In a recent edition of AP&T, Sunwoo and colleagues published the results of a phase 1 study of DWP14012, a new potassium-competitive acid blocker (P-CAB) (1). The advent of the P-CABs marks the latest development in our ability to control gastric acid secretion.
The dawn of the 20th century began with the statement “No acid, no ulcer” (2) heralding massive efforts to control acid secretion by the stomach since peptic ulcer was then a major cause of morbidity and mortality. Surgical approaches were reasonably successful, starting with vagotomy (3).
There were no well tolerated drugs available until the work of Black and colleagues who developed the first H2-receptor antagonist (H2RA), burimamide (4), which was modified to generate cimetidine, the first marketed medical therapy for peptic ulcer disease. Although the H2RAs revolutionised the medical treatment of peptic ulcer disease, they all showed tolerance where their efficacy dropped by 50% after 1 week’s treatment.
Serendipitously, a compound, timoprazole was found to be a potent inhibitor of gastric acid secretion although it affected the thyroid and the thymus. A modification of this to omeprazole (4-methoxy-3,5-dimethylpyridyl, 5-methoxybenzimidazole) ablated the thyroid and thymus effects, and was the first proton pump inhibitor (PPI) (5).
All the PPIs are weak bases with a pyridine pKa between 4 and 5 and thus accumulate in the luminal compartment of the parietal cell’s secretory canaliculus. The benzimidazole N has a pKa of ~1 and is protonated only at the pH of the active gastric acid pump, converting the sulfinyl moiety to a sulfenic acid or a sulfenamide (6) allowing covalent reaction with cysteine 813 in the proton channel of the α-subunit of the gastric H, K ATPase. However, the short half-life (60–90 minutes) allows gastric pH to drop to about 3.0 at night time even at twice-daily dosing. A pH of 3.0 prevents growth of H. pylori and generates resistance to amoxicillin that requires growth for bactericidal efficacy (7).
In 1983, Schering-Plough synthesised SCH28080. This was a potassium-competitive antagonist of the gastric H, K ATPase with a short duration of action, but some hepatotoxicity (8). This compound was the first Figure 1). Subsequent compounds had a short duration of action and never reached market until Takeda introduced vonoprazan (1-[5-(2-fluorophenyl)-1-pyridine-3-ylsulfonyl pyrrol-3-yl] N methyl methanamine fumarate), which had a long duration of action and showed excellent efficacy at treating GERD and, in combination with antibiotics, eradication of H. pylori (9). Analysis of the crystal structure of the gastric H, K ATPase with bound vonoprazan (10) showed that the methylmethaneamine moiety (pKa 9.4) bound at glutamine 343, the potassium-binding site of the pump.
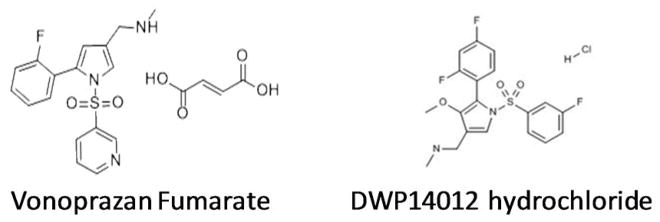
Figure 1.
Structures of vonoprazan fumarate and DWP14012 hydrochloride. The benefits of these two P-CABs are predicted to be: (1) Elevation of pH to >5.0, 24 hours from a single dose; (2) Improved treatment of acid-related diseases; (3) Eradication of H. pylori in combination with amoxicillin only; (4) Immediate onset of action; (5) No requirement for enteric coating; (6) Meal independence; (7) Easy solution formulation. A dose of 40 mg once a day would maintain a pH >5.0 for 24 hours per day.
Currently, a homolog of vonoprazan, DWP14012 (1-(5-2,1-4-difluorophenyl)-1-3-((3-fluorophenyl)sulfonyl)-4-methoxy-1H-pyrrol -3-yl)-N-methylmethanamine hydrochloride) is being developed in Korea (1). This compound has remarkably similar properties to vonoprazan; it is potassium-competitive with a long duration of action (the pyridine is replaced by a fluorophenyl) and promises a successful launch.
Acknowledgments
This work was supported in part by NIH grants DK46917, DK53462 and DK58333 to GS and K08DK100661, R03DK110579, UCLA Children’s Discovery and Innovation Institute and Today’s and Tomorrow’s Children Fund to EAM.
References
- 1.Sunwoo J, Oh J, Moon SJ, Ji SC, Lee SH, Yu K-S, et al. Randomised Clinical Trial: safety tolerability pharmacodynamics and pharmacokinetics of DWP14012 a novel potassium competitive acid blocker in healthy male subjects. Aliment Pharmacol Ther. 2018 doi: 10.1111/apt.14818. [DOI] [PubMed]
- 2.Schwartz K. Ueber penetrierende magen - und jejunalgeschure. Bruns Beitr Klin Chir. 1910;67:96–128. [Google Scholar]
- 3.Dragstedt LR, Harper PV, Jr, et al. Section of the vagus nerves to the stomach in the treatment of peptic ulcer complications and end results after four years. Ann Surg. 1947 Nov;126(5):687–708. [PubMed] [Google Scholar]
- 4.Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–90. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- 5.Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjostrand SE, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+, K+)ATPase. Nature. 1981 Mar 12;290(5802):159–61. doi: 10.1038/290159a0. [DOI] [PubMed] [Google Scholar]
- 6.Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013 Jan;19(1):25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther [Research Support, N.I.H., Extramural] 2012 Nov;36(10):972–9. doi: 10.1111/apt.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallmark B, Briving C, Fryklund J, Munson K, Jackson R, Mendlein J, et al. Inhibition of gastric H+,K+-ATPase and acid secretion by SCH 28080, a substituted pyridyl(1,2a)imidazole. J Biol Chem. 1987 Feb 15;262(5):2077–84. [PubMed] [Google Scholar]
- 9.Hori Y, Matsukawa J, Takeuchi T, Nishida H, Kajino M, Inatomi N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther [Comparative Study] 2011 Jun;337(3):797–804. doi: 10.1124/jpet.111.179556. [DOI] [PubMed] [Google Scholar]
- 10.Abe K, Irie K, Nakanishi H, Suzuki H, Fujiyoshi Y. Crystal structures of the gastric proton pump. Nature. 2018 Apr;556(7700):214–8. doi: 10.1038/s41586-018-0003-8. [DOI] [PubMed] [Google Scholar]