Abstract
In order to overcome the main disadvantages of conventional cancer therapies, which prove to be inadequate because of their lack of selectivity, the development of targeted delivery systems is one of the main focuses in anticancer research.
It has been repeatedly shown that decorating the surface of nanocarriers with high-affinity targeting ligands, such as peptides or small molecules, is an effective way to selectively deliver therapeutics by enhancing their specific cellular uptake via the binding between a specific receptor and the nanosystems.
Nowadays, the need of finding new potential biological targets with high endocytic efficiency as well as low tendency to mutate is urgent and, in this context, mannose and mannose-6-phosphate receptors appear promising to target anticancer drugs to cells where their expression is up-regulated. Moreover, they open the path to encouraging applications in immune based and gene therapies as well as in theragnostic purposes.
In this work, the potential of mannose and mannose-6-phosphate targeted delivery systems in cancer therapy is discussed, emphasizing their broad application both in direct treatments against cancer cells with conventional chemotherapeutics or by gene therapy and also their encouraging capabilities in immunotherapy and diagnostics purposes.
Keywords: Cancer, mannose, mannose-6-phosphate, immunotherapy, targeted drug delivery
1. Introduction
Despite significant progress in medicine, most tumors remain incurable and cancer is the second leading cause of death globally. According to the World Health Organization (WHO), 8.8 million deaths were associated with cancer in 2015, which means that, globally, nearly 1 in 6 deaths is caused by tumors.[1]
Many of the current available therapies are still inadequate and, despite the last advances in understanding biological mechanisms behind cancer progression, many patients have relapses due to the proliferation of residual malignant cells [2] or because cancer cells mutate and develop resistance, which renders also the most recent treatments ineffective.[3] Moreover, the shortage of selective delivery of anti-cancer drugs to tumor tissues is still one of the major limitations to overcome in order to reduce toxicity of chemotherapeutics and increase the efficiency of many treatments.[4]
Therefore, the need of finding new potential targets to address anticancer drugs is urgent, and in this regard would be desirable to take especially biological markers into account which have a lower tendency to mutate.[3] Moreover, combining conventional chemotherapeutic cancer therapy with more innovative approaches such as immunotherapy or theragnostics would potentially result in a more durable remission.[5]
Nowadays, carbohydrates such as mannose and mannose-6-phosphate are acquiring more and more interest as promising targeting ligands in cancer therapy. Since lectin type receptors are over-expressed in many tumors, they can be used to selectively target and deliver drugs, or diagnostic probes to cancer cells by receptor-mediated endocytosis, providing better efficacy and lower toxicity in healthy tissues.[3] Moreover, the presence of mannose receptor (MR) on the surface of professional antigen presenting cells (APCs) involved in immune responses, such as macrophages (MCs) and dendritic cells (DCs), would allow their targeting and subsequent modulation, in order to induce a specific and long-lasting anticancer response.[6]
It has been widely shown that decorating nanocarriers with targeting agents, such as peptides or folic acid, is an effective way to deliver drugs by enhancing specific cellular uptake via the binding between specific cells and the nanocarriers.[7] In this regard, nanomedicine products decorated with mannose and mannose-6-phosphate represent a promising opportunity to overcome the main current issues in cancer therapy, such as lack of selectivity for tumor cells and cytotoxicity for healthy tissues, and to achieve an efficient targeted delivery to specific cell populations. Moreover, nanocarriers can enhance the pharmacokinetic and pharmacodynamic profiles of conventional drugs and may thus optimize the efficacy of already existing anti-cancer compounds. A wide range of materials and methods have been developed so far to formulate nano-sized therapeutics for targeted drug delivery. The latter may be categorized as conjugates, lipid-based or polymeric carriers.[4] Moreover, some of them have already obtained regulatory approval and are available on the market for tumor treatments, while a number of nanotechnology-enabled therapeutic modalities are currently tested in clinical trials.[8]
In this work, we discuss the potential of mannose and mannose-6-phosphate targeted delivery systems in cancer therapy, and we emphasize on their potential broad application both in direct treatments against cancer cells with conventional chemotherapeutics or by gene therapy as well as their encouraging capabilities in immunotherapy and diagnostics purposes.
2. Nanomedicine: an overview
Nanomedicine represents one of the most promising strategies in cancer therapy. Many nano-sized carriers have been developed, and several of them have been confirmed to be beneficial in phase 3 clinical trials.[4]
Nanocarriers have unique features such as nanoscale size, high surface-to-volume ratio and conducive physico-chemical properties. Moreover, they can overcome solubility problems that prevent the use of many drugs in clinical settings, modulating both their pharmacokinetic and pharmacodynamic profiles and improving their therapeutic index.[4] At the same time, loading of therapeutics into nanocarriers may increase their chemical stability and protect drugs from enzymatic degradation, extending their blood circulation time. Furthermore, these systems can be tailored to achieve a controlled release and to address the delivery on a specific target, leading to a higher therapeutic effect and a decreased development of tumor resistance.[9]
Along with the diversity of available materials, several nanosystems have been developed so far to deliver therapeutics, and they can be broadly classified as conjugates, lipid-based carriers or polymer-based carriers. Furthermore, beside these organic carriers, also inorganic nanoparticles are currently explored and have been found to be useful to target cancer cells, mostly for diagnostic purposes or phototherapy.[4]
Classic conjugates consist of an active pharmaceutical agent or carrier material covalently linked to a ligand that can improve the selectivity to a desired target without changing physical and chemical properties of the drug.[10] In contrast, carriers composed of lipids or polymers commonly encapsulate the active compound that then does not need to be modified, minimizing the risk of losing its activity. In this regard, both approaches present advantages and disadvantages, and the choice of a system rather than another often depends on the chosen cargo and the therapeutic use. In fact, while lipid carrier such as liposomes are usually biocompatible and well tolerated, they present shorter stability, due to their rapid clearance, compared to many synthetic polymers. However, many polymer-based nanocarriers are not fully biodegradable and do not allow complete release of the load and, thus, only a few polymers are currently approved for clinical use. The major benefit of synthetic delivery systems is that they can be easily manipulated using surface modifications in order to overcome their intrinsic limitations such as the need to improve the circulation time [11], enhance their mucus penetration ability [12], enhance intracellular delivery and, in particular, target specific cell types.[13] This is an important aspect especially in cancer therapies where the reduction of side effects is particularly required. In fact, even if nanocarriers, upon systemic administration, are expected to accumulate preferably within the tumor because of their hypothesized enhanced permeability and retention effect (EPR), passive targeting was shown not to be adequate to reduce the side effects of cytotoxic therapeutics compared to active receptor-mediated targeted drug delivery. As a matter of fact, this effect is often oversimplified since many events can occur along the blood stream, such as interactions between the nanosystems and plasmatic proteins, which can interfere with EPR targeting. In this context, even if some challenges have still to be overcome before bringing this kind of nanomedicine into the clinical setting, the idea of a fully selective targeted drug delivery is very appealing and holds big hopes. In this regard, the development of nano-sized delivery systems able to target mannose and mannose-6-phosphate receptors expressed on selected cells surface may increase the efficacy of the treatments and also limit possible side effects of the therapy.
3. Mannose Receptor-targeted drug delivery systems in anticancer therapy
3.1. The Mannose Receptor
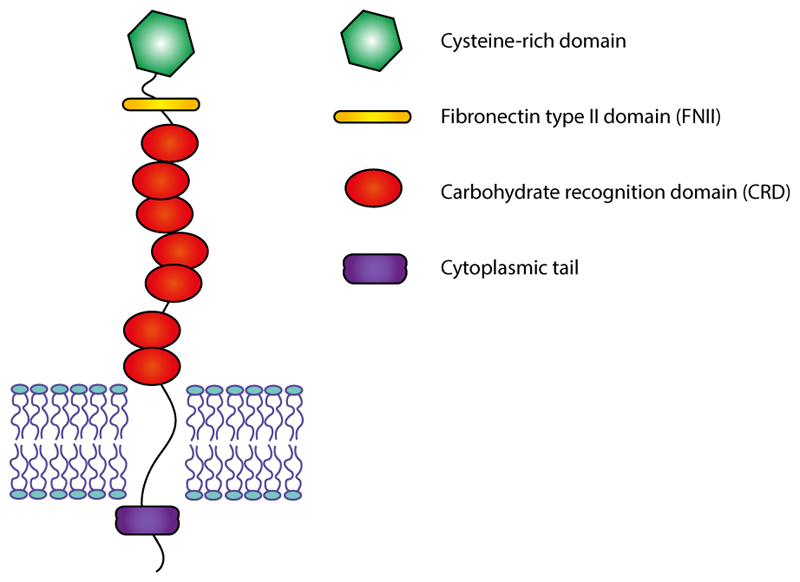
The mannose receptor family belongs to the C-type lectin superfamily and comprises four glycoproteins, the mannose receptor (MR), the M-type receptor for secretory phospholipases A2 (PLA2R), DEC-205/gp200-MR6 and Endo180/uPARAP. They are type I transmembrane receptors with a large extracellular domain composed of an N-terminal cysteine-rich domain, followed by a fibronectin type II (FNII) domain, 8 to 10 carbohydrate recognition domains (CRDs) and a short cytoplasmic tail containing two endocytosis motives (Figure 1). CRDs 4 and 5 bind mannose, fucose, and N-acetylglucosamine residues, while the cysteine-rich N-terminal binds sulfated sugars. Characteristically, these proteins are able to recycle between the plasma membrane and the endosomal apparatus, that allows them to transport extracellular ligands to the cells.[14]
Figure 1.
The structure of Mannose Receptor (MR) family. The MR is a transmembrane receptor containing a large extracellular domain composed of an N-terminal cysteine-rich domain, followed by a fibronectin type II (FNII) domain, 8 to 10 carbohydrate recognition domains (CRDs) and a short cytoplasmic tail.
The mannose receptor MR (cluster of differentiation number CD206) is also known as the macrophage mannose receptor (MMR), as in fact it is mostly expressed by macrophages, dendritic cells and endothelial cells.[6] This receptor contains eight CRDs, and three of them cooperate to reach high-affinity binding to multivalent glycoconjugates. Thus, it can distinguish between self and non-self and efficiently binds to mannosylated ligands present at the surface of various pathogens. Accordingly, MR can mediate the phagocytosis or endocytosis of these compounds, playing an important role in host defense, both providing immediate defense of the host from invading microorganisms (innate immunity) and also in inducing specific long-lasting immune responses, through the uptake and presentation of antigens (adaptive immunity).[6]
In the context of innate immunity, the protection of the host in the first hours of infection is primarily achieved by leukocytes, such as natural killer cells, and professional phagocytic cells such as macrophages and dendritic cells, that take up the invading organisms and degrade them within the phagolysosome/lysosome. In fact, the CRDs of the MR on these phagocytic cells selectively bind to glycans associated with microorganisms pathogens, including Candida albicans, Pneumocystis carinii, Leishmania donovani, Mycobacterium tuberculosis and Klebsiella pneumoniae, leading to their rapid internalisation and subsequent phagocytosis.[16] In addition to defense against pathogens, macrophages are able to induce the complete resolution of inflammation since their MR can also bind and thus degrade self-glycoproteins released in response to pathological events, such as, sulfated hormones, lysosomal hydrolases, tissue plasminogen activator or neutrophil myeloperoxidase.[17]. For this reason, MR has been postulated to be associated with autoimmunity and the preservation of host homeostasis, by mediating the clearance of some highly regulated glycoproteins, such as the ones named above. On the other hand, MR expressed on endothelial cells has been proposed to mediate the attachment and trafficking of lymphocytes and also cancer cells through its specific interaction with L-selectin.[6]
Moreover, as previously mentioned, MR plays also a crucial role in the adaptive immune system especially on immature and mature dendritic cells which internalize antigens and deliver them to the major histocompatibility complex (MHC) class compartments.[15] In particular, this receptor exists both as a membrane-bound and as a soluble protein, shed by proteolytic clipping. However, it is reported that the form that is involved the most in humoral immune responses is the soluble form which has a higher ability in capturing antigens and may facilitate their transport to immune cells as it contains a cysteine-rich domain that has been shown to bind them specifically. [18][16]
As already described above, the MR was originally termed the macrophage mannose receptor because, it was initially believed to be restricted to macrophages. On macrophages, it is actually restricted to steady state conditions which is in agreement with its role in the clearance of hormones and self-proteins.[6] Macrophages are large, motile cells of the mononuclear phagocyte series. Macrophages are formed through differentiation of circulating monocytes which leave the bloodstream through the blood vessel wall and migrate into the adjacent tissues, becoming macrophages. These cells have a critical role in the immune system and, as described before, are implicated in the innate and adaptive responses, mediated by MRs expressed on their surface. Moreover, they have also been involved in pathogenic phenomena, such as wound repair, angiogenesis, and the promotion of cancer progression, as well as tissue morphogenesis during development. Therefore, macrophages are an interesting therapeutic target that can be exploited not only in the treatment of cancer, but also in the prevention of tumor progression. They also mediate vaccines-based therapies and, MR-mediated targeting appears a promising strategy to deliver therapeutics to these cells.[19]
As described before, the MR is also expressed on the surface of the other APCs, such as dendritic cells. Dendritic cells (DCs) are a heterogeneous population of leukocytes remarkably potent at initiating and directing innate and adaptive immunity.[20] DCs are crucially important in T- and B-cell responses against bacterial and viral infections, since they are involved in internalizing and processing antigens which in turn are presented as antigenic peptides to CD4+ and CD8+ T lymphocytes.[21] Therefore, since DCs are the most effective APCs and are able to direct a significant part of the adaptive immune response, the MR appears a promising target to develop selective and efficient DC-delivered vaccine platforms that can be tailored against pathogens and cancer cells as well.
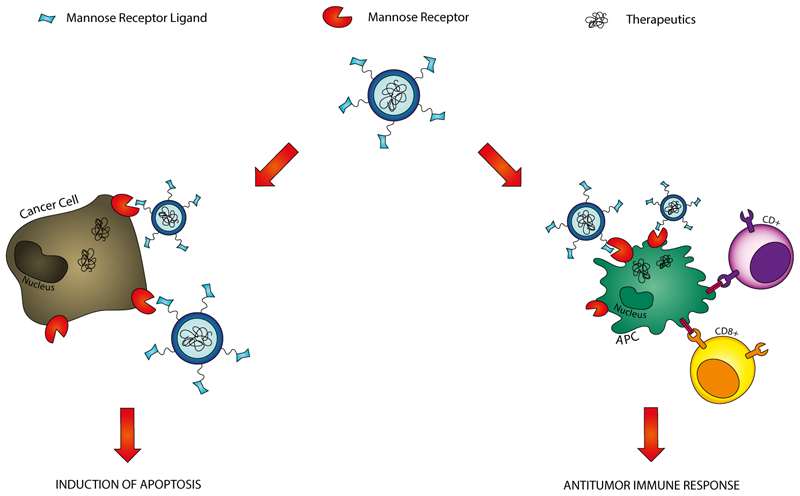
At the same time, many human malignant tumors over express the MR, since cancer cells have very high affinity for carbohydrate molecules compared to the normal cells due to high demand of nutrients for their rapid proliferation.[22] Accordingly, the MR can be used as potential target to distinguish tumors from healthy cells, achieving more selectivity and consequently lower toxicity. Because of the expression of MR both on the surface of many malignant tumors and APCs, it is clear that a MR-targeted nanocarrier can open the way to a number of applications in cancer therapy. In this way, MR-targeted delivery systems hold many promises in the field of a combination therapy where, as summarized in Figure 2, conventional chemotherapy and innovative immunotherapy are expected to be employed together to achieve the complete cancer remission. As a matter of fact, a direct induction of apoptosis in cancer cells can be obtained by the specific delivery of cytotoxic drugs to the tumor. On the other hand, in those tumors where MR expression is not up-regulated, this receptor can be the basis for engineered APCs which can induce CD4+ and CD8+ T lymphocyte-mediated antitumor immune response, that would result in antitumor vaccination and relapse prevention. Therefore, in the following sections a number of MR-mediated delivery systems are described in order to highlight their potentialities in improving the efficiency of current cancer therapy.
Figure 2.
Applications of Mannose Receptor-targeted drug delivery systems in anticancer therapy. The MR is a potential target to delivery anticancer drugs selectively to tumors, since its expression is up-regulated in several malignant cancers. Moreover, MR-targeted carriers can be used to deliver antigens or antigen-encoded nucleic acids to APCs and induce CD4+ and CD8+ T lymphocyte-mediated antitumor immune response.
3.2. APC targeting for anticancer immunotherapy
The majority of patients that survive a cancer has a high probability to have a relapse later, mostly because of the malignant cells which were able to escape the conventional treatments and can proliferate later. Therefore, the development of more effective cancer therapy is still a challenge, and the complete eradication of the complete population of malignant cells would be essential to prevent a relapse and reach a long term remission.[2]
Nowadays, there is an increase in the number of evidence supporting the idea that the immune system is able to trigger responses against tumors, that can thus be boosted using several, immunotherapy strategies, including cytokine therapy, immune-activating antibodies and APC cell–based immunotherapy.[23] Along these lines, active immunotherapy, such as cancer vaccination, using professional antigen presenting cells (APCs), such as dendritic cells (DCs) and macrophages (MCs), has emerged as a novel effective therapeutic option to achieve the complete remission from cancerous diseases. In fact, in contrast to passive immunotherapy with monoclonal antibodies, active immunotherapy is expected to induce long-term specific anticancer responses through immune memory cells, which could promote an effective and lasting elimination of cancer cells.[2] Moreover, another important advantage that distinguishes vaccination from traditional anticancer therapy is its ability to destroy malignant cells, without damaging healthy tissues. In this regard, driven by the discoveries of tumor-associated antigens (TAA) and the recognition of tumor specific CD8+ cytolytic T cells and CD4+ helper T cells as the major effector elements against cancer, a number of new vaccines have been developed so far to specifically induce and activate these cells directed against tumors.[24] As a matter of fact, activated CD8+ T-cells are able to directly attack malignant cells and also obtain a persisting phenotype memory, essential in order to avoid cancer relapses.[25] Moreover, CD4+ helper T cells are cruical in achieving an effective anti-tumor response by enhancing clonal expansion of Cytotoxic T lymphocytes (CTLs) at the tumor site and promoting the generation and maintenance of a memory phenotype.[26] Nevertheless, to stimulate both CD8+ and CD4+ T-cells against tumors, TAAs have to be presented through major histocompatibility complex (MHC) class I and II molecules in an immunostimulatory context.[27]
In order to achieve the antigen presentation to APCs and activate the immune response, two main strategies have been developed so far: (I) the direct delivery of antigens or (II) the delivery of antigen-encoding nucleic acids. Usually, extracellular antigens are internalized by APCs and presented via the major histocompatibility complex MHC class II (MHC-II) molecules to activate CD4+ T cells-mediated humoral immunity. However, as soon as antigens are delivered into the cytoplasm, they can be degraded in the proteasomes and thus presented by major histocompatibility complex class I (MHC-I) molecules, that leads to cellular immunity and induction of antigen-specific CTLs, which is essential for effective immune cell-based cancer therapy.[28] A number of peptides and proteins have been employed so far as antigens to induce antigen-specific immune responses. Even if they are usually safe and well tolerated, they unfortunately cannot generate high levels of immune responses because of their low internalization by APCs.[29] In order to overcome this hurdle, the delivery of antigen-encoding DNA has been developed. As a matter of fact, after administration of plasmid DNA (pDNA) encoding a specific antigen, a process of cellular internalization and processing takes place, that leads to potent protective responses and long-lasting humoral and cellular immunity avoiding the direct delivery of the antigens.[30] Furthermore, it has additional advantages such as cost-effective manufacturing and stability during storage.[21]
However, the delivery of antigen-encoded genetic material still remains an important impediment due to its low internalization, rapid enzymatic degradation and ineffective release from endosomes, which results in inefficient antigen presentation.[21] In light of these considerations, the development of a suitable delivery system to target and enhance the uptake of antigens or antigen-encoding genes to APCs is the main challenge that needs to be overcome in order to lead cancer immunotherapy to clinical applications. In this process, the initial antigen uptake and processing by APCs is the precondition for inducing immune responses which is closely related to successful vaccination.[21]
Targeting of APCs can be achieved using cell-specific endocytic receptors, such as the mannose receptor. As explained above, the MR is expressed on APCs and recognizes carbohydrates including mannose and is a key factor in antigen processing and presentation because MR-mediated endocytosis permits quick accumulation of mannosylated antigens for subsequent MHC presentation.[30] Consequently, targeted and successful delivery of antigen and also DNA vaccines into APCs could be achieved through MR-mediated endocytosis and, therefore, the use of delivery systems decorated with mannosylated moieties results a promising strategy. Accordingly, different methods developed so far for the delivery of therapeutics to APCs via MR are described below.
3.2.1. Strategy and carriers for antigen delivery to APCs via MR
Antigen targeting to MR is a promising method to develop new immunotherapeutic treatments in which antigen presentation on APCs is involved. In such cases, proteins or peptides that can activate the immune system are encapsulated or conjugated with a moiety that can provide binding and consequent uptake by MR-mediated endocytosis in APCs in order to obtain successful immunization.[15]
In this regard, a promising approach to improve the efficiency of protein- and peptide-based vaccine is delivery by mannose-decorated particulate delivery systems. In addition to the selectivity for MR on the surface of APCs given by the mannose moiety, these carriers also protect antigens from adverse physiologic conditions that can negatively affect the antigen presentation process, such as enzyme digestion and nonspecific interaction with other compounds in the extracellular matrix. Moreover, mannosylated-nanoparticles can also be tailored to release antigens in a controlled way, leading to increased cellular and humoral immune response, depending on the carrier material used to formulate the system.[29]
In this regard, Zhang and co-workers [29] developed a pH sensitive mannosylated nanosystem able to target DCs. The carrier was prepared by cross-linking mannose-modified alginate (MAN-ALG) and a conjugate (ALG=OVA) between alginate (ALG) and Ovalbumin (OVA), that was selected as model antigen. Thus, the obtained nanoparticles (MAN-ALG/ALG=OVA NPs) were tested in vitro using mouse bone marrow dendritic cells (BMDCs). The results clearly showed that the MAN-ALG/ALG=OVA NPs significantly enhanced DC antigen uptake and cytosolic release, in comparison to free OVA, suggesting that the system facilitated and improved selective antigen transport. Moreover the mannosylated system was also found to induce DCs maturation, which is a preliminary step in initiating an immune response. In fact, MAN-ALG/ALG=OVA NPs produced up-regulation of expression of co-stimulatory molecules CD40, CD80, CD86 and chemokine receptor 7 (CCR7) and increased cytokine secretion, compared to DCs treated with free OVA. As further demonstration of the increased uptake of MAN-ALG/ALG=OVA NPs, they showed enhanced MHC-1 antigen cross-presentation compared to free OVA. Moreover, the distribution of the nanoparticles after administration was evaluated in in vivo studies. The results showed that MAN-ALG/ALG=OVA NPs transported more antigen from the injection site to the draining lymph nodes than free OVA. Finally, anti-tumor effectiveness of MAN-ALG/ALG=OVA NPs was evaluated using OVA-expressing E.G7-OVA cells in mice, using free OVA and PBS as control. The in vivo data generated were consistent with the previous results and demonstrated that the MAN-ALG/ALG=OVA NPs induced major cytotoxic T lymphocyte response accompanied by inhibition of E.G7 tumor growth in C57BL/6 mice. Taken together, this novel delivery system efficiently delivered antigens and elicited a strong tumor-specific immunity. However, it is worth noting that the authors never included, as control, a non-mannosylated analogue of the nanosystems, which would be useful to further highlight the undeniable contribution of the mannose moiety in the improvement of the carrier efficiency. Moreover, an important restriction of this carrier is that its application is limited to the delivery of antigens with reactive amino groups, prior to the Schiff base binding with the alginate moiety. In consideration of this drawback, using a more versatile polymer that allows the encapsulation of antigens regardless of their structure would be desirable.[29]
On the same subject, Silva and co-workers [31] developed a reproducible formulation using mannose-functionalized aliphatic polyester copolymers to produce biodegradable polymeric nanoparticles loaded with melanoma associated antigens (TAAs) and the toll-like receptor (TLR) ligands oligonucleotide CpG and dsRNA analog Poly(I:C). Nanoparticles were formulated of a mixture of three aliphatic polyester copolymers: poly(D,L-lactic-co-glycolide) (PLGA); Poly(D,L-lactic-co-glycolide-b-ethylene glycol) (PEG-b-PLGA) and mannose-grafted poly(ε-caprolactone-b-ethylene glycol) (man-PEG-b-PCL). PEG-b-PLGA and PEG-b-PCL were added to the formulation to functionalize the polymers with the mannose residue and improve the stability of the system because of PEG’s repulsive properties. Thanks to this functionalized copolymer, the carrier showed great flexibility in terms of their selectivity for MR on the surface of APC and capability to entrap different molecules. In addition, since it is widely known that the presentation of antigens is more efficient in an immunostimulatory environment, TLR ligands such as oligonucleotide CpG and dsRNA analog Poly(I:C) were co-entrapped with TAAs. The anti-tumor therapeutic effect of this system was evaluated in B16F10-bearing mice. Altogether, the in vivo results showed that the mannose functionalization improved the performance of the nanoparticles in terms of the desired Th1/anti-tumor immune response and, moreover, the co-entrapment of antigens and immunopotentiators in the nanosystems was as well crucial to promote a long lasting Th1 immune response.[31]
In addition to the delivery of singular proteins, the use of whole tumor cell lysates (TCL) appears promising in anticancer immunotherapy because it has been recently shown that TCL preparation can present a complete source of possible tumor antigens and therefore induce CTL responses and CD4+ T helper cell activation.[32] However, soluble tumor lysates containing antigens and cytokines are naturally unstable and may result in low DC internalization, ineffective antigen cross-presentation, and induction of inadequate CTL response.[33] In this regard, their encapsulation into mannose-decorated nanosystems could improve both the selectivity of the delivery and the efficiency of a TCL-based vaccine.
On this subject, in a recent and very interesting report [34], Shi and co-workers designed chitosan-based nanoparticles surface-decorated with mannose (Man-CTS NPs) for specific MR targeting. TCL generated from B16 melanoma cells was encapsulated into the system, that was evaluated in in vitro and in vivo studies, using uncoated chitosan nanoparticles (CTS-TCL NPs) and free TCL as control. In vitro studies were performed in BMDCs and showed that, compared to TCL and non-mannosylated nanoparticles, Man-CTS-TCL NPs significantly increased antigen uptake in BMDCs. Moreover, exposure to Man-CTS-TCL NPs significantly increased expression levels of CD80, CD86 and CD40 surface markers and of MHC I, MHC II, and CCR7, which indicates DCs maturation. In vivo results were consistent with the in vitro data and showed that the mannosylated system had a higher antitumor efficacy in terms of TCL-induced antitumor immune response, compared to uncoated NPs and TCL alone: Man-CTS-TCL NPs were immediately internalized by endogenous DCs within the draining lymph node (DLN) and increased IFN-g and IL-4 levels in serum. Additionally, tumor growth was considerably delayed in mice treated with Man-CTS-TCL NPs vaccine. This result was, at least in part, attributed to a cytotoxic T lymphocyte response. Furthermore, the Man-CTS-TCL NPs vaccine showed also therapeutic effects in mice with melanoma as it was observed that vaccination with the mannosylated delivery system notably inhibited B16 tumor growth compared with controls.[34]
In addition to nanoparticulate carriers where antigens are loaded, another option to target MR on APCs is through the direct delivery of antigens functionalized with mannose residues or other ligands that have high affinity for MR. In this regard, novel fusion proteins can be designed in order to modify antigen structure and achieve a targeted delivery to APCs’ MR.
To this extent, a fusion protein made out of oxidized mannan conjugated to Mucin 1 (MUC1), a large complex glycoprotein widely expressed in adenocarcinoma and in particular in breast cancer, reached clinical phase and was used in a pilot Phase III clinical trial for immunotherapy against early breast cancer (stage II).[35] Upon the finding that mannan-MUC1 could generate CTL and give tumor protection unlike its non-modified analogue, over the last 20 years numerous preclinical and clinical studies were in accordance with the effectiveness of this conjugate. The overall results confirmed that targeting antigens to APCs via C-type lectin receptors, such as the MR, results in efficient stimulation of immunity in patients with cancer and confers protection against cancer recurrence.[35]
In addition to the chemical modification of the antigen with a small molecule that shows affinity for the MR, another possible strategy is achieving the targeting by the conjugation with a specific antibody (mAb) against MR.
Accordingly, Tsuji and co-workers [36] generated a novel fusion protein based on the full-length antigen NY-ESO-1 fused to human mAbs specific for MR (MR-NY-ESO). NY-ESO-1, a cancer-testis antigen expressed in a number of tumor types and broadly used in clinical cancer vaccine trials, was chosen and the conjugate was assessed for the ability to trigger NY-ESO-1–specific human CD4+ and CD8+ T cells. In vitro results showed that MR-NY-ESO rapidly bound the target MR on APCs, and its selective delivery was achieved. Moreover, whereas non-targeted and MR-ESO proteins comparably activated CD4+ T cells, cross-presentation to CD8+ T cells was only efficiently driven by targeted NY-ESO-1, resulting in an enhancement of the immunization efficiency.[36]
3.2.2. Mannosylated carriers for gene delivery to APCs
In addition to the delivery of antigens, it has been previously been shown that it is possible to use mannosylated lipoplexes and polyplexes for gene delivery into APCs and therefore to deliver efficient anticancer DNA vaccines. [37]
Indeed, DNA vaccination offers many practical advantages that cannot easily be achieved with the other currently available forms of vaccines, such as recombinant protein vaccines or whole tumor cells. For instance, DNA vaccines are easy to design, and their large-scale manufacturing is more economical than for recombinant proteins. Moreover, their storage and global delivery are more straightforward because plasmid DNAs are rather stable at room temperature.[38] Another distinguishing feature of DNA vaccines is that this genetic immunization is often able to induce a cellular immune response and, therefore, it is thought to be crucial for achieving an effective long-lasting vaccination.[39] In spite of all these advantages, the poor in vivo cell transfection efficiencies that characterizes naked plasmid DNA and difficulties in APC targeting are impeding their clinical success. Therefore, mannose decorated polymers able to complex, deliver, and release antigen-encoding DNA into the cytoplasm represent promising carrier material for APC-targeted delivery systems via MR. In light of these considerations, many known polycations were chemically mannose-functionalized or synthesized ex novo, in order to elicit specificity and potency in leading APC targeting and subsequent immune response.
The synthesis of completely new polymers is time consuming, therefore, coupling mannose residues with polycations with a well-known transfection profile is expected to be the easiest option to improve the transfection efficacy. For instance, many Mannose-PEI conjugate formulations have been designed and applied in MR-targeted gene delivery into APCs. However, while some of these studies demonstrate that Man-PEI conjugates prove very efficient in gene delivery via MR, their translational applicability is limited because of their relative toxicity at the optimal polycation/DNA ratio.[40] In this regard, Raviv and co-workers [41] successfully developed a method to formulate mannosylated polyion complexes (PICs) made of cationic linear polyethylenimine (PEI) and hydrophilic polyethylene glycol (PEG) segments, also carrying mono- and trivalent mannose as ligands for targeting DCs. Thus, the lower cellular uptake ability of PEG-PEI in comparison to PEI alone was compensated for by the mannose-functionalization. Amino-terminated mannose (Man)-containing ligands in mono- and trivalent presentations (Man- and Man3-, respectively) were formulated and conjugated to PEG via an N-hydroxysuccinimide (NHS)-activated terminal. Thiolated PEI was conjugated to the mannosylated PEG via the maleimide (MAL)-activated terminal. The obtained positively charged diblock copolymers carrying mannose residues (Man-PEG-b-PEI and Man3-PEG-b-PEI) were self-assembled with DNA to form polyion complexes (PICs). Monovalent- and trivalent-mannosylated ligands (Man-PAP and Man3-AHT, respectively) were used to increase the binding affinity and selectivity to DCs. It was found that mannosylation provides surface shielding of the PICs contributing to higher stability and at the same time improving water solubility, as additional advantage. Transfection efficiency was evaluated in the MR-positive murine dendritic cell line DC2.4 as an APC cell line model. Cells were transfected with Man3-PEG-b-PEI and Man-PEG-b-PEI loaded with luciferase or green fluorescent protein (GFP) expressing plasmids and PEG-b-PEI and PEI were used as controls. Man3-PEG-b-PEI demonstrated a higher in vitro transfection efficiency compared to Man-PEG-b-PEI, confirming correlation between the number of mannose targeting moieties and internalization efficacy. On the other hand, Man3-PEG-b-PEI exhibited almost equal efficiency as compared to the positive control PEI/DNA, but with the additional advantage of its lower toxicity.
In vivo CD11c+ transfection efficiency was evaluated in C57/BL6 mice, and the results were consistent with in vitro observations. Man3-PEG-b-PEI/DNA showed the highest transfection efficiency as compared to the other systems and, in this case, it was found to be also more effective than the positive control PEI/DNA complexes. A possible explanation for the results obtained is that the PEI/DNA complexes could adhere to the cell membranes at the site of injection, while the mannose functionalization, allowed the mannosylated systems to be efficiently delivered and internalized.
All these data support the idea that this mannosylated system improves the selectivity and the safety profile of the carrier. Despite the promising results obtained so far, further studies using an antigen-encoding DNA are required to confirm the ability of the system in inducing a valuable immune response.[41]
As previously widely discussed, mannose ligands can increase the binding affinity and selectivity to APCs but, in general, a targeted system cannot assure the successful escape of a nanocarrier from endosomes and the subsequent release of the cargo into the cytoplasm, which are crucial prerequisites for inducing an effective immune response. Hence, many current scientific approaches are focusing on overcoming this hurdle by the development of new systems and biomaterials that can be tailored to achieve this aim. Even nowadays, the efficacy of most nanocarriers in releasing nucleic acids is still too low. Despite much progress that has been achieved, nanomedicine still has a long way to go and, in this context innovative strategies and further studies are needed in order to guarantee a successful endosomal escape. Endosomal release is a prerequisite for targeting intracellular organelles, as for instance the nucleus. This necessity represents one of the main drawbacks that keep nanomedicine often far from clinical translation. In this regard, Jones and coworkers [30] developed a new set of mannosylated poly(beta-amino esters) (PBAEs) that represents a novel class of APC-targeting cationic polymers that are, furthermore, easy to synthesize. Gene delivery assessment in vitro in RAW264.7 cells, that are known for their poor transfectability, exhibited significant improvement upon PBAE mannosylation and implied that mannose-mediated internalization and processing is involved in the efficiency of gene delivery. Furthermore, they used an ovalbumin mouse immunization model in order to test the capabilities of the delivery systems in an in vivo setting. As results, when it is compared to genetic and protein control antigens, the mannosylated PBAEs showed a robust, efficient, and safe in vivo humoral immune response without using any adjuvant.[30]
In another recent work, Xu and co-workers [21] developed a method to manufacture novel pH-responsive multi-layer nanocomplexes (PDMD) for DNA delivery to APCs thanks to the mannosylated chitosan used to encapsulate the genetic material. Dendritic lipopeptide, charge-reversible polymer and mannosylated chitosan, as APC-targeted material, were successfully integrated into this delivery system through layer-by-layer (LBL) assembly. Amphiphilic dendritic lipopeptide DSPE-G2, engineered as virus inspired building blocks, was used to condense pDNA forming virus-inspired nanovectors (VINs). A charge-reversible polymer poly(allylamine hydrochloride)-citraconic anhydride (PAH-Cit) was deposited onto the positively charged core complexes to facilitate the release of pDNA, upon exposure to the mildly acidic environment in endosomes.[21]
The mannose moiety of the system was expected to assure targeting and MR-mediated endocytosis of the carrier and, on the other hand, the LBL assembly was hypothesized to further increase gene transfection and better control the incorporation and release of DNA.
Confirming the hypothesis, in vitro studies in DCs showed that this novel mannosylated nanocarrier has a higher transfection efficiency as well as a lower toxicity compared to the positive control made of GFP expressing plasmid (pGFP) and PEI (pGFP/PEI). Nonetheless, the promising results of this work should be further confirmed by in vivo data and the encapsulation of a tumor-antigen-encoding pDNA.[21]
In order to improve the stability of the system, an alternative approach to the direct decoration of the nanocarrier with mannose moieties, is the functionalization of polymers with different ligands of MR.
In this regard, in a recent promising work, Garu and co-workers [39] formulated liposomes made from three novel different cationic amphiphiles containing mannose-mimicking shikimoyl (1), quinoyl (2) head-group, and their mannosyl analogue (3), respectively. Their transfection efficacy was evaluated in primary mouse bone marrow-derived DCs (mbmDCs) and it was found that the lipoplexes of lipid 1 with mannose-mimicking shikimoyl head-group were the most efficient compared to liposomes made with the other two lipids. These data were consistent with in vivo experiments where C57BL/6J mice were treated with lipoplexes of the three amphipiles and encapsulating a melanoma antigen (MART1)-encoded DNA vaccine (p-CMV-MART1). Finally, the system bearing the mannose-mimicking shikimoyl amphiphilic compound was again the most efficient in terms of transfection efficiency and resulted in the highest MART1 expression levels. Subsequently, a further experiment with direct in vivo immunization of mice treated with the cationic liposome bearing the mannose-mimicking shikimoyl and p-CMV-MART1 showed that this system generated long-lasting anti-melanoma primary immune response with remarkable memory response, compared to the group treated with control lipoplexes and vehicle only. Therefore, this work [39] showed that the development and evaluation of other ligands in addition to natural mannose can further help improve selectivity and efficiency of gene delivery systems to target APCs.
In addition to improving the efficiency and selectivity of the delivery system, it would be desirable also to overcome many limitations of conventional vaccine immunization such the need of invasive administration such as the intramuscular route. In this regard, an interesting approach was recently developed by Hu and co-workers.[42] They designed a novel trans-cutaneous DNA vaccine delivery system in order to achieve mannose-mediated epidermal DC-targeting and mannosylated low molecular weight PEI for copolymer-mediated gene transfection. Transcutaneous immunization (TCI) is an appealing vaccination strategy since it is non-invasive or minimally invasive and can go beyond the limitations of usual vaccine formulations administered by intramuscular injection. Moreover, the skin is highly immunoreactive because the epidermis is rich in dendritic cells, the Langerhans cells (LCs) that are involved in capturing, processing, and presenting foreign antigens to lymph nodes. Thus, LCs induce effective immune responses and also express MR.[43] Therefore, the skin appears an ideal and attractive avenue for vaccine administration. Moreover, MR targeting to APCs can help improve the efficiency of potential carriers for transcutaneous immunization. Furthermore, to improve the percutaneous delivery of vaccines through the stratum corneum and reduce the dose, microneedle-mediated TCI has been developed. It was shown that a mannosylated grafted vector was more efficient in delivering DNA when applied through microneedles for targeting dermal DCs in draining lymphoid tissues compared to naked DNA and PEI/DNA. Therefore, the antitumural immunity induced by the system was evaluate in BALB/c mice treated with nanoparticles made of a cell penetrating peptide (CPP), linear 800 Da PEI (PEIl800), and mannose (Man), abbreviated CPP-PEI1800-Man. The nanoparticles were loaded with a plasmid DNA encoding the tumor antigen of the fused tyrosinase-related protein 2 (Trp-2), a melanogenic protease overexpressed in both melanocytes and melanomas which was selected as Malignant Melanoma antigen.[42] The in vivo studies showed that the Man-PEI1800-CPP/DNA complexes were taken up by the epidermal DCs more efficiently than PEI complexes and naked DNA used as control groups, and that they moved into the lymph nodes with subsequent expression of the tumor-associated antigens and higher production of IL-12. Moreover, the mannosylated system generated relevant therapeutic anti-tumor immunity, with prolonged survival time compared to the non-APC targeting method. Thus, this nanocarrier represents a promising TCI approach with DC-targeting DNA delivery for priming cellular immune responses for cancer immunotherapy.[42]
Additionally to this novel transcutaneous route, nowadays mucosal immunization is attracting an increasing level of attention. In fact, it is thought to be the more successful strategy for the induction of local mucosal and efficient systemic immune response. Moreover, it is noninvasive, more cost-effective and less painful during the administration, therefore resulting in higher patient compliance.[44] Interestingly, nasal mucosa incorporates more lymphoid cells than muscular tissues and therefore it represents a promising route of immunization via MR mediated endocytosis. [45]
In light of this consideration, Yao and coworkers [46] designed a novel intranasal mucosal delivery protocol and developed mannosylated chitosan nanoparticles loaded with an anti-gastrin-releasing peptide (GRP) DNA vaccine (pCR3.1-VS-HSP65-TP-GRP6-M2, short pGRP), that were intranasally administered in a subcutaneous mouse prostate carcinoma model. GRP is a 27-amino acid peptide that acts as a gastrointestinal hormone and is connected with the growth of tumors. [47] It was shown that, following intramuscular injection, pGRP inhibited the growth of prostate carcinoma and melanoma cells in a mouse model by producing anti-GRP antibodies.[48] First, the authors confirmed the higher uptake of the mannosylated nanoparticles compared to uncoated ones in RAW 264.7 macrophages, which was mediated by an endocytotic process via MR. Subsequently, the efficiency of intranasal immunization was evaluated in RM-1 mice in terms of induction of specific anti-GRP antibodies, using saline, pGRP solution and non-mannosylated nanoparticles as control. In vivo studies confirmed the potential of the system, that induced higher levels of antitumor antibodies. Moreover, the contribution of the mannose functionalization to the anti-prostate carcinoma immunization was confirmed as well: both an evident reduction in the magnitude of tumor growth and the higher level of antibodies until 14 days after tumor cell challenge were associated with mannosylated-chitosan nanosystem treatments.[46]
In addition to antigens or antigen-encoded DNA, an emerging strategy to treat cancer is through the delivery of RNA. As a matter of fact, Total Tumor RNA (TTRna) isolated from different cancer cells encodes many tumor antigens that may induce DC-mediated generation of tumor-specific CTLs. Also in this case, a mannose decorated delivery system may help to enhance the efficiency of the carrier to deliver the RNA to APCs.[49]
Addressing this goal, Markov and co-workers [50] designed novel mannosylated liposomes (ML) and showed their ability to deliver model plasmid DNA encoding EGFP and TTRna into murine BMDCs by MR-mediated endocytosis, enhancing the efficiency of the anti-tumor response. TTRna encoding a variety of tumor antigens was isolated from B16 murine melanoma cells (TTRna -B16). Their ML consist of spermine-based cationic lipid (1,26-Bis(cholest-5-en-3β-yloxycarbonylamino)-7,11,16,20-tetraazahexacosan tetrahydrochloride, short 2X3), helper lipid (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, short DOPE), and novel mannosylated lipoconjugates, in different ratios. Therefore, ML naturally form complexes with plasmid DNA and RNA because of their electrostatic interactions. ML demonstrated better pDNA and RNA delivery into immature DCs via MR-mediated endocytosis, as compared to the control liposomes. ML containing mannosylated lipoconjugate with diethylsquarate linker showed some additional advantages over the lipoconjugate with the succinyl linker and, therefore, they were selected for in vivo studies. ML lipoplexes containing TTRna -B16 were intravenously injected into male C57Bl/6 mice, and ML were found to generate melanoma B16-specific cytotoxic T-lymphocytes, which showed a two-fold higher efficiency in B16 cell killing than those from the control liposome group. Markov and co-workers found that the best in vivo DC-targeting was achieved by ML containing 10% mol of the mannosylated lipoconjugate with diethylsquarate linker (M6), which resulted in DC-mediated generation of CTLs that induced higher levels of melanoma B16 cell killing as compared to non-specific control and control liposomes made from 2X3 and DOPE. In light of the promising results obtained in this study, it is likely that these novel mannosylated liposomes provided high levels of RNA delivery into DCs by MR-mediated endocytosis and therefore induced immunization and anti-metastatic immune responses.[50]
In addition to DCs, macrophages as well can behave as APCs and they represent another important therapeutic target. Moreover, MR was confirmed to be up regulated in tumor-associated macrophages (TAMs) and therefore it may constitute a useful target for cancer therapy through MR targeting.
In a number of cancer types, high level of resident macrophages have been observed and associated with poor prognoses.[51] These TAMs are hypothesized to promote tumor growth and invasiveness and therefore reprogramming their behavior through the use of RNA interference therapy appears a promising strategy.[52] One therapeutic approach consists of delivering small interfering RNA (siRNA). Upon its internalization, siRNA is processed by the inherent transcriptional regulation apparatus of the target cell, with the final effect of gene silencing through cleavage and inactivation of mRNA complementary to the antisense strand of the administrated therapeutic siRNA duplex.[53] By silencing genes that regulate unfavorable macrophage activity, RNA interference (RNAi) treatment has the potential to precisely block macrophage functions that often cause disease progression. In this regard, the delivery of siRNA by MR mediated endocytosis might allow to bypass the major hurdles in MC transfection, such as their extremely degradative phagocytic, endosomal and lysosomal compartments, as well as favor the delivery and cytoplasmic release of genetic material.[54] In light of these considerations the mannosylated polymeric micelles designed by Yu and coworkers [54] appear to be promising to achieve this aim. Indeed, they developed a pH responsive mannosylated system using “click chemistry” (ManNPs) that was found to improve the transport of siRNA into primary murine macrophages and induced robust knockdown of a model gene, while the non-targeted NPs delivered considerably smaller amounts of siRNA into the cells. Moreover, since the ManNPs are internalized via an endocytotic receptor, the novel carrier can successfully facilitate escape from the endosomal pathway through a mechanism that is probably due to the pH-responsive of the core-forming terpolymer block.[54] Lastly, these nanoparticles were also avidly identified and taken up by human macrophages and mediated the delivery of 13-fold more siRNA into these cells than into model breast cancer cell lines. Therefore, the ManNPs showed cell selectivity, and the results suggested that in a tumor environment where cancer cells cohabit with a markedly smaller population of macrophages, the ManNPs would be internalized by macrophages significantly faster than the cancer cells. Thus, these MR-targeted, endosomolytic siRNA delivery nanosystems represent a promising technology for targeting APC cells such as MCs.[54]
3.3. Mannose-functionalized Tumor-targeted Drug Delivery Systems
The development of targeted carriers able to recognize and deliver drugs at a sustained rate directly to cancer cells could results in higher efficacy and lower toxicity in cancer therapy.[3] In this regard, mannose-targeted systems may represent a possible strategy to overcome the problem of poor specificity and dose-limiting toxicity of traditional chemotherapeutics agents.[3] As a matter of fact, it was found that MR is over expressed in many tumors because, generally, cancer cells have very high affinity for carbohydrate molecules compared to the normal cells due to high demand of nutrients for their rapid proliferation.[55] Moreover, while tumor cells have high tendencies to mutate and develop multiple resistance mechanisms, the MR appears a more stable marker that can therefore bypass the need of identifying specific and changing tumor antigens. Therefore, mannose-mediated nanovectors are expected to increasingly influence cancer treatment approaches, improving the efficiency of many therapeutics or diagnostics already in use. Moreover, since the interaction between a single mannose and the MR is weak, nanoparticles bearing multiple mannose residues are expected to provide a higher presentation of mannose ligands and a resulting stronger binding with the receptor.[7] Therefore, encapsulating anticancer drugs or imaging probes in mannose-decorated carriers may potentially increase their uptake in cancer cells that overexpress MR.[56] In addition, the encapsulating of a drug in mannosylated delivery systems would have the additional advantage of not only reducing the drug’s cytotoxicity but also improving chemical-physical properties of the compound that could limit its bioavailability.
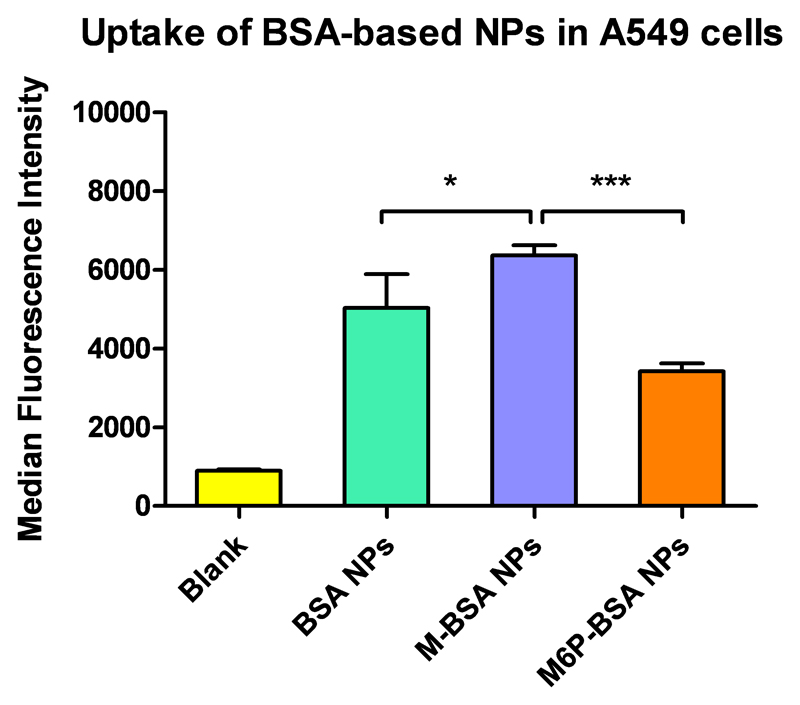
To this extent, Guo and coworkers [55] developed a mannosylated lipid nano-emulsion formulation in order to deliver the anticancer drug lycorine while also improving its poor solubility both in lipids and water and avoiding undesired toxicity due to off-target delivery. Lycorine (LYC) is an isoquinoline alkaloid extracted from Lycoris radiate, a medicinal plant traditionally used in Chinese medicine. Recent studies revealed that it mediates antitumor effects such as high antiproliferative activity in cancer cell.[55] In this context, a mannosylated lipid nano-emulsion (M-LYC-OA-LNE) could thus be an attractive and balanced formulation for tumor-targeted delivery of LYC by parenteral administration. Indeed, nano-emulsions (LNEs) show a number of advantages for intravenous administration, because of the strict prerequisites of this route of administration, mainly the need for a formulation droplet size lower than 1 μm.[57] Moreover, although lectin receptors can be found also on the surface of other cells rather than just tumor cells, mannose modified LNEs can still be targeted to the tumor site since such nanosystems can potentially passively accumulate in tumor tissue though the enhanced permeability and retention (EPR) effect, followed by MR-mediated tumor cell uptake. In this formulation, oleic acid (OA) was used as the lipophilic complexing agent to make a lycorine-oleic acid ionic complex (LYC-OA) in order to increase the lipophilicity of lycorine. First, the in vitro growth inhibition activity and cellular internalization of mannosylated and uncoated LNEs were evaluated in A549 lung epithelial cancer cell line and CHO cells as control. Consistent with the hypothesis, uptake studies showed that mannosylated LNEs were internalized more efficiently than the uncoated LNEs in A549 cells, while in CHO they showed no significant differences. Subsequently, a growth inhibition activity study was performed by MTT assay and the results confirmed those from the uptake study with M-LYC-OA-LNEs showing best activity compared with free LYC and uncoated LNEs. It was thus concluded that the mannose ligands on the nanoparticles promoted better internalization with in A549 cells via MR-mediate endocytosis. In accordance with this conclusion, also our results, shown in Figure 3, report that nanoparticles made of mannosylated bovine serum albumin (BSA) were significantly more efficiently internalized in A549 cells, as compared to non-modified BSA and mannose-6-phosphate-functionalized nanoparticles. Nevertheless, further studies are required to evaluate if mannosylated BSA particles could also efficiently mediate higher therapeutic efficacy than unmodified BSA particles.
Figure 3.
Cellular uptake of BSA-based nanoparticles in A549 lung epithelial cancer cell line. Internalization of mannosylated nanoparticles (M-BSA NPs) was significantly higher compared to non-modified BSA (BSA NPs) and mannose-6-phospate functionalized nanoparticles (M6P-BSA NPs (data points indicate mean ± SD; One-way ANOVA, *p < 0.05, ***p < 0.0001, n = 3).
In order to reach an optimal release of the payload from the carrier, it is worth to take into account that the environment of tumor tissues is often more acidic than under normal physiological conditions, suggesting that the pH-triggered drug release of a targeted-delivery system would be much more efficient for tumor therapy. Taking this condition into account, Ye and co-workers [7] developed a new generation of self-assembled nanoparticles for both mannose receptor-mediated cancer cell targeting and pH-controlled drug delivery. Amphiphilic β-Cyclodextrin (β–CD), a cyclic oligosaccharide with a lipophilic central cavity, was used as carrier material of the system. [58] From this starting material, a novel amphiphilic heptamannosylated β-CD with propargyl α-D-mannopyranoside (C3-CD-Man7) was synthesized to form nanoparticles by self-assembly in aqueous solution. Then, doxorubicin (DOX), a chemotherapeutic drug that is widely used to treat various solid malignant tumors, was chosen as the model anticancer drug for loading into the C3-CD-Man7 nanoparticles (abbreviated DOX-C3-CD-Man7 NPs) via hydrophobic interactions between the drug and the hydrophobic cavity of the carrier. Moreover, DOX allows easy observation of its cellular internalization and intracellular release behavior, due to its intrinsic red fluorescence. In in vitro studies, DOX-C3-CD-Man7 NPs were efficiently internalized by MDA-MB-231 breast cancer cells, compared with HEK293 normal cells, via MR-mediated endocytosis, resulting in intracellular pH-triggered DOX release and subsequent apoptosis. Moreover, considering the acidic tumor tissue environment and the lower pH of endosomes and lysosomes, these results indicate that the pH-triggered drug release of DOX-C3-CD-Man7 NPs is suitable for tumor therapy. Furthermore, the multivalent mannose nanoparticles markedly increased the targeting of MDA-MB-231 cancer cells such that the efficacy of the anticancer drug delivery of DOX-C3-CD-Man7 NPs was enhanced both in vitro and in vivo. Indeed, in vitro results displayed that the cytotoxic efficacy of DOX-C3-CD-Man7 NPs was higher than that of free DOX towards MDA-MB-231 cancer cells. Moreover the in vivo antitumor efficacy of DOX-C3-CD-Man7 NPs was further assessed using a mouse model, which was established by injecting MDA-MB-231 cancer cells into subcutaneous tissues and treated using saline, C3-CD-Man7, free DOX and a glucosylated analogue (DOX-C3-CD -Glu7 NPs) as control. The data demonstrated a greater tumor growth inhibition achieved by DOX-C3-CD-Man7 NPs than could be attributed to the cooperative effects of the enhanced tumor accumulation and mannose-mediated targeting of the system towards MDA-MB-231 cancer cells.
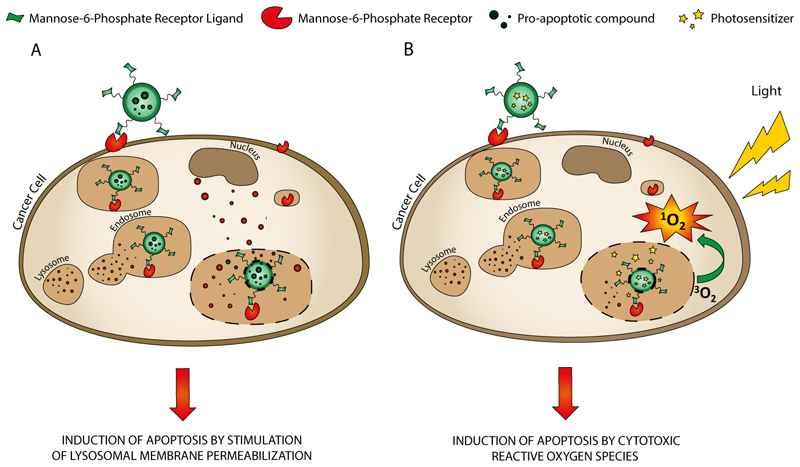
A similar formulation was developed by the same group to deliver the photosensitizer boron dipyrromethene (BODIPY) into cancer cells to boost the efficiency and reduce the side effects of photodynamic therapy.[59] Because the mannose receptor is overexpressed on the surfaces of many malignant tumors, such as MDA-MB-231 breast cancer cells [60], it is expected that mannosylated carriers will be selectively trapped through MR-mediated endocytosis, minimizing the phototoxicity to healthy cells during photodynamic therapy treatment. Photodynamic therapy (PDT) is a promising local and minimally invasive method to treat a number of cancers that combines three non-toxic components: light, oxygen and a photosensitizer (PS).[61] After PS activation by irradiation with light of the proper wavelength, the excited PS preferentially transfers energy to molecular oxygen and produces highly cytotoxic reactive oxygen species, primarily singlet oxygen (1O2), inducing irreversible damage to surrounding cells. Therefore, the incorporation of PSs into targeted nanoparticles can improve bioavailability while retaining the desirable photoactivity and minimizing undesired adverse effects observed when delivered without tumor selectivity.[62] In this regard, in a recent report, Zhang and coworkers [59] used a heptamannosylated β-cyclodextrin (CD-Man7) complex with an adamantane (Ad)-functionalized BODIPY conjugate (BTA). Because of the presence of supramolecular host−guest interactions between β-cyclodextrin and adamantane units, CD-Man7 can be immobilized onto the surface of BTA aggregates to stabilize them in aqueous suspension. Therefore CD-Man7 behaves as a modulator and stabilizer during the formation of mannose-functionalized nanoparticles (BTA-CD-Man7 NPs) in aqueous solution.[59] These nanosystems show excellent targeted delivery of the PS and consequent cancer cell death after irradiation both in vitro and in vivo. In vivo experiments showed that mannose groups greatly enhance the specific targeting of BTA-CD-Man7 NPs and they are selectively taken up by MR-rich MDA-MB-231 breast cancer cells via receptor-mediated endocytosis rather than by healthy MCF-10A cells, thereby accelerating singlet oxygen generation to trigger apoptosis in cancer cells upon red light irradiation. Accordingly, the MR-mediated PS delivery system for targeted PDT against breast cancer cells selectively killed cancer cells but prevented damage to normal cells. In vivo efficacy of the system was evaluated using a mouse model that was established by injecting MDA-MB-231 cancer cells into subcutaneous tissues, and the data confirmed the remarkable tumor inhibition effect of BTA-CD-Man7 NPs under irradiation, due to the selectivity of the mannosylated system for tumor cells.[59]
Moreover, the same group recently developed a three-arm distyryl BODIPY derivative conjugated with mannose units (denoted by BTM) able to co-assemble with Tween 80 to form nanomicelles (BTM-NMs) as possible carrier for targeted PDT against MDA-MB-231 breast cancer cells.[63] In vitro studies confirmed that MDA-MB-231 breast cancer cells recognized and selectively internalized BTM-NMs via MR-mediated endocytosis with preferential accumulation in the lysosomes. The NMs disassembled in cell lysosomes and subsequently induce highly efficient singlet oxygen generation upon light irradiation. 1O2 disrupted the lysosomal membrane and promoted the escape of BTM from the lysosome into the cytoplasm, thereby resulting in the efficient and selective killing of cancer cells through PDT. The excellent targeted delivery of the BTM-NMs was demonstrated through their phototoxicity toward MDA-MB-231 cancer cells and lack of toxicity to MCF-10A cells.
PDT in combination with two-photon excitation (TPE) in the near-IR region suggests new perspectives for the treatment of solid tumors because of its higher penetration depth and exclusive spatial resolution. However, the use of conventional photosensitizers requires very high excitation powers (close to the threshold of tissue photodamage) due to the low two-photon absorption (TPA) cross-sections (σ2) in the biological spectral window (i.e. 700–1000 nm).[64] Therefore, also in this case a functionalization with mannose moieties on the surface of the delivery system could provide further enhancement of the potential of this strategy. In this regard, Gary-Bobo and co-workers [60] developed fluorescent mesoporous silica nanoparticles (MSNs) with giant two-photon absorption (TPA) cross-sections, for efficient TPE-PDT. MSNs appear already very promising for tumor treatments as they are biocompatible and preferentially are internalized and accumulate in tumors[65]. Additionally, their surface was post-functionalized with a mannose derived compound in order to increase specific targeting to the MR over-expressed by cancer cells. TPE-PDT with these MSNs was evaluated in vitro on human breast and colon cancer cell lines, and the results clearly showed that MSNs functionalized with mannose were more efficient than non-functionalized ones. Moreover, in vivo experiments completed in athymic mice bearing xenografted tumors from colon cancer cells were consistent with in vitro results. Authors showed that a single injection was sufficient to target these nanosystems to the tumor area while two-photon irradiation in the near IR generated a major reduction of the tumor size. Moreover, this protocol demonstrated to hamper the development of metastases associated with the spread of cancer without evident systemic toxicity and without toxicity under standard (daylight) illumination. This therapeutic approach appears promising and especially suitable for minimally invasive ablation of small localized solid tumors such as in prostate and colon cancers, retino-blastoma, head and neck cancers. A targeted TPE-PDT may therefore give an alternative to conventional more invasive therapeutic approaches, such as surgery and radiotherapy for restricted organs and low-grade diseases. Mannose targeting appears to be an efficient way to optimize the delivery systems for these approaches. [66]
Mannose functionalization of gene carriers can also be used to target and transfect tumors directly since gene therapy is another promising strategy to cope with cancer diseases as discussed above. In this regard, Pan and coworkers [67] developed a mannose-modified, bioreducible hybrid polymer of low-molecular-weight PEI, mannose, and a cell-penetrating peptide (termed Man-PEI5k–CPP) as a vector to deliver TRAIL plasmid (pTRAIL) for colorectal cancer treatment in MR overexpressing colon cancer cells. The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor super family and causes extrinsic apoptosis by binding the death receptors (DR4 and DR5) in cancer cells but sparing normal cells. Therefore, the delivery of pTRAIL can potentially address the problematic short half-life of the recombinant human TRAIL protein, considering that transfected cells continually produce TRAIL for extended periods, thus inhibiting tumor growth.[67] With the combined functions of the tumor-targeting mannose moieties and the CPP, Man-PEI5k–CPP was able to improve the cellular uptake efficiency, and also increased the effectiveness of transfection, hence resulting in higher growth inhibition efficacy on HCT116 human colon cancer cells, in comparison with conventional PEI25k. In vivo experiments were consistent with cell culture results and further validated the treatment success of Man-PEI5k–CPP/pTRAIL in mice bearing xenografted HCT116 tumors and revealed minimal systemic toxicity.[67]
In addition to the delivery of DNA, recent studies have demonstrated that therapeutics containing other nucleic acid, such as siRNA, constitute an emerging strategy with a great potential in cancer treatment, due to the efficient gene knockdown that can be achieved. Accordingly, a mannosylated targeted delivery system can help overcome the major hurdles that prevent siRNA therapeutics from reaching clinical phases, such as the poor of cellular uptake and nonspecific off-target delivery. In this regard in a recent study, Li and co-workers [22] have successfully prepared a novel nanocarrier (termed Man-SiO2@LDH NPs) by grafting mannose onto SiO2-coated sheet-like layered double hydroxide nanoparticles (LDH NPs). Indeed, LDH NPs are considered a promising candidate for drug and siRNA delivery because of their features such as high ionic exchange property, controlled release, low cytotoxicity and biocompatibility.[68] Moreover, due to mannosylation they were more efficiently delivered to osteosarcoma (U2OS) cells compared to non-mannosylated LDH NPs. The results of this study showed that Man-SiO2@LDH NPs enhanced MR-mediated siRNA-delivery leading to a higher inhibition of cancer cell growth compared to their non-mannosylated analogues, which was attributed to their quicker uptake and siRNA release.
4. Mannose-6-Phosphate- Receptor-targeted drug delivery systems in anticancer therapy
4.1. The Mannose-6-Phosphate Receptors
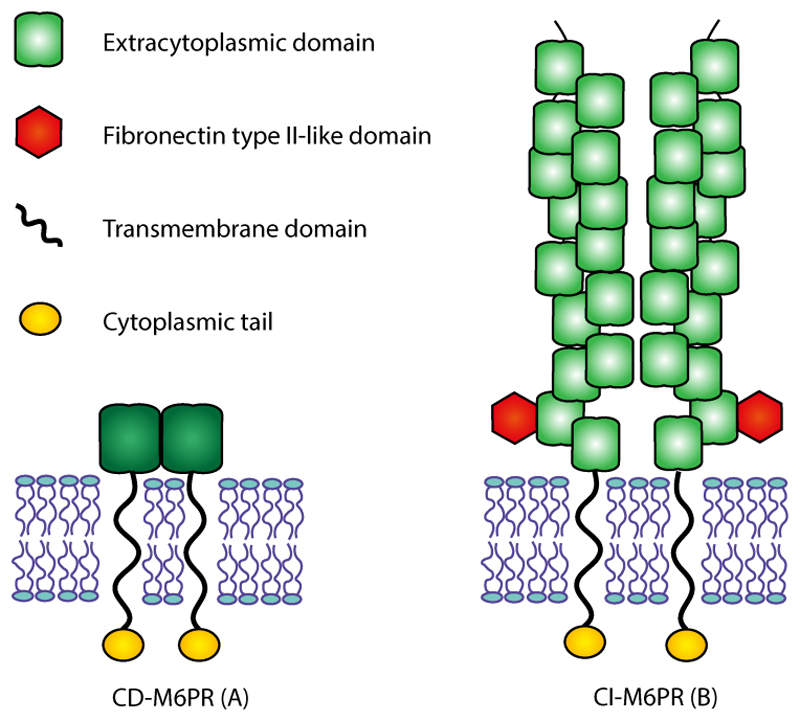
In contrast to the mannose receptor, two mannose-6-phosphate receptors (M6PRs) have been characterized: they both are type I transmembrane glycoproteins and the only members of the p-type lectin family (Figure 4). They are (I) the cation-dependent mannose-6-phosphate receptor (CD-M6PR), that requires divalent cations to bind M6P ligands and does not bind insulin-like growth factor 2, and (II) the mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2-R), that binds both M6P ligands and IGF2.[69] The M6P/IGF2-R is also termed cation-independent M6P receptor (CI-M6PR) since it does not require ions to bind its ligands
Figure 4.
The structure of Mannose-6-Phosphate Receptors. The two mannose-6-phosphate receptors (M6PRs) are transmembrane glycoproteins and the only members of the p-type lectin family. The cation-dependent mannose-6-phosphate receptor (CD-M6PR) (A), appears to be a homodimer at the membrane, and it consists of an N-terminal extracytoplasmic region, a transmembrane domain anda C-terminal cytoplasmic region. The cation-independent mannose-6-phosphate receptor (CI-M6PR) (B), is also called mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2-R), because it binds both M6P ligands and IGF2. CI-M6PR seems to behave as homodimer in the membrane, and it contains a large N-terminal extracytoplasmic domain organized in 15 repeats, a short transmembrane region and a small intracellular C-terminal domain.
The CD-M6PR is a 46 kDa single polypeptide chain which consists of a transmembrane domain and a supposed signal sequence. The transmembrane domain is a single membrane-spanning domain, that divides an N-terminal extracytoplasmic region with 5 potential Asparagine-linked glycosylation sites from a C-terminal cytoplasmic region without glycosylation sites (Figure 4.A). Moreover, it was found to be highly conserved from mouse to human (93% homology), and this receptor seems to be a homodimer at the membrane, and either a dimer or a tetramer in solution.[70]
The second receptor, CI-M6PR, is a large 300 kDa single-chain glycoprotein consisting of 2491amino acids. It contains an N-terminal signal sequence, a large extracytoplasmic domain (with 19 potential glycosylation sites) organized in 15 repeats, a short transmembrane region and a small intracellular domain which constitutes the C-terminal domain (Figure 4.B). Even if the quaternary structure of this protein has not been fully clarified, some data have provided a description of the dimerization of its membrane. Furthermore, an additional soluble form of the CI-M6PR receptor is spontaneously released by proteolytic cleavage from the integral cellular receptor, and this soluble protein has been found at low concentrations in serum of many mammalian species. Moreover, it was also reported that the nucleotide sequence of the full-length cDNA and the corresponding amino acid sequence for the CI-M6PR are extremely similar to those reported for the human insulin-like growth factor II (IGF-II) receptor from HepG2 hepatoma cells, suggesting that CI-M6PR is a multifunctional binding protein, identical to the IGF-II receptor, and that is able to bind both to M6P and IGF-II. In fact, within the extracytoplasmic domain, the CI-M6PR can bind two molecules of M6P and one molecule of IGF-II per molecule of receptor.[70]
Here we will focus on CI-M6PR (that will be above termed M6PR) and its role in tumor progression, and the main strategies to use this receptor as a gate in cancer therapy will be explored.
One of the most important and evident function of M6PR is the uptake of M6P-bearing ligands and their transport to the lysosomes.[70] M6P containing ligands bind to domains 3 and 9 and their affinity for the receptor depends on their structures. In this regard, molecules that contains phosphonate, carboxylate or malonate groups can bind the receptor as well and, moreover, they show a stronger stability in human serum.[70]
One of M6PR’s major roles is directing the intracellular trafficking of newly synthesized lysosomal enzymes, bearing M6P moiety, from the trans-Golgi apparatus to the late endosomes. Moreover, M6PR is also mainly involved in the cellular internalization of secreted lysosomal enzyme precursors.[71] Since lysosomal immature proenzymes contains M6P signals they are recognized and bind to M6PR. Then, the complexes of proenzyme/receptor are first internalized in clathrin-coated vesicles to acidic endosomal compartments where the low pH induces the dissociation of the enzyme from the receptor.[71] Subsequently, the enzymes are released into the lysosomes, their final destination, for their ultimate maturation, while the M6PR are either transported to the cell membrane to be recycled or moved back to the trans-Golgi network to participate in other transport cycles.
In light of these considerations, M6PR has evident application in targeting lysosomes and constitutes a potential target for enzyme replacement therapy (ERT) for lysosomal diseases. However, M6PR could also be exploited to deliver and release specific therapeutics that are able to induce apoptosis into lysosomes.
Another important function of M6PR is mediating endocytosis and clearance of IGF-II.[72] As a matter of fact, the best described non M6P-bearing ligand of CI-M6PR is IGF II. This hormone has critical importance in metabolic regulation and fetal growth since it binds two tyrosine kinase receptors (IGF-I receptor and insulin receptor isoform A), inducing a protein phophorylation cascade which leads to relevant biological effects, mainly insulin-mediated growth.[73] Therefore, internalization by M6PR and the subsequent lysosomal degradation of IGF-II is essential, since high levels of IGF-II cause overgrowth. Since the lack of M6PR increases IGF-II levels, with consequent tissues proliferation, while physiological concentrations of this receptor can block tumor growth mediated by IGF-II, it has been suggested that M6PR plays a role as tumor suppressor.[74] Therefore, M6PR represents a promising marker for understanding tumor prognosis.
M6PR has also been found to bind a third type of ligand with high affinity, the retinoic acid at a site that is different from those of M6P and IGF-II. Because of its binding to the nuclear retinoid receptors, retinoic acid is primary involved in development, cellular metabolism, and regulation of cell proliferation and oncogenesis. In this regard, retinoic acid has been found to stimulate M6P/IGF2 receptor-mediated uptake of IGF2 and to enhance lysosomal enzymes sorting.[71] Therefore, M6P/IGF2-R has been suggested as important actor in mediating retinoid-induced apoptosis/growth inhibition.[71]
In addition to IGF-II, retinoic acid and lysosomal enzymes carrying M6P, M6PR has been found to bind many other extracellular ligands carrying the M6P recognition tag in order to activate or degrade them. Some multi-protein complexes, for instance, containing latent-TGFβ or urokinase-type plasminogen activator receptor (uPAR), bind to the membrane M6PR in order to be activated extracellularly. Other proteins, such as the cytokine leukemia inhibitory factor (LIF) bind to M6PR to be taken up or directed to the lysosomes and then degraded.[71]
Since membrane M6PR plays a crucial role in some types of cancer and in sorting of lysosomal enzymes, here we will focus on different potential strategies to use this receptor in cancer therapy both evaluating its role as tumor suppressor and the up-regulation of its expression on some types of cancer cells.
M6PR is present ubiquitously in the Golgi apparatus and endosomal compartments, and therefore M6PR-mediated targeting has been extensively explored for the treatment of lysosomal storage diseases and enzyme replacement therapy.[70] The M6PR primarily and constantly moves and recycles between endomembrane compartments and cell surface. At a steady state, it is mainly localized in trans-Golgi network (TGN) and endosomal compartments and present on the plasma membrane only to a low extent. Compared to MR, it is not characteristic of any cell populations and it is present in several human tissues such as kidney, liver, spleen, lung but also in heart and skeletal muscle.[75] Normally it is highly expressed in fetal and neonatal tissues but it decreases postnatally, however it is over-expressed in some pathological conditions, such as fibrosis, Alzheimer’s and some cancerous diseases.[76]
For instance, its expression is up-regulated in hepatic stellate cells (HSCs) upon their activation due to acute or chronic liver injury, such as fibrosis.[77] Moreover, over-expression of this receptor in the central nervous system may play a role in the production of amyloid precursor protein, that leads to the generation of β-amyloid peptide, which is widely associated with neurodegenerative diseases.[76] Furthermore, in adults M6PR has been shown to be induced in a number of human carcinoma cells, such as breast cancer, pancreatic cancer, gastric cancer, melanoma and prostate cancer.[78] Therefore it is emerging as a potential target for tumor-specific delivery of anticancer therapeutics.
4.2. The role of Mannose-6-Phosphate Receptor in tumor progression
The gene encoding M6PR in frequently down-regulated or inactivated by mutations in a broad range of malignant human cancers, such as ovarian cancer, liver cancer and squamous cell carcinoma, compared to normal cells and less aggressive forms of cancer.[69] Thus, it has been suggested that it acts as tumor suppressor and that it can have a potential role as a cancer prognostic marker.
As a matter of fact, it has been stated that M6PR may act as a potential tumor suppressor for instance in 70% of hepatocarcinomas, where over-expression of M6PR induced growth inhibition of cancer cells and regression of tumors in mice.[70] These effects of M6PR on tumor progression can be explained by the different roles played by this receptor in controlling the cell growth. In fact, M6PR is closely involved in the regulation of the cellular life cycle since it is responsible for uptake and degradation of many growth-promoting factors, such as IGF-II, glycosylated LIF, and other M6P-containing cytokines, such as the macrophage colony-stimulating factor. Moreover, it is directly involved in the regulation of cellular death, by binding and internalizing granzyme B, which is a crucial factor for T cell-mediated apoptosis. Lastly, M6PR mediates also the regulation of the level of secreted lysosomal enzymes which are key factors in extracellular matrix degradation and tumor dissemination. In addition, it is reported that many of its ligands are involved in cancer development and metastasis.[70]
In light of these considerations, M6PR appears a promising cancer prognostic marker and, therefore, also a possible target for gene therapy. In this regard, Puxbaum and co-workers [79] evaluated the role of M6PR expression in a receptor-negative transformed fetal rat liver cell line (FRL14), inducing the expression of the receptor after transfection with cDNA that encoded the human wild type receptor gene, in order to evaluate differences in invasiveness and tumorigenic nature. Consistent with the tumor suppressor hypothesis, they found that invasiveness and motility, that are factors strongly involved in tumorigenicity, of receptor-expressing cells was reduced compared to non-transfected cells. Vice versa, in another experiment, they also confirmed that the migratory and invasive potential of receptor-positive mouse hepatocytes was increased upon RNAi-mediated M6PR knock-down.[79] Therefore a connection between dysfunctional M6PR expression and cancer disease progression is clearly highlighted by these results and is consistent with the activities of the receptor as tumor suppressor in other types of cancer.[80] In this consideration, identification of receptor status would result in a better prediction of cancer prognosis. On the other hand, pharmacological intervention on the protein expression level, by pDNA or iRNA transfection, can represent a promising complementary approach to cancer therapy.
In addition to a lower expression of the receptor, some cancer-associated mutations can inactivate the expressed receptors, preventing their ability to properly bind ligands.[81] Because of the mutagenic properties of IGF-II, it is widely thought that M6PR participates in tumor progression because it mediates internalization and subsequent degradation of IGF-II. As a matter of fact, it has been confirmed that, in this way, M6PR is able to prevent abnormal cellular growth and following cancer development, by down-regulating the growth-promoting and anti-apoptotic effects of the mitogen IGF-II.[81]
Nevertheless, in another interesting study, Probst and co-worker [81] demonstrated that the capacity to reduce cellular motility and invasiveness is not due to dysfunctional IGF-II internalization and subsequent degradation. As a matter of fact, it was reported that, in an experiment performed in squamous cell carcinoma (SCC-VII) cells, the anti-invasive activity of M6PR does not depend on the presence of a functional binding site for IGF-II. On the contrary, they found that the ability of the receptor to suppress growth and invasiveness relies mainly on the functionality of M6P-binding site, since the receptor possibly facilitates tumor progression and metastasis largely by mediating the release of M6P-bearing proteins such as acid hydrolases (for instance, lysosomal proteinases) into the extracellular space.
Although the role of M6PR in cellular growth suppression is well recognized and reported, its impact on tumorigenicity, invasiveness, and metastasis development is still not completely understood and therefore further studies are necessary to establish proper strategies and method to use the potential of this target in cancer therapy. Nevertheless, it is broadly accepted that finding specific prognostic markers able to predict the cancer progression would be a cornerstone in cancer therapy.
4.3. Mannose-6-Phosphate Receptor-targeted drug delivery systems in cancer therapy
The overexpression of M6PR in several human cancer cells, such as in breast cancers [69], pancreatic cancer [82], gastric cancer [83], melanoma [84] and prostate cancer [78] indicates that this receptor could be also considered a potential target to selectively deliver chemotherapeutics, however it is also described as tumor suppressor and it was found to be decreased or mutated in some malignancies such as hepatocarcinomas.[79] An explanation for the higher selectivity of M6PR targeting of cancer cells rather than normal cells could be, on the one hand, the higher expression of M6PR on the cell surface, but also, on the other hand, it is the higher affinity that the receptor presents at the weakly acidic pH (pH 6-6.5) found in the extracellular environment of solid tumors. In this context and since anti-neoplastic drugs, for instance vinca alkaloids or anthracyclines (e.g., doxorubicin), are slightly basic compounds developed to target the neutral pH compartment of tumors, but are inactivated in a more acidic environment, targeted delivery via M6PR could improve their activity by preventing their inactivation.[70]
The first report concerning the delivery of anticancer drugs to tumor cells through M6PR was published by Prakash and co-workers.[76] M6P-decorated Human Serum Albumin (HSA-M6P) conjugated with the traditional anticancer drug Dox was used as targeted delivery system (Dox-HSA-M6P). First, the authors confirmed the upregulated expression of the receptor in B16 melanoma and C26 colon carcinoma cells and in their subcutaneous tumors compared to normal organs, such as kidney, lung, heart. Moreover, they demonstrated that M6P-HSA also accumulated in fibroblast-like stromal cells, that can be further beneficial since they promote the proliferation and invasion of cancer cells. Subsequently, further in vitro studies confirmed higher uptake of M6P-HSA compared to unmodified HSA in tumor cells and that also Dox-HSA-M6P was selectively internalized via M6PR-mediated endocytosis. Finally, the efficacy of Dox-HSA-M6P inhibiting tumor growth was evaluated in B16-F10 subcutaneous tumors in mice. Altogether, the data clearly showed that the conjugate was stable enough to be delivered and accumulate in tumors, which was further linked with an increased anti-cancer effect of Dox-HSA-M6P in comparison with free doxorubicin, that was not measurable in tumors but distrubuted into most of the organs. However, data from a further in vivo study using Dox-HSA as control are missing in this report, while it would be worth to confirm that the higher efficiency of the system is due to the M6P functionalization and not to the encapsulation into the HSA-based carrier.
Moreover, M6PR can be targeted not only to deliver cytotoxic drugs into cancer cells but also to specifically address them to lysosomes. Thus, the routing of these therapeutics via M6PR may induce the lysis of lysosomes and subsequently mediate cell death, due to the release of lysosomal enzymes.[70] From this perspective, lysosomes could be an interesting therapeutic target useful for the induction of apoptotic pathways in cancer cells that express M6PR (Figure 5.A).
Figure 5.
Applications of Mannose-6-Phosphate Receptor-targeted Drug Delivery Systems in anticancer therapy. M6PR-targeted nanosystems allow specific receptor-mediated endocytosis in cancer cells that overexpress the receptor and then are able to specifically transport and release their cargo into lysosomes. Lysosomotropic targeted nanocarriers can be used in cancer therapy A) for loading compounds that can induce lysosomal membrane permeabilization and lead to apoptosis, upon release of hydrolytic enzymes, such as cathepsins; and B) for loading photosensitizers (PSs) that, after activation by irradiation with light of the appropriate wavelength, result in generation of singlet oxygen (1O2) and/or other reactive oxygen species (ROS). Thus, the consequent oxydative stress leads to cell death.
Regarding this approach, in a recent and very interesting work, Minnelli and co-workers [85] developed M6P-decorated liposomes loaded with C6-ceramide (C6-cer), which behaves as a detergent and was found to induce lysosomal membrane permeabilization (LMP), in order to target lysosomes in cancer cells and induce apoptosis. Ceramide has a very low solubility in aqueous environment and tends to form micelles that prevent its cellular uptake. Moreover, since it is a natural cellular lipid, it is susceptible to enzymatic degradation. Therefore, the design of a proper carrier is necessary to overcome these issues, and the lysosome targeting would maximize the therapeutic efficiency. The group successfully achieved this aim by designing liposomes made of a M6P cholesteryl conjugate (Chol-M6P) where a sufficiently rigid aryl-incorporated linker connects the M6P moiety to a steroid structure and ensured exposure of the M6P function to favor tight association of the liposome with the receptor.[86] After confirming the overexpression of M6PR in human breast adenocarcinoma cell line (MCF7) compared to Human Dermal Fibroblasts (HDF), they confirmed the enhanced uptake of M6P-decorated liposomes in MCF7 compared to HDF cells. The authors first confirmed the higher delivery capacity in MCF7 than in HDF cells by specific uptake of M6P liposomes loaded with a calcein probe. In order to exclude non-specific endocytosis, free calcein and calcein-loaded non-functionalized liposomes (NFL) were used as control and no significant calcein uptake was observed. Then, the cytotoxic potential of ceramide-loaded M6P-liposomes (M6P-C6-cer) was evaluated in MCF7 and in HDF cells and results showed that the functionalized system had a strong cytotoxicity effect in MCF7 cell lines at all tested concentrations but not in HDF cells. Moreover, in MCF7 cells the treatment with M6P-C6-cer raised the level of apoptotic cells compared with uncoated analogue, whereas not substantial differences were observed for the HDF cells. This observation can be explained by the lack of cellular internalization. Since these results show that liposomes could selectively target cancer cells expressing M6PR but that they were not readily internalized by cells in the absence of M6P targeting ligand, these M6P-functionalized liposomes loaded with cytotoxic molecules, could potentially also be used as delivery systems in other tumor cells over-expressing M6PR.
Interestingly, the phosphate moiety at the 6-position of mannose is thought to specifically address the delivery system exclusively to lysosomes while the carbohydrate structure is not enough to achieve the internalization into these organelles.[87] In this regard, Das and co-workers [87] confirmed the selectivity of an end-functionalized polyvalent mannose-6-phosphate glycopolypeptides (M6P-GPs) for trafficking into lysosomes, in comparison with the corresponding mannose-GP polymer. They showed that an alteration of the trafficking route of the system is achievable by introducing the phosphate moiety at the 6-position of mannose and thus made the carrier absolutely lysosome specific, while the mannosylated analogue was more evenly distributed inside the cells. Therefore, M6P-decorated biomaterials, such as their novel polymer that mimics proteins conjugated with phosphomannosyl residues, appear to be a very promising carrier system to load cytotoxic drugs and deliver them directly into lysosomes, through M6PR-mediated endocytosis.
M6PR targeting, additionally to improving the delivery and the activity of traditional anticancer drugs, is expected to play an interesting role also for theragnostic applications, for example by encapsulating a photon sensitizer (Figure 5.B). In this regard, Vaillant and co-workers [78] suggested M6PR as a possible target for prostate cancer due to its over-expression in prostate cancer tissues with no expression in normal tissues or non-cancerous hypertrophy of prostate. In their recent work, they synthesized a M6P analogue (M6C) and grafted it onto the surface of mesoporus silica nanoparticles (MSNs) covalently incorporating one- or two-photon sensitizers, for the treatment of prostate cancer by photodynamic therapy. First, they confirmed the low expression of the receptor in normal prostate tissues compared to the over-expression in malignant cells, evaluating samples from 126 patients. The potential of M6PR for theragnostic applications was then assessed on prostate cancer cell lines and primary culture cells directly isolated from tissues, treated with nanoparticles containing one- or two-photon sensitizers, and the difference between M6C-decorated (M6C- MSNs) and uncoated nanosystems was evaluated. The in vitro results confirmed that M6C- MSNs were internalized by receptor-mediated endocytosis, and the photodynamic therapeutic effect was higher than after treatment with uncoated nanoparticles. Interestingly, the same treatment did not result in any cell death in normal fibroblasts, therefore suggesting that M6C- MSNs was not taken up in normal cells. Moreover, the functionalization of the system with an analogue of M6P (M6C) bearing a lateral chain with a carboxylate function bioisostere of the phosphate group, instead of the natural ligand, resulted in the additional advantage of improving the stability of the system, since the isostere made the compound insensitive to serum phosphatases. Thus, M6PR seems to hold promising hopes as new target for specifically directed medicine applications in prostate cancer as well as in many other tumors that over-express the receptor. Moreover, other ligands that could have higher affinity for the receptor or that could confer longer stability are worth to be explored in order to achieve further improvement in M6PR-targeted anticancer therapy efficiency.
5.1. Challenges and current limits in the application of Mannose and Mannose-6-Phosphate receptors mediated targeted anticancer therapy
Over the last decades, much progress has been achieved in anticancer therapy. Some of these advancements are due to the introduction of nanotechnology in cancer treatments. Nanosystems already introduced into clinical settings have allowed many improvements both in achieving remission and an earlier diagnosis. Nevertheless, long lasting and complete remission from several malignancies is still a challenging hurdle that needs to be overcome.
There is no doubt that the application of mannose and mannose-6-phosphate receptor targeting offers many possibilities in anticancer therapy and, as reported in most of the cited articles, these strategies have already given promising results both in in vitro and in vivo studies.
The preferential expression of mannose and mannose-6-phosphate receptors on the surface of several aggressive cancer cells represents a benefit to develop receptor-targeted drug delivery systems for chemotherapeutic treatments. Overall, we have shown several successful approaches where mannose and mannose-6-phosphate targeted drug delivery provides more efficient and less harmful anticancer treatments, as targeted therapies are supposed to exhibit high specificity in reaching tumor tissue. Thus, as assessed in many reported studies, these receptor-targeted approaches could prevent major drawbacks of conventional chemotherapy, such as lack of selectivity, cytotoxicity for healthy cells and the need to limit the dose of the active compound, which often leads to therapeutic failure. There is no doubt that targeted nanomedicine is, in general a step forward compared to passive targeting. However, the mere addition of targeting ligands often neither prevents unwanted interactions of the delivery system in the blood circulation nor assures the release of the cargo at the proper site and with proper timing. Additionally, in case of mannose and mannose-6-phosphate receptor targeting, a deeper knowledge about these receptors has to be accrued in order to, for example, optimize the density of the sugar moieties and ensure the optimal internalization without undesired interactions with plasmatic proteins. Moreover, as earlier described, the insertion of a targeting ligand does not ensure escape from the endosomes nor the complete release of the payload. Additionally, all the physiological barriers have to be considered, especially in the context of nucleic acids delivery, where either nuclear internalization or cytoplasmic accumulation are prerequisites for therapeutic effects.
Beside the application of both receptors in targeted delivery of chemotherapeutics to cancer cells in order to treat the tumor directly, here we have reported additional potential application fields of these receptors in cancer treatment: (I) immunotherapy, due to the preferential expression of MR on APCs, and (II) cancer prognosis, because of the oncosuppressor nature of M6PR. In the context of aiming for a complete remission and the improvement of the patient life span, both an early diagnosis of the tumor and an efficient therapy that eradicates all the residual malignant cells are crucial factors. In this regard, it is clear that these two receptors are very appealing in cancer therapy, although more research is needed before achieving a better understanding of their potentialities. In case of MR targeting, for example, it is clear that there is a positive influence of mannosylation on effectively targeting APCs. In most of the discussed work, protective tumor immunity and modulation of the immunosuppressive tumor environment was achieved, leading to successful inhibition of tumor growth alongside opening the way to a longer cancer remission as already demonstrated in at least one clinical study. However, one of the biggest hurdles is the design of the best combination of immunotherapy and traditional chemotherapy. As a matter of fact, many variables have to be considered before translating this approach into clinical trials. First of all, the reaction of the human organism to all compounds of a combination therapy, especially in terms of immune-response and toxicity would need to be studied. While, among the lectin-type receptors, MR is a deeply studied target, M6PR is gaining a growing interest only in the last years in the context of cancer therapy since its application has been restricted to lysosomic diseases for a long time. For this reason, fewer data are available at the moment, as compared to MR, but the results reported in this work are clearly very promising, since evidence in several studies supports its role as tumor suppressor. Even though its expression is up-regulated in some malignancies, in other types of cancer the loss of its function is associated with invasiveness and tumor progression and therefore it could both represent a promising prognostic marker as well as a potential target for corrective gene therapy. Nevertheless, further efforts are required to better elucidate the feasibility of its application. Additionally, its dichotomy in cancer disease needs to be clarified, where it acts sometimes as tumosuppressor and it is overexpressed in other type of cancers. Moreover, a number of questions have been put forward since the discovery that this receptor is involved in many physiological processes, beside the trafficking of lysosomal enzymes. Therefore, the consequences of its targeting or eventual gene-mediated regulation on other physiological functions, such as embryogenesis, have to be deeply understood before translating its application to clinical settings.
5. Conclusion
Beside the effective application of mannose and mannose-6-phosphate receptors themselves, it is important to keep in mind that further studies have to be performed to confirm the feasibility and the effective potential of targeted nanotechnology in cancer therapy. The main challenges intrinsic to nanomedicine are the achievement of a reproducible and large-scale manufacturing and the inadequacy of the current pre-clinical models in fully mimicking the human organism. Indeed, achieving monodisperse systems that meet proper physicochemical features is not only a key factor for its preclinical and clinical application, but also a prerequisite to the translation of nanomedicine into the pharmaceutical industry. Much progress has been achieved in the last years thanks to technical development involving microfluidic and other approaches, but the manufacturing of more complex nanosystems, which often involves several steps, is still a challenge. Moreover, though the results from several in vitro and in vivo studies appear to be promising, these models are still inadequate to fully mimic the heterogenic tumor progression in a human patient. As a matter of fact, while cell culture and engineered animal models can helping to better understand interactions and some effects of the systems, they cannot provide a full view of the human body’s complexity.
Although nowadays still many drawbacks prevent the widespread application of nanotechnology in the clinic, in this promising area of anticancer targeted-nanomedicine, mannose and mannose-6-phosphate targeting systems could doubtless play a key role in the next-generation clinical therapeutics and, with this in mind, a big effort is required to afford cancer patients the most rapid access to these innovative treatment options.
Acknowledgements
This work was supported by the European Research Council [grant number ERC-2014-StG – 63783]; the Nanosystems Initiative Munich (NIM); and ERASMUS+ fellowship to Elena Dalle Vedove.
Biographies
Elena Dalle Vedove received her MS in Chemistry and Pharmaceutical Technology from Università degli Studi di Camerino, Italy, in 2016, following which she won an ERASMUS+ scholarship and was recruited as research intern at Ludwig-Maximilians-Universität München, Germany. Her research focuses on development of Mannose and Mannose-6-Phosphate functionalized nanocarries for gene delivery. 
Dr. Gabriella Costabile received her MS in Chemistry and Pharmaceutical Technology in 2012 and her PhD in Pharmaceutical Sciences in 2016, both at the University of Napoli Federico II, Italy. Following her PhD, she joined Ludwig-Maximilians-Universität München, Germany, in 2017, where she is currently Postdoctoral Group Leader. Dr. Costabile’s the research interest is focused on the design and development of innovative delivery systems for low molecular weight drugs and biomacromolecules (i.e., peptides, proteins and nucleic acid therapeutics), with special regard to particulate carriers for lung delivery. 
Prof. Dr. Olivia Merkel received her degree in pharmacy from the Philipps-Universität Marburg, Germany, in 2004, her MS in Pharmaceutical Technology from Martin-Luther-Universität Halle-Wittenberg, her PhD in Pharmaceutical Technology from the Philipps-Universität Marburg, Germany, in 2009, and was a postdoctoral associate and lecturer until 2011 when she accepted a Tenure Track Assistant Professorship in Pharmaceutical Sciences at Wayne State University, Detroit, MI. She became Associate Faculty of Oncology at WSU in 2012 and accepted a Professorship for Drug Delivery at Ludwig-Maximilians-Universität München, Germany, in 2015. Her research interest focuses on topical drug and gene delivery using novel and smart dosage forms. 
References
- [1].WHO. Cancer (Fact sheet) [accessed: February];2017 http://www.who.int/mediacentre/factsheets/fs297/en/
- [2].Rolinski J, Hus I. Transplant Proc. 2010;42:3306. doi: 10.1016/j.transproceed.2010.07.033. [DOI] [PubMed] [Google Scholar]
- [3].Ahire JH, Behray M, Webster CA, Wang Q, Sherwood V, Saengkrit N, Ruktanonchai U, Woramongkolchai N, Chao Y. Adv Healthc Mater. 2015;4:1877. doi: 10.1002/adhm.201500298. [DOI] [PubMed] [Google Scholar]
- [4].Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. J Control Release. 2015;200:138. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- [5].Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, Engelman JA, et al. Nat Rev Cancer. 2017;17:286. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- [6].Keler T, Ramakrishna V, Fanger MW. Expert Opin Biol Ther. 2004;4:1953. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]
- [7].Ye Z, Zhang Q, Wang S, Bharate P, Varela-Aramburu S, Lu M, Seeberger PH, Yin J. Chemistry (Easton) 2016;22:15216. doi: 10.1002/chem.201603294. [DOI] [PubMed] [Google Scholar]
- [8].Shi J, Kantoff PW, Wooster R, Farokhzad OC. Nat Rev Cancer. 2017;17:20. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. Adv Drug Deliv Rev. 2013;65:1284. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- [10].Duncan R. Nature Reviews Cancer. 2006;6:688. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- [11].Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. Adv Drug Deliv Rev. 1995;16:215. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boylan NJ, Suk JS, Lai SK, Jelinek R, Boyle MP, Cooper MJ, Hanes J. J Control Release. 2012;157:72. doi: 10.1016/j.jconrel.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bareford LM, Swaan PW. Adv Drug Deliv Rev. 2007;59:748. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].East L, Isacke CM. Biochim Biophys Acta. 2002;1572:364. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- [15].Irache JM, Salman HH, Gamazo C, Espuelas S. Expert opinion on drug delivery. 2008;5:703. doi: 10.1517/17425247.5.6.703. [DOI] [PubMed] [Google Scholar]
- [16].Regnier-Vigouroux A. Int Rev Cytol. 2003;226:321. doi: 10.1016/s0074-7696(03)01006-4. [DOI] [PubMed] [Google Scholar]
- [17].Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC. Science. 2002;295:1898. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- [18].Martínez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S, Bonnefoy JY, Gordon S. The Journal of Experimental Medicine. 1996;184:1927. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sturge J, Todd SK, Kogianni G, McCarthy A, Isacke CM. Biomacromolecules. 2007;82:585. doi: 10.1189/jlb.0107053. [DOI] [PubMed] [Google Scholar]
- [20].Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Proc Natl Acad Sci U S A. 2010;107:4287. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu M, Chen Y, Banerjee P, Zong L, Jiang L. AAPS PharmSciTech. 2017 doi: 10.1208/s12249-017-0741-1. [DOI] [PubMed] [Google Scholar]
- [22].Li L, Zhang R, Gu W, Xu ZP. Nanomedicine. 2017 [Google Scholar]
- [23].Begley J, Ribas A. Clin Cancer Res. 2008;14:4385. doi: 10.1158/1078-0432.CCR-07-4804. [DOI] [PubMed] [Google Scholar]
- [24].Melssen M, Slingluff CL., Jr Curr Opin Immunol. 2017;47:85. doi: 10.1016/j.coi.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arens R, Schoenberger SP. Immunol Rev. 2010;235:190. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shedlock DJ, Shen H. Science. 2003;300:337. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- [27].Kadowaki N. Allergol Int. 2007;56:193. doi: 10.2332/allergolint.R-07-146. [DOI] [PubMed] [Google Scholar]
- [28].Joffre OP, Segura E, Savina A, Amigorena S. Nat Rev Immunol. 2012;12:557. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- [29].Zhang C, Shi G, Zhang J, Song H, Niu J, Shi S, Huang P, Wang Y, Wang W, Li C, Kong D. J Control Release. 2017;256:170. doi: 10.1016/j.jconrel.2017.04.020. [DOI] [PubMed] [Google Scholar]
- [30].Jones CH, Chen M, Ravikrishnan A, Reddinger R, Zhang G, Hakansson AP, Pfeifer BA. Biomaterials. 2015;37:333. doi: 10.1016/j.biomaterials.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Silva JM, Zupancic E, Vandermeulen G, Oliveira VG, Salgado A, Videira M, Gaspar M, Graca L, Preat V, Florindo HF. J Control Release. 2015;198:91. doi: 10.1016/j.jconrel.2014.11.033. [DOI] [PubMed] [Google Scholar]
- [32].Chiang CL, Benencia F, Coukos G. Semin Immunol. 2010;22:132. doi: 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Mol Pharm. 2007;4:47. doi: 10.1021/mp060107e. [DOI] [PubMed] [Google Scholar]
- [34].Shi GN, Zhang CN, Xu R, Niu JF, Song HJ, Zhang XY, Wang WW, Wang YM, Li C, Wei XQ, Kong DL. Biomaterials. 2017;113:191. doi: 10.1016/j.biomaterials.2016.10.047. [DOI] [PubMed] [Google Scholar]
- [35].Vassilaros S, Tsibanis A, Tsikkinis A, Pietersz GA, McKenzie IF, Apostolopoulos V. Immunotherapy. 2013;5:1177. doi: 10.2217/imt.13.126. [DOI] [PubMed] [Google Scholar]
- [36].Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, Keler T, Odunsi K, Old LJ, Ritter G, Gnjatic S. J Immunol. 2011;186:1218. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- [37].Li M, Jiang Y, Xu C, Zhang Z, Sun X. International journal of nanomedicine. 2013;8:1843. doi: 10.2147/IJN.S43827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Quaak SG, Haanen JB, Beijnen JH, Nuijen B. AAPS PharmSciTech. 2010;11:344. doi: 10.1208/s12249-010-9391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garu A, Moku G, Gulla SK, Chaudhuri A. Mol Ther. 2016;24:385. doi: 10.1038/mt.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Diebold SS, Kursa M, Wagner E, Cotten M, Zenke M. J Biol Chem. 1999;274:19087. doi: 10.1074/jbc.274.27.19087. [DOI] [PubMed] [Google Scholar]
- [41].Raviv L, Jaron-Mendelson M, David A. Mol Pharm. 2015;12:453. doi: 10.1021/mp5005492. [DOI] [PubMed] [Google Scholar]
- [42].Xu J, Xu B, Tao J, Yang Y, Hu Y, Huang Y. Small. 2017;13 doi: 10.1002/smll.201700666. [DOI] [PubMed] [Google Scholar]
- [43].Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Clin Exp Immunol. 2007;147:176. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang X, Xu J, Qiu C, Ren L, Liu L, Wan Y, Zhang N, Peng H, Shao Y. Vaccine. 2007;25:2620. doi: 10.1016/j.vaccine.2006.12.020. [DOI] [PubMed] [Google Scholar]
- [45].Verheul RJ, Slutter B, Bal SM, Bouwstra JA, Jiskoot W, Hennink WE. J Control Release. 2011;156:46. doi: 10.1016/j.jconrel.2011.07.014. [DOI] [PubMed] [Google Scholar]
- [46].Yao W, Peng Y, Du M, Luo J, Zong L. Mol Pharm. 2013;10:2904. doi: 10.1021/mp4000053. [DOI] [PubMed] [Google Scholar]
- [47].Patel O, Shulkes A, Baldwin GS. Biochim Biophys Acta. 2006;1766:23. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [48].Lu Y, Mekoo DJ, Ouyang K, Hu X, Liu Y, Lin M, Jin L, Cao R, Li T, Zhang Y, Fan H, et al. Endocr Relat Cancer. 2009;16:1171. doi: 10.1677/ERC-09-0058. [DOI] [PubMed] [Google Scholar]
- [49].Vichchatorn P, Wongkajornsilp A, Petvises S, Tangpradabkul S, Pakakasama S, Hongeng S. J Neurooncol. 2005;75:111. doi: 10.1007/s11060-005-2317-2. [DOI] [PubMed] [Google Scholar]
- [50].Markov OV, Mironova NL, Shmendel EV, Serikov RN, Morozova NG, Maslov MA, Vlassov VV, Zenkova MA. J Control Release. 2015;213:45. doi: 10.1016/j.jconrel.2015.06.028. [DOI] [PubMed] [Google Scholar]
- [51].Lewis CE, Pollard JW. Cancer Res. 2006;66:605. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- [52].Vasievich EA, Huang L. Mol Pharm. 2011;8:635. doi: 10.1021/mp1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Nature. 1998;391:806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- [54].Yu SS, Lau CM, Barham WJ, Onishko HM, Nelson CE, Li H, Smith CA, Yull FE, Duvall CL, Giorgio TD. Mol Pharm. 2013;10:975. doi: 10.1021/mp300434e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guo Y, Liu X, Sun X, Zhang Q, Gong T, Zhang Z. Theranostics. 2012;2:1104. doi: 10.7150/thno.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lim YB, Lee M. Org Biomol Chem. 2007;5:401. doi: 10.1039/b615744k. [DOI] [PubMed] [Google Scholar]
- [57].Sadurni N, Solans C, Azemar N, Garcia-Celma MJ. Eur J Pharm Sci. 205;26:438. doi: 10.1016/j.ejps.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [58].Dan Z, Cao H, He X, Zeng L, Zou L, Shen Q, Zhang Z. Int J Pharm. 2015;483:63. doi: 10.1016/j.ijpharm.2015.01.035. [DOI] [PubMed] [Google Scholar]
- [59].Zhang Q, Cai Y, Wang XJ, Xu JL, Ye Z, Wang S, Seeberger PH, Yin J. ACS Appl Mater Interfaces. 2016;8:33405. doi: 10.1021/acsami.6b13612. [DOI] [PubMed] [Google Scholar]
- [60].Gary-Bobo M, Mir Y, Rouxel C, Brevet D, Basile I, Maynadier M, Vaillant O, Mongin O, Blanchard-Desce M, Morere A, Garcia M, et al. Angew Chem Int Ed Engl. 2011;50:11425. doi: 10.1002/anie.201104765. [DOI] [PubMed] [Google Scholar]
- [61].Castano AP, Mroz P, Hamblin MR. Nat Rev Cancer. 2006;6:535. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lucky SS, Soo KC, Zhang Y. Chem Rev. 2015;115:1990. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- [63].Zhang Q, Cai Y, Li QY, Hao LN, Ma Z, Wang XJ, Yin J. Chemistry (Easton) 2017;23:14307. doi: 10.1002/chem.201702935. [DOI] [PubMed] [Google Scholar]
- [64].Robertson CA, Evans DH, Abrahamse H. J Photochem Photobiol B. 2009;96:1. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [65].Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, Fortelius LE, Landor S, Toivola DM, Linden M, Sahlgren C. Mol Ther. 2011;19:1538. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ahmed HU, Moore C, Emberton M. Surg Oncol. 2009;18:219. doi: 10.1016/j.suronc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- [67].Pan Z, Kang X, Zeng Y, Zhang W, Peng H, Wang J, Huang W, Wang H, Shen Y, Huang Y. Polymer Chemistry. 2017;8:5275. [Google Scholar]
- [68].Hu H, Xiu KM, Xu SL, Yang WT, Xu FJ. Bioconjug Chem. 2013;24:968. doi: 10.1021/bc300683y. [DOI] [PubMed] [Google Scholar]
- [69].Berthe ML, Esslimani Sahla M, Roger P, Gleizes M, Lemamy GJ, Brouillet JP, Rochefort H. Eur J Cancer. 2003;39:635. doi: 10.1016/s0959-8049(02)00627-5. [DOI] [PubMed] [Google Scholar]
- [70].Gary-Bobo M, Nirde P, Jeanjean A, Morere A, Garcia M. Curr Med Chem. 2007;14:2945. doi: 10.2174/092986707782794005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lemamy GJ, Ndeboko B, Omouessi ST, Mouecoucou J. Restricted Growth-Clinical, Genetic and Molecular Aspects. 2016 InTech. [Google Scholar]
- [72].Oka Y, Rozek L, Czech M. J Biol Chem. 1985;260:9435. [PubMed] [Google Scholar]
- [73].Han VK, D'Ercole AJ, Lund PK. Science. 1987;236:193. doi: 10.1126/science.3563497. [DOI] [PubMed] [Google Scholar]
- [74].Scott CD, Weiss J. J Cell Physiol. 2000;182:62. doi: 10.1002/(SICI)1097-4652(200001)182:1<62::AID-JCP7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [75].Funk B, Kessler U, Eisenmenger W, Hansmann A, Kolb HJ, Kiess W. The journal of clinical endocrinology & metabolism. 1992;75:424. doi: 10.1210/jcem.75.2.1379254. [DOI] [PubMed] [Google Scholar]
- [76].Prakash J, Beljaars L, Harapanahalli AK, Zeinstra-Smith M, de Jager-Krikken A, Hessing M, Steen H, Poelstra K. Int J Cancer. 2010;126:1966. doi: 10.1002/ijc.24914. [DOI] [PubMed] [Google Scholar]
- [77].Ye Z, Cheng K, Guntaka RV, Mahato RI. Biochemistry. 2005;44:4466. doi: 10.1021/bi047529j. [DOI] [PubMed] [Google Scholar]
- [78].Vaillant O, El Cheikh K, Warther D, Brevet D, Maynadier M, Bouffard E, Salgues F, Jeanjean A, Puche P, Mazerolles C, Maillard P, et al. Angew Chem Int Ed Engl. 2015;54:5952. doi: 10.1002/anie.201500286. [DOI] [PubMed] [Google Scholar]
- [79].Puxbaum V, Nimmerfall E, Bauerl C, Taub N, Blaas PM, Wieser J, Mikula M, Mikulits W, Ng KM, Yeoh GC, Mach L. J Hepatol. 2012;57:337. doi: 10.1016/j.jhep.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li J, Sahagian GG. Oncogene. 2004;23:9359. doi: 10.1038/sj.onc.1208039. [DOI] [PubMed] [Google Scholar]
- [81].Probst OC, Karayel E, Schida N, Nimmerfall E, Hehenberger E, Puxbaum V, Mach L. Biochem J. 2013;451:91. doi: 10.1042/BJ20121422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jonson T, Albrechtsson E, Axelson J, Heidenblad M, Gorunova L, Johansson B, Hoglund M. Int J Oncol. 2001;19:71. [PubMed] [Google Scholar]
- [83].Pavelić K, Kolak T, Kapitanović S, Radošević S, Spaventi Š, Krušlin B, Pavelić J. The Journal of pathology. 2003;201:430. doi: 10.1002/path.1465. [DOI] [PubMed] [Google Scholar]
- [84].Laube F. Anticancer Res. 2009;29:1383. [PubMed] [Google Scholar]
- [85].Minnelli C, Cianfruglia L, Laudadio E, Galeazzi R, Pisani M, Crucianelli E, Bizzaro D, Armeni T, Mobbili G. J Drug Target. 2017;1 doi: 10.1080/1061186X.2017.1365873. [DOI] [PubMed] [Google Scholar]
- [86].Crucianelli E, Bruni P, Frontini A, Massaccesi L, Pisani M, Smorlesi A, Mobbili G. RSC Advances. 2014;4:58204. [Google Scholar]
- [87].Das S, Parekh N, Mondal B, Gupta SS. ACS Macro Letters. 2016;5:809. doi: 10.1021/acsmacrolett.6b00297. [DOI] [PubMed] [Google Scholar]