48 ± 7 and 49 ± 18% of CD25+ cells were Foxp3+ in the spleen or MLN, respectively. In contrast, only 0.7 ± 0.2 and 0.6 ± 0.4% of CD25− cells were Foxp3+ (Figure 1j). This enrichment of Foxp3+ cells within the CD25+ pool is in accordance with the mRNA expression of these cells (Figure 1k). The frequency of Foxp3+ cells among CD25+ cells in the colon, at 20 ± 18%, was lower than in the spleen and MLN. Whether this represents a real reduction in the frequency of TR cells or is secondary to an increased frequency of activated CD4+CD25+ T cells is not known. Costaining of CD25, Foxp3 and MHC-II indicated that 60–90% of CD25+Foxp3+ cells were in contact with MHC- II+ cells (Figure 1f–i), including in the intestine where they were preferentially contained within organized leukocytic clusters (Figure 1h and i). The presence of Foxp3 cells in colonic lymphoid structures argues for immunosuppressive activity of TR cells not only in the spleen and MLN, but also in the colon.
Figure 1. Expression of Foxp3 by CD4+CD25+ T cells in the lymphoid organs and colon of wild-type BALB/c mice.
(a) Wild-type MLN stained for Foxp3 and DAPI. (b) Wild-type spleen and (c) Foxp3−/− spleen stained for CD4 and Foxp3. (d) Overview and (e) high power magnification of wild-type MLN stained for CD4, CD25, and Foxp3 and analysed using laser-scanning microscopy. (f–i) Wild-type spleen (f), MLN (g), and colon (h) stained for Foxp3, CD25+, and MHC-II and analysed using conventional fluorescence microscopy. (h) and (i) show the same section of colon at different magnifications. (j) Percentage of Foxp3+ among CD25+ cells (filled symbols) and Foxp3+ among CD25− cells (open symbols) in the T cell area of spleen, MLN, and colon of BALB/c mice. The quantification is based on the approximate number of cells per area of lymphoid organ as indicated by the DAPI nuclear staining. Statistical significance was tested using the Mann- Whitney U test. Similar expression patterns of Foxp3 were found in spleen, MLN, and colon of C57BL/6 mice. k, Expression of Foxp3 mRNA by splenic CD4+CD25+CD45RBlow and CD4+CD25−CD45RBlow T cells. Foxp3 mRNA expression was normalized to CD3γ expression. Data from RNA expression analysis of three independent FACS sorts were pooled.
In situ distribution of Foxp3+CD4+CD25+ T cells during cure of colitis
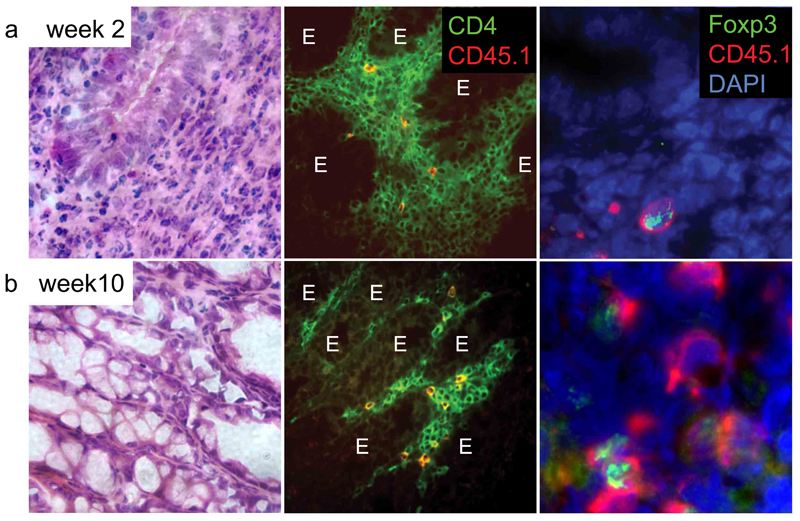
We next investigated the distribution of Foxp3+ cells following cure of colitis by transfer of CD4+CD25+ T cells. Colitis was induced in immune-deficient (C57BL/6J Rag−/−) mice by transfer of wild-type (CD45.2+) CD4+CD45RBhigh T cells. After development of the first signs of colitis, indicated by weight loss and diarrhea, a group of mice received a second transfer of congenic CD45.1+CD4+CD25+ T cells. As previously described (20) this second transfer led to increased weight and resolution of colitis (Figure 2). At the time of transfer, 90% of CD25+ T cells expressed Foxp3 (data not shown).
Figure 2. Presence of Foxp3+CD4+CD25+ T cells in the colons of mice with colitis and mice cured of colitis.
Rag-1−/− mice were injected with 4 × 105 wild-type CD45.2+CD4+CD45RBhigh T cells. Four weeks after the T cell transfer, wasting disease and colitis were evident, and a second transfer of 1 × 106 congenic CD45.1+CD4+CD25+ T cells was performed. Sections of colon taken either 2 (a) or 10 (b) weeks after the second T cell transfer were stained with H&E, Abs to CD4 and CD45.1, or Abs to Foxp3 and CD45.1. DAPI counterstaining was performed to visualize the tissue structure. E indicates epithelium. The presented micrographs are representative of three to five mice analysed.
Two weeks after transfer of CD4+CD25+ T cells, when colitis was still present, 50% of the CD45.1+ progeny of the CD4+CD25+ T cells expressed Foxp3 irrespective of whether spleen, MLN, or colon were analysed (Figure 2a and data not shown). Our data suggest that Foxp3+CD4+CD25+ regulatory cells migrate not only to lymphoid organs but also into the inflamed LP. Following resolution of colitis, 5–10 wk after the secondary transfer of CD4+CD25+ T cells, Foxp3 expression was still primarily restricted to the CD25+ progeny and still detectable within 50% of these cells (Figure 2b). Less than 2% of the CD4+CD45RBhigh progeny expressed Foxp3 at this time (n = 4, data not shown), compared with <1% of donor CD45RBhigh T cells directly ex vivo (n = 3, data not shown). Furthermore, in similar experiments performed in mice on the BALB/c background, we again saw no increase in the percentage of Foxp3+ cells among the CD45RBhigh progeny. This suggests that cure of colitis in this model situation is not associated with the induction of Foxp3 in the progeny of CD4+CD45RBhigh T cells.
Presence of Foxp3+IL-10+ cells in the LP of the human colon
The T cell transfer model of colitis reflects several features of the human IBDs, UC and CD. However, the human diseases present distinct histological morphology and are of heterogeneous pathogenicity. Consequently, we investigated the distribution of Foxp3+ cells in human tissue. Within lymphoid organs, as well as in the appendix, Foxp3 expression was confined to the nuclei of CD4+ and CD3+ cells (Figure 3a and e) and present predominantly in the T cell areas. In tissue samples from noninflamed human colons, and in patients with intestinal inflammation, nuclear Foxp3-positive cells of lymphocyte morphology were present with the highest density in lymphoid follicles, although there were also scattered cells within the LP (Figure 3b). The total number of Foxp3+ cells was greater in inflamed tissue than in normal controls (Figure 3c). This in part reflected the increase in CD3+ cells and lymphoid follicles in the inflamed colon (Figure 3c and d). There was no increase in the actual density of Foxp3+ cells in the T cell areas in inflamed vs uninflamed samples. By contrast there was a higher density of Foxp3+ cells in the inflamed LP compared with controls. Together, the data suggest that inflammation is driving the accumulation of regulatory cells, particularly in effector sites.
Figure 3. Presence of Foxp3-positive cells in the mucosa and intestinal lymphoid follicles of patients with IBD.
Colon samples from normal controls and patients with ulcerative colitis (UC), Crohn’s disease (CD), non-IBD inflammations of the colon (diverticulitis (D), pseudomembranous colitis (PC), and CMV-induced colitis (CMV)) were analysed for CD3, CD4, and Foxp3. Sections from tonsil were analysed for CD4 and Foxp3. (a) Co-staining for CD4 and Foxp3 on human tonsil. Overview (×100) and high-power magnification. (b) CD3 and Foxp3 staining on colon tissue. (c) Density of CD3+ and Foxp3+ cells within colon. Numbers of cells were quantified per area at ×400 magnification. Each data point represents one patient. (d) Density of Foxp3+ cells within colonic LP, as well as T and B cell areas of mucosa- associated lymphoid tissue. Each data point represents one patient. (e) Co-staining of Foxp3 and IL-10 on human appendix tissue. Left, Co-staining of Foxp3, IL-10, and DAPI showing IL-10-positive cells within the germinal center (GC) and subepithelial dome (SED) close to the epithelium (E). Detail of Foxp3+IL-10+ cells within the subepithelial dome area are also shown. Right, Co-staining of Foxp3, IL-10, and CD3. Detail shows IL-10+CD3+Foxp3+ cells as well CD3+Foxp3+ cells that are in close contact with IL- 10+CD3−Foxp3− cells.
To analyse whether the accumulation of Foxp3+ cells in inflamed IBD LP is a specific feature of CD or UC or is due to a general accumulation of Foxp3+ cells in inflamed intestinal tissue, we compared the density of Foxp3+ cells in colon sections of patients with IBD with those with diverticulitis, pseudomembranous colitis, or CMV-induced colitis (CMV) (Figure 3b–d). In all cases we found an increased density of Foxp3+ cells indicating that the accumulation of Foxp3+ cells is driven by inflammation in the intestine and is not a specific feature of IBD. The presence of Foxp3+ cells in patients with IBD argues against a simple lack of Foxp3+ TR as a cause of the intestinal inflammation, but for ineffective activity of these cells, or nonresponsiveness to their activity, under conditions of chronic intestinal inflammation.
To investigate whether there is an association of Foxp3 positive cells with IL-10 secretion within the human intestine, we stained human appendix for IL-10 and Foxp3. IL-10-producing cells were found in high density within the germinal center as well as in the subepithelial dome area (Figure 3e). We show the presence of IL-10-positive CD3+Foxp3+ TR cells as well CD3+Foxp3+ cells that are IL-10 negative but are in close contact with IL-10-positive CD3−Foxp3− cells (Figure 3e). This indicates that some Foxp3+ cells secrete IL-10 within the intestine but that these cells are frequently in close contact with IL-10-producing non-T cells (CD3−Foxp3− cells).
Cure of colitis is dependent on IL-10
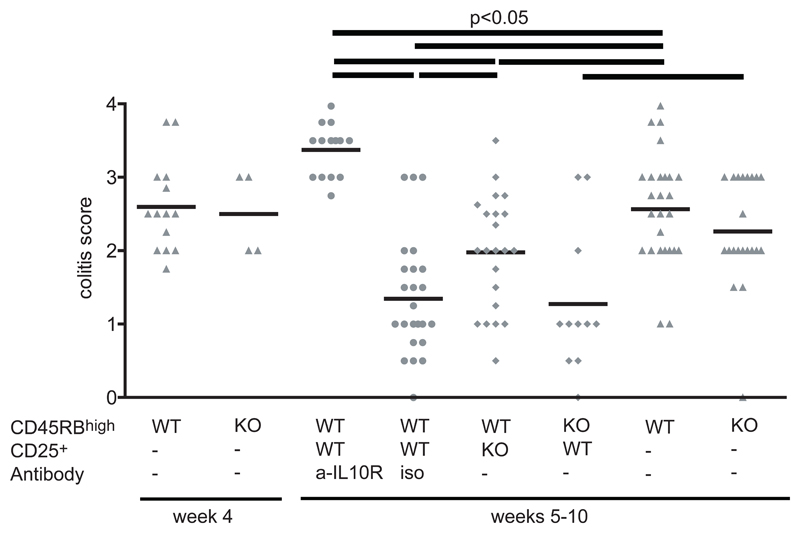
We have described the presence of Foxp3+CD4+CD25+ T cells in both the secondary lymphoid organs and colonic LP during cure of experimental colitis, suggesting that regulation occurs at both sites. We next decided to further investigate the mechanism by which CD4+CD25+ T cells cure colitis. Again, we transferred CD4+CD25+ cells into animals with established T cell- induced colitis. However, if the transfer of CD4+CD25+ cells was combined with injection of a neutralizing anti-IL-10R mAb, mice continued to lose weight and remained colitic (Figure 4). Many different cell types have been shown to be able to produce IL-10. To investigate whether the IL-10 needs to be secreted by the CD4+CD25+ T cell progeny a third group of colitic mice received CD4+CD25+ T cells from IL-10−/− mice. Interestingly, although CD4+CD25+IL-10−/− T cells were less effective at controlling intestinal inflammation than wild-type CD4+CD25+ cells they did lead to some amelioration of disease (Figure 4). The data demonstrates that the presence of IL-10 is required for treatment of colitis, and also suggests that functionally relevant IL-10 may derive from both TR and non-TR sources. However, wild-type CD4+CD25+ cells were equally effective at curing colitis induced by wild-type or IL-10−/− CD4+CD45RBhigh cells, suggesting that IL-10 production by CD4+CD45RBhigh progeny is not critical for cure of colitis.
Figure 4. Cure of colitis depends on the presence of IL-10 from CD4+CD25+ T cells, as well as other cell types.
Colitis was induced by transfer of 4 × 105 CD4+CD45RBhigh T cells into CB17 SCID mice. After development of colitis, mice were injected with 1 × 106 CD4+CD25+ T cells from wild- type mice together with anti-IL-10R mAb or isotype control Abs. Further groups of colitic mice received either CD45RBhigh or CD4+CD25+ T cells from IL-10−/− mice. Sections of colon were scored for the severity of colitis. Score values above 2 indicate mice with pronounced colitis. Data are pooled from four independent experiments.
IL-10-producing CD4+CD25+ T cells enrich within the colon
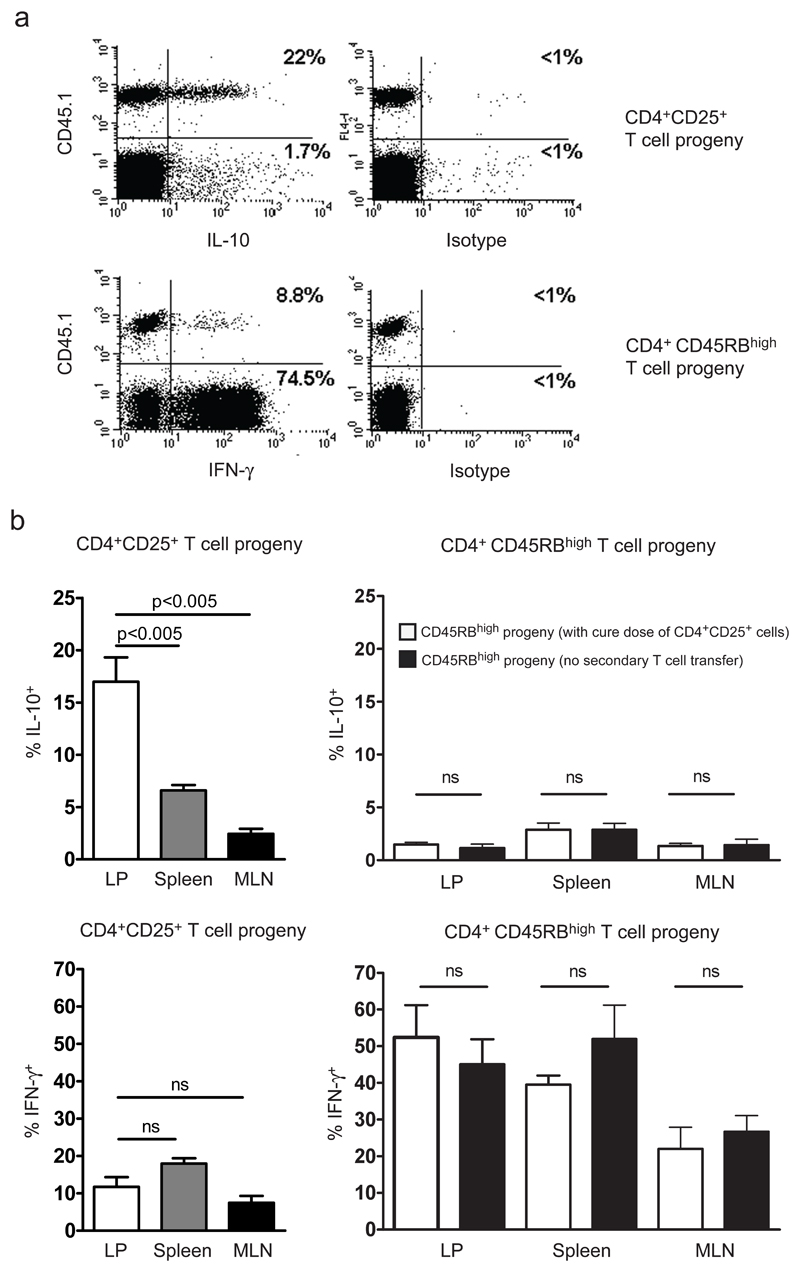
To further investigate the source of IL-10 and the frequency of IL-10-producing T cells within the CD4+CD25+ T cell progeny during cure of colitis, we used the congenic system described above. T cells were isolated from colitic and cured mice and activated using PMA/ionomycin. This permitted analysis of the potential of the progeny of the CD4+CD45RBhigh cells, and the progeny of the CD4+CD25+ cells, to produce IL-10 and IFN-γ (Figure 5). A significant proportion of T cells derived from the CD4+CD45RBhigh progeny produced IFN-γ, irrespective of whether they were isolated from spleen, MLN, or colonic LP (Figure 5). Indeed the frequency of IFN-γ+ cells among the CD4+CD45RBhigh progeny in the LP was similar in colitic mice and mice with TR-mediated resolution of colitis (Figure 5b). Within this population there was no significant induction of IL-10 production, indicating that cure of colitis does not involve immune deviation of the colitogenic T cell population into one producing immune suppressive cytokines (Figure 5b). A significantly higher proportion of the progeny of CD4+CD25+ cells produced IL-10 than the progeny of CD4+CD45RBhigh cells (p < 0.05). Strikingly, IL-10- producing CD4+CD25+ progeny were selectively enriched within the colonic LP (Figure 5b). To test whether the enrichment of IL-10-producing cells within the colonic LP was a general feature of CD4+CD25+ T cells, or due to the specific inflammatory conditions present during the cure of colitis, we analysed IL-10 production by CD4+CD25+ T cells from the colons of wt mice. Consistent with the results obtained in the T cell transfer model, we were able to identify IL-10-producing CD4+CD25+ cells in wild-type mice that selectively enriched within the colonic LP (data not shown). We were also able to show that in wt mice the IL-10 secreting T cells isolated from the colonic LP expressed Foxp3 (Figure 6).
Figure 5. Accumulation of the IL-10-producing progeny of CD4+CD25+ T cells within the colon.
Rag-1−/− mice were injected with 4 × 105 wild-type CD45.2+CD4+CD45RBhigh T cells. After development of colitis, mice received a second transfer of 1 × 106 congenic CD45.1+ CD4+CD25+ T cells. After 4 wk, the ability of CD4+CD45RBhigh (n = 6) and CD4+CD25+ (n = 6) progeny to produce IL-10 and IFN-γ was analysed. Lymphocytes were prepared from spleen, MLN, and the colonic LP and stimulated with PMA/ionomycin and brefeldin A. The results of two independent experiments are pooled. (a) Representative FACS plot showing preferential IL-10 production by LP CD4+CD25+ T cell progeny (CD45.1+) and preferential IFN-γ production by the CD4+CD45RBhigh T cell progeny (CD45.1−). (b) Production of IL-10 and IFN-γ by CD4+CD25+ T cell progeny and comparison of IL-10 and IFN-γ production by CD4+CD45RBhigh T cell progeny in the presence or absence of CD4+CD25+ T cells. Data are shown for spleen, MLN, and LP. Significance was tested using the Mann-Whitney U test; n.s., not significant.
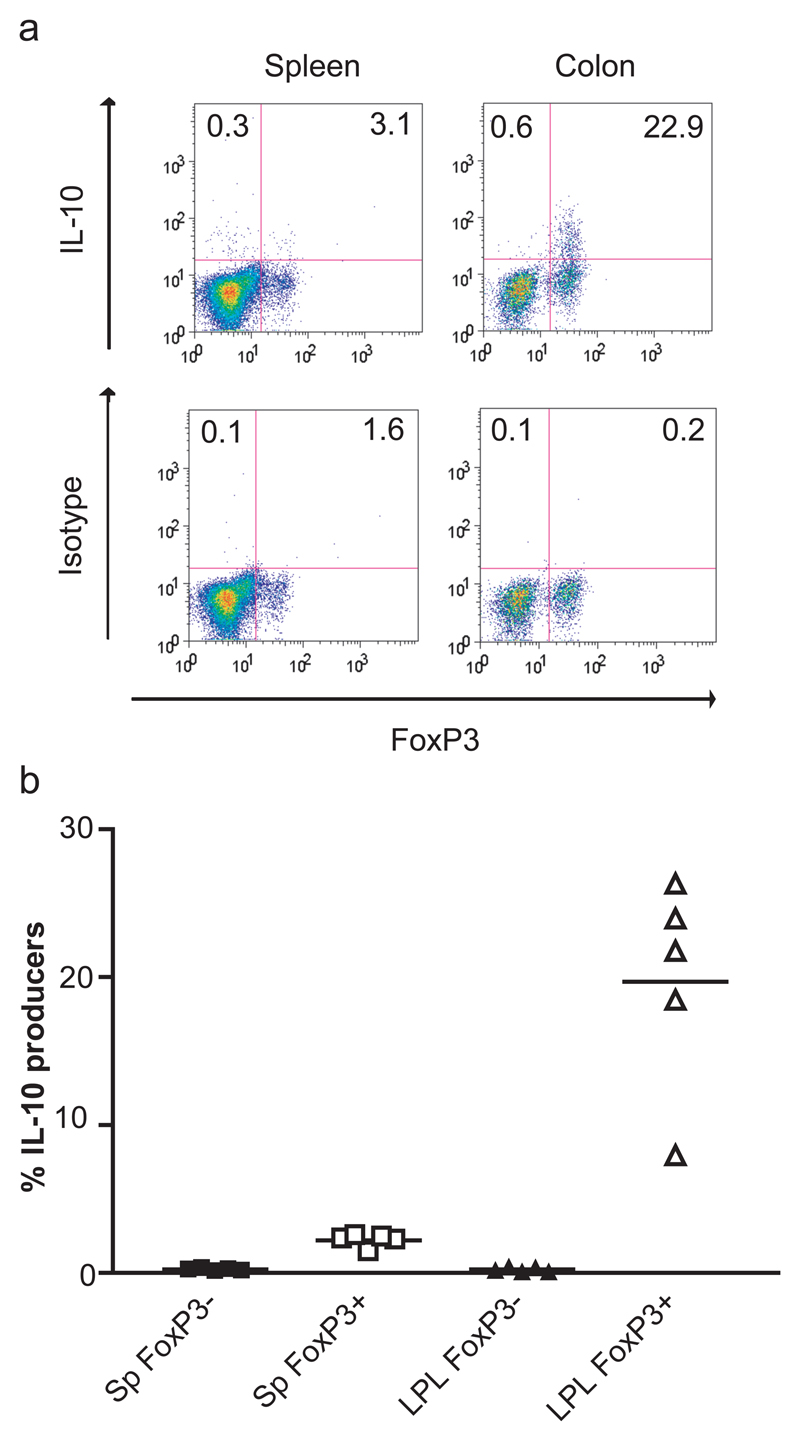
Figure 6. IL-10 secretion by Foxp3+ cells in the LP of wild-type mice.
Lamina propria and spleen cell suspensions were restimulated with PMA/ionomycin as described in Materials and Methods. (a) Representative dot plots showing Foxp3 expression against IL-10 or an isotype control in spleen and LP of B6 SJL CD45 congenic mice. Plots are gated on CD4+ cells. The numbers indicate the percentage of IL-10+ cells in the Foxp3− or Foxp3+ populations. (b) Percentage of IL-10-secreting cells in the Foxp3− (filled symbols) and Foxp3+ (open symbols) populations from spleen (Sp) and LP CD4+ lymphocytes (LPL) from B6 SJL CD45 congenic mice. Each symbol represents data from an individual mouse. In all cases, the isotype control stained <1% of total cells. Similar results were obtained in an experiment using BALB/c mice.
Discussion
In previous studies, we and others have shown that transfer of CD4+CD25+ TR cells to colitic mice is sufficient to resolve the inflammatory response leading to restoration of normal intestinal architecture (19–20). Here we have analysed the anatomical location and mechanism by which small numbers of TR cells are able to overcome sustained activation of the innate and adaptive immune response. Using expression of Foxp3 protein as a marker of bona fide naturally arising TR cells, we show that cure of colitis involves the accumulation of Foxp3+ TR cells in the secondary lymphoid tissue as well as in the colonic LP. Such cells are also present in the intestine of normal mice and are increased in number in the intestine of patients with IBD. We also show that cure of colitis by CD4+CD25+ TR cells involves an IL-10-dependent mechanism, and that CD4+CD25+ TR cells are able to secrete IL-10. Strikingly, IL-10-producing CD4+CD25+ T cells were most prominent in the colonic LP of both colitic mice and unmanipulated wild-type mice suggesting further functional specialization of TR cells in effector sites.
Although naturally occurring TR cells enrich within the CD4+CD25+ subset, CD25 itself is a marker of activation and therefore unsuitable as an indicator of T cells with regulatory potential. However, the transcription factor, Foxp3, is highly expressed among the CD4+CD25+ T cell pool, and is in fact required for regulatory T cell development and function (12–14). Foxp3 is therefore a useful and specific marker for naturally occurring TR. Elegant studies by Fontenot et al. (23) using mice with a GFP-Foxp3 fusion protein-reporter knockin allele have shown that approximately half of the CD4+CD25+ population isolated from lymph nodes express Foxp3. However, detailed analysis of Foxp3 expression in situ, and in pathogenic situations, was not performed in that study. Our results using in situ analysis with a Foxp3 polyclonal Ab showed that Foxp3+ cells were located in the T cell areas in close contact with APCs. Foxp3-expressing CD4+CD25+ T cells were also found in the colon under normal physiological conditions suggesting an in situ role for TR cells in intestinal homeostasis.
Transfer of naive CD45RBhigh cells into immunodeficient mice leads to the development of a severe colitis, but it is now well-established that cotransfer of CD4+CD25+ TR can prevent disease. More recently, we showed that CD4+CD25+ TR can also bring an existing inflammatory response under control. It can be envisaged that the focus of TR activity may differ in these two situations, with the need for TR activity in the periphery being greater during cure of colitis when T cells have already become activated and migrated into peripheral effector sites. Consistent with this, during and after cure of colitis the progeny of the CD4+CD25+ cells were found both in the secondary lymphoid organs and the LP and follicles of the inflamed colon (20). CD4+CD25+ TR have also been shown to localize to colonic follicles when cotransferred with CD4+ T cells reactive to bacterially expressed OVA (28). Using Foxp3 as a more accurate indicator of regulatory activity, we now show that Foxp3+ cells are also found in both the lymphoid tissue and intestine of colitic mice. By contrast with normal physiological conditions, where Foxp3+ cells account for 20% of CD4+CD25+ cells in the colon, during cure of experimental colitis, Foxp3+ cells accounted for some 50% of the CD25+ progeny. This could indicate that the accumulation of Foxp3+CD4+CD25+ T cells may be in part driven by intestinal inflammation, or may simply reflect an even distribution of the transferred splenic CD4+CD25+ T cells in all of the organs analysed.
We have also investigated the role of IL-10 in the cure of colitis by CD4+CD25+ T cells. Our previous studies showed that mice typically started to recover, by around two weeks after TR cell transfer. At this time point, effector T cells, as well as other inflammatory cells, greatly outnumber the CD4+CD25+ progeny. This indicates that CD4+CD25+ TR suppressor mechanisms are locally very potent. This potency may be a consequence of the secretion of high concentrations of immunosuppressive cytokines, or instruction of effector cells to produce such cytokines. Indeed, previous studies have demonstrated the necessity of IL-10 for the cure of T cell-induced colitis, and of CD4+CD25+ T cell-derived IL-10 for inhibition of colonic inflammation and dysplasia (19,29). Our own results confirm IL-10 is required for the resolution of established colitis, and furthermore demonstrate that a proportion of the CD4+CD25+ cell progeny in the colons of treated mice produced IL-10. Similarly, recent studies have demonstrated the presence of IL-10-producing CD4+CD25+ T cells in prediabetic and Leishmania major lesions, and in the CNS in EAE (30–32). However, we also show that IL-10−/− CD4+CD25+ T cells are, although less potent, able to cure colitis. It would appear that although TR are a major source of the IL-10 required for cure of colitis, other cell types, possibly under the instruction of TR, can make an important contribution. Indeed, experiments investigating the protective role of IL-10 in schistosomiasis have shown that both innate immune cells and T cells contribute to IL-10 production (33). Many different cell types, including epithelial cells, macrophages, DC, and other T cells subsets, have been shown to produce IL-10. For example, naive T cells can differentiate into IL-10 producers with regulatory function both in vitro and in vivo (2). Importantly, such cells are also able to cure colitis in the T cell transfer model (21). However, we do not consider the naive T cell population to be an essential source of IL-10 in our model. This is based on the finding that colitis induced by IL-10−/−CD45RBhigh T cells was efficiently cured by CD4+CD25+TR cells.
Foxp3+ TR cells can also arise from naive progenitors in vivo following chronic exposure to low doses of Ag (34) or in vitro when activated in the presence of TGF-β (24, 35–37). However we found no evidence that resolution of intestinal inflammation, at least in the T cell transfer model, was associated with significant immune deviation of CD4+CD45RBhigh progeny into either Foxp3+- or IL-10-producing TR cells. Cure of colitis was also not dependent on a reduction in the proportion of IFN-γ-secreting cells among the CD4+CD45RBhigh progeny, although, as reported previously, the total number of CD4+ T cells present in the colon was greatly reduced (20).
We have previously demonstrated that IL-10 is not required for the prevention of colitis by CD4+CD25+ TR. However, when the whole CD4+CD45RBlow fraction is transferred instead of CD4+CD25+ TR, prevention of colitis becomes dependent on IL-10. In this situation, TR-derived IL-10 appears to be essential for the control of colitogenic cells contained within the Ag-experienced CD45RBlow pool, but not for control of naive T cells (27). The finding that IL- 10 is essential for cure of colitis, where a large population of Ag-experienced cells are present, is consistent with this.
We have shown that the IL-10-producing subset of CD25+ selectively enriches within the colon of cured mice. The population of TR used in our model was isolated from the spleen and as such expressed only low levels of IL-10 upon transfer. It is therefore possible that the gut environment conditioned the TR to further differentiate into IL-10-producing cells. Alternatively, the accumulation of IL-10+ TR in the colon could be attributed to the preferential expansion and homing of a minor subset of IL-10 producers contained within the transferred population. The inability of CD4+CD25+ cells isolated from peripheral lymphoid organs to secrete IL-10 in vitro has led to the notion that naturally arising Foxp3+ TR cells function via cytokine independent mechanisms (38). However, our finding that IL-10+Foxp3+ cells are enriched within tissue sites illustrates the problems with extrapolating observations in vitro to predicted function in vivo. The identification of IL-10-producing CD4+CD25+ T cells in the normal colon suggests a constitutive role for those cells in the prevention of intestinal inflammatory responses. Our data is supported by the earlier observation by Cong et al. (39) that bacterially reactive IL-10-secreting CD4+ T cells are present in the colons of normal mice. However, that study did not establish whether such bacterially reactive cells belonged to the naturally occurring Foxp3+ TR pool, or acquired regulatory activity in the periphery. We now show that IL-10-secreting CD4+CD25+ TR in the normal colon are contained within the Foxp3+ subset. It is however likely that under certain conditions, other IL-10-producing TR cells that do not express Foxp3 may be induced (40).
Human IBDs consist of two dominant disease subtypes. CD is largely Th1 in nature (41), while IL-5-, and IL-13-producing T or NKT cells are found in UC (42). Although there is evidence for T cell-mediated immune dysregulation in human IBD, there is limited evidence that disease results from a primary lack or dysfunction of TR cells. However, clear evidence that a lack of functionally sufficient TR cells can lead to chronic intestinal inflammation in humans does come from the study of patients with immune polyendocrine X-linked enteropathy syndrome (17–18). CD4+CD25+ Foxp3 mRNA expressing T cells have recently been isolated from human intestinal tissue samples, and CD4+CD25+ T cells from the colons of IBD patients have been shown to be suppressive in vitro (43–44). Our data show that Foxp3-positive cells are present within the non-inflamed LP, and are also present in increased numbers in patients with acute UC and CD. The increase in Foxp3+ cell density was seen largely in the LP, rather than in the T cell areas of isolated lymphoid follicles. This can be explained by considering the T cell areas of the isolated lymphoid follicles to be sites of induction of immune responses, analogous to those in the lymph node. Therefore, during an active inflammatory response, TR may be expected to be preferentially recruited to the site of inflammation. A recent study also found an increase in CD25+Foxp3+ cells in the colon of UC and CD patients, although this increase was modest compared with that seen in diverticulitis (45). In our study, a similar increase in Foxp3+ cells is seen in UC, CD, and diverticulitis, but also in CMV colitis and pseudomembranous colitis, strongly suggesting that chronic intestinal inflammation is not simply a consequence of the absence of Foxp3+CD4+ TR cells at the site of inflammation. Whether the TR cells present have, at least in some patients, impaired intrinsic immunosuppressive activity, or whether the inflamed tissue environment is resistant to immunosuppression by TR cells remains to be investigated. In any case, our data on human Foxp3+ cells supports the hypothesis that intestinal immune regulation in mice and humans may share common features. Consequently, further investigation of the mechanisms by which TR can cure experimental colitis may give us insight into how regulatory T cell function might be enhanced in human IBD.
Materials and Methods
Mouse and human tissue samples
BALB/cJ, C57BL/6J, congenic C57BL/6.SJL.CD45, C57BL/6 recombinase-activating gene 1- deficient (rag1−/−), CB17 SCID, IL-10-knockout (IL-10−/−), and Foxp3−/− mice (12) were bred under specific pathogen-free conditions. All mice used were >6 wk old.
Colonic tissue sections from patients with Crohn’s disease (CD) and ulcerative colitis (UC) as well as inflamed and noninflamed human colon, appendix, and tonsil tissue were obtained from the Department of Pathology and from the Department of Ear Nose and Throat diseases of the University of Leipzig. All tissues were obtained for therapeutic and diagnostic reasons. The studies were approved by the local ethical review committee (HU242/04).
Cell purification and flow cytometry
CD4+ T cell subsets were isolated from spleens as described (20). Essentially, erythrocyte- depleted spleen cell suspensions were enriched for CD4+ cells by negative selection following incubation with anti-CD8 (clone YTS169 (25)), anti-B220 (clone RA3-6B2 (26)), anti-CD11b (clone TIB128; American Type Culture Collection (ATCC)) and anti-MHC-II Abs (TIB120; ATCC). Ag-positive cells were depleted using sheep anti-rat-IgG Dynal beads (Dynal Biotech).
For MACS sorting of CD4+CD25+ T cells, CD4+-enriched cells were incubated with biotinylated anti-CD25 (clone 7D4), followed by streptavidin MACS beads, and sorted on an AutoMACS (Miltenyi Biotec).
For FACS sorting of CD4+CD45RBhigh and CD4+CD25+ T cells, CD4+-enriched cells were stained with anti-CD45RB (clone 16A), anti-CD25 (clone 7D4), and anti-CD4 (clone H129.19; all BD Biosciences), and sorted on a MoFlo (DakoCytomation). The purity of MACS- and FACS-sorted cells was >90 and >99%, respectively. Because similar results were obtained using MACS or FACS sorting, data were pooled.
Unless otherwise stated, all Abs were obtained from BD Biosciences.
FACS analysis of Foxp3 expression was performed using the Foxp3 staining kit from eBioscience.
T cell transfer experiments
SCID and rag1−/− mice were injected i.p. with 4 × 105 syngeneic CD4+CD45RBhigh T cells. Mice developed colitis 3.5–4.5 wk posttransfer. Mice with clinical signs of disease were either sacrificed to assess the severity of colitis or received 106 CD4+CD25+ T cells i.p., or no treatment. A further group of mice received weekly injections of 0.5 mg of anti-IL-10R Ab (clone 1B1.2). Mice were observed daily and weighed weekly. All animal experiments were performed according to the home office guidelines and the United Kingdom Animals Scientific Procedures Act of 1986.
Histology
Tissue sections were stained with H&E and colitis severity was graded semiquantitatively from 0 to 4, as outlined in (27).
Mouse tissue samples were snap-frozen and cryocut. Slides were acetone-fixed and blocked with donkey serum (Sigma-Aldrich). The endogenous peroxidase (POD) activity was inhibited using H2O2, sodium azide, and in some cases glucose oxidase. Foxp3 staining was performed using rabbit polyclonal anti-mouse Foxp3 Abs (Celltech R&D) followed by FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories). The Foxp3 staining was combined either with staining for CD4, CD25, MHC class II (MHC-II) or CD45.1 and 4′,6′- diamidino-2-phenylindole (DAPI). Directly labeled anti-CD4-FITC (clone RM4-5; BD Biosciences) was used. Anti-CD25 (clone PC61) was detected using anti-mouse POD (Jackson ImmunoResearch Laboratories), and biotinylated anti-CD45.1 (A20; BD Biosciences) was detected using avidin-POD (Vector Laboratories). The POD activity was visualized by tyramide amplification (NEN Life Science Products) as described previously (20). Biotinylated anti-MHC-II (I-A/I-E, clone 2G9) was detected using directly labeled streptavidin fluorescence conjugates (BD Biosciences). Some tissue sections were counterstained for 3–5 min with 0.1–1 μg/ml DAPI (Sigma Aldrich). Fluorescence was detected using appropriate filter combinations for DAPI, FITC, Texas Red/Cy3, and Cy5.
Human formalin-fixed paraffin-embedded tissue samples were deparaffinized, microwave pressure cooked to demask fixed Ags, and stained for CD3 (clone F7.2.38; DakoCytomation), and Foxp3 (rabbit polyclonal serum against human Foxp3; Celltech R&D) followed by anti-mouse or anti-rabbit POD-labeled Abs and routine POD staining. For costaining experiments, frozen tissue sections of human tonsil or appendix were cryocut, fixed in formalin, and incubated at 85°C in Ag retrieval buffer. After blocking the endogenous POD activity, sections were stained using mouse anti-CD4 (clone 1F6; Serotec) and donkey anti-mouse-POD (Jackson ImmunoResearch Laboratories) followed by tyramide amplification. After subsequent blocking of POD activity, Foxp3 was stained using polyclonal anti-Foxp3 followed by donkey anti-rabbit-POD (Jackson ImmunoResearch Laboratories) and tyramide amplification. DAPI counterstaining was performed to visualize the tissue structure and to ensure the nuclear localization of the Foxp3 signal. Similarly, costaining for IL-10, CD3, and Foxp3 was performed using rat anti-IL-10 (clone JES3-9D7; Caltag Laboratories), polyclonal anti-CD3 (A0452; DakoCytomation) and mouse anti-Foxp3 (clones 105/221D/D3 and 236A/E7; Abcam). Sections were subsequently incubated with appropriate donkey POD- labeled Abs (Jackson ImmunoResearch Laboratories) followed by tyramide amplification.
Quantitative real-time PCR analysis
Cells were FACS sorted to >99% purity and RNA extracted using the RNeasy minikit (Qiagen), including a DNase digestion step. cDNA was transcribed with Superscript II (Invitrogen Life Technologies) and used as a template for real-time quantitative PCR. A multiplex reaction for CD3γ and Foxp3 was preformed using the chromo4 (MJ Research) machine and results expressed relative to an arbitrary standard of CD4+ T cell cDNA. Foxp3 expression was then normalized to the internal standard, CD3γ. The following primers and TaqMan probe sequences were used: CD3γ probe VIC-ACATAGGCACCATATCCGGCTTTATCTTCGTAMRA CD3γ 5′-TTACAGAATGTGTGAAAACTGCATTG CD3γ 3′-CACCAAGAGCAAGGAAGAAGATG Foxp3 probe FAM-ATCCTACCCACTGCTGGCAAATGGAGTC-TAMRA Foxp3 5′-CCCAGGAAAGACAGCAACCTT Foxp3 3′-TTCTCACAACCAGGCCACTTG
Intracellular cytokine staining
Cell suspensions were prepared from spleen, MLN, and the LP. For preparation of LP cell suspensions, colons were cut into 0.5-cm pieces and incubated in RPMI 1640 containing 10% heat-inactivated FCS and 5 mM EDTA for 15 min to remove epithelial cells. This step was performed in a shaking incubator at 37°C, and was repeated a further two times, with all supernatant being discarded. The remaining tissue was digested twice using RPMI 1640 containing 10% FCS, 15 mM HEPES, and 0.3 mg/ml Type II Collagenase/Dispase (Worthington Biochemical) for 1 h in a 37°C shaking incubator. LP cells were collected and layered on a Percoll gradient (Amersham Biosciences) and centrifuged at 600 × g for 20 min. The lymphocyte-enriched population was recovered from the 40 to 75% interface. Cell suspensions were stimulated with 100 ng/ml PMA and 1 μg/ml ionomycin for 4 h in the presence of 20 μg/ml brefeldin A. After this in vitro stimulation, cells were stained for CD4 (clone H129.19) and CD45.1 (clone A20) followed by fixation in 2% paraformaldehyde and permeabilization in 0.5% saponin. This was followed by staining for intracellular IL-10 (clone JES5-16E3), IFN-γ (clone XMG1.2), or appropriate isotype controls (clones A95.1 and R3-34, respectively). For IL-10 and Foxp3 staining, cells were fixed in eBioscience Fix/Perm buffer after the in vitro stimulation. This was followed by permeabilization in 0.5% saponin and staining for IL-10 as indicated above. Finally, the cells were permeabilized in eBioscience buffer and stained for Foxp3 according to the manufacturer’s instructions. Cells were analysed using a FACSCalibur or FACSort (BD Biosciences).
Statistics
Two-tailed Mann-Whitney U test was performed using GraphPad Prism 3.00 (GraphPad). Values of p < 0.05 were regarded as significant. Data are presented as mean ± SD.
Acknowledgments
We thank animal facility personnel, Liz Darley, Sandy Laue, and Martina Fuegenschuh for histology, Nigel Rust for cell sorting, and Kevin Maloy for critical reading of the manuscript.
This work was supported by the Wellcome trust (FP and CT), the MRC (JC), the Deutsche Forschungsgemeinschaft (HU 128-2), the University of Leipzig (HU, F1-49), the Swiss National Fund (CM), and training grants from the National Institutes of Health (NIH) and the Cancer Research Institute (JDF).
Non Standard Abbreviations
- IPEX
immune polyendocrinopathy X-linked enteropathy
- TR
regulatory T cell
- IBD
inflammatory bowel disease
- MLN
mesenteric lymph node
- LP
lamina propria
- Rag
recombinase activating gene
- CD
Crohn’s disease
- UC
ulcerative colitis
- POD
peroxidase
References
- 1.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 2.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor FOXP3. Science. 2003 doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 14.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337. [PubMed] [Google Scholar]
- 15.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379. [PMC free article] [PubMed] [Google Scholar]
- 16.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 17.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 18.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 20.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 21.Foussat A, Cottrez F, Brun V, Fournier N, Breittmayer JP, Groux H. A comparative study between T regulatory type 1 and CD4+CD25+ T cells in the control of inflammation. J Immunol. 2003;171:5018. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]
- 22.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 26.Coffman RL. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 27.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Yamori M, Kita T, Chiba T, Wakatsuki Y. CD4+CD25+ T cells regulate colonic localization of CD4 T cells reactive to a microbial antigen. Inflamm Bowel Dis. 2005;11:541. doi: 10.1097/01.mib.0000163696.26969.e4. [DOI] [PubMed] [Google Scholar]
- 29.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4+CD25+ regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042. [PubMed] [Google Scholar]
- 30.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 31.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 33.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 34.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 38.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 39.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 40.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 41.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 42.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 44.Kelsen J, Agnholt J, Hoffmann HJ, Romer JL, Hvas CL, Dahlerup JF. FoxP3+CD4+CD25+ T cells with regulatory properties can be cultured from colonic mucosa of patients with Crohn’s disease. Clin Exp Immunol. 2005;141:549. doi: 10.1111/j.1365-2249.2005.02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]