Abstract
Naturally occurring CD4+ TR cells that express CD25 and the transcription factor FoxP3 play a key role in immune homeostasis preventing immune pathological responses to self and foreign antigens. CTLA-4 is expressed by a high percentage of these cells, and is often considered as a marker for TR in experimental and clinical analysis. However, it has not yet been proven that CTLA-4 has a direct role in TR function. Using a colitis transfer model, we previously showed that anti-CTLA-4 mAb treatment abrogates suppression of colitis mediated by CD4+ CD25+ TR. Here we demonstrate that anti-CTLA-4 mAb treatment inhibits TR function via direct effects on CTLA-4 expressing TR cells, and not via hyper-activation of colitogenic T cells. Although anti-CTLA-4 mAb treatment completely inhibits TR function, it does not affect TR cell expansion, persistence or homing to the gut-associated lymphoid tissue, indicative of the blockade of a signal required for TR cell activity. In contrast to the striking effect of the antibody, CTLA-4 deficient mice can produce functional TR cells, suggesting that compensatory mechanisms can develop. This study provides direct evidence that CTLA-4 has a specific, non-redundant role in the function of normal regulatory T cells. This role has to be taken into account when targeting CTLA-4 for therapeutic purposes, as such a strategy will not only boost effector T cell responses, but might also break TR-mediated self-tolerance.
Keywords: CD4+ T Lymphocyte, Inflammatory Bowel Disease, Th1 cell, immunoregulation, mucosal immunity
Introduction
Immune responses to specific pathogens incorporate multiple regulatory mechanisms that have evolved to restrict reactivity to self-Ags, while allowing both the clearance of the pathogen and the development of long-term immunological memory (1, 2). This is especially critical in the intestine, where it is necessary to mount protective immune responses against pathogens while limiting responses to commensal bacteria and dietary and self-Ags, in the presence of a large antigenic load. Indeed, a breakdown in intestinal homeostasis and the development of aberrant inflammatory responses to intestinal bacteria have been associated with the pathogenesis of inflammatory bowel disease (IBD) in humans (3, 4).
Studies using mouse models of IBD have provided convincing evidence that functionally specialized populations of regulatory T cells (TR) play an important role in the control of intestinal inflammation (5). Some of the best studied are the naturally arising CD4+CD25+ TR that have been shown to prevent and even cure colitis in the T cell transfer model (6–8). In addition to their role in intestinal homeostasis, CD4+CD25+TR play a key role in dominant tolerance to self-Ags and can also impede host protective immune responses to tumors and pathogens (9–11). Recently, expression of the transcription factor FoxP3 has been shown to be a useful marker for naturally arising TR. FoxP3 plays a key functional role in CD4+CD25+TR development, as mice with natural or induced mutations in this gene lack TR and develop a fatal multiorgan inflammatory disease (12–14). Similarly, loss of function mutations in FOXP3 have been shown to be responsible for the human autoimmune and inflammatory disease, immune polyendocrine X-linked enteropathy. Diabetes and chronic intestinal inflammation with several features resembling IBD are found in nearly all patients, and gastrointestinal symptoms are typically the reason for the initial clinical presentation, providing evidence that TR also contribute to intestinal homeostasis in humans (15, 16).
Recently, it has been proposed that the inhibitory receptor CTLA-4 plays a functional role in TR activity (6, 17, 18). This receptor belongs to the same family as CD28 and binds to the same ligands, B7-1 and B7-2. CTLA-4 is up-regulated upon T cell activation, and its activity as a negative regulator of T cell responses is now well-described (19). In vitro, ligation of CTLA-4 on activated CD4+ T cells suppresses IL-2 production and limits cell cycle progression (20, 21). In vivo, blockade of CTLA-4 leads to increased T cell-mediated immunity in a number of model systems including Ag-specific responses (22), parasitic infection (23), and autoimmune disease (24–26). Manipulation of the B7:CTLA-4 pathway is also an attractive target for stimulating antitumor immunity (27–29). In a recent clinical trial, metastatic melanoma patients were treated with a humanized anti-CTLA-4 mAb in conjunction with two modified gp100 melanoma-associated Ags; this led to cancer regression in a proportion of patients (3 out of 14). However, anti-CTLA-4 treatment also resulted in autoimmune disease (6 out of 14) including the development of enterocolitis (30). These findings are consistent with work in animal models, demonstrate a critical role for CTLA-4 in the regulation of peripheral tolerance in humans, and give further impetus to understanding how CTLA-4 may be important for regulating tolerance to colonic Ags.
Among resting CD4+ T cells, CTLA-4 is expressed primarily by CD4+CD25+ TR, being detectable on ~50% of these cells as compared with <1% of naive CD4+CD45RBhigh cells (6, 17). The expression of CTLA-4 on TR has been linked to regulation of organ-specific autoimmune disease in vivo, and there is some evidence to suggest that CTLA-4 is required for the suppressive function of this population in vitro (17). In the T cell transfer model of colitis, administration of anti-CTLA-4 mAb to mice that received both CD4+CD45RBhigh and CD4+CD25+ populations led to development of colitis, suggesting a key role for CTLA-4 in TR-mediated control of intestinal homeostasis (6, 18). As CTLA-4 is induced on naive T cells following activation (31), anti-CTLA-4 mAb treatment may abrogate suppression indirectly via hyperactivation of colitogenic T cells or directly via effects on the CD4+CD25+ TR population. In this report, we have used CTLA-4-deficient mice and anti-CTLA-4 mAb to dissect how CTLA-4 influences the balance between effector and TR cells in the intestine.
Materials and Methods
Mice
BALB/c wild-type (WT), B7-1/B7-2-deficient (B7-1/B7-2 knockout (KO)), and B7-1/B7-2/CTLA-4-deficient (B7-1/B7-2/CTLA-4 KO) mice were maintained in accordance with the institutional guidelines of Brigham and Women’s Hospital and Harvard Medical School (Boston, MA; accredited by the American Association of Accreditation of Laboratory Animal Care (AALAC)). C.B-17 scid mice were purchased from Taconic Farms. For some experiments, BALB/c, C.B-17 scid, BALB/c.C57B10D2.Ly9.2 congenic, BALB/c.CTLA-4-deficient (CTLA-4 KO), and BALB/c.RAG2-deficient (RAG KO) mice were maintained in specific pathogen-free conditions at the Sir William Dunn School of Pathology (University of Oxford, Oxford, U.K.) and were used at 6–10 wk of age. All procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986.
Generation of mixed bone marrow chimeras
Bone marrow isolated from 2- to 3-wk-old BALB/c.CTLA-4 KO was depleted of T cells using anti-CD4 and anti-CD8 Abs together with anti-rat coated Dynabeads (Dynal). CTLA-4 KO bone marrow was then mixed in a 1:1 ratio with bone marrow taken from BALB/c.C57B10D2.Ly9.2 mice and injected i.v. into gamma-irradiated (5.5 Gy, 550 rad) BALB/c.C57B10D2.Ly9.2 mice. Eight weeks later, T cell reconstitution was assessed by analysis of expression of the Ly9 allele in peripheral blood. For additional experiments, CTLA-4−/− TR were sorted based on expression of CD4, CD25, and Ly9.1.
Purification of CD4+ T cells
CD4+ T cells were purified from spleens using anti-mouse CD4 (clone L3T4) coated MACS beads (Miltenyi Biotec) in accordance with the manufacturer’s instructions. Alternatively, non-CD4+ cells were depleted using anti-CD8, anti-B220, anti-H-2, and anti-Mac-1 Abs, together with anti-rat coated Dynabeads (Dynal). Purified CD4+ T cells were stained with anti-mouse CD4-CyChrome (clone RM4.5; BD Biosciences), anti-mouse CD45RB-FITC (clone 16A; BD Biosciences), and anti-mouse CD25-PE (clone PC61; BD Biosciences). Subpopulations of CD4+ cells were sorted by using three-color sorting on a FACSVantage (BD Biosciences) or MoFlo (DakoCytomation) cell sorter. T cells were sorted into CD4+(CD45RBlow)CD25+ and CD4+CD45RBhigh(CD25−) subpopulations. Sorted populations were >98.5% positive on reanalysis. FACS analysis of the sorted CD4+CD45RBhigh population showed <1% FoxP3+ cells (anti-mouse FoxP3 staining set; eBioscience).
Generation of mAb used in vivo
Anti-mouse CTLA-4 mAb (clone UC10-4F10-11) (32) and anti-mouse IL-10R mAb (clone 1B1.2) (33) were purified from hybridoma supernatant by affinity chromatography and shown to contain <1.0 endotoxin units per milligram of protein. Purified hamster IgG was used as a control (Jackson ImmunoResearch Laboratories). Fab were generated using immobilized papain (Perbio) in accordance with the manufacturer’s instructions. HPLC analysis of purified Fab before use indicated that <0.5% of the material existed in a nonmonomeric form. Surface plasmon resonance was used to confirm the binding activity of the anti-CTLA-4 Fab using a BIAcore1000 instrument (see Figure 5A). Briefly, CTLA-4.Ig (24 ng, 1399RU) was captured onto a sensor chip (CM5) using a covalently bound anti-human Ig Ab. Anti-CTLA-4 or control Fab (20 ng/µl, 5 µl/min) was then passed through the cell and binding monitored. Nonspecific binding was assessed by measuring anti-CTLA-4 Fab binding to a control cell lacking CTLA-4-Ig.
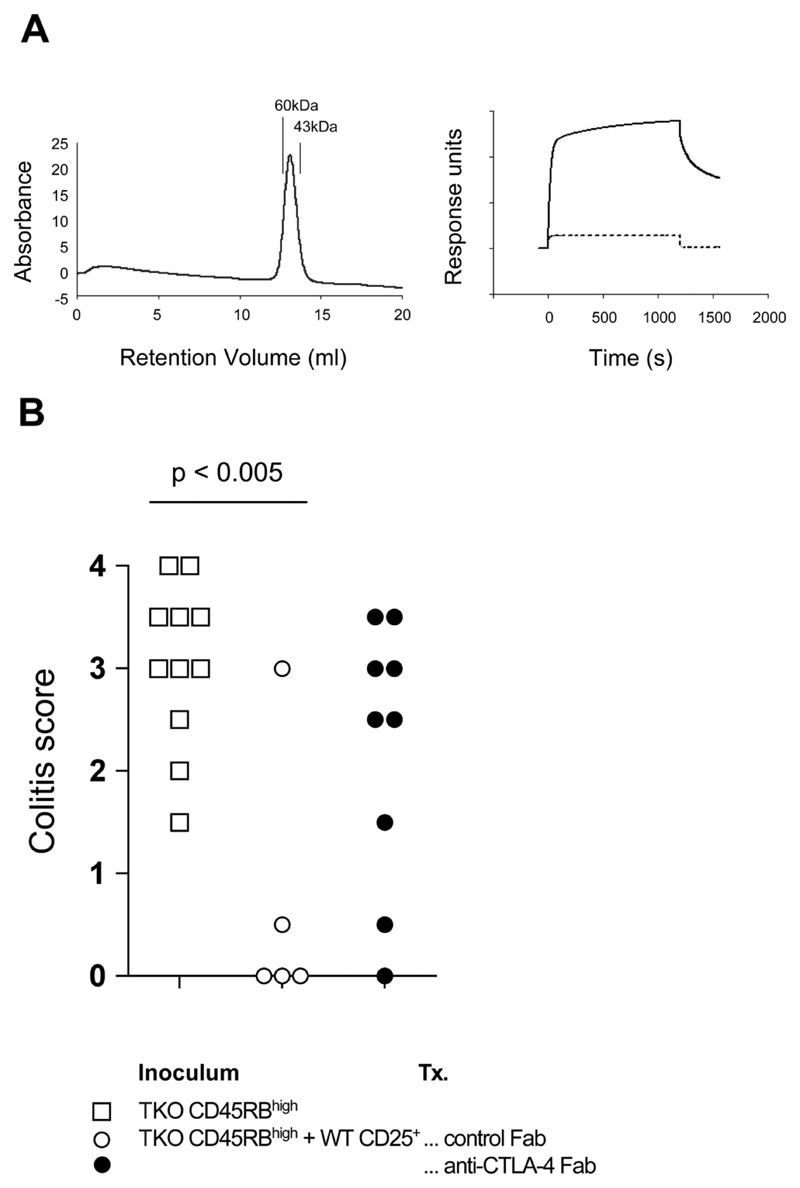
Figure 5. Administration of anti-CTLA-4 Fab is sufficient to abrogate CD4+CD25+ T cell-mediated control of colitis.
(A) Biochemical analyses of anti-CTLA-4 Fab. Left: size-exclusion chromatography indicates that the purified anti-CTLA-4 Fab exists primarily as a monomer. Purified Fab was passed over a Superdex 200 column and eluted as a single peak consistent with a monomer of ~50 kDa. The column was calibrated with molecular mass standards as indicated. In addition, anti-CTLA-4 Fab retained the ability to bind a CTLA-4.Ig fusion protein as measured by surface plasmon resonance (right). The plot shows binding of anti-CTLA-4 Fab to immobilized CTLA-4.Ig as compared with a control reference surface (dotted line). A control Fab failed to bind the immobilized CTLA-4.Ig, giving a trace identical with the reference surface (data not shown). (B) C.B-17 scid mice received B7-1/B7-2/CTLA-4 KO (triple KO (TKO)) CD4+CD45RBhigh cells alone or in combination with CD4+CD25+ cells. Mice also received either anti-CTLA-4 Fab or a control hamster IgG Fab. Mice were sacrificed 6–8 wk after transfer and colons taken for histological analysis. Data show colitis scores for individual mice taken from two independent experiments. In the presence of anti-CTLA-4 Fab, CD4+CD25+ cells failed to confer significant protection from colitis induced by B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh cells.
T cell Reconstitution
Immune-deficient mice, either C.B-17 scid or BALB/c.RAG2 KO mice, were injected i.p. with the sorted T cell populations. No differences were observed in the induction of colitis, or the protection from colitis between experiments using scid and RAG2 KO recipients (our unpublished observations). Mice received 4 × 105 CD4+CD45RBhigh cells alone or in combination with 1 × 105 CD4+CD25+ cells. Control mice received 1 × 105 CD4+CD25+ alone. In experiments where the pathogenic population came from B7-deficient mice, only 1 × 105 CD4+CD45RBhigh cells were transferred. Following T cell transfer, some mice received anti-CTLA-4 mAb (clone UC10-4F10-11) or a control hamster IgG; 200 µg of purified IgG were injected i.p. in PBS the day after T cell reconstitution and then on alternate days for 6–8 wk. Similarly, some mice received purified anti-CTLA-4 Fab or control Fab (100 µg) daily from the day after T cell transfer for 6–8 wk. In other experiments, mice were injected with 500 µg of anti-IL-10R mAb twice a week from the day after transfer until the end of the experiment. Mice were weighed weekly and monitored for clinical signs of colitis. Mice losing in excess of 20% of initial body weight or showing signs of severe disease were sacrificed.
Histological Examination
Colons were removed from mice 6–8wk after T cell reconstitution and fixed in buffered 10% formalin. Six-micrometer paraffin-embedded sections were cut and stained with H&E. Inflammation was scored in a blinded fashion, on a scale of 0–4 where a grade of 0 was given when there were no changes observed (34). Changes associated with other grades were as follows: grade 1, minimal scattered mucosal inflammatory cell infiltrates, with or without minimal epithelial hyperplasia; grade 2, mild scattered to diffuse inflammatory cell infiltrates, sometimes extending into the submucosa and associated with erosions, with mild to moderate epithelial hyperplasia and mild to moderate mucin depletion from goblet cells; grade 3, moderate inflammatory cell infiltrates that were sometimes transmural, with moderate to severe epithelial hyperplasia and mucin depletion; grade 4, marked inflammatory cell infiltrates that were often transmural and associated with crypt abscesses and occasional ulceration, with marked epithelial hyperplasia, mucin depletion and loss of intestinal glands.
Immunofluorescence
Tissue samples were snap-frozen, cryosectioned and fixed using acetone. Sections were blocked with donkey serum (Sigma-Aldrich) and then stained with biotinylated anti-mouse CD3 (clone 145-2C11; BD Biosciences) plus streptavidin-Cy5 (Jackson ImmunoResearch Laboratories). FoxP3 staining was performed using rabbit polyclonal anti-mouse FoxP3 Abs (generously provided by F. Ramsdell, Zymogenetics, Seattle, WA) and donkey anti-rabbit IgG FITC (Jackson ImmunoResearch Laboratories). The specificity of FoxP3 staining was confirmed by the absence of nuclear staining in organs from FoxP3–/– mice (62).
Statistical analysis
Colitis scores were compared using the Mann-Whitney U test and differences were considered statistically significant with p < 0.05.
Results
CD4+CD25+ T cells are present in mice lacking B7-1, B7-2, and CTLA-4
It has been previously shown that administration of anti-CTLA-4 is able to abrogate suppression of colitis mediated by CD4+CD25+ TR in the T cell transfer model of colitis (6, 18). To dissect the mechanism by which anti-CTLA-4 Ab administration results in a loss of immune regulation, CD4+ T cell populations were isolated from CTLA-4-deficient mice and analysed for their ability to inhibit colitis. Due to the aberrant T cell activation, lymphoproliferation and early mortality that occurs in CTLA-4-deficient mice, it was not possible to use these mice as a source of T cells for transfer experiments (35–36). Therefore, the CTLA-4-deficient mice used in this study were maintained on a B7-1/B7-2 KO background. The absence of B7-1/B7-2 expression prevents ligation of CD28, which has been shown to be critical for activation of naive T cells, and the lymphoproliferative phenotype is avoided (37). In this respect, the B7-1/B7-2CTLA-4 KO mouse strain provides a unique tool to analyze the function of CTLA-4 on both regulatory and colitogenic T cells during the development of colitis.
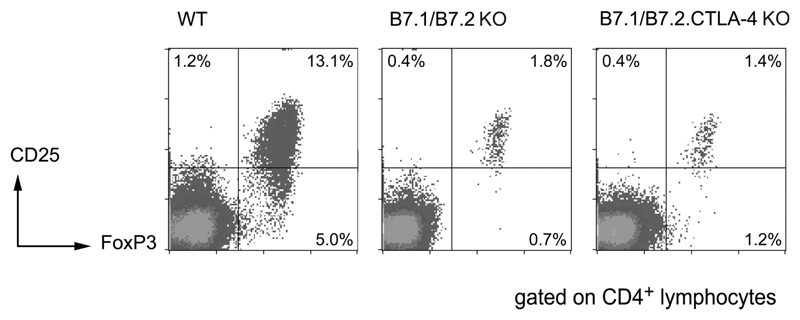
To confirm that CD25+ TR can be generated in the absence of CTLA-4, splenocytes were isolated from WT, B7-1/B7-2 KO, and B7-1/B7-2/CTLA-4 KO mice and stained for expression of CD4, CD25, and FoxP3. In WT BALB/c mice, CD4+CD25+ cells comprise around 10% of the CD4+ T cell population (Figure 1). FoxP3, a more definitive marker of TR is expressed by ~15% of CD4+ cells, with the majority of CD25+ cells also expressing FoxP3 (Figure 1). In contrast, in both B7-1/B7-2 KO and B7-1/B7-2/CTLA-4 KO mice, only 1–2% of CD4+ cells express CD25 (Figure 1). In the same way the frequency of FoxP3+ cells is significantly reduced (2.5–3.0% of CD4+ cells) but again the majority of CD4+CD25+ cells still express FoxP3, confirming the suitability of CD25 as a TR marker in these mice. The reduction in CD4+CD25+ TR frequency in B7-1/B7-2 KO mice is most likely due to the lack of CD28 ligation, as previous studies have shown that blockade of B7-1/B7-2 or loss of CD28 resulted in a reduction in both thymic and peripheral CD4+CD25+TR (38–40).
Figure 1. CD4+CD25+FoxP3+ cells are present in both B7-1/B7-2 KO and B7-1/B7-2/CTLA-4 KO mice.
Unfractionated splenocytes from WT, B7-1/B7-2 KO, and B7-1/B7-2/CTLA-4 KO mice were analysed by flow cytometry for the expression of CD4, CD25, and FoxP3. Mice were analysed at 6–8 wk of age. Representative plots show log10 fluorescence and are gated on CD4+ lymphocytes.
CTLA-4-deficient TR prevent colitis
We next determined whether CD4+CD25+ TR from CTLA-4-deficient mice retain functional activity. This would not directly exclude a role of CTLA-4 on WT TR, as genetically modified mice often develop alternative mechanisms to compensate for the loss of a key molecule. To check for CD4+CD25+ TR function, this population was isolated from WT, B7-1/B7-2 KO, and B7-1/B7-2/CTLA-4 KO mice and transferred alone or in combination with WT CD4+CD45RBhigh cells to SCID mice. None of the isolated CD25+ populations were pathogenic, since transfer of WT, or B7-1/B7-2 KO, or B7-1/B7-2/CTLA-4 KO CD4+CD25+ cells alone to SCID recipients did not elicit any colonic inflammation (Table I). As previously described, WT CD25+ TR inhibited the development of colitis when cotransferred with WT CD4+CD45RBhigh cells (Table I, Figure 2) (6). Similarly, CD4+CD25+ TR from both B7-1/B7-2 KO and B7-1/B7-2/CTLA-4 KO mice were able to protect mice from the induction of colitis by WT CD4+CD45RBhigh. Reducing the number of CD4+CD25+ T cells transferred failed to reveal any difference in the potency of these populations (data not shown). Thus, CTLA-4-deficient CD4+CD25+ cells retain the ability to prevent disease.
Table 1. Induction of, and protection from, colitis by CD4+ T cell subsets lacking expression of CTLA-4.
| Phenotype of Cells Injected | Incidence of colitis (n) | Mean colitis score | |
|---|---|---|---|
| CD4+CD45RBhigh | CD4+CD25+ | ||
| WT | none | 16 (16) | 3.6 ± 0.6 |
| B7-1-/-B7-2-/- | none | 9 (9) | 3.3 ± 0.7 |
| B7-1-/-B7-2-/-CTLA-4-/- | none | 4 (4) | 3.1 ± 0.6 |
| none | WT | 0 (8) | - |
| none | B7-1-/-B7-2-/- | 0 (5) | - |
| none | B7-1-/-B7-2-/-CTLA-4-/- | 0 (5) | - |
| WT | WT | 0 (9) | - |
| WT | B7-1-/-B7-2-/- | 0 (4) | - |
| WT | B7-1-/-B7-2-/-CTLA-4-/- | 0 (7) | - |
| B7-1-/-B7-2-/- | B7-1-/-B7-2-/-CTLA-4-/- | 0 (10) | - |
| B7-1-/-B7-2-/-CTLA-4-/- | B7-1-/-B7-2-/- | 1 (5) | 2.5 |
| B7-1-/-B7-2-/-CTLA-4-/- | B7-1-/-B7-2-/-CTLA-4-/- | 5 (6) | 3.0 ± 0.0 |
C.B-17.scid mice received CD4+ T cell subsets as described. Mice were sacrificed 6–8 wk after T cell transfer and colons were taken for histological analysis. Data show incidence of colitis (colitis score ≥2) and mean colitis score of those mice with colitis. Data are pooled from four independent experiments.
Figure 2. Effects of CTLA-4 on development and prevention of colitis.
The figure shows representative photomicrographs of the distal colon of C.B-17 scid mice following transfer of CD4+ T cells. CD4+CD45RBhigh cells isolated from either WT (A) or B7-1/B7-2/CTLA-4 KO mice (B) were able to induce colitis in C.B-17 scid recipients. Cotransfer of WT CD4+CD25+ cells was able to prevent colitis induced by either WT (C) or B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh cells (D). However, CD4+CD25+ cells from B7-1/B7-2/CTLA-4 KO mice could inhibit colitis induced by WT CD4+ CD45RBhigh cells (E) but not that induced by the equivalent population from B7-1/B7-2/CTLA-4 KO mice (F). Original magnification: ×250 (H&E).
Induction of, and protection from, colitis by CD4+ T cellsubsets lacking expression of CTLA-4a
The role of CTLA-4 expression on the potentially pathogenic CD4+CD45RBhigh cells was also investigated. In early experiments we noted a rapid but transient wasting disease following transfer of B7-deficient CD45RBhigh cells that did not occur in recipients of WT CD45RBhigh cells (data not shown). There was no correlation between this early wasting and the presence or absence of CD25+ TR, or the later development of colitis, and in later experiments it was avoided by reducing the number of CD45RBhigh cells transferred. This modification did not affect the incidence or severity of colitis.
Both WT and B7-1/B7-2 KO CD4+CD25+TR were able to inhibit colitis when cotransferred with B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh cells (Table I, Figure 2). Similarly, B7-1/B7-2/CTLA-4 KO CD4+CD25+ cells could mediate protection from colitis induced by B7-1/B7-2 KO CD45RBhigh cells. However, when CTLA-4 was absent on both colitogenic and regulatory cells the majority of recipient mice went on to develop disease (Table I, Figure 2). This would suggest that CTLA-4 expressed by both the colitogenic T cells and by the TR has an impact on the protection from colitis. This is consistent with multiple roles for CTLA-4 in both the activation of effector T cells and in mediating TR activity.
B7-sufficient CTLA-4 KO TR retain the ability to prevent colitis
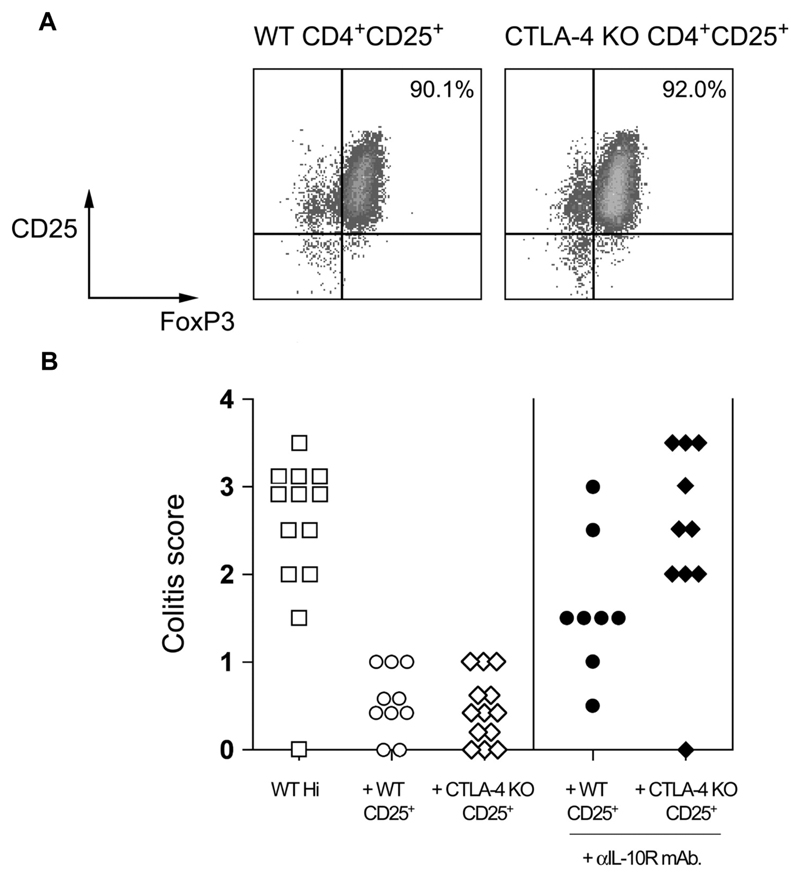
The absence of B7.1/B7.2 has profound effects on peripheral T cell homeostasis, and may alter the activity of the CD4+CD25+ population taken from B7.1/B7.2/CTLA-4 KO mice. To rule out any effect related to the lack of B7 molecules, mixed bone marrow chimeras were generated using CTLA-4 KO (Ly9.1+) and BALB/c.C57B10D2.Ly9.2 congenic donors. As has been reported previously (41), these animals do not develop the lymphoproliferative pathology that is characteristic of intact CTLA-4 KO mice. CTLA-4 KO CD4+CD25+ TR could be recovered from these mice using expression of the congenic marker Ly9.1. The sorted CTLA-4 KO CD4+CD25+ TR contained a similar frequency of FoxP3+ cells as the counterpart WT CD4+CD25+ TR and were also able to prevent colitis induced by transfer of WT CD4+CD45RBhigh cells to BALB/c.RAG2 KO mice (Figure 3). This confirms our observation that TR that cannot use CTLA-4 are still able to prevent colitis, irrespective of expression of B7.
Figure 3. CTLA-4 KO CD4+CD25+ T cells express FoxP3 and retain the ability to prevent colitis.
(A) WT and CTLA-4 KO TR were isolated from CTLA-4 KO mixed bone marrow chimeras by sorting for Ly9.1+CD4+CD25+ (CTLA-4 KO) and Ly9.1−CD4+CD25+ (CTLA-4-sufficient) populations as described above. The resulting populations were stained for expression of FoxP3 and analyzed by flow cytometry. Plots are gated on CD4+ lymphocytes, and percentages refer to the percentage of CD25+ FoxP3+ cells. (B) BALB/c RAG KO mice received WT CD4+CD45RBhigh cells alone or in combination with WT or CTLA-4 KO CD4+CD25+ cells. Some mice also received anti-IL10R mAb. Mice were sacrificed 8–10 wk after transfer and colons taken for histological analysis. Data are pooled from two independent experiments. In the presence of anti-IL-10R mAb, WT CD4+CD25+ TR mediated significant protection from colitis (p < 0.05), however, CTLA-4 KO CD4+CD25+ TR provided no significant protection from colitis (p > 0.05).
It has been reported that CD4+ CD25+ cells from CTLA-4 KO mice express increased levels of IL-10 (42). We have previously reported that protection from colitis by WT CD4+CD25+ TR is largely independent of IL-10 (43), as IL-10-deficient TR retained the ability to prevent disease and blockade of IL-10 signaling using an anti-IL-10R mAb resulted in only a marginal loss of protection mediated by WT CD25+ TR (Figure 3 and Ref. 43). By contrast, administration of an anti-IL-10R mAb completely abrogated suppression mediated by CTLA-4-deficient CD4+CD25+ TR (Figure 3). So, although TR that lack CTLA-4 can still prevent colitis, they appear to do so by using alternative immune suppressive pathways to those used by WT TR.
Anti-CTLA-4 mAb treatment targets CD4+CD25+ TR and not colitogenic T cells to abrogate suppression
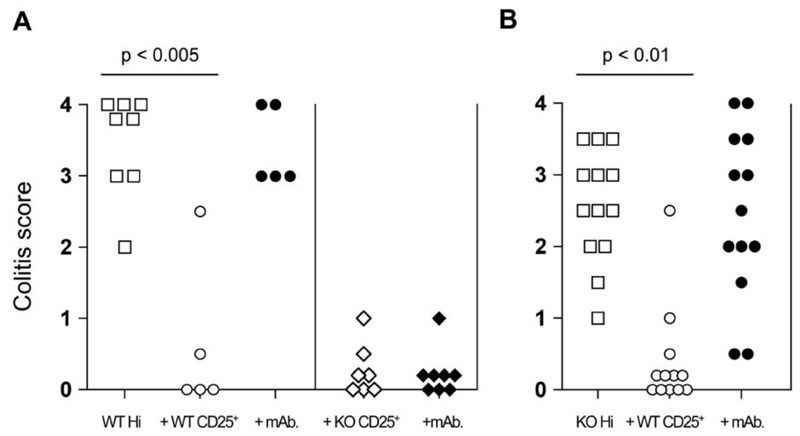
We have previously reported that administration of anti-CTLA-4 mAb abrogates CD4+CD25+ TR-mediated suppression of colitis (6, 18). However, whether the Ab functions via effects on effector T cells, TR, or both is not known. To investigate this issue, the outcome of anti-CTLA-4 mAb administration was examined in transfer experiments where expression of CTLA-4 was restricted to the colitogenic or TR cells. Suppression of colitis mediated by B7-1/B7-2/CTLA-4 KO CD4+CD25+ TR was not affected by anti-CTLA4 mAb treatment (Figure 4A). In addition, while WT CD4+CD25+ TR were able to prevent colitis induced by CD4+CD45RBhigh cells from B7-1/B7-2/CTLA-4 KO mice, the addition of anti-CTLA-4 mAb led to a loss of protection and the development of disease (Figure 4B). Together, these data indicate that anti-CTLA-4 mAb abrogates TR-mediated control of colitis via its effects on TR and not colitogenic effector cells.
Figure 4. Anti-CTLA-4 acts to inhibit CD4+CD25+ TR activity by interacting with CTLA-4 expressed by CD4+CD25+ cells.
(A) C.B-17.scid mice received WT CD4+CD45RBhigh cells alone or in combination with either WT or B7-1/B7-2/CTLA-4 KO (triple KO (TKO)) CD4+CD25+ cells. In addition, some mice also received anti-CTLA-4 mAb. (B) In similar experiments, C.B-17.scid mice received B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh cells alone or in combination with WT CD4+CD25+ cells. Again some mice also received anti-CTLA-4 mAb. Mice were sacrificed 6–8 wk after transfer and colons taken for histological analysis. Data show colitis scores for individual mice taken from two to three independent experiments.
Anti-CTLA-4 Fab retains the ability to disrupt the function of WT TR
Next, we investigated the possibility that the anti-CTLA-4 treatment was somehow eliminating the TR population. Anti-CTLA-4 mAb bound to the surface of TR might lead to deletion of this population, or it might cross-link CTLA-4 providing an agonistic signal, inhibiting TR expansion. To explore these possibilities, anti-CTLA-4 Fab were generated and used for in vivo studies (Figure 5A). Administration of anti-CTLA-4 Fab to SCID mice cotransferred with B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh cells and WT CD4+CD25+ cells led to a loss of suppression of colitis similar to that seen in mice injected with intact anti-CTLA-4 mAb (Figure 5B). Protection from colitis was not affected in similarly transferred mice that received a control Fab. These results indicate that the functional effects of anti-CTLA-4 administration are independent of the Fc portion of the Ab, ruling out Ab-induced cross-linking of CTLA-4 and generation of an agonistic signal, as well as Fc-mediated cellular depletion, as mechanisms of action.
Accumulation of CD4+CD25+TR is not inhibited by the presence of anti-CTLA-4
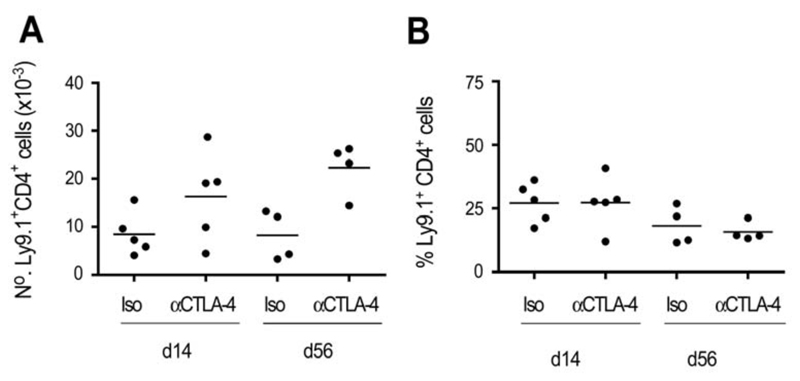
It was possible that blockade of CTLA-4 on TR inhibited a positive signal required for TR proliferation and accumulation. To assess the effect of the mAb on TR accumulation, expression of an allotypic marker was used to distinguish the progeny of the CD4+CD25+ and CD4+CD45RBhigh populations following transfer. CD4+CD25+ TR were purified from WT BALB/c (Ly9.1) mice and transferred together with CD4+CD45RBhigh cells from BALB/c.C57B10D2.Ly9.2 congenic mice to immunodeficient recipients. After 2 wk, CD4+ T cells from the spleen were analysed for expression of allotypic markers. Mice that received anti-CTLA-4 mAb had an increased number of total CD4+ T cells, with both Ly9.1+ and Ly9.2+ cells being increased ~2-fold (Figure 6A, data not shown). However, the proportion of CD4+CD25+ progeny (Ly9.1+CD4+ cells) vs CD4+CD45RBhigh progeny was similar in mice that had received anti-CTLA-4 to that seen in control mice, a pattern that was maintained at later time points (Figure 6B). These data show that the ability of anti-CTLA-4 to suppress TR activity is not the result of impaired accumulation of the CD4+CD25+population.
Figure 6. Anti-CTLA-4 mAb does not inhibit CD4+CD25+ TR accumulation in vivo.
RAG2 KO mice received BALB/c.C57B10D2.Ly9.2 congenic CD4+CD45RBhigh cells and BALB/c (Ly9.1+) CD4+CD25+ cells together with anti-CTLA-4 or control mAb. Mice were sacrificed and the number and proportion of CD4+Ly9.1+ cells determined at the time points indicated. Data show absolute numbers of CD4+Ly9.1+ cells (A) and Ly9.1+ cells as a proportion of total CD4+ cells (B) in the spleens of individual mice, and are representative of three independent experiments.
CD4+CD25+ TR are present in GALT of colitic mice
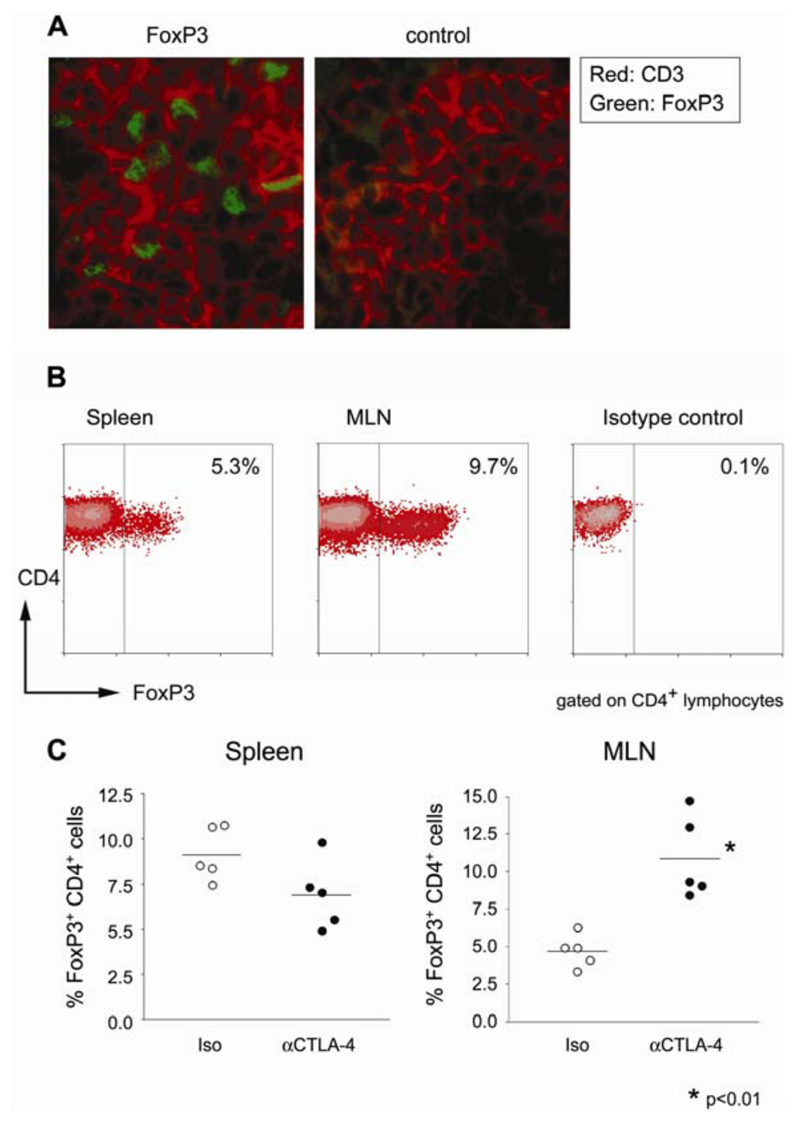
It remained possible that the presence of anti-CTLA-4 was in some way able to disrupt the homing of CD4+CD25+ TR. To determine whether this was the case, the GALT from T cell-transferred SCID mice was analysed for the presence of TR. Frozen mesenteric lymph node sections from mice that had received CD4+CD45RBhigh cells in combination with CD4+CD25+ cells and anti-CTLA-4 mAb were stained for the presence of CD3+ cells. The same sections were also stained for the presence of FoxP3+ cells, a definitive marker of TR (Figure 7A). In all mice analysed, it was possible to identify FoxP3+CD3+ cells in the mesenteric lymph node (MLN), even though these mice had ongoing colitis. In addition, FACS analysis was performed to quantify FoxP3 expressing cells in spleen and MLN of these mice (Figure 7B). FoxP3+CD4+ T cells could be found in both the spleen and MLN of T cell transferred mice, with no significant reduction in the frequency of FoxP3+ cells in anti-CTLA-4-treated mice compared with the controls (Figure 7C). Together, these data demonstrate that FoxP3+CD4+ T cells are able to access the GALT in anti-CTLA-4-treated mice and yet fail to control the colitogenic T cell response.
Figure 7. CD4+FoxP3+ T cells accumulate in the GALT of diseased mice receiving anti-CTLA-4 mAb.
RAG2 KO mice received CD4+CD45RBhigh and CD4+CD25+ T cells together with anti-CTLA-4 mAb. Eight weeks later, mice were sacrificed and tissues taken for analysis. (A) Sections from mesenteric lymph nodes were stained for CD3 (red) and FoxP3 (green), or CD3 and a control rabbit Ig. Images are representative of the analysis of four mice from two independent experiments. Original magnification: ×630. (B) Representative analysis of FoxP3 expression by transferred CD4+ T cells from anti-CTLA-4-treated mice. Single-cell suspensions from spleen and mesenteric lymph nodes were stained for flow cytometric analysis. Plots show log10 fluorescence and are gated on CD3+CD4+ lymphocytes. (C) Proportion of CD4+ T cells expressing FoxP3 in the spleen and mesenteric lymph nodes of anti-CTLA-4 or control mice. Each point represents a single mouse analyzed by flow cytometry as in (B).
Discussion
CTLA-4 has long been known to play an important role in controlling immune responses (19). Although many mechanisms have been reported for CTLA-4 function in vitro, evidence for their presence in vivo is still contradictory. One of the reasons for this uncertainty is that CTLA-4 may fulfill a variety of functions, as it is expressed by different T cell subpopulations at various time points. CTLA-4 was first described to be up-regulated by naive CD4+ T cells upon activation (31). More recently, CTLA-4 has been shown to be specifically expressed on CD4+CD25+ TR populations, and administration of anti-CTLA-4 mAb has been linked to a loss of TR-mediated suppression, both in vitro and in vivo (6, 17–18). Polymorphisms in CTLA-4 have also been associated with autoimmune disorders in humans (44) and susceptibility to autoimmune disease in mice (45). Based on these and other reports, CTLA-4 expression is now widely used as a marker of TR. Despite this, its precise role in these systems remains controversial, in part due to expression of CTLA-4 on effector T cell populations, but also due to conflicting results that have been obtained in vitro (46–47). Here, using the T cell transfer model of colitis, we show that anti-CTLA-4 mAb disrupts TR activity in vivo by targeting TR, not by exacerbating the activity of pathogenic T cells. This effect is mediated by blockade of the interaction between CTLA-4 and its ligands, and not depletion of CTLA-4 expressing regulatory cells, as it has been previously suggested (46). Our results demonstrate that CTLA-4 expression is not only a phenotypic characteristic of TR, but that its presence on CD4+CD25+ TR plays an important role in the functional activity of this population.
In this study, we have used T cells lacking CTLA-4 as a tool to investigate the role of CTLA-4 in CD4+CD25+ TR function and to clarify the effects of the anti-CTLA-4 mAb. Mice deficient in B7-1 and B7-2 as well as CTLA-4 were used as donors (37), thus avoiding the problems associated with isolating CD4+CD25+ T cells from CTLA-4-deficient mice (35–36). Previous studies have shown that administration of anti-CTLA-4 mAb overcame the ability of CD4+CD25+ TR to protect from colitis (6, 18). Here, we demonstrate that protection is dependent upon CTLA-4 expression by TR but is independent of CTLA-4 expression by the colitogenic CD4+CD45RBhigh population, indicating that the effects of the Ab are mediated through CTLA-4 expressed on the CD4+CD25+ TR.
The mode of action of the anti-CTLA-4 mAb was analysed by comparing the activity of intact IgG and Fab. Fab were as effective as intact Ab, indicating that the Ab does not cause Fc-mediated deletion of the TR; nor does it cross-link CTLA-4 providing an agonistic signal. Ligation of CTLA-4 has been reported to inhibit activation induced cell death in certain T cell populations (48–49); it was thus possible that the effect of the mAb was due to the inhibition of a similar survival signal. However, anti-CTLA-4 did not reduce the accumulation of TR progeny following transfer in vivo. Instead, absolute numbers of CD25+ TR were increased along with splenomegaly, although the frequency of TR progeny as a percentage of the transferred CD4+ cells remained similar. Comparable results have been observed in a clinical trial in humans, where anti-CTLA-4 treatment, despite inducing antitumor responses and autoimmunity, did not reduce the frequency of FoxP3 expression in PBMC (50). Furthermore, in our system, it was possible to detect the FoxP3+ progeny of transferred CD4+CD25+ TR in the GALT of anti-CTLA-4-treated mice, consistent with these cells migrating to the lymphoid organs that drain the diseased tissue but being unable to prevent the development of disease.
B7-1/B7-2/CTLA-4 KO mice contained a reduced frequency of peripheral CD4+CD25+ TR, consistent with a role for B7:CD28-mediated costimulation in the generation and maintenance of these cells (38–40). The additional deficiency in CTLA-4 did not alter the frequency CD4+CD25+ cells. Significantly, this population retained FoxP3 expression and TR function in the absence of CTLA-4, indicating that the receptor is not absolutely required for the development of CD4+CD25+ TR. Importantly, administration of the anti-CTLA-4 mAb did not overcome the regulatory activity of CTLA-4 deficient CD4+CD25+ TR, showing that ligation of CTLA-4 on the colitogenic population had limited impact on the regulation of colitis in this system.
Recent reports have shown that the interaction of CTLA-4 with B7 ligands expressed by APCs may modulate immune responses. This raises the possibility that anti-CTLA-4 mAb may disrupt TR function by preventing a CTLA-4-mediated signal through B7-1/B7-2 expressed on dendritic cells (DC). The data show that binding of a CTLA-4.Ig fusion protein to the surface of DC induces expression of indoleamine 2,3 dioxygenase, leading to the depletion of tryptophan and inhibition of T cell function (51). CTLA-4 expressed by CD25+ TR has similar effects, suggesting that this interaction may be important for the suppressive activity of these cells (52). Our findings that CD4+CD25+ TR are able to suppress colitis induced by CTLA-4-deficient CD4+CD45RBhigh cells in a CTLA-4-dependent manner are in line with that data, raising the possibility that it is ligation of B7-1/B7-2 on DC by CTLA-4 expressed by TR that is crucial for TR-mediated control of colitis. Additional experiments are required to test this hypothesis.
As not only CTLA-4, but also B7 is up-regulated on T cells upon activation, the interaction of CTLA-4 with B7 expressed on effector T cells may also play a role in CD4+CD25+ cell-mediated suppression. In a recent report, B7-deficient CD4+CD25− cells were found to be refractory to TR-mediated suppression in vitro and in vivo (53). Whether CTLA-4 expressed by TR was important in these interactions is not clear. Indeed, while this may represent another mechanism by which CD4+CD25+ TR influence T cell responses, it does not appear to be essential, since in our model B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh T cells remained susceptible to suppression by WT TR, through a CTLA-4 dependent mechanism.
PD-1, another member of the CD28/CTLA-4 family, has also been linked to TR-mediated prevention of colitis. CD4+CD25−PD-1+ T cells expressing high levels of FoxP3 and CTLA-4 have been shown to prevent colitis in the CD4+CD45RBhigh transfer model (54). This protection, like that mediated by CD4+CD25+ T cells, was overcome by anti-CTLA-4 but not by anti-PD-1 Abs. Although the role of PD-1 in TR function remains elusive, this report further highlights the functional importance of CTLA-4 in protection from colitis in a different model.
The data presented here demonstrate that signalling through CTLA-4 is required for WT CD25+ TR to exert their suppressive phenotype. However, TR generated in the absence of CTLA-4 retain the ability to suppress colitis, suggesting that they are able to compensate for the loss of this receptor. The functionality of CTLA-4-deficient CD25+ TR cells in vitro has been linked to an increased production of the immune suppressive cytokines IL-10 and TGF-β (42). In vivo, CTLA-4-deficient TR cells rely on IL-10 more heavily than the WT TR population, as administration of an anti-IL-10R mAb abrogated the protection mediated by these cells. Although CTLA-4-deficient TR can compensate for the absence of CTLA-4, this compensation is not complete. Thus, CD4+CD25+ T cells from B7-1/B7-2/CTLA-4 KO mice failed to suppress effector T cells of the same genotype, although they were effective in controlling both WT and B7-1/B7-2 KO CD4+CD45RBhigh cells. By contrast, B7-1/B7-2/CTLA-4 KO CD4+CD45RBhigh cells were susceptible to regulation by WT and B7-1/B7-2 KO CD4+CD25+ cells. One explanation could be that the CD4+CD45RBhigh population from B7.1/B7.2/CTLA-4 KO mice is less susceptible to regulation than that from WT or B7.1/B7.2 KO mice. Thus, the combination of CTLA-4 deficiency in both effector and TR populations is sufficient to tip the balance away from a regulated immune response and toward the development of inflammatory pathology. These data are consistent with a growing acceptance that immune regulation is mediated by multiple mechanisms and that removal of one or other pathway may or may not result in a loss of suppression as a function of the particular assay.
In clinical studies, anti-CTLA-4 mAb has been developed as a reagent to enhance T cell immunity (55). Early results with anti-CTLA-4 mAb in humans have suggested that this reagent may be an effective means to enhance antitumor immunity; however, the treatment has also lead to transient negative side effects, including the development of enterocolitis (30). More recent trials with the same anti-CTLA-4 mAb have seem similar autoimmune and gastrointestinal perturbances (56–58). As FoxP3 expression was not perturbed by the therapy, the effects have been ascribed to the interaction of anti-CTLA-4 mAb with effector cells (50). The data presented herein offer an alternative interpretation, indicating that anti-CTLA-4 can target CD4+CD25+ TR function without changes in FoxP3 expression.
The clinical studies illustrate that the anti-CTLA-4 mAb treatment may also have an impact on TR-mediated suppression of T cell responses and alter the balance of immune regulation, especially in the gut. Although the studies show that manipulation of CTLA-4 signalling is useful as a mechanism to enhance immune responses in a therapeutic setting, they also highlight the need to separate its useful and harmful effects. This is particularly significant at this time, with the recent approval of CTLA-4 Ig therapy for the treatment of rheumatoid arthritis by the Food and Drug Administration (59). In this article, we present a model where CTLA-4 mediates two different effects. On one hand, it reduces the pathogenicity of effector cells (60, 61). On the other, it is required for TR-mediated control of immune responses. Both roles have to be taken into account when designing clinical trials, as the stimulation of antitumor effector cells by blocking CTLA-4 could be accompanied by the breakdown of TR-mediated self-tolerance.
Acknowledgments
We thank B. Chang and N. Rust for their excellent technical assistance, L. Darley for processing of histological samples, and Dr. S. Clark and staff for care of experimental animals. We gratefully acknowledge Dr. F. Ramsdell for the provision of polyclonal anti-FoxP3 Ab. We also thank Dr. K. Maloy and J. Coombes for critical review of this manuscript.
Funding
S.R., N.R., and F.P. were funded by the Wellcome Trust. A.I. is a recipient of a postdoctoral grant from the Spanish Ministerio de Educación y Ciencia. A.H.S. is the recipient of National Institutes of Health Grants R01 AI40614 and AI38310.
Abbreviations
- H&E
hematoxylin and eosin
- TR
regulatory T
Bibliography
- 1).Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 2).Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 3).Pirzer U, Schonhaar A, Fleischer B, Hermann E, Meyer zum Buschenfelde KH. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn’s disease. Lancet. 1991;338:1238–1239. doi: 10.1016/0140-6736(91)92104-a. [DOI] [PubMed] [Google Scholar]
- 4).Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 6).Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 8).Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-β, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 9).Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 11).Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 12).Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13).Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 14).Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 15).Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16).Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 17).Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Liu Z, Geboes K, Hellings P, Maerten P, Heremans H, Vandenberghe P, Boon L, van Kooten P, Rutgeerts P, Ceuppens JL. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J Immunol. 2001;167:1830–1838. doi: 10.4049/jimmunol.167.3.1830. [DOI] [PubMed] [Google Scholar]
- 19).Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 20).Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 23).McCoy K, Camberis M, Gros GL. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 26).Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 28).van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Kwon ED, Foster BA, Hurwitz AA, Madias C, Allison JP, Greenberg NM, Burg MB. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 33).O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3 dependent and -independent pathways. EMBO J. 1998;17:1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Leach MW, Bean AG, Mauze S, Coffman RL, Powrie F. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am J Pathol. 1996;148:1503–1515. [PMC free article] [PubMed] [Google Scholar]
- 35).Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 36).Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 37).Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 39).Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 40).Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 41).Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–1131. [PubMed] [Google Scholar]
- 42).Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 43).Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971–978. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 44).Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 45).Wicker LS, Chamberlain G, Hunter K, Rainbow D, Howlett S, Tiffen P, Clark J, Gonzalez-Munoz A, Cumiskey AM, Rosa RL, et al. Fine mapping, gene content, comparative sequencing, and expression analyses support CTLA4 and Nramp1 as candidates for Idd5.1 and Idd5.2 in the nonobese diabetic mouse. J Immunol. 2004;173:164–173. doi: 10.4049/jimmunol.173.1.164. [DOI] [PubMed] [Google Scholar]
- 46).Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 47).Kataoka H, Takahashi S, Takase K, Yamasaki S, Yokosuka T, Koike T, Saito T. CD25+CD4+ regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17:421–427. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 48).Pandiyan P, Gartner D, Soezeri O, Radbruch A, Schulze-Osthoff K, Brunner-Weinzierl MC. CD152 (CTLA-4) determines the unequal resistance of Th1 and Th2 cells against activation-induced cell death by a mechanism requiring PI3 kinase function. J Exp Med. 2004;199:831–842. doi: 10.1084/jem.20031058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).da Rocha Diasqq S, Rudd CE. CTLA-4 blockade of antigen-induced cell death. Blood. 2001;97:1134–1137. doi: 10.1182/blood.v97.4.1134. [DOI] [PubMed] [Google Scholar]
- 50).Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 52).Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 53).Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Totsuka T, Kanai T, Makita S, Fujii R, Nemoto Y, Oshima S, Okamoto R, Koyanagi A, Akiba H, Okumura K, et al. Regulation of murine chronic colitis by CD4+CD25− programmed death-1+ T cells. Eur J Immunol. 2005;35:1773–1785. doi: 10.1002/eji.200425109. [DOI] [PubMed] [Google Scholar]
- 55).Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 56).Dranoff G. CTLA-4 blockade: unveiling immune regulation. J Clin Oncol. 2005;23:662–664. doi: 10.1200/JCO.2005.09.923. [DOI] [PubMed] [Google Scholar]
- 57).Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 58).Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Bluestone JA, Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 60).Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 61).Eggena MP, Walker LS, Nagabhushanam V, Barron L, Chodos A, Abbas AK. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdeld F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10 secreting CD4+CD25+r T cells during cure of colitis. J Immunol. doi: 10.4049/jimmunol.177.9.5852. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]