Abstract
The Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome 1 (MCAHS1) has been described in two families to date. We describe a 2-year-old Mexican American boy with the syndrome and additional manifestations not yet reported as part of the phenotype. The patient presented with severe hypotonia, microphallus and left cryptorchidism, and was later diagnosed with epilepsy and severe cortical visual impairment. He also had supernumerary nipples, pectus excavatum, a short upturned nose, fleshy ear lobes and a right auricular pit. Massively parallel exome sequencing and analysis revealed two novel compound heterozygous missense (Trp136Gly and Ser859Thr) variants in the PIGN gene. This report extends and further defines the phenotype of this syndrome.
Keywords: Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome-1(MCAHS1), PIGN Gene, North Carolina Clinical Genomic Evaluation by NextGen Exome Sequencing (NCGENES), Exome sequencing
INTRODUCTION
The Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome (MCAHS) is an autosomal recessive disorder comprising neonatal hypotonia, seizures, minor anomalies, delayed or lack of psychomotor development, and various congenital organ anomalies [Maydan et al., 2011]. Genetic heterogeneity has been described, as MCAHS1 is caused by mutations in the PIGN gene, MCAHS2 by mutations in the PIGA gene, and MCAHS3 by mutations in the PIGT gene [Belet et al., 2014; Kato et al., 2014; Kvarnung et al., 2013; Maydan et al., 2011; van der Crabben et al., 2014].
The PIGN gene maps to chromosome band 18q21.33 and encodes glycosylphosphatidylinositol (GPI) ethanolamine phosphate transferase 1, a protein involved in GPI-anchor biosynthesis [Gaynor et al., 1999; Hong et al., 1999]. The PIGN protein is expressed in different tissues, and mutations cause multiple physical anomalies and severe developmental disability. We present a patient with two novel compound heterozygous missense variants c.406T>G [p.Trp136Gly]/c.2576G>C [p.Ser859Thr] in the PIGN gene identified by massively parallel exome sequencing and analysis, confirmed by Sanger DNA sequencing analysis and interpreted as providing a potential diagnosis for the patient’s phenotype. The aim of reporting this patient is to further define and extend the phenotypic characterization of this rare syndrome that, to our knowledge, has only been reported in two families to date.
CLINICAL REPORT
The patient is a 2-year-old Mexican American boy with severe hypotonia and minor anomalies (Fig. 1). He was born at term after an uncomplicated pregnancy to healthy 34-year-old non-consanguinous Hispanic parents. He was delivered by repeat cesarean section without complication. Apgar scores were 8 and 9 at 1 and 5 minutes, and the infant had congenital hypotonia and reduced oxygen saturation, microphallus and left cryptorchidism. His birth weight was 4.271 kg (>90th centile), length 53.97 cm (75th centile), and head circumference (OFC) 36.8 cm (>90th centile) (Table I). Brain MRI at 4 days was normal. He had a patent foramen ovale with no additional structural heart anomalies. Lobulated kidneys with a duplicated collecting system and hydronephrosis were present. Infantile spasms characterized by tonic flexion and eye blinking episodes began at age 9 months. At age 12 months, glasses were prescribed for visual developmental delay, esotropia, and high hypermetropia. At age 17 months, a gastrostomy tube was placed for repeated aspiration.
Figure 1.
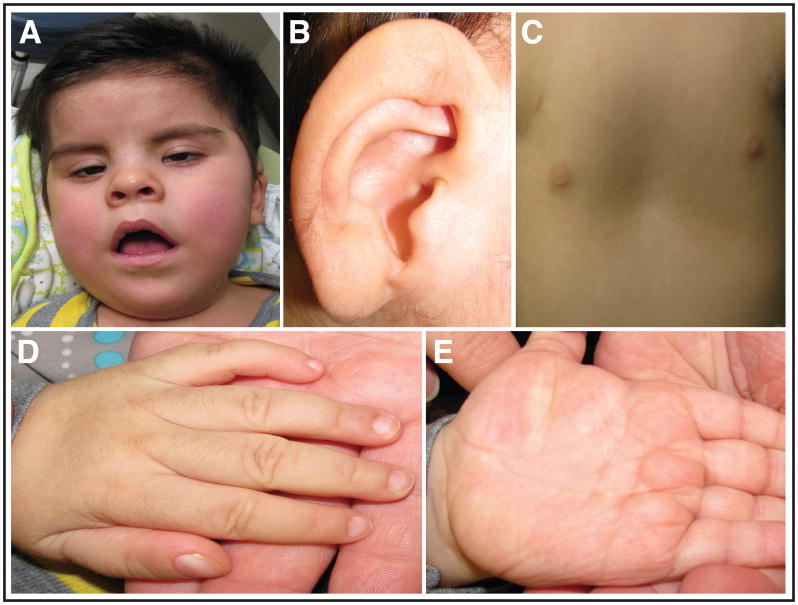
Clinical features of the patient with MCAHS1 at age 2 years. A. Note the patient’s low anterior hairline, epicanthal folds, and short upturned nose. B. Right ear revealing the fleshy ear lobe. C. The patient also has supernumerary nipples and pectus excavatum. D and E. Hands and digits had broad short thumbs and ventral swellings, likely representing a normal familial variant and remnants of fetal lymphedema, respectively.
Table I.
Characteristics of MCASH1 found in all patients published to date
| Demographic and Birth Characteristics of Patients with MCAHS1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Source | Maydan et. al., 2011 | Maydan et. al., 2011 | Maydan et. al., 2011 | Maydan et. al., 2011 | Maydan et. al., 2011 | Maydan et. al., 2011 | Maydan et. al., 2011 | Ohba et. al., 2013 | Ohba et. al., 2013 | Couser et. al., 2015 |
| Sex | M | M | M | F | F | F | M | F | M | M |
| Race | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Japanese | Japanese | Mexican American |
| Birth Age (weeks) | 36 | 38 | 38 | 42 | 38 | 39 | NA | 39 | 37 | 39 |
| Birth Weight (kg) | 3.566 | 4.065 | 3.850 | 3.410 | 4.250 | 4.300 | 4.800 | 3.390 | 3.252 | 4.271 |
| Birth Length (cm) | NA | NA | NA | NA | NA | NA | NA | 49 | 50 | 53.97 |
| Head Circumference (cm) | 37 | 37 | 35.5 | 34.5 | NA | NA | NA | 35 | 35 | 36.8 |
| Sex | M | M | M | F | F | F | M | F | M | M |
| Race | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Israeli Arab | Japanese | Japanese | Mexican American |
| Prominent Clinical Features of Patients with MCAHS1 | ||||||||||
| CNS | Hypotonia, developmental delay, seizures, hyporeflexia, tremor, hoarse cry, choreoathetosis | Hypotonia, developmental delay, hyperreflexia, tremor, hoarse cry, choreoathetosis, mastication | Hypotonia, developmental delay, seizures, hyporeflexia, tremor | Hypotonia, developmental delay, seizures, hyperreflexia, tremor, hoarse cry, mastication | Hypotonia, developmental delay, seizures, hyporeflexia, tremor, hoarse cry | Hypotonia, developmental delay, seizures, hyporeflexia | Hypotonia, developmental delay, seizures, hyporeflexia | Hypotonia, developmental delay, seizures, tremors | Hypotonia, developmental delay, seizures, tremors | Hypotonia, developmental delay, seizures, hyporeflexia |
| CV | Patent foramen ovale, pulmonary stenosis | Atrial septal defect | Patent foramen ovale | Atrial septal defect, patent formaen ovale, patent ductus arteriosus | Normal | Patent ductus arteriosus, pulmonary hypertension, right ventrical enlarged | Normal | Normal | Normal | Patent foramen ovale |
| GI/GU | Hydrocele, dilation of renal collecting system, gastroesophageal reflux | Hydrocele, hydronephrosis, trabecular urinary bladder, gastroesophageal reflux | Right kidney dysplastic, left hydroureter and hydronephrosis, trabecular urinary bladder, anal stenosis | Anus imperforate, ano-vestibular fistula | Normal | Swallowing problems | Swallowing problems | Vesicoureteral reflux, gastroesophageal reflux | Normal | Swallowing problems, lobulated kidneys, duplicated collecting system, hydronephrosis |
| Head/Face | Prominent occiput, bitemporal narrowing, coarse facies, tented upper lip, high arched palate, micrognathia | Prominent occiput, bitemporal narrowing, metopic and sagittal ridges, fair hair, flat philtrum, thin upper lip, retrognathia, high arached palate | NA | Thin lips | Low forehead | Coarse facies, small nose, large mouth | Face elongated, upturned nose | Prominent occiput, bitemporal narrowing, micrognathia, high arached palate, tented upper lid | Prominent occiput, bitemporal narrowing, micrognathia, high arached palate, tented upper lid | Coarse facies, low hairline |
| Eyes | Wandering eyes, nystagmus | Wandering eyes, nystagmus, strabismus | Normal | Nystagmus | Rolling eyes | Nystagmus | Nystagmus, suspected blindness | Vertical ystagmus, No eye pursuit, epicanthal folds | Vertical nystagmus | Wandering eyes, strabismus, high hypermetropia |
| Ears | Fleshy ear lobes, overfolded right helix | Large ears, overfolded right helix | Ears low set | Left ear overfolded | Ears small | Large ears | Large ears | Normal | Normal | Fleshy ear lobes, right auricular pit |
| Chest/Back | Chest narrow | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Supernumerary nipples and pectus excavatum | |
| Extremities | Brachydactyly, deep plantar groove | Fingers tapered, deep plantar groove | Deep plantar groove | Normal | Normal | Deep plantar groove | Small feet, flexion of toes, pes cavus | Deep plantar groove | Deep plantar groove | Broad short thumbs, ventral swellings on hand |
| Brain MRI | Enlargement extra axial spaces and posterior fossa, loss vermis parenchyma | NA | NA | Delayed white matter maturation, cerebrospinal fluid space enlargement, thin corpus callosum | NA | NA | NA | Cerebellar atrophy, delayed myelination, ventricle enlargement | Normal | Normal |
| PIGN gene mutation | Homozygous p.R709Q | Homozygous p.R709Q | Homozygous p.R709Q | Homozygous p.R709Q | Homozygous p.R709Q | Homozygous p.R709Q | Homozygous p.R709Q | Heterozygous p.S270P/c.963G>A | Heterozygous p.S270P/c.963G>A | Heterozygous p.W136G/p.S859T |
At his last evaluation at age 2 years, the patient had profound global developmental delays and had developed intractable seizures. He was unable to sit or control head movements without support, did not make eye contact or track objects with or without spectacles, did not respond to spoken request or utter words. All feeding took place through the gastrostomy tube. His weight was 12.882 kg (55th centile), length 90.50 cm (87th centile), and OFC 47.3 cm (17th centile). He was normocephalic with a low anterior hairline (Figure 1A). He had epicanthal folds, fleshy ear lobes, and an upturned nose (Figure 1A, B). Positive visual responses to an opticokinetic drum and eye blinking to a bright fixation light were present. Variable ocular balance misalignment and wandering eye movements were observed in the absence of involuntary rapid ocular oscillations. He had supernumerary nipples, broad short thumbs and prominent ventral swellings (Fig. 1 C, D). Hyporeflexia was present.
SEQUENCING ANALYSIS
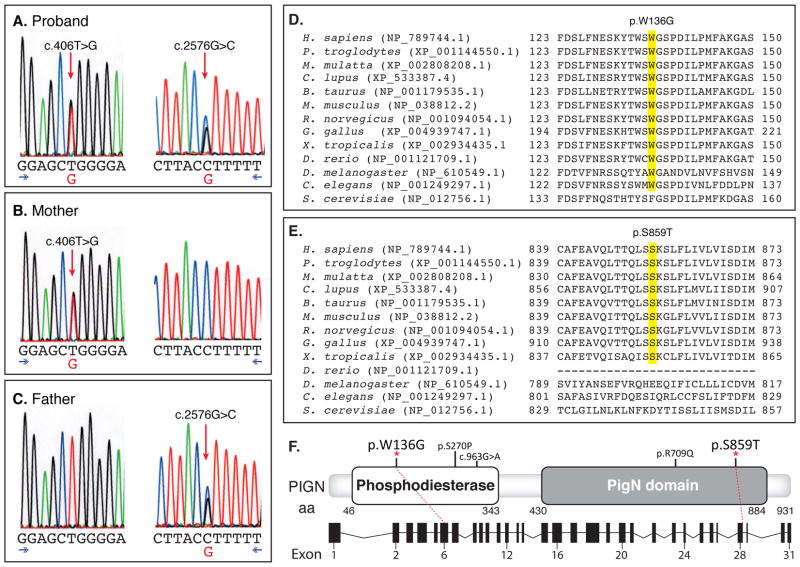
Using a peripheral blood sample and extracted DNA, massively parallel exome sequencing was performed under the North Carolina Clinical Genomic Evaluation by NextGen Exome Sequencing (NCGENES) NHGRI-funded Clinical Sequencing Exploratory Research study. Two sequence variants, c.406T>G [p.Trp136Gly]/c.2576G>C [p.Ser859Thr], in the PIGN gene (phosphatidylinositol glycan anchor biosynthesis, class N;NM_176787) were detected. The two PIGN variants were confirmed by bidirectional Sanger sequencing in the University of North Carolina (UNC) Hospitals CLIA-certified Clinical Molecular Genetics Laboratory (Fig. 2). Both of the identified variants are apparently novel missense variants. Parental follow-up studies determined that the p.Trp136Gly variant was inherited from the unaffected mother and the p.Ser859Thr variant was inherited from the unaffected father, consistent with a trans allelic pattern of inheritance and compound heterozygosity for these variants. Both variants are highly or mostly conserved evolutionarily and located within defined functional domains of the PIGN gene. Neither variant has been observed in a collection of >60,000 exomes (http://exac.broadinstitute.org), indicating that these are rare. These variants were not found in the LOVD 3.0 PIGN-specific variant database, ClinVar, or by a pubmed search. In addition, although there were 7 rare missense variants identified in the PIGN gene among >400 NCGENES participants, this patient is the only participant with two variants in this gene.
Figure 2.
A. Sanger sequence data for proband. Proband PIGN genotype, c.406T>G [p.Trp136Gly] and proband PIGN genotype, c.2576G>C [p.Ser859Thr] (reverse reaction). B. Proband mother positive for c.406T>G, negative for c.2576G>C. C. Proband father positive for c.2576G>C, negative for c.406T>G. D. Conserved domains, c.406T>G. E. Conserved domains, c.2576G>C. F. Genomic structure of the PIGN gene with reported variants, along with a depiction of the gene structure with dashed lines linking the coding exons to their respective locations in the cDNA figure.
DISCUSSION
MCAHS1 was described in 2011 in 7 patients from a large consanguineous Israeli-Arab family [Maydan et al., 2011]. The phenotype included increased birth weight, large head circumference, hypotonia, severe developmental delay, seizures, tremors, nystagmus, ear anomalies, gastrointestinal tract abnormalities, cardiovascular defects and early deaths. Minor anomalies included prominent occiput, bitemporal narrowness, coarse facies, micrognathia, retrognathia, hypertelorism, long philtrum, upturned nose, and a high arched palate.
In 2013 the syndrome was reported in two Japanese sibs born to non-consanguineous healthy parents [Ohba et al., 2014]. The older sib was a 9-year-old girl with hypotonia, severe developmental delay, nystagmus, and complex partial seizures that developed at 8 months. She had a prominent occiput, bitemporal narrowness, micrognathia, high arched palate and epicanthal folds. Brain MRI was normal at age 6 months, but later revealed cerebellar atrophy and delayed myelination. The younger sib, a 2-year-old boy, also was hypotonic and had severe delays, nystagmus, complex partial seizures that developed at 5 months of age, and similar minor anomalies. Brain MRI was normal at age 2 months.
Our patient has findings similar to those previously reported. (Table I). He has profound developmental delay, hypotonia and seizures; ear, eye, and limb anomalies; along with cardiac and genitourinary abnormalities. He also had coarse features with an expressionless face reflecting his severe congenital hypotonia and seizure condition. The patient presented with additional anomalies not present in the previously described cases of this disorder. Cryptorchidism was noted after birth of the patient along with renal anomalies including lobulated kidneys, duplicated ureter and hydronephrosis, which was resolved after 14 months. Additional he had a right preauricular pit, broad thumbs, and prominent ventral swellings which were not described in the previous cases. However, it should be noted that the mother also had prominent knuckle pads and the father and other paternal relatives had short, broad thumbs, thus these may be familial traits unrelated to his syndromic condition. The ventral swellings are remnants of fetal lymphedema and its severity directly proportional to the infant’s hypotonia. Additionally, high hypermetropia was noted. No nystagmus or tremors were present, which were common features in some of the other reported cases. MRI shortly after birth revealed normal brain structure. Previous cases were notable for brain abnormalities in some affected family members including cerebellar atrophy and delayed myelination.
PIGN encodes a protein necessary for GPI anchor biosynethsis. Genetic analysis of the case of the Israeli Arab family revealed a homozygous mutation in the PIGN gene of c.2126G→A (p.Arg709Gln). The patients from Japan had heterozygous PIGN mutations [c.808T >C (p.Ser270Pro) and c.963G >A]. The mutations resulted in a greatly decreased expression of GPI anchored proteins such as CD16, CD24, and CD59. Genetic analysis of our patient revealed novel heterozygous mutations of c.406T>G (p.Trp136Gly)/c.2576G>C (p.Ser859Thr) in the PIGN gene. There have been no reported cases of this particular genetic disorder in the United States.
In summary, we present a patient with compound heterozygous mutations in the PIGN gene inherited from heterozygous carrier parents with a phenotype consistent with the Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome 1 (MCAHS1). The rarity of the disorder provides an incentive to further investigate the cause, phenotype and prognosis of the disorder.
Acknowledgments
This work was supported by the NHGRI, Award 1UO1HG006487-01
Footnotes
-
Natario L. Couser, MD, MS:1) Consultant for NovaBay Pharmaceuticals from 2013–20142) Consultant for Vertex Pharmaceutical from 2013–2014
-
Maheer M. Masood, BA:Nothing to declare
-
Natasha T. Strande, PhD:Nothing to declare
-
Ann Katherine M. Foreman, MS, CGC:Nothing to declare
-
Kristy Crooks, PhD:Nothing to declare
-
Karen E. Weck, MD:Nothing to declare
-
Mei Lu, MD, MS:Nothing to declare
-
Kirk C. Wilhelmsen, MD, PhD:Nothing to declare
-
Myra Roche, MS, CGC:Nothing to declare
-
James P. Evans, MD, PhD:Nothing to declare
-
Jonathan S. Berg, MD, PhD:Nothing to declare
-
Cynthia M. Powell, MD:Nothing to declare
References
- Belet S, Fieremans N, Yuan X, Van Esch H, Verbeeck J, Ye Z, Cheng L, Brodsky BR, Hu H, Kalscheuer VM, Brodsky RA, Froyen G. Early frameshift mutation in PIGA identified in a large XLID family without neonatal lethality. Hum Mutat. 2014;35:350–355. doi: 10.1002/humu.22498. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Mondésert G, Grimme SJ, Reed SI, Orlean P, Emr SD. MCD4 encodes a conserved endoplasmic reticulum membrane protein essential for glycosylphosphatidylinositol anchor synthesis in yeast. Mol Biol Cell. 1999;10:627–48. doi: 10.1091/mbc.10.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Maeda Y, Watanabe R, Ohishi K, Mishkind M, Riezman H, Kinoshita T. Pig-n, a mammalian homologue of yeast Mcd4p, is involved in transferring phosphoethanolamine to the first mannose of the glycosylphosphatidylinositol. J Biol Chem. 1999;274:35099–106. doi: 10.1074/jbc.274.49.35099. [DOI] [PubMed] [Google Scholar]
- Kato M, Saitsu H, Murakami Y, Kikuchi K, Watanabe S, Iai M, Miya K, Matsuura R, Takayama R, Ohba C, Nakashima M, Tsurusaki Y, Miyake N, Hamano S, Osaka H, Hayasaka K, Kinoshita T, Matsumoto N. PIGA mutations cause early-onset epileptic encephalopathies and distinctive features. Neurology. 2014;82:1587–96. doi: 10.1212/WNL.0000000000000389. [DOI] [PubMed] [Google Scholar]
- Kvarnung M, Nilsson D, Lindstrand A, Korenke GC, Chiang SC, Blennow E, Bergmann M, Stödberg T, Mäkitie O, Anderlid BM, Bryceson YT, Nordenskjöld M, Nordgren A. A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J Med Genet. 2013;50:521–8. doi: 10.1136/jmedgenet-2013-101654. [DOI] [PubMed] [Google Scholar]
- Maydan G, Noyman I, Har-Zahav A, Neriah ZB, Pasmanik-Chor M, Yeheskel A, Albin-Kaplanski A, Maya I, Magal N, Birk E, Simon AJ, Halevy A, Rechavi G, Shohat M, Straussberg R, Basel-Vanagaite L. Multiple congenital anomalies-hypotonia-seizures syndrome is caused by a mutation in PIGN. J Med Genet. 2011;48:383–9. doi: 10.1136/jmg.2010.087114. [DOI] [PubMed] [Google Scholar]
- Ohba C, Okamoto N, Murakami Y, Suzuki Y, Tsurusaki Y, Nakashima M, Miyake N, Tanaka F, Kinoshita T, Matsumoto N, Saitsu H. PIGN mutations cause congenital anomalies, developmental delay, hypotonia, epilepsy, and progressive cerebellar atrophy. Neurogenetics. 2014;15:85–92. doi: 10.1007/s10048-013-0384-7. [DOI] [PubMed] [Google Scholar]
- van der Crabben SN, Harakalova M, Brilstra EH, van Berkestijn FM, Hofstede FC, van Vught AJ, Cuppen E, Kloosterman W, Ploos van Amstel HK, van Haaften G, van Haelst MM. Expanding the spectrum of phenotypes associated with germline PIGA mutations: a child with developmental delay, accelerated linear growth, facial dysmorphisms, elevated alkaline phosphatase, and progressive CNS abnormalities. Am J Med Genet A. 2014;164A:29–35. doi: 10.1002/ajmg.a.36184. [DOI] [PubMed] [Google Scholar]