Summary
For well over half of the 150 years since the discovery of the neural crest, the special ability of these cells to function as a source of species‐specific pattern has been clearly recognized. Initially, this observation arose in association with chimeric transplant experiments among differentially pigmented amphibians, where the neural crest origin for melanocytes had been duly noted. Shortly thereafter, the role of cranial neural crest cells in transmitting species‐specific information on size and shape to the pharyngeal arch skeleton as well as in regulating the timing of its differentiation became readily apparent. Since then, what has emerged is a deeper understanding of how the neural crest accomplishes such a presumably difficult mission, and this includes a more complete picture of the molecular and cellular programs whereby neural crest shapes the face of each species. This review covers studies on a broad range of vertebrates and describes neural‐crest‐mediated mechanisms that endow the craniofacial complex with species‐specific pattern. A major focus is on experiments in quail and duck embryos that reveal a hierarchy of cell‐autonomous and non‐autonomous signaling interactions through which neural crest generates species‐specific pattern in the craniofacial integument, skeleton, and musculature. By controlling size and shape throughout the development of these systems, the neural crest underlies the structural and functional integration of the craniofacial complex during evolution.
Keywords: cranial neural crest, craniofacial development, evolutionary‐developmental biology, quail‐duck chimeras, quck, species‐specific pattern, tissue‐interactions
1. INTRODUCTION
The notion that neural crest cells generate species‐specific pattern has a long and colorful history. Some of the earliest indications first arose from surgical transplantation experiments designed to exploit pigment variations in amphibian embryos. Around the beginning of the 20th Century, embryologists such as Born (1896), Harrison (1898, 1903), and Spemann (1918) pioneered the use of chimeras, that is combining embryonic components from distinct animal species, to follow the movements and fates of cells, and understand the inductive properties of tissues (Harrison, 1935; Mangold, 1923; Mangold & Seidel, 1927; Noden, 1984; Spemann, 1938; Spemann & Mangold, 1924). Their reliance on intrinsic differences in the number, distribution, and color of intracellular pigment granules as a means to keep track of donor versus host tissues was actually a proxy for a neural crest‐derived lineage (i.e., melanocytes), something which was suggested by Harrison (1910) and others but which remained debatable at the time (Dorris, 1938; DuShane, 1934, 1935, 1938, 1939; Harrison, 1969; Holtfreter, 1933; Raven, 1931). Soon thereafter, numerous efforts were underway to determine the extent to which neural crest cells establish inter‐ and intra‐specific pigment patterns and to sort out the effects and/or role of interactions with epidermis (Clark Dalton, 1950; Harrison, 1935; Hörstadius, 1950; Macmillan, 1976). For example, neural crest transplants among the tiger salamander, spotted salamander, or white and black strains of the Mexican salamander revealed that the “characteristic adult spots of the graft are in most cases distinctly different from those of the host, and are similar to those of donor adults” (DuShane, 1935, p. 25). Other interspecific transplants also confirmed this finding (Twitty, 1936, 1945; Twitty & Bodenstein, 1939). Thus, what became evident was that “the type of pattern as a whole depends upon qualities intrinsic to the crest‐cells” (Hörstadius, 1950, p. 75). What remained unknown was if the neural crest was playing a comparable role in providing species‐specific patterning information for any of its other derivatives, something that chimeras could help resolve.
Chimerism was all the rage at the dawn of experimental embryology given its great potential to reveal morphogenetic mechanisms during normal development that lead to the progressive integration of ectoderm, mesoderm, and endoderm; but also, because chimerism could provide a window into the developmental basis for evolutionary variation among species. In his comprehensive review, “Heteroplastic Grafting in Embryology” Harrison (1935) claimed with regard to making chimeras that, “the applicability of the method rests upon the fact that there are related species that differ from one another in pigmentation, rate of growth and development, ultimate size, relative time of appearance of organs, or even in the presence or absence of organs, while at the same time their tissues show a mutual tolerance when combined in one organism” (Harrison, 1969, p. 216). Results of these chimeric transplants among different taxa (i.e., species, genera, families, and even orders such as frogs and salamanders) indicated that some donor tissues did not convey species‐specific information.
Harrison (1935) further described conclusions from a broad range of studies and stated that, “In general, inducers are not specific.…But the induced organ has entirely the character of the species from which it is developed” (Harrison, 1969, p. 217). For example, in classic “organizer” studies (Spemann, 1918, 1938; Spemann & Schotte, 1932), where some of the donor tissues were from salamander endoderm, the induced mouth parts remained frog‐like leading to Spemann's purported description of the conversation between the host ectoderm tissue and its endoderm inducer, “you tell me to make a mouth; all right, I'll do so, but I can't make your kind of mouth; I can make my own and I'll do that” (Harrison, 1933, p. 318).
In contrast, when entire limb buds were exchanged between two species of salamanders with very different rates of development or where the limbs themselves varied greatly in size, the resultant chimeras always had limbs like that of the donor in terms of timing of maturation and morphology (Detwiler, 1930; Harrison, 1915, 1917, 1924; Schwind, 1932; Swett, 1930). Grafts of other embryonic rudiments and whole organs such as the eye, ear, heart, teeth, or gills produced equivalent results (Copenhaver, 1930; Harrison, 1929, 1935; Huxley, 1932; Kaan, 1930; Richardson, 1932; Stone, 1930; Twitty, 1930, 1932, 1934; Twitty & Schwind, 1931), with the conclusion being that somewhere within a composite organ resided the source of species‐specific pattern.
Subsequent explorations of the relative contributions of the constituent parts of these composite organs began to shed light on this issue, particularly with regard to the requisite role for derivatives of each of the three germ layers. In the case of the eye, the lens (from surface ectoderm) and the optic cup (from neural ectoderm) were mutually regulating (Harrison, 1929, 1935; Stone & Dinnean, 1940; Twitty, 1930, 1932), whereas host eye muscles (from paraxial mesoderm) were subservient to the eye itself and would accommodate the size, orientation, and location of the donor eye (Twitty, 1930, 1932, 1934, 1966). In the case of the forelimb, the mesoderm, which produces the muscles and skeleton, would always determine its size, shape, and growth rate, whereas the ectoderm had limited influence (Harrison, 1935; Rotmann, 1931, 1933; Schwind, 1931, 1932).
Like what had been observed for pigment patterns, the gills or branchial system of the pharynx provided clear evidence for a dominant role of neural crest‐derived mesenchyme not only in generating the jaw and gill cartilages themselves (Landacre, 1921; Platt, 1891, 1893, 1898; Stone, 1922, 1926, 1929) but also in determining their species‐specific pattern (De Beer, 1947; Harrison, 1935). In addition to the neural crest skeletal derivatives, the gill arches contain a pouch lined by endoderm, muscles from the mesoderm, and an outer epithelium from the ectoderm (De Beer, 1937; Goodrich, 1913, 1918, 1930). Numerous grafting experiments of each of these constituents either separately or in various combinations (Adams, 1931; Harrison, 1921, 1935; Holtfreter, 1936; Rotmann, 1931, 1933; Severinghaus, 1930; Spemann, 1921; Stone, 1932) showed that the ectoderm, mesoderm, and endoderm had mixed and inconsistent effects on the size, shape, and rate of development of the external gills.
In stark contrast, were the neural crest cells, which according to Harrison (1935) were “Far more conclusive” and had “a profound effect upon the development of the branchial system, particularly the visceral skeleton” (Harrison, 1969, p. 233). For example, transplants of neural crest from a larger species of salamander in place of neural crest from a smaller species gave rise to pharyngeal arches with the size and shape of the donor species (De Beer, 1947; Harrison, 1933; Hörstadius, 1950). Other neural crest transplants among frogs and salamanders also produced donor‐like cartilages in the pharyngeal arches and jaw skeleton (Andres, 1949; Fassler, 1996; Hall & Hörstadius, 1988; Hörstadius & Sellman, 1941, 1946; Noden & Schneider, 2006; Raven, 1931, 1933, 1935; Spemann & Schotte, 1932; Wagner, 1959). Early on, such work suggested to scientists like Raven (1933), as conveyed by Hörstadius (1950) that, “the neural crest in the head might have a special task in connection with its movements, as a carrier of inductive influences” (p. 93). This classic body of literature demonstrated clearly that, “transplanted neural crest cells express a species‐specific patterning that is an intrinsic property of the skeletogenic cells” (Hall, 1999, p. 71). Moreover, this initial work garnered a much deeper appreciation for the hierarchical levels of organization within these complex developmental organ systems and pointed to the key role for neural crest cells in the evolution of species‐specific morphology.
By the turn of the 21th Century, other transplant experiments in non‐amphibian taxa such as among mouse, human, chick, or quail (Cohen et al., 2016; Fontaine‐Perus, 2000; Fontaine‐Perus & Cheraud, 2005; Fontaine‐Perus, Cheraud, & Halgand, 1996; Fontaine‐Perus et al., 1997; Kirby, Stadt, Kumiski, & Herlea, 2000; Lwigale & Schneider, 2008; Mitsiadis, Caton, & Cobourne, 2006; Mitsiadis, Cheraud, Sharpe, & Fontaine‐Perus, 2003; Pudliszewski & Pardanaud, 2005; Serbedzija & McMahon, 1997); among divergent species of birds including quail, chick, duck, and emu (Ealba et al., 2015; Eames & Schneider, 2005, 2008; Fish & Schneider, 2014a, 2014b; Fish, Sklar, Woronowicz, & Schneider, 2014; Hall et al., 2014; Jheon & Schneider, 2009; Le Douarin, Dieterlen‐Lievre, Teillet, & Ziller, 2000; Merrill, Eames, Weston, Heath, & Schneider, 2008; Schneider, 2005, 2015; Schneider & Helms, 2003; Sohal, 1976; Solem, Eames, Tokita, & Schneider, 2011; Tokita & Schneider, 2009; Tucker & Lumsden, 2004; Woronowicz, Gline, Herfat, Fields, & Schneider, 2018; Yamashita & Sohal, 1986); as well as between Mexican cavefish and surface fish (Yoshizawa, Hixon, & Jeffery, 2018), reinforced the conclusion that species‐specific pattern in the craniofacial complex is largely driven by the neural crest. Harrison (1969) argued that such an ability is due to “congenital specific factors” that “control the relative growth rate” (p. 31) of grafts. Noden (1984) correspondingly observed that, “while quail tissues differentiate more rapidly, they generally form smaller skeletal structures than do chick tissues” (p. 274), which in the case of quail‐chick chimeras leads to the formation of shorter quail‐like upper and lower portions of the beak, depending on from where along the neural tube the quail donor neural crest cells are derived. In other words, donor neural crest cells keep track of their intrinsic rates of development, and that appears to have direct implications for the generation of species‐specific size and shape in the craniofacial complex.
Discerning exactly how neural crest accomplishes such a seemingly complicated task and pinpointing precise morphogenetic mechanisms that ultimately function as determinants of species‐specific pattern, has been a goal of work from our lab over the past 15 years. Somewhat systematically, we have been investigating the extent to which the cranial neural crest directs the patterning of its own derivatives (e.g., cartilages, bones, tendons), as well as those arising from ectoderm (e.g., feathers and egg teeth) and mesoderm (e.g., muscles, blood vessels, osteoclasts) in order to understand how the major systems of the craniofacial complex become structurally and functionally integrated during development and how they become modified during evolution (Figure 1a). Such work, which has helped illuminate the origin of species‐specific pattern, is summarized and contextualized in the sections below.
Figure 1.
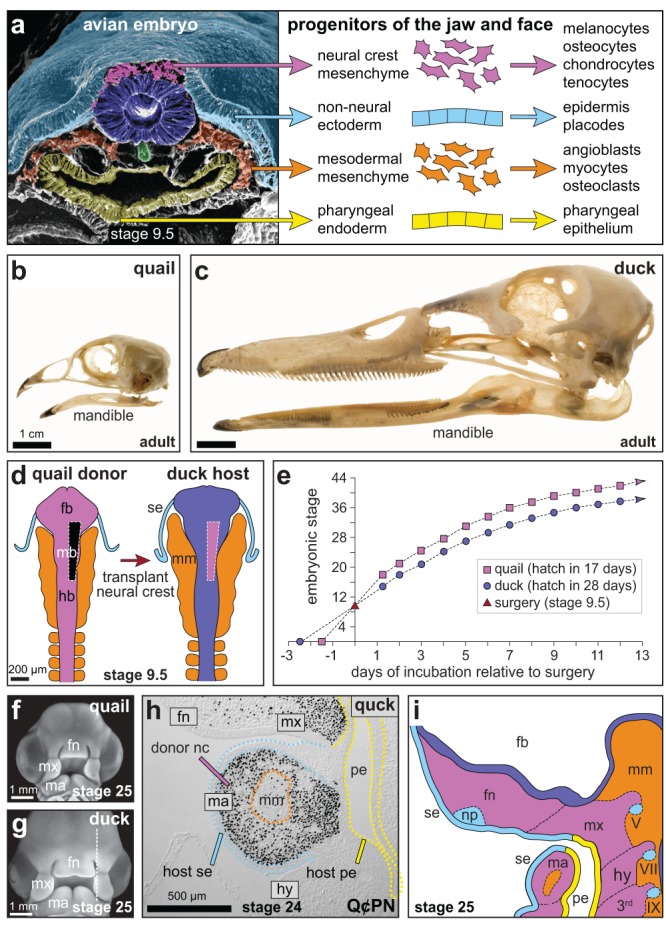
(a) Pseudocolored scanning electron micrograph of a chick in cross‐section [modified from Tosney (1982)] through the midbrain/hindbrain boundary and first pharyngeal arch showing progenitors of tissues in the jaw and face. Modified from Ealba et al. (2015). (b) Skull and lower jaw (mandible) of an adult quail and (c) duck in lateral view. Modified from Tokita and Schneider (2009). (d) Unilateral transplant of presumptive quail neural crest from the posterior forebrain (fb), midbrain (mb), and anterior hindbrain (hb) into a duck host. Non‐neural surface ectoderm (se) and mesodermal mesenchyme (mm) are shown in dorsal view. Modified from Eames and Schneider (2008). (e) Distinct maturation rates of quail (pink squares) versus duck (purple circles) after being stage‐matched at HH9.5 for surgery (red triangle on the Y‐axis) result in quail donor cells remaining accelerated by approximately three stages within 2 days after surgery relative to the duck host [modified from Eames & Schneider (2005)]. (f) By stage 25, the frontonasal (fn), maxillary (mx), and mandibular (ma) primordia of quail and (g) duck appear similar in shape but not in size (frontal view). Modified from Schneider (2005). (h) Sagittal section (in plane of white dashed line in panel g) in a chimeric quck through the maxillary (mx) and mandibular (ma) region showing quail donor cells labeled with Q¢PN (black nuclei). Duck‐host surface ectoderm (se), pharyngeal endoderm (pe), mesodermal mesenchyme (mm) are unlabeled. The hyoid arch (hy) is also negative since its precursors were not transplanted. Modified from Ealba and Schneider (2013). (i) By HH25, the frontonasal (fn), maxillary (mx), mandibular (ma), and hyoid (hy) primordia (sagittal view) are surrounded by surface ectoderm (se), pharyngeal endoderm (pe) and forebrain neuroepithelium (fb), and contain contributions from the neural crest, nasal placode (np), and cranial ganglia (V, VII, IX). Mesodermal mesenchyme (mm) produces muscles, vascular endothelium, and some skeletal tissues. Modified from Schneider (2005)
2. ORIGIN OF SPECIES‐SPECIFIC VERSUS SPECIES‐GENERIC ASPECTS OF PATTERN
When describing species‐specific pattern, what is typically meant are those relatively unique morphological or behavioral features of an organism that often appear well‐suited to meet certain functional, ecological, sexual, or other kinds of selective pressures. Moreover, such features can be defining and used to distinguish one species (or higher taxonomic level) from another. In this context, and in terms of morphology, a type of species‐specific pattern that has long been of central concern pertains to changes in size and shape during development and evolution (Fish & Schneider, 2014b; Schneider, 2015, 2018). This focus was most significantly catalogued and detailed over 100 years ago by Thompson (1917) in his celebrated tome, On Growth and Form. Using a geometric system of Cartesian coordinates, Thompson strove to describe transformations in the size and shape of organs and organisms during the growth of individuals and across different species. In so doing, he helped spawn an entire discipline of morphometrics that continues to this day (Arthur, 2006; Benson, Chapman, & Siegel, 1982; Bookstein, 1978, 1990; Gayon, 2000; Hallgrimsson et al., 2015; Marcus, 1996; Schneider, 2018; Siegel & Benson, 1982; Stern & Emlen, 1999; Zelditch, 2004).
Since Thompson, many other scientists have endeavored to address the origins of species‐specific size and shape through mathematical, theoretical, and experimental means, ultimately in search of underlying genetic, molecular, cellular, or other developmental mechanisms including allometry and heterochrony (Alberch, 1982a, 1985, 1989; Alberch, Gould, Oster, & Wake, 1979; Anderson & Busch, 1941; Atchley, Rutledge, & Cowley, 1981; Bertalanffy & Pirozynski, 1952; Clark & Medawar, 1945; Coppinger & Coppinger, 1982; Coppinger & Schneider, 1995; De Beer, 1930; De Renzi, 2009; Drake, 2011; Godfrey & Sutherland, 1995; Gould, 1966, 1971, 1977; Hersh, 1934; Huxley, 1932, 1950; Huxley & Teissier, 1936; Kermack & Haldane, 1950; Klingenberg, 1998; Lande, 1979; Lord, Schneider, & Coppinger, 2016; Lumer, 1940; Minot, 1908; Needham & Lerner, 1940; Oster & Alberch, 1982; Oster, Shubin, Murray, & Alberch, 1988; Reeve, 1950; Rensch, 1948; Roth & Mercer, 2000; Shea, 1985; Smith et al., 2015; Smith, 2003; Stern & Emlen, 1999; Von Bonin, 1937; Waddington, 1950, 1957). A common theme for much of the research on size and shape relates to those changes that occur with respect to developmental time either as a function of age or growth. Minot (1908) laid the groundwork for this perspective by emphasizing the importance of cell number, differentiation, and rates of growth in the regulation of the size of animals and/or their organs. Thompson (1952) later elaborated on this idea when stating that, “the form of an organism is determined by its rate of growth in various directions; hence rate of growth deserves to be studied as a necessary preliminary to the theoretical study of form, and organic form itself is found, mathematically speaking, to be a function of time” (p. 79). Thus, given that the neural crest generates species‐specific pattern in the craniofacial complex, and this pattern can be defined primarily as the size and shape of structures, then a critical insight could be gained by understanding the extent to which the neural crest controls the timing of events during development. A further question also remains, which is from where do other aspects of craniofacial pattern (i.e., those that are not necessarily species‐specific) arise?
In addition to their species‐specific pattern, structures likewise possess many more “species‐generic” aspects of pattern. These include their axial orientation (e.g., dorsal‐ventral, medial‐lateral, proximal‐distal, oral‐aboral), anatomical identity (e.g., upper versus lower jaw, eye versus ear), and tissue type (e.g., cartilage, bone, muscle, tendon, nerve). For the most part, epithelia in the craniofacial complex appear to supply the cues required for the establishment of generic pattern and express the factors necessary to maintain outgrowth of individual components. For example, signaling by ectodermal epithelium around the frontonasal process (i.e., the primordium that gives rise to the mid‐ and upper‐face) is essential for proper expansion and orientation of skeletal elements along the dorsoventral, mediolateral, and proximodistal axes (Foppiano, Hu, & Marcucio, 2007; Hu & Marcucio, 2009b; Hu, Marcucio, & Helms, 2003). Experimentally rotating epithelium in the frontonasal process can lead to mirror image duplications of upper beak structures along the dorsal–ventral axis (Helms & Schneider, 2003; Hu et al., 2003; Marcucio, Cordero, Hu, & Helms, 2005).
Similarly, endodermal epithelium that lines the pharynx is needed for the proper axial orientation, anatomical identity, and growth of cartilage and bone in the lower jaw and hyoid skeleton (Brito, Teillet, & Le Douarin, 2006; Couly, Creuzet, Bennaceur, Vincent, & Le Douarin, 2002; Crump, Maves, Lawson, Weinstein, & Kimmel, 2004; David, Saint‐Etienne, Tsang, Schilling, & Rosa, 2002; Delloye‐Bourgeois, Rama, Brito, Le Douarin, & Mehlen, 2014; Graham, 2003; Haworth et al., 2007; Kikuchi et al., 2001; Kimmel et al., 1998; Miller, Schilling, Lee, Parker, & Kimmel, 2000; Piotrowski & Nusslein‐Volhard, 2000; Ruhin et al., 2003; Veitch, Begbie, Schilling, Smith, & Graham, 1999). When endodermal epithelium is rotated surgically or removed, the associated neural crest‐derived skeleton follows accordingly (Couly et al., 2002; Haworth et al., 2007). Therefore, both ectodermal and endodermal epithelia function as local sources of signals for generic pattern that elicit and/or maintain programmatic responses from the adjacent neural crest‐derived mesenchyme (Creuzet, Couly, & Le Douarin, 2005; Ferguson, Tucker, & Sharpe, 2000; Higashihori, Buchtova, & Richman, 2010; Langille & Hall, 1993; Le Douarin, Creuzet, Couly, & Dupin, 2004; Mitsiadis et al., 2003; Richman & Tickle, 1989; Santagati & Rijli, 2003; Tak, Park, Piao, & Lee, 2017; Tucker, Yamada, Grigoriou, Pachnis, & Sharpe, 1999; Wilson & Tucker, 2004). As will be discussed later, such programmatic responses are in fact species‐specific, and they also reciprocally influence the temporal and spatial domains of expression in adjacent epithelia (Eames & Schneider, 2005; Schneider & Helms, 2003).
Pharyngeal endoderm, and neural and non‐neural ectoderm function as key epithelial signaling centers by releasing complex combinations of secreted molecules from well‐characterized pathways including Bone Morphogenetic Protein (BMP), Sonic Hedgehog (SHH), Fibroblast Growth Factor (FGF), and Wingless‐Related (WNT) that are indispensable to the proper patterning and differentiation of neural crest mesenchyme (Alvarado‐Mallart, 2005; Anderson, Lawrence, Stottmann, Bachiller, & Klingensmith, 2002; Barlow & Francis‐West, 1997; Benouaiche, Gitton, Vincent, Couly, & Levi, 2008; Cela et al., 2016; Crump et al., 2004; Ekker et al., 1995; Francis‐West, Ladher, Barlow, & Graveson, 1998; Gitton et al., 2010; Graham, 2003; Marcucio et al., 2005; Marcucio, Young, Hu, & Hallgrimsson, 2011; Pera, Stein, & Kessel, 1999; Piotrowski & Nusslein‐Volhard, 2000; Sasai & De Robertis, 1997; Schneider, Hu, Rubenstein, Maden, & Helms, 2001; Shimamura, Hartigan, Martinez, Puelles, & Rubenstein, 1995; Veitch et al., 1999; Wilson & Tucker, 2004; Withington, Beddington, & Cooke, 2001; Xu et al., 2015). Various members and targets of these pathways also become differentially regulated not only as a mechanism to support the outgrowth of the jaw and facial skeletons (Abzhanov & Tabin, 2004; Ashique, Fu, & Richman, 2002a; Chong et al., 2012; Cordero, Schneider, & Helms, 2002; Couly et al., 2002; Doufexi & Mina, 2008; Geetha‐Loganathan, Nimmagadda, Fu, & Richman, 2014; Havens et al., 2008; Helms & Schneider, 2003; Hu, Colnot, & Marcucio, 2008; Hu et al., 2003; Liu et al., 2005; MacDonald, Abbott, & Richman, 2004; Melnick, Witcher, Bringas, Carlsson, & Jaskoll, 2005; Miller et al., 2000; Mina, Wang, Ivanisevic, Upholt, & Rodgers, 2002; Nimmagadda et al., 2015; Richman, Herbert, Matovinovic, & Walin, 1997; Rowe, Richman, & Brickell, 1992; Schneider, Hu, & Helms, 1999; Schneider et al., 2001; Szabo‐Rogers, Geetha‐Loganathan, Nimmagadda, Fu, & Richman, 2008; Wada et al., 2005; Young, Chong, Hu, Hallgrimsson, & Marcucio, 2010) but also as a component of regulating species‐specific size and shape (Abramyan, Leung, & Richman, 2014; Abzhanov et al., 2006; Abzhanov, Protas, Grant, Grant, & Tabin, 2004; Bhullar et al., 2015; Brugmann et al., 2007; Brugmann et al., 2010; Cheng et al., 2017; Foppiano et al., 2007; Grant, Grant, & Abzhanov, 2006; Hu & Marcucio, 2009b, 2012; Hu, Young, Li, et al., 2015; Hu, Young, Xu, et al., 2015; Wu, Jiang, Shen, Widelitz, & Chuong, 2006; Wu, Jiang, Suksaweang, Widelitz, & Chuong, 2004; Young et al., 2014).
Other epithelial tissues that are derived from surface (i.e., non‐neural) ectoderm also function as critical patterning centers in conjunction with cranial neural crest. In particular, cranial placodes that contribute to sensory ganglia and sense organs such as the olfactory, optic, otic, trigeminal, and epibranchial systems require repeated and reciprocal interactions with adjacent neural crest mesenchyme for their proper morphogenesis (Baker & Bronner‐Fraser, 2001; Bancroft & Bellairs, 1977; Couly & Le Douarin, 1988; Francis‐West, Ladher, & Schoenwolf, 2002; Ladher, O'Neill, & Begbie, 2010; Lwigale, 2001; Pispa & Thesleff, 2003; Song, Hui, Fu, & Richman, 2004; Szabo‐Rogers et al., 2008; Webb & Noden, 1993).
Members and targets of the FGF, BMP, WNT, and other pathways mediate complex signaling interactions among the developing placodes, mesoderm, endoderm, and the neural crest, which in turn lead to the differential activation of placode‐specific sets of transcription factors (Anwar, Tambalo, Ranganathan, Grocott, & Streit, 2017; Baker, Stark, Marcelle, & Bronner‐Fraser, 1999; Brunskill et al., 2014; Depew et al., 1999; Grocott, Johnson, Bailey, & Streit, 2011; Groves & Bronner‐Fraser, 2000; Hintze et al., 2017; Jourdeuil & Taneyhill, 2018; Ladher, 2017; Ladher, Wright, Moon, Mansour, & Schoenwolf, 2005; McLarren, Litsiou, & Streit, 2003; Moody & LaMantia, 2015; Saint‐Jeannet & Moody, 2014; Steventon, Mayor, & Streit, 2014; Yang et al., 2013). In almost all of these cases, the neural crest plays an obligatory role during proper patterning and differentiation.
As neural crest cells migrate throughout the craniofacial complex and settle adjacent to these different types of epithelia they respond by expressing a broad range of transcription factors and other genes that affect their anatomical identity (Balling, Mutter, Gruss, & Kessel, 1989; Clouthier et al., 2000; Creuzet, Couly, Vincent, & Le Douarin, 2002; Depew, Lufkin, & Rubenstein, 2002; Gendron‐Maguire, Mallo, Zhang, & Gridley, 1993; Grammatopoulos, Bell, Toole, Lumsden, & Tucker, 2000; Hunt, Clarke, Buxton, Ferretti, & Thorogood, 1998; Kimmel et al., 2005; Lufkin et al., 1992; Pasqualetti, Ori, Nardi, & Rijli, 2000; Qiu et al., 1997; Rijli et al., 1993; Ruest, Xiang, Lim, Levi, & Clouthier, 2004; Schilling, 1997; Smith & Schneider, 1998; Tavares, Cox, Maxson, Ford, & Clouthier, 2017). Modulating the levels of various molecules expressed by these epithelia, such as retinoic acid and the BMP antagonist Noggin, can for example, transform one facial primordium into another (Lee, Fu, Hui, & Richman, 2001; Richman & Lee, 2003) ostensibly by altering the gene regulatory networks within the responding neural crest cells.
Moreover, combinatorial expression of homeobox genes such as those in the Hox cluster and other transcription factors affect the ability of neural crest cells from the posterior hindbrain to form appropriate anatomical pattern in the hyoid and subsequent arches (Couly & Le Douarin, 1990; Trainor & Krumlauf, 2000; Trainor & Krumlauf, 2001). In contrast, neural crest cells from the midbrain and anterior hindbrain that migrate into the frontonasal, maxillary, and mandibular primordia do not rely on Hox genes (Couly et al., 2002; Couly, Grapin‐Botton, Coltey, Ruhin, & Le Douarin, 1998; Hunt & Krumlauf, 1991; Hunt, Wilkinson, & Krumlauf, 1991). If these midbrain and anterior hindbrain populations of neural crest cells are surgically rotated by 180° in order to transpose frontonasal and mandibular precursors, they generate facial and jaw skeletons that are appropriate for their new location, which reinforces the idea that anatomical identity is established locally (Noden, 1983) in response to epithelial signals.
Along similar lines, if the Hox code is deleted from neural crest cells destined to form the hyoid arch either by grafting non‐Hox‐expressing mandibular or frontonasal neural crest in place of hyoid arch neural crest cells, or by knocking down Hoxa2, then hyoid arch skeletal elements become replaced by mandibular structures (Gendron‐Maguire et al., 1993; Noden, 1983; Rijli et al., 1993; Trainor, Ariza‐McNaughton, & Krumlauf, 2002; Trainor, Melton, & Manzanares, 2003). Conversely, over‐expressing Hoxa2 in mandibular arch neural crest cells gives rise to hyoid skeletal structures instead of mandibular ones (Grammatopoulos et al., 2000; Pasqualetti et al., 2000). Also illustrating the necessity of signaling interactions between the neural ectoderm and the adjacent neural crest, Hoxa2 is downregulated by FGF8, and ectopic expression of Fgf8 in the hindbrain disrupts the pattern of hyoid arch structures (Creuzet et al., 2002; Trainor, Ariza‐McNaughton, et al., 2002). Thus, ongoing and reciprocal interactions between epithelia derived from the ectoderm and endoderm, and neural crest mesenchyme lead to the activation of intrinsic transcription factor modules that establish a more species‐generic type of pattern, specifically the axial orientation and anatomical identity of craniofacial structures.
Such a conclusion is further supported by experiments that alter combinatorial codes of transcription factors including the Dlx genes, and that genetically manipulate signaling pathways such as endothelin, which affect the axial pattern, outgrowth, and in some instances switch the anatomical identify of the maxillary and the mandibular primordia (Depew et al., 2002; Kuraku, Takio, Sugahara, Takechi, & Kuratani, 2010; Miller, Yelon, Stainier, & Kimmel, 2003; Sato et al., 2008; Tavares et al., 2012; Tavares et al., 2017). Notably, these molecular mechanisms and gene regulatory networks that pattern the axes and impart anatomical identity within the pharyngeal arches and other regions of the craniofacial complex have remained highly conserved across vertebrates (Cerny, Lwigale, et al., 2004; Cerny, Meulemans, et al., 2004; Depew & Compagnucci, 2008; Kuraku et al., 2010; Kuratani, 2004, 2005a, 2005b, 2012; Kuratani, Adachi, Wada, Oisi, & Sugahara, 2013; Kuratani, Nobusada, Horigome, & Shigetani, 2001; Kuratani, Oisi, & Ota, 2016; Medeiros & Crump, 2012; Minarik et al., 2017; Myojin et al., 2001; Nikitina, Sauka‐Spengler, & Bronner‐Fraser, 2008; Oisi, Ota, Fujimoto, & Kuratani, 2013; Oisi, Ota, Kuraku, Fujimoto, & Kuratani, 2013; Olsson, Ericsson, & Cerny, 2005; Ota, Kuraku, & Kuratani, 2007; Sauka‐Spengler, Meulemans, Jones, & Bronner‐Fraser, 2007; Shigetani et al., 2002; Shone & Graham, 2014; Square, Jandzik, Romasek, Cerny, & Medeiros, 2017; Sugahara et al., 2011; Takio et al., 2004; Yao, Ohtani, Kuratani, & Wada, 2011). This level of conservation indicates that all vertebrates more or less deploy the same gene regulatory networks, signaling pathways, and developmental modules to specify their axes and determine the anatomical identity of the homologous structures from which their craniofacial complexes get built.
Epithelial–mesenchymal interactions also contribute to another generic aspect of pattern, which is the establishment of tissue type, and in particular the differentiation of craniofacial cartilage and bone (Balczerski et al., 2012; Bee & Thorogood, 1980; Cela et al., 2016; Couly et al., 2002; Dunlop & Hall, 1995; Ferguson et al., 2000; Francis‐West, Robson, & Evans, 2003; Hall, 1980, 1982, 1987; MacDonald & Hall, 2001; Merrill et al., 2008; Richman & Tickle, 1989; Richman & Tickle, 1992; Schowing, 1968; Shigetani, Nobusada, & Kuratani, 2000; Shigetani et al., 2002; Thorogood, 1987; Thorogood, Bee, & von der Mark, 1986; Tyler, 1978, 1983). For example one prominent hypothesis, originally deemed the “flypaper model” (Garrod, 1986; Thorogood, 1988, 1993) suggested that epithelial‐mesenchymal interactions promote the production of extracellular matrix, which acts as an adhesive that “traps” migrating neural crest cells at their site of differentiation and results in mesenchymal condensation and cartilage induction. Such epithelia would include the pharyngeal endoderm and surface ectoderm around the facial primordia, as well as the brain and sensory capsules, all of which have been shown to initiate and/or direct chondrogenesis at various stages of development (Hall, 1980, 1981, 2000, 2005; Hall & Miyake, 2000; Mina, Upholt, & Kollar, 1994; Miyake, Cameron, & Hall, 1996; Thorogood et al., 1986).
What has become apparent from the many types of experimental strategies undertaken and vertebrate models studied, is that the reciprocal interactions during craniofacial development between neural crest mesenchyme and surrounding epithelia are highly dynamic, hierarchical, and likely involve both cell‐autonomous and non‐autonomous signals (Noden & Schneider, 2006). But the penultimate morphological outcome for a majority of components throughout the craniofacial complex, especially in the jaws and face, arises as a function of the special intrinsic ability of neural crest cells to propagate species‐specific pattern by superimposing parameters like size and shape onto more generic aspects of pattern such as axial orientation, anatomical identity, and tissue type (Fish & Schneider, 2014b). This capacity has most likely enhanced the evolutionary plasticity and potential (i.e., adaptability) of structures that contain or rely upon neural crest derivatives (Donoghue, Graham, & Kelsh, 2008; Jheon & Schneider, 2009; Le Douarin et al., 2004; Schneider, 2005; Young et al., 2014) in the pharyngeal and rostral regions of the vertebrate head (Gans & Northcutt, 1983; Northcutt, 2005; Northcutt & Gans, 1983), the lateral wall of the mammalian skull (Schneider, 1999; Smith & Schneider, 1998), and during the process of domestication (Lord et al., 2016; Sanchez‐Villagra, Geiger, & Schneider, 2016; Wilkins, Wrangham, & Fitch, 2014).
In fact, as described below, the degree to which neural crest cells convey species‐specific patterning information, and the intrinsic mechanisms that they use, have been made most evident by leveraging chimeric transplant systems that exploit evolutionary differences among birds. Recent research in this area has begun to paint a clearer picture of how individual structures within the craniofacial complex acquire their species‐specific pattern, and notably, such work illustrates how developmental programs can become modified internally on the molecular and cellular levels so that morphological variation can be generated in a manner essential for evolution.
3. ORIGIN OF SPECIES‐SPECIFIC PATTERN AS REVEALED BY AVIAN CHIMERAS
Much like prior studies involving chimeras between different amphibian species, a well‐established and very useful experimental approach for investigating the developmental origins and patterning of craniofacial structures in amniote embryos has been the use of the quail‐chick chimeric system (Baker, Bronner‐Fraser, Le Douarin, & Teillet, 1997; Borue & Noden, 2004; Cobos, Shimamura, Rubenstein, Martinez, & Puelles, 2001; Couly & Le Douarin, 1990; Couly, Coltey, & Le Douarin, 1992; Couly, Coltey, & Le Douarin, 1993; Köntges & Lumsden, 1996; Le Douarin, 1973; Le Lièvre, 1978; Le Lièvre & Le Douarin, 1975; Noden, 1978b, 1983, 1986a; Noden & Schneider, 2006; Olivera‐Martinez, Coltey, Dhouailly, & Pourquie, 2000; Schneider, 1999; Schneider et al., 2001; Selleck & Bronner‐Fraser, 1995). Quail and chick are closely related birds with similar rates of growth and morphology (Ainsworth, Stanley, & Evans, 2010; Fitzgerald, 1969; Hamburger & Hamilton, 1951; Nakane & Tsudzuki, 1999; Padgett & Ivey, 1960; Smith et al., 2015; Zacchei, 1961). Surgical transplants between them have enabled the fates, functions, and behaviors of different cells and tissues to be observed throughout embryogenesis, and this has been indispensable to understanding countless facets of developmental biology (Abramyan & Richman, 2018; Le Douarin & McLaren, 1984; Le Douarin & Dieterlen, 2013; Le Douarin, Dieterlen‐Lievre, & Teillet, 1996; Noden, 1984; Noden & Schneider, 2006).
The success of the quail‐chick chimeric system stems from the fact that in general, avian embryos are easily accessible in ovo for all kinds of experimental manipulations. This includes grafting or extirpation of tissues through microsurgery, labeling of cells for lineage analysis, implantation of reagent‐soaked beads, injection of biochemicals, and manipulation of gene expression via retroviral infection or electroporation (Cerny, Lwigale, et al., 2004; Chen et al., 1999; Ealba et al., 2015; Eichele, Tickle, & Alberts, 1984; Fekete & Cepko, 1993; Fish & Schneider, 2014a; Fish et al., 2014; Hall et al., 2014; Johnston, 1966; Krull, 2004; Kulesa, Bronner‐Fraser, & Fraser, 2000; Larsen, Zeltser, & Lumsden, 2001; Logan & Tabin, 1998; Lwigale, Conrad, & Bronner‐Fraser, 2004; Lwigale, Cressy, & Bronner‐Fraser, 2005; Lwigale & Schneider, 2008; Momose et al., 1999; Nakamura & Funahashi, 2001; Noden, 1975; Schneider et al., 2001; Serbedzija, Bronner‐Fraser, & Fraser, 1989; Stocker, Brown, & Ciment, 1993; Woronowicz et al., 2018). One advantage of working in birds is that after surgery or other manipulations, eggs can simply be resealed and incubated until the embryos reach stages appropriate for further analysis.
Another factor contributing to the success of avian chimeric systems is that embryos from different avian species can be readily stage‐matched using an approach that relies on external morphological characters and that is independent of body size and incubation time (Hamilton, 1965; Ricklefs & Starck, 1998; Starck & Ricklefs, 1998). The Hamburger and Hamilton (HH) staging system, initially created for chick, is the accepted standard (Hamburger & Hamilton, 1951). Other staging systems have been published for quail (Ainsworth et al., 2010; Nakane & Tsudzuki, 1999; Padgett & Ivey, 1960; Zacchei, 1961) and duck (Koecke, 1958), but embryos of these birds can also be staged using the HH system for chicken (Ainsworth et al., 2010; Le Douarin et al., 1996; Lwigale & Schneider, 2008; Mitgutsch, Wimmer, Sanchez‐Villagra, Hahnloser, & Schneider, 2011; Schneider & Helms, 2003; Smith et al., 2015; Starck, 1989; Yamashita & Sohal, 1987; Young et al., 2014). The ease at which embryos of diverse types of birds can be stage‐matched has advanced the study of species‐specific patterning.
During her early and pioneering neural crest transplant work Le Douarin observed that, “Quail and chick cells, experimentally associated, definitively retain their species characteristics in the chimaera” (Le Douarin & Teillet, 1974, p. 163). Yet, while some examples related to patterning and pigmentation of epidermal appendages, as well as hatching behavior continued to be noted (Balaban, 1997; Balaban, Teillet, & Le Douarin, 1988; Sengel, 1990), for the most part because quail and chick are relatively similar, subtle species‐specific differences that may have been induced by donor cells have gone largely undetected. Moreover, determining mechanisms of species‐specific pattern was not really the primary goal of most studies that employed quail‐chick chimeras. In contrast, other experiments using avian chimeras have included domestic duck as a way to identify those patterning mechanisms that generate species‐specific differences (Dhouailly, 1967, 1970; Hampe, 1957; Lwigale & Schneider, 2008; Pautou, 1968; Schneider & Helms, 2003; Sohal, 1976; Sohal et al., 1990; Sohal et al., 1985; Tucker & Lumsden, 2004; Waddington, 1930; Waddington, 1932; Yamashita & Sohal, 1986, Yamashita & Sohal, 1987; Zwilling, 1959). Additional studies on species‐specific size and control of scaling have also included chimeras between quail and emu (Hall et al., 2014), quail and zebra finch (Chen, Balaban, & Jarvis, 2012), and chick and zebra finch (Uygur et al., 2016).
The quail‐duck chimeric transplant system has been especially useful for identifying the molecular and cellular basis for species‐specific aspects of pattern and for illuminating the mechanistic contributions of neural crest cells during tissue interactions that facilitate the structural and functional integration of the craniofacial complex (Figure 1b,c). The system itself combines classical grafting techniques and tools in vertebrate embryology that have already been mentioned (e.g., Andres, 1949; Hamburger, 1942; Harrison, 1917, 1921, 1924, 1929, 1935; Spemann, 1918, 1921, 1938; Spemann & Mangold, 1924; Spemann & Schotte, 1932; Twitty, 1934, 1945; Twitty & Schwind, 1931; Waddington, 1930, 1932; Wagner, 1959) with modern molecular and cellular methods and assays (Ealba & Schneider, 2013; Fish & Schneider, 2014a; Lwigale & Schneider, 2008). In short, presumptive neural crest cells from the midbrain and anterior hindbrain are transplanted from either quail to duck to create chimeric “quck” or from duck to quail to make chimeric “duail” (Ealba & Schneider, 2013; Fish & Schneider, 2014a; Lwigale & Schneider, 2008; Schneider & Helms, 2003) (Figure 1d). In this experimental framework, the ability to exploit chimeras between quail and duck embryos is predicated on three features that distinguish these species of birds.
First, quail and duck embryos and their constituent parts are noticeably different in size and shape, which offers a direct way to resolve if species‐specific features are mediated by donor‐ or host‐derived tissues. Second, quail and duck embryos develop at distinct rates (17 versus 28 days) (Figure 1e), which allows the effects of donor cells on the host to be readily assessed simply by looking for species‐specific changes to the timing of gene expression, tissue differentiation, and/or other events throughout the embryogenesis of the facial primordia (Figure 1f,g). Moreover, by examining the effects of intrinsic rates of maturation (i.e., differences in developmental time) on changes in morphology (i.e., evolutionary differences in size and shape), the quail‐duck chimeric system can help advance the study of the relationship between ontogeny and phylogeny, vis‐à‐vis the cranial neural crest (Schneider, 2018). Third, as is the case for the quail‐chick chimeric system (Le Douarin et al., 1996), there is an antibody (Q¢PN) that recognizes only quail cells, which permanently discriminates donor versus host derivatives (i.e., Q¢PN‐positive versus Q¢PN‐negative). Among other neural crest‐mediated outcomes, detection of this antibody enables species‐specific changes in gene expression patterns to be correlated with the distribution of donor cells in both donor‐ and host‐derived tissues (Figure 1h,i). Similarly, using species‐specific primers to amplify ubiquitously expressed housekeeping genes allows the ratio of quail versus duck cells, as well as any neural crest‐dependent changes to genes of interest, to be quantified on the molecular level using a PCR‐based strategy (Ealba & Schneider, 2013). Therefore, as designed, the quail‐duck chimeric system permits the role of the neural crest (and other cell populations) to be characterized during development and provides a powerful tool for ascertaining precisely when and where morphogenetic events and gene regulatory networks become modified as a means to generate species‐specific pattern in the craniofacial complex.
4. ORIGIN OF SPECIES‐SPECIFIC PATTERN IN THE CRANIOFACIAL INTEGUMENT
Like what has been described earlier for studies on the pigmentation of amphibians, the ability of neural crest cells to mediate species‐specific pattern is most readily apparent in the integument of quail‐duck chimeras (Eames & Schneider, 2005; Schneider, 2005; Schneider & Helms, 2003). Moreover, in many ways the integument can serve as a microcosm for understanding how reciprocal epithelial‐mesenchymal interactions drive the patterning of a broad range of vertebrate organ systems including the limbs, facial primordia, hair, glands, teeth, and bone (Dunlop & Hall, 1995; Fisher, 1987; Francis‐West et al., 1998; Hu et al., 2003; Hughes et al., 2018; Lumsden, 1988; Mitsiadis, Hirsinger, Lendahl, & Goridis, 1998; Pispa & Thesleff, 2003; Richman & Tickle, 1992; Salaun, Salzgeber, & Guenet, 1986; Saunders & Gasseling, 1968; Schneider et al., 1999; Schneider et al., 2001; Sharpe & Ferguson, 1988; Shigetani et al., 2000; Thesleff & Sharpe, 1997; Tonegawa, 1973; Tucker, Al Khamis, & Sharpe, 1998; Wang, Upholt, Sharpe, Kollar, & Mina, 1998; Wedden, 1987). The integument is composed partly of epidermis, which is derived from the non‐neural ectoderm and is stratified into multiple layers (Hamilton, 1965; Romanoff, 1960; Yasui & Hayashi, 1967). In amniotes, the uppermost layer of epidermis generally produces the keratinized components associated with epidermal appendages such as feathers, scales, hair, horns, beaks, and egg teeth (Couly & Le Douarin, 1988; Kingsbury, Allen, & Rotheram, 1953; Lucas & Stettenheim, 1972; Pera et al., 1999; Pispa & Thesleff, 2003; Sawyer, O'Guin, & Knapp, 1984; Yu et al., 2004).
Beneath the epidermis lies the dermis, which in the trunk and posterior regions of the head comes from mesodermal mesenchyme, whereas in the face and aspects of the neck the dermis originates from neural crest mesenchyme (Couly et al., 1992; Matsuoka et al., 2005; Noden, 1978b, 1986b, 1988; Olivera‐Martinez et al., 2000; Olivera‐Martinez, Thelu, & Dhouailly, 2004). As discussed already, neural crest cells also generate the pigment‐producing melanocytes that infiltrate the epidermis and supply color to the skin and epidermal appendages (Bronner‐Fraser, 1994; Cramer, 1991; Hirobe, 1995; Le Douarin & Dupin, 1993; Noden, 1978a; Rawles, 1944, 1948). Patterning and differentiation of the integument relies upon a series of reciprocal signaling interactions between the mesenchyme of the dermis and the epithelium of the epidermis (Dhouailly, 1973, 1975; Lucas & Stettenheim, 1972; Pispa & Thesleff, 2003; Widelitz & Chuong, 1999; Widelitz et al., 1997). Feather development starts when mesenchyme aggregates into a thin layer called “dense dermis” beneath the epithelium (Brotman, 1977; Mayerson & Fallon, 1985; Wessells, 1965). Subsequently, the overlying epithelium thickens into a series of epidermal placodes, the dense dermis forms discrete mesenchymal condensations, the mesenchyme and placodes emerge out of the surface of the integument as feather buds (Figure 2a), and both tissues grow and differentiate into discrete feathers (Olivera‐Martinez, Viallet, Michon, Pearton, & Dhouailly, 2004; Pispa & Thesleff, 2003). Together, feathers form in continuous rows that make up tracts (Lucas & Stettenheim, 1972).
Figure 2.

(a) Cranial feather buds form through interactions between neural crest‐derived dermis and overlying epidermis, which in chimeras are derived from donor and host, respectively. At stage 33, there is little evidence for feather development, but by stage 36, feather buds contain dermal condensations and they begin to rise above the surface of the integument. (b) Quail cranial feather buds are large and widely spaced whereas those of (c) duck are smaller and more closely spaced. (d) In chimeric quck, quail‐like feathers appear at the long bud stage while those derived from the duck host are still short buds. (e) At stage 33, Bmp2 is not expressed in either the dermis or epidermis. However, in (f) chimeric quck at stage 33 Bmp2 is expressed prematurely in donor‐derived dermis as well as in host‐derived epidermis like what is observed three stages later in (g) control quail (and duck). Modified from Schneider (2005), Eames and Schneider (2005), and Fish and Schneider (2014b). (h) The beaks of quail embryos are short and blunt whereas those of (i) duck are long and broad. (j) Transplants of presumptive cranial neural crest cells, which are destined to form the beak, from quail donors to duck hosts produce chimeric “quck” embryos with quail‐like beak size and shape (asterisk). Note that the quail‐like quck has webbed feet (arrow), which is indicative of the duck host. Modified from Schneider (2005). (k) The extent of transformation in chimeras corresponds to the boundary that exists in the skull between those bones and cartilages derived from the cranial neural crest and those formed from mesoderm. Based on a drawing from D. Noden. (l) By stage 35, Meckel's cartilage and the lower jaw are slightly curved in duck as seen in dorsal view. (m) This curved morphology is maintained on the duck host side of chimeric quck whereas Meckel's cartilage and the lower jaw appear to straighten out on the donor side and achieve a larger size like that observed in a (n) quail embryo three stages later at stage 38. Modified from Fish and Schneider (2014b)
The timing of induction as well as species‐specific characteristics such as the location, number, distribution, pigmentation, and size of epidermal appendages like feathers is determined by dermis (Cairns & Saunders, 1954; Dhouailly, 1967, 1970, 1973; Dhouailly & Sawyer, 1984; Eames & Schneider, 2005; Fliniaux, Viallet, & Dhouailly, 2004; Linsenmayer, 1972; Prin & Dhouailly, 2004; Rawles, 1963; Saunders & Gasseling, 1957; Schneider, 2005; Song & Sawyer, 1996; Wessells, 1965). In other instances and depending on the stage of development, the epithelium can provide patterning information on anatomical identity such as specifying whether epidermis produces feathers or scales (Chuong, Chodankar, Widelitz, & Jiang, 2000; Prin & Dhouailly, 2004; Rawles, 1963; Widelitz, Jiang, Lu, & Chuong, 2000) or in controlling late‐stage branching patterns (Harris, Fallon, & Prum, 2002; Yu, Wu, Widelitz, & Chuong, 2002). But both tissues collaborate to form feathers. For instance, when dermis and epidermis from different‐staged wild type and featherless chicken mutants are recombined, the dermis is able to induce epidermal placodes, although this ability goes away quickly without proper epidermal interactions (Viallet et al., 1998). The dominant role of the dermis can be most clearly appreciated using quail‐duck chimeras.
The embryos of Japanese quail (Coturnix coturnix japonica) have large, widely spaced, and pigmented feather buds whereas the embryos of white Pekin duck (Anas platyrhynchos) have relatively smaller, tightly arranged, and un‐pigmented feather buds (Lucas & Stettenheim, 1972; Schneider, 2005) (Figure 2b,c). In quail‐duck chimeras, where quail donor neural crest cells generate the dermis and duck host ectoderm gives rise to the epidermis, cranial feather pattern acquires the identity of the donor species (Eames & Schneider, 2005). Coincident with the distribution of quail donor neural crest‐derived dermis, these chimeric “quck” contain long brown and black quail‐like feathers assembled among short white duck host feather buds (Figure 2d). Conversely, when duck donor neural crest cells are transplanted into quail hosts, the chimeric “duail” embryos have unpigmented duck‐like feathers. Thus, quail‐duck chimeras corroborate the role of the neural crest (and by extension the dermis throughout the integument) as the principal source of species‐specific patterning information for cranial feathers. These results align with data from other tissue recombination experiments in the trunk between duck and chick, which also indicated that the dermis was a source of species‐specific pattern for the feathers (Dhouailly, 1967, 1970). But exactly how does neural crest‐derived dermis impose its species‐specific will on the epidermis?
To accomplish this task, neural crest‐derived dermis executes an autonomous molecular feather program that is not only intrinsic to the donor genome, but also that overrides the epidermal feather program of the host. This phenomenon becomes most readily apparent when examining changes to the spatial and temporal domains of gene expression, and rates of feather bud development in quck and duail chimeras (Eames & Schneider, 2005). In particular, donor neural crest modifies the expression of members and targets of the SHH, BMP, and Delta/Notch pathways, which are well known to regulate normal feather morphogenesis (Ashique, Fu, & Richman, 2002b; Chuong et al., 2001; Chuong, Patel, Lin, Jung, & Widelitz, 2000; Crowe, Henrique, Ish‐Horowicz, & Niswander, 1998; Morgan, Orkin, Noramly, & Perez, 1998; Patel, Makarenkova, & Jung, 1999; Pispa & Thesleff, 2003; Ting‐Berreth & Chuong, 1996; Widelitz et al., 1997; Yu et al., 2002). In chimeras, each of these signaling pathways shows a significant change in the timing and domains of expression in both the dermis and epidermis corresponding to the species and stage of the donor neural crest.
For example, Bmp2 is one of the earliest genes to be expressed wherever the epidermis begins to thicken into placodes along the presumptive feather tracts as well as in the underlying condensations of dermis; likewise, Bmp4 expression is restricted to the dermis as the mesenchyme begins to condense and thereafter (Chuong, Widelitz, Ting‐Berreth, & Jiang, 1996; Jung et al., 1998; Nohno et al., 1995; Noramly & Morgan, 1998; Scaal et al., 2002; Widelitz et al., 1997). In chimeric quck, the timing of Bmp2 and Bmp4 expression is accelerated by three stages not only in the faster‐developing quail donor‐derived dermis, but also Bmp2 is expressed prematurely in the normally slower‐developing duck host‐derived epidermis (Figure 2e–g). The same is observed for all other genes examined, which become spatially and temporally regulated by donor dermis. In other words, the dermis specifies the pattern through which epidermis forms feather buds by adhering to the timetable of the donor species and by defining the expression domains of members and targets of the SHH, BMP, and Delta/Notch pathways (Eames & Schneider, 2005).
This remarkable capacity holds true in reverse as evidenced by duail chimeras where slower‐developing duck dermis acts out its feather program on a delayed timetable relative to quail host epidermis and produces duck‐like feathers. Extrapolating these results, we would predict that the dermis (of both neural crest and mesodermal origin) most likely regulates in a species‐specific manner many other genes known to function during feather morphogenesis such as members and targets of the FGF, Epidermal Growth Factor, and WNT pathways (Atit, Conlon, & Niswander, 2003; Chang et al., 2004; Chodankar et al., 2003; Mandler & Neubuser, 2004; Noji et al., 1993; Noramly, Freeman, & Morgan, 1999; Olivera‐Martinez, Thelu, Teillet, & Dhouailly, 2001; Rouzankina, Abate‐Shen, & Niswander, 2004; Song, Wang, & Goetinck, 1996; Song, Lee, & Goetinck, 2004; Tanda, Ohuchi, Yoshioka, Noji, & Nohno, 1995; Tao et al., 2002; Widelitz, Jiang, Chen, Stott, & Chuong, 1999; Widelitz, Jiang, Noveen, Chen, & Chuong, 1996). Thus, neural crest cells exert their species‐specific will by presiding at the top of hierarchical conversations with their neighbors (e.g., epidermis), autonomously implementing their own molecular and cellular agendas, and dictating the terms of morphogenetic events in space and time.
5. ORIGIN OF SPECIES‐SPECIFIC PATTERN IN THE BEAK
As is the case for the integument, quail‐duck transplants likewise demonstrate that neural crest cells provide species‐specific information for patterning the beak and underlying jaw skeleton, which differ substantially between quail and duck in conjunction with their highly specialized modes of feeding (Figure 2h,i). Quail neural crest cells destined to form the beak skeleton make quail‐like beaks on duck hosts and reciprocal transplants of duck neural crest cells generate duck‐like bills on quail hosts (Schneider & Helms, 2003) (Figure 2j,k). Equivalent species‐specific transformations are observed when neural crest cells fated to become cartilages in the jaw joint are transplanted between quail and duck (Tucker & Lumsden, 2004). Overall, these studies using quail‐duck chimeras reinforce the key role for neural crest in establishing species‐specific morphology of the beak and jaw apparatus.
However, such results are not really surprising given that the jaw skeleton is derived entirely from the neural crest (Couly et al., 1993; Köntges & Lumsden, 1996; Le Lièvre, 1978; Le Lièvre & Le Douarin, 1975; Noden, 1978b), and also because the long history of chimeric grafting experiments discussed earlier had already revealed the special species‐specific properties of this lineage. But gaining the ability to distinguish between beak tissues that arise from the donor versus the host with a high degree of certainty, as well as possessing tools to assay for donor‐mediated changes in gene expression, is what sets this modern chimeric strategy apart from earlier studies (Ealba & Schneider, 2013; Fish & Schneider, 2014a; Lwigale & Schneider, 2008; Schneider & Helms, 2003). A first critical insight in this regard came from examining changes to beak tissues derived from the host. For instance, at the tip of their bill, duck have an egg tooth that is a flat epidermal nail, whereas quail develop an egg tooth that is a conical protrusion of hard keratin (Lucas & Stettenheim, 1972). The quck egg tooth, despite arising entirely from non‐transplanted duck host epidermis, resembles that found in quail. Similarly, the duail egg tooth looks like that of the duck. This clear transfer of patterning information from donor neural crest to non‐neural crest host‐derived tissues reveals that the transformation of the beak in chimeras is more‐or‐less comprehensive and helps explain how the beak can become modified as an integrated morphological unit in its entirety during the course of evolution (Schneider, 2005).
A second important discovery arose after analyzing genes that are known to pattern the face and that also show well‐defined periods of expression during development. Because quail and duck have distinct rates of maturation, the initiation and cessation of expression of such genes differ in absolute time. For example, twenty‐four hours after surgery, control quail embryos express the transcription factors Barx1 and Msx1 in neural crest‐derived mesenchyme of the developing beak primordia, but control duck embryos do not yet express these genes because they require a longer period of time to reach an equivalent stage. Correspondingly and quite strikingly in quck chimeras, these genes are expressed in mesenchyme derived from the quail donor but not from the duck host. Similarly, 48 hr after surgery, control quail express Shh but not Pax6 in facial ectoderm whereas control duck express Pax6 but not Shh. In quck chimeras, Shh is found in duck host facial ectoderm but Pax6 is not, which is the pattern observed in quails. As already discussed, Shh expression in the facial ectoderm for instance, is not only mediated by the neural crest (Hu & Marcucio, 2012; Schneider et al., 2001), but also plays a critical role in specifying the axial orientation and maintaining the outgrowth of the facial skeleton (Abzhanov & Tabin, 2004; Ahlgren & Bronner‐Fraser, 1999; Chong et al., 2012; Delloye‐Bourgeois et al., 2014; Helms et al., 1997; Hu & Helms, 1999; Hu & Marcucio, 2009a; Hu, Young, Li, et al., 2015; Jeong, Mao, Tenzen, Kottmann, & McMahon, 2004; Lan & Jiang, 2009; Young et al., 2010). Thus, such temporal shifts in the onsets and offsets of gene expression supply stark evidence that quail neural crest cells produce quail‐like beaks on duck by sustaining their own molecular programs and by modulating the spatial and temporal patterns of gene expression in non‐neural crest host tissues such as the adjacent epithelium.
Again, as noted by Raven (1933) and conveyed by Hörstadius (1950), part of the special ability of neural crest to regulate species‐specific pattern likely arises “in connection with its movements, as a carrier of inductive influences” (p. 93). Simple parameters such as the amount and distribution of donor neural crest cells throughout the facial primordia appears to modulate gene expression in host epithelium in a dose‐dependent manner (Ealba & Schneider, 2013; Merrill et al., 2008; Schneider & Helms, 2003). Once a certain threshold is reached (Woronowicz et al., 2018), neural crest cells ultimately endow structures with species‐specific size and shape presumably because their “inductive influences” are mediated at the population level. This scenario is also substantiated by our observation that significant differences exist in the number of jaw precursors that migrate into the mandibular primordia of duck versus quail (Fish & Schneider, 2014c; Fish et al., 2014). During neurulation, duck generate about 15% more pre‐migratory neural crest cells at the levels of the midbrain and rostral hindbrain, and these are the cells that will ultimately enable them to build their long bills. Only a few stages later, duck have twice as many cells in their mandibular primordia as do quail due to specific‐specific variation in cell proliferation dynamics and cell cycle length.
Cell cycle length in duck mandibular mesenchyme is longer (13.5 hr) than in quail (11 hr), and this might seem counterintuitive given that duck make more cells, but when the total duration of each embryonic stage during this developmental window is considered in terms of absolute time (i.e., 45 hr for duck versus 32 hr for quail), then duck cells wind up proliferating more in total than those of quail. Therefore, by sustaining their slower intrinsic maturation rate over a longer period of time, duck implement a cellular mechanism that progressively increases jaw size during development (Fish & Schneider, 2014b; Fish et al., 2014; Schneider, 2015, 2018). In this way, duck seem to rely on time as one means to control size, which supports prior observations in birds on the correlation between innate rates of growth and body size (Ricklefs & Starck, 1998; Starck, 1989; Starck & Ricklefs, 1998).
6. ORIGIN OF SPECIES‐SPECIFIC PATTERN IN THE CARTILAGINOUS SKELETON
To explain more precisely how differences in the rates of development and the numbers of neural crest cells that get allocated to the mandibular primordia become species‐specific determinants of size and shape, we focused on the differentiation and growth of Meckel's cartilage in the lower jaw skeleton (Eames & Schneider, 2008). Meckel's cartilage develops from neural crest mesenchyme into a cylindrical rod that rarely ever ossifies except in the proximal‐most region(De Beer, 1937; Eames, Sharpe, & Helms, 2004; Ekanayake & Hall, 1994; Helms & Schneider, 2003; Kavumpurath & Hall, 1990; Noden, 1978b). During cartilage formation, pre‐chondrogenic cells first undergo condensation and then begin overt differentiation, where they secrete extracellular matrix (Eames, de la Fuente, & Helms, 2003; Hall, 2005). There is a well‐documented relationship between condensation size and skeletal size (Hall & Miyake, 1992, 1995, 2000; Miyake et al., 1996; Smith & Schneider, 1998), and from the earliest stages of chondrogenesis, we observe smaller condensations in quail relative to duck (Eames & Schneider, 2008). When we transplant presumptive neural crest from quail embryos into stage‐matched duck we do so unilaterally (Figure 1d), which allows these quail donor neural crest cells to fill one side of the duck host mandible and enables us to compare the development of donor quail‐derived versus host duck‐derived Meckel's cartilage in the same chimeric quck. While the sequential stages of chondrogenesis are comparable in quail and duck, in quck chimeras, we find that quail donor neural crest cells make smaller condensations and differentiate into cartilage on a faster timetable (i.e., three stages ahead of the duck).
Accompanying these changes in quck chimeras is the premature expression of chondrogenic genes by quail donor cells relative to duck host cells on the contralateral side. For example, Sox9, which is an early molecular marker of chondrogenic condensations (Eames et al., 2003; Eames et al., 2004; Healy, Uwanogho, & Sharpe, 1996; Zhao, Eberspaecher, Lefebvre, & De Crombrugghe, 1997), and Col2a1, which is directly regulated by Sox9 (Bell et al., 1997) are both upregulated coincident with the presence of quail donor neural crest mesenchyme. Additionally, we find that FGF signaling, which operates upstream of Sox9 and chondrogenesis (Bobick, Thornhill, & Kulyk, 2007; De Crombrugghe et al., 2000; Eames et al., 2004; Govindarajan & Overbeek, 2006; Healy, Uwanogho, & Sharpe, 1999; Murakami, Kan, McKeehan, & de Crombrugghe, 2000; Petiot, Ferretti, Copp, & Chan, 2002) is also regulated by neural crest mesenchyme as evidenced by analyzing expression of the ligands Fgf4 and Fgf8, and the receptor Fgfr2. While FGF ligands are known to be expressed continuously in mandibular epithelium from the earliest embryonic stages onward (Havens, Rodgers, & Mina, 2006; Mina et al., 2002; Shigetani et al., 2000; Wall & Hogan, 1995), we find that in chimeras the receptor Fgfr2 is expressed three stages earlier by quail donor neural crest mesenchyme. If FGF signaling is blocked during this discrete temporal window when Fgfr2 becomes activated, then Meckel's cartilage fails to form.
Ultimately, by exerting control over the timing of FGF signaling and the expression of downstream targets such as Sox9 and Col2a1, neural crest mesenchyme likely provides cues on the molecular level that impart Meckel's cartilage with species‐specific size and shape. Such a conclusion is also based on our observation that on the morphological level Meckel's cartilage displays obvious stage‐specific and species‐specific differences in size and shape throughout development in quail and duck, and that these differences are maintained by quail donor neural crest mesenchyme in quck chimeras (Figure 2l–n). For example, Meckel's initially forms in both duck and quail as a slightly curved cartilage that becomes more S‐shaped. Shortly thereafter, Meckel's in duck remains curved while Meckel's in quail straightens out. Meckel's continues to grow in both quail and duck, but increasingly gets larger in duck. In quck chimeras, quail donor neural crest maintains its faster maturation rate within the relatively slower duck host, and the differentiation of Meckel's cartilage gets accelerated by approximately three stages on the donor side. Furthermore, the size and shape of Meckel's cartilage on the donor side becomes consistently more quail‐like compared with that observed on the contralateral duck host side.
So, while surrounding endodermal and ectodermal epithelia seem to define “where” cartilage condensations form along an axis, which is most likely equivalent between quail and duck, our chimeric transplant experiments reveal that neural crest defines “when” and “what” by responding through intrinsic programs that control both stage‐specific and species‐specific size and shape. Significantly, this ability to keep track of stage‐specific and species‐specific size and shape simultaneously indicates that the neural crest cells themselves can function as a potent mechanism linking ontogeny and phylogeny. Such were the predictions made by proponents of heterochrony who argued that changes in the timing of developmental events and/or rates of growth had direct implications for the evolution of size and shape (Alberch et al., 1979; De Beer, 1930; Foster & Kaesler, 1988; Gould, 1977; Hall, 1984; Klingenberg & Spence, 1993; McKinney, 1988; Raff, 1996; Roth, 1984; Russell, 1916; Schneider, 2018). In this regard, faster‐developing quail donor neural crest mesenchyme not only induces a heterochrony by altering the rates of growth in chimeras, but also, the presence of these cells appears to introduce shifts in the relative onsets, cessations, or durations of molecular and cellular events, which is an additional process through which changes in time can affect size and shape (Smith, 2001, 2002, 2003), especially in the context of reciprocal epithelial–mesenchymal interactions underlying skeletal evolution (Smith & Hall, 1990). Our transplants in birds with even larger disparities in growth rates like quail and emu (i.e., 17 versus 58 days from fertilization to hatching), which are separated by about seven embryonic stages during chondrogenesis, reveal that there are very few developmental constraints that prevent the host from supporting the execution of neural crest mesenchyme‐dependent programs for skeletogenesis (Hall et al., 2014).
7. ORIGIN OF SPECIES‐SPECIFIC PATTERN IN THE BONY SKELETON
Akin to what we have found with cartilage, neural crest mesenchyme likewise communicates species‐specific information on size and shape to bone in the jaws and facial skeleton by establishing the timing of major events during osteogenesis. In quck chimeras, quail donor neural crest mesenchyme upholds its faster timetable for maturation and autonomously executes molecular and cellular programs that promote and orchestrate each individual step of osteogenesis including the induction, proliferation, differentiation, mineralization, and remodeling of bone (Ealba et al., 2015; Hall et al., 2014; Merrill et al., 2008). Such a capacity by neural crest mesenchyme to function as a developmental timekeeper supports earlier theoretical predictions (Alberch, 1982a; Oster & Alberch, 1982; Oster et al., 1988) about how quantitative changes to parameters within ontogenetic systems can drive morphological evolution (Schneider, 2018). For example, genetic or epigenetic modifications to “biochemical, cell–cell, or tissue interactions” (Alberch, 1985, p. 50) can in turn alter “rates of diffusion, mitotic rate, cell adhesion, etc.” (Alberch, 1989, p. 27), which can then cause evolutionary changes in size and shape. Similarly, our work reveals that such quantitative changes to cellular parameters like neural crest‐mediated differences between quail and duck in the number of progenitors, rate of proliferation, length of the cell cycle, and timing of differentiation leads to morphological outcomes in the bony skeleton that are species‐specific.
Along these lines and as a case in point, we find that neural crest mesenchyme establishes the timing of osteogenesis in the jaw by regulating cell cycle progression (Hall et al., 2014). Seemingly, neural crest mesenchyme controls the cell cycle through stage‐ and species‐specific expression of cyclin and cyclin‐dependent kinase inhibitors (CKI) including p27 (cdkn1b), which is a CKI that can decrease proliferation in differentiating osteoblasts; Cyclin E (Ccne1), which is needed for G1/S phase transition; and Cyclin B1 (Ccnb1), which is essential for G2/M phase transition (Coats, Flanagan, Nourse, & Roberts, 1996; Drissi et al., 1999; Zavitz & Zipursky, 1997). We find species‐specific differences in the expression and post‐translational processing of these cell cycle regulators, which we predict could permit birds like quail to shorten their period of mesenchymal proliferation and lead to faster‐differentiating and smaller beak skeletons. For example, in quail and on the donor side of quck, we find that p27 is up‐regulated relative to that observed in duck. Previous experiments have demonstrated that p27 is associated with size, including p27‐deficient mice, which are much larger than their wild‐type littermates yet have no obvious defects in their skeletons (Drissi et al., 1999). Likewise, the frontonasal process of duck has lower p27 levels than that observed in chick (Powder et al., 2012), and furthermore the mandibular primordia shows tissue‐specific post‐translational regulation of p27, like what has been reported in other systems (Hirano et al., 2001; Zhang, Bergamaschi, Jin, & Lu, 2005). Therefore, changing p27 levels may affect tissue‐ and species‐specific size and/or total growth. This direct connection between the regulation of cell cycle progression and the timing of events throughout bone development offers a mechanism through which neural crest mesenchyme might be able to generate changes in skeletal size and shape during evolution.
The potential for such a mechanism is further supported by experiments in which we can mimic the results observed in quck chimeras by prematurely inducing cell cycle exit. In this scenario, we find that the molecular program for osteogenesis becomes accelerated (Hall et al., 2014). Specifically, we observe early and elevated expression of genes such as Runx2, which is known to be a “master regulator” of bone formation that can direct osteoblast differentiation, influence skeletal size, and control the timing of mineralization (Ducy et al., 1999; Ducy, Zhang, Geoffroy, Ridall, & Karsenty, 1997; Eames et al., 2004; Galindo et al., 2005; Komori et al., 1997; Maeno et al., 2011; Otto et al., 1997; Pratap et al., 2003; Thomas et al., 2004). Neural crest mesenchyme in the mandibular primordia normally expresses Runx2 during a tightly controlled temporal window and at well‐defined levels (Eames et al., 2004; Merrill et al., 2008), but by over‐expressing Runx2 prematurely and at higher levels in chick embryos, we can markedly reduce the size of the beak skeleton (Hall et al., 2014). This in effect, reflects the relationship normally observed between endogenous Runx2 levels and species‐specific beak size. In fact, when the quail jaw skeleton begins to mineralize, its Runx2 levels are more than twice that found in duck. Along these lines, other studies have hypothesized that there is a mechanistic link between predicted differential levels of Runx2 expression (based on ratios of tandem repeats in DNA) and the length of the face among dogs and other mammals (Fondon and Garner, 2004; Pointer et al., 2012; Sears, Goswami, Flynn, & Niswander, 2007).
That neural crest mesenchyme regulates the timing and levels of Runx2 expression, and that this in turn has a direct effect on skeletal size, fulfills predictions made more than 75 years ago by embryologists such as Huxley (1932) and Goldschmidt (1938, 1940) with regard to the existence of genes that establish the time and rate of development (Schneider, 2018). Along similar lines, De Beer (1954) argued that, “by acting at different rates, the genes can alter the time at which certain structures appear” (p. 20). Data from in vitro experiments also help explain how Runx2 could function in this capacity whereby Runx2 expression both depends upon and regulates cell cycle progression through mechanisms such as the repression of rRNA synthesis and the up‐regulation of p27 (Galindo et al., 2005; Pratap et al., 2003; Thomas et al., 2004; Young et al., 2007). Collectively, these observations indicate that neural crest mesenchyme establishes species‐specific size and shape in the bony skeleton by mediating the timing of the transition from proliferation to differentiation and by modulating the expression levels of osteogenic transcription factors such as Runx2.
Neural crest mesenchyme also appears to exert control over species‐specific size and shape during osteogenesis upstream of Runx2 by governing the temporal and spatial expression of members and targets of the BMP pathway (Merrill et al., 2008). BMP ligands can induce bone formation both embryonically (Kingsley et al., 1992; Luo et al., 1995; Solloway et al., 1998) and postnatally (Urist, 1965; Wang et al., 1990; Wozney et al., 1988). During jaw development, Bmp2, Bmp4, and Bmp7, as well as their receptors (Bmpr1a, Bmpr1b, and Alk2) are expressed in mandibular mesenchyme and/or epithelium (Ashique et al., 2002a; Bennett, Hunt, & Thorogood, 1995; Francis‐West, Tatla, & Brickell, 1994; Wall & Hogan, 1995), and they play critical roles during osteogenesis (Ashique et al., 2002b; Francis‐West et al., 1998; Wang et al., 1998). For example, BMP4 helps neural crest mesenchyme differentiate into bone (Abzhanov, Rodda, McMahon, & Tabin, 2007) and the lower jaw fails to form when Bmp4 is conditionally eliminated from mandibular epithelium (Liu et al., 2005). BMP signaling regulates osteogenesis via a highly conserved pathway (Derynck, Piek, Schneider, Choy, & Alliston, 2008; Heldin, Miyazono, & ten Dijke, 1997; Kawabata, Imamura, & Miyazono, 1998; Massague & Wotton, 2000) involving Smad activation, which in turn affects Runx2 expression (Ducy, 2000; Ducy et al., 1997; Kang, Alliston, Delston, & Derynck, 2005; Karsenty et al., 1999; Komori et al., 1997) and mandibular osteogenesis (Otto et al., 1997). Moreover, physical interactions between SMAD proteins and Runx2 drive osteoblast‐specific gene expression (Alliston, Choy, Ducy, Karsenty, & Derynck, 2001; Ito et al., 2002; Lee et al., 2000). Other targets of BMP signaling including Msx1 (Tribulo, Aybar, Nguyen, Mullins, & Mayor, 2003) play a role during the epithelial–mesenchymal interactions of the mandible (Bei & Maas, 1998; Chen & Struhl, 1996; Han et al., 2007), are neural crest‐mediated (Schneider & Helms, 2003), and affect bone formation (Roybal et al., 2010; Satokata & Maas, 1994).
As part of its osteo‐inductive role, BMP signaling likely shapes the avian beak by creating domains of differential growth within the mesenchyme. For instance, distinct domains of Bmp4 expression in the frontonasal primordium contribute to beak width and depth among birds including Darwin's finches, cockatiels, chicks, and ducks (Abzhanov et al., 2004; Schneider, 2007; Wu et al., 2006, 2004). Likewise, over‐expressing Bmp4 in cichlid fish that usually form elongated jaws, shortens and widens the jaw and in effect phenocopies features that are coupled with the evolution of distinct feeding strategies (Albertson, Streelman, Kocher, & Yelick, 2005). This salient ability of neural crest mesenchyme to control the timing of osteogenesis by autonomously executing molecular programs involving BMP signaling as well as transcriptional targets such as Msx1 and Runx2, likely serves as a key developmental mechanism facilitating the evolution of species‐specific size and shape in the craniofacial skeleton.
8. ORIGIN OF SPECIES‐SPECIFIC PATTERN DURING BONE RESORPTION
While much of the work we have performed has demonstrated that neural crest mesenchyme conveys species‐specific size and shape to the craniofacial skeleton by regulating molecular and cellular programs for the induction and deposition of cartilage and bone, we have also discovered that a previously underappreciated but potentially just as important mechanism affecting species‐specific size and shape lies in the ability of neural crest mesenchyme to direct the process of bone resorption (Ealba et al., 2015; Schneider, 2015). Usually, bone resorption is tied to bone deposition as a metabolic function for maintaining homeostasis in the adult skeleton (Buckwalter, Glimcher, Cooper, & Recker, 1996; Filvaroff & Derynck, 1998; Hall, 2005; Nguyen, Tang, Nguyen, & Alliston, 2013; O'Brien et al., 2008; Teitelbaum, 2000; Teitelbaum, Tondravi, & Ross, 1997). In contrast, little is known about the role of resorption during skeletal patterning in embryos, except for a few hypotheses about the effects of differential fields of bone resorption on the size and shape of the developing human jaw skeleton (Enlow, Moyers, & Merow, 1975; Moore, 1981; Radlanski & Klarkowski, 2001; Radlanski, Renz, Lajvardi, & Schneider, 2004) and recent work on the remodeling of Meckel's cartilage by chondroclasts in mammals (Anthwal, Urban, Luo, Sears, & Tucker, 2017).
When we assay for molecular and enzymatic markers of bone resorption we observe significantly higher levels and distinct spatial domains in quail versus duck that correlate with species‐specific differences in beak size and shape. There are two populations of cells that resorb bone in the craniofacial skeleton. Osteoclasts arise from the mesodermal hematopoietic lineage (Couly, Coltey, Eichmann, & Le Douarin, 1995; Couly et al., 1992; Jotereau & Le Douarin, 1978; Kahn et al., 2009), and osteocytes (Akil et al., 2014; Belanger, 1969; Fowler et al., 2017; Jauregui et al., 2016; O'Brien et al., 2008; Qing et al., 2012; Tang, Herber, Ho, & Alliston, 2012; Xiong & O'Brien, 2012; Xiong et al., 2014) are derived entirely from neural crest mesenchyme (Helms & Schneider, 2003; Le Lièvre, 1978; Noden, 1978b). Therefore, in our quail‐duck chimeras, all osteoclasts form exclusively from the host mesoderm whereas all osteocytes come from the donor neural crest.
Osteoclasts and osteocytes secrete tartrate‐resistant acid phosphatase (TRAP) when they are actively resorbing bone (Minkin, 1982; Qing et al., 2012; Tang et al., 2012). Also, osteoclasts express Matrix Metalloproteinase 9 (Mmp9) (Engsig et al., 2000; Reponen, Sahlberg, Munaut, Thesleff, & Tryggvason, 1994) and osteocytes express Mmp13 (Behonick et al., 2007; Johansson et al., 1997; Sasano et al., 2002). Accordingly, in the lower jaw of chimeric quck, Mmp9 is expressed by duck host‐derived osteoclasts while Mmp13 is expressed by quail donor‐derived osteocytes. We find that control quail express substantially higher levels of TRAP, Mmp9, and Mmp13 than do duck, suggesting that increased resorption may relate to shorter beaks (Ealba et al., 2015). Similarly, chimeric quck have greatly elevated TRAP, Mmp9, and Mmp13 expression in association with the donor‐mediated transformation into quail‐like beaks, which implies that quail donor neural crest mesenchyme executes an autonomous species‐specific program that controls bone resorption by its own derivatives (i.e., osteoclasts) as well as by those of the duck host (i.e., osteoclasts). This provides another neural crest‐dependent mechanism that contributes to the shorter beaks of quail and chimeric quck. In support of this conclusion, we find that when we experimentally apply small molecules, pharmacologic agents, or recombinant proteins to inhibit resorption we can lengthen the beak, whereas if we activate resorption we can shorten the beak.
By extension then, our work reveals that beak size in birds is inversely proportional to levels of bone resorption, and that such levels are established by neural crest mesenchyme. Prior work on Darwin's finches and other species, have correspondingly argued that the calcium binding protein Calmodulin is a key determinant of beak length (Abzhanov et al., 2006; Gunter, Koppermann, & Meyer, 2014; Schneider, 2007). Quite interestingly, Calmodulin is known to regulate osteoclasts and osteocytes (Choi, Ann, et al., 2013; Choi, Choi, Oh, & Lee, 2013; Seales, Micoli, & McDonald, 2006; Zayzafoon, 2006), calcium signaling is important for bone resorption (Hwang & Putney, 2011; Kajiya, 2012; Xia & Ferrier, 1996; Xiong et al., 2014), and this pathway can affect jaw size (Gunter et al., 2014; Parsons & Albertson, 2009). Thus, taken together, all of these studies imply that bone resorption may function like a rheostat during skeletal evolution, and in this capacity might be especially tuned to the availability of dietary calcium, to the effects of calcium‐dependent hormones, and to gradients of calcium signaling within the beak primordia (Schneider, 2007, 2018). Such spatial and temporal regulation of resorption by neural crest mesenchyme likely acts as a determinant of species‐specific size and shape by creating local zones of resorption in quail versus duck that more‐or‐less sculpt the bone and inhibit or promote directional growth.
Overall, we have discovered that neural crest mesenchyme wields precise spatial and temporal control over each step of osteogenesis including the induction, differentiation, deposition, mineralization, and resorption of bone (Ealba et al., 2015; Eames & Schneider, 2008; Hall et al., 2014; Merrill et al., 2008; Schneider & Helms, 2003). This control appears to be integrated and implemented on multiple interacting genetic and epigenetic levels (Alberch, 1982a) such that neural crest can orchestrate species‐specific programs for skeletal size and shape throughout development and serve as a source for morphological variation in the craniofacial complex during evolution. Most likely, the mechanisms that distinguish the species‐specific programs of quail from those of duck are multifactorial and based on intrinsic and emergent differences in genome organization, cis‐regulation of individual genes, epigenetic activities of non‐coding RNA at the transcriptional and post‐transcriptional level, connectivity at nodes within gene regulatory networks, biochemical interactions among gene products (e.g., enzymes and other proteins), post‐translational modification of proteins, diffusion‐reaction gradients and thresholds that affect induction and developmental potential, properties and movements of cells, and/or physical and signaling interactions among tissues (Schneider, 2018). Changes at any of these hierarchical levels of organization during development could undoubtedly be a means to affect species‐specific morphology. By investigating such changes in quail versus duck we aspire to rise to the challenge set forth by Alberch et al. (1979) when they expressed their hope that their “attempts to construct a quantitative theory will stimulate others to delve more deeply below the level of pure phenomenology and come to grips with the central issue underlying evolutionary diversification of size and shape—that is, the morphogenetic unfolding of genetic programs in ontogeny and their alteration in the course of phyletic evolution” (p. 297).
9. ORIGIN OF SPECIES‐SPECIFIC PATTERN IN THE JAW MUSCULATURE
In addition to cartilage and bone, cranial neural crest mesenchyme also produces skeletal and muscle connective tissues such as tendons, ligaments, fascia, and epi‐ and endomysia (Couly et al., 1993; Köntges & Lumsden, 1996; Le Lièvre & Le Douarin, 1975; Noden, 1978b, 1983; Noden & Schneider, 2006). Head and jaw muscles however, form from mesodermal mesenchyme (Couly et al., 1992; Evans & Noden, 2006; Noden, 1983; Noden & Francis‐West, 2006; Noden & Trainor, 2005; Scaal & Marcelle, 2018; Wachtler & Jacob, 1986). Given these differences in embryonic origin, the quail‐duck chimeric system provides a means to examine the extent to which donor neural crest mesenchyme regulates species‐specific pattern in host muscle (Fish & Schneider, 2014b; Solem et al., 2011; Tokita & Schneider, 2009). A broad range of prior investigations have revealed that cranial neural crest mesenchyme plays a critical role during muscle development. In particular, the early migration, differentiation, and spatial patterning of myogenic mesenchyme in the head relies on interactions with surrounding muscle connective tissues (Borue & Noden, 2004; Ericsson, Cerny, Falck, & Olsson, 2004; Francis‐West et al., 2003; Grammatopoulos et al., 2000; Grenier, Teillet, Grifone, Kelly, & Duprez, 2009; Hall, 1950; Knight, Mebus, & Roehl, 2008; Knight & Schilling, 2006; Köntges & Lumsden, 1996; McGurk et al., 2017; Nassari, Duprez, & Fournier‐Thibault, 2017; Noden, 1983, 1986a, 1988; Noden, Marcucio, Borycki, & Emerson, 1999; Noden & Schneider, 2006; Noden & Trainor, 2005; Olsson, Falck, Lopez, Cobb, & Hanken, 2001; Pasqualetti et al., 2000; Rinon et al., 2007; Schilling et al., 1996; Schnorrer & Dickson, 2004; Subramanian & Schilling, 2015; Sugii et al., 2017; Tokita, Nakayama, Schneider, & Agata, 2013; Trainor & Krumlauf, 2000; Trainor, Sobieszczuk, Wilkinson, & Krumlauf, 2002; Tzahor et al., 2003).
With regard to the species‐specific patterning of the muscles, a clear example of the effects of neural crest mesenchyme can be seen in the resultant jaw complex of quail‐duck chimeras. Quail and duck have highly specialized jaw morphologies associated with their species‐specific modes of feeding. Quail use their sharp, pointed beaks like forceps to peck at seed on the ground whereas duck use a suction‐pump mechanism and apply leverage across their long, broad bills to strain water and sediment. Such differences in feeding behavior are mirrored in the size, shape, and attachment sites of their jaw muscles as well as in their jaw kinetics and mechanics (Bout & Zweers, 2001; Dawson, Metzger, Baier, & Brainerd, 2011; Fisher, 1955; Soni, 1979; Zweers, 1974; Zweers, Gerritsen, & Kranenburg‐Voogd, 1977; Zweers, Kunz, & Mos, 1977) (Figure 3a–d). In quail‐duck chimeras we find that quail donor neural crest mesenchyme imparts quail‐like pattern on the duck host mesoderm‐derived jaw muscles (Solem et al., 2011; Tokita & Schneider, 2009). These transformations are not only species‐specific but also stage‐specific, in that the muscle anatomy on the donor side is more like that found in control quail three stages later. For example, in duck, the mandibular adductor muscle inserts on the lateral side of the mandible (Zweers, 1974; Zweers, Kunz, et al., 1977), whereas in quail, the same muscle inserts on the dorsal surface of the mandible (Baumel, 1993; Van den Heuvel, 1992). In quck chimeras, these species‐specific differences in the shape, orientation, and insertion point of the mandibular adductor muscle are patterned by neural crest mesenchyme. In particular, we find that quail donor neural crest mesenchyme is distributed throughout the skeletal and muscular connective tissues that surround the developing host muscle precursors and causes the duck host jaw muscles to elongate rostrally and attach dorsally as in quail (Solem et al., 2011; Tokita & Schneider, 2009; Woronowicz et al., 2018) (Figure 3e–j).
Figure 3.

(a) Adult quail and (b) duck heads in lateral view showing the mandibular adductor muscles that close the jaw (yellow dashed lines). The duck mandibular adductor inserts more laterally and proximally, and integrates into a pronounced coronoid process along the side of the lower jaw (black arrow) whereas the quail mandibular adductor inserts more dorsally and distally. Modified from Tokita and Schneider (2009). (c) Lateral view of cleared and stained quail and (d) duck embryos at stage 41. Cartilage is blue and bone is red. In duck, the coronoid process forms as a secondary cartilage (white arrow) on the lateral side of the surangular bone along the lower jaw skeleton. A corresponding cartilage is absent in quail (white asterisk). (e) In quck chimeras, jaw muscles come from the duck host whereas skeletal and connective tissues come from quail donor neural crest. Jaw anatomy on the donor side is transformed to something more like that found in quail. The mandibular adductor is narrower, inserts dorsally along the surangular, and as in quail, does not contain secondary cartilage. (f) In contrast, on the host side of quck, the mandibular adductor muscle is broader and inserts laterally on the surangular bone, and secondary cartilage forms within the insertion (white arrow) like that normally observed in duck. (g) Trichrome‐stained section of duck in lateral view showing the mandibular adductor muscle at its insertion (black arrow and stained purple), which is wide and triangular shaped along the surangular bone (stained blue). (h) On the host side of quck, the insertion looks the same as in duck whereas (i) on the donor side of quck (white asterisk) and in (j) quail the insertion is relatively thin. (k) Coincident with the eventual formation of secondary cartilage in duck but not quail, the chondrogenic transcription factor Sox9 is expressed highly in the insertion (white arrow) of duck and (l) on the host side of quck, but not in the insertion (m) on the donor side of quck (white asterisk) or (n) in quail. Modified from Solem et al. (2011)
Preceding these dramatic morphological transformations are changes to the spatial and temporal patterns of gene expression for muscle connective tissue markers such as Tcf4, which is a transcription factor that functions downstream of the WNT pathway, and which plays an essential role during muscle development (Anakwe et al., 2003; Bonafede, Kohler, Rodriguez‐Niedenfuhr, & Brand‐Saberi, 2006; Mathew et al., 2011; Miller et al., 2007) including ordaining the spatial pattern of limb muscles (Kardon, Harfe, & Tabin, 2003). Another transcription factor, Scleraxis (Scx), which is expressed by and required for the differentiation of tendon and ligament progenitors in the trunk and head (Berthet et al., 2013; Blitz, Sharir, Akiyama, & Zelzer, 2013; Blitz et al., 2009; Brent, Braun, & Tabin, 2005; Cserjesi et al., 1995; Grenier et al., 2009; Murchison et al., 2007; Pryce, Brent, Murchison, Tabin, & Schweitzer, 2007; Schweitzer et al., 2001; Shukunami, Takimoto, Oro, & Hiraki, 2006), is also up‐regulated on the donor side of quck chimeras in quail‐like patterns. Thus, these neural crest‐mediated changes in gene expression appear to direct the species‐specific shape and attachment sites of the jaw muscles. This in turn, alters the functional morphology and the associated mechanical force environment such that the duck host side of quck chimeras induces Sox9 expression and a robust secondary cartilage at the insertion of the mandibular adductor muscle on the coronoid process of the mandible, whereas the quail donor side fails to express Sox9 or form a secondary cartilage just like in control quail (Solem et al., 2011; Woronowicz et al., 2018) (Figure 3k–n).
Interestingly, donor neural crest mesenchyme does not seem to alter the early programs for host myogenic specification or muscle differentiation, which based on our expression analyses of early muscle markers and structural proteins continue to follow the timetable of the host (Tokita & Schneider, 2009). Such a result is not entirely surprising given that muscle is an ancient mesodermal lineage that evolved long before the appearance of the cranial neural crest and therefore, likely executes aspects of its own developmental program rather autonomously. This also appears to be the case for the mesodermally‐derived blood vessels of the host, which are similarly unaffected in chimeras (Hall et al., 2014). However, this situation is quite unlike what we have observed for the donor‐derived programs for cartilage, bone, and tendon, and the host‐derived programs for epidermis (i.e., feathers and egg teeth) and osteoclasts, which in chimeras all follow in lockstep with the quail donor timetable and become accelerated by three stages (Ealba et al., 2015; Eames & Schneider, 2005, Eames & Schneider, 2008; Hall et al., 2014; Merrill et al., 2008; Schneider & Helms, 2003).
In sum, neural‐crest mesenchyme and its muscle connective tissue derivatives transmit species‐specific patterning information to craniofacial muscles by executing autonomous molecular programs and by dominating interactions with their partners from the mesoderm. Such mechanistic insights help explain how skeletal and muscular components in the jaw complex have so intimately co‐evolved as species radiate into new niches and their mouthparts become adapted in various ways. This can be seen clearly in birds such as parrots, where the number and organization of jaw muscles have been extremely modified, and most likely in close association with changes to neural crest (Tokita, 2004, 2006; Tokita, Kiyoshi, & Armstrong, 2007; Tokita et al., 2013; Zusi, 1993). The fact that neural crest mesenchyme establishes a direct relationship between skeletal anatomy, muscle architecture, and feeding mechanics suggests that the capability of a given species to modify its jaw complex rapidly during evolution, which is critical to accommodate novel ecological conditions, resides in the cranial neural crest. Thus, the neural crest has played a leading role in dictating species‐specific pattern and in directing the structural and functional integration of the craniofacial complex during the course of vertebrate evolution.
10. CONCLUSION
The vertebrate craniofacial complex displays tremendous conservation in its anatomical organization but remarkable diversity in its species‐specific size and shape. Such dualism mirrors the need for the craniofacial complex to satisfy essential requirements for survival like feeding, breathing, and sensing, yet simultaneously generate enough morphological variation to allow for adaptive evolution. Conspicuously, much of the evolutionary diversity in the craniofacial complex has appeared in those structures derived from the cranial neural crest, suggesting a high degree of plasticity (Eames & Schneider, 2005; Fish & Schneider, 2014b; Gans & Northcutt, 1983; Hanken & Gross, 2005; Jheon & Schneider, 2009; Le Douarin et al., 2004; Noden & Schneider, 2006; Northcutt, 2005; Schlosser & Wagner, 2004; Schneider, 1999, 2005, 2015; Trainor et al., 2003; West‐Eberhard, 1989, 2003; Young et al., 2014).
To this point, one might ask, what endows the cranial neural crest with both the plasticity to drive evolutionary diversification and the regulatory abilities to put them near the top of hierarchies in developmental programs for species‐specific pattern? Contributing factors may include gene duplication events, which are believed to have capacitated the evolution of neural crest development via co‐option and novel gene function (Green & Bronner, 2013; Meulemans & Bronner‐Fraser, 2005). Similarly, results from transcriptional profiling studies point to novel signaling pathways and suites of transcription factors that are enriched in sub‐populations of neural crest (Lumb, Buckberry, Secker, Lawrence, & Schwarz, 2017; Simoes‐Costa & Bronner, 2016; Simoes‐Costa, Tan‐Cabugao, Antoshechkin, Sauka‐Spengler, & Bronner, 2014). Moreover, given that cranial neural crest derivatives participate deeply in the development and patterning of multiple systems including the nervous, neuroendocrine, integumentary, and skeletal, regulatory changes to the neural crest can be a major source of simultaneous evolutionary transformations in behavior, pigmentation, as well as the size and shape of cartilage and bone in the face (Lord et al., 2016; Sanchez‐Villagra et al., 2016; Schneider, 2005, 2018; Singh et al., 2017; Wilkins et al., 2014).
Without a doubt, data from a wide variety of experimental systems and strategies performed in diverse vertebrate taxa, demonstrate that neural crest mesenchyme controls species‐specific pattern. Work from our lab takes this conclusion one step further and reveals that neural crest does so by autonomously executing molecular and cellular programs for making neural crest‐derived structures, as well as by governing the required interactions with adjacent tissues (e.g., ectodermal epithelia and mesodermal mesenchyme). Furthermore, the precise origin of species‐specific pattern is rooted in the fact that epithelia are generally permissive and supply generic anatomical information (e.g., “form a lower jaw”) while neural crest mesenchyme contains instructive information for size and shape (e.g., “form a quail‐like lower jaw” versus “form a duck‐like lower jaw”). In other words, the list of parts is more or less the same but the species‐specific differences that arise during the construction process appear to stem from where, when, and for how long neural crest mesenchyme autonomously activates and executes intrinsic molecular programs including the expression of receptors that allow for signal transduction to begin and end, as well as a variety of transcription factors that modulate gene regulatory networks. Ultimately, the developmental fate of each species is determined by the differential unfurling of each genome in three dimensions as a function of absolute and/or relative time (e.g., cell cycle dynamics and maturation rates).
Most significantly, the propensity of neural crest mesenchyme to keep track concurrently of stage‐specific and species‐specific time, size, and shape provides a potent mechanism linking ontogeny and phylogeny in the integumentary and musculoskeletal systems, both of which have been critical to the success of vertebrates. Such findings about the integration of these systems vis‐à‐vis the cranial neural crest also reveal the many ways development can play a “generative and regulatory” role in the evolution of species‐specific pattern (Alberch, 1982b), and they help substantiate heterochrony as a viable developmental mechanism whereby species‐specific transformations in size and shape can come about via changes in the timing of developmental events (Alberch et al., 1979; De Beer, 1930; Fish & Schneider, 2014b; Hall, 1984; Schneider, 2018; Smith, 2003).
In this framework, a major remaining question for future research involves identifying on a much larger scale (i.e., systems level) where, when, and how variation in the molecular and cellular programs that are directed by the neural crest leads to species‐specific changes in size and shape. Addressing this question has important implications for understanding both evolution and the etiologies of craniofacial birth defects (Schneider, 2015). Furthermore, heterochrony may be an oversimplification of the many processes at work and instead a more multidimensional strategy that accounts for the effects of complex changes in facets such as the levels and spatial distribution of gene expression over developmental time (Depew & Simpson, 2006) may be necessary. Additional approaches that incorporate genome‐wide differences in the regulation of neural crest‐mediated programs among divergent taxa, have great potential to elucidate these issues (Betancur, Bronner‐Fraser, & Sauka‐Spengler, 2010; Long, Prescott, & Wysocka, 2016; Nikitina et al., 2008; Prescott et al., 2015; Rebeiz & Tsiantis, 2017; Sauka‐Spengler & Bronner‐Fraser, 2008; Sauka‐Spengler et al., 2007; Trinh et al., 2017; Williams et al., 2018). One can only imagine what the next 150 years of neural crest biology will uncover.
ACKNOWLEDGMENTS
I thank my collaborators and trainees whose work over the years forms the basis for much of what I have reviewed here (I have tried to cite them whenever possible). I am also very grateful to my long‐time mentor Drew Noden, who took me under his wing and got me hooked on crest while I was a fledgling graduate student more than two decades ago.
Schneider RA. Neural crest and the origin of species‐specific pattern. genesis. 2018;56:e23219. 10.1002/dvg.23219
Funding Information NIH/NIDCR; Grant/Award Numbers: R01 DE016402 and R01 DE025668 (RAS)
REFERENCES
- Abramyan, J. , Leung, K. J. , & Richman, J. M. (2014). Divergent palate morphology in turtles and birds correlates with differences in proliferation and BMP2 expression during embryonic development. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 322, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramyan, J. , & Richman, J. M. (2018). Craniofacial development: Discoveries made in the chicken embryo. The International Journal of Developmental Biology, 62, 97–107. [DOI] [PubMed] [Google Scholar]
- Abzhanov, A. , Kuo, W. P. , Hartmann, C. , Grant, B. R. , Grant, P. R. , & Tabin, C. J. (2006). The calmodulin pathway and evolution of elongated beak morphology in Darwin's finches. Nature, 442, 563–567. [DOI] [PubMed] [Google Scholar]
- Abzhanov, A. , Protas, M. , Grant, B. R. , Grant, P. R. , & Tabin, C. J. (2004). Bmp4 and morphological variation of beaks in Darwin's finches. Science (New York, N.Y.), 305, 1462–1465. [DOI] [PubMed] [Google Scholar]
- Abzhanov, A. , Rodda, S. J. , McMahon, A. P. , & Tabin, C. J. (2007). Regulation of skeletogenic differentiation in cranial dermal bone. Development (Cambridge, England), 134, 3133–3144. [DOI] [PubMed] [Google Scholar]
- Abzhanov, A. , & Tabin, C. J. (2004). Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Developmental Biology, 273, 134–148. [DOI] [PubMed] [Google Scholar]
- Adams, A. E. (1931). Some effects of removal of endoderm from the mouth region of early Amblystoma punctatum embryos. Journal of Experimental Zoology, 58, 147–163. [Google Scholar]
- Ahlgren, S. C. , & Bronner‐Fraser, M. (1999). Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Current Biology: Cb, 9, 1304–1314. [DOI] [PubMed] [Google Scholar]
- Ainsworth, S. J. , Stanley, R. L. , & Evans, D. J. (2010). Developmental stages of the Japanese quail. Journal of Anatomy, 216, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil, O. , Hall‐Glenn, F. , Chang, J. , Li, A. , Chang, W. , Lustig, L. R. , … Hsiao, E. C. (2014). Disrupted bone remodeling leads to cochlear overgrowth and hearing loss in a mouse model of fibrous dysplasia. PLoS One, 9, e94989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberch, P. (1982a). Developmental constraints in evolutionary processes In Bonner J. T. (Ed.), Evolution and development (pp. 313–332). Dahlem: Springer Verlag. [Google Scholar]
- Alberch, P. (1982b). The generative and regulatory roles of development in evolution In Mossakowski D., & Roth G. (Eds.), Environmental adaptation and evolution: A theoretical and empirical approach (pp. 19–36). Stuttgart: G. Fischer‐Verlag. [Google Scholar]
- Alberch, P. (1985). Problems with the interpretation of developmental sequences. Systematic Zoology, 34, 46–58. [Google Scholar]
- Alberch, P. (1989). The logic of monsters: Evidence for internal constraint in development and evolution. Geobios, 22, 21–57. [Google Scholar]
- Alberch, P. , Gould, S. J. , Oster, G. F. , & Wake, D. B. (1979). Size and shape in ontogeny and phylogeny. Paleobiology, 5, 296–317. [Google Scholar]
- Albertson, R. C. , Streelman, J. T. , Kocher, T. D. , & Yelick, P. C. (2005). Integration and evolution of the cichlid mandible: The molecular basis of alternate feeding strategies. Proceedings of the National Academy of Sciences of the United States of America, 102, 16287–16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliston, T. , Choy, L. , Ducy, P. , Karsenty, G. , & Derynck, R. (2001). TGF‐beta‐induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. The Embo Journal, 20, 2254–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado‐Mallart, R. M. (2005). The chick/quail transplantation model: Discovery of the isthmic organizer center. Brain Research. Brain Research Reviews, 49, 109–113. [DOI] [PubMed] [Google Scholar]
- Anakwe, K. , Robson, L. , Hadley, J. , Buxton, P. , Church, V. , Allen, S. , … Francis‐West, P. (2003). Wnt signalling regulates myogenic differentiation in the developing avian wing. Development (Cambridge, England), 130, 3503–3514. [DOI] [PubMed] [Google Scholar]
- Anderson, B. G. , & Busch, H. L. (1941). Allometry in normal and regenerating antennal segments in daphnia. Biological Bulletin, 81, 119–126. [Google Scholar]
- Anderson, R. M. , Lawrence, A. R. , Stottmann, R. W. , Bachiller, D. , & Klingensmith, J. (2002). Chordin and noggin promote organizing centers of forebrain development in the mouse. Development (Cambridge, England), 129, 4975–4987. [DOI] [PubMed] [Google Scholar]
- Andres, G. (1949). Untersuchungen an Chimären von Triton und Bombinator. Genetica, 24, 387–534. [Google Scholar]
- Anthwal, N. , Urban, D. J. , Luo, Z. X. , Sears, K. E. , & Tucker, A. S. (2017). Meckel's cartilage breakdown offers clues to mammalian middle ear evolution. Nature Ecology & Evolution, 1, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar, M. , Tambalo, M. , Ranganathan, R. , Grocott, T. , & Streit, A. (2017). A gene network regulated by FGF signalling during ear development. Scientific Reports, 7, 6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W. (2006). D'Arcy Thompson and the theory of transformations. Nature Reviews. Genetics, 7, 401–406. [DOI] [PubMed] [Google Scholar]
- Ashique, A. M. , Fu, K. , & Richman, J. M. (2002a). Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development, 129, 4647–4660. [DOI] [PubMed] [Google Scholar]
- Ashique, A. M. , Fu, K. , & Richman, J. M. (2002b). Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. International Journal of Developmental Biology, 46, 243–253. [DOI] [PubMed] [Google Scholar]
- Atchley, W. R. , Rutledge, J. J. , & Cowley, D. E. (1981). Genetic components of size and shape. II. Multivariate covariance patterns in the rat and mouse skull. Evolution; International Journal of Organic Evolution, 35, 1037–1055. [DOI] [PubMed] [Google Scholar]
- Atit, R. , Conlon, R. A. , & Niswander, L. (2003). EGF signaling patterns the feather array by promoting the interbud fate. Developmental Cell, 4, 231–240. [DOI] [PubMed] [Google Scholar]
- Baker, C. V. , & Bronner‐Fraser, M. (2001). Vertebrate cranial placodes I. Embryonic induction. Developmental Biology, 232, 1–61. [DOI] [PubMed] [Google Scholar]
- Baker, C. V. , Bronner‐Fraser, M. , Le Douarin, N. M. , & Teillet, M. A. (1997). Early‐ and late‐migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development (Cambridge, England), 124, 3077–3087. [DOI] [PubMed] [Google Scholar]
- Baker, C. V. , Stark, M. R. , Marcelle, C. , & Bronner‐Fraser, M. (1999). Competence, specification and induction of Pax‐3 in the trigeminal placode. Development (Cambridge, England), 126, 147–156. [DOI] [PubMed] [Google Scholar]
- Balaban, E. (1997). Changes in multiple brain regions underlie species differences in a complex, congenital behavior. Proceedings of the National Academy of Sciences of the United States of America, 94, 2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban, E. , Teillet, M. A. , & Le Douarin, N. (1988). Application of the quail‐chick chimera system to the study of brain development and behavior. Science, 241, 1339–1342. [DOI] [PubMed] [Google Scholar]
- Balczerski, B. , Zakaria, S. , Tucker, A. S. , Borycki, A. G. , Koyama, E. , Pacifici, M. , & Francis‐West, P. (2012). Distinct spatiotemporal roles of hedgehog signalling during chick and mouse cranial base and axial skeleton development. Developmental Biology, 371, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balling, R. , Mutter, G. , Gruss, P. , & Kessel, M. (1989). Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox‐1.1 in transgenic mice. Cell, 58, 337–347. [DOI] [PubMed] [Google Scholar]
- Bancroft, M. , & Bellairs, R. (1977). Placodes of the chick embryo studied by SEM. Anatomy and Embryology, 151, 97–108. [DOI] [PubMed] [Google Scholar]
- Barlow, A. J. , & Francis‐West, P. H. (1997). Ectopic application of recombinant BMP‐2 and BMP‐4 can change patterning of developing chick facial primordia. Development (Cambridge, England), 124, 391–398. [DOI] [PubMed] [Google Scholar]
- Baumel, J. J. (1993). Handbook of avian anatomy: Nomina anatomica avium. Cambridge, MA: Nuttall Ornithological Club. [Google Scholar]
- Bee, J. , & Thorogood, P. (1980). The role of tissue interactions in the skeletogenic differentiation of avian neural crest cells. Developmental Biology, 78, 47–66. [DOI] [PubMed] [Google Scholar]
- Behonick, D. J. , Xing, Z. , Lieu, S. , Buckley, J. M. , Lotz, J. C. , Marcucio, R. S. , … Colnot, C. (2007). Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PloS One, 2, e1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei, M. , & Maas, R. (1998). FGFs and BMP4 induce both Msx1‐independent and Msx1‐dependent signaling pathways in early tooth development. Development (Cambridge, England), 125, 4325–4333. [DOI] [PubMed] [Google Scholar]
- Belanger, L. F. (1969). Osteocytic osteolysis. Calcified Tissue Research, 4, 1–12. [DOI] [PubMed] [Google Scholar]
- Bell, D. M. , Leung, K. K. , Wheatley, S. C. , Ng, L. J. , Zhou, S. , Ling, K. W. , … Cheah, K. S. (1997). SOX9 directly regulates the type‐II collagen gene. Nature Genetics, 16, 174–178. [DOI] [PubMed] [Google Scholar]
- Bennett, J. H. , Hunt, P. , & Thorogood, P. (1995). Bone morphogenetic protein‐2 and −4 expression during murine orofacial development. Archives of Oral Biology, 40, 847–854. [DOI] [PubMed] [Google Scholar]
- Benouaiche, L. , Gitton, Y. , Vincent, C. , Couly, G. , & Levi, G. (2008). Sonic hedgehog signalling from foregut endoderm patterns the avian nasal capsule. Development (Cambridge, England), 135, 2221–2225. [DOI] [PubMed] [Google Scholar]
- Benson, R. H. , Chapman, R. E. , & Siegel, A. F. (1982). On the measurement of morphology and its change. Paleobiology, 8, 328–339. [Google Scholar]
- Bertalanffy, L. V. , & Pirozynski, W. J. (1952). Ontogenetic and evolutionary allometry. Evolution, 6, 387–392. [Google Scholar]
- Berthet, E. , Chen, C. , Butcher, K. , Schneider, R. A. , Alliston, T. , & Amirtharajah, M. (2013). Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 31, 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur, P. , Bronner‐Fraser, M. , & Sauka‐Spengler, T. (2010). Assembling neural crest regulatory circuits into a gene regulatory network. Annual Review of Cell and Developmental Biology, 26, 581–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar, B. A. , Morris, Z. S. , Sefton, E. M. , Tok, A. , Tokita, M. , Namkoong, B. , … Abzhanov, A. (2015). A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution; International Journal of Organic Evolution, 69, 1665–1677. [DOI] [PubMed] [Google Scholar]
- Blitz, E. , Sharir, A. , Akiyama, H. , & Zelzer, E. (2013). Tendon‐bone attachment unit is formed modularly by a distinct pool of Scx‐ and Sox9‐positive progenitors. Development (Cambridge, England), 140, 2680–2690. [DOI] [PubMed] [Google Scholar]
- Blitz, E. , Viukov, S. , Sharir, A. , Shwartz, Y. , Galloway, J. L. , Pryce, B. A. , … Zelzer, E. (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon‐skeleton junction. Developmental Cell, 17, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobick, B. E. , Thornhill, T. M. , & Kulyk, W. M. (2007). Fibroblast growth factors 2, 4, and 8 exert both negative and positive effects on limb, frontonasal, and mandibular chondrogenesis via MEK‐ERK activation. Journal of Cellular Physiology, 211, 233–243. [DOI] [PubMed] [Google Scholar]
- Bonafede, A. , Kohler, T. , Rodriguez‐Niedenfuhr, M. , & Brand‐Saberi, B. (2006). BMPs restrict the position of premuscle masses in the limb buds by influencing Tcf4 expression. Developmental Biology, 299, 330–344. [DOI] [PubMed] [Google Scholar]
- Bookstein, F. L. (1978). The measurement of biological shape and shape change. New York, NY: Springer‐Verlag. [Google Scholar]
- Bookstein, F. L. (1990). Multivariate methods In Rohlf F. J., & Bookstein F. L. (Eds.), Proceedings of the Michgan morphometrics workshop (pp. 75–76). Ann Arbor: University of Michigan Museum of Zoology. [Google Scholar]
- Born, G. (1896). Über Verwachsungsversuche mit Amphibienlarven. Archiv Für Mikroskopische Anatomie, 4, 349–465. [Google Scholar]
- Borue, X. , & Noden, D. M. (2004). Normal and aberrant craniofacial myogenesis by grafted trunk somitic and segmental plate mesoderm. Development (Cambridge, England), 131, 3967–3980. [DOI] [PubMed] [Google Scholar]
- Bout, R. G. , & Zweers, G. A. (2001). The role of cranial kinesis in birds. Comparative Biochemistry and Physiology. Part A, Molecular &Amp; Integrative Physiology, 131, 197–205. [DOI] [PubMed] [Google Scholar]
- Brent, A. E. , Braun, T. , & Tabin, C. J. (2005). Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development (Cambridge, England), 132, 515–528. [DOI] [PubMed] [Google Scholar]
- Brito, J. M. , Teillet, M. A. , & Le Douarin, N. M. (2006). An early role for sonic hedgehog from foregut endoderm in jaw development: Ensuring neural crest cell survival. Proceedings of the National Academy of Sciences of the United States of America, 103, 11607–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner‐Fraser, M. (1994). Neural crest cell formation and migration in the developing embryo. Faseb Journal, 8, 699–706. [DOI] [PubMed] [Google Scholar]
- Brotman, H. F. (1977). Abnormal morphogenesis of feather structures and pattern in the chick embryo integument. II. Histological description. The Journal of Experimental Zoology, 200, 107–124. [DOI] [PubMed] [Google Scholar]
- Brugmann, S. A. , Goodnough, L. H. , Gregorieff, A. , Leucht, P. , ten Berge, D. , Fuerer, C. , … Helms, J. A. (2007). Wnt signaling mediates regional specification in the vertebrate face. Development (Cambridge, England), 134, 3283–3295. [DOI] [PubMed] [Google Scholar]
- Brugmann, S. A. , Powder, K. E. , Young, N. M. , Goodnough, L. H. , Hahn, S. M. , James, A. W. , … Lovett, M. (2010). Comparative gene expression analysis of avian embryonic facial structures reveals new candidates for human craniofacial disorders. Human Molecular Genetics, 19, 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill, E. W. , Potter, A. S. , Distasio, A. , Dexheimer, P. , Plassard, A. , Aronow, B. J. , & Potter, S. S. (2014). A gene expression atlas of early craniofacial development. Developmental Biology, 391, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter, J. A. , Glimcher, M. J. , Cooper, R. R. , & Recker, R. (1996). Bone biology. II: Formation, form, modeling, remodeling, and regulation of cell function. Instructional Course Lectures, 45, 387–399. [PubMed] [Google Scholar]
- Cairns, J. M. , & Saunders, J. W. (1954). The influence of embryonic mesoderm on the regional specification of epidermal derivatives in the chick. Journal of Experimental Zoology, 127, 221–248. [Google Scholar]
- Cela, P. , Buchtova, M. , Vesela, I. , Fu, K. , Bogardi, J. P. , Song, Y. , … Richman, J. M. (2016). BMP signaling regulates the fate of chondro‐osteoprogenitor cells in facial mesenchyme in a stage‐specific manner. Developmental Dynamics, 245, 947–962. [DOI] [PubMed] [Google Scholar]
- Cerny, R. , Lwigale, P. , Ericsson, R. , Meulemans, D. , Epperlein, H. H. , & Bronner‐Fraser, M. (2004). Developmental origins and evolution of jaws: New interpretation of “maxillary” and “mandibular”. Developmental Biology, 276, 225–236. [DOI] [PubMed] [Google Scholar]
- Cerny, R. , Meulemans, D. , Berger, J. , Wilsch‐Brauninger, M. , Kurth, T. , Bronner‐Fraser, M. , & Epperlein, H. H. (2004). Combined intrinsic and extrinsic influences pattern cranial neural crest migration and pharyngeal arch morphogenesis in axolotl. Developmental Biology, 266, 252–269. [DOI] [PubMed] [Google Scholar]
- Chang, C. H. , Jiang, T. X. , Lin, C. M. , Burrus, L. W. , Chuong, C. M. , & Widelitz, R. (2004). Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mechanisms of Development, 121, 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. C. , Balaban, E. , & Jarvis, E. D. (2012). Interspecies avian brain chimeras reveal that large brain size differences are influenced by cell‐interdependent processes. PLoS One, 7, e42477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. M. , Smith, D. M. , Peters, M. A. , Samson, M. E. , Zitz, J. , Tabin, C. J. , & Cepko, C. L. (1999). Production and design of more effective avian replication‐incompetent retroviral vectors. Developmental Biology, 214, 370–384. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , & Struhl, G. (1996). Dual roles for patched in sequestering and transducing Hedgehog. Cell, 87, 553–563. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Gao, B. , Wang, H. , Han, N. , Shao, S. , Wu, S. , … Lei, F. (2017). Evolution of beak morphology in the Ground Tit revealed by comparative transcriptomics. Frontiers in Zoology, 14, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodankar, R. , Chang, C. H. , Yue, Z. , Jiang, T. X. , Suksaweang, S. , Burrus, L. , … Widelitz, R. (2003). Shift of localized growth zones contributes to skin appendage morphogenesis: Role of the Wnt/beta‐catenin pathway. The Journal of Investigative Dermatology, 120, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. H. , Ann, E. J. , Yoon, J. H. , Mo, J. S. , Kim, M. Y. , & Park, H. S. (2013). Calcium/calmodulin‐dependent protein kinase IV (CaMKIV) enhances osteoclast differentiation via the up‐regulation of Notch1 protein stability. Biochimica et Biophysica Acta, 1833, 69–79. [DOI] [PubMed] [Google Scholar]
- Choi, Y. H. , Choi, J. H. , Oh, J. W. , & Lee, K. Y. (2013). Calmodulin‐dependent kinase II regulates osteoblast differentiation through regulation of Osterix. Biochemical and Biophysical Research Communications, 432, 248–255. [DOI] [PubMed] [Google Scholar]
- Chong, H. J. , Young, N. M. , Hu, D. , Jeong, J. , McMahon, A. P. , Hallgrimsson, B. , & Marcucio, R. S. (2012). Signaling by SHH rescues facial defects following blockade in the brain. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 241, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong, C. M. , Chodankar, R. , Widelitz, R. B. , & Jiang, T. X. (2000). Evo‐devo of feathers and scales: Building complex epithelial appendages. Current Opinion in Genetics & Development, 10, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong, C. M. , Hou, L. , Chen, P. J. , Wu, P. , Patel, N. , & Chen, Y. (2001). Dinosaur's feather and chicken's tooth? Tissue engineering of the integument. European Journal of Dermatology: Ejd, 11, 286–292. [PMC free article] [PubMed] [Google Scholar]
- Chuong, C. M. , Patel, N. , Lin, J. , Jung, H. S. , & Widelitz, R. B. (2000). Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: Perspectives in development and evolution. Cellular and Molecular Life Sciences, 57, 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong, C. M. , Widelitz, R. B. , Ting‐Berreth, S. , & Jiang, T. X. (1996). Early events during avian skin appendage regeneration: Dependence on epithelial‐mesenchymal interaction and order of molecular reappearance. The Journal of Investigative Dermatology, 107, 639–646. [DOI] [PubMed] [Google Scholar]
- Clark Dalton, H. (1950). Comparison of white and black axolotl chromatophores in vitro. Journal of Experimental Zoology, 115, 17–35. [Google Scholar]
- Clark, W. E. L. G. , & Medawar, P. B. (1945). Essays on growth and form presented to D'Arcy Wentworth Thompson. Oxford: Clarendon Press. [Google Scholar]
- Clouthier, D. E. , Williams, S. C. , Yanagisawa, H. , Wieduwilt, M. , Richardson, J. A. , & Yanagisawa, M. (2000). Signaling pathways crucial for craniofacial development revealed by endothelin‐A receptor‐deficient mice. Developmental Biology, 217, 10–24. [DOI] [PubMed] [Google Scholar]
- Coats, S. , Flanagan, W. M. , Nourse, J. , & Roberts, J. M. (1996). Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science (New York, N.Y.), 272, 877–880. [DOI] [PubMed] [Google Scholar]
- Cobos, I. , Shimamura, K. , Rubenstein, J. L. , Martinez, S. , & Puelles, L. (2001). Fate map of the avian anterior forebrain at the four‐somite stage, based on the analysis of quail‐chick chimeras. Developmental Biology, 239, 46–67. [DOI] [PubMed] [Google Scholar]
- Cohen, M. A. , Wert, K. J. , Goldmann, J. , Markoulaki, S. , Buganim, Y. , Fu, D. , & Jaenisch, R. (2016). Human neural crest cells contribute to coat pigmentation in interspecies chimeras after in utero injection into mouse embryos. Proceedings of the National Academy of Sciences of the United States of America, 113, 1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver, W. M. (1930). Results of heteroplastic transplantation of anterior and posterior parts of the heart rudiment in Amblystoma embryos. Journal of Experimental Zoology, 55, 293–318. [Google Scholar]
- Coppinger, L. , & Coppinger, R. P. (1982). Livestock‐guarding dogs that wear sheep's clothing. Smithsonian, 13, 64–73. [Google Scholar]
- Coppinger, R. P. , & Schneider, R. A. (1995). Evolution of working dogs In Serpell J. (Ed.) The domestic dog (pp. 21–47). Cambridge: Cambridge University Press. [Google Scholar]
- Cordero, D. R. , Schneider, R. A. , & Helms, J. A. (2002). Morphogenesis of the face In Lin K. Y., Ogle R. C., & Jane J. A. (Eds.), Craniofacial surgery: Science & surgical technique (pp. 75–83). Philadelphia: W. B. Saunders Company. [Google Scholar]
- Couly, G. , Coltey, P. , Eichmann, A. , & Le Douarin, N. M. (1995). The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mechanisms of Development, 53, 97–112. [DOI] [PubMed] [Google Scholar]
- Couly, G. , Creuzet, S. , Bennaceur, S. , Vincent, C. , & Le Douarin, N. M. (2002). Interactions between Hox‐negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development (Cambridge, England), 129, 1061–1073. [DOI] [PubMed] [Google Scholar]
- Couly, G. , Grapin‐Botton, A. , Coltey, P. , Ruhin, B. , & Le Douarin, N. M. (1998). Determination of the identity of the derivatives of the cephalic neural crest: Incompatibility between Hox gene expression and lower jaw development. Development (Cambridge, England), 125, 3445–3459. [DOI] [PubMed] [Google Scholar]
- Couly, G. , & Le Douarin, N. M. (1988). The fate map of the cephalic neural primordium at the presomitic to the 3‐somite stage in the avian embryo. Development, 103, 101–113. [DOI] [PubMed] [Google Scholar]
- Couly, G. , & Le Douarin, N. M. (1990). Head morphogenesis in embryonic avian chimeras: Evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development (Cambridge, England), 108, 543–558. [DOI] [PubMed] [Google Scholar]
- Couly, G. F. , Coltey, P. M. , & Le Douarin, N. M. (1992). The developmental fate of the cephalic mesoderm in quail‐chick chimeras. Development (Cambridge, England), 114, 1–15. [DOI] [PubMed] [Google Scholar]
- Couly, G. F. , Coltey, P. M. , & Le Douarin, N. M. (1993). The triple origin of skull in higher vertebrates: A study in quail‐chick chimeras. Development (Cambridge, England), 117, 409–429. [DOI] [PubMed] [Google Scholar]
- Cramer, S. F. (1991). The origin of epidermal melanocytes. Implications for the histogenesis of nevi and melanomas. Archives of Pathology &Amp; Laboratory Medicine, 115, 115–119. [PubMed] [Google Scholar]
- Creuzet, S. , Couly, G. , & Le Douarin, N. M. (2005). Patterning the neural crest derivatives during development of the vertebrate head: Insights from avian studies. Journal of Anatomy, 207, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet, S. , Couly, G. , Vincent, C. , & Le Douarin, N. M. (2002). Negative effect of Hox gene expression on the development of the neural crest‐derived facial skeleton. Development (Cambridge, England), 129, 4301–4313. [DOI] [PubMed] [Google Scholar]
- Crowe, R. , Henrique, D. , Ish‐Horowicz, D. , & Niswander, L. (1998). A new role for Notch and Delta in cell fate decisions: Patterning the feather array. Development (Cambridge, England), 125, 767–775. [DOI] [PubMed] [Google Scholar]
- Crump, J. G. , Maves, L. , Lawson, N. D. , Weinstein, B. M. , & Kimmel, C. B. (2004). An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development (Cambridge, England), 131, 5703–5716. [DOI] [PubMed] [Google Scholar]
- Cserjesi, P. , Brown, D. , Ligon, K. L. , Lyons, G. E. , Copeland, N. G. , Gilbert, D. J. , … Olson, E. N. (1995). Scleraxis: A basic helix‐loop‐helix protein that prefigures skeletal formation during mouse embryogenesis. Development (Cambridge, England), 121, 1099–1110. [DOI] [PubMed] [Google Scholar]
- David, N. B. , Saint‐Etienne, L. , Tsang, M. , Schilling, T. F. , & Rosa, F. M. (2002). Requirement for endoderm and FGF3 in ventral head skeleton formation. Development (Cambridge, England), 129, 4457–4468. [DOI] [PubMed] [Google Scholar]
- Dawson, M. M. , Metzger, K. A. , Baier, D. B. , & Brainerd, E. L. (2011). Kinematics of the quadrate bone during feeding in mallard ducks. The Journal of Experimental Biology, 214, 2036–2046. [DOI] [PubMed] [Google Scholar]
- De Beer, G. R. (1930). Embryology and evolution. Oxford: Clarendon Press. [Google Scholar]
- De Beer, G. R. (1937). The development of the vertebrate skull (p. 554). Chicago: University of Chicago Press. [Google Scholar]
- De Beer, G. R. (1947). The differentiation of neural crest cells into visceral cartilages and odontoblasts in amblystoma, and a re‐examination of the germ‐layer theory. Proceedings of the Royal Society of London. Series B, Biological Sciences, 134, 377–398. [PubMed] [Google Scholar]
- De Beer, G. R. (1954). Embryos and ancestors. Oxford: Clarendon Press. [Google Scholar]
- De Crombrugghe, B. , Lefebvre, V. , Behringer, R. R. , Bi, W. , Murakami, S. , & Huang, W. (2000). Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol, 19, 389–394. [DOI] [PubMed] [Google Scholar]
- De Renzi, M. (2009). Developmental and historical patterns at the crossroads in the work of Pere Alberch In Rasskin‐Gutman D., & De Renzi M. (Eds.), Pere Alberch: The creative trajectory of an evo‐devo biologist (pp. 45–66). Valencia: Universidad de València. [Google Scholar]
- Delloye‐Bourgeois, C. , Rama, N. , Brito, J. , Le Douarin, N. , & Mehlen, P. (2014). Sonic Hedgehog promotes the survival of neural crest cells by limiting apoptosis induced by the dependence receptor CDON during branchial arch development. Biochemical and Biophysical Research Communications, 452, 655–660. [DOI] [PubMed] [Google Scholar]
- Depew, M. J. , & Compagnucci, C. (2008). Tweaking the hinge and caps: Testing a model of the organization of jaws. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 310, 315–335. [DOI] [PubMed] [Google Scholar]
- Depew, M. J. , Liu, J. K. , Long, J. E. , Presley, R. , Meneses, J. J. , Pedersen, R. A. , & Rubenstein, J. L. (1999). Dlx5 regulates regional development of the branchial arches and sensory capsules. Development (Cambridge, England), 126, 3831–3846. [DOI] [PubMed] [Google Scholar]
- Depew, M. J. , Lufkin, T. , & Rubenstein, J. L. (2002). Specification of jaw subdivisions by Dlx genes. Science (New York, N.Y.), 298, 381–385. [DOI] [PubMed] [Google Scholar]
- Depew, M. J. , & Simpson, C. A. (2006). 21st century neontology and the comparative development of the vertebrate skull. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 235, 1256–1291. [DOI] [PubMed] [Google Scholar]
- Derynck, R. , Piek, E. , Schneider, R. A. , Choy, L. , & Alliston, T. (2008). TGF‐β family signalling in mesenchymal differentiation In Derynck R., & Miyazono K. (Eds.), The TGF‐beta family (pp. 613–666). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Detwiler, S. R. (1930). Observations upon the growth, function, and nerve supply of limbs when grafted to the head of salamander embryos. Journal of Experimental Zoology, 55, 319–379. [Google Scholar]
- Dhouailly, D. (1967). Analysis of the factors in the specific differenciation of the neoptile feathers in the duck and chicken. Journal of Embryology and Experimental Morphology, 18, 389–400. [PubMed] [Google Scholar]
- Dhouailly, D. (1970). The determination of specific differentiation of neoptile and teleoptile feathers in the chick and the duck. Journal of Embryology and Experimental Morphology, 24, 73–94. [PubMed] [Google Scholar]
- Dhouailly, D. (1973). Dermo‐epidermal interactions between birds and mammals: Differentiation of cutaneous appendages. Journal of Embryology and Experimental Morphology, 30, 587–603. [PubMed] [Google Scholar]
- Dhouailly, D. (1975). Formation of cutaneous appendages in dermo‐epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux's Archives of Developmental Biology, 177, 323–340. [DOI] [PubMed] [Google Scholar]
- Dhouailly, D. , & Sawyer, R. H. (1984). Avian scale development. XI. Initial appearance of the dermal defect in scaleless skin. Developmental Biology, 105, 343–350. [DOI] [PubMed] [Google Scholar]
- Donoghue, P. C. , Graham, A. , & Kelsh, R. N. (2008). The origin and evolution of the neural crest. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 30, 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris, F. (1938). The production of pigment in vitro by chick neural crest. Wilhelm Roux' Archiv Fur Entwicklungsmechanik Der Organismen, 138, 323–334. [DOI] [PubMed] [Google Scholar]
- Doufexi, A. E. , & Mina, M. (2008). Signaling pathways regulating the expression of Prx1 and Prx2 in the chick mandibular mesenchyme. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 237, 3115–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, A. G. (2011). Dispelling dog dogma: An investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape. Evolution & Development, 13, 204–213. [DOI] [PubMed] [Google Scholar]
- Drissi, H. , Hushka, D. , Aslam, F. , Nguyen, Q. , Buffone, E. , Koff, A. , … Stein, G. S. (1999). The cell cycle regulator p27kip1 contributes to growth and differentiation of osteoblasts. Cancer Research, 59, 3705–3711. [PubMed] [Google Scholar]
- Ducy, P. (2000). Cbfa1: A molecular switch in osteoblast biology. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 219, 461–471. [DOI] [PubMed] [Google Scholar]
- Ducy, P. , Starbuck, M. , Priemel, M. , Shen, J. , Pinero, G. , Geoffroy, V. , … Karsenty, G. (1999). A Cbfa1‐dependent genetic pathway controls bone formation beyond embryonic development. Genes & Development, 13, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy, P. , Zhang, R. , Geoffroy, V. , Ridall, A. L. , & Karsenty, G. (1997). Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell, 89, 747–754. [DOI] [PubMed] [Google Scholar]
- Dunlop, L. L. , & Hall, B. K. (1995). Relationships between cellular condensation, preosteoblast formation and epithelial‐mesenchymal interactions in initiation of osteogenesis. The International Journal of Developmental Biology, 39, 357–371. [PubMed] [Google Scholar]
- DuShane, G. P. (1934). The origin of pigment cells in amphibia. Science (New York, N.Y.), 80, 620–621. [DOI] [PubMed] [Google Scholar]
- DuShane, G. P. (1935). An experimental study of the origin of pigment cells in Amphibia. Journal of Experimental Zoology, 72, 1–31. [Google Scholar]
- DuShane, G. P. (1938). Neural fold derivatives in the amphibia: Pigment cells, spinal ganglia and Rohon‐Beard cells. Journal of Experimental Zoology, 78, 485–503. [Google Scholar]
- DuShane, G. P. (1939). The role of embryonic ectoderm and mesoderm in pigment production in amphibia. Journal of Experimental Zoology, 82, 193–215. [Google Scholar]
- Ealba, E. L. , Jheon, A. H. , Hall, J. , Curantz, C. , Butcher, K. D. , & Schneider, R. A. (2015). Neural crest‐mediated bone resorption is a determinant of species‐specific jaw length. Developmental Biology, 408, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealba, E. L. , & Schneider, R. A. (2013). A simple PCR‐based strategy for estimating species‐specific contributions in chimeras and xenografts. Development (Cambridge, England), 140, 3062–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames, B. F. , de la Fuente, L. , & Helms, J. A. (2003). Molecular ontogeny of the skeleton. Birth Defects Research. Part C, Embryo Today: Reviews, 69, 93–101. [DOI] [PubMed] [Google Scholar]
- Eames, B. F. , & Schneider, R. A. (2005). Quail‐duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development (Cambridge, England), 132, 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames, B. F. , & Schneider, R. A. (2008). The genesis of cartilage size and shape during development and evolution. Development (Cambridge, England), 135, 3947–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames, B. F. , Sharpe, P. T. , & Helms, J. A. (2004). Hierarchy revealed in the specification of three skeletal fates by Sox9 and Runx2. Developmental Biology, 274, 188–200. [DOI] [PubMed] [Google Scholar]
- Eichele, G. , Tickle, C. , & Alberts, B. M. (1984). Microcontrolled release of biologically active compounds in chick embryos: Beads of 200‐microns diameter for the local release of retinoids. Analytical Biochemistry, 142, 542–555. [DOI] [PubMed] [Google Scholar]
- Ekanayake, S. , & Hall, B. K. (1994). Hypertrophy is not a prerequisite for type X collagen expression or mineralization of chondrocytes derived from cultured chick mandibular ectomesenchyme. The International Journal of Developmental Biology, 38, 683–694. [PubMed] [Google Scholar]
- Ekker, S. C. , Ungar, A. R. , Greenstein, P. , von Kessler, D. P. , Porter, J. A. , Moon, R. T. , & Beachy, P. A. (1995). Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Current Biology: Cb, 5, 944–955. [DOI] [PubMed] [Google Scholar]
- Engsig, M. T. , Chen, Q. J. , Vu, T. H. , Pedersen, A. C. , Therkidsen, B. , Lund, L. R. , … Delaisse, J. M. (2000). Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. The Journal of Cell Biology, 151, 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow, D. H. , Moyers, R. E. , & Merow, W. W. (1975). Handbook of facial growth. Philadelphia, PA: Saunders. [Google Scholar]
- Ericsson, R. , Cerny, R. , Falck, P. , & Olsson, L. (2004). Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 231, 237–247. [DOI] [PubMed] [Google Scholar]
- Evans, D. J. , & Noden, D. M. (2006). Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 235, 1310–1325. [DOI] [PubMed] [Google Scholar]
- Fassler, P. E. (1996). Hans Spemann (1869–1941) and the Freiburg School of Embryology. International Journal of Developmental Biology, 40, 49–57. [PubMed] [Google Scholar]
- Fekete, D. M. , & Cepko, C. L. (1993). Retroviral infection coupled with tissue transplantation limits gene transfer in the chicken embryo. Proceedings of the National Academy of Sciences of the United States of America, 90, 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, C. A. , Tucker, A. S. , & Sharpe, P. T. (2000). Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development (Cambridge, England), 127, 403–412. [DOI] [PubMed] [Google Scholar]
- Filvaroff, E. , & Derynck, R. (1998). Bone remodelling: A signalling system for osteoclast regulation. Current Biology: Cb, 8, R679–R682. [DOI] [PubMed] [Google Scholar]
- Fish, J. L. , & Schneider, R. A. (2014a). Assessing species‐specific contributions to craniofacial development using quail‐duck chimeras. Journal of Visualized Experiments, 87, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish, J. L. , & Schneider, R. A. (2014b). Chapter 6 ‐ neural crest‐mediated tissue interactions during craniofacial development: The origins of species‐specific pattern In Trainor P. A. (Ed.), Neural crest cells (pp. 101–124). Boston: Academic Press. [Google Scholar]
- Fish, J. L. , & Schneider, R. A. (2014c). On the origins of species‐specific size. Cambridge: The Company of Biologists. [Google Scholar]
- Fish, J. L. , Sklar, R. S. , Woronowicz, K. C. , & Schneider, R. A. (2014). Multiple developmental mechanisms regulate species‐specific jaw size. Development (Cambridge, England), 141, 674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, C. (1987). Abnormal development in the skin of the pupoid fetus (pf/pf) mutant mouse: Abnormal keratinization, recovery of a normal phenotype, and relationship to the repeated epilation (Er/Er) mutant mouse. Current Topics in Developmental Biology, 22, 209–234. [DOI] [PubMed] [Google Scholar]
- Fisher, H. I. (1955). Some aspects of the kinetics in the jaws of birds. The Wilson Bulletin 67, 175–188. [Google Scholar]
- Fitzgerald, T. C. (1969). The Coturnix quail; anatomy and histology. Ames: Iowa State University Press. [Google Scholar]
- Fliniaux, I. , Viallet, J. P. , & Dhouailly, D. (2004). Ventral vs. dorsal chick dermal progenitor specification. The International Journal of Developmental Biology, 48, 103–106. [DOI] [PubMed] [Google Scholar]
- Fondon, J. W. , & Garner, H. R. (2004). Molecular origins of rapid and continuous morphological evolution. Proceedings of the National Academy of Sciences of the United States of America, 101, 18058–18063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine‐Perus, J. (2000). Interspecific mouse‐chick chimeras. Methods in Molecular Biology, 135, 443–446. [DOI] [PubMed] [Google Scholar]
- Fontaine‐Perus, J. , & Cheraud, Y. (2005). Mouse‐chick neural chimeras. The International Journal of Developmental Biology, 49, 349–353. [DOI] [PubMed] [Google Scholar]
- Fontaine‐Perus, J. , Cheraud, Y. , & Halgand, P. (1996). Developmental potentialities of the mouse neural tube grafted in chick embryo. The International Journal of Developmental Biology, Suppl 1, 135S. [PubMed] [Google Scholar]
- Fontaine‐Perus, J. , Halgand, P. , Cheraud, Y. , Rouaud, T. , Velasco, M. E. , Cifuentes Diaz, C. , & Rieger, F. (1997). Mouse‐chick chimera: A developmental model of murine neurogenic cells. Development (Cambridge, England), 124, 3025–3036. [DOI] [PubMed] [Google Scholar]
- Foppiano, S. , Hu, D. , & Marcucio, R. S. (2007). Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Developmental Biology, 312, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, D. W. , & Kaesler, R. L. (1988). Shape analysis: Ideas from the Ostracoda In McKinney M. L. (Ed.), Heterochrony in evolution: A multidisciplinary approach (pp. 53–69). New York, NY: Plenum Press. [Google Scholar]
- Fowler, T. W. , Acevedo, C. , Mazur, C. M. , Hall‐Glenn, F. , Fields, A. J. , Bale, H. A. , … Alliston, T. (2017). Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Scientific Reports, 7, 44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis‐West, P. , Ladher, R. , Barlow, A. , & Graveson, A. (1998). Signalling interactions during facial development. Mechanisms of Development, 75, 3–28. [DOI] [PubMed] [Google Scholar]
- Francis‐West, P. H. , Ladher, R. K. , & Schoenwolf, G. C. (2002). Development of the sensory organs. Science Progress, 85, 151–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis‐West, P. H. , Robson, L. , & Evans, D. J. (2003). Craniofacial development: The tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol, 169, 1–138. [DOI] [PubMed] [Google Scholar]
- Francis‐West, P. H. , Tatla, T. , & Brickell, P. M. (1994). Expression patterns of the bone morphogenetic protein genes Bmp‐4 and Bmp‐2 in the developing chick face suggest a role in outgrowth of the primordia. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 201, 168–178. [DOI] [PubMed] [Google Scholar]
- Galindo, M. , Pratap, J. , Young, D. W. , Hovhannisyan, H. , Im, H. J. , Choi, J. Y. , … van Wijnen, A. J. (2005). The bone‐specific expression of Runx2 oscillates during the cell cycle to support a G1‐related antiproliferative function in osteoblasts. The Journal of Biological Chemistry, 280, 20274–20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans, C. , & Northcutt, R. G. (1983). Neural crest and the origin of vertebrates: A new head. Science (New York, N.Y.), 220, 268–274. [DOI] [PubMed] [Google Scholar]
- Garrod, D. R. (1986). Specific inductive flypaper. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 5, 172–173. [DOI] [PubMed] [Google Scholar]
- Gayon, J. (2000). History of the concept of allometry. American Zoologist, 40, 748–758. [Google Scholar]
- Geetha‐Loganathan, P. , Nimmagadda, S. , Fu, K. , & Richman, J. M. (2014). Avian facial morphogenesis is regulated by c‐Jun N‐terminal kinase/planar cell polarity (JNK/PCP) wingless‐related (WNT) signaling. The Journal of Biological Chemistry, 289, 24153–24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron‐Maguire, M. , Mallo, M. , Zhang, M. , & Gridley, T. (1993). Hoxa‐2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell, 75, 1317–1331. [DOI] [PubMed] [Google Scholar]
- Gitton, Y. , Heude, E. , Vieux‐Rochas, M. , Benouaiche, L. , Fontaine, A. , Sato, T. , … Levi, G. (2010). Evolving maps in craniofacial development. Seminars in Cell & Developmental Biology, 21, 301–308. [DOI] [PubMed] [Google Scholar]
- Godfrey, L. R. , & Sutherland, M. R. (1995). What's growth got to do with it? Process and product in the evolution of ontogeny. Journal of Human Evolution, 29, 405–431. [Google Scholar]
- Goldschmidt, R. (1938). Physiological genetics. New York, NY: McGraw Hill. [Google Scholar]
- Goldschmidt, R. (1940). The material basis of evolution. New Haven: Yale University Press. [Google Scholar]
- Goodrich, E. S. (1913). Metameric Segmentation and Homology. Quarterly Journal of Microscopical Science, 59, 227–248. [Google Scholar]
- Goodrich, E. S. (1918). On the development of the segments of the head in Scyllium . Quarterly Journal of Microscopical Science, 63, 1–30. [Google Scholar]
- Goodrich, E. S. (1930). Studies on the structure and development of vertebrates. Chicago: University of Chicago Press. [Google Scholar]
- Gould, S. J. (1966). Allometry and size in ontogeny and phylogeny. Biological Reviews of the Cambridge Philosophical Society, 41, 587–640. [DOI] [PubMed] [Google Scholar]
- Gould, S. J. (1971). Geometric similarity in allometric growth: A contribution to the problems of scaling in the evolution of size. The American Naturalist, 105, 113–136. [Google Scholar]
- Gould, S. J. (1977). Ontogeny and phylogeny (p. 501). Cambridge: Harvard University Press. [Google Scholar]
- Govindarajan, V. , & Overbeek, P. A. (2006). FGF9 can induce endochondral ossification in cranial mesenchyme. BMC Developmental Biology, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, A. (2003). Development of the pharyngeal arches. American Journal of Medical Genetics, 119A, 251–256. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos, G. A. , Bell, E. , Toole, L. , Lumsden, A. , & Tucker, A. S. (2000). Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development (Cambridge, England), 127, 5355–5365. [DOI] [PubMed] [Google Scholar]
- Grant, P. R. , Grant, B. R. , & Abzhanov, A. (2006). A developing paradigm for the development of bird beaks. Biological Journal of the Linnean Society, 88, 17–22. [Google Scholar]
- Green, S. A. , & Bronner, M. E. (2013). Gene duplications and the early evolution of neural crest development. Seminars in Cell & Developmental Biology, 24, 95–100. [DOI] [PubMed] [Google Scholar]
- Grenier, J. , Teillet, M. A. , Grifone, R. , Kelly, R. G. , & Duprez, D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS One, 4, e4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott, T. , Johnson, S. , Bailey, A. P. , & Streit, A. (2011). Neural crest cells organize the eye via TGF‐beta and canonical Wnt signalling. Nature Communications, 2, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves, A. K. , & Bronner‐Fraser, M. (2000). Competence, specification and commitment in otic placode induction. Development (Cambridge, England), 127, 3489–3499. [DOI] [PubMed] [Google Scholar]
- Gunter, H. M. , Koppermann, C. , & Meyer, A. (2014). Revisiting de Beer's textbook example of heterochrony and jaw elongation in fish: Calmodulin expression reflects heterochronic growth, and underlies morphological innovation in the jaws of belonoid fishes. Evodevo, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, B. K. (1980). Tissue interactions and the initiation of osteogenesis and chondrogenesis in the neural crest‐derived mandibular skeleton of the embryonic mouse as seen in isolated murine tissues and in recombinations of murine and avian tissues. Journal of Embryology and Experimental Morphology, 58, 251–264. [PubMed] [Google Scholar]
- Hall, B. K. (1981). The induction of neural crest‐derived cartilage and bone by embryonic epithelia: An analysis of the mode of action of an epithelial‐mesenchymal interaction. Journal of Embryology and Experimental Morphology, 64, 305–320. [PubMed] [Google Scholar]
- Hall, B. K. (1982). The role of tissue interactions in the growth of bone In Dixon A. D., & Sarnat B. G. (Eds.), Factors and mechanisms influencing bone growth (pp. 205–215). New York, NY: Alan R. Liss, Inc. [Google Scholar]
- Hall, B. K. (1984). Developmental processes underlying heterochrony as an evolutionary mechanism. Canadian Journal of Zoology, 62, 1–7. [Google Scholar]
- Hall, B. K. (1987). Tissue interactions in the development and evolution of the vertebrate head In Maderson P. F. A. (Ed.), Developmental and evolutionary aspects of the neural crest (pp. 215–260). New York, NY: John Wiley & Sons. [Google Scholar]
- Hall, B. K. (1999). The neural crest in development and evolution. New York, Berlin: Springer. [Google Scholar]
- Hall, B. K. (2000). Epithelial‐mesenchymal interactions. Methods in Molecular Biology (Clifton, N.J.), 137, 235–243. [DOI] [PubMed] [Google Scholar]
- Hall, B. K. (2005). Bones and cartilage: Developmental and evolutionary skeletal biology. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Hall, B. K. , & Hörstadius, S. (1988). The neural crest. London: Oxford University Press. [Google Scholar]
- Hall, B. K. , & Miyake, T. (1992). The membranous skeleton: The role of cell condensations in vertebrate skeletogenesis. Anatomy and Embryology, 186, 107–124. [DOI] [PubMed] [Google Scholar]
- Hall, B. K. , & Miyake, T. (1995). Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. The International Journal of Developmental Biology, 39, 881–893. [PubMed] [Google Scholar]
- Hall, B. K. , & Miyake, T. (2000). All for one and one for all: Condensations and the initiation of skeletal development. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 22, 138–147. [DOI] [PubMed] [Google Scholar]
- Hall, E. K. (1950). Experimental modifications of muscle development in Amblystoma puncatum. Journal of Experimental Zoology, 113, 355–377. [Google Scholar]
- Hall, J. , Jheon, A. H. , Ealba, E. L. , Eames, B. F. , Butcher, K. D. , Mak, S. S. , … Schneider, R. A. (2014). Evolution of a developmental mechanism: Species‐specific regulation of the cell cycle and the timing of events during craniofacial osteogenesis. Developmental Biology, 385, 380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson, B. , Percival, C. J. , Green, R. , Young, N. M. , Mio, W. , & Marcucio, R. (2015). Morphometrics, 3D imaging, and craniofacial development. Current Topics in Developmental Biology, 115, 561–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger, V. (1942). A manual of experimental embryology. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Hamburger, V. , & Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. Journal of Morphology, 88, 49–92. [PubMed] [Google Scholar]
- Hamilton, H. L. (1965). Lillie's development of the chick: An introduction to embryology. New York, NY: Holt, Rinehart and Winston. [Google Scholar]
- Hampe, A. (1957). Role of mesoderm and ectoderm of leg bud in exchanges between duck and chicken. Comptes Rendus de l'Académie des Sciences, 244, 3179–3181. [PubMed] [Google Scholar]
- Han, J. , Ishii, M. , Bringas, P., Jr. , Maas, R. L. , Maxson, R. E., Jr. , & Chai, Y. (2007). Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mechanisms of Development, 124, 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken, J. , & Gross, J. B. (2005). Evolution of cranial development and the role of neural crest: Insights from amphibians. Journal of Anatomy, 207, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M. P. , Fallon, J. F. , & Prum, R. O. (2002). Shh‐Bmp2 signaling module and the evolutionary origin and diversification of feathers. The Journal of Experimental Zoology, 294, 160–176. [DOI] [PubMed] [Google Scholar]
- Harrison, R. G. (1898). The growth and regeneration of the tail of the frog larva. Roux's Archives of Developmental Biology, 7, 430–485. [Google Scholar]
- Harrison, R. G. (1903). Experimentelle Untersuchungen Über die Entwicklung der Sinnesorgane der Seitenlinie bei den Ampkibien. Archiv Für Mikroskopische Anatomie, 63, 35–149. [Google Scholar]
- Harrison, R. G. (1910). The outgrowth of the nerve fiber as a mode of protoplasmic movement. Journal of Experimental Zoology, 9, 787–846. [DOI] [PubMed] [Google Scholar]
- Harrison, R. G. (1915). Experiments on the development of the limbs in amphibia. Proceedings of the National Academy of Sciences of the United States of America, 1, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, R. G. (1917). Transplantation of limbs. Proceedings of the National Academy of Sciences of the United States of America, 3, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, R. G. (1921). Experiments on the development of the gills in the amphibian embryo. The Biological Bulletin, 41, 156. [Google Scholar]
- Harrison, R. G. (1924). Some unexpected results of the heteroplastic transplantation of limbs. Proceedings of the National Academy of Sciences, 10, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, R. G. (1929). Correlation in the development and growth of the eye studied by means of heteroplastic transplantation. Wilhelm Roux' Archiv Für Entwicklungsmechanik Der Organismen, 120, 1–55. [DOI] [PubMed] [Google Scholar]
- Harrison, R. G. (1933). Some difficulties of the determination problem. American Naturalist, 67, 306–321. [Google Scholar]
- Harrison, R. G. (1935). Heteroplastic grafting in embryology. Baltimore: Williams and Wilkins. [Google Scholar]
- Harrison, R. G. (1969). Organization and development of the embryo. New Haven: Yale University Press. [Google Scholar]
- Havens, B. A. , Rodgers, B. , & Mina, M. (2006). Tissue‐specific expression of Fgfr2b and Fgfr2c isoforms, Fgf10 and Fgf9 in the developing chick mandible. Archives of Oral Biology, 51, 134–145. [DOI] [PubMed] [Google Scholar]
- Havens, B. A. , Velonis, D. , Kronenberg, M. S. , Lichtler, A. C. , Oliver, B. , & Mina, M. (2008). Roles of FGFR3 during morphogenesis of Meckel's cartilage and mandibular bones. Developmental Biology, 316, 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth, K. E. , Wilson, J. M. , Grevellec, A. , Cobourne, M. T. , Healy, C. , Helms, J. A. , … Tucker, A. S. (2007). Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Developmental Biology, 303, 244–258. [DOI] [PubMed] [Google Scholar]
- Healy, C. , Uwanogho, D. , & Sharpe, P. T. (1996). Expression of the chicken Sox9 gene marks the onset of cartilage differentiation. Annals of the New York Academy of Sciences, 785, 261–262. [DOI] [PubMed] [Google Scholar]
- Healy, C. , Uwanogho, D. , & Sharpe, P. T. (1999). Regulation and role of Sox9 in cartilage formation. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 215, 69–78. [DOI] [PubMed] [Google Scholar]
- Heldin, C. H. , Miyazono, K. , & ten Dijke, P. (1997). TGF‐beta signalling from cell membrane to nucleus through SMAD proteins. Nature, 390, 465–471. [DOI] [PubMed] [Google Scholar]
- Helms, J. A. , Kim, C. H. , Hu, D. , Minkoff, R. , Thaller, C. , & Eichele, G. (1997). Sonic hedgehog participates in craniofacial morphogenesis and is down‐regulated by teratogenic doses of retinoic acid. Developmental Biology, 187, 25–35. [DOI] [PubMed] [Google Scholar]
- Helms, J. A. , & Schneider, R. A. (2003). Cranial skeletal biology. Nature, 423, 326–331. [DOI] [PubMed] [Google Scholar]
- Hersh, A. H. (1934). Evolutionary allometric growth in the titanotheres. American Naturalist, 68, 537–561. [Google Scholar]
- Higashihori, N. , Buchtova, M. , & Richman, J. M. (2010). The function and regulation of TBX22 in avian frontonasal morphogenesis. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 239, 458–473. [DOI] [PubMed] [Google Scholar]
- Hintze, M. , Prajapati, R. S. , Tambalo, M. , Christophorou, N. A. D. , Anwar, M. , Grocott, T. , & Streit, A. (2017). Cell interactions, signals and transcriptional hierarchy governing placode progenitor induction. Development (Cambridge, England), 144, 2810–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, K. , Hirano, M. , Zeng, Y. , Nishimura, J. , Hara, K. , Muta, K. , … Kanaide, H. (2001). Cloning and functional expression of a degradation‐resistant novel isoform of p27Kip1. The Biochemical Journal, 353, 51–57. [PMC free article] [PubMed] [Google Scholar]
- Hirobe, T. (1995). Structure and function of melanocytes: Microscopic morphology and cell biology of mouse melanocytes in the epidermis and hair follicle. Histology and Histopathology, 10, 223–237. [PubMed] [Google Scholar]
- Holtfreter, J. (1933). Der Einfluss von Wirtsalter und verschiedenen Organbezirken auf die Differenzierung von angelagertem Gastrulaektoderm. Wilhelm Roux' Archiv fur Entwicklungsmechanik Der Organismen, 127, 619–775. [DOI] [PubMed] [Google Scholar]
- Holtfreter, J. (1936). Regionale Induktionen in xenoplastisch zusammengesetzten Explantaten. Wilhelm Roux' Archiv fur Entwicklungsmechanik Der Organismen, 134, 466–550. [DOI] [PubMed] [Google Scholar]
- Hörstadius, S. (1950). The neural crest; its properties and derivatives in the light of experimental research. London, New York: Oxford University Press. [Google Scholar]
- Hörstadius, S. , & Sellman, S. (1941). Experimental studies on the determination of the chondrocranium in Amblystoma mexicanum . Niederländisches Archiv Für Zoologie, 33, 1–8. [Google Scholar]
- Hörstadius, S. , & Sellman, S. (1946). Experimentelle untersuchungen über die determination des knorpeligen kopfskelettes bei urodelen. Nova Acta Regiae Societatis Scientiarum Upsaliensis Ser, IV, 1–170. [Google Scholar]
- Hu, D. , Colnot, C. , & Marcucio, R. S. (2008). Effect of bone morphogenetic protein signaling on development of the jaw skeleton. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 237, 3727–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , & Helms, J. A. (1999). The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development (Cambridge, England), 126, 4873–4884. [DOI] [PubMed] [Google Scholar]
- Hu, D. , & Marcucio, R. S. (2009a). A SHH‐responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development, 136, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , & Marcucio, R. S. (2009b). Unique organization of the frontonasal ectodermal zone in birds and mammals. Developmental Biology, 325, 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , & Marcucio, R. S. (2012). Neural crest cells pattern the surface cephalic ectoderm during FEZ formation. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 241, 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , Marcucio, R. S. , & Helms, J. A. (2003). A zone of frontonasal ectoderm regulates patterning and growth in the face. Development (Cambridge, England), 130, 1749–1758. [DOI] [PubMed] [Google Scholar]
- Hu, D. , Young, N. M. , Li, X. , Xu, Y. , Hallgrimsson, B. , & Marcucio, R. S. (2015). A dynamic Shh expression pattern, regulated by SHH and BMP signaling, coordinates fusion of primordia in the amniote face. Development, 142, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , Young, N. M. , Xu, Q. , Jamniczky, H. , Green, R. M. , Mio, W. , Marcucio, R. S. , & Hallgrimsson, B. (2015). Signals from the brain induce variation in avian facial shape. Developmental Dynamics, 244, 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, A. J. , Miyazaki, H. , Coyle, M. C. , Zhang, J. , Laurie, M. T. , Chu, D. , … Gartner, Z. J. (2018). Engineered tissue folding by mechanical compaction of the mesenchyme. Developmental Cell, 44, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, P. , Clarke, J. D. , Buxton, P. , Ferretti, P. , & Thorogood, P. (1998). Stability and plasticity of neural crest patterning and branchial arch Hox code after extensive cephalic crest rotation. Developmental Biology, 198, 82–104. [DOI] [PubMed] [Google Scholar]
- Hunt, P. , & Krumlauf, R. (1991). Deciphering the hox code: Clues to patterning branchial regions of the head. Cell, 66, 1075–1078. [DOI] [PubMed] [Google Scholar]
- Hunt, P. , Wilkinson, D. , & Krumlauf, R. (1991). Patterning the vertebrate head: Murine Hox 2 genes mark distinct subpopulations of premigratory and migrating cranial neural crest. Development (Cambridge, England), 112, 43–50. [DOI] [PubMed] [Google Scholar]
- Huxley, J. S. (1932). Problems of relative growth. London: Methuen. [Google Scholar]
- Huxley, J. S. (1950). Relative growth and form transformation. Proceedings of the Royal Society of London. Series B, Biological Sciences, 137, 465–469. [DOI] [PubMed] [Google Scholar]
- Huxley, J. S. , & Teissier, G. (1936). Terminology of relative growth. Nature, 137, 780–781. [Google Scholar]
- Hwang, S. Y. , & Putney, J. W. Jr. (2011). Calcium signaling in osteoclasts. Biochimica Et Biophysica Acta, 1813, 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, Y. , Bringas, P., Jr. , Mogharei, A. , Zhao, J. , Deng, C. , & Chai, Y. (2002). Receptor‐regulated and inhibitory Smads are critical in regulating transforming growth factor beta‐mediated Meckel's cartilage development. Developmental Dynamics, 224, 69–78. [DOI] [PubMed] [Google Scholar]
- Jauregui, E. J. , Akil, O. , Acevedo, C. , Hall‐Glenn, F. , Tsai, B. S. , Bale, H. A. , … Alliston, T. (2016). Parallel mechanisms suppress cochlear bone remodeling to protect hearing. Bone, 89, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, J. , Mao, J. , Tenzen, T. , Kottmann, A. H. , & McMahon, A. P. (2004). Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes & Development, 18, 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheon, A. H. , & Schneider, R. A. (2009). The cells that fill the bill: Neural crest and the evolution of craniofacial development. Journal of Dental Research, 88, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, N. , Saarialho‐Kere, U. , Airola, K. , Herva, R. , Nissinen, L. , Westermarck, J. , … Kähäri, V. M. (1997). Collagenase‐3 (MMP‐13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 208, 387–397. [DOI] [PubMed] [Google Scholar]
- Johnston, M. C. (1966). A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. The Anatomical Record, 156, 143–155. [DOI] [PubMed] [Google Scholar]
- Jotereau, F. V. , & Le Douarin, N. M. (1978). The development relationship between osteocytes and osteoclasts: A study using the quail‐chick nuclear marker in endochondral ossification. Developmental Biology, 63, 253–265. [DOI] [PubMed] [Google Scholar]
- Jourdeuil, K. , & Taneyhill, L. A. (2018). Spatiotemporal expression pattern of Connexin 43 during early chick embryogenesis. Gene Expression Patterns, 27, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H. S. , Francis‐West, P. H. , Widelitz, R. B. , Jiang, T. X. , Ting‐Berreth, S. , Tickle, C. , … Chuong, C. M. (1998). Local inhibitory action of BMPs and their relationships with activators in feather formation: Implications for periodic patterning. Developmental Biology, 196, 11–23. [DOI] [PubMed] [Google Scholar]
- Kaan, H. W. (1930). The relation of the developing auditory vesicle to the formation of the cartilage capsule in Amblystoma punctatum. Journal of Experimental Zoology, 55, 263–291. [Google Scholar]
- Kahn, J. , Shwartz, Y. , Blitz, E. , Krief, S. , Sharir, A. , Breitel, D. A. , … Zelzer, E. (2009). Muscle contraction is necessary to maintain joint progenitor cell fate. Developmental Cell, 16, 734–743. [DOI] [PubMed] [Google Scholar]
- Kajiya, H. (2012). Calcium signaling in osteoclast differentiation and bone resorption. Advances in Experimental Medicine and Biology, 740, 917–932. [DOI] [PubMed] [Google Scholar]
- Kang, J. S. , Alliston, T. , Delston, R. , & Derynck, R. (2005). Repression of Runx2 function by TGF‐beta through recruitment of class II histone deacetylases by Smad3. The Embo Journal, 24, 2543–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon, G. , Harfe, B. D. , & Tabin, C. J. (2003). A Tcf4‐positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Developmental Cell, 5, 937–944. [DOI] [PubMed] [Google Scholar]
- Karsenty, G. , Ducy, P. , Starbuck, M. , Priemel, M. , Shen, J. , Geoffroy, V. , & Amling, M. (1999). Cbfa1 as a regulator of osteoblast differentiation and function. Bone, 25, 107–108. [DOI] [PubMed] [Google Scholar]
- Kavumpurath, S. , & Hall, B. K. (1990). Lack of either chondrocyte hypertrophy or osteogenesis in Meckel's cartilage of the embryonic chick exposed to epithelia and to thyroxine in vitro. Journal of Craniofacial Genetics and Developmental Biology, 10, 263–275. [PubMed] [Google Scholar]
- Kawabata, M. , Imamura, T. , & Miyazono, K. (1998). Signal transduction by bone morphogenetic proteins. Cytokine & Growth Factor Reviews, 9, 49–61. [DOI] [PubMed] [Google Scholar]
- Kermack, K. A. , & Haldane, J. B. S. (1950). Organic correlation and allometry. Biometrika, 37, 30–41. [PubMed] [Google Scholar]
- Kikuchi, Y. , Agathon, A. , Alexander, J. , Thisse, C. , Waldron, S. , Yelon, D. , … Stainier, D. Y. (2001). casanova encodes a novel Sox‐related protein necessary and sufficient for early endoderm formation in zebrafish. Genes & Development, 15, 1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel, C. B. , Miller, C. T. , Kruze, G. , Ullmann, B. , BreMiller, R. A. , Larison, K. D. , & Snyder, H. C. (1998). The shaping of pharyngeal cartilages during early development of the zebrafish. Developmental Biology, 203, 245–263. [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B. , Ullmann, B. , Walker, C. , Wilson, C. , Currey, M. , Phillips, P. C. , … Cresko, W. A. (2005). Evolution and development of facial bone morphology in threespine sticklebacks. Proceedings of the National Academy of Sciences of the United States of America, 102, 5791–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury, J. W. , Allen, V. G. , & Rotheram, B. A. (1953). The histological structure of the beak in the chick. The Anatomical Record, 116, 95–115. [DOI] [PubMed] [Google Scholar]
- Kingsley, D. M. , Bland, A. E. , Grubber, J. M. , Marker, P. C. , Russell, L. B. , Copeland, N. G. , & Jenkins, N. A. (1992). The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell, 71, 399–410. [DOI] [PubMed] [Google Scholar]
- Kirby, M. L. , Stadt, H. , Kumiski, D. , & Herlea, V. (2000). Interspecific chimeras. Transplantation of neural crest between mouse and chick embryos. Methods in Molecular Biology (Clifton, N.J.), 135, 439–442. [DOI] [PubMed] [Google Scholar]
- Klingenberg, C. P. (1998). Heterochrony and allometry: The analysis of evolutionary change in ontogeny. Biological Reviews of the Cambridge Philosophical Society, 73, 79–123. [DOI] [PubMed] [Google Scholar]
- Klingenberg, C. P. , & Spence, J. R. (1993). Heterochrony and allometry: Lessons from the water strider genus Limnoporus . Evolution, 47, 1834–1853. [DOI] [PubMed] [Google Scholar]
- Knight, R. D. , Mebus, K. , & Roehl, H. H. (2008). Mandibular arch muscle identity is regulated by a conserved molecular process during vertebrate development. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 310B, 355–369. [DOI] [PubMed] [Google Scholar]
- Knight, R. D. , & Schilling, T. F. (2006). Cranial neural crest and development of the head skeleton. Advances in Experimental Medicine and Biology, 589, 120–133. [DOI] [PubMed] [Google Scholar]
- Koecke, H. (1958). Normalstadien der Embryonalentwicklung bei der Hausente (Anas boschas domestica). Embryologia, 4, 55–78. [Google Scholar]
- Komori, T. , Yagi, H. , Nomura, S. , Yamaguchi, A. , Sasaki, K. , Deguchi, K. , … Kishimoto, T. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 89, 755–764. [DOI] [PubMed] [Google Scholar]
- Köntges, G. , & Lumsden, A. (1996). Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development (Cambridge, England), 122, 3229–3242. [DOI] [PubMed] [Google Scholar]
- Krull, C. E. (2004). A primer on using in ovo electroporation to analyze gene function. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 229, 433–439. [DOI] [PubMed] [Google Scholar]
- Kulesa, P. , Bronner‐Fraser, M. , & Fraser, S. (2000). In ovo time‐lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest. Development (Cambridge, England), 127, 2843–2852. [DOI] [PubMed] [Google Scholar]
- Kuraku, S. , Takio, Y. , Sugahara, F. , Takechi, M. , & Kuratani, S. (2010). Evolution of oropharyngeal patterning mechanisms involving Dlx and endothelins in vertebrates. Developmental Biology, 341, 315–323. [DOI] [PubMed] [Google Scholar]
- Kuratani, S. (2004). Evolution of the vertebrate jaw: Comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. Journal of Anatomy, 205, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani, S. (2005a). Cephalic neural crest cells and the evolution of craniofacial structures in vertebrates: Morphological and embryological significance of the premandibular‐mandibular boundary. Zoology, 108, 13–25. [DOI] [PubMed] [Google Scholar]
- Kuratani, S. (2005b). Developmental studies of the lamprey and hierarchical evolutionary steps toward the acquisition of the jaw. Journal of Anatomy, 207, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani, S. (2012). Evolution of the vertebrate jaw from developmental perspectives. Evolution & Development, 14, 76–92. [DOI] [PubMed] [Google Scholar]
- Kuratani, S. , Adachi, N. , Wada, N. , Oisi, Y. , & Sugahara, F. (2013). Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: Revising the heterotopy scenario of gnathostome jaw evolution. Journal of Anatomy, 222, 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani, S. , Nobusada, Y. , Horigome, N. , & Shigetani, Y. (2001). Embryology of the lamprey and evolution of the vertebrate jaw: Insights from molecular and developmental perspectives. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 356, 1615–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani, S. , Oisi, Y. , & Ota, K. G. (2016). Evolution of the vertebrate cranium: Viewed from hagfish developmental studies. Zoological Science, 33, 229–238. [DOI] [PubMed] [Google Scholar]
- Ladher, R. K. (2017). Changing shape and shaping change: Inducing the inner ear. Seminars in Cell & Developmental Biology, 65, 39–46. [DOI] [PubMed] [Google Scholar]
- Ladher, R. K. , O'Neill, P. , & Begbie, J. (2010). From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development (Cambridge, England), 137, 1777–1785. [DOI] [PubMed] [Google Scholar]
- Ladher, R. K. , Wright, T. J. , Moon, A. M. , Mansour, S. L. , & Schoenwolf, G. C. (2005). FGF8 initiates inner ear induction in chick and mouse. Genes & Development, 19, 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, Y. , & Jiang, R. (2009). Sonic hedgehog signaling regulates reciprocal epithelial‐mesenchymal interactions controlling palatal outgrowth. Development (Cambridge, England), 136, 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landacre, F. L. (1921). The fate of the neural crest in the head of the urodeles. The Journal of Comparative Neurology, 33, 1–43. [Google Scholar]
- Lande, R. (1979). Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution, 33, 402–416. [DOI] [PubMed] [Google Scholar]
- Langille, R. M. , & Hall, B. K. (1993). Pattern formation and the neural crest In Hanken J., & Hall B. K. (Eds.), The skull (pp. 77–111). Chicago: University of Chicago Press. [Google Scholar]
- Larsen, C. W. , Zeltser, L. M. , & Lumsden, A. (2001). Boundary formation and compartition in the avian diencephalon. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21, 4699–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin, N. M. (1973). A biological cell labelling technique and its use in experimental embryology. Developmental Biology, 30, 217–222. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N. , & McLaren, A. (1984). Chimeras in developmental biology. London; Orlando: Academic Press. [Google Scholar]
- Le Douarin, N. M. , Creuzet, S. , Couly, G. , & Dupin, E. (2004). Neural crest cell plasticity and its limits. Development (Cambridge, England), 131, 4637–4650. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N. M. , & Dieterlen, L. F. (2013). How studies on the avian embryo have opened new avenues in the understanding of development: A view about the neural and hematopoietic systems. Development, Growth & Differentiation, 55, 1–14. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N. M. , Dieterlen‐Lievre, F. , & Teillet, M. (1996). Quail‐chick transplantations In Bronner‐Fraser M. (Ed.), Methods in avian embryology (pp. 23–59). San Diego: Academic Press. [PubMed] [Google Scholar]
- Le Douarin, N. M. , Dieterlen‐Lievre, F. , Teillet, M. A. , & Ziller, C. (2000). Interspecific chimeras in avian embryos. Methods in Molecular Biology (Clifton, N.J.), 135, 373–386. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N. M. , & Dupin, E. (1993). Cell lineage analysis in neural crest ontogeny. Journal of Neurobiology, 24, 146–161. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N. M. , & Teillet, M. M. (1974). Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Developmental Biology, 41, 162–184. [DOI] [PubMed] [Google Scholar]
- Le Lièvre, C. S. (1978). Participation of neural crest‐derived cells in the genesis of the skull in birds. Journal of Embryology and Experimental Morphology, 47, 17–37. [PubMed] [Google Scholar]
- Le Lièvre, C. S. , & Le Douarin, N. M. (1975). Mesenchymal derivatives of the neural crest: Analysis of chimaeric quail and chick embryos. Journal of Embryology and Experimental Morphology, 34, 125–154. [PubMed] [Google Scholar]
- Lee, K. S. , Kim, H. J. , Li, Q. L. , Chi, X. Z. , Ueta, C. , Komori, T. , … Bae, S. C. (2000). Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast‐specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Molecular and Cellular Biology, 20, 8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. H. , Fu, K. K. , Hui, J. N. , & Richman, J. M. (2001). Noggin and retinoic acid transform the identity of avian facial prominences. Nature, 414, 909–912. [DOI] [PubMed] [Google Scholar]
- Linsenmayer, T. F. (1972). Control of integumentary patterns in the chick. Developmental Biology, 27, 244–271. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Selever, J. , Murali, D. , Sun, X. , Brugger, S. M. , Ma, L. , … Martin, J. F. (2005). Threshold‐specific requirements for Bmp4 in mandibular development. Developmental Biology, 283, 282–293. [DOI] [PubMed] [Google Scholar]
- Logan, M. , & Tabin, C. (1998). Targeted gene misexpression in chick limb buds using avian replication‐competent retroviruses. Methods (San Diego, Calif.), 14, 407–420. [DOI] [PubMed] [Google Scholar]
- Long, H. K. , Prescott, S. L. , & Wysocka, J. (2016). Ever‐changing landscapes: Transcriptional enhancers in development and evolution. Cell, 167, 1170–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, K. , Schneider, R. A. , & Coppinger, R. P. (2016). Evolution of working dogs In Serpell J. (Ed.), The domestic dog (pp. 42–68). Cambridge: Cambridge University Press. [Google Scholar]
- Lucas, A. M. , & Stettenheim, P. R. (1972). Avian anatomy: Integument. Washington, D.C: United States Department of Agriculture. [Google Scholar]
- Lufkin, T. , Mark, M. , Hart, C. , Dollé, P. , Lemeur, M. , & Chambon, P. (1992). Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature, 359, 835–841. [DOI] [PubMed] [Google Scholar]
- Lumb, R. , Buckberry, S. , Secker, G. , Lawrence, D. , & Schwarz, Q. (2017). Transcriptome profiling reveals expression signatures of cranial neural crest cells arising from different axial levels. BMC Developmental Biology, 17, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumer, H. (1940). Evolutionary allometry in the skeleton of the domesticated dog. The American Naturalist, 74, 439–467. [Google Scholar]
- Lumsden, A. G. (1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development (Cambridge, England), 103 Suppl, 155–169. [DOI] [PubMed] [Google Scholar]
- Luo, G. , Hofmann, C. , Bronckers, A. L. , Sohocki, M. , Bradley, A. , & Karsenty, G. (1995). BMP‐7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes & Development, 9, 2808–2820. [DOI] [PubMed] [Google Scholar]
- Lwigale, P. Y. (2001). Embryonic origin of avian corneal sensory nerves. Developmental Biology, 239, 323–337. [DOI] [PubMed] [Google Scholar]
- Lwigale, P. Y. , Conrad, G. W. , & Bronner‐Fraser, M. (2004). Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development (Cambridge, England), 131, 1979–1991. [DOI] [PubMed] [Google Scholar]
- Lwigale, P. Y. , Cressy, P. A. , & Bronner‐Fraser, M. (2005). Corneal keratocytes retain neural crest progenitor cell properties. Developmental Biology, 288, 284–293. [DOI] [PubMed] [Google Scholar]
- Lwigale, P. Y. , & Schneider, R. A. (2008). Other chimeras: Quail‐duck and mouse‐chick. Methods in Cell Biology, 87, 59–74. [DOI] [PubMed] [Google Scholar]
- MacDonald, M. E. , Abbott, U. K. , & Richman, J. M. (2004). Upper beak truncation in chicken embryos with the cleft primary palate mutation is due to an epithelial defect in the frontonasal mass. Developmental Dynamics, 230, 335–349. [DOI] [PubMed] [Google Scholar]
- MacDonald, M. E. , & Hall, B. K. (2001). Altered timing of the extracellular‐matrix‐mediated epithelial‐ mesenchymal interaction that initiates mandibular skeletogenesis in three inbred strains of mice: Development, heterochrony, and evolutionary change in morphology. The Journal of Experimental Zoology, 291, 258–273. [DOI] [PubMed] [Google Scholar]
- Macmillan, G. J. (1976). Melanoblast‐tissue interactions and the development of pigment pattern in Xenopus larvae. Journal of Embryology and Experimental Morphology, 35, 463–484. [PubMed] [Google Scholar]
- Maeno, T. , Moriishi, T. , Yoshida, C. A. , Komori, H. , Kanatani, N. , Izumi, S. , … Komori, T. (2011). Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone, 49, 673–682. [DOI] [PubMed] [Google Scholar]
- Mandler, M. , & Neubuser, A. (2004). FGF signaling is required for initiation of feather placode development. Development (Cambridge, England), 131, 3333–3343. [DOI] [PubMed] [Google Scholar]
- Mangold, O. (1923). Transplantationsversuche zur Frage der Spezifität und der Bildung der Keimblätter. Archiv Für Mikroskopische Anatomie Und Entwicklungsmechanik, 100, 198–301. [Google Scholar]
- Mangold, O. , & Seidel, F. (1927). Homoplastische und heteroplastische verschmelzung ganzer tritonkeime. Wilhelm Roux' Archiv Für Entwicklungsmechanik Der Organismen, 111, 593–665. [DOI] [PubMed] [Google Scholar]
- Marcucio, R. S. , Cordero, D. R. , Hu, D. , & Helms, J. A. (2005). Molecular interactions coordinating the development of the forebrain and face. Developmental Biology, 284, 48–61. [DOI] [PubMed] [Google Scholar]
- Marcucio, R. S. , Young, N. M. , Hu, D. , & Hallgrimsson, B. (2011). Mechanisms that underlie co‐variation of the brain and face. Genesis (New York, N.Y. : 2000), 49, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, L. F. (1996). Advances in morphometrics. New York, NY: Plenum Press. [Google Scholar]
- Massague, J. , & Wotton, D. (2000). Transcriptional control by the TGF‐beta/Smad signaling system. The Embo Journal, 19, 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew, S. J. , Hansen, J. M. , Merrell, A. J. , Murphy, M. M. , Lawson, J. A. , Hutcheson, D. A. , … Kardon, G. (2011). Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development (Cambridge, England), 138, 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, T. , Ahlberg, P. E. , Kessaris, N. , Iannarelli, P. , Dennehy, U. , Richardson, W. D. , … Koentges, G. (2005). Neural crest origins of the neck and shoulder. Nature, 436, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerson, P. L. , & Fallon, J. F. (1985). The spatial pattern and temporal sequence in which feather germs arise in the white Leghorn chick embryo. Developmental Biology, 109, 259–267. [DOI] [PubMed] [Google Scholar]
- McGurk, P. A.‐O. , Swartz, M. A.‐O. , Chen, J. W. , Galloway, J. L. , & Eberhart, J. K. (2017). In vivo zebrafish morphogenesis shows Cyp26b1 promotes tendon condensation and musculoskeletal patterning in the embryonic jaw. PLoS Genetics, 13, e1007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney, M. L. (1988). Classifying heterochrony: Allometry, size, and time In McKinney M. L. (Ed.), Heterochrony in evolution: A multidisciplinary approach (pp. 17–34). New York, NY: Plenum Press. [Google Scholar]
- McLarren, K. W. , Litsiou, A. , & Streit, A. (2003). DLX5 positions the neural crest and preplacode region at the border of the neural plate. Developmental Biology, 259, 34–47. [DOI] [PubMed] [Google Scholar]
- Medeiros, D. M. , & Crump, J. G. (2012). New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Developmental Biology, 371, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick, M. , Witcher, D. , Bringas, P., Jr. , Carlsson, P. , & Jaskoll, T. (2005). Meckel's cartilage differentiation is dependent on hedgehog signaling. Cells Tissues Organs, 179, 146–157. [DOI] [PubMed] [Google Scholar]
- Merrill, A. E. , Eames, B. F. , Weston, S. J. , Heath, T. , & Schneider, R. A. (2008). Mesenchyme‐dependent BMP signaling directs the timing of mandibular osteogenesis. Development (Cambridge, England), 135, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans, D. , & Bronner‐Fraser, M. (2005). Central role of gene cooption in neural crest evolution. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 304, 298–303. [DOI] [PubMed] [Google Scholar]
- Miller, C. T. , Schilling, T. F. , Lee, K. , Parker, J. , & Kimmel, C. B. (2000). sucker encodes a zebrafish Endothelin‐1 required for ventral pharyngeal arch development. Development (Cambridge, England), 127, 3815–3828. [DOI] [PubMed] [Google Scholar]
- Miller, C. T. , Yelon, D. , Stainier, D. Y. , & Kimmel, C. B. (2003). Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development (Cambridge, England), 130, 1353–1365. [DOI] [PubMed] [Google Scholar]
- Miller, K. A. , Barrow, J. , Collinson, J. M. , Davidson, S. , Lear, M. , Hill, R. E. , & Mackenzie, A. (2007). A highly conserved Wnt‐dependent TCF4 binding site within the proximal enhancer of the anti‐myogenic Msx1 gene supports expression within Pax3‐expressing limb bud muscle precursor cells. Developmental Biology, 311, 665–678. [DOI] [PubMed] [Google Scholar]
- Mina, M. , Upholt, W. B. , & Kollar, E. J. (1994). Enhancement of avian mandibular chondrogenesis in vitro in the absence of epithelium. Archives of Oral Biology, 39, 551–562. [DOI] [PubMed] [Google Scholar]
- Mina, M. , Wang, Y. H. , Ivanisevic, A. M. , Upholt, W. B. , & Rodgers, B. (2002). Region‐ and stage‐specific effects of FGFs and BMPs in chick mandibular morphogenesis. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 223, 333–352. [DOI] [PubMed] [Google Scholar]
- Minarik, M. , Stundl, J. , Fabian, P. , Jandzik, D. , Metscher, B. D. , Psenicka, M. , … Cerny, R. (2017). Pre‐oral gut contributes to facial structures in non‐teleost fishes. Nature, 547, 209–212. [DOI] [PubMed] [Google Scholar]
- Minkin, C. (1982). Bone acid phosphatase: Tartrate‐resistant acid phosphatase as a marker of osteoclast function. Calcified Tissue International, 34, 285–290. [DOI] [PubMed] [Google Scholar]
- Minot, C. S. (1908). The problem of age, growth, and death: A study of cytomorphosis, based on lectures at the Lowell Institute, March, 1907. London: J. Murray. [Google Scholar]
- Mitgutsch, C. , Wimmer, C. , Sanchez‐Villagra, M. R. , Hahnloser, R. , & Schneider, R. A. (2011). Timing of ossification in duck, quail, and zebra finch: Intraspecific variation, heterochronies, and life history evolution. Zoological Science, 28, 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiadis, T. A. , Caton, J. , & Cobourne, M. (2006). Waking‐up the sleeping beauty: Recovery of the ancestral bird odontogenic program. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 306, 227–233. [DOI] [PubMed] [Google Scholar]
- Mitsiadis, T. A. , Cheraud, Y. , Sharpe, P. , & Fontaine‐Perus, J. (2003). Development of teeth in chick embryos after mouse neural crest transplantations. Proceedings of the National Academy of Sciences of the United States of America, 100, 6541–6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiadis, T. A. , Hirsinger, E. , Lendahl, U. , & Goridis, C. (1998). Delta‐notch signaling in odontogenesis: Correlation with cytodifferentiation and evidence for feedback regulation. Developmental Biology, 204, 420–431. [DOI] [PubMed] [Google Scholar]
- Miyake, T. , Cameron, A. M. , & Hall, B. K. (1996). Stage‐specific onset of condensation and matrix deposition for Meckel's and other first arch cartilages in inbred C57BL/6 mice. Journal of Craniofacial Genetics and Devlopmental Biology, 16, 32–47. [PubMed] [Google Scholar]
- Momose, T. , Tonegawa, A. , Takeuchi, J. , Ogawa, H. , Umesono, K. , & Yasuda, K. (1999). Efficient targeting of gene expression in chick embryos by microelectroporation. Development, Growth & Differentiation, 41, 335–344. [DOI] [PubMed] [Google Scholar]
- Moody, S. A. , & LaMantia, A. S. (2015). Transcriptional regulation of cranial sensory placode development. Current Topics in Developmental Biology, 111, 301–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, W. J. (1981). The mammalian skull (p. 369). Cambridge: Cambridge University Press. [Google Scholar]
- Morgan, B. A. , Orkin, R. W. , Noramly, S. , & Perez, A. (1998). Stage‐specific effects of sonic hedgehog expression in the epidermis. Developmental Biology, 201, 1–12. [DOI] [PubMed] [Google Scholar]
- Murakami, S. , Kan, M. , McKeehan, W. L. , & de Crombrugghe, B. (2000). Up‐regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen‐activated protein kinase pathway. Proceedings of the National Academy of Sciences of the United States of America, 97, 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison, N. D. , Price, B. A. , Conner, D. A. , Keene, D. R. , Olson, E. N. , Tabin, C. J. , & Schweitzer, R. (2007). Regulation of tendon differentiation by scleraxis distinguishes force‐transmitting tendons from muscle‐anchoring tendons. Development (Cambridge, England), 134, 2697–2708. [DOI] [PubMed] [Google Scholar]
- Myojin, M. , Ueki, T. , Sugahara, F. , Murakami, Y. , Shigetani, Y. , Aizawa, S. , … Kuratani, S. (2001). Isolation of Dlx and Emx gene cognates in an agnathan species, Lampetra japonica, and their expression patterns during embryonic and larval development: Conserved and diversified regulatory patterns of homeobox genes in vertebrate head evolution. Journal of Experimental Zoology, 291, 68–84. [DOI] [PubMed] [Google Scholar]
- Nakamura, H. , & Funahashi, J. (2001). Introduction of DNA into chick embryos by in ovo electroporation. Methods (San Diego, Calif.), 24, 43–48. [DOI] [PubMed] [Google Scholar]
- Nakane, Y. , & Tsudzuki, M. (1999). Development of the skeleton in Japanese quail embryos. Development, Growth & Differentiation, 41, 523–534. [DOI] [PubMed] [Google Scholar]
- Nassari, S. , Duprez, D. , & Fournier‐Thibault, C. (2017). Non‐myogenic contribution to muscle development and homeostasis: The role of connective tissues. Frontiers in Cell and Developmental Biology, 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham, J. , & Lerner, M. I. (1940). Terminology of relative growth‐rates. Nature, 146, 618. [Google Scholar]
- Nguyen, J. , Tang, S. Y. , Nguyen, D. , & Alliston, T. (2013). Load regulates bone formation and Sclerostin expression through a TGFbeta‐dependent mechanism. PLoS One, 8, e53813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina, N. , Sauka‐Spengler, T. , & Bronner‐Fraser, M. (2008). Dissecting early regulatory relationships in the lamprey neural crest gene network. Proceedings of the National Academy of Sciences of the United States of America, 105, 20083–20088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmagadda, S. , Buchtova, M. , Fu, K. , Geetha‐Loganathan, P. , Hosseini‐Farahabadi, S. , Trachtenberg, A. J. , … Richman, J. M. (2015). Identification and functional analysis of novel facial patterning genes in the duplicated beak chicken embryo. Developmental Biology, 407, 275–288. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. (1975). An analysis of the migratory behavior of avian cephalic neural crest cells. Developmental Biology, 42, 106–130. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. (1978a). The control of avian cephalic neural crest cytodifferentiation: II. Neural tissues. Developmental Biology, 67, 313–329. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. (1978b). The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Developmental Biology, 67, 296–312. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. (1983). The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Developmental Biology, 96, 144–165. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. (1984). The use of chimeras in analyses of craniofacial development In Le Douarin N., & McLaren A. (Eds.), Chimeras in developmental biology (pp. 241–280). London; Orlando: Academic Press. [Google Scholar]
- Noden, D. M. (1986a). Origins and patterning of avian craniofacial muscles. Developmental Biology, 116, 347–356. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. (1986b). Origins and patterning of craniofacial mesenchymal tissues. Journal of Craniofacial Genetics and Developmental Biology, 2, 15–31. [PubMed] [Google Scholar]
- Noden, D. M. (1988). Interactions and fates of avian craniofacial mesenchyme. Development, 103, 121–140. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. , & Francis‐West, P. (2006). The differentiation and morphogenesis of craniofacial muscles. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 235, 1194–1218. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. , Marcucio, R. , Borycki, A. G. , & Emerson, C. P., Jr. (1999). Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Developmental Dynamics, 216, 96–112. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. , & Schneider, R. A. (2006). Neural crest cells and the community of plan for craniofacial development: Historical debates and current perspectives. Advances in Experimental Medicine and Biology, 589, 1–23. [DOI] [PubMed] [Google Scholar]
- Noden, D. M. , & Trainor, P. A. (2005). Relations and interactions between cranial mesoderm and neural crest populations. Journal of Anatomy, 207, 575–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohno, T. , Kawakami, Y. , Ohuchi, H. , Fujiwara, A. , Yoshioka, H. , & Noji, S. (1995). Involvement of the Sonic hedgehog gene in chick feather formation. Biochemical and Biophysical Research Communications, 206, 33–39. [DOI] [PubMed] [Google Scholar]
- Noji, S. , Koyama, E. , Myokai, F. , Nohno, T. , Ohuchi, H. , Nishikawa, K. , & Taniguchi, S. (1993). Differential expression of three chick FGF receptor genes, FGFR1, FGFR2 and FGFR3, in limb and feather development. Progress in Clinical and Biological Research, 383B, 645–654. [PubMed] [Google Scholar]
- Noramly, S. , Freeman, A. , & Morgan, B. A. (1999). beta‐catenin signaling can initiate feather bud development. Development (Cambridge, England), 126, 3509–3521. [DOI] [PubMed] [Google Scholar]
- Noramly, S. , & Morgan, B. A. (1998). BMPs mediate lateral inhibition at successive stages in feather tract development. Development (Cambridge, England), 125, 3775–3787. [DOI] [PubMed] [Google Scholar]
- Northcutt, R. G. (2005). The new head hypothesis revisited. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 304B, 274–297. [DOI] [PubMed] [Google Scholar]
- Northcutt, R. G. , & Gans, C. (1983). The genesis of neural crest and epidermal placodes. The Quarterly Review of Biology, 58, 1–27. [DOI] [PubMed] [Google Scholar]
- O'Brien, C. A. , Plotkin, L. I. , Galli, C. , Goellner, J. J. , Gortazar, A. R. , Allen, M. R. , … Bellido, T. (2008). Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One, 3, e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oisi, Y. , Ota, K. G. , Fujimoto, S. , & Kuratani, S. (2013). Development of the chondrocranium in hagfishes, with special reference to the early evolution of vertebrates. Zoological Science, 30, 944–961. [DOI] [PubMed] [Google Scholar]
- Oisi, Y. , Ota, K. G. , Kuraku, S. , Fujimoto, S. , & Kuratani, S. (2013). Craniofacial development of hagfishes and the evolution of vertebrates. Nature, 493, 175–180. [DOI] [PubMed] [Google Scholar]
- Olivera‐Martinez, I. , Coltey, M. , Dhouailly, D. , & Pourquie, O. (2000). Mediolateral somitic origin of ribs and dermis determined by quail‐chick chimeras. Development (Cambridge, England), 127, 4611–4617. [DOI] [PubMed] [Google Scholar]
- Olivera‐Martinez, I. , Thelu, J. , & Dhouailly, D. (2004). Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. The International Journal of Developmental Biology, 48, 93–101. [DOI] [PubMed] [Google Scholar]
- Olivera‐Martinez, I. , Thelu, J. , Teillet, M. A. , & Dhouailly, D. (2001). Dorsal dermis development depends on a signal from the dorsal neural tube, which can be substituted by Wnt‐1. Mechanisms of Development, 100, 233–244. [DOI] [PubMed] [Google Scholar]
- Olivera‐Martinez, I. , Viallet, J. P. , Michon, F. , Pearton, D. J. , & Dhouailly, D. (2004). The different steps of skin formation in vertebrates. The International Journal of Developmental Biology, 48, 107–115. [DOI] [PubMed] [Google Scholar]
- Olsson, L. , Ericsson, R. , & Cerny, R. (2005). Vertebrate head development: Segmentation, novelties, and homology. Theory in Biosciences = Theorie in Den Biowissenschaften, 124, 145–163. [DOI] [PubMed] [Google Scholar]
- Olsson, L. , Falck, P. , Lopez, K. , Cobb, J. , & Hanken, J. (2001). Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Developmental Biology, 237, 354–367. [DOI] [PubMed] [Google Scholar]
- Oster, G. , & Alberch, P. (1982). Evolution and bifurcation of developmental programs. Evolution, 36, 444–459. [DOI] [PubMed] [Google Scholar]
- Oster, G. F. , Shubin, N. , Murray, J. D. , & Alberch, P. (1988). Evolution and morphogenetic rules ‐ the shape of the vertebrate limb in ontogeny and phylogeny. Evolution, 42, 862–884. [DOI] [PubMed] [Google Scholar]
- Ota, K. G. , Kuraku, S. , & Kuratani, S. (2007). Hagfish embryology with reference to the evolution of the neural crest. Nature, 446, 672–675. [DOI] [PubMed] [Google Scholar]
- Otto, F. , Thornell, A. P. , Crompton, T. , Denzel, A. , Gilmour, K. C. , Rosewell, I. R. , … Owen, M. J. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 89, 765–771. [DOI] [PubMed] [Google Scholar]
- Padgett, C. S. , & Ivey, W. D. (1960). The normal embryology of the Coturnix quail. The Anatomical Record, 137, 1–11. [DOI] [PubMed] [Google Scholar]
- Parsons, K. J. , & Albertson, R. C. (2009). Roles for Bmp4 and CaM1 in shaping the jaw: Evo‐devo and beyond. Annual Review of Genetics, 43, 369–388. [DOI] [PubMed] [Google Scholar]
- Pasqualetti, M. , Ori, M. , Nardi, I. , & Rijli, F. M. (2000). Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development (Cambridge, England), 127, 5367–5378. [DOI] [PubMed] [Google Scholar]
- Patel, K. , Makarenkova, H. , & Jung, H. S. (1999). The role of long range, local and direct signalling molecules during chick feather bud development involving the BMPs, follistatin and the Eph receptor tyrosine kinase Eph‐A4. Mechanisms of Development, 86, 51–62. [DOI] [PubMed] [Google Scholar]
- Pautou, M. P. (1968). Determining role of the mesoderm in the specific differentiation of the leg in birds. Archives D'anatomie Microscopique et de Morphologie Experimentale, 57, 311–328. [PubMed] [Google Scholar]
- Pera, E. , Stein, S. , & Kessel, M. (1999). Ectodermal patterning in the avian embryo: Epidermis versus neural plate. Development (Cambridge, England), 126, 63–73. [DOI] [PubMed] [Google Scholar]
- Petiot, A. , Ferretti, P. , Copp, A. J. , & Chan, C. T. (2002). Induction of chondrogenesis in neural crest cells by mutant fibroblast growth factor receptors. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 224, 210–221. [DOI] [PubMed] [Google Scholar]
- Piotrowski, T. , & Nusslein‐Volhard, C. (2000). The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Developmental Biology, 225, 339–356. [DOI] [PubMed] [Google Scholar]
- Pispa, J. , & Thesleff, I. (2003). Mechanisms of ectodermal organogenesis. Developmental Biology, 262, 195–205. [DOI] [PubMed] [Google Scholar]
- Platt, J. B. (1891). A contribution to the morphology of the vertebrate head, based on a study of Acanthias vulgaris . Journal of Morphology, 5, 79–112. [Google Scholar]
- Platt, J. B. (1893). Ectodermic origin of the cartilages of the head. Anatomischer Anzeiger, 8, 506–509. [Google Scholar]
- Platt, J. B. (1898). The development of the cartilaginous skull and of the branchial and hypoglossal musculature in Necturus. Morphologisches Jahrbuch, 25, 377–467. [Google Scholar]
- Pointer, M. A. , Kamilar, J. M. , Warmuth, V. , Chester, S. G. , Delsuc, F. , Mundy, N. I. , … Bradley, B. J. (2012). RUNX2 tandem repeats and the evolution of facial length in placental mammals. BMC Evolutionary Biology, 12, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powder, K. E. , Ku, Y. C. , Brugmann, S. A. , Veile, R. A. , Renaud, N. A. , Helms, J. A. , & Lovett, M. (2012). A cross‐species analysis of microRNAs in the developing avian face. PLoS One, 7, e35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap, J. , Galindo, M. , Zaidi, S. K. , Vradii, D. , Bhat, B. M. , Robinson, J. A. , … van Wijnen, A. J. (2003). Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Research, 63, 5357–5362. [PubMed] [Google Scholar]
- Prescott, S. L. , Srinivasan, R. , Marchetto, M. C. , Grishina, I. , Narvaiza, I. , Selleri, L. , … Wysocka, J. (2015). Enhancer divergence and cis‐regulatory evolution in the human and chimp neural crest. Cell, 163, 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prin, F. , & Dhouailly, D. (2004). How and when the regional competence of chick epidermis is established: Feathers vs. scutate and reticulate scales, a problem en route to a solution. The International Journal of Developmental Biology, 48, 137–148. [DOI] [PubMed] [Google Scholar]
- Pryce, B. A. , Brent, A. E. , Murchison, N. D. , Tabin, C. J. , & Schweitzer, R. (2007). Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 236, 1677–1682. [DOI] [PubMed] [Google Scholar]
- Pudliszewski, M. , & Pardanaud, L. (2005). Vasculogenesis and angiogenesis in the mouse embryo studied using quail/mouse chimeras. The International Journal of Developmental Biology, 49, 355–361. [DOI] [PubMed] [Google Scholar]
- Qing, H. , Ardeshirpour, L. , Pajevic, P. D. , Dusevich, V. , Jahn, K. , Kato, S. , … Bonewald, L. F. (2012). Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 27, 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, M. , Bulfone, A. , Ghattas, I. , Meneses, J. J. , Christensen, L. , Sharpe, P. T. , … Rubenstein, J. L. (1997). Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: Mutations of Dlx‐1, Dlx‐2, and Dlx‐1 and −2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Developmental Biology, 185, 165–184. [DOI] [PubMed] [Google Scholar]
- Radlanski, R. J. , & Klarkowski, M. C. (2001). Bone remodeling of the human mandible during prenatal development. Journal of Orofacial Orthopedics = Fortschritte Der Kieferorthopadie: Organ/Official Journal Deutsche Gesellschaft Fur Kieferorthopadie, 62, 191–201. [DOI] [PubMed] [Google Scholar]
- Radlanski, R. J. , Renz, H. , Lajvardi, S. , & Schneider, R. A. (2004). Bone remodeling during prenatal morphogenesis of the human mental foramen. European Journal of Oral Sciences, 112, 301–310. [DOI] [PubMed] [Google Scholar]
- Raff, R. A. (1996). The shape of life: Genes, development, and the evolution of animal form. Chicago: University of Chicago Press. [Google Scholar]
- Raven, C. P. (1931). Zur entwicklung der Ganglienleiste. I. Die Kinematik der Ganglienleistenentwicklung bei den Urodelen. Wilhelm Roux' Archiv Fur Entwicklungsmechanik Der Organismen, 125, 210–292. [DOI] [PubMed] [Google Scholar]
- Raven, C. P. (1933). Zur Entwicklung der Ganglienleiste III. Die Induktionsfähigkeit des Kopfganglienleistenmaterials von Rana fusca. Wilhelm Roux' Archiv Fur Entwicklungsmechanik Der Organismen, 130, 517–561. [DOI] [PubMed] [Google Scholar]
- Raven, C. P. (1935). Zur Entwicklung der Ganglienleiste. IV. Wilhelm Roux' Archiv Fur Entwicklungsmechanik Der Organismen, 132, 509–575. [DOI] [PubMed] [Google Scholar]
- Rawles, M. E. (1944). The migration of melanoblasts after hatching into pigment‐free skin grafts of the common fowl. Physiological Zoology, 17, 167–183. [Google Scholar]
- Rawles, M. E. (1948). Origin of melanophores and their role in the development of color pattern in vertebrates. Physiological Reviews, 28, 383–408. [DOI] [PubMed] [Google Scholar]
- Rawles, M. E. (1963). Tissue interactions in scale and feather development as studied in dermal‐epidermal recombinations. Journal of Embryology and Experimental Morphology, 11, 765–789. [PubMed] [Google Scholar]
- Rebeiz, M. , & Tsiantis, M. (2017). Enhancer evolution and the origins of morphological novelty. Current Opinion in Genetics & Development, 45, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve, E. C. R. (1950). Genetical aspects of size allometry. Proceedings of the Royal Society of London. Series B, Biological Sciences, 137, 515–518. [DOI] [PubMed] [Google Scholar]
- Rensch, B. (1948). Histological changes correlated with evolutionary changes of body size. Evolution, 2, 218–230. [DOI] [PubMed] [Google Scholar]
- Reponen, P. , Sahlberg, C. , Munaut, C. , Thesleff, I. , & Tryggvason, K. (1994). High expression of 92‐kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. The Journal of Cell Biology, 124, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, D. (1932). Some effects of heteroplastic transplantation of the ear vesicle in Amblystoma. Journal of Experimental Zoology, 63, 413–445. [Google Scholar]
- Richman, J. M. , Herbert, M. , Matovinovic, E. , & Walin, J. (1997). Effect of fibroblast growth factors on outgrowth of facial mesenchyme. Developmental Biology, 189, 135–147. [DOI] [PubMed] [Google Scholar]
- Richman, J. M. , & Lee, S. H. (2003). About face: Signals and genes controlling jaw patterning and identity in vertebrates. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 25, 554–568. [DOI] [PubMed] [Google Scholar]
- Richman, J. M. , & Tickle, C. (1989). Epithelia are interchangeable between facial primordia of chick embryos and morphogenesis is controlled by the mesenchyme. Developmental Biology, 136, 201–210. [DOI] [PubMed] [Google Scholar]
- Richman, J. M. , & Tickle, C. (1992). Epithelial‐mesenchymal interactions in the outgrowth of limb buds and facial primordia in chick embryos. Developmental Biology, 154, 299–308. [DOI] [PubMed] [Google Scholar]
- Ricklefs, R. E. & Starck, J. M. (1998). Embryonic growth and development In Starck J. M., & Ricklefs R. E. (Ed.), Avian growth and development: Evolution within the altricial‐precocial spectrum (pp. 31–58). New York, NY: Oxford University Press. [Google Scholar]
- Rijli, F. M. , Mark, M. , Lakkaraju, S. , Dierich, A. , Dolle, P. , & Chambon, P. (1993). A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa‐2, which acts as a selector gene. Cell, 75, 1333–1349. [DOI] [PubMed] [Google Scholar]
- Rinon, A. , Lazar, S. , Marshall, H. , Buchmann‐Moller, S. , Neufeld, A. , Elhanany‐Tamir, H. , … Tzahor, E. (2007). Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development (Cambridge, England), 134, 3065–3075. [DOI] [PubMed] [Google Scholar]
- Romanoff, A. L. (1960). The avian embryo. New York, NY: The Macmillan Company. [Google Scholar]
- Roth, V. L. (1984). How elephants grow: Heterochrony and the calibration of developmental stages in some living and fossil species. Journal of Vertebrate Paleontology, 4, 126–145. [Google Scholar]
- Roth, V. L. , & Mercer, J. M. (2000). Morphometrics in development and evolution. American Zoologist, 40, 801–810. [Google Scholar]
- Rotmann, E. (1931). Die rolle des ektoderms und mesoderms bei der formbildung der kiemen und extremitäten von triton. Wilhelm Roux' Archiv Für Entwicklungsmechanik Der Organismen, 124, 747–794. [DOI] [PubMed] [Google Scholar]
- Rotmann, E. (1933). Die rolle des ektoderms und mesoderms bei der formbildung der extremitäten von triton. Wilhelm Roux' Archiv fur Entwicklungsmechanik Der Organismen, 129, 85–119. [DOI] [PubMed] [Google Scholar]
- Rouzankina, I. , Abate‐Shen, C. , & Niswander, L. (2004). Dlx genes integrate positive and negative signals during feather bud development. Developmental Biology, 265, 219–233. [DOI] [PubMed] [Google Scholar]
- Rowe, A. , Richman, J. M. , & Brickell, P. M. (1992). Development of the spatial pattern of retinoic acid receptor‐beta transcripts in embryonic chick facial primordia. Development (Cambridge, England), 114, 805–813. [DOI] [PubMed] [Google Scholar]
- Roybal, P. G. , Wu, N. L. , Sun, J. , Ting, M. C. , Schafer, C. A. , & Maxson, R. E. (2010). Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Developmental Biology, 343, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest, L. B. , Xiang, X. , Lim, K. C. , Levi, G. , & Clouthier, D. E. (2004). Endothelin‐A receptor‐dependent and ‐independent signaling pathways in establishing mandibular identity. Development (Cambridge, England), 131, 4413–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhin, B. , Creuzet, S. , Vincent, C. , Benouaiche, L. , Le Douarin, N. M. , & Couly, G. (2003). Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 228, 239–246. [DOI] [PubMed] [Google Scholar]
- Russell, E. S. (1916). Form and function: A contribution to the history of animal morphology. London: John Murray Publishers Ltd. [Google Scholar]
- Saint‐Jeannet, J. P. , & Moody, S. A. (2014). Establishing the pre‐placodal region and breaking it into placodes with distinct identities. Developmental Biology, 389, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun, J. , Salzgeber, B. , & Guenet, J. L. (1986). The differentiation of repeated epilation (Er/Er) mouse mutant skin in organ culture and in grafts. Anatomy and Embryology, 174, 195–205. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Villagra, M. R. , Geiger, M. , & Schneider, R. A. (2016). The taming of the neural crest: A developmental perspective on the origins of morphological covariation in domesticated mammals. Royal Society Open Science, 3, 160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagati, F. , & Rijli, F. M. (2003). Cranial neural crest and the building of the vertebrate head. Nature Reviews. Neuroscience, 4, 806–818. [DOI] [PubMed] [Google Scholar]
- Sasai, Y. , & De Robertis, E. M. (1997). Ectodermal patterning in vertebrate embryos. Developmental Biology, 182, 5–20. [DOI] [PubMed] [Google Scholar]
- Sasano, Y. , Zhu, J. X. , Tsubota, M. , Takahashi, I. , Onodera, K. , Mizoguchi, I. , & Kagayama, M. (2002). Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society, 50, 325–332. [DOI] [PubMed] [Google Scholar]
- Sato, T. , Kurihara, Y. , Asai, R. , Kawamura, Y. , Tonami, K. , Uchijima, Y. , … Kurihara, H. (2008). An endothelin‐1 switch specifies maxillomandibular identity. Proceedings of the National Academy of Sciences of the United States of America, 105, 18806–18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata, I. , & Maas, R. (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nature Genetics, 6, 348–356. [DOI] [PubMed] [Google Scholar]
- Sauka‐Spengler, T. , & Bronner‐Fraser, M. (2008). A gene regulatory network orchestrates neural crest formation. Nature Reviews. Molecular Cell Biology, 9, 557–568. [DOI] [PubMed] [Google Scholar]
- Sauka‐Spengler, T. , Meulemans, D. , Jones, M. , & Bronner‐Fraser, M. (2007). Ancient evolutionary origin of the neural crest gene regulatory network. Developmental Cell, 13, 405–420. [DOI] [PubMed] [Google Scholar]
- Saunders, J. W. , & Gasseling, M. T. (1957). The origin of pattern and feather germ tract specificity. Journal of Experimental Zoology, 135, 503–528. [Google Scholar]
- Saunders, J. W. J. , & Gasseling, M. T. (1968). Ectoderm‐mesenchymal interactions in the origin of wing symmetry In Fleischmajer R., & Billingham R. E. (Eds.), Epithelial‐mesenchymal interactions (pp. 78–97). Baltimore, MD: Williams and Wilkins. [Google Scholar]
- Sawyer, R. H. , O'Guin, W. M. , & Knapp, L. W. (1984). Avian scale development. X. Dermal induction of tissue‐specific keratins in extraembryonic ectoderm. Developmental Biology, 101, 8–18. [DOI] [PubMed] [Google Scholar]
- Scaal, M. , & Marcelle, C. (2018). Chick muscle development. The International Journal of Developmental Biology, 62, 127–136. [DOI] [PubMed] [Google Scholar]
- Scaal, M. , Prols, F. , Fuchtbauer, E. M. , Patel, K. , Hornik, C. , Kohler, T. , … Brand‐Saberi, B. (2002). BMPs induce dermal markers and ectopic feather tracts. Mechanisms of Development, 110, 51–60. [DOI] [PubMed] [Google Scholar]
- Schilling, T. F. (1997). Genetic analysis of craniofacial development in the vertebrate embryo. BioEssays, 19, 459–468. [DOI] [PubMed] [Google Scholar]
- Schilling, T. F. , Piotrowski, T. , Grandel, H. , Brand, M. , Heisenberg, C. P. , Jiang, Y. J. , … Nüsslein‐Volhard, C. (1996). Jaw and branchial arch mutants in zebrafish I: Branchial arches. Development (Cambridge, England), 123, 329–344. [DOI] [PubMed] [Google Scholar]
- Schlosser, G. , & Wagner, G. P. (2004). Modularity in development and evolution. Chicago: University of Chicago Press. [Google Scholar]
- Schneider, R. A. (1999). Neural crest can form cartilages normally derived from mesoderm during development of the avian head skeleton. Developmental Biology, 208, 441–455. [DOI] [PubMed] [Google Scholar]
- Schneider, R. A. (2005). Developmental mechanisms facilitating the evolution of bills and quills. Journal of Anatomy, 207, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, R. A. (2007). How to tweak a beak: Molecular techniques for studying the evolution of size and shape in Darwin's finches and other birds. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 29, 1–6. [DOI] [PubMed] [Google Scholar]
- Schneider, R. A. (2015). Regulation of jaw length during development, disease, and evolution. Current Topics in Developmental Biology, 115, 271–298. [DOI] [PubMed] [Google Scholar]
- Schneider, R. A. (2018). Cellular control of time, size, and shape in development and evolution In Hall B. K., & Moody S. (Eds.), Cells in evolutionary biology: Translating genotypes into phenotypes – past, present, future (pp. 167–212). Boca Raton: CRC Press, Taylor & Francis Group. [Google Scholar]
- Schneider, R. A. , & Helms, J. A. (2003). The cellular and molecular origins of beak morphology. Science (New York, N.Y.), 299, 565–568. [DOI] [PubMed] [Google Scholar]
- Schneider, R. A. , Hu, D. , & Helms, J. A. (1999). From head to toe: Conservation of molecular signals regulating limb and craniofacial morphogenesis. Cell and Tissue Research, 296, 103–109. [DOI] [PubMed] [Google Scholar]
- Schneider, R. A. , Hu, D. , Rubenstein, J. L. , Maden, M. , & Helms, J. A. (2001). Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development (Cambridge, England), 128, 2755–2767. [DOI] [PubMed] [Google Scholar]
- Schnorrer, F. , & Dickson, B. J. (2004). Muscle building; mechanisms of myotube guidance and attachment site selection. Developmental Cell, 7, 9–20. [DOI] [PubMed] [Google Scholar]
- Schowing, J. (1968). Influence inductrice de l'encéphale embryonnaire sur le développement du crâne chez le Poulet. Journal of Embryology and Experimental Morphology, 19, 9–32. [PubMed] [Google Scholar]
- Schweitzer, R. , Chyung, J. H. , Murtaugh, L. C. , Brent, A. E. , Rosen, V. , Olson, E. N. , … Tabin, C. J. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development (Cambridge, England), 128, 3855–3866. [DOI] [PubMed] [Google Scholar]
- Schwind, J. L. (1931). Heteroplastic experiments on the limb and shoulder girdle of Amblystoma. Journal of Experimental Zoology, 59, 265–295. [Google Scholar]
- Schwind, J. L. (1932). Further experiments on limbs containing tissue of two species. Journal of Experimental Zoology, 63, 365–369. [Google Scholar]
- Seales, E. C. , Micoli, K. J. , & McDonald, J. M. (2006). Calmodulin is a critical regulator of osteoclastic differentiation, function, and survival. Journal of Cellular Biochemistry, 97, 45–55. [DOI] [PubMed] [Google Scholar]
- Sears, K. E. , Goswami, A. , Flynn, J. J. , & Niswander, L. A. (2007). The correlated evolution of Runx2 tandem repeats, transcriptional activity, and facial length in carnivora. Evolution &Amp; Development, 9, 555–565. [DOI] [PubMed] [Google Scholar]
- Selleck, M. A. J. , & Bronner‐Fraser, M. (1995). Origins of the avian neural crest: The role of neural plate‐epidermal interactions. Development, 121, 525–538. [DOI] [PubMed] [Google Scholar]
- Sengel, P. (1990). Pattern formation in skin development. The International Journal of Developmental Biology, 34, 33–50. [PubMed] [Google Scholar]
- Serbedzija, G. N. , Bronner‐Fraser, M. , & Fraser, S. E. (1989). A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development (Cambridge, England), 106, 809–816. [DOI] [PubMed] [Google Scholar]
- Serbedzija, G. N. , & McMahon, A. P. (1997). Analysis of neural crest cell migration in Splotch mice using a neural crest‐specific LacZ reporter. Developmental Biology, 185, 139–147. [DOI] [PubMed] [Google Scholar]
- Severinghaus, A. E. (1930). Gill development in Amblystoma punctatum. Journal of Experimental Zoology, 56, 1–29. [Google Scholar]
- Sharpe, P. M. , & Ferguson, M. W. (1988). Mesenchymal influences on epithelial differentiation in developing systems. Journal of Cell Science. Supplement, 10, 195–230. [DOI] [PubMed] [Google Scholar]
- Shea, B. T. (1985). Ontogenetic allometry and scaling: A discussion based on the growth and form of the skull in African apes In Jungers W. L. (Ed.), Size and scaling in primate biology (pp. 175–205). New York, NY: Springer. [Google Scholar]
- Shigetani, Y. , Nobusada, Y. , & Kuratani, S. (2000). Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: Epithelial‐mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Developmental Biology, 228, 73–85. [DOI] [PubMed] [Google Scholar]
- Shigetani, Y. , Sugahara, F. , Kawakami, Y. , Murakami, Y. , Hirano, S. , & Kuratani, S. (2002). Heterotopic shift of epithelial‐mesenchymal interactions in vertebrate jaw evolution. Science (New York, N.Y.), 296, 1316–1319. [DOI] [PubMed] [Google Scholar]
- Shimamura, K. , Hartigan, D. J. , Martinez, S. , Puelles, L. , & Rubenstein, J. L. (1995). Longitudinal organization of the anterior neural plate and neural tube. Development (Cambridge, England), 121, 3923–3933. [DOI] [PubMed] [Google Scholar]
- Shone, V. , & Graham, A. (2014). Endodermal/ectodermal interfaces during pharyngeal segmentation in vertebrates. Journal of Anatomy, 225, 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukunami, C. , Takimoto, A. , Oro, M. , & Hiraki, Y. (2006). Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental Biology, 298, 234–247. [DOI] [PubMed] [Google Scholar]
- Siegel, A. F. , & Benson, R. H. (1982). A robust comparison of biological shapes. Biometrics, 38, 341–350. [PubMed] [Google Scholar]
- Simoes‐Costa, M. , & Bronner, M. E. (2016). Reprogramming of avian neural crest axial identity and cell fate. Science (New York, N.Y.), 352, 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes‐Costa, M. , Tan‐Cabugao, J. , Antoshechkin, I. , Sauka‐Spengler, T. , & Bronner, M. E. (2014). Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Research, 24, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , Albert, F. W. , Plyusnina, I. , Trut, L. , Pӓӓbo, S. , & Harvati, K. (2017). Facial shape differences between rats selected for tame and aggressive behaviors. PLoS One, 12, e0175043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, F. J. , Percival, C. J. , Young, N. M. , Hu, D. , Schneider, R. A. , Marcucio, R. S. , & Hallgrimsson, B. (2015). Divergence of craniofacial developmental trajectories among avian embryos. Developmental Dynamics, 244, 1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. K. (2001). Heterochrony revisited: The evolution of developmental sequences. Biological Journal of the Linnean Society, 73, 169–186. [Google Scholar]
- Smith, K. K. (2002). Sequence heterochrony and the evolution of development. Journal of Morphology, 252, 82–97. [DOI] [PubMed] [Google Scholar]
- Smith, K. K. (2003). Time's arrow: Heterochrony and the evolution of development. The International Journal of Developmental Biology, 47, 613–621. [PubMed] [Google Scholar]
- Smith, K. K. , & Schneider, R. A. (1998). Have gene knockouts caused evolutionary reversals in the mammalian first arch?. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 20, 245–255. [DOI] [PubMed] [Google Scholar]
- Smith, M. M. , & Hall, B. K. (1990). Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biological Reviews of the Cambridge Philosophical Society, 65, 277–373. [DOI] [PubMed] [Google Scholar]
- Sohal, G. S. (1976). Effects of reciprocal forebrain transplantation on motility and hatching in chick and duck embryos. Brain Research, 113, 35–43. [DOI] [PubMed] [Google Scholar]
- Sohal, G. S. , Bal, H. S. , Campbell, L. R. , Husain, I. , Arumugam, T. , & Kumaresan, K. (1990). Synapse formation on quail trochlear neurons transplanted in duck embryos before naturally occurring motor neuron death. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 8, 9–16. [DOI] [PubMed] [Google Scholar]
- Sohal, G. S. , Knox, T. S. , Allen, J. C., Jr. , Arumugam, T. , Campbell, L. R. , & Yamashita, T. (1985). Development of the trochlear nucleus in quail and comparative study of the trochlear nucleus, nerve, and innervation of the superior oblique muscle in quail, chick, and duck. The Journal of Comparative Neurology, 239, 227–236. [DOI] [PubMed] [Google Scholar]
- Solem, R. C. , Eames, B. F. , Tokita, M. , & Schneider, R. A. (2011). Mesenchymal and mechanical mechanisms of secondary cartilage induction. Developmental Biology, 356, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway, M. J. , Dudley, A. T. , Bikoff, E. K. , Lyons, K. M. , Hogan, B. L. , & Robertson, E. J. (1998). Mice lacking Bmp6 function. Developmental Genetics, 22, 321–339. [DOI] [PubMed] [Google Scholar]
- Song, H. K. , Lee, S. H. , & Goetinck, P. F. (2004). FGF‐2 signaling is sufficient to induce dermal condensations during feather development. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 231, 741–749. [DOI] [PubMed] [Google Scholar]
- Song, H. K. , & Sawyer, R. H. (1996). Dorsal dermis of the scaleless (sc/sc) embryo directs normal feather pattern formation until day 8 of development. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 205, 82–91. [DOI] [PubMed] [Google Scholar]
- Song, H. , Wang, Y. , & Goetinck, P. F. (1996). Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proceedings of the National Academy of Sciences of the United States of America, 93, 10246–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Hui, J. N. , Fu, K. K. , & Richman, J. M. (2004). Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton. Developmental Biology, 276, 313–329. [DOI] [PubMed] [Google Scholar]
- Soni, V. C. (1979). The role of kinesis and mechanical advantage in the feeding apparatus of some partridges and quails. Annals of Zoology, 15, 103–110. [Google Scholar]
- Spemann, H. (1918). Über die determination der ersten organanlagen des amphibienembryo I–VI. Archiv Für Entwicklungsmechanik Der Organismen, 43, 448–555. [Google Scholar]
- Spemann, H. (1921). Die erzeugung tierischer chimären durch heteroplastische embryonale transplantation zwischen triton cristatus und taeniatus. Archiv Für Entwicklungsmechanik Der Organismen, 48, 533–570. [Google Scholar]
- Spemann, H. (1938). Embryonic development and induction. New Haven: Yale University Press. [Google Scholar]
- Spemann, H. , & Mangold, H. (1924). Induction of embryonic primordia by implantation of organizers from a different species (pp. 144–184). New York, NY: Hafner. [PubMed] [Google Scholar]
- Spemann, H. , & Schotte, O. (1932). Über xenoplastische Transplantation als Mittel zur Analyse der embryonvalen Induktion. Die Naturwissenschaften, 20, 463–467. [Google Scholar]
- Square, T. , Jandzik, D. , Romasek, M. , Cerny, R. , & Medeiros, D. M. (2017). The origin and diversification of the developmental mechanisms that pattern the vertebrate head skeleton. Developmental Biology, 427, 219–229. [DOI] [PubMed] [Google Scholar]
- Starck, J. M. (1989). Zeitmuster der Ontogenesen bei nestflüchtenden und nesthockenden Vögeln. Cour Forsch‐Inst Senckenberg, 114, 1–319. [Google Scholar]
- Starck, J. M. , & Ricklefs, R. E. (1998). Avian growth and development: Evolution within the altricial‐precocial spectrum. New York, NY: Oxford University Press. [Google Scholar]
- Stern, D. L. , & Emlen, D. J. (1999). The developmental basis for allometry in insects. Development (Cambridge, England), 126, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Steventon, B. , Mayor, R. , & Streit, A. (2014). Neural crest and placode interaction during the development of the cranial sensory system. Developmental Biology, 389, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, K. M. , Brown, A. M. C. , & Ciment, G. (1993). Gene transfer of lacz into avian neural tube and neural crest by retroviral infection of grafted embryonic tissues. Journal of Neuroscience Research, 34, 135–145. [DOI] [PubMed] [Google Scholar]
- Stone, L. S. (1922). Experiments on the development of the cranial ganglia and the lateral line sense organs in Amblystoma punctatum. Journal of Experimental Zoology, 35, 420–496. [Google Scholar]
- Stone, L. S. (1926). Further experiments on the extirpation and transplantation of mesectoderm in Amblystoma punctatum . Journal of Experimental Zoology, 44, 95–131. [Google Scholar]
- Stone, L. S. (1929). Experiments showing the role of migrating neural crest (mesectoderm) in the formation of head skeleton and loose connective tissue in Rana palustris. Wilhelm Roux' Archiv Für Entwicklungsmechanik Der Organismen, 118, 40–77. [DOI] [PubMed] [Google Scholar]
- Stone, L. S. (1930). Heteroplastic transplantation of eyes between the larvae of two species of Amblystoma. Journal of Experimental Zoology, 55, 193–261. [Google Scholar]
- Stone, L. S. (1932). Transplantation of hyobranchial mesentoderm, including the right lateral anlage of the second basibranchium, in Amblystoma punctatum. Journal of Experimental Zoology, 62, 109–123. [Google Scholar]
- Stone, L. S. , & Dinnean, F. L. (1940). Experimental studies on the relation of the optic vesicle and cup to lens formation in Amblystoma punctatum. Journal of Experimental Zoology, 83, 95–125. [Google Scholar]
- Subramanian, A. , & Schilling, T. F. (2015). Tendon development and musculoskeletal assembly: Emerging roles for the extracellular matrix. Development (Cambridge, England), 142, 4191–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara, F. , Aota, S. , Kuraku, S. , Murakami, Y. , Takio‐Ogawa, Y. , Hirano, S. , & Kuratani, S. (2011). Involvement of Hedgehog and FGF signalling in the lamprey telencephalon: Evolution of regionalization and dorsoventral patterning of the vertebrate forebrain. Development (Cambridge, England), 138, 1217–1226. [DOI] [PubMed] [Google Scholar]
- Sugii, H. , Grimaldi, A. , Li, J. , Parada, C. , Vu‐Ho, T. , Feng, J. , … Chai, Y. (2017). The Dlx5‐FGF10 signaling cascade controls cranial neural crest and myoblast interaction during oropharyngeal patterning and development. Development (Cambridge, England), 144, 4037–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett, F. H. (1930). The permanence of limb‐axis polarity. Journal of Experimental Zoology, 55, 87–99. [Google Scholar]
- Szabo‐Rogers, H. L. , Geetha‐Loganathan, P. , Nimmagadda, S. , Fu, K. K. , & Richman, J. M. (2008). FGF signals from the nasal pit are necessary for normal facial morphogenesis. Developmental Biology, 318, 289–302. [DOI] [PubMed] [Google Scholar]
- Tak, H. J. , Park, T. J. , Piao, Z. , & Lee, S. H. (2017). Separate development of the maxilla and mandible is controlled by regional signaling of the maxillomandibular junction during avian development. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 246, 28–40. [DOI] [PubMed] [Google Scholar]
- Takio, Y. , Pasqualetti, M. , Kuraku, S. , Hirano, S. , Rijli, F. M. , & Kuratani, S. (2004). Evolutionary biology: Lamprey Hox genes and the evolution of jaws. Nature, 429, 1. [DOI] [PubMed] [Google Scholar]
- Tanda, N. , Ohuchi, H. , Yoshioka, H. , Noji, S. , & Nohno, T. (1995). A chicken Wnt gene, Wnt‐11, is involved in dermal development. Biochemical and Biophysical Research Communications, 211, 123–129. [DOI] [PubMed] [Google Scholar]
- Tang, S. Y. , Herber, R. P. , Ho, S. P. , & Alliston, T. (2012). Matrix metalloproteinase‐13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 27, 1936–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, H. , Yoshimoto, Y. , Yoshioka, H. , Nohno, T. , Noji, S. , & Ohuchi, H. (2002). FGF10 is a mesenchymally derived stimulator for epidermal development in the chick embryonic skin. Mechanisms of Development, 116, 39–49. [DOI] [PubMed] [Google Scholar]
- Tavares, A. L. P. , Cox, T. C. , Maxson, R. M. , Ford, H. L. , & Clouthier, D. E. (2017). Negative regulation of endothelin signaling by SIX1 is required for proper maxillary development. Development (Cambridge, England), 144, 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares, A. L. , Garcia, E. L. , Kuhn, K. , Woods, C. M. , Williams, T. , & Clouthier, D. E. (2012). Ectodermal‐derived Endothelin1 is required for patterning the distal and intermediate domains of the mouse mandibular arch. Developmental Biology, 371, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum, S. L. (2000). Bone resorption by osteoclasts. Science (New York, N.Y.), 289, 1504–1508. [DOI] [PubMed] [Google Scholar]
- Teitelbaum, S. L. , Tondravi, M. M. , & Ross, F. P. (1997). Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. Journal of Leukocyte Biology, 61, 381–388. [DOI] [PubMed] [Google Scholar]
- Thesleff, I. , & Sharpe, P. (1997). Signalling networks regulating dental development. Mechanisms of Development, 67, 111–123. [DOI] [PubMed] [Google Scholar]
- Thomas, D. M. , Johnson, S. A. , Sims, N. A. , Trivett, M. K. , Slavin, J. L. , Rubin, B. P. , … Hinds, P. W. (2004). Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. The Journal of Cell Biology, 167, 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, D. W. ( 1917). On growth and form. Cambridge: University Press. [Google Scholar]
- Thompson, D. W. ( 1952). On growth and form. Cambridge: University Press; (1116 pages). [Google Scholar]
- Thorogood, P. (1987). Mechanisms of morphogenetic specification in skull development In Wolff J. R. (Ed.), Mesenchymal‐epithelial interactions in neural development (pp. 141–152). Berlin: Springer‐Verlag. [Google Scholar]
- Thorogood P. ( 1988). The developmental specification of the vertebrate skull. Development 103, 141–153. [DOI] [PubMed] [Google Scholar]
- Thorogood, P. (1993). Differentiation and morphogenesis of cranial skeletal tissues In Hanken J., & Hall B. K. (Eds.), The skull (pp. 112–152). Chicago: University of Chicago Press. [Google Scholar]
- Thorogood, P. , Bee, J. , & von der Mark, K. (1986). Transient expression of collagen type II at epitheliomesenchymal interfaces during morphogenesis of the cartilaginous neurocranium. Developmental Biology, 116, 497–509. [DOI] [PubMed] [Google Scholar]
- Ting‐Berreth, S. A. , & Chuong, C. M. (1996). Sonic Hedgehog in feather morphogenesis: Induction of mesenchymal condensation and association with cell death. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 207, 157–170. [DOI] [PubMed] [Google Scholar]
- Tokita, M. (2004). Morphogenesis of parrot jaw muscles: Understanding the development of an evolutionary novelty. Journal of Morphology, 259, 69–81. [DOI] [PubMed] [Google Scholar]
- Tokita, M. (2006). Cranial neural crest cell migration in cockatiel Nymphicus hollandicus (Aves: Psittaciformes). Journal of Morphology, 267, 333–340. [DOI] [PubMed] [Google Scholar]
- Tokita, M. , Kiyoshi, T. , & Armstrong, K. N. (2007). Evolution of craniofacial novelty in parrots through developmental modularity and heterochrony. Evolution & Development, 9, 590–601. [DOI] [PubMed] [Google Scholar]
- Tokita, M. , Nakayama, T. , Schneider, R. A. , & Agata, K. (2013). Molecular and cellular changes associated with the evolution of novel jaw muscles in parrots. Proceedings of Biological Sciences, 280, 20122319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita, M. , & Schneider, R. A. (2009). Developmental origins of species‐specific muscle pattern. Developmental Biology, 331, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa, Y. (1973). Inductive tissue interactions in beak of a chick‐embryo. Development, Growth and Differentiation, 15, 57–71. [DOI] [PubMed] [Google Scholar]
- Tosney, K. W. (1982). The segregation and early migration of cranial neural crest cells in the avian embryo. Developmental Biology, 89, 13–24. [DOI] [PubMed] [Google Scholar]
- Trainor, P. , & Krumlauf, R. (2000). Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nature Cell Biology, 2, 96–102. [DOI] [PubMed] [Google Scholar]
- Trainor, P. A. , Ariza‐McNaughton, L. , & Krumlauf, R. (2002). Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science (New York, N.Y.), 295, 1288–1291. [DOI] [PubMed] [Google Scholar]
- Trainor, P. A. , & Krumlauf, R. (2001). Hox genes, neural crest cells and branchial arch patterning. Current Opinion in Cell Biology, 13, 698–705. [DOI] [PubMed] [Google Scholar]
- Trainor, P. A. , Melton, K. R. , & Manzanares, M. (2003). Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. The International Journal of Developmental Biology, 47, 541–553. [PubMed] [Google Scholar]
- Trainor PA, Sobieszczuk D, Wilkinson D, Krumlauf R. ( 2002b). Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development, 129, 433–442. [DOI] [PubMed] [Google Scholar]
- Tribulo, C. , Aybar, M. J. , Nguyen, V. H. , Mullins, M. C. , & Mayor, R. (2003). Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development (Cambridge, England), 130, 6441–6452. [DOI] [PubMed] [Google Scholar]
- Trinh, L. A. , Chong‐Morrison, V. , Gavriouchkina, D. , Hochgreb‐Hägele, T. , Senanayake, U. , Fraser, S. E. , & Sauka‐Spengler, T. (2017). Biotagging of specific cell populations in zebrafish reveals gene regulatory logic encoded in the nuclear transcriptome. Cell Reports, 19, 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, A. S. , Al Khamis, A. , & Sharpe, P. T. (1998). Interactions between Bmp‐4 and Msx‐1 act to restrict gene expression to odontogenic mesenchyme. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 212, 533–539. [DOI] [PubMed] [Google Scholar]
- Tucker, A. S. , & Lumsden, A. (2004). Neural crest cells provide species‐specific patterning information in the developing branchial skeleton. Evolution & Development, 6, 32–40. [DOI] [PubMed] [Google Scholar]
- Tucker, A. S. , Yamada, G. , Grigoriou, M. , Pachnis, V. , & Sharpe, P. T. (1999). Fgf‐8 determines rostral‐caudal polarity in the first branchial arch. Development (Cambridge, England), 126, 51–61. [DOI] [PubMed] [Google Scholar]
- Twitty, V. C. (1930). Regulation in the growth of transplanted eyes. Journal of Experimental Zoology, 55, 43–52. [Google Scholar]
- Twitty, V. C. (1932). Influence of the eye on the growth of its associated structures, studied by means of heteroplastic transplantation. Journal of Experimental Zoology, 61, 333–374. [Google Scholar]
- Twitty, V. C. (1934). Growth correlations in amphibia studied by the method of transplantation. Cold Spring Harbor Symposia on Quantitative Biology, 2, 148–156. [Google Scholar]
- Twitty, V. C. (1936). Correlated genetic and embryological experiments on Triturus. I and II. Journal of Experimental Zoology, 74, 239–302. [Google Scholar]
- Twitty, V. C. (1945). The developmental analysis of specific pigment patterns. Journal of Experimental Zoology, 100, 141–178. [Google Scholar]
- Twitty, V. C. ( 1966). Of Scientists and Salamanders. San Francisco: W. H. Freeman and Company. [Google Scholar]
- Twitty, V. C. , & Bodenstein, D. (1939). Correlated genetic and embryological experiments on Triturus. III. Further transplantation experiments on pigment development. IV. The study of pigment cell behavior in vitro. Journal of Experimental Zoology, 81, 357–398. [Google Scholar]
- Twitty, V. C. , & Schwind, J. L. (1931). The growth of eyes and limbs transplanted heteroplastically between two species of Amblystoma. Journal of Experimental Zoology, 59, 61–86. [Google Scholar]
- Tyler, M. S. (1978). Epithelial influences on membrane bone formation in the maxilla of the embryonic chick. The Anatomical Record, 192, 225–233. [DOI] [PubMed] [Google Scholar]
- Tyler, M. S. (1983). Development of the frontal bone and cranial meninges in the embryonic chick: An experimental study of tissue interactions. The Anatomical Record, 206, 61–70. [DOI] [PubMed] [Google Scholar]
- Tzahor, E. , Kempf, H. , Mootoosamy, R. C. , Poon, A. C. , Abzhanov, A. , Tabin, C. J. , … Lassar, A. B. (2003). Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes & Development, 17, 3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist, M. R. (1965). Bone: Formation by autoinduction. Science (New York, N.Y.), 150, 893–899. [DOI] [PubMed] [Google Scholar]
- Uygur, A. , Young, J. , Huycke, T. R. , Koska, M. , Briscoe, J. , & Tabin, C. J. (2016). Scaling pattern to variations in size during development of the vertebrate neural tube. Developmental Cell, 37, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel, W. F. (1992). Kinetics of the skull in the chicken (Gallus Gallus Domesticus). Netherlands Journal of Zoology, 42, 561–582. [Google Scholar]
- Veitch, E. , Begbie, J. , Schilling, T. F. , Smith, M. M. , & Graham, A. (1999). Pharyngeal arch patterning in the absence of neural crest. Current Biology: Cb, 9, 1481–1484. [DOI] [PubMed] [Google Scholar]
- Viallet, J. P. , Prin, F. , Olivera‐Martinez, I. , Hirsinger, E. , Pourquié, O. , & Dhouailly, D. (1998). Chick Delta‐1 gene expression and the formation of the feather primordia. Mechanisms of Development, 72, 159–168. [DOI] [PubMed] [Google Scholar]
- Von Bonin, G. (1937). Brain‐weight and body‐weight of mammals. Journal of General Psychology, 16, 379–389. [Google Scholar]
- Wachtler, F. , & Jacob, M. (1986). Origin and development of the cranial skeletal muscles. Bibliotheca Anatomica, 29, 24–46. [PubMed] [Google Scholar]
- Wada, N. , Javidan, Y. , Nelson, S. , Carney, T. J. , Kelsh, R. N. , & Schilling, T. F. (2005). Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development (Cambridge, England), 132, 3977–3988. [DOI] [PubMed] [Google Scholar]
- Waddington, C. H. (1930). Developmental mechanics of chick and duck embryos. Nature, 125, 924–925. [Google Scholar]
- Waddington, C. H. (1932). Experiments on the development of chick and duck embryos, cultivated in vitro. Philosophical Transactions of the Royal Society of London, 221, 179–230. [Google Scholar]
- Waddington, C. H. (1950). The Biological foundations of measurements of growth and form. proceedings of the royal society of London. Proceedings of the Royal Society of London. Series B, Biological Sciences, 137, 509–515. [DOI] [PubMed] [Google Scholar]
- Waddington, C. H. (1957). The strategy of the genes. London: George Allan Unwin. [Google Scholar]
- Wagner, G. (1959). Untersuchungen an Bombinator‐Triton‐Chimaeren. Wilhelm Roux' Archiv Fur Entwicklungsmechanik Der Organismen, 151, 136–158. [DOI] [PubMed] [Google Scholar]
- Wall, N. A. , & Hogan, B. L. (1995). Expression of bone morphogenetic protein‐4 (BMP‐4), bone morphogenetic protein‐7 (BMP‐7), fibroblast growth factor‐8 (FGF‐8) and sonic hedgehog (SHH) during branchial arch development in the chick. Mechanisms of Development, 53, 383–392. [DOI] [PubMed] [Google Scholar]
- Wang, E. A. , Rosen, V. , D'Alessandro, J. S. , Bauduy, M. , Cordes, P. , Harada, T. , … LaPan, P. (1990). Recombinant human bone morphogenetic protein induces bone formation. Proceedings of the National Academy of Sciences of the United States of America, 87, 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. H. , Upholt, W. B. , Sharpe, P. T. , Kollar, E. J. , & Mina, M. (1998). Odontogenic epithelium induces similar molecular responses in chick and mouse mandibular mesenchyme. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 213, 386–397. [DOI] [PubMed] [Google Scholar]
- Webb, J. F. , & Noden, D. M. (1993). Ectodermal placodes: Contributions to the development of the vertebrate head. American Zoologist, 33, 434–447. [Google Scholar]
- Wedden, S. E. (1987). Epithelial‐mesenchymal interactions in the development of chick facial primordia and the target of retinoid action. Development (Cambridge, England), 99, 341–351. [DOI] [PubMed] [Google Scholar]
- Wessells, N. K. (1965). Morphology and proliferation during early feather development. Developmental Biology, 12, 131–153. [DOI] [PubMed] [Google Scholar]
- West‐Eberhard, M. J. (1989). Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics, 20, 249–278. [Google Scholar]
- West‐Eberhard, M. J. (2003). Developmental plasticity and evolution. Oxford; New York: Oxford University Press. [Google Scholar]
- Widelitz, R. B. , & Chuong, C. M. (1999). Early events in skin appendage formation: Induction of epithelial placodes and condensation of dermal mesenchyme. The Journal of Investigative Dermatology. Symposium Proceedings, 4, 302–306. [DOI] [PubMed] [Google Scholar]
- Widelitz, R. B. , Jiang, T. X. , Chen, C. W. , Stott, N. S. , & Chuong, C. M. ( 1999). Wnt‐7a in feather morphogenesis: Involvement of anterior‐posterior asymmetry and proximal‐distal elongation demonstrated with an in vitro reconstitution model. Development, 126, 2577–2587. [DOI] [PubMed] [Google Scholar]
- Widelitz, R. B. , Jiang, T. X. , Lu, J. , & Chuong, C. M. (2000). beta‐catenin in epithelial morphogenesis: Conversion of part of avian foot scales into feather buds with a mutated beta‐catenin. Developmental Biology, 219, 98–114. [DOI] [PubMed] [Google Scholar]
- Widelitz, R. B. , Jiang, T. X. , Noveen, A. , Chen, C. W. , & Chuong, C. M. (1996). FGF induces new feather buds from developing avian skin. The Journal of Investigative Dermatology, 107, 797–803. [DOI] [PubMed] [Google Scholar]
- Widelitz, R. B. , Jiang, T. X. , Noveen, A. , Ting‐Berreth, S. A. , Yin, E. , Jung, H. S. , & Chuong, C. M. (1997). Molecular histology in skin appendage morphogenesis. Microscopy Research and Technique, 38, 452–465. [DOI] [PubMed] [Google Scholar]
- Wilkins, A. S. , Wrangham, R. W. , & Fitch, W. T. (2014). The “domestication syndrome” in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics, 197, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, R. M. , Senanayake, U. , Artibani, M. , Taylor, G. , Wells, D. , Ahmed, A. A. , & Sauka‐Spengler, T. (2018). Genome and epigenome engineering CRISPR toolkit for in vivo modulation of cis‐regulatory interactions and gene expression in the chicken embryo. Development, 145, dev160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J. , & Tucker, A. S. (2004). Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Developmental Biology, 266, 138–150. [DOI] [PubMed] [Google Scholar]
- Withington, S. , Beddington, R. , & Cooke, J. (2001). Foregut endoderm is required at head process stages for anteriormost neural patterning in chick. Development (Cambridge, England), 128, 309–320. [DOI] [PubMed] [Google Scholar]
- Woronowicz, K. C. , Gline, S. E. , Herfat, S. T. , Fields, A. J. , & Schneider, R. A. (2018). FGF and TGFβ signaling link form and function during jaw development and evolution. Developmental Biology, 10.1016/j.ydbio.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney, J. M. , Rosen, V. , Celeste, A. J. , Mitsock, L. M. , Whitters, M. J. , Kriz, R. W. , … Wang, E. A. (1988). Novel regulators of bone formation: Molecular clones and activities. Science (New York, N.Y.), 242, 1528–1534. [DOI] [PubMed] [Google Scholar]
- Wu, P. , Jiang, T. X. , Shen, J. Y. , Widelitz, R. B. , & Chuong, C. M. (2006). Morphoregulation of avian beaks: Comparative mapping of growth zone activities and morphological evolution. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 235, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, P. , Jiang, T. X. , Suksaweang, S. , Widelitz, R. B. , & Chuong, C. M. (2004). Molecular shaping of the beak. Science (New York, N.Y.), 305, 1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, S. L. , & Ferrier, J. (1996). Localized calcium signaling in multinucleated osteoclasts. Journal of Cellular Physiology, 167, 148–155. [DOI] [PubMed] [Google Scholar]
- Xiong, J. , & O'Brien, C. A. (2012). Osteocyte RANKL: New insights into the control of bone remodeling. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 27, 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, J. , Piemontese, M. , Thostenson, J. D. , Weinstein, R. S. , Manolagas, S. C. , & O'Brien, C. A. (2014). Osteocyte‐derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone, 66, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q. , Jamniczky, H. , Hu, D. , Green, R. M. , Marcucio, R. S. , Hallgrimsson, B. , & Mio, W. (2015). Correlations between the morphology of sonic hedgehog expression domains and embryonic craniofacial shape. Evolutionary Biology, 42, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, T. , & Sohal, G. S. (1986). Development of smooth and skeletal muscle cells in the iris of the domestic duck, chick and quail. Cell and Tissue Research, 244, 121–131. [DOI] [PubMed] [Google Scholar]
- Yamashita, T. , & Sohal, G. S. (1987). Embryonic origin of skeletal muscle cells in the iris of the duck and quail. Cell and Tissue Research, 249, 31–37. [DOI] [PubMed] [Google Scholar]
- Yang, L. , O'Neill, P. , Martin, K. , Maass, J. C. , Vassilev, V. , Ladher, R. , & Groves, A. K. (2013). Analysis of FGF‐dependent and FGF‐independent pathways in otic placode induction. PLoS One, 8, e55011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, T. , Ohtani, K. , Kuratani, S. , & Wada, H. (2011). Development of lamprey mucocartilage and its dorsal‐ventral patterning by endothelin signaling, with insight into vertebrate jaw evolution. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 316, 339–346. [DOI] [PubMed] [Google Scholar]
- Yasui, K. , & Hayashi, Y. (1967). Morphogenesis of the beak of the chick embryo: Histological, histochemical and autoradiographic studies. Embryologia, 10, 42–74. [DOI] [PubMed] [Google Scholar]
- Yoshizawa, M. , Hixon, E. , & Jeffery, W. R. (2018). Neural crest transplantation reveals key roles in the evolution of cavefish development. Integrative and Comparative Biology, 52, icy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, N. M. , Chong, H. J. , Hu, D. , Hallgrimsson, B. , & Marcucio, R. S. (2010). Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development (Cambridge, England), 137, 3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, D. W. , Hassan, M. Q. , Pratap, J. , Galindo, M. , Zaidi, S. K. , Lee, S. H. , … Stein, G. S. (2007). Mitotic occupancy and lineage‐specific transcriptional control of rRNA genes by Runx2. Nature, 445, 442–446. [DOI] [PubMed] [Google Scholar]
- Young, N. M. , Hu, D. , Lainoff, A. J. , Smith, F. J. , Diaz, R. , Tucker, A. S. , … Marcucio, R. S. (2014). Embryonic bauplans and the developmental origins of facial diversity and constraint. Development (Cambridge, England), 141, 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M. , Wu, P. , Widelitz, R. B. , & Chuong, C. M. (2002). The morphogenesis of feathers. Nature, 420, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M. , Yue, Z. , Wu, P. , Wu, D. Y. , Mayer, J. A. , Medina, M. , … Chuong, C. M. (2004). The developmental biology of feather follicles. The International Journal of Developmental Biology, 48, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchei, A. M. (1961). The embryonic development of the Japanese quail (Coturnix coturnix japonica). Archivio Italiano Di Anatomia e Di Embriologia. Italian Journal of Anatomy and Embryology, 66, 36–62. [PubMed] [Google Scholar]
- Zavitz, K. H. , & Zipursky, S. L. (1997). Controlling cell proliferation in differentiating tissues: Genetic analysis of negative regulators of G1–>S‐phase progression. Current Opinion in Cell Biology, 9, 773–781. [DOI] [PubMed] [Google Scholar]
- Zayzafoon, M. (2006). Calcium/calmodulin signaling controls osteoblast growth and differentiation. Journal of Cellular Biochemistry, 97, 56–70. [DOI] [PubMed] [Google Scholar]
- Zelditch, M. (2004). Geometric morphometrics for biologists: A primer. Amsterdam; Boston: Elsevier Academic Press. [Google Scholar]
- Zhang, W. , Bergamaschi, D. , Jin, B. , & Lu, X. (2005). Posttranslational modifications of p27kip1 determine its binding specificity to different cyclins and cyclin‐dependent kinases in vivo. Blood, 105, 3691–3698. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Eberspaecher, H. , Lefebvre, V. , & De Crombrugghe, B. (1997). Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 209, 377–386. [DOI] [PubMed] [Google Scholar]
- Zusi, R. L. (1993). Patterns of diversity in the avian skull In Hanken J., & Hall B. K. (Eds.), The skull (pp. 391–437). Chicago: University of Chicago Press. [Google Scholar]
- Zweers, G. (1974). Structure, movement, and myography of the feeding apparatus of the mallard (Anas platyrhynchos L.). A study in functional anatomy. Netherlands Journal of Zoology, 24, 323–467. [Google Scholar]
- Zweers, G. A. , Gerritsen, A. F. C. , & Kranenburg‐Voogd, P. J. (1977). Mechanics of feeding of the mallard (Anas platyrhynchos L.; Aves, Anseriformes): The lingual apparatus and the suction‐pressure pump mechanism of straining. Basel; New York: S. Karger. [Google Scholar]
- Zweers, G. A. , Kunz, G. , & Mos, J. ( 1977). Functional anatomy of the feeding apparatus of the mallard (Anas platyrhynchos L.) structure, movement, electro‐myography and electro‐neurography. Annals of Anatomy, 142, 10–20. [PubMed] [Google Scholar]
- Zwilling, E. (1959). Interaction between ectoderm and mesoderm in duck‐chicken limb bud chimaeras. The Journal of Experimental Zoology, 142, 521–532. [DOI] [PubMed] [Google Scholar]


