Abstract
Purpose
We assess parent-child agreement regarding child's health-related quality of life (HRQoL) in children operated for congenital glaucoma (CG).
Methods
A total of 121 children aged 8 to 18 years (mean age, 11.8 years) operated for CG (mean duration since surgery, 10.2 years) and their parents (mean age, 36.5 years) completed the child and parent versions of the Kidscreen-27 questionnaire, respectively. Psychometric properties of Kidscreen-27 were assessed using Rasch analysis, and child–parent agreement regarding child's HRQoL was investigated using the Bland-Altman limits of agreement (LoA) method.
Results
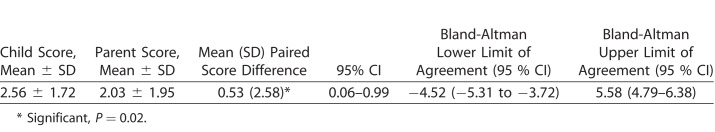
Minor modifications in the rating scale and deletion of few misfitting items resulted in a psychometrically robust Kidscreen-23 questionnaire. Average parental HRQoL score was higher than the child's own ratings, with a significant difference between their scores (mean ± standard deviation [SD] difference = 0.53 ± 2.58 logits, P = 0.02; lower LoA [95% CI], −4.52 [−5.31 to −3.72] and upper LoA [95% CI], 5.58 [4.79–6.38]). The range of child–parent agreement was wide and bidirectional, with parents tending to underestimate and overestimate their child's HRQoL. Younger children and girls showed greater discordance in their HRQoL with parental reports than adolescents and boys, respectively.
Conclusions
Discordance between CG child's self-report of HRQoL and parent's report indicate that both groups perceive the broader impact of living with CG very differently.
Translational Relevance
The HRQoL as reported by the child with CG and by his/her parent should be viewed as being complementary, rather than interchangeable. Both assessments should be taken into account in clinical practice and research studies.
Keywords: congenital glaucoma, health-related quality of life, Kidscreen-27, agreement, Bland-Altman
Introduction
Congenital glaucoma (CG) accounts for 4% of blindness in children,1 with a prevalence of 1 per 3300 live births in the Indian state of Andhra Pradesh,2,3 and is much higher than the prevalence of 1 per 10,000 births in the Caucasian populations.4 Surgical management is the mainstay of treatment of CG, and the goal of surgery is to reduce intraocular pressure (IOP) and restore corneal transparency.5,6 Although untreated CG often leads to blindness, a proportion of treated children with severe forms of the disease also have poor visual outcomes.6,7 Given the chronic nature of the disease, children with CG require extended hospital follow-up visits with the ophthalmologist, and some may have to undergo repeated glaucoma surgeries or use topical medications for control of IOP. Symptoms of CG, such as photophobia, epiphora, and impaired vision, coupled with other issues, such as frequent absenteeism from school, travel cost, medication adverse effects, as well as the treatment itself, are challenging for the patients and parents.8 Studies of CG management typically have focused on clinical measures of efficacy related to control of IOP and restoration of corneal transparency.5–7,9–15 The significance of assessment of the patient perspective as an important outcome in medicine and ophthalmology is increasingly recognized. The impact on quality of life (QoL) during the critical period of development and adolescence, including education, often is considerable. Therefore, assessment of the patient's own estimation of their QoL is essential and may have an impact on treatment strategies. QoL is a multidimensional psychologic construct encompassing the physical, mental, social, emotional, and functional aspects of health and well-being, all considered essentially from the person's perspective.16,17
Over the years, a number of health-related quality of life (HRQoL) instruments have been developed for children and adolescents. Children as young as 8 years can fully understand the content, recall period, and response scales of QoL instruments,18–20 making children's ratings a valid representation of their QoL status. Despite this, most instruments are designed to collect information from proxies,16 mainly parents, given the belief that children are too young to respond by themselves, and/or lack of cognitive and linguistic skills necessary for self-completion of HRQoL measures. Given the subjective nature of the concept of QoL, assessment of HRQoL by proxies is controversial and proxy-respondent bias represents a limitation on the assessment of HRQoL by parents. Nevertheless, parents' perspective frequently is necessary given that they are responsible for the children and the ones who decide on their health needs and use of health care services. In the literature, discrepancies between children's self-report and parents' proxy reports are well documented, but self-reports and proxy reports constitute important complementary information concerning children's QoL.17,21,22
Over the last few years, a review21 and several studies have examined the agreement in QoL reports between parents and their children from the general population18,19,23,24 and with various health conditions.25–29 However, the evidence is mixed, with some parents rating their children's HRQoL worse than the children themselves,27,29–31 and some studies reporting lower child–parent agreement for specific domains, such as emotional and social functioning (subjective domains), and higher agreement for physical functioning (objective domain).21,32,33 By comparison, some researchers reported no such difference.18,23,26 Whether differences exist between child and parent–proxy reports exist in CG is not yet known. Several investigators argue that pediatric HRQoL instruments should include children and proxy versions as they cover different perspectives.21,34–36 Intuitively, it would seem highly unlikely that parents would know exactly all of their children's problems, especially when problems typically occur at the time the children are not with their parents; for example, in school or when with friends.
Gaining this information from the perspective of the child is vital because children may differ from adults in their preparedness to undergo treatments and their expectations about long-term success. In addition, more insight into child–parent (dis)agreement is particularly valuable in the field of pediatric glaucoma. Given that children with CG require life-long ophthalmic follow-up, an important goal for care of these children is preparing the transition from pediatric/adolescent to adult care. Transition is fraught with several developmental challenges and requires good self-management competencies and skills.37 A first step in enhancing these children/adolescents' self-reliance is to explore how they evaluate themselves given their ocular condition. It also is important to find out how parents think about their children's ocular condition and overall health because parental perception can influence the child's use of health services38 and parents are expected gradually to relinquish their caregiving responsibilities to their child.37,39 Therefore, we assessed the agreement (concordance) between children/adolescents' (operated for CG) own ratings of HRQoL and their parents' proxy reports using the Kidscreen-27 questionnaire. We hypothesized that children with CG and their parents would not agree on some of the domains regarding the child's HRQoL.
Methods
Participants comprised a consecutive series of children aged 8 to 11 years and adolescents aged 12 to 18 years (hereafter, children and adolescents are referred to as “children”) at the time of our study (2013–2016) and were operated for CG by a single surgeon between 1997 and 2012 at the L V Prasad Eye Institute, Hyderabad, India. Primary congenital glaucoma (PGC) was diagnosed according to generally accepted clinical signs. Primary combined trabeculotomy-trabeculectomy (CTT) was performed, and simultaneous surgery was performed in both eyes in cases of bilateral PCG.6,13,15,40 Parents and their children with CG were invited to participate in the study by the treating glaucoma specialist (AKM) during their routine follow-up clinic visit. After explanation of the nature and possible consequences of the study, children provided assent and their parents provided an informed consent. Parent–child dyads unable to speak or read Hindi, Telugu, or English were excluded, as also were those children who had additional disabilities (such as hearing, cognitive impairment). The study was approved by the ethics committee for Human Research at the L V Prasad Eye Institute (LVPEI), Hyderabad, India, and followed the tenets of the Declaration of Helsinki.
Kidscreen-27 Questionnaire
We chose to use the Kidscreen-27 questionnaire (child and adolescent version, and parent version) to assess HRQoL rather than Kidscreen-52 because of its excellent psychometric properties and to reduce the respondent burden. It is a generic HRQoL questionnaire that is a psychometrically robust version of the original Kidscreen-52 version assessing children and adolescents' QoL.41 The instrument has been found to have excellent cross-cultural comparative validity.42 The 27-item questionnaire consists of five health-related QoL dimensions: physical wellbeing (five items), psychological wellbeing (seven items), parent relations and autonomy (seven items), social support and peers (four items), and school (four items). The items use a five-point Likert-type scale with three different sets of responses: (1) poor, fair, good, very good, excellent; (2) not at all, slightly, moderately, very, extremely; (3) never, seldom, quite often, very often, always. The response polarity was reversed according to standard procedures for three items (items 9–11) to render them consistent with other items in the questionnaire whereby a higher score meant worse performance.43 Higher Kidscreen-27 scores indicate worse HRQoL. The recall period is 1 week and responses measure how the respondent experienced each item during the previous week. As noted earlier, we used the Kidscreen-27 parent version for the parent/caregiver in which they were asked to assess the degree of HRQoL in different dimensions from the perspective of their child with CG. This parent version corresponds in its scale structure to the version for children and adolescents. It consists of similar items, but asks the parents to respond by indicating how they think their children feel. Given that translations of the Kidscreen-27 questionnaire were not available in Telugu and Hindi (local languages) from the instrument's developers, these were done according to international guidelines for use in our study.44
Procedure
We provided the Kidscreen-27 questionnaire to the child and accompanying parent (if both parents accompanied, then the parent who spent the maximum time with the child was included) who were seated in separate rooms so as to avoid any contamination of responses during administration. Children were encouraged to complete the questionnaire by themselves; however, the researcher was available for any clarifications. If the child or parent could not complete the questionnaire by him/herself, then the researcher verbally administered the Kidscreen-27 to each of them separately in a face-to-face interview.
We collected sociodemographic variables that included: current age of the child, relationship of the parent with child (mother/father), age, highest educational level of the parent, number of children in the family, order of birth of the child with glaucoma, and number of children with glaucoma. Data collected from the medical records included: age of the child at surgery, date and type of glaucoma surgery, laterality of PCG, number and type of antiglaucoma medications at follow-up (if any), clinical data, such as visual acuity, and IOP. Average time for administration of the questionnaires was 30 minutes.
Assessment of Measurement Properties of Kidscreen-27
When measuring HRQoL (as in our study), the investigator relied on subjective reports from the patient's perspective. However, the investigator could not be confident that the trait (HRQoL in the present case) being assessed was valid, meaningful, or accurate unless the outcome measure under consideration was optimal in psychometric content and validity. A modern psychometric approach, Rasch analysis, tests this assumption and provides a comprehensive psychometric validation. Although the Kidscreen-27 questionnaire has undergone extensive testing of its measurement properties in several populations, albeit using CTT most often, it is imperative that the questionnaire be tested in the population in which it is intended to be used. Moreover, the limitations of CTT in instrument validation are well recognized.45 Given that the questionnaire has not been used previously in our population, we sought to investigate its measurement properties using Rasch analysis before using it for further analyses.
We performed Rasch analysis on the Kidscreen-27 and its five subscales using Winsteps software, version 3.74 (available in the public domain at http://www.winsteps.com/index.htm).46 The Andrich rating scale model was used.47 Rasch analysis is a psychometric method that mathematically describes the interaction between the participants and items in a questionnaire and applies a probabilistic model that the pattern of participants' responses should satisfy. Details of Rasch analysis have been described in previously.8,48–50 Briefly, Rasch analysis converts raw questionnaire scores into data that approximate interval-level measurement expressed in log of the odds units (logits). Rasch analysis also provides greater insight into the measurement properties of a questionnaire compared with CTT.51,52 Specifically, Rasch analysis permits investigation of the functioning of rating scale categories (i.e., if higher categories represented better functioning); the validity (i.e., does the questionnaire measure what it purports to measure) of a measure by evaluating the fit of individual items to the underlying construct (i.e., how well an individual item is in tandem with the whole group); and determining whether the items measure a unidimensional construct, which is required to justify the summation of scores.53 Rasch scores were generated after the structure of the responses and items satisfied the requirements of the Rasch model.
Statistical Analysis
Descriptive statistical analyses were performed to characterize the participants' sociodemographic, clinical, and Kidscreen-27 data using the Statistical Package for Social Sciences for Windows, version 19.0 (IBM SPSS, Inc., Armonk, NY). Data were summarized as number and percentages for categorical variables, and as mean, median, and range for those measured on a continuous variable. P < 0.05 was considered statistically significant.
Agreement between ratings of HRQoL from the child and parent on the Kidscreen-27 was analyzed using the Bland-Altman method of limits of agreement (LoAs) in the MedCalc software (MedCalc version 12.5.0.0; Acacialaan 22, B-8400, Ostend, Belgium).54 In a Bland-Altman plot, the difference between the two methods of measurements (plotted on the y-axis) is plotted against their mean (plotted on the x-axis).54 The LoA technique describes by how much the scores from the two groups differ, and if this difference is small enough the measurements may be used interchangeably. In brief, this technique depicts mean difference between two methods of measurement (the “bias”), and 95% LoA (prediction interval) as mean difference (1.96 standard deviation [SD]). The pattern of the data points identifies agreement, types of bias, and outliers. The 95% LoA (mean difference ± 1.96 SD) provide the distance between the measurements by the methods with 95% confidence. It would be expected that the 95% confidence intervals (CI) include 95% of the data point differences between the two measurements, that is, approximately 95% of the points should lie within the interval. The presence of significant proportional bias indicates that the methods do not agree equally through the range of measurements.54
We used the independent samples t-test to assess the differences in child–parent scores for the sociodemographic and clinical variables: child's age (8–11/12–18 years), sex, laterality of CG (unilateral/bilateral), time of onset (congenital/ infantile and juvenile), parent age (median age <36/≥ 36 years), visual status (no visual impairment/visual impairment), parent education (<secondary school vs. ≥ secondary school), and number of children with glaucoma (one vs. more than one child). We used Cohen's effect size (ES) to evaluate differences between children and parent responses in reporting HRQoL, and ES of 0.21 to 0.5 was considered small, 0.51 to 0.8 moderate, and >0.8 large.55
Results
Participant Characteristics
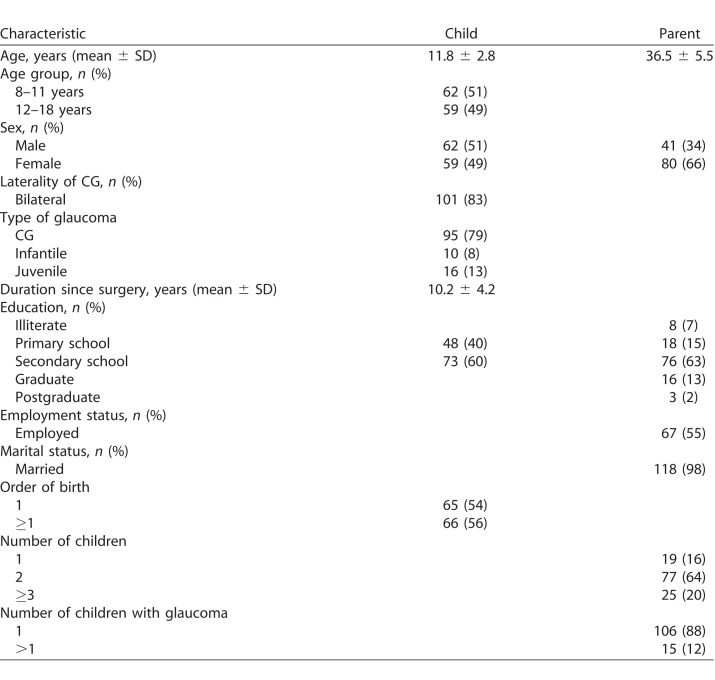
We included 121 (13.3%) child–parent dyads in this study. Table 1 shows the sociodemographic characteristics of the children and their parents. Mean (SD) age of the parents was 36.5 (5.5) years and two-thirds (66%) of the parent responders were mothers. Mean (SD) age of the children was 11.8 (2.8) years. The majority had bilateral PCG (83%) with congenital onset (79%). Presenting acuity ranged from 20/20 to light perception (median, 20/30). A little over half of the eyes (57%) with CG did not need any antiglaucoma medication for control of IOP, 92 eyes (41%) required 1 medication, and three eyes (1%) underwent repeat trabeculectomy since their first glaucoma surgery.
Table 1.
Demographic Characteristics of Children and Parents (n = 121)
Psychometric Evaluation of Kidscreen-27 Questionnaire
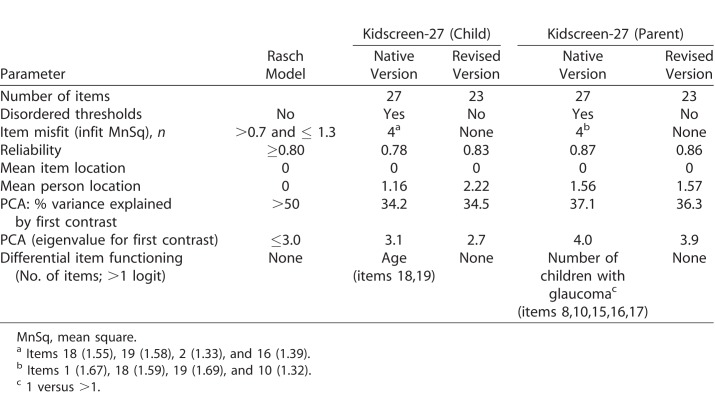
Table 2 outlines the results of Rasch analysis of the Kidscreen-27 questionnaire in children with CG and their parents. In children with CG, all the three types of rating scales displayed disordered thresholds, but merging adjacent response categories subsequently resulted in ordered thresholds for all. Principal components analysis of residuals established unidimensionality. Four items demonstrated misfit (i.e., infit mean square >1.3), so they were iteratively deleted to improve fit statistics. Of these four misfitting items, two also demonstrated large differential item functioning (DIF) by age (1.61 and 1.73 logits, respectively). Both items were rated easier by children aged 12 to 18 years compared to their younger counterparts. Following deletion of four misfitting items, all remaining 23 items fit the Rasch model and the psychometric properties of the revised questionnaire were adequate. However, all five subscales demonstrated inadequate measurement precision rendering them dysfunctional. In parents of children with CG, two of the three types of rating scales displayed disordered thresholds, but merging adjacent response categories subsequently resulted in ordered thresholds for all. Four items misfit that were deleted iteratively and following this step, the remaining 23 items fit the Rasch model with satisfactory psychometric properties. However, principle component analysis (PCA) of residuals revealed lack of unidimensionality and this was perhaps expected given that Kidscreen-27 is a measure of HRQoL—a multidimensional construct, and is composed of several but correlated domains. In such cases a multidimensional Rasch model is considered more appropriate than a unidimensional Rasch model.56 For our study purposes, however, we used the unidimensional Rasch model. All five subscales demonstrated inadequate measurement precision.
Table 2.
Overall Performance of the Kidscreen-27 in 121 Children With CG and Their Parents
Child–Parent Agreement
Mean difference, SD of the difference, and LoA with their associated 95% CIs were calculated for the HRQoL score (i.e., Kidscreen score), and the results are displayed in Table 3. The standard graphical representation of the LoA (Bland-Altman plot) between the child–parent dyads is displayed in Figure 1. As can be seen from Figure 1, the level of agreement varied according to HRQoL level, and there was a statistically significant difference between mean HRQoL scores of children with CG and their parents (Table 3). Furthermore, the range of child–parent agreement was wide and bidirectional; parents tended to underestimate and overestimate their child's HRQoL. A total of 45 (37%) parents rated their child's HRQoL much lower than the child's self-reported HRQoL. Two (1.6%) child–parent dyads lie above the upper limits (i.e., the child rated their HRQoL lower than their parent), while four (3.3%) child–parent dyads had the parent rate lower than their child. Visual inspection of the Bland-Altman plot also revealed that the agreement between the child–parent dyad was greater for higher or better (i.e., low positive scores on the x-axis) for better (or higher) levels of HRQoL.
Table 3.
Mean Difference, Standard Deviation of the Difference, and the Limits of Agreement With Their Associated 95% CI for HRQoL Scores From Child–Parent Dyads on Kidscreen-27 Questionnaire
Figure 1.
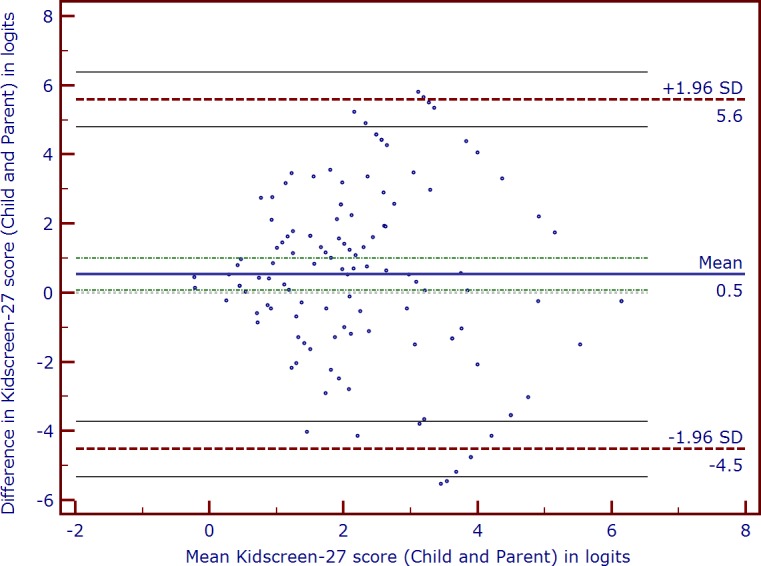
Bland-Altman plot of child–parent dyad health-related quality of life scores (in logits) using Kidscreen-27 for children/adolescents with congenital glaucoma and their parents. Positive scores indicate lower health-related quality of life. The horizontal solid line (center) represents mean difference between the scores or bias (0.5 logits) and the 95% limits of agreement (lower −1.96 SD and upper +1.96 SD) are represented by the dashed lines.
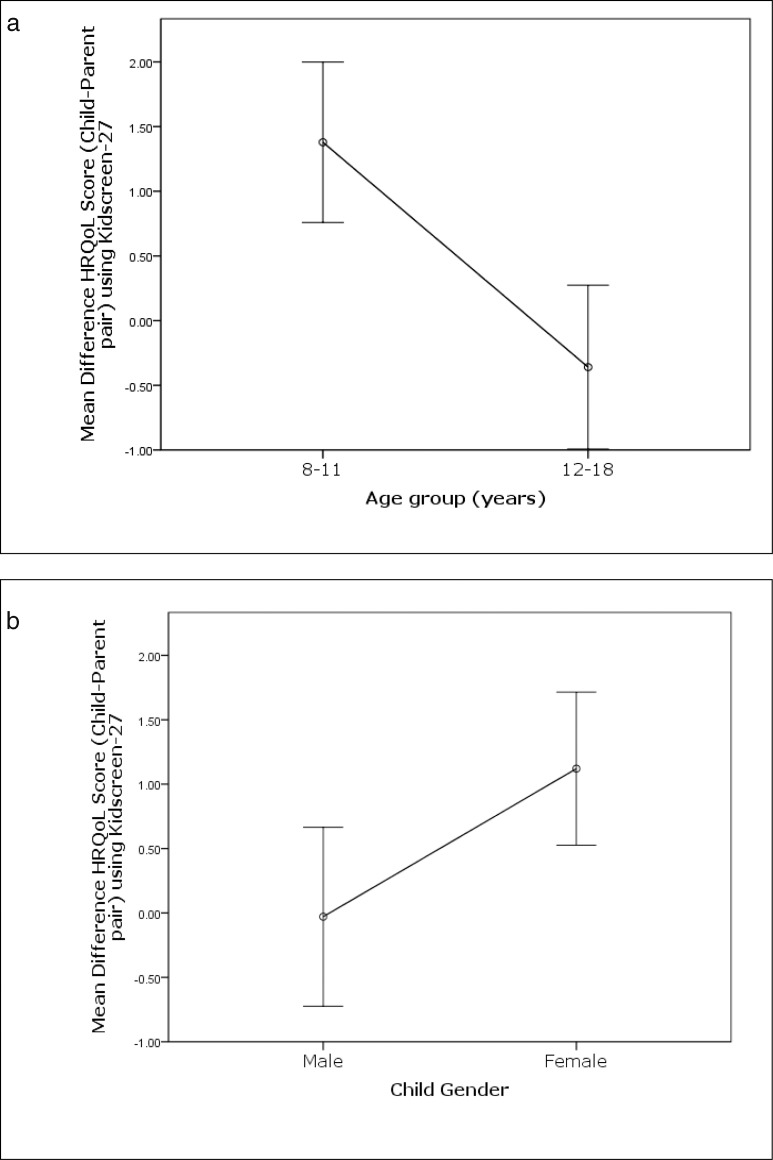
Of the variables examined, age and sex of the child influenced the concordance between the child's self-report and parent report of child's HRQoL. Younger children (8–11 years) showed a significantly larger difference from their parental reports compared to adolescents (mean difference = 1.38 ± 2.44 vs. −0.36 ± 2.43, P < 0.0001; ES = 0.71 [moderate]); younger children reported significantly worse HRQoL scores than their parents (Fig. 2a). Similarly, girls showed a significantly larger difference in HRQoL scores from parental reports compared to boys (mean difference = 1.12 ± 2.28 vs. −0.03 ± 2.73, P = 0.01; ES = 0.42 [small]); girls reported significantly worse HRQoL scores from their parents (Fig. 2b).
Figure 2.
Bar chart shows mean difference in health-related quality of life score (in logits) of child–parent dyad using Kidscreen-27 stratified by (a) age group of children (8–11 and 12–18 years), and (b) child's sex. Positive scores indicate lower health-related quality of life.
Discussion
Our results demonstrated that the perspectives of children with CG and their parents regarding the child's HRQoL vary widely, with parents underestimating and overestimating their child's own report. Overall, our results of disagreement between the child's self-report and parental report of the child's HRQoL are consistent with those of prior reports.19,21 To the best of our knowledge, this is the first such study to have been conducted among children treated for CG and their parents.
Several studies have reported increasing discordance between child and parental reports with increasing child's age.57–59 However, we found the reverse, that is, the discordance was larger for younger children with CG (mean difference = 1.38 logits) than for adolescents (mean difference = 0.35 logits). In a sample of 2505 healthy (93%) children and adolescents as well as those with special needs across seven European countries, Robitail et al.19 reportedly found closer agreement across all 10 domains between child's self-report and proxy-reported HRQoL (than for adolescents), albeit using Kidscreen-52 (native longer version). Although we could not evaluate the disagreement domain-wise (given the poor measurement precision of subscales in our sample), we believe that a complex interplay in the interrater agreement between the developmental level of the child and HRQoL in some domains may be the reason for our findings of discordance in younger children. For example, younger children may find it more difficult to express their emotional needs to their parents than school-aged children, leading to greater discordance between them and their parents. By comparison, older children, with greater language skills, might be able to better articulate their experiences, emotions, and concerns and therefore, may have greater concordance with parental reports.17 Some investigators have demonstrated parental HRQoL indicators to be more highly associated with clinical indicators of the child's condition,60 a finding that has led to the suggestion that parent reports may be more accurate (in terms of disease status) than the child's self-reported HRQoL. However, there has been mixed evidence regarding concordance between child's self-report and parental report of child's HRQoL in chronic health conditions.38 For example, Robitail et al.19 reported greater concordance between children/adolescents with “special health care needs” and their parents, compared to healthy children. By comparison, Walker et al.61 reported lower concordance between children and their parents for healthy children than for those with abdominal pain.
We found statistically significant differences (small ES) in the child's HRQoL scores between girls and their parents than between boys and their parents, albeit using mean difference in HRQoL scores. However, it has been suggested for a meaningful interpretation of mean differences, an essential assumption known as measurement invariance (respondents from different groups interpret the concept of particular similarly62) should be established.63 Very few studies have investigated the impact of sex on the concordance and the results have been inconsistent.38,64 For example, several studies have reported higher concordance among girls and their parents in all 10 domains of the Kidscreen-52.65 By comparison, other studies showed significant differences only in some aspects of HRQoL.23 Factors, such as type and severity of the child's condition, disease status (active versus inactive), duration of illness, type of condition, number of siblings, parental age, well-being, health, and educational level, also are likely to influence the nature and extent of discordance between child's self-reported HRQoL and that reported their parents.66 We found no influence of other demographic or clinical factors assessed, such as parental age or education, laterality of CG, visual status, and time of onset of CG, on the level of concordance.
As noted earlier, we could not evaluate the different domains of HRQoL in our sample given that they were inadequate in distinguishing between participants with high and low HRQoL (i.e., poor measurement precision). The main problem was inadequate measurement precision, which could not be remediated without the addition of items. However, addition of items is beyond the scope of our study. Among the desirable features of an optimally functioning instrument include an ability to discriminate as many groups of participant ability as possible, simulating the gradations on a ruler; the finer the gradations, the better the measurement properties.49 All five dysfunctional subscales of Kidscreen-27 lacked adequate discriminative ability in that they could distinguish only between two strata (i.e. high versus low) of participant's HRQoL. Given that person separation is sample-dependent, the finding of dysfunctional subscales, therefore, will only be applicable in similar populations. Therefore, subscales should be tested in other populations. Assuming this sample is typical of a CG in the developing world, the likelihood of finding adequately performing subscales would be low.
Patterns of concordance/discordance in our study are similar to those in other studies on child–parent concordance on child's health outcomes in other pediatric populations.38,66 The range of discordance in the child's HRQoL as evidenced from the Bland-Altman plots was wide for our cohort of children and parents (95% CI, 0.06–0.99; lower LoA, −4.52 and upper LoA, 5.58 logits). However, Bland-Altman plots based on the total Kidscreen-27 interval score showed that the concordance between child and parental report was better for higher (better) levels of HRQoL. Our study extends the findings of earlier studies by examining concordance between children with CG and their parents using a generic HRQoL measure, the Kidscreen-27. Children with CG as well as other chronic disease states, such as epilepsy and obesity,58 are at risk of experiencing lower HRQoL than their healthy-peers. However, given that we did not compare patients with their healthy peers, we were unable to confirm this observation. The assessment of QoL, including aspects of physical, psychologic, and social functioning, have an important role in the evaluation of the burden of this chronic disease. Involvement of parents in the treatment process is essential for optimal adaptation of medical treatment and psychologic support. Therefore, it is important to know how parents estimate the HRQoL of their child with CG. The difference in perception of the child's HRQoL between the children themselves and their parents might partially be due to underreporting or minimizing of symptoms by young patients with CG, as a result of an adaptive reaction to deny the full extent of the disease, or by their inability to report psychologic symptoms. It also is possible that children report symptoms quite accurately to their parents, who then pathologize normal behavior and overreport symptoms compared to parents of typically developing peers. Since HRQoL is subjective and individual, a certain difference may be normal and given this, the inclusion of patients' and parents' measures of HRQoL should be considered so as to obtain complimentary perspectives.67 Nonetheless, the discussion of differing results also might enable constructive communication between parents and children.
Our study has important strengths, but some limitations as well. Strengths of the study are the prospective cohort design, which included a relatively large homogenous sample (operated upon by a single surgeon) of stable Indian CG patients, use of an internationally accepted HRQoL measure, the Kidscreen-27, parallel completion of child and parent versions of the Kidscreen questionnaire, and use of modern psychometric technique (Rasch analysis) to validate the instrument so as to produce linear interval overall measure of HRQoL. Use of recommended statistical techniques, such as Bland-Altman plots, for assessing agreement is another major strength of our study. Among the limitations, firstly, is the cross-sectional nature of our data, so we were unable to confirm whether the instrument is sensitive enough to pick-up changes in the child's health condition. Secondly, given that patients under the care of a single surgeon only were included, we cannot exclude an undefined selection bias. Thirdly, we used mean scores to assess difference in HRQoL scores by sex, but it is difficult to determine if the observed differences are true differences or a result of differences in interpretation; however, for a meaningful interpretation of mean differences, measurement invariance should be established and we suggest applying a hierarchical ordinal logistic regression model68 or multilevel multiple-indicator multiple-cause approach69 in future studies to examine the measurement invariance of the Kidscreen-27 across sexes.
In conclusion, our study showed that Indian patients with CG and their parents have significantly different perspectives on how the child/adolescent feels and functions. In the context of HRQoL assessment and use of this information in the decision-making process, it is important to realize that the child and parental perspectives are required, and each perspective potentially provides unique information in developing a treatment plan. In the absence of a “gold standard,” however, discordant child–parent reports should currently be considered as a parallel source of information on patient functioning. Our study also provided significant new information in response to the need for instruments that are valid and reliable for measuring HRQoL in children with CG, and supported the continued use of Kidscreen-27 questionnaire, albeit with some modifications in Indian children with CG and their parents. Future studies should attempt to determine the size of clinical meaningfulness of the discrepancy between the HRQoL reports and also replicate or extend our findings in a larger sample from the analysis of Kidscreen-27 in other cultures and in other pediatric conditions.
Acknowledgments
Supported in part by the Hyderabad Eye Research Foundation, Hyderabad, India.
Disclosure: V.K. Gothwal, None; S. Bharani, None; A.K. Mandal, None
References
- 1.Franks W, Taylor D. Congenital glaucoma--a preventable cause of blindness. Arch Dis Child. 1989;64:649–650. doi: 10.1136/adc.64.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandal A, Netland PA. Epidemiology and genetics of developmental glaucomas. In: Mandal A, Netland P, editors. The Pediatric Glaucomas. Philadelphia, PA: Elsevier Butterworth Heinemann;; 2006. pp. 19–22. [Google Scholar]
- 3.Dandona L, Williams J, Williams B, Rao GN. Population-based assessment of childhood blindness in southern India. Arch Ophthalmol. 1998;116:545–546. [PubMed] [Google Scholar]
- 4.Francois J. Congenital glaucoma and its inheritance. Ophthalmologica. 1972;181:61–73. doi: 10.1159/000309028. [DOI] [PubMed] [Google Scholar]
- 5.Mandal A, Naduvilath T, Jayagandan A. Surgical results of combined trabeculotomy-trabeculectomy for developmental glaucoma. Ophthalmology. 1998;105:974–982. doi: 10.1016/S0161-6420(98)96022-5. [DOI] [PubMed] [Google Scholar]
- 6.Mandal A, Gothwal V, Nutheti R. Surgical outcome of primary developmental glaucoma: a single surgeon's long-term experience from a tertiary eye care centre in India. Eye. 2007;21:764–774. doi: 10.1038/sj.eye.6702324. [DOI] [PubMed] [Google Scholar]
- 7.Biglan A, Hiles DA. The visual results following infantile glaucoma surgery. J Pediatr Ophthalmol Strabismus. 1979;16:377–381. doi: 10.3928/0191-3913-19791101-10. [DOI] [PubMed] [Google Scholar]
- 8.Gothwal V, Bharani S, Mandal AK. Quality of life of caregivers of children with congenital glaucoma: development and validation of a novel questionnaire (CarCGQoL) Invest Ophthalmol Vis Sci. 2015;56:770–777. doi: 10.1167/iovs.14-15905. [DOI] [PubMed] [Google Scholar]
- 9.Broughton W, Parks MM. An analysis of treatment of congenital glaucoma by goniotomy. Am J Ophthalmol. 1981;91:566–572. doi: 10.1016/0002-9394(81)90054-4. [DOI] [PubMed] [Google Scholar]
- 10.Richardson KT, Jr, Ferguson WJ, Jr, Shaffer RN. Long-term functional results in infantile glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1967;71:833–837. [PubMed] [Google Scholar]
- 11.Morgan K, Black B, Ellis F, Helveston EM. Treatment of congenital glaucoma. Am J Ophthalmol. 1981;92:799–803. doi: 10.1016/s0002-9394(14)75633-8. [DOI] [PubMed] [Google Scholar]
- 12.Elder MJ. Combined trabeculotomy-trabeculectomy compared with primary trabeculectomy for congenital glaucoma. Br J Ophthalmol. 1994;78:745–748. doi: 10.1136/bjo.78.10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal A, Bhatia P, Bhaskar A, Nutheti R. Long-term surgical and visual outcomes in Indian children with developmental glaucoma operated on within 6 months of birth. Ophthalmology. 2004;111:283–290. doi: 10.1016/j.ophtha.2003.05.027. [DOI] [PubMed] [Google Scholar]
- 14.McPherson SD, Jr, McFarland D. External trabeculotomy for developmental glaucoma. Ophthalmology. 1980;87:302–305. doi: 10.1016/s0161-6420(80)35233-0. [DOI] [PubMed] [Google Scholar]
- 15.Mandal A, Gothwal V, Bagga H, Nutheti R, Mansoori T. Outcome of surgery on infants younger than 1 month with congenital glaucoma. Ophthalmology. 2003;110:1909–1915. doi: 10.1016/S0161-6420(03)00671-7. [DOI] [PubMed] [Google Scholar]
- 16.Bullinger M, Ravens-Sieberer U. Health-related QOL assessment in children: a review of the literature. Eur Rev Appl Psychol. 1995;45:245–254. [Google Scholar]
- 17.Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess. 2001;5:1–157. doi: 10.3310/hta5040. [DOI] [PubMed] [Google Scholar]
- 18.Cremeens J, Eiser C, Blades M. Factors influencing agreement between child self-report and parent proxy-reports on the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes. 2006;4:58. doi: 10.1186/1477-7525-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robitail S, Simeoni M, Erhart M, Ravens-Sieberer U, Bruil J, Auquier P. Validation of the European proxy KIDSCREEN-52 pilot test health-related quality of life questionnaire: first results. J Adolesc Health. 2006;39:596, e591–e510. doi: 10.1016/j.jadohealth.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Gothwal V, Lovie-Kitchin J, Nutheti R. The development of the LV Prasad-Functional Vision Questionnaire: a measure of functional vision performance of visually impaired children. Invest Ophthalmol Vis Sci. 2003;44:4131–4139. doi: 10.1167/iovs.02-1238. [DOI] [PubMed] [Google Scholar]
- 21.Eiser C, Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10:347–357. doi: 10.1023/a:1012253723272. [DOI] [PubMed] [Google Scholar]
- 22.De Civita M, Regier D, Alamgir A, Anis A, Fitzgerald M, Marra CA. Evaluating health-related quality-of-life studies in paediatric populations: some conceptual, methodological and developmental considerations and recent applications. Pharmacoeconomics. 2005;23:659–685. doi: 10.2165/00019053-200523070-00003. [DOI] [PubMed] [Google Scholar]
- 23.Theunissen N, Vogels T, Koopman H, et al. The proxy problem: child report versus parent report in health-related quality of life research. Qual Life Res. 1998;7:387–397. doi: 10.1023/a:1008801802877. [DOI] [PubMed] [Google Scholar]
- 24.Waters E, Stewart-Brown S, Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child: Care, Health Dev. 2003;29:501–509. doi: 10.1046/j.1365-2214.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 25.April K, Feldman D, Platt R, Duffy CM. Comparison between Children with Juvenile Idiopathic Arthritis (JIA) and their parents concerning perceived Quality of Life. Qual Life Res. 2006;15:655–661. doi: 10.1007/s11136-005-3690-1. [DOI] [PubMed] [Google Scholar]
- 26.Chang P, Yeh CH. Agreement between child self-report and parent proxy-report to evaluate quality of life in children with cancer. Psycho-Oncol. 2005;14:125–134. doi: 10.1002/pon.828. [DOI] [PubMed] [Google Scholar]
- 27.Forinder U, Lof C, Winiarski J. Quality of life following allogeneic stem cell transplantation, comparing parents' and children's perspective. Ped Transplant. 2006;10:491–496. doi: 10.1111/j.1399-3046.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 28.Jokovic A, Locker D, Stephens M, Guyatt G. Agreement between mothers and children aged 11-14 years in rating child oral health-related quality of life. Comm Dent Oral Epidemiol. 2003;31:335–343. doi: 10.1034/j.1600-0528.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 29.Havermans T, Vreys M, Proesmans M, De Boeck C. Assessment of agreement between parents and children on health-related quality of life in children with cystic fibrosis. Child: Care, Health, Dev. 2006;32:1–7. doi: 10.1111/j.1365-2214.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- 30.Britto M, Kotagal U, Chenier T, Tsevat J, Atherton H, Wilmott RW. Differences between adolescents' and parents' reports of health-related quality of life in cystic fibrosis. Ped Pulmonol. 2004;37:165–171. doi: 10.1002/ppul.10436. [DOI] [PubMed] [Google Scholar]
- 31.Klassen A, Miller A, Fine S. Agreement between parent and child report of quality of life in children with attention-deficit/hyperactivity disorder. Child: Care, Health, Dev. 2006;32:397–406. doi: 10.1111/j.1365-2214.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 32.Levi R, Drotar D. Health-related quality of life in childhood cancer: discrepancy in parent-child reports. Int J Cancer Suppl. 1999;12:58–64. doi: 10.1002/(sici)1097-0215(1999)83:12+<58::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Verrips G, Vogels A, den Ouden A, Paneth N, Verloove-Vanhorick SP. Measuring health-related quality of life in adolescents: agreement between raters and between methods of administration. Child: Care, Health, Dev. 2000;26:457–469. doi: 10.1046/j.1365-2214.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 34.Connolly M, Johnson JA. Measuring quality of life in paediatric patients. Pharmacoeconomics. 1999;16:605–625. doi: 10.2165/00019053-199916060-00002. [DOI] [PubMed] [Google Scholar]
- 35.Landgraf J, Abetz LN. Measuring Health Outcomes in Pediatric Populations: Issues in Psychometrics and Applications. Philadelphia, PA: Lippincott-Raven;; 1996. [Google Scholar]
- 36.Goodwin D, Boggs S, Graham-Pole J. Development and validation of the Pediatric Oncology Quality of Life Scale. Psychol Assess. 1994;6:321–328. [Google Scholar]
- 37.Viner RM. Transition of care from paediatric to adult services: one part of improved health services for adolescents. Arch Dis Child. 2008;93:160–163. doi: 10.1136/adc.2006.103721. [DOI] [PubMed] [Google Scholar]
- 38.Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17:895–913. doi: 10.1007/s11136-008-9350-5. [DOI] [PubMed] [Google Scholar]
- 39.Sawyer M, Reynolds K, Couper J, et al. A two-year prospective study of the health-related quality of life of children with chronic illness--the parents' perspective. Qual Life Res. 2005;14:395–405. doi: 10.1007/s11136-004-0786-y. [DOI] [PubMed] [Google Scholar]
- 40.Mandal A, Netland P. The Pediatric Glaucomas. Philadelphia: Elsevier;; 2006. [Google Scholar]
- 41.Ravens-Sieberer U, Auquier P, Erhart M, et al. The KIDSCREEN-27 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Qual Life Res. 2007;16:1347–1356. doi: 10.1007/s11136-007-9240-2. [DOI] [PubMed] [Google Scholar]
- 42.Ravens-Sieberer U, Herdman M, Devine J, et al. The European KIDSCREEN approach to measure quality of life and well-being in children: development, current application, and future advances. Qual Life Res. 2014;23:791–803. doi: 10.1007/s11136-013-0428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The KIDSCREEN Group Europe. The KIDSCREEN Questionnaires Quality of Life Questionnaires for Children and Adolescents Handbook. Lengerich, Germany: Pabt Science Publishers;; 2006. [Google Scholar]
- 44.Beaton D, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 45.DeVellis RF. Classical test theory. Med Care. 2006;44:S50–59. doi: 10.1097/01.mlr.0000245426.10853.30. [DOI] [PubMed] [Google Scholar]
- 46.Linacre JM. A User's Guide to Winsteps/Ministeps Rasch Model Computer Programs. Chicago, IL: MESA Press;; 2014. [Google Scholar]
- 47.Andrich DA. A rating scale formaultion for ordered response categories. Psychometrika. 1978;43:361–373. [Google Scholar]
- 48.Massof RW. The measurement of vision disability. Optom Vis Sci. 2002;79:516–552. doi: 10.1097/00006324-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Pesudovs K, Burr J, Harley C, Elliott DB. The development, assessment, and selection of questionnaires. Optom Vis Sci. 2007;84:663–674. doi: 10.1097/OPX.0b013e318141fe75. [DOI] [PubMed] [Google Scholar]
- 50.Gothwal V, Wright T, Lamoureux E, Pesudovs K. Activities of Daily Vision Scale: what do the subscales measure? Invest Ophthalmol Vis Sci. 2010;51:694–700. doi: 10.1167/iovs.09-3448. [DOI] [PubMed] [Google Scholar]
- 51.Bond T, Fox CM. Applying the Rasch Model: Fundamental Measurement in the Human Sciences. London: Lawrence Erlbaum Associates;; 2001. [Google Scholar]
- 52.Wright B, Masters GN. Rating Scale Analysis. Chicago: MESA Press;; 1982. [Google Scholar]
- 53.Emberston S, Reise SP. Item Response Theory for Psychologists. Marwah, NJ: Erlbaum Associates;; 2000. [Google Scholar]
- 54.Bland J, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 55.Cohen JW. Statistical Power Analysis for the Behavioural Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates;; 1988. [Google Scholar]
- 56.Adams R, Wilson M, Wang WC. The multidimensional random coefficient multinomial logit model. Appl Psychol Meas. 1997;21:1–23. [Google Scholar]
- 57.Rajmil L, Lopez A, Lopez-Aguila S, Alonso J. Parent-child agreement on health-related quality of life (HRQOL): a longitudinal study. Health Qual Life Outcomes. 2013;11:101. doi: 10.1186/1477-7525-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreira H, Carona C, Silva N, Frontini R, Bullinger M, Canavarro MC. Psychological and quality of life outcomes in pediatric populations: a parent-child perspective. J Ped. 2013;163:1471–1478. doi: 10.1016/j.jpeds.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 59.Parsons S, Fairclough D, Wang J, Hinds PS. Comparing longitudinal assessments of quality of life by patient and parent in newly diagnosed children with cancer: the value of both raters' perspectives. Qual Life Res. 2012;21:915–923. doi: 10.1007/s11136-011-9986-4. [DOI] [PubMed] [Google Scholar]
- 60.Czyzewski D, Mariotto M, Bartholomew L, LeCompte S, Sockrider MM. Measurement of quality of well being in a child and adolescent cystic fibrosis population. Med Care. 1994;32:965–972. doi: 10.1097/00005650-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Walker L, Heflinger CA. Quality of Life Predictors of Outcome in Pediatric Abdominal Pain Patients: Findings at Initial Assessment and 5-Years Follow-Up. Hillsdale, NJ: Lawrence Erlbaum;; 1998. [Google Scholar]
- 62.Huang I, Shenkman E, Leite W, Knapp C, Thompson L, Revicki DA. Agreement was not found in adolescents' quality of life rated by parents and adolescents. J Clin Epidemiol. 2009;62:337–346. doi: 10.1016/j.jclinepi.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teresi J, Fleishman JA. Differential item functioning and health assessment. Qual Life Res. 2007;16(suppl)1):33–42. doi: 10.1007/s11136-007-9184-6. [DOI] [PubMed] [Google Scholar]
- 64.Eiser C, Morse R. A review of measures of quality of life for children with chronic illness. Arch Dis Child. 2001;84:205–211. doi: 10.1136/adc.84.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robitail S, Simeoni M, Ravens-Sieberer U, Bruil J, Auquier P, Group K. Children proxies' quality-of-life agreement depended on the country using the European KIDSCREEN-52 questionnaire. J Clin Epidemiol. 2007;60:469–478. doi: 10.1016/j.jclinepi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Eiser C, Varni JW. Health-related quality of life and symptom reporting: similarities and differences between children and their parents. Eur J Pediatr. 2013;172:1299–1304. doi: 10.1007/s00431-013-2049-9. [DOI] [PubMed] [Google Scholar]
- 67.Varni J, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 68.French B, Finch HW. Hierarchical logistic regression: accounting for multilevel data in DIF detection. J Educ Meas. 2010;47:299–317. [Google Scholar]
- 69.Finch H, French BF. Detecting differential item functioning of a course satisfaction instrument in the presence of multilevel data. J First-Year Ex Stud Trans. 2010;22:27–47. [Google Scholar]