Highlights
-
•
Muscularis macrophages densily colonize the outermost layer of the gastrointestinal tract.
-
•
Muscularis macrophages communicate with enteric neurons in a bidirectional matter.
-
•
Muscularis macrophages are tissue-protective but can contribute to disease.
-
•
Current challenges are to decipher therapeutic potentials of muscularis macrophages.
Abbreviations: Mφ, Macrophage; LpMφ, Lamina propria macrophage; MMφ, Muscularis macrophage; ENS, Enteric nervous system; DCs, Dendritic cells; β2-AR, ß2-adrenergic receptors; POI, Postoperative ileus; α7nAChR, α7 nicotinic receptor; CNS, Central nervous system; I/R, Ischemia-reperfusion; ICC, Interstitial cells of Cajal; VNS, Vagus nerve stimulation; CAIP, Cholinergic anti-inflammatory pathway
Keywords: Intestinal muscularis externa, Intestinal macrophage, Enteric nervous system, Neuro-immune interactions, Gastrointestinal disorders, Tissue-macrophage ontogeny
Abstract
Macrophages residing in the muscularis externa of the gastrointestinal tract are highly specialized cells that are essential for tissue homeostasis during steady-state conditions as well as during disease. They are characterized by their unique protective functional phenotype that is undoubtedly a consequence of the reciprocal interaction with their environment, including the enteric nervous system. This muscularis macrophage-neuron interaction dictates intestinal motility and promotes tissue-protection during injury and infection, but can also contribute to tissue damage in gastrointestinal disorders such as post-operative ileus and gastroparesis. Although the importance of muscularis macrophages is clearly recognized, different aspects of these cells remain largely unexplored such their origin, longevity and instructive signals that determine their function and phenotype. In this review, we will discuss the phenotype, functions and origin of muscularis macrophages during steady-state and disease conditions. We will highlight the bidirectional crosstalk with neurons and potential therapeutic strategies that target and manipulate muscularis macrophages to restore their protective signature as a treatment for disease.
Tissue-resident macrophages (Mφ) are highly specialized phagocytes that actively contribute to organ homeostasis. This delicate task relies on their ability to sense and respond to challenges including metabolic changes, tissue damage and microbial insults, while performing tissue-specific functions to support surrounding cells and structures [1]. Depending on the tissue in which they reside, Mφ may have to fulfill completely different tasks. For example, lung alveolar Mφ are specialized in removal and recycling of surfactant molecules produced by alveolar epithelial cells, while in the intestinal lamina propria, Mφ contribute to the local tolerogenic milieu [2], [3]. In the brain, resident Mφ, i.e. microglia, assist in synaptic pruning and provide neurotrophic factors such as brain-derived neurotrophic factor [4]. Yet, resident Mφ populations are highly heterogeneous and can acquire distinct phenotypes in response to the dynamic environment within different tissues, paralleled by distinct gene-expression programs [5]. Indeed, even within the same organ, a variety of different Mφ subtypes can be identified. In the intestine, Mφ residing in the muscular layer have a different gene-expression profile compared to those residing in the lamina propria [6], while the genetic signature of lung alveolar Mφ differs from that of interstitial Mφ residing in the lung parenchyma [7]. Also in the brain, different subpopulations of Mφ subtypes can be identified, including microglia, perivascular, meningeal and choroid plexus Mφ, undoubtedly each with a different and specific function [8].
In the gastrointestinal (GI) tract, most studies have focused on the Mφ population present in the lamina propria (LpMφ). These immune cells play a crucial role in protecting the host against harmful micro-organisms and continuously phagocytose and clear luminal antigens that occasionally breach the epithelial layer. Furthermore, LpMφ express receptors for anti-inflammatory cytokines such as IL-10 that prevent unnecessary inflammation towards harmless commensal bacteria and install tolerance to harmless dietary antigens [3], [9]. Hence, loss of tolerance towards commensal bacteria or food antigens is believed to underlie chronic inflammation of the intestine, which can lead to inflammatory bowel diseases. However, the GI tract contains another important yet largely understudied subpopulation of resident Mφ that resides within the muscularis externa (MMφ). The recent awareness that these MMφ have a distinct gene expression profile and morphology compared to LpMφ provided evidence of the strong heterogeneity among intestinal Mφ [6]. Although MMφ have important functions in GI motility during homeostasis and disease, they are largely neglected and far less defined compared to their lamina propria counterparts. In this review, we will focus on the current knowledge of MMφ and discuss their functions and phenotypes during homeostasis and pathological conditions. We will speculate on the unexplored topics of origin and longevity of MMφ, which are highly relevant factors to understand the complexity and the heterogeneity of the intestinal Mφ compartment.
1. Phenotypic characterization of muscularis macrophages
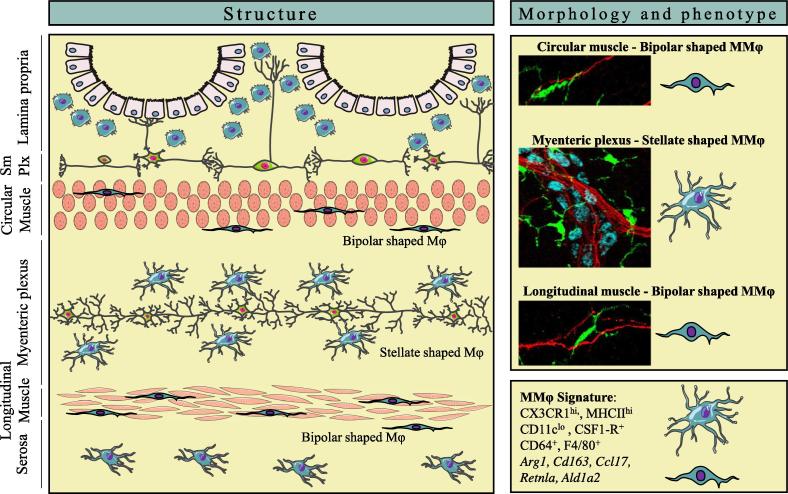
Intestinal Mφ are highly heterogeneous cells that are abundantly present in different layers of the GI tract as shown in Fig. 1. The lamina propria contains the largest number of Mφ within the intestine and are mostly found in close proximity to the intestinal epithelium, where they phagocytose bacterial antigens and produce mediators that drive epithelial cell renewal [10]. In contrast, Mφs in the muscularis externa are located distant from the intestinal lumen and are found in a dense network within the myenteric plexus, part of the enteric nervous system (ENS). Mφs are also present in lower numbers within the circular and longitudinal muscle layers of the muscularis externa and within the serosal layer, that separates the intestine from the peritoneum [6]. Of note, a CX3CR1hi Mφ population is also positioned within the submucosal plexus, located immediately below the lamina propria [6]. Earlier histological studies confirmed the distribution of “macrophage-like” cells that were able to endocytose FITC-conjugated dextran particles in the muscularis externa of both mice and human [11]. Depending on the position within the muscularis externa, CX3CR1hi MMφ indeed display either a bipolar or stellate morphology, what could suggest that these Mφ represent at least two phenotypically different subsets (Fig. 1) [6]. Interestingly, a similar stellate morphology is seen by microglia in the brain, that use their highly ramified filopodia to survey the brain parenchyma and actively communicate with surrounding neurons [12].
Fig. 1.
The localization and phenotype of muscularis macrophages. (Left panel) Anatomical overview of macrophage (Mφ) distribution in different layers of the gastrointestinal tract. (Upper right panel) Mφ (green, CX3CR1) located in the muscularis externa (MMφ) have a distinct morphology dependent on their position within circular and longitudinal muscle layers or myenteric plexus. MMφ in the myenteric plexus resemble microglia with a ramified, stellate shaped morphology and closely contact enteric neurons (red, TUBB3) and myenteric ganglia (blue, HUC/D). (Lower right panel) MMφ express typical Mφ-specific surface markers such as CX3CR1, CD64 and F4/80. They are characterized by their tissue-protective genetic signature, such as the expression of Arg1, Cd163, Ccl17, Retnla and Ald1a2. SmPlx = submucosal plexus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Phagocytes in the muscularis externa were initially identified based on their high expression levels of MHC class II and together with their antigen-presenting functions, they were originally classified as dendritic cells (DCs) [13]. Defining the exact nature of these cells in the muscularis externa was stifled by the lack of a clear panel of surface markers and the phenotypic similarities with LpMφ in whole-intestinal tissue preparations. To date, MMφ are typically identified based on flow cytometric analysis. Single cell suspensions can be obtained from the muscularis externa by using a protocol to mechanically separate and enzymatically digest the different layers of the intestinal tract [14]. This approach demonstrated that high expression levels of CX3CR1, F4/80 and CSF1-R and the absence of CD103 comprehensively distinguish Mφ from DCs in separate layers of the intestinal tract, including the muscularis externa. In contrast to the lamina propria, mainly populated by MHCIIhiCX3CR1hiCD11chi Mφ, the muscularis externa is exclusively populated by MHCIIhi CX3CR1hi Mφ that express low levels of CD11c [14]. However, the presence of a minor population of CD11c−/low cells within the lamina propria does not allow the use of CD11c to distinguish MMφ in whole-intestinal tissue preparations (own observations). Of note, MHCIIhiCX3CR1hi cells within the muscularis externa also express the Mφ-specific gene marker Fcgr1 (encoding for CD64). CSF-1 is crucial for the differentiation and maintenance of MMφ, which seem to be more dependent on the CSF-1R than LpMφ as indicated by the nearly absence of MMφ compared to the significant reduction in LpMφ in Csf1r−/− mice [14], [15]. Finally, together with their characteristic large vesicular cytoplasm, MMφ can be defined as bona fide Mφ. Altogether, despite their original classification as DCs, the Mφ phenotype within the muscularis externa is now well-defined based on a panel of Mφ -specific surface markers. Nevertheless, a clear phenotypic discrimination from LpMφ requires additional surface markers, yet further characterization of MMφ is highly awaited especially as this cell population appears to be heterogeneous.
The genetic signature of MMφ appears to differ from their lamina propria counterparts [6; own unpublished results]. Gabanyi and colleagues demonstrated that LpMφ express higher expression levels pro-inflammatory genes including Il1b and Il12b, likely instructed by signals from the microbial lumen or epithelial cells [6]. In contrast, MMφ exhibit a tissue-protective phenotype that includes the expression of M2-associated genes such as Arg1, Chi3l3 and Cd163. Interestingly, this signature is further upregulated in response to bacterial infection as a consequence of neuro-immune crosstalk between MMφ and enteric neurons [6]. Thus, MMφ are functionally adapted to and most likely imprinted by their surrounding environment, primarily consisting of the ENS. The mediators involved however remain to be identified.
2. Muscularis macrophage function: A ‘Give-and-Take’ relationship with the ENS
The ENS consists over 100 million neurons and glial cells that reside in the myenteric and submucosal plexus of the gut [17]. Hence, this “little brain of the gut” conducts many vital GI functions including the regulation of secretion and absorption and modulation of blood flow, coordinated by sensory neurons, motor neurons and interneurons. Additionally, the ENS closely communicates with various immune cells including MMφ by both intrinsic and extrinsic neural mechanisms, to ensure appropriate tissue-protective reactions to pathogens and inflammatory stimuli (Fig. 2) [18].
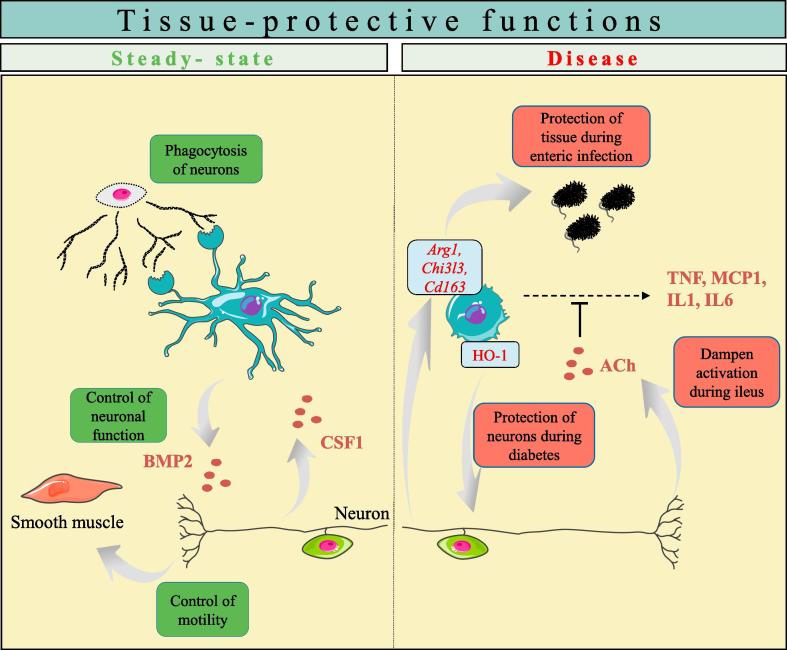
Fig. 2.
Muscularis macrophages contribute to tissue-protective functions during steady-state and disease. (Left) Muscularis macrophages (MMφ) are actively communicating with surrounding neurons, being imprinted by neuronal-derived CSF-1 for their maintenance and development. Conversely, MMφ sustain neuronal function and motility via BMP2 secretion. In addition, MMφ participate in the phagocytosis of surrounding neurons. (Right) MMφ are involved in tissue protection during disease conditions. During postoperative ileus, vagus nerve stimulation can activate enteric neurons, leading to increased production of acetylcholine, and dampening of MMφ. This reduces inflammation and accelerates the recovery of motility. Moreover, MMφ exert a M2-like signature that is enhanced upon enteric infection and express the enzyme heme oxygenase-1 (HO-1), that exerts a protective effect on neurons during diabetes.
2.1. Crosstalk between muscularis macrophages and intrinsic neurons
In a murine model of postoperative ileus (POI), a condition characterized by impaired contractility of the intestine due to inflammation of the muscularis externa, it was shown by our group that vagus nerve stimulation reduced intestinal inflammation by activation of cholingeric enteric neuron that are in close contact with MMφ [17]. This MMφ-dampening effect is most likely coordinated by intrinsic neurons, since vagal efferents in the myenteric plexus synapse with cholinergic myenteric neurons but not directly with MMφ [19]. Also under steady-state conditions, enteric neurons closely interact with Mφ and contribute to their survival and maintenance. Enteric neurons indeed constitutively produce the growth factor CSF-1, necessary for MMφ survival and maintenance, as shown by the lack of MMφ in genetically-engineered Csf1op/op mice, which possess an inactivating mutation in the csf-1 gene [16]. CSF-1 mediated control of Mφ survival was related to commensal bacteria colonization as microbial-derived signals stimulated enteric neuron expression of CSF-1. Conversely, MMφ have a supportive M2-like phenotype that supports neuronal function whereby they actively contribute to proper neuronal function and gut motility during homeostasis through the secretion of BMP2, that binds to its receptor BMPRII expressed on enteric neurons. The importance of this MMφ-secreted factor was demonstrated in mice treated with an inhibitor of BMP2, that exhibit altered intestinal contractility correlated with decreased nuclear staining for phosphorylated SMAD1/5/8 in enteric neurons. Moreover, the absence of MMφ in Csf1op/op mice is associated with an increased density of enteric neurons, and a less organized architecture of the myenteric plexus [16]. This is in line with recent data showing phagocytosis of neuronal debris by MMφ during steady-state, suggesting a role for MMφ in actively shaping the ENS [21]. Intriguingly, this suggests that MMφ exert a similar function as microglia in the brain, known to be involved in removing apoptotic neurons from both the developing and adult central nervous system (CNS) [22], [23], [24]. This process is detrimental to avoid inflammation in the adult brain or to regulate neurogenesis in the developing brain. During development, microglial phagocytic capacity is dependent on TREM2 expressed by microglial cells, since TREM2 deficiency resulted in impaired clearance of apoptotic neurons and induced the expression of TNF-α and IL-1β [25]. In contrast, phagocytosis of apoptotic neurons during adult CNS neurogenesis is dependent on MERTK [24]. Of interest, TREM2 and MERTK are also highly expressed by MMφ and LpMφ (own observations). Apart from their global role in the removal of dead neurons, microglia directly contact neuronal synapses to mediate a process termed ‘synaptic pruning’, the selective elimination of synapses and axon branches mainly during postnatal development [26]. Interestingly, deficiency in Cx3cr1, the chemokine receptor that is highly expressed by both microglia and intestinal Mφ, resulted in impaired synaptic pruning associated with a deficient CNS circuitry [26]. Whether MMφ are involved in shaping the ENS remains to be identified, but it is not unlikely that the mechanisms used by microglia to affect neuronal development in the CNS can be extrapolated to MMφ in the ENS. Taken together, the above summarized observations clearly indicate that MMφ and intrinsic enteric neurons bidirectionally communicate and support each other’s function. Whether such neuro-immune communication analogous to the muscularis externa holds true for Mφ in the submucosal plexus remains to be identified.
2.2. Extrinsic innervation modulates muscularis macrophages
In addition to intrinsic innervation, the gastrointestinal tract is extrinsically innervated by the sympathetic and parasympathetic system. MMφ express receptors for the neurotransmitter norepinephrine, in particular the adrenoreceptor beta2 (Adrb2). Moreover, MMφ reside in close approximation to tyrosine hydroxylase+ adrenergic fibers in the myenteric plexus, suggesting the possible involvement of adrenergic immune-modulation in the modulation of MMφ (as discussed below) [27].
3. Origin and turnover of intestinal macrophages: What about the muscularis externa?
Most tissue-resident Mφ are derived from yolk sac or fetal liver embryonic precursors that seed the tissues prenatally and differentiate into Mφ [28]. During adulthood, depending on the tissue, these progenitors maintain the Mφ population by local proliferation or alternatively, they are replaced by circulating bone marrow-derived monocytes. Microglia represent one extreme of the ontological spectrum, as they are almost exclusively derived from embryonic precursors that persist throughout adulthood without contribution of circulating monocytes, at least in steady state conditions [28]. Other tissue-resident Mφ, for example Langerhans cells in the skin and cardiac Mφ, represent a more mixed origin and are derived from both embryonic and adult bone marrow-derived monocytes [29], [30].
In the lamina propria, commensal bacteria facing the lumen cause an ongoing low-grade inflammation that is counterbalanced by the tolerogenic phenotype of the Mφ. This homeostatic challenge is associated with a high Mφ turnover rate demanding a continuously replacement by incoming short-lived bone marrow-derived monocytes [31]. In contrast, studies on the origin and turnover of MMφ are largely lacking. Earlier imaging studies in embryos and newborn mice demonstrate the presence of MHCII+ Mφ in different layers of the muscularis externa, including the myenteric plexus and external muscular layers [32]. Moreover, CX3CR1+ embryonic MMφ appear as early as 8.5 days post-conception, before the onset of neurogenesis and the formation of a complete functional myenteric plexus (own unpublished results). Interestingly, these embryonic progenitors persist for at least 6 weeks after birth and are located in close contact to myenteric neurons. In contrast, MMφ that are associated with the longitudinal and circular muscle seem to arise from postnatal bone marrow-derived monocytes. Through the use of elegant fate mapping studies, the intriguing notion of mixed Mφ origins during adulthood was already investigated in several other organs including the brain and the heart. These studies are mainly based on transgenic mice that harbor promotor-specific tamoxifen-inducible Cre recombinase activity under the control of Mφ-specific promotors (Cx3cr1 or Csf1r) and are crossed to transgenic mice that express a fluorescent reporter under the control of the constitutive promotor Rosa26 [27]. By using this strategy, researchers can track and trace embryonic progenitors and resident Mφ and discriminate them from rapidly replaced bone marrow monocytes. This approach revealed that microglia in the brain are exclusively derived from embryonic progenitors, with an exception to Mφ in the choroid-plexus that are partially replaced by monocytes over time [8], [28]. As myenteric plexus-residing MMφ are also settled in close contact to enteric neurons, it is tempting to speculate that these cells might have a similar origin as microglia. In contrast, fate-mapping studies indicated that cardiac Mφ are embryonically-derived and present in newborn mice but are slowly replaced by monocyte-derived Mφ during adulthood. This increasing contribution of monocyte-derived Mφ to the resident Mφ pool might be explained by the inability of resident embryonic Mφ to cope with mechanical stress induced over time by the contracting cardiac muscle, thus providing a stimulus for monocyte recruitment in order to maintain organ homeostasis. These examples demonstrate how the longevity of Mφ is determined by the tissue context, either vulnerable tissues such as neurons that need continuous protective support by a steady population, or tissues that require freshly recruited monocytes to ensure tissue homeostasis as exemplified by the heart and lamina propria. It is tempting to speculate that such a concept holds true for the muscularis externa.
4. Muscularis macrophages have detrimental roles in gastrointestinal disorders
Although insight into the physiological role of MMφ is still rather limited, several studies clearly indicate a key role in GI disorders such as postoperative and septic ileus, intestinal ischemia-reperfusion (I/R) damage and gastroparesis [33]. These disorders are characterized by impaired GI motility and/or transit, evoked by the activation of MMφ or loss of their tolerogenic phenotype. Not only smooth muscle function, but also interstitial cells of Cajal (ICC), the pacemaker cells of the gut, and neurons are affected by this process, further emphasizing their crucial role in maintaining tissue homeostasis and function. Hence, MMφ are increasingly recognized as an interesting target for treatment, urging the need for more insight in their function, both in health and disease.
4.1. Postoperative ileus
POI is a condition characterized by transient impaired GI motility [34], [35] following each abdominal surgical procedure. This leads to clinical symptoms such as nausea, vomiting, intolerance to solids, inability to defecate and ultimately prolonged hospitalization [36], [37]. Although minimal invasive surgery and multimodal enhanced recovery programs have reduced the duration of POI, it still occurs after each abdominal surgical procedure with an estimated socio-economical burden of 1.4 billion dollar per year in the USA alone [38], [39], [40]. Nevertheless, treatment of POI remains merely symptomatic as efficient therapies restoring gut motility are lacking, largely due to a limited insight in its pathophysiology.
Bauer [34] and co-workers were the first to recognize that POI resulted from a subtle inflammatory response localized in the muscularis externa leading to a prolonged inhibition of muscle contractility. Of note, they showed that surgical manipulation of the intestine leads to activation of MMφ initiating an inflammatory cascade of events in the muscularis externa [34], [41]. This included the upregulation of several transcription factors (i.e. NF-kB, STAT3 [42], [43] and p38-MAPK [44], early growth response protein 1 [45]) in MMφ, with subsequent induction of pro-inflammatory gene expression and the release of chemokines, cytokines (i.e. IL1, MCP1, IL6, TNFα) ([42], [46], [47] and kinetically active substances (i.e. NO and prostaglandins) [48], [49], [50], [51], [52], [53]. This inflammatory process contributes to the upregulation of adhesion molecules and recruitment of circulating leukocytes such as neutrophils and monocytes, a finding temporally correlated with impaired intestinal motility in both mice and human [34], [35], [41], [54], [55]. Previous reports showed that depletion of MMφ by clodronate liposome abrogated leukocyte recruitment and prevented POI. In line, Csf1op/op mice were also shown to be protected against POI, further underscoring the crucial role of MMφ in the pathogenesis of POI [56], [57]. Interestingly, we have recently shown that monocyte-derived MMφ have a pro-resolving function after the induction of POI, essential to abrogate neutrophil recruitment and the induction of collagen deposition [58]. The accumulation and differentiation of these pro-resolving MMφ are crucial for a correct resolution of the inflammatory process and restoration of tissue homeostasis, since blocking of monocyte migration in C–C motif chemokine receptor 2 (CCR2)-deficient mice has dramatic effects on the recovery of the GI transit after manipulation-induced inflammation. Of note, these findings were also associated with alterations in enteric ganglia and persistent impaired neuromuscular function [58]. Interestingly, ICC are also closely associated with MMφ. The former cells are negatively affected by the inflammatory response during POI. Indeed, IL6 released by MMφ upregulated miR-19a, an inflammation-related miRNA, during GI surgery which was associated with a decreased number of ICC, suggesting that impaired motility during POI can also be the result of damage to ICCs [59]. To date, it however remains to be elucidated whether a bidirectional interaction exists between MMφ and ICC.
4.2. Gastroparesis
Gastroparesis is a condition characterized by delayed emptying of the stomach in the absence of an organic cause. It mainly occurs in patients suffering from diabetes or patients who underwent esophageal or gastric surgery, although in approximately one-third no cause can be identified. Its prevalence was reported to be as high as 40% in patients with type I diabetes and 20% in patients with type II diabetes, leading to a socio-economical burden of 3500 million/year in the USA alone [60], [61]. Gastroparetic patients present with symptoms such as weight loss, nausea, early satiation, abdominal bloating and postprandial fullness. Until recently, the mechanisms contributing to the inability of the stomach to properly handle its contents were far from understood. Emerging evidence however has revealed a major role for MMφ. Under steady state conditions, the MMφ in the stomach have a typical M2-like phenotype, as evidenced by expression of CD206. Farrugia and colleagues however provided compelling evidence that they lose their tolerogenic phenotype in response to oxidative stress, a phenomena associated with diabetes [62]. Loss of M2-like MMφ, especially those expressing heme oxygenase-1 (HO-1), has been associated with the loss of ICC and neuronal nitric oxide synthase-expressing neurons [63]. As a result, gastric motility is severely disturbed resulting in delayed gastric emptying [60]. Strikingly, the depletion of MMφ using Csf1op/op mice was protective against the development of gastroparesis in diabetic mice [64]. These findings suggest that although anti-inflammatory M2-like MMφ protect against gastroparesis, M1-like MMφ cause significant damage to the key players in gastric motility, i.e. ICC and enteric neurons [64], [65], [66]. Also in patients with diabetic gastroparesis, ICCs are reduced in number, a finding that is associated with a reduction in number of CD206+ MMφ. This clearly illustrates the role of MMφ in the pathogenesis of gastroparesis [67], [68], [69].
4.3. Intestinal ischemia-reperfusion injury
Intestinal I/R injury is a severe condition resulting from hemorrhagic shock, cardiac arrest or arterial occlusion, but can also be a complication of surgical procedures such as intestinal transplantation or abdominal aortic surgery [70]. The ischemic event causes severe cellular damage and organ injury leading to disruption of the intestinal barrier. This enables bacterial translocation, a potent trigger for the activation of innate immune system, in particular MMφ [71], [72]. Intestinal I/R injury has been shown to switch MMφ towards a pro-inflammatory phenotype [73]. Similar to POI, these classically M1-like Mφ release pro-inflammatory cytokines (i.e. IL6, IL8, MCP-1) causing the recruitment of circulating leukocytes that subsequently reduce smooth muscle function and impair gut motility through nitric oxide release [72], [74], [75]. The impaired spontaneous mechanical contractile activity was also associated with functional changes of ICC networks, most likely caused by the inflammatory response in intestinal I/R injury [76]. Of note, the phenotypic alterations of ICC following I/R injury were only transient, indicating a central role in both disrupting and restoring gut motility in I/R injury. Similar to POI and diabetic gastroparesis, future research should further focus on the existence of a bidirectional interaction between MMφ and ICC.
5. Potential therapeutic strategies to target muscularis macrophages
Given that MMφ are the orchestrators of the inflammatory cascade in the muscularis externa, inhibition/prevention of their activation or restoration of their M2-like tolerogenic phenotype may proof an interesting novel approach to treat gastroparesis and prevent POI or I/R damage [77], [78].
5.1. The vagal or cholinergic anti-inflammatory pathway and resident muscularis macrophages
The group of Tracey was the first showing that Mφ can be modulated by the nervous system, in particular by the vagus nerve. In a model of sepsis, electrical activation of the vagus nerve (VNS) improved survival by dampening TNF production in the spleen. This effect was shown to be mediated by acetylcholine interacting with alpha7 nicotinic receptors (α7nAChR) expressed by splenic Mφ. These observations lead to the introduction of the cholinergic anti-inflammatory pathway (CAIP) and was proposed as a novel additional mechanism to control the immune system [79]. Since then, given the role of Mφ, including MMφ, in a variety of diseases, the therapeutic potential of the vagus nerve or the CAIP has been a novel and exciting area of research. In the following paragraphs, the evidence supporting MMφ as potential target for modulation by the CAIP is briefly summarized.
5.2. Postoperative ileus
Our group and others have convincingly demonstrated that also in the gut, VNS has anti-inflammatory properties via modulation of MMφ (reviewed in [33], [80]). Indeed, we found that VNS reduces muscular inflammation and improves POI [43], [81], an effect mediated by the release of acetylcholine acting on α7nAChR-positive MMφ [20], [82]. Especially as we showed that vagal nerve endings synapse with enteric neurons, but not directly with MMφ, the effect of VNS in the intestine seems to be rather mediated by cholinergic enteric neurons [19]. Ongoing work has indeed revealed immunomodulatory properties of enteric neurons dampening the activation of MMφ (unpublished data). Of interest, the therapeutic effect of VNS can be mimicked by pharmacological or nutritional (i.e. enteral feeding) activation of the vagus nerve. Of note, activation of vagal afferents by high fat enteral feeding stimulates the vagal anti-inflammatory pathway, an effect mediated by the release of cholecystokinin [83]. This intervention reduces manipulation-induced muscular inflammation and accelerates the recovery of GI motility in POI [84]. Also in clinical practice, early enteral feeding improves recovery of gut motility, as shown by a reduction in the time to first defecation and hospital stay in patients undergoing major abdominal surgery [85]. In line, sham feeding and gum chewing seems to have therapeutic potential in patients undergoing abdominal surgery [86]. However, larger clinical studies are required to confirm these findings.
5.3. Diabetic gastroparesis
To date, effective therapies for diabetic gastroparesis are largely lacking. Based on the above, targeting MMφ might however be a novel therapeutic approach. Of interest, HO-1 is induced in M2-like MMφ to protect against the oxidative stress during diabetes. HO-1 is a rate-limiting enzyme catalyzing heme into CO and biliverdin, both compounds with known anti-inflammatory properties. Loss of HO-1+ M2-like MMφ was shown to be strongly correlated with the development of gastroparesis. In this regard, induction of HO-1 using various pharmacological approaches might prevent the development of gastroparesis. Indeed, administration of hemin, a potent inducer of HO-1, was able to sustain the expression of HO-1 in MMφ and subsequently reverse delayed gastric emptying in mice [62], [63]. Of interest, hemin administration was also able to induce the expression of HO-1 in healthy volunteers [87]. In patients with diabetic gastroparesis however, this upregulation of HO-1 did not result in the acceleration of gastric emptying [88]. As CO is the end-product of HO-1 activity, inhalation of CO was also applied to mice with diabetic gastroparesis [89]. Similar to POI, CO-based treatments prevented gastroparesis via upregulation of HO-1 and IL-10 in MMφ and possibly also by inhibition of the p38 MAPK pathway [62], [90], [91]. The advantage of these CO-based treatments is that it could be administered acutely in the vicinity of the target site by intraperitoneal injection [92].
5.4. Intestinal ischemia-reperfusion injury
Intestinal transplantation is more challenging than transplantations of other solid organs due to the high immunogenicity of the gut [93]. Even though recent advances in surgical techniques and immunosuppressive therapy have improved the outcome of intestinal transplantation over the last decade, acute graft rejection in the first 90 days still occurs in about 30–50% of graft recipients and about 15% of the patients continue to experience chronic rejection. Accordingly, this is accompanied by a poor 5-year patient survival of approximately 50% [94].
One of the main challenges of intestinal transplantation is graft injury, which is initiated, in addition to other factors, by brain death of the donor, surgical manipulation of the intestine and inevitable I/R injury [95], [96]. As discussed previously, these events activate MMφ inducing intestinal inflammation and disruption of the intestinal barrier. Of note, Schaefer and colleagues demonstrated that graft MMφ are indeed major players orchestrating the inflammatory response after intestinal transplantation. Isogenic transplantation of an intestinal graft in which MMφ are depleted by clodronate liposomes in combination with gadoliniumchloride, reduced the recruitment of leukocytes and improved the gut motility [71]. Of note, the authors were not able to completely deplete the MMφ. Nevertheless, restricting the graft MMφ population did result in a significant alleviation of molecular and cellular inflammatory events within the graft muscularis and subsequent improvement of graft smooth muscle contractility. Moreover, administration of CPSI-2364, known to centrally activate the CAIP, also reduced muscularis inflammation and improved graft motility [96]. Interestingly, preclinical evidence in other models of I/R injury suggests that electrical stimulation of the vagus nerve [97], [98], [99] and administration of α7nAChR agonists [100], [101], [102] could also be potential therapeutic approaches for intestinal I/R injury after intestinal transplantation, but further research is definitely warranted.
Similar to POI and diabetic gastroparesis, induction of endogenous HO-1 using various pharmacological approaches ameliorates intestinal I/R injury. Indeed, preoperative intervention with hemin, the substrate of HO-1, resulted in reduced intestinal inflammation and improved gut motility after intestinal I/R injury. In line, inhalation of CO also protected the intestine from I/R injury following intestinal transplantation [103]. Of interest, ex vivo perfusion of the intestinal graft with 5% CO significantly reduced the intestinal I/R injury [104]. These data provide strong evidence that targeting MMφ is an extremely exciting approach to prevent the development of I/R injury, improve the quality and survival of the intestinal graft and thus may significantly impact on the survival of patients undergoing intestinal transplantation.
5.5. Extrinsic adrenergic (sympathetic) innervation in GI disorders
As mentioned earlier, under steady state conditions, MMφ preferentially express M2-like tissue-protective and wound healing genes. Of interest, during enteric infection with Salmonella typhimurium, the M2-like genes Arg1 and Chi3l3 were further upregulated, an effect mediated by norepinephrine acting on Adrb2 [6]. In line, β2-AR agonists (i.e. noradrenaline and salbutamol) also mediate the induction of M2-like genes in Adrb2-postive MMφ, whilst β2-AR antagonists (i.e. butaxamine) and the genetic ablation of Adrb2 impair the upregulation of M2-related genes both in steady state and during enteric infection [6], [105]. These findings provide evidence that MMφ are modulated by sympathetic neural signals, most likely to protect the ENS from further damage. Nevertheless, it remains to be elucidated whether β2-AR agonists could affect the clinical outcome in Mφ-mediated GI disorders, such as POI, gastroparesis and intestinal I/R injury. Interestingly, however, preclinical evidence in other disease models suggests that selective activation of β2-adrenergic signaling may indeed have therapeutic benefits by inhibition of M1-like MMφ or re-polarization into M2-like MMφ. In more detail, β2-AR agonists exhibited protective effects against kidney and cardiovascular complications of diabetes mediated by their Mφ-modulating properties [106]. In line, β2-AR antagonists reduced survival and promoted tissue injury in animal models of endotoxemia and LPS-induced acute lung injury [107]. Further studies are however required to evaluate their potential therapeutic properties in MMφ -mediated disorders.
6. Conclusions
Altogether, muscularis Mφ are specialized phagocytes that fulfill crucial roles in the maintenance of intestinal homeostasis and motility. They exist in different subpopulations dependent on their location within the muscularis externa and are intimately communicating with their microenvironment to acquire a tissue-protective profile and dampened activation state. These unique features are detrimental to control local inflammatory responses that could lead to nervous tissue damage and loss of intestinal motility. Nevertheless, the secreted factors involved in neuron-Mφ crosstalk in the muscularis externa remain largely unknown. Moreover, many other important features such as origin and longevity remain to be fully explored. Indeed, it is unclear whether the paradigm of continuous monocyte replacement in the intestine is applicable to MMφ residing in the myenteric plexus. Nevertheless, genetic fate-mapping tools are now available to define the exact origin of the MMφ, to further elucidate Mφ heterogeneity within the gastrointestinal tract and to understand their functions during health and disease.
Funding
GEB is supported by the European Research Council (ERC) Advanced Grant (ERC-2013-Adg: 340,101 Cholstim).
Conflicts of interest
None.
Contributor Information
Sebastiaan De Schepper, Email: sebastiaan.deschepper@kuleuven.be.
Nathalie Stakenborg, Email: nathalie.stakenborg@kuleuven.be.
Gianluca Matteoli, Email: gianluca.matteoli@kuleuven.be.
Simon Verheijden, Email: simon.verheijden@ucb.com.
Guy E. Boeckxstaens, Email: guy.boeckxstaens@kuleuven.be.
References
- 1.Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussell T., Bell T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 3.Rivollier A., He J., Kole A., Valatas V., Kelsall B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., 3rd, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosselin D., Link V.M., Romanoski C.E., Fonseca G.J., Eichenfield D.Z., Spann N.J., Stender J.D., Chun H.B., Garner H., Geissmann F., Glass C.K. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabanyi I., Muller P.A., Feighery L., Oliveira T.Y., Costa-Pinto F.A., Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbings S.L., Goyal R., Desch A.N., Leach S.M., Prabagar M., Atif S.M., Bratton D.L., Janssen W., Jakubzick C.V. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood. 2015;126:1357–1366. doi: 10.1182/blood-2015-01-624809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldmann T., Wieghofer P., Jordao M.J., Prutek F., Hagemeyer N., Frenzel K., Amann L., Staszewski O., Kierdorf K., Krueger M., Locatelli G., Hochgerner H., Zeiser R., Epelman S., Geissmann F., Priller J., Rossi F.M., Bechmann I., Kerschensteiner M., Linnarsson S., Jung S., Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Muller W., Sparwasser T., Forster R., Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen H.B., Thuneberg L., Rumessen J.J., Thorball N. Macrophage-like cells in the muscularis externa of mouse small intestine. Anatom. Record. 1985;213:77–86. doi: 10.1002/ar.1092130111. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 13.Flores-Langarica A., Meza-Perez S., Calderon-Amador J., Estrada-Garcia T., Macpherson G., Lebecque S., Saeland S., Steinman R.M., Flores-Romo L. Network of dendritic cells within the muscular layer of the mouse intestine. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19039–19044. doi: 10.1073/pnas.0504253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koscso B., Gowda K., Schell T.D., Bogunovic M. Purification of dendritic cell and macrophage subsets from the normal mouse small intestine. J. Immunol. Meth. 2015;421:1–13. doi: 10.1016/j.jim.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., Stanley E.R., Nussenzweig M., Lira S.A., Randolph G.J., Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller P.A., Koscso B., Rajani G.M., Stevanovic K., Berres M.L., Hashimoto D., Mortha A., Leboeuf M., Li X.M., Mucida D., Stanley E.R., Dahan S., Margolis K.G., Gershon M.D., Merad M., Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furness J.B., Callaghan B.P., Rivera L.R., Cho H.J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Experim. Med. Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 18.Yoo B.B., Mazmanian S.K. The enteric network: interactions between the immune and nervous systems of the gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cailotto C., Costes L.M., van der Vliet J., van Bree S.H., van Heerikhuize J.J., Buijs R.M., Boeckxstaens G.E. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 2012;24:191–200. doi: 10.1111/j.1365-2982.2011.01824.x. e93. [DOI] [PubMed] [Google Scholar]
- 20.Matteoli G., Gomez-Pinilla P.J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S.H., Michel K., Tracey K.J., Schemann M., Boesmans W. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2013 doi: 10.1136/gutjnl-2013-304676. gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L., Li Q., Saha M., Li C., Enikolopov G., Becker L., Rakhilin N., Anderson M., Shen X., Dong X., Butte M.J., Song H., Southard-Smith E.M., Kapur R.P., Bogunovic M., Pasricha P.J. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res. Develop. Brain Res. 1990;55:219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- 23.Sierra A., Encinas J.M., Deudero J.J., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fourgeaud L., Traves P.G., Tufail Y., Leal-Bailey H., Lew E.D., Burrola P.G., Callaway P., Zagorska A., Rothlin C.V., Nimmerjahn A., Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K., Rochford C.D., Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 27.Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D.A., Perlman H., Malissen B., Zelzer E., Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginhoux F., Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol. Cell Biol. 2010;88:387–392. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- 30.Epelman S., Lavine K.J., Beaudin A.E., Sojka D.K., Carrero J.A., Calderon B., Brija T., Gautier E.L., Ivanov S., Satpathy A.T., Schilling J.D., Schwendener R., Sergin I., Razani B., Forsberg E.C., Yokoyama W.M., Unanue E.R., Colonna M., Randolph G.J., Mann D.L. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bain C.C., Bravo-Blas A., Scott C.L., Perdiguero E.G., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., Mowat A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikkelsen H.B., Garbarsch C., Tranum-Jensen J., Thuneberg L. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J. Mol. Histol. 2004;35:377–387. doi: 10.1023/b:hijo.0000039840.86420.b7. [DOI] [PubMed] [Google Scholar]
- 33.Browning K.N., Verheijden S., Boeckxstaens G.E. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152:730–744. doi: 10.1053/j.gastro.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalff J.C., Schraut W.H., Simmons R.L., Bauer A.J. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann. Surg. 1998;228:652–663. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eskandari M.K., Kalff J.C., Billiar T.R., Lee K.K., Bauer A.J. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am. J. Physiol. 1997;273:G727–G734. doi: 10.1152/ajpgi.1997.273.3.G727. [DOI] [PubMed] [Google Scholar]
- 36.Livingston E.H., Passaro E.P., Jr. Postoperative ileus. Digest. Dis. Sci. 1990;35:121–132. doi: 10.1007/BF01537233. [DOI] [PubMed] [Google Scholar]
- 37.van Bree S.H., Nemethova A., Cailotto C., Gomez-Pinilla P.J., Matteoli G., Boeckxstaens G.E. New therapeutic strategies for postoperative ileus. Nat. Rev. Gastroenterol. Hepatol. 2012;9:675–683. doi: 10.1038/nrgastro.2012.134. [DOI] [PubMed] [Google Scholar]
- 38.Wolthuis A.M., Bislenghi G., Fieuws S., de Buck van Overstraeten A., Boeckxstaens G., D'Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis. 2016;18:O1–O9. doi: 10.1111/codi.13210. [DOI] [PubMed] [Google Scholar]
- 39.Iyer S., Saunders W.B., Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J. Manage Care Pharm. 2009;15:485–494. doi: 10.18553/jmcp.2009.15.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Bree S., Vlug M., Bemelman W., Hollmann M., Ubbink D., Zwinderman K., de Jonge W., Snoek S., Bolhuis K., van der Zanden E., The F., Bennink R., Boeckxstaens G. Faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology. 2011;141 doi: 10.1053/j.gastro.2011.05.034. 872-U594. [DOI] [PubMed] [Google Scholar]
- 41.Kalff J.C., Buchholz B.M., Eskandari M.K., Hierholzer C., Schraut W.H., Simmons R.L., Bauer A.J. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126:498–509. [PubMed] [Google Scholar]
- 42.Wehner S., Schwarz N.T., Hundsdoerfer R., Hierholzer C., Tweardy D.J., Billiar T.R., Bauer A.J., Kalff J.C. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137:436–446. doi: 10.1016/j.surg.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 43.de Jonge W.J., van der Zanden E.P., The F.O., Bijlsma M.F., van Westerloo D.J., Bennink R.J., Berthoud H.R., Uematsu S., Akira S., van den Wijngaard R.M., Boeckxstaens G.E. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 44.Wehner S., Straesser S., Vilz T.O., Pantelis D., Sielecki T., de la Cruz V.F., Hirner A., Kalff J.C. Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology. 2009;136:619–629. doi: 10.1053/j.gastro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt J., Stoffels B., Moore B.A., Chanthaphavong R.S., Mazie A.R., Buchholz B.M., Bauer A.J. Proinflammatory role of leukocyte-derived Egr-1 in the development of murine postoperative ileus. Gastroenterology. 2008;135:926–936. doi: 10.1053/j.gastro.2008.05.079. 936 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turler A., Schwarz N.T., Turler E., Kalff J.C., Bauer A.J. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G145–G155. doi: 10.1152/ajpgi.00263.2001. [DOI] [PubMed] [Google Scholar]
- 47.Farro G., Gomez-Pinilla P.J., Di Giovangiulio M., Stakenborg N., Auteri M., Thijs T., Depoortere I., Matteoli G., Boeckxstaens G.E. Smooth muscle and neural dysfunction contribute to different phases of murine postoperative ileus. Neurogastroenterol. Motil. 2016;28:934–947. doi: 10.1111/nmo.12796. [DOI] [PubMed] [Google Scholar]
- 48.Turler A., Kalff J.C., Moore B.A., Hoffman R.A., Billiar T.R., Simmons R.L., Bauer A.J. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann. Surg. 2006;244:220–229. doi: 10.1097/01.sla.0000229963.37544.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eskandari M.K., Kalff J.C., Billiar T.R., Lee K.K., Bauer A.J. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am. J. Physiol. 1999;277:G478–G486. doi: 10.1152/ajpgi.1999.277.2.G478. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz N.T., Kalff J.C., Turler A., Engel B.M., Watkins S.C., Billiar T.R., Bauer A.J. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 51.Kreiss C., Birder L.A., Kiss S., VanBibber M.M., Bauer A.J. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52:527–534. doi: 10.1136/gut.52.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalff J.C., Turler A., Schwarz N.T., Schraut W.H., Lee K.K., Tweardy D.J., Billiar T.R., Simmons R.L., Bauer A.J. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann. Surg. 2003;237:301–315. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Winter B.Y., Boeckxstaens G.E., De Man J.G., Moreels T.G., Herman A.G., Pelckmans P.A. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br. J. Pharmacol. 1997;120:464–468. doi: 10.1038/sj.bjp.0700913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalff J.C., Carlos T.M., Schraut W.H., Billiar T.R., Simmons R.L., Bauer A.J. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117:378–387. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- 55.Bauer A.J. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol. Motil. 2008;20(Suppl 1):81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 56.Wehner S., Behrendt F.F., Lyutenski B.N., Lysson M., Bauer A.J., Hirner A., Kalff J.C. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantelis D., Kabba M.S., Kirfel J., Kahl P., Wehner S., Buettner R., Hirner A., Kalff J.C. Transient perioperative pharmacologic inhibition of muscularis macrophages as a target for prophylaxis of postoperative ileus does not affect anastomotic healing in mice. Surgery. 2010;148:59–70. doi: 10.1016/j.surg.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Farro G., Stakenborg M., Gomez-Pinilla P.J., Labeeuw E., Goverse G., Giovangiulio M.D., Stakenborg N., Meroni E., D'Errico F., Elkrim Y., Laoui D., Lisowski Z.M., Sauter K.A., Hume D.A., Van Ginderachter J.A., Boeckxstaens G.E., Matteoli G. CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut. 2017;66:2098–2109. doi: 10.1136/gutjnl-2016-313144. [DOI] [PubMed] [Google Scholar]
- 59.Deng J., Yang S., Yuan Q., Chen Y., Li D., Sun H., Tan X., Zhang F., Zhou D. Acupuncture Ameliorates Postoperative Ileus via IL-6-miR-19a-KIT Axis to Protect Interstitial Cells of Cajal. Am. J. Chin. Med. 2017;45:737–755. doi: 10.1142/S0192415X17500392. [DOI] [PubMed] [Google Scholar]
- 60.Neshatian L., Gibbons S.J., Farrugia G. Macrophages in diabetic gastroparesis–the missing link? Neurogastroenterol. Motil. 2015;27:7–18. doi: 10.1111/nmo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y.R., Fisher R.S., Parkman H.P. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am. J. Gastroenterol. 2008;103:313–322. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 62.Choi K.M., Gibbons S.J., Nguyen T.V., Stoltz G.J., Lurken M.S., Ordog T., Szurszewski J.H., Farrugia G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. doi: 10.1053/j.gastro.2008.09.003. 2064 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi K.M., Kashyap P.C., Dutta N., Stoltz G.J., Ordog T., Shea Donohue T., Bauer A.J., Linden D.R., Szurszewski J.H., Gibbons S.J., Farrugia G. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409. doi: 10.1053/j.gastro.2010.02.014. 2409 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cipriani G., Gibbons S.J., Verhulst P.J., Choi K.M., Eisenman S.T., Hein S.S., Ordog T., Linden D.R., Szurszewski J.H., Farrugia G. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol. Gastroenterol. Hepatol. 2016;2:40–47. doi: 10.1016/j.jcmgh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eisenman S.T., Gibbons S.J., Verhulst P.J., Cipriani G., Saur D., Farrugia G. Tumor necrosis factor alpha derived from classically activated “M1” macrophages reduces interstitial cell of Cajal numbers. Neurogastroenterol. Motil. 2017;29 doi: 10.1111/nmo.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan P.Q., Tache Y. Abdominal surgery induced gastric ileus and activation of M1 like macrophages in the gastric myenteric plexus: prevention by central vagal activation in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2017 doi: 10.1152/ajpgi.00121.2017. ajpgi 00121 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grover M., Bernard C.E., Pasricha P.J., Lurken M.S., Faussone-Pellegrini M.S., Smyrk T.C., Parkman H.P., Abell T.L., Snape W.J., Hasler W.L., McCallum R.W., Nguyen L., Koch K.L., Calles J., Lee L., Tonascia J., Unalp-Arida A., Hamilton F.A., Farrugia G., N.G.C.R. Consortium Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol. Motil. 2012;24:531–539. doi: 10.1111/j.1365-2982.2012.01894.x. e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grover M., Bernard C.E., Pasricha P.J., Parkman H.P., Gibbons S.J., Tonascia J., Koch K.L., McCallum R.W., Sarosiek I., Hasler W.L., Nguyen L.A.B., Abell T.L., Snape W.J., Kendrick M.L., Kellogg T.A., McKenzie T.J., Hamilton F.A., Farrugia G., N.G.C.R. Consortium Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol. Motil. 2017;29 doi: 10.1111/nmo.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernard C.E., Gibbons S.J., Mann I.S., Froschauer L., Parkman H.P., Harbison S., Abell T.L., Snape W.J., Hasler W.L., McCallum R.W., Sarosiek I., Nguyen L.A., Koch K.L., Tonascia J., Hamilton F.A., Kendrick M.L., Shen K.R., Pasricha P.J., Farrugia G., N.G.C.R. Consortium Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol. Motil. 2014;26:1275–1284. doi: 10.1111/nmo.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaefer N., Tahara K., Pech T., Websky M.V., Fujishiro J., Pantelis D., Abu-Elmagd K., Kalff J.C., Hirner A., Turler A. Inducible nitric oxide synthase expression in the intestinal muscularis mediates severe smooth muscle dysfunction during acute rejection in allogenic rodent small bowel transplantation. J. Surg. Res. 2008;150:159–168. doi: 10.1016/j.jss.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Schaefer N., Tahara K., Schmidt J., Wehner S., Kalff J.C., Abu-Elmagd K., Hirner A., Turler A. Resident macrophages are involved in intestinal transplantation-associated inflammation and motoric dysfunction of the graft muscularis. Am. J. Transplant. 2007;7:1062–1070. doi: 10.1111/j.1600-6143.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 72.Schaefer N., Tahara K., von Websky M., Wehner S., Pech T., Tolba R., Abu-Elmagd K., Kalff J.C., Hirner A., Turler A. Role of resident macrophages in the immunologic response and smooth muscle dysfunction during acute allograft rejection after intestinal transplantation. Transpl. Int. 2008;21:778–791. doi: 10.1111/j.1432-2277.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- 73.Liu W.F., Wen S.H., Zhan J.H., Li Y.S., Shen J.T., Yang W.J., Zhou X.W., Liu K.X. Treatment with Recombinant Trichinella spiralis Cathepsin B-like Protein Ameliorates Intestinal Ischemia/Reperfusion Injury in Mice by Promoting a Switch from M1 to M2 Macrophages. J. Immunol. 2015;195:317–328. doi: 10.4049/jimmunol.1401864. [DOI] [PubMed] [Google Scholar]
- 74.Vane D.W., Grosfeld J.L., Moore W., Abu-Dalu K., Hurwitz A. Impaired bowel motility following small intestinal transplantation. J. Surg. Res. 1989;47:288–291. doi: 10.1016/0022-4804(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 75.Turler A., Kalff J.C., Heeckt P., Abu-Elmagd K.M., Schraut W.H., Bond G.J., Moore B.A., Brunagel G., Bauer A.J. Molecular and functional observations on the donor intestinal muscularis during human small bowel transplantation. Gastroenterology. 2002;122:1886–1897. doi: 10.1053/gast.2002.33628. [DOI] [PubMed] [Google Scholar]
- 76.Shimojima N., Nakaki T., Morikawa Y., Hoshino K., Ozaki H., Hori M., Kitajima M. Interstitial cells of Cajal in dysmotility in intestinal ischemia and reperfusion injury in rats. J. Surg. Res. 2006;135:255–261. doi: 10.1016/j.jss.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 77.Stakenborg N., Gomez-Pinilla P.J., Boeckxstaens G.E. Postoperative ileus: pathophysiology current therapeutic approaches. Handb. Exp. Pharmacol. 2017;239:39–57. doi: 10.1007/164_2016_108. [DOI] [PubMed] [Google Scholar]
- 78.Verheijden S., Boeckxstaens G.E. Neuro-immune interaction and the regulation of intestinal immune homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017 doi: 10.1152/ajpgi.00425.2016. ajpgi 00425 2016. [DOI] [PubMed] [Google Scholar]
- 79.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 80.Stakenborg N., Di Giovangiulio M., Boeckxstaens G.E., Matteoli G. The versatile role of the vagus neve in the gastrointestinal tract. EMJ Gastroenterol. 2013;1:106–114. [Google Scholar]
- 81.Stakenborg N., Wolthuis A.M., Gomez-Pinilla P.J., Farro G., Di Giovangiulio M., Bosmans G., Labeeuw E., Verhaegen M., Depoortere I., D'Hoore A., Matteoli G., Boeckxstaens G.E. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol. Motil. 2017 doi: 10.1111/nmo.13075. [DOI] [PubMed] [Google Scholar]
- 82.The F., Cailotto C., van der Vliet J., de Jonge W.J., Bennink R.J., Buijs R.M., Boeckxstaens G.E. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br. J. Pharmacol. 2011;163:1007–1016. doi: 10.1111/j.1476-5381.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luyer M.D., Greve J.W., Hadfoune M., Jacobs J.A., Dejong C.H., Buurman W.A. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lubbers T., Luyer M.D., de Haan J.J., Hadfoune M., Buurman W.A., Greve J.W. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann. Surg. 2009;249:481–487. doi: 10.1097/SLA.0b013e318194d187. [DOI] [PubMed] [Google Scholar]
- 85.Boelens P.G., Heesakkers F.F., Luyer M.D., van Barneveld K.W., de Hingh I.H., Nieuwenhuijzen G.A., Roos A.N., Rutten H.J. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann. Surg. 2014;259:649–655. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 86.Short V., Herbert G., Perry R., Atkinson C., Ness A.R., Penfold C., Thomas S., Andersen H.K., Lewis S.J. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD006506.pub3. CD006506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bharucha A.E., Kulkarni A., Choi K.M., Camilleri M., Lempke M., Brunn G.J., Gibbons S.J., Zinsmeister A.R., Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin. Pharmacol. Ther. 2010;87:187–190. doi: 10.1038/clpt.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bharucha A.E., Daley S.L., Low P.A., Gibbons S.J., Choi K.M., Camilleri M., Saw J.J., Farrugia G., Zinsmeister A.R. Effects of hemin on heme oxygenase-1, gastric emptying, and symptoms in diabetic gastroparesis. Neurogastroent. Motil. 2016;28:1731–1740. doi: 10.1111/nmo.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alcaraz M.J., Fernandez P., Guillen M.I. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Des. 2003;9:2541–2551. doi: 10.2174/1381612033453749. [DOI] [PubMed] [Google Scholar]
- 90.Stoffels B., Turler A., Schmidt J., Nazir A., Tsukamoto T., Moore B.A., Schnurr C., Kalff J.C., Bauer A.J. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol. Motil. 2011;23:76–87. doi: 10.1111/j.1365-2982.2010.01603.x. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kashyap P., Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716–1726. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakao A., Schmidt J., Harada T., Tsung A., Stoffels B., Cruz R.J., Jr., Kohmoto J., Peng X., Tomiyama K., Murase N., Bauer A.J., Fink M.P. A single intraperitoneal dose of carbon monoxide-saturated ringer's lactate solution ameliorates postoperative ileus in mice. J. Pharmacol. Exp. Ther. 2006;319:1265–1275. doi: 10.1124/jpet.106.108654. [DOI] [PubMed] [Google Scholar]
- 93.Di Giovangiulio M., Verheijden S., Bosmans G., Stakenborg N., Boeckxstaens G.E., Matteoli G. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front. Immunol. 2015;6:590. doi: 10.3389/fimmu.2015.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fishbein T.M. Intestinal transplantation. New England J. Med. 2009;361:998–1008. doi: 10.1056/NEJMra0804605. [DOI] [PubMed] [Google Scholar]
- 95.Schaefer N., Tahara K., Schmidt J., Zobel S., Kalff J.C., Hirner A., Turler A. Mechanism and impact of organ harvesting and ischemia-reperfusion injury within the graft muscularis in rat small bowel transplantation. Transplant. Proc. 2006;38:1821–1822. doi: 10.1016/j.transproceed.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 96.Websky M., Fujishiro J., Ohsawa I., Praktiknjo M., Wehner S., Abu-Elmagd K., Kitamura K., Kalff J.C., Schaefer N., Pech T. The novel guanylhydrazone CPSI-2364 ameliorates ischemia reperfusion injury after experimental small bowel transplantation. Transplantation. 2013;95:1315–1323. doi: 10.1097/TP.0b013e31828e72fa. [DOI] [PubMed] [Google Scholar]
- 97.Guarini S., Altavilla D., Cainazzo M.M., Giuliani D., Bigiani A., Marini H., Squadrito G., Minutoli L., Bertolini A., Marini R., Adamo E.B., Venuti F.S., Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 98.Altavilla D., Guarini S., Bitto A., Mioni C., Giuliani D., Bigiani A., Squadrito G., Minutoli L., Venuti F.S., Messineo F., De Meo V., Bazzani C., Squadrito F. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 99.Inoue T., Abe C., Sung S.S., Moscalu S., Jankowski J., Huang L., Ye H., Rosin D.L., Guyenet P.G., Okusa M.D. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J. Clin. Invest. 2016;126:1939–1952. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ge J., Tian J., Yang H., Hou L., Wang Z., He Z., Wang X. Alpha7 nicotine acetylcholine receptor agonist PNU-282987 attenuates acute lung injury in a cardiopulmonary bypass model in rats. Shock. 2017;47:474–479. doi: 10.1097/SHK.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 101.Li F., Chen Z., Pan Q., Fu S., Lin F., Ren H., Han H., Billiar T.R., Sun F., Li Q. The protective effect of PNU-282987, a selective alpha7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor kappaB activation in mice. Shock. 2013;39:197–203. doi: 10.1097/SHK.0b013e31827aa1f6. [DOI] [PubMed] [Google Scholar]
- 102.Li H., Zhang Z.Z., Zhan J., He X.H., Song X.M., Wang Y.L. Protective effect of PNU-120596, a selective alpha7 nicotinic acetylcholine receptor-positive allosteric modulator, on myocardial ischemia-reperfusion injury in rats. J. Cardiovasc. Pharmacol. 2012;59:507–513. doi: 10.1097/FJC.0b013e31824c86c3. [DOI] [PubMed] [Google Scholar]
- 103.Nakao A., Moore B.A., Murase N., Liu F., Zuckerbraun B.S., Bach F.H., Choi A.M., Nalesnik M.A., Otterbein L.E., Bauer A.J. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278–1285. doi: 10.1136/gut.52.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakao A., Toyokawa H., Tsung A., Nalesnik M.A., Stolz D.B., Kohmoto J., Ikeda A., Tomiyama K., Harada T., Takahashi T., Yang R., Fink M.P., Morita K., Choi A.M., Murase N. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am. J. Transplant. 2006;6:2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 105.Spengler R.N., Chensue S.W., Giacherio D.A., Blenk N., Kunkel S.L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 1994;152:3024–3031. [PubMed] [Google Scholar]
- 106.Noh H., Yu M.R., Kim H.J., Lee J.H., Park B.W., Wu I.H., Matsumoto M., King G.L. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 2017;92:101–113. doi: 10.1016/j.kint.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grailer J.J., Haggadone M.D., Sarma J.V., Zetoune F.S., Ward P.A. Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J. Innate Immun. 2014;6:607–618. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]