Abstract
Background:
Multiple Northeast U.S. communities have discovered per- and polyfluoroalkyl substances (PFASs) in drinking water aquifers in excess of health-based regulatory levels or advisories. Regional stakeholders (consultants, regulators, and others) need technical background and tools to mitigate risks associated with exposure to PFAS-affected groundwater.
Objectives:
The aim was to identify challenges faced by stakeholders to extend best practices to other regions experiencing PFAS releases and to establish a framework for research strategies and best management practices.
Methods and Approach:
Management challenges were identified during stakeholder engagement events connecting attendees with PFAS experts in focus areas, including fate/transport, toxicology, and regulation. Review of the literature provided perspective on challenges in all focus areas. Publicly available data were used to characterize sources of PFAS impacts in groundwater and conduct a geospatial case study of potential source locations relative to drinking water aquifers in Rhode Island.
Discussion:
Challenges in managing PFAS impacts in drinking water arise from the large number of relevant PFASs, unconsolidated information regarding sources, and limited studies on some PFASs. In particular, there is still considerable uncertainty regarding human health impacts of PFASs. Frameworks sequentially evaluating exposure, persistence, and treatability can prioritize PFASs for evaluation of potential human health impacts. A regional case study illustrates how risk-based, geospatial methods can help address knowledge gaps regarding potential sources of PFASs in drinking water aquifers and evaluate risk of exposure.
Conclusion:
Lessons learned from stakeholder engagement can assist in developing strategies for management of PFASs in other regions. However, current management practices primarily target a subset of PFASs for which in-depth studies are available. Exposure to less-studied, co-occurring PFASs remains largely unaddressed. Frameworks leveraging the current state of science can be applied toward accelerating this process and reducing exposure to total PFASs in drinking water, even as research regarding health effects continues. https://doi.org/10.1289/EHP2727
Introduction
Per- and polyfluoroalkyl substances (PFASs) exhibit unique chemistry that makes them favorable for use in a wide variety of consumer and industrial products and applications (Kissa 2001). This same chemistry has led to limitations in using traditional environmental chemistry and engineering principles and techniques to understand and manage risks associated with their environmental releases. For example, unlike many neutral organic contaminants, in organisms PFASs are not lipophilic and are known to bind to proteins such as serum albumin (Conder et al. 2008). Additionally, some PFASs are environmentally persistent with no significant natural pathways for complete degradation following release. PFAS chemistry is largely attributable to the strength and low polarizability of the carbon-fluorine covalent bond (Banks et al. 1994; Kissa 2001). PFAS characteristics include thermal stability, chemical stability, surfactant behavior, and stain-resistant properties (Banks et al. 1994; Kissa 2001). Because of these characteristics, PFASs are used in products and applications such as firefighting foams, fluoropolymer manufacturing, stain-resistant coatings, and electroplating. These uses have contributed to their global distribution in organisms and the environment. At the same time, knowledge regarding human health impacts is quite limited, and because of their unique properties, conventional water-treatment techniques do not fully mitigate exposure (DeWitt 2015; Eschauzier et al. 2012; Giesy and Kannan 2001).
Recent studies estimate as many as 3,000 PFASs are now or have been on the global market (Wang et al. 2017). Within this group are perfluoroalkyl substances, which contain an alkyl tail with all carbons bonded to fluorine and which are persistent in the environment (Buck et al. 2011). These perfluoroalkyl substances include PFASs such as perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS), which have been the subject of much of the PFAS research to date. PFOA, PFOS, and their homologues (i.e., shorter and/or longer perfluoroalkyl carboxylates and perfluoroalkyl sulfonates) are often collectively referred to as perfluoroalkyl acids (PFAAs) (Buck et al. 2011). Polyfluoroalkyl substances have at least one perfluoroalkyl moiety but elsewhere in the structure also contain carbons bonded to hydrogen. These compounds are capable of transformation in the environment (Buck et al. 2011). The terminal degradation products of polyfluoroalkyl substances include PFAAs (e.g., PFOA). So, they are often referred to as precursors and thus still represent a source of recalcitrant PFAAs in the environment (Harding-Marjanovic et al. 2015; Mejia Avendaño and Liu 2015). Examples include fluorotelomer sulfonates, some of which are capable of transforming to PFOA (Harding-Marjanovic et al. 2015).
There are concerns about the human health impacts of some PFASs, particularly PFOA, PFOS, and other perfluoroalkyl substances. Briefly, in rodent studies, these compounds are known to affect lipid metabolism (e.g., Das et al. 2017) and liver weight (e.g., Loveless et al. 2006), decrease birth weight/increased resorptions (e.g., Lau et al. 2006), delay hind/fore limb phalanges ossification sites in offspring (Lau et al. 2006), delay mammary gland development in offspring (e.g., Tucker et al. 2015), and induce immunosuppression (e.g., DeWitt et al. 2008). Notably, these effects have provided the basis for regulation of PFOA/PFOS. In epidemiological studies conducted by the C8 Science Panel (C8 Science Panel 2017) and others, PFASs have shown positive associations with adverse outcomes, including elevated cholesterol (Frisbee et al. 2010; Nelson et al. 2010), ulcerative colitis (Steenland et al. 2013), thyroid disease (Lopez-Espinosa et al. 2012), testicular and kidney cancer (Barry et al. 2013), childhood adiposity (Braun et al. 2016), decreased duration of breast feeding in infants (Romano et al. 2016), and possibly preeclampsia (Savitz et al. 2012b, 2012a).
Routes of exposure to PFASs include diet (Fromme et al. 2007), dust (Shoeib et al. 2005), and drinking water (Hu et al. 2016). This exposure has led to development of a U.S. Environmental Protection Agency (U.S. EPA) drinking water lifetime health advisory (LHA) for the sum of PFOA and PFOS of [70 parts per trillion (ppt)] (U.S. EPA 2016b, 2016a), and also regional drinking water standards in the low ppt range (NJDWQI 2016; VTDOH 2016). The U.S. EPA recently completed a national survey of six PFASs in U.S. drinking water that was targeted primarily at large, public, drinking water systems serving more than 10,000 people (U.S. EPA 2012). Studies analyzing these publicly available data (under the Third Unregulated Contaminant Monitoring Rule or UCMR3) have concluded that 6 million U.S. residents are served by systems exceeding the LHA (Hu et al. 2016; U.S. EPA 2012). The survey sampled equal numbers of systems sourced from surface water and groundwater, but approximately 72% of PFAS detections occurred in groundwater (Guelfo and Adamson 2018). Groundwater is the water source for 33% of public supplies in the U.S., and 90% of supplies in rural regions that rely on smaller (i.e., private) wells (USGS 2016). Collectively, health concerns and rates of occurrence highlight the important role of groundwater in human health risks associated with PFAS releases.
Numerous communities in the Northeast U.S. are currently assessing and managing risks due to PFAS-affected groundwater used as drinking water at the private, community, and public scales (e.g., Cape Cod, Massachusetts; Merrimack, New Hampshire; Portsmouth, New Hampshire; Hoosick Falls, New York; Paulsboro, New Jersey; and Bennington, Vermont). In many cases, discovery of these impacts occurred almost simultaneously in a period beginning in 2015, as a result of investigations initiated when residents and regulators learned of potential PFAS sources proximal to drinking water wells. Discovery of PFAS impacts led to a large group of regulators, consultants, analytical laboratories, and responsible parties (herein referred to collectively as stakeholders) with an immediate need to understand and manage risks associated with PFAS-affected groundwater and associated exposure. To determine risks associated with affected groundwater aquifers, stakeholders need information regarding sources, fate and transport pathways, affected receptors, sampling/analytical tools, health-based regulations, and water treatment technologies.
The Brown University Superfund Research Program (SRP) actively engaged with over a thousand stakeholders through PFAS workshops, analytical guidance, and other research translation efforts targeted at communicating the state of the science in the areas of PFAS chemistry, uses, sources, sampling/analysis, fate/transport, remediation, toxicology, regulation, and case studies. These efforts also provided an opportunity to hear diverse perspectives and learn of the key challenges faced by the broader scientific community in managing PFAS impacts. The objectives of this commentary are to (1) compile, critically evaluate, and share research translation findings to rationalize extrapolation of findings to other regions experiencing PFAS releases and (2) provide a framework to guide research and management strategies by prioritizing those PFASs that represent the highest risk of occurrence in treated drinking water. We conclude with an illustrative case study to demonstrate methods that can be used to address knowledge gaps regarding PFASs in drinking water to more effectively evaluate and mitigate risk.
Methods and Approach
The current evaluation utilizes a combination of research translation approaches, review of information in traditional publication outlets, review of publicly available data, and assimilation of select data sources into a limited case study of the risk of PFAS drinking water aquifer impacts in the State of Rhode Island.
Research Translation
To address stakeholder needs in the Northeast, a series of research translation activities were implemented beginning in 2016 to connect stakeholders (primarily from Connecticut, Massachusetts, Maine, New Hampshire, New Jersey, New York, and Vermont) with PFAS experts in various focus areas to communicate relevant aspects of research and current state-of-science (Table S1). Following these efforts, PFAS experts who participated in these events collaborated to document and review the challenges and knowledge gaps that are summarized in the current commentary. A literature review was used to evaluate the knowledge gaps in the framework of the current state-of-science. This review included web searches in the Web of Science database for all English language peer-reviewed articles (e.g., primary data, reviews, editorials) published 1995–present using title and topic search string PFOA OR PFOS OR PFAS in titles and topics from 1995–present .
Illustrative Case Study
This commentary presents a geospatial case study to predict risks of PFAS impacts in drinking water aquifers. The approach required an inventory of potential PFAS release sites, which are defined for the case study as facilities that may be associated with the synthesis, use, or disposal of PFASs. We first reviewed peer-reviewed literature and regulatory data to understand potential PFAS source types and associated characteristics (Table 1). Next, we reviewed publicly available regional geospatial coverages and manufacturing directories related to these source types to build a database of facilities in the state of Rhode Island (Table S2). Hard-copy archives of historical manufacturing directories were converted into a digital database using tools described in Berenbaum et al. 2016. Briefly, an open-source data processing tool named GEOREG was created to process the scanned images of Rhode Island manufacturing directories and convert the text into geocoded historical industrial and manufacturing locations from 1950s–present. GEOREG also extracts additional information regarding a facility’s name, address, standard industrial classification (SIC) code (i.e., manufacturing type), and number of employees. Resulting data include more than 11,000 unique historical and contemporary manufacturing sites. From this database, we selected only sites that matched the SIC codes (Table S2) and time frame (1960s–present) relevant to PFASs. The application of potential PFAS sources in a geospatial risk evaluation of potential PFAS impacts in Rhode Island groundwater is further discussed in the case study.
Table 1.
Groundwater concentrations, compounds, relevant groundwater pathways, and affected receptors resulting from groundwater PFAS source types summarized from peer-reviewed literature and regulatory reports.
| Source type | Magnitude of [PFAS] (µg/L) | Max PFAS | PFASs detected | Ground water pathways | Receptors impacted | Ref. cited |
|---|---|---|---|---|---|---|
| PFAS/FP manufacturing | PFOA | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFBS, PFHxS, PFOS | VZ to GW Atm SW to GW | DW, GW, SW, B | MDOH 2012; Davis et al. 2007; Bach et al. 2017; Dauchy et al. 2012; Weston Solutions 2009 | |
| AFFF use (DoD)a | 6:2 FtS | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFDoA, PFEtS, PFPrS, PFBS, PFPeS, PFHxS, PFHpS, PFOS, PFDS, 4:2 FtS, 6:2 FtS, 8:2 FtS, FHxSA, FOSA, 4:2 FtTAoS, 6:2 FtTAoS, PFBSaAm, PFPeSaAm, PFHxSaAm, PFHxSaAmA | VZ to GW | DW, GW, SW, B | Houtz et al. 2013; McGuire et al. 2014; Schultz et al. 2004; Moody et al. 2003; MDHHS 2016; Hull et al 2017; Moody and Field 1999; Barzen-Hanson and Field 2015; Backe et al. 2013 | |
| AFFF use (airport) | PFOA | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFBS, PFHxS, PFOS | VZ to GW | DW, GW, SW, B | Ahrens et al. 2015; Awad et al. 2011; Yingling 2016; Antea Group 2011; Delta Consultants 2010; Horsley Witten Group, Inc., 2016 | |
| AFFF use (fire training area)b | PFOS | PFBA, PFPeA, PFHxA, PFOA, PFDoA, PFTriA, PFTreA, PFBS, PFHxS, PFOS, EtFASE, MeFASE | VZ to GW | DW, GW, SW | Antea Group 2011; Cape Cod Commission 2016 | |
| AFFF use (petroleum) | PFOS | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFBS, PFHxS, PFOS, FOSA | VZ to GW | DW, GW | Antea Group 2011 | |
| FP coating (e.g. plastics, textiles, metals) | PFOA | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFBS, PFHxS, PFHpS, PFOS, FOSA, 6:2 FtS, 8:2 FtS | Not specified | DW, GW | U.S. EPA 2016c; NHDES 2017a | |
| Electronics | PFOA | PFHpA, PFOA, PFOS | Not specified | DW, GW | Unicorn Mgmt. Consultants 2016 | |
| Waste streams (landfills) | PFBA | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFBS, PFHxS, PFOS, 6:2 FtS | VA to GW Atm | DW, GW | NHDES 2017a; Weston Solutions 2016; VTDEC 2016; Oliaei et al. 2006; Oliaei et al. 2013 | |
| Waste streams (biosolids) | PFOA | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFBS, PFHxS, PFOS | VZ to GW | DW, GW, SW, B | Lindstrom et al. 2011 | |
| Waste streams (septic systems) | PFHxS | PFHxA, PFHpA, PFOA, PFBS, PFHxS, PFOS | VZ to GW | DW, GW | Schaider et al. 2016 |
Note: PFBA, Perfluorobutanoate; PFPeA, perfluoropentanoate; PFHxA, perfluorohexanoate; PFHpA, perfluoroheptanoate; PFOA, perfluorooctanoate; PFNA, perfluorononanoate; PFDA, perfluorodecanoate; PFUnA, perfluoroundecanoate; PFDoA, perfluorododecanoate; PFTriA, perfluorotridecanoate; PFTreA, perfluorotetradecanoate; PFEtS, perfluoroethane sulfonate; PFPrS, perfluoropropane sulfonate; PFBS, perfluorobutane sulfonate; PFPeS, perfluoropentane sulfonate; PFHxS, perfluorohexane sulfonate; PFHpS, perfluoroheptane sulfonate; PFOS, perfluorooctane sulfonate; PFDS, perfluorodecane sulfonate; 4:2 FtS, 4:2 fluorotelomer sulfonate; 6:2 FtS, 6:2 fluorotelomer sulfonate; 8:2 FtS, 8:2 fluorotelomer sulfonate; FHxSA, perfluorohexane sulfonamide; FOSA, perfluorooctane sulfonamide; 4:2 FtTAoS, 4:2 fluorotelomer thioether amido sulfonate; 6:2 FtTAoS, 6:2 fluorotelomer thioether amido sulfonate; 8:2 fluorotelomerthioether amido sulfonate (8:2 FtTAoS); PFBSaAM, perfluorobutane sulfonamido amine; PFBSaAM, perfluoropentane sulfonamido amine; PFHxSaAm, perfluoropentane sulfonamido amine; PFHxSaAmA, perfluorohexane sulfonamide amino carboxylate; EtFASE, N-ethyl perfluoroalkane sulfonamidoethanol; MeFASE, N-methyl perfluoroalkane sulfonamidoethanol; VZ, vadose zone; GW, groundwater; SW, surface water; (Atm.) atmospheric deposition and migration through the vadose zone; DW, drinking water, B, biota; DoD, Department of Defense; FP, fluoropolymer.
Recent studies have identified 11 new classes of PFASs comprising 50 individual compounds in AFFF-impacted groundwater from DoD facilities (Barzen-Hanson et al. 2017b); these compounds are not listed here because quantification of their concentrations is not yet available.
Represents fire training areas at municipal or private fire training institutions.
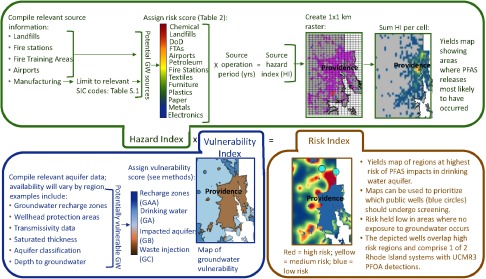
We used the characteristics listed in Table 1 to rank PFAS source types according to associated risk for causing groundwater PFAS impacts. First, PFAS source data coverages identified or developed for Rhode Island were matched to a source type (i.e., Table 1 Source Type in Table 2). Next, Table 1 was used to determine the number of PFAS compounds known to be present and upper magnitude of PFAS concentrations measured in affected groundwater of each source type. Then, each source type was assigned a risk score of 25, 50, 75, or 100, with the maximum value assigned to those sites yielding highest PFAS groundwater concentrations and number of PFASs (Table 2). The risk score values themselves are arbitrary values used as multipliers along with the duration of operation for each identified facility in calculating a hazard index (HI). A raster of one by one km cells was overlaid on the sources in Rhode Island, and the HI values within a cell were summed (Figure 1). Next, each cell was assigned a groundwater vulnerability index (VI) based on Rhode Island aquifer classifications GAA, GA, GB, and GC (RIDEM 2012). We further divided GAA into groundwater recharge zones and wellhead protection areas. These classifications were given VI values of 100 (GAA, recharge zones), 80 (GAA wellhead protection areas), 60 (GA), 40 (GB) and 20 (GC). Summed HI values were multiplied by VI values to assign a risk index (RI) to each raster cell. Finally, we used a simple universal kriging procedure on the raster cell centroids to smooth RI values across the raster surface and provide some conservative interpolation of the RI values. We used the results to generate risk maps of the Rhode Island region. The risk scores, HI, VI, RI, and groundwater classifications are conceptually described in the discussion.
Table 2.
Risk scores utilized for calculation of the PFAS source hazard index (HI).
| PFAS source | Upper magnitude | No. PFASs | Risk score | Table 1 source type |
|---|---|---|---|---|
| DoD facilities | 10,000 | 28 | 100 | AFFF use (DoD) |
| Chemical manufacturing | 1,000 | 13 | 100 | PFAS/FP manufacturing |
| Landfills | 1,000 | 11 | 100 | Waste streams (landfills) |
| Airports | 100 | 28 | 75 | AFFF use (Airports)a |
| Fire training areas | 100 | 28 | 75 | AFFF use (fire training areas)a |
| Petroleum refineries | 10 | 28 | 75 | AFFF use (petroleum refineries)a |
| Textiles | 10 | 13 | 50 | FP coating (plastics, textiles, metals) |
| Furniture | 10 | 13 | 50 | FP coating (plastics, textiles, metals) |
| Paper | 10 | 13 | 50 | FP coating (plastics, textiles, metals) |
| Rubber/plastics | 10 | 13 | 50 | FP coating (plastics, textiles, metals) |
| Fire Stations | N/A | 28 | 25 | N/Aa,b |
| Fabricated metal | N/A | 11 | 25 | N/Ac |
The number of PFASs reported for this source type was lower in the literature or no data were available (Table 1). A value of 28 was applied because this is the number of PFASs quantified at DoD AFFF-impacted facilities, and it is assumed that an equal number of PFASs may be present at all AFFF-impacted facilities.
There were no data available on groundwater impacts due to fire stations, but fire stations were indicated as a probable source of groundwater impacts during stakeholder engagement. The overall risk score was presumed to be low because many fire stations do not store or use AFFF, and those that do have AFFF do not typically discharge the foams onsite. In personal communications with industry, municipal, and volunteer firefighters, some report that equipment cleaning may occur on site following AFFF use (oral communications, July 2014–July 2017).
There were no data available on groundwater impacts due to electroplating, but data were available on PFASs in waste streams in the chrome plating process (U.S. EPA 2009). These data were used to determine the number of PFASs, and the upper concentration magnitude was the average of the magnitudes from other manufacturing sources.
Figure 1.
Overview of Rhode Island case study that utilizes a systematic approach to conduct a geospatial risk assessment of potential PFAS impacts in drinking water aquifers. Wells are shown with 1-mile buffers.
Results
Key Information Gaps
Sample collection.
Investigation of human health and environmental impacts of any compound requires reliable sampling techniques suitable for concentration levels that may represent health concerns. For PFASs, the challenge arises because of the low regulatory limits (U.S. EPA 2016b, 2016a), their ubiquitous nature (Prevedouros et al. 2006), and their use in the manufacturing process for some types of polytetrafluoroethylene (PTFE), which is a common laboratory material (Kissa 2001). Stakeholders are adopting special precautions (e.g., use of high- or low-density polyethylene containers and silicon tubing) when collecting samples for PFAS analysis (e.g., MassDEP 2017; NHDES 2017b). However, some stakeholders are adopting sampling and analysis protocols that include lists of unallowable items for which the need for prohibition is uncertain or not supported by scientific studies. These protocols include avoiding use of waterproof field notebooks, waterproof clothing, clothing laundered fewer than six times or laundered with fabric softener, cosmetics, and certain sunscreens (MassDEP 2017; NHDES 2017b). There is only limited evidence documenting the presence of PFASs in some of these products (Fujii et al. 2013; Keawmanee et al. 2015), and no cited published data measuring the potential for transfer of PFASs from these materials into samples during collection are available. Therefore, we conclude that the precautions represent an extremely conservative approach to avoid products and materials that include even trace amounts of PFASs. Data are needed to support prioritization of these precautions to avoid unnecessary inconvenience to field sampling personnel. When regulating in the low ppt level, we propose that understanding potential sources of background in samples is also key to differentiating between PFAS-affected drinking water and cross-contamination of samples.
Targeted PFAS analysis.
Despite notable progress over the past 20 y in utilizing liquid chromatography tandem mass spectrometry (LC-MS/MS) techniques (and gas chromatographic techniques for volatile PFASs such as fluorotelomer alcohols) for PFAS analysis (e.g., Mahmoud et al. 2009; Moody et al. 2001; Schultz et al. 2004), significant challenges remain. Many obstacles stem from the fact that the complete list of PFASs relevant to environmental and human health exposure scenarios is still unknown and ever increasing as more studies are completed identifying novel PFASs and precursor transformation products (e.g., Barzen-Hanson et al. 2017b). Therefore, though the majority of PFASs are suitable for LC-MS/MS analysis, standards needed to quantify them are not currently available, and it is difficult to keep pace with the increasing number of relevant compounds. It should also be noted that even implementation of targeted LC-MS/MS analysis may represent a challenge in terms of instrument expense, effort, and elimination of PFAS background issues (i.e., from aforementioned laboratory materials such as PTFE) for laboratories that are being required to address the issue. PFAS standards are commercially available (not including special order synthesis) for approximately 70 PFASs, and of those also have available isotopically labeled versions for use in isotope dilution approaches. It should be noted that in cases where a compound standard is available but a matching labeled standard is not, the labeled version of another PFAS may be used as an internal standard. For example, 1,802- PFHxS has been used for analysis of PFBS (e.g., Guelfo and Higgins 2013; McGuire et al. 2014). In our view, these challenges make it virtually impossible for any regulatory authority to comprehensively specify which PFASs need to be investigated at a potentially affected site.
Additional analytical tools.
Tools are available that can help to characterize the PFAS fraction not quantified during targeted LC-MS/MS analysis. These tools include the total oxidizable precursor (TOP) assay, high-resolution mass spectrometry (HRMS) analysis, particle induced gamma ray emission (PIGE) and adsorbable organofluorine (AOF) analysis. However, these methods are still limited in their availability and ability to quantify concentrations of individual PFASs present at a site. Detailed descriptions of TOP (Houtz and Sedlak 2012), PIGE (Ritter et al. 2017), and AOF (Wagner et al. 2013) are available elsewhere, but briefly, they enable measurement of total precursors, total fluorine, and total organic fluorine, respectively. Coupling TOP, PIGE, or AOF with targeted LC-MS/MS analysis can help researchers understand the total PFAS load present in a sample but does not result in identification of all individual PFASs present. High-resolution mass spectrometry (HRMS) using technology such as quadrupole time of flight generates high mass accuracy data that can be used in identification of unknown compounds (Barzen-Hanson et al. 2017b; Strynar et al. 2015), but quantification of PFASs without standards remains a challenge. TOP has begun to emerge as a commercially used technique, but availability of PIGE, AOF, and HRMS is often limited to noncommercial research laboratories, leaving limited access for regulators and other practitioners who want to implement these tools. During research translation events, stakeholders reported that these challenges prevent them from developing conceptual models of affected sites that include a complete list of PFASs to which environmental and human receptors may be exposed.
Source zone identification.
Another key knowledge gap is PFAS source zone identification, which can be illustrated through comparison with the legacy groundwater contaminant, methyl tert-butyl ether (MTBE). MTBE was historically used as a gasoline fuel additive with peak use occurring from 1992–2005 (U.S. EPA 2016d). MTBE has high aqueous solubility , has low soil sorption, and is slow to degrade, leading to the potential for more extensive groundwater plumes relative to other legacy contaminants, such as benzene (Squillace et al. 1996). MTBE had a single primary use (U.S. EPA 2016d), so point sources were commonly facilities or infrastructure associated with retail gasoline supply and distribution for which current and historical information is typically available. Though indirect sources of MTBE groundwater contamination such as atmospheric this deposition are possible, this would primarily contribute to disperse, low-level background concentrations (Squillace et al. 1997) not likely to pose a threat to human health or the environment.
When considering the sources of PFASs and comparing with the example of MTBE, we conclude the following. PFASs also may be highly water soluble with weak soil sorption and exhibit recalcitrance to natural degradation, leading to the potential for large groundwater plumes. However, unlike MTBE, there are many relevant PFASs and diverse products and applications with which they are associated (Table 1). Additionally, although PFASs may be associated with a particular process or product, such as textiles manufacturing, it cannot be concluded that all products and manufacturers in a relevant industrial category utilized PFASs. Finally, due to regulatory limits in the low ppt range, indirect sources of PFASs (e.g., groundwater impacts due to leaching of atmospheric deposition or land application of composts and wastewater biosolids) have led to environmentally relevant groundwater impacts (Lindstrom et al. 2011; Shin et al. 2011) and cannot be discounted as important PFAS sources. Assembling information on all potential PFAS sources in a particular region is further complicated because information on current and historical locations of both direct and indirect sources is often missing or unconsolidated. For stakeholders who need to identify sources of known PFAS impacts or to design targeted screening programs to assess if releases have occurred, unconsolidated source data may lead to inefficient or untargeted sampling plans, a failure to identify all sources relevant to a particular release, or the inability to determine a source, thereby increasing time required to reduce risks to public health and the environment.
Subsurface fate and transport.
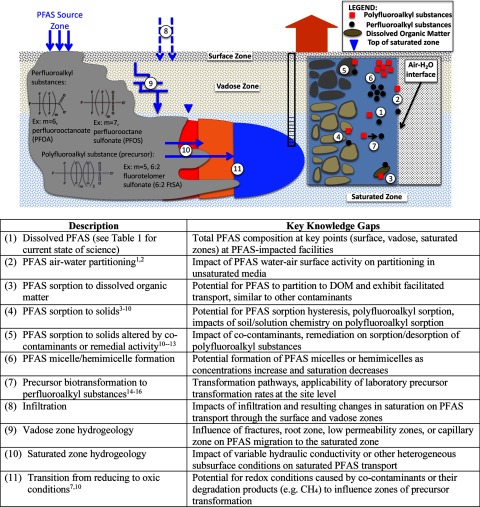
Although studies have investigated PFAS fate and transport, significant knowledge gaps remain (Figure 2). There are knowledge gaps in the general areas of PFAS composition [Figure 2 (1)] partitioning [Figure 2 (2–6)] transformation [Figure 2 (7)], and the influence of site hydrogeology and geochemistry [Figure 2 (8–11)]. An understanding of fate and transport requires knowledge of both compound-specific (e.g., sorption, transformation) and site-specific (e.g., geology, geochemistry) factors (Fetter 1999). PFAAs are recalcitrant (Prevedouros et al. 2006), so the primary compound-specific factors that need to be understood are sorption and potential for generation from precursors. Polyfluoroalkyl substances (i.e., precursors) can transform (Harding-Marjanovic et al. 2015; Mejia Avendaño and Liu 2015; Weiner et al. 2013), so in addition to sorption, knowledge of transformation rates and pathways (e.g., intermediate products) is required. These processes rely on compound-specific properties, such as sorbate structure, but are also influenced by site-specific properties, such as sorbent type, solution chemistry, and cocontaminants (Guelfo and Higgins 2013; Higgins and Luthy 2006; Weber et al. 2017). Further, PFAS distribution at the field scale also depends on subsurface hydrogeologic conditions, including groundwater flow direction, velocity, and influence of heterogeneous geology (Fetter 1999). Finally, user-friendly groundwater modeling tools for legacy contaminants have been developed to assist stakeholders with decision points, such as site prioritization, monitoring plans and duration, and design of aquifer remediation alternatives (Aziz and Newell 2000; Newell et al. 1996), but comparable tools are currently not available for PFASs. In our opinion, these challenges in understanding PFAS fate and transport limit the ability to identify at-risk receptors, understand the probability for a source to affect those receptors, and to help identify potential sources of newly discovered groundwater releases.
Figure 2.
Conceptual model of micro and macroscale PFAS fate/transport processes and associated knowledge gaps. Superscripted numbers refer to the following references: 1Kissa 2001; 2Banks et al. 1994; 3Higgins and Luthy 2006; 4Liu and Lee 2007; 5Liu and Lee 2005; 6Ferrey et al. 2012; 7Ololade et al. 2016; 8Tang et al. 2010; 9Barzen-Hanson et al. 2017a; 10Weber et al. 2017; 11Guelfo and Higgins 2013; 12McKenzie et al. 2015; 13McKenzie et al. 2016; 14Harding-Marjanovic et al. 2015; 15Mejia Avendaño and Liu 2015; 16Weiner et al. 2013.
PFAS toxicology and use in regulation.
Other efforts have summarized the current state of science regarding PFAS toxicology (ASTDR 2015; DeWitt et al. 2009, 2015; Lau et al. 2007; Negri et al. 2017) and epidemiology (Bach et al. 2015, 2016, Chang et al. 2014; Negri et al. 2017; Steenland et al. 2010). In our view, results of toxicology studies highlight several challenges related to understanding health effects of PFASs. First, studies address the toxicology of only a subset of PFASs. Outside of PFOA/PFOS, toxicology studies are available for other PFAAs, such as PFBA mouse studies (Das et al. 2008; Foreman et al. 2009), PFBS rat studies (Lieder et al. 2009a, 2009b), a PFHxA rat study (Loveless et al. 2009), PFNA mouse studies (Das et al. 2015; Fang et al. 2008), a PFNA rat study (Feng et al. 2010), and a PFHxS rat study (Butenhoff et al. 2009). Studies are also generally limited for polyfluoroalkyl compounds (Buck 2015), especially recent replacement products, although some data are available for PFOA replacement products GenX (Beekman et al. 2016; Caverly Rae et al. 2015; Gannon et al. 2016) and ADONA (Gordon 2011). Second, the mechanism of action for PFAA-associated toxicity is not well understood, although peroxisome proliferator-activated receptor alpha () activation is often implicated (Das et al. 2015; Lau et al. 2007). Third, although animal studies are useful in elucidating target organs, there are notable differences in how humans and animals interact with PFAAs. For example, PFAAs are documented to have half-lives on the order of years in humans (Li et al. 2018; Worley et al. 2017) but only hours to days in laboratory animals (Lau et al. 2007). Long half-lives indicate human serum concentrations will remain elevated, suggesting toxicity in humans may persist even after environmental PFAS levels decrease. Further, applying activation in animal studies to humans is complicated by several species differences that are well described elsewhere (Post et al. 2017). Fourth, the potential for synergistic toxicity is not well characterized, despite human exposure to PFAS mixtures. Two related in vitro studies found that mixtures of 2–4 PFASs yielded additive, not synergistic, activation of murine (Carr et al. 2013; Wolf et al. 2014). However, as noted, questions remain regarding the role of and the applicability of animal studies to humans. Additionally, in vitro studies are unable to capture pharmacokinetics alterations that may lead to synergistic toxicity.
Knowledge gaps in toxicology pose challenges for regulators and other stakeholders tasked with managing PFAS releases. Although data are available for PFOS and PFOA, there is still a lack of consensus regarding which toxicological end point and subpopulations should be targeted in development of drinking water standards. This lack of consensus can be illustrated through comparison of the U.S. EPA LHAs (U.S. EPA 2016b, 2016a) to standards developed for PFOA in New Jersey (NJDWQI 2016) and Vermont (VTDOH 2016). Drinking water quality standards (DWQS) are generally calculated as follows (e.g., U.S. EPA 2016b, 2016a):
| [1] |
where RfD is the reference dose (i.e., the maximum daily dose for which no adverse health effects are expected to occur), BW is the body weight, DWI is the drinking water ingestion rate, and RSC is the relative source contribution, or proportion of PFAS exposure from drinking water. The LHA (U.S. EPA 2016b, 2016a) and DWQS differ between New Jersey (NJDWQI 2016) and Vermont (VTDOH 2016) in part because of key differences in the values used to calculate these standards (Table 3). In standards development, values for DWI may change, depending on the subpopulation considered (e.g., adults, children), and, in the case of PFOA agencies, differ on which rates are deemed adequately protective: lactating women (U.S. EPA), BW adjusted rate for the first year of life (Vermont), or adult water intake (New Jersey). Table 3 also illustrates that there is debate regarding the most appropriate RfD. Specifically, the NJDWQI believes that the EPA failed to consider more sensitive end points, such as the liver and immune effects for which the NJ RfD is considered protective (NJDWQI 2016). The NJDWQI also expressed concerns, which we share, regarding low-dose findings, such as lack of repetition, nonmonotonic data (delay in phalanges ossification and mammary gland development), and unknown clinical significance (mammary gland development without disruption of lactation, delay in phalanges ossification without malformations, mild reductions in immune factors without increase incident of infection) (NJDWQI 2016).
Table 3.
Values used in development of PFOA advisories and standards and associated maximum recommended levels in drinking water.
| Agency | Advisory or standard | RfD | DWI/BW | RSC | Toxicological end point | Reference |
|---|---|---|---|---|---|---|
| USEPA | 70 | 2.E-05 | 0.054 | 0.2 | delay in phalanges ossification, mice | U.S. EPA 2016b, 2016a |
| NJDWQI | 14 | 2.E-06 | 0.029 | 0.2 | Hepatoxicity, mice | NJDWQI 2016 |
| VTDOH | 20 | 2.E-05 | 0.175 | 0.2 | delay in phalanges ossification, mice | VTDOH 2016 |
Note: NJDWQI, New Jersey Drinking Water Quality Institute; VTDOH, Vermont Department of Health; DWI, drinking water ingestion rate; BW, body weight; RSC, relative source contribution.
Although all approaches result in standards in the low ppt range, these variations lead to different interpretations of what would be considered an affected drinking water system. For example, the U.S. EPA UCMR3 efforts sampled public drinking water systems in the U.S. for six PFASs, including PFOS, PFOA, and PFNA (U.S. EPA 2016e). The number of systems in the data set that would be considered problematic based on the U.S. EPA LHAs more than doubles if the New Jersey or Vermont standards are applied to the data set (Guelfo and Adamson 2018). Additionally, different standards may be applied to drinking water of adjacent communities separated by state lines. Such is the case in New York and Vermont, raising questions about why a community in one state may continue to drink groundwater that would be considered unsafe by an adjacent community across a state line.
Groundwater remediation.
Conventional treatment techniques are ineffective for removal or destruction of the full suite of PFASs present in affected water (e.g., Rahman et al. 2014; Schultz et al. 2006). For example, processes relying on in situ chemical oxidation cannot fully destroy all PFASs but can enhance oxidation of precursors to end point PFAAs (Houtz and Sedlak 2012). Additionally, they may destroy perfluoroalkyl carboxylates (e.g., PFOA) under some conditions but are ineffective at degrading perfluoroalkyl sulfonates (e.g., PFOS) (Bruton and Sedlak 2017; Park et al. 2016). Filtration with granular activated carbon (GAC) and anion exchange resins (AER) have been shown to remove PFOS and PFOA but may not be as effective for treatment of short chain PFAAs and precursors (Appleman et al. 2013; Xiao et al. 2017; Yu et al. 2009; Zaggia et al. 2016). Further, filtration does not achieve compound destruction, so additional treatment or disposal of spent media is required. There has been some success at the bench and pilot scale using advanced oxidation processes that rely on electrochemical or plasma-based techniques to destroy PFASs in extracted, affected groundwater (Chaplin 2014; Stratton et al. 2017). Despite significant progress, these techniques are generally not ready for full-scale implementation, and key concerns include potential treatment-rate limitations and energy requirements. Last, design of any treatment technique may also need to account for cocontaminants (e.g., hydrocarbon constituents) that may be present in some aquifers (McGuire et al. 2014).
Despite knowledge gaps, filtration with GAC is a common technique used to address PFAS in affected drinking water systems (e.g., Damon 2016; NYDEC 2016; Weston & Sampson 2016). Although effective for treatment of PFASs currently targeted for regulation (i.e., PFOS, PFOA), these systems are not optimized for removal of the full suite of PFASs present in some affected groundwater. In our view, this suggests exposure to PFASs for which toxicological outcomes are not yet fully understood may be ongoing. In the event that water quality standards are developed for additional PFASs, we also point to the potential scenario that sites formerly remediated for PFOS/PFOA will need to be revisited for treatment of PFASs not previously considered. To minimize these potential risks, we agree with others that it may be necessary to implement combined remedies or treatment trains (Crimi et al. 2017; Kucharzyk et al. 2017) and argue that these approaches should target removal or destruction of total PFASs present.
Discussion
Often during site assessment, evaluation of levels of exposure and potential human health consequences are of paramount concern (U.S. EPA 1989). In our view, even when areas such as occurrence, fate/transport, and remediation are well understood, the health consequences of PFASs will remain uncertain. Although the volume of research in both toxicology and human health studies has increased markedly in the last decade, firm conclusions relating individual PFASs to specific health outcomes have remained elusive. As noted, a wide range of potential links between PFAS exposure and health outcomes have been reported (e.g., ASTDR 2015; Steenland et al. 2010), but the uncertainties remain substantial. As one extends that interest into other legacy PFASs and particularly into the newer generation of PFASs, the empirical evidence guiding interpretation of health effects declines substantially and is virtually absent for many of the compounds, suggesting a need for strategies to prioritize PFASs for further study.
PFAS Framework
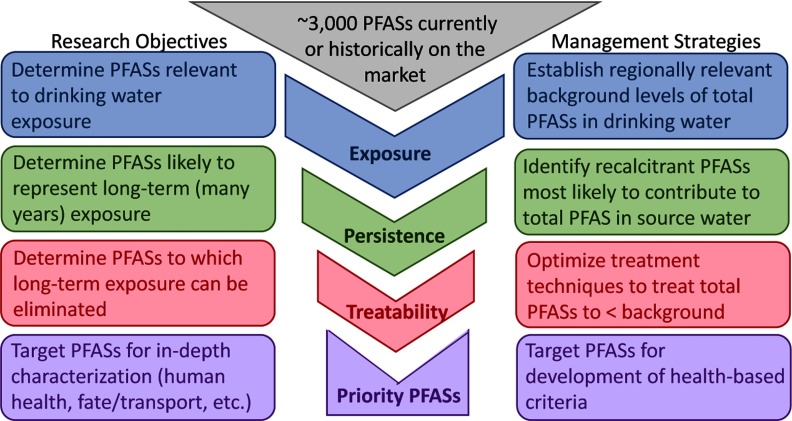
Frameworks have been developed in previous studies to evaluate large groups of compounds and prioritize those that should be targeted for further research on factors such as analysis, occurrence, fate and transport, and treatability (de Voogt et al. 2009; Howard and Muir 2010, 2011, 2013; Kumar and Xagoraraki 2010; Strempel et al. 2012), but these approaches often rely at least in part on toxicity information, the limitations of which have already been discussed for PFASs. Nevertheless, we propose that additional criteria used in these approaches, such as occurrence, persistence, and treatability, might be coupled with evaluation of exposure into a framework to guide future research and inform best management practices (Figure 3). Despite challenges outlined herein, progress has been made in understanding drinking water occurrence, persistence, and treatability, such that it is possible to begin identifying PFASs that should be targeted for further study. That is particularly the case for evaluation of exposure (Step 1), and here we present a limited case study illustrating a risk-based evaluation of the potential for PFAS exposure in drinking water due to presence of potential source zones. Last, we note that the body of publicly available information regarding aspects such as compound persistence and toxicity continues to grow as part of legislative efforts, such as the European Union's Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) (ECHA 2017a). REACH requires industry to identify and communicate risks associated with substances they use (ECHA 2017b), creating a valuable resource that can be applied toward compound prioritization using this framework.
Figure 3.
Framework for research and management strategies that prioritize PFASs based on highest risk of exposure in drinking water. It should be noted that here exposure refers only to drinking water; other routes of ingestion, such as food, are not considered.
Geospatial Evaluation of PFAS Exposure Risk
As discussed, there are challenges in identifying sources of and exposure to PFAS groundwater impacts on a regional scale. We present a case study of potential PFAS groundwater impacts in the state of Rhode Island to illustrate methods that can be used to overcome these challenges. Previous studies have assessed regional risks of degraded groundwater quality due to other classes of contaminants by compiling information on potential sources of contamination (e.g., population-dense regions, landfills, gas stations) and comparing their location with groundwater that is vulnerable to impacts (Babiker et al. 2005; Rahman 2008). Groundwater vulnerability may be evaluated through hydrogeologic characteristics, such as depth to groundwater and transmissivity (Gemitzi et al. 2006; Wang et al. 2012). A similar approach can be adopted for PFASs by compiling data on potential PFAS sources and groundwater vulnerability and applying a risk-based system to evaluate the potential for PFAS impacts (Figure 1).
As described in the Methods section, risk scores were assigned to each PFAS source type (Table 2). This score was based on the fact that not all PFAS sources are likely to be associated with the same (or any) severity of groundwater effects. A limitation to the calculation of the risk scores is that the number of PFASs detected at a given type of site may be a product of the limited PFASs that were analyzed. For example, many precursors could be present that have never been investigated. Additionally, some source types. such as electronics facilities and metal plating. had limited or no groundwater data from affected sites, so the upper magnitude of concentrations may not be representative. Nevertheless, the data are considered to be representative in terms of understanding the different source types relative to each other. Further, the resulting source ranking that reflects Department of Defense (DoD), chemicals manufacturing, and landfills as the highest risk sites is consistent with literature and regulatory reports in terms of capturing release types that are known to have caused significant drinking water aquifer PFAS impacts (Davis et al. 2007; Moody et al. 2003; NH DHHS 2016; Oliaei et al. 2013). Risk scores were used to calculate HI values (see Methods) which, when mapped, depict areas where PFAS releases are most likely to have occurred (Figure 1).
The HI, or likelihood of PFAS release, should not be used as the sole indicator for potential aquifer impacts and subsequent exposure because releases that occur must also be transported through the subsurface to the saturated zone in order to affect aquifers, and certain hydrogeologic conditions make this more likely (i.e., higher groundwater vulnerability) in some regions (Fetter 1999). Rhode Island aquifer classifications already represent an evaluation of groundwater vulnerability. The State of Rhode Island classifications are based on locations of shallow zones of recharge to deeper aquifers and wellhead protection areas (GAA), drinking water aquifers evaluated based on transmissivity and saturated aquifer thickness (GA), aquifers presumed or known to be degraded (GB), and groundwater where waste injection is permitted (GC) (RIDEM 2012). VI values based on these classifications (see Methods) define the risk of environmental releases affecting usable groundwater when such events occur (Figure 1). When the HI and VI are combined to calculate RI, the highest RI values represent areas at highest risk for PFAS impacts in drinking water and subsequent exposure (Figure 1).
Results of the evaluation of groundwater PFAS impacts in Rhode Island reveal high HI values centered around regions with a high density of former manufacturing facilities that are situated in the population-dense region of Providence. Although there is a high likelihood of a PFAS release having occurred in this region, the RI values near Providence are low due to depressed VI values. Aquifers in the immediate vicinity of Providence are classified as GB, indicating that there is no use of the groundwater (RIDEM 2012), so there should be no drinking water exposure to PFAS impacts present. The highest RI values occurred in more rural regions with a lower density of sources (i.e., lower HI) but higher VI values due to proximity to groundwater recharge areas and drinking water aquifers. RI maps can be applied towards understanding potential sources of known PFAS groundwater impacts or in prioritizing drinking water wells that should be targeted in sampling programs, with the ultimate goal of understanding and mitigating risks associated with PFAS exposure. In Rhode Island, the majority of wells in high RI regions are private or small community wells, which were not screened as part of U.S. EPA UCMR3 screening efforts. Notably, two PFOA detections were discovered in Rhode Island as part of UCMR3, with concentrations of . The geospatial evaluation of PFASs in Rhode Island aquifers found that wells in both of these systems (some systems are sourced from multiple wells) have overlap with areas of high RI (Figure 1). Finally, it should be noted that this approach represents a limited case study for illustrative purposes, and efforts to further ground truth, refine the geospatial approach, and characterize which (if any) PFASs are present are part of ongoing research.
Conclusions
In summary, interactions with stakeholders from affected communities in the Northeast U.S. have identified a number of key knowledge gaps in several areas, including sampling and analysis, fate and transport, toxicology, regulation, and water treatment. An important result is a lack of consensus regarding management and regulation of PFASs in drinking water. Both laboratory and epidemiological studies support the potential for negative health outcomes due to PFAS exposure. In response, water quality regulations for PFASs are starting to emerge, but these regulations primarily apply to PFOS/PFOA. Regulatory levels are based solely on extrapolation from mechanistic studies in animal models and incorporate substantial uncertainty factors as a margin of safety. We conclude that these recently recommended standards and advisories for select PFASs should not be interpreted as indicating that health will be adversely affected if levels are exceeded. Rather, in explaining the health implications of elevated levels of PFASs in water sources to various stakeholders, it is important to be clear that knowledge is limited, in most cases severely so, and that declaration of safe or harmful levels of contamination is not possible.
Despite this uncertainty, we believe that it is important not to be complacent about human exposure to PFASs via drinking water, and strategies are needed to begin addressing water quality impacts even as research is ongoing. Therefore, we conclude that understanding knowledge gaps will help to guide investigation, management, and mitigation of specific releases, and that the framework developed here can be used to facilitate broader strategies for research and management focused on total PFASs in drinking water. The latter will help accelerate the process of mitigating exposure to PFASs for which detailed studies are lacking.
Results presented herein suggest that it is possible to begin implementing a comprehensive strategy towards PFAS management despite the considerable gaps in current knowledge, particularly regarding toxicity. In particular, this work compiles an already a large body of evidence related to potential PFAS sources and occurrence in groundwater (Table 1) that can be applied toward understanding exposure. We illustrated this by performing a risk-based, geospatial case study of potential PFAS source zones in Rhode Island drinking water aquifers. When compared with limited groundwater aquifer results, high-risk zones identified in the geospatial evaluation were proximal to drinking water wells with detectable PFAS concentrations (Figure 1). Further, new regulations such as REACH have led to increased sharing of industry data related to compound behavior in the environment, and this sharing helps build connections between research in academia, industry, and government. In our view, growing such partnerships facilitates effective management of chemical use and release. This commentary focuses on streamlined research strategies and best management practices for PFASs in drinking water, and this focus could be extended to evaluate other routes of exposure. Similar approaches might also be applied to other complex mixtures of aqueous contaminants with the overall effect of leveraging the current state of the science towards understanding drinking water impacts and reducing risks to human health.
Supplemental Material
Acknowledgments
We thank the Northeast Waste Management Officials Association (NEWMOA) for their efforts in coordinating PFAS workshops and webinars that provided the basis for many of the regional stakeholder engagement opportunities. We acknowledge financial support from the Superfund Research Program of the NIEHS grant 2P42 ES013660 and from the Institute at Brown for Environment and Society, which funds a Research Assistantship for T.M.
References
- Ahrens L, Norström K, Viktor T, Cousins AP, Josefsson S. 2015. Stockholm Arlanda Airport as a source of per- and polyfluoroalkyl substances to water, sediment and fish. Chemosphere 129:33–38, PMID: 24821232, 10.1016/j.chemosphere.2014.03.136. [DOI] [PubMed] [Google Scholar]
- Antea Group. 2011. Perfluorocarbon (PFC)-containing firefighting foams and their use in Minnesota: Survey and sampling activities, state fiscal year 2011. https://www.pca.state.mn.us/sites/default/files/c-pfc1-20.pdf [accessed 11 May 2017].
- Appleman TD, Dickenson ERV, Bellona C, Higgins CP. 2013. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J Hazard Mater 260:740–746, PMID: 23846124, 10.1016/j.jhazmat.2013.06.033. [DOI] [PubMed] [Google Scholar]
- ASTDR (Agency for Toxic Substances and Disease Registry). 2015. Draft Toxicological Profile for Perfluoroalkyls. U.S. Department of Health and Human Services Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [accessed 10 March 2018].
- Awad E, Zhang X, Bhavsar SP, Petro S, Crozier PW, Reiner EJ. 2011. Long-term environmental fate of perfluorinated compounds after accidental release at Toronto airport. Environ Sci Technol 45(19):8081–8089, PMID: 21774496, 10.1021/es2001985. [DOI] [PubMed] [Google Scholar]
- Aziz C, Newell C. 2000. BIOCHLOR Natural Attenuation Decision Support System. Natl. Risk Manag. Lab. Off. Res. Dev. U.S. EPA EPA/600/R-00/008. [Google Scholar]
- Bach C, Dauchy X, Boiteux V, Colin A, Hemard J, Sagres V, et al. 2017. The impact of two fluoropolymer manufacturing facilities on downstream contamination of a river and drinking water resources with per- and polyfluoroalkyl substances. Environ Sci Pollut Res Int 24(5):4916–4925, PMID: 27988902, 10.1007/s11356-016-8243-3. [DOI] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JPE, Henriksen TB. 2015. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol 45(1):53–67, PMID: 25372700, 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- Bach CC, Vested A, Jørgensen KT, Bonde JPE, Henriksen TB, Toft G. 2016. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: a systematic review. Crit Rev Toxicol 46(9):735–755, PMID: 27268162, 10.1080/10408444.2016.1182117. [DOI] [PubMed] [Google Scholar]
- Backe WJ, Day TC, Field JA. 2013. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ Sci Technol 47(10):5226–5234, PMID: 23590254, 10.1021/es3034999. [DOI] [PubMed] [Google Scholar]
- Banks RE, Smart BE, Tatlow JC. 1994. Organofluorine Chemistry Principles and Applications. New York, NY:Plenum Press. [Google Scholar]
- Barry V, Winquist A, Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect, PMID: 24007715, 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen-Hanson KA, Davis SE, Kleber M, Field JA. 2017a. Sorption of fluorotelomer sulfonates, fluorotelomer sulfonamido betaines, and a fluorotelomer sulfonamido amine in national foam aqueous film-forming foam to soil. Environ Sci Technol 51(21):12394–12404, PMID: 28968065, 10.1021/acs.est.7b03452. [DOI] [PubMed] [Google Scholar]
- Barzen-Hanson KA, Field JA. 2015. Discovery and implications of C2 and C3 perfluoroalkyl sulfonates in aqueous film-forming foams and groundwater. Environ Sci Technol Lett 2(4):95–99, 10.1021/acs.estlett.5b00049. [DOI] [Google Scholar]
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N, et al. 2017b. Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ Sci Technol 51(4):2047–2057, PMID: 28098989, 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Beekman M, Zweers P, Muller A, de Vries W, Janssen P, Zeilmaker M. 2016. Evaluation of substances used in the GenX technology by Chemours, Dordrecht. Dutch Natl. Inst. Public Health Environ. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=4&ved=0ahUKEwisvZ38ps7XAhXq4IMKHQjjC6AQFghCMAM&url=http%3A%2F%2Frvlvy.com%2Fmain%2Findex.php%3Fs%3DNetherlands%2520National%2520Institute%2520for%2520Public%2520Health%2520and%2520the%2520Environment%26item_type%3Dtopic&usg=AOvVaw2dnkakh-pGSN5Rkh7tiepC [accessed 20 November 17].
- Berenbaum D, Deighan D, Marlow T, Lee A, Frickel S, Howison M. 2016. Mining Spatio-temporal Data on Industrialization from Historical Registries. CoRR abs/1612.00992.
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study: Prenatal PFAS Exposure and Child Adiposity. Obesity (Silver Spring) 24(1):231–237, PMID: 26554535, 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton TA, Sedlak DL. 2017. Treatment of aqueous film-forming foam by heat-activated persulfate under conditions representative of in situ chemical oxidation. Environ Sci Technol 51(23):13878–13885, PMID: 29164864, 10.1021/acs.est.7b03969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: 21793199, 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC. 2015. Toxicology Data for Alternative “Short-Chain” Fluorinated Substances. In: Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. DeWitt JC, ed. Cham, Switzerland:Springer International Publishing; 451–477. [Google Scholar]
- Butenhoff JL, Chang S-C, Ehresman DJ, York RG. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod Toxicol 27(3–4):331–341, PMID: 19429404, 10.1016/j.reprotox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- C8 Science Panel. 2017. C8 Science Panel. http://www.c8sciencepanel.org [accessed 12 December 2017].
- Cape Cod Commission. 2016. Immediate Response Action Plan Barnstable County Fire and Rescue Training Academy RTN:4-26179 RTN: 4-190. http://public.dep.state.ma.us/fileviewer/Default.aspx?formdataid=0&documentid=367910 [accessed 12 May 2017].
- Carr CK, Watkins AM, Wolf CJ, Abbott BD, Lau C, Gennings C. 2013. Testing for departures from additivity in mixtures of perfluoroalkyl acids (PFAAs). Toxicology 306:169–175, PMID: 23470359, 10.1016/j.tox.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverly Rae JM, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL. 2015. Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague–Dawley rats. Toxicol Rep 2:939–949, PMID: 28962433, 10.1016/j.toxrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ET, Adami H-O, Boffetta P, Cole P, Starr TB, Mandel JS. 2014. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit Rev Toxicol 44(suppl1):1–81, PMID: 24793953, 10.3109/10408444.2014.905767. [DOI] [PubMed] [Google Scholar]
- Chaplin BP. 2014. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ Sci Process Impacts 16(6):1182, PMID: 24549240, 10.1039/C3EM00679D. [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, de Wolf W, Russell MH, Buck RC. 2008. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ Sci Technol 42(4):995–1003, PMID: 18351063, 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- Crimi M, Holsen T, Bellona CL, Divine C, Dickenson ERV. 2017. In Situ Treatment Train for Remediation of Perfluoroalkyl Contaminated Groundwater: In Situ Chemical Oxidation of Sorbed Contaminants (ISCO-SC). SERDP Project ER-2423.
- Damon E. 2016. No-drink order lifted on a Pownal public water system; Shumlin to visit on Monday. Bennington Banner. http://www.benningtonbanner.com/stories/no-drink-order-lifted-on-a-pownal-public-water-system-shumlin-to-visit-on-monday,117918 [accessed 24 July 2017].
- Das KP, Grey BE, Rosen MB, Wood CR, Tatum-Gibbs KR, Zehr RD, et al. 2015. Developmental toxicity of perfluorononanoic acid in mice. Reprod Toxicol 51:133–144, PMID: 25543169, 10.1016/j.reprotox.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Das KP, Grey BE, Zehr RD, Wood CR, Butenhoff JL, Chang S-C, et al. 2008. Effects of perfluorobutyrate exposure during pregnancy in the mouse. Toxicol Sci 105(1):173–181, PMID: 18511431, 10.1093/toxsci/kfn099. [DOI] [PubMed] [Google Scholar]
- Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, et al. 2017. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 378:37–52, PMID: 28049043, 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy X, Boiteux V, Rosin C, Munoz J-F. 2012. Relationship between industrial discharges and contamination of raw water resources by perfluorinated compounds. Part I: Case study of a fluoropolymer manufacturing plant. Bull Environ Contam Toxicol 89(3):525–530, PMID: 22706876, 10.1007/s00128-012-0704-x. [DOI] [PubMed] [Google Scholar]
- Davis KL, Aucoin MD, Larsen BS, Kaiser MA, Hartten AS. 2007. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 67(10):2011–2019, PMID: 17250873, 10.1016/j.chemosphere.2006.11.049. [DOI] [PubMed] [Google Scholar]
- de Voogt P, Janex-Habibi M-L, Sacher F, Puijker L, Mons M. 2009. Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci Technol 59(1):39, PMID: 19151484, 10.2166/wst.2009.764. [DOI] [PubMed] [Google Scholar]
- Delta Consultants. 2010. Report of Investigation Activities at Select Firefighting Foam Training Areas and Foam Discharge Sites in Minnesota. https://www.pca.state.mn.us/sites/default/files/c-pfc1-09.pdf [accessed 11 May 2017].
- DeWitt JC, Copeland CB, Strynar MJ, Luebke RW. 2008. Perfluorooctanoic acid–induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ Health Perspect 116(5):644–650, PMID: 18470313, 10.1289/ehp.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, et al. 2009. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol 39(1):76–94, PMID: 18802816, 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- DeWitt JC. 2015. Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances. Switzerland, Switzerland:Springer International Publishing. [Google Scholar]
- ECHA (European Chemicals Agency). 2017a. European Chemicals Agency Chemicals Search. https://echa.europa.eu [accessed 23 April 2018].
- ECHA. 2017b. Understanding REACH. https://echa.europa.eu/regulations/reach/understanding-reach [accessed 24 April 2018].
- Eschauzier C, Beerendonk E, Scholte-Veenendaal P, De Voogt P. 2012. impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain. Environ Sci Technol 46(3):1708–1715, PMID: 22201258, 10.1021/es201662b. [DOI] [PubMed] [Google Scholar]
- Fang X, Zhang L, Feng Y, Zhao Y, Dai J. 2008. Immunotoxic effects of perfluorononanoic acid on BALB/c mice. Toxicol Sci 105(2):312–321, PMID: 18583369, 10.1093/toxsci/kfn127. [DOI] [PubMed] [Google Scholar]
- Feng Y, Fang X, Shi Z, Xu M, Dai J. 2010. Effects of PFNA exposure on expression of junction-associated molecules and secretory function in rat Sertoli cells. Reprod Toxicol 30(3):429–437, PMID: 20580666, 10.1016/j.reprotox.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Ferrey ML, Wilson JT, Adair C, Su C, Fine DD, Liu X, et al. 2012. Behavior and fate of PFOA and PFOS in sandy aquifer sediment. Groundwater Monit R 32: 63–71, 10.1111/j.1745-6592.2012.01395.x. [DOI] [Google Scholar]
- Fetter CW. 1999. Contaminant Hydrogeology, 2nd Edition Waveland Press, Inc:Long Grove, IL. [Google Scholar]
- Foreman JE, Chang S-C, Ehresman DJ, Butenhoff JL, Anderson CR, Palkar PS, et al. 2009. Differential hepatic effects of perfluorobutyrate mediated by mouse and human PPAR-α. Toxicol Sci 110(1):204–211, PMID: 19359353, 10.1093/toxsci/kfp077. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. 2010. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med 164(9), PMID: 20819969, 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Schlummer M, Möller A, Gruber L, Wolz G, Ungewiss J, et al. 2007. Exposure of an adult population to perfluorinated substances using duplicate diet portions and biomonitoring data. Environ Sci Technol 41(22):7928–7933, PMID: 18075110, 10.1021/es071244n. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Harada KH, Koizumi A. 2013. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere 93(3):538–544, PMID: 23932147, 10.1016/j.chemosphere.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, et al. 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340:1–9, PMID: 26743852, 10.1016/j.tox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Gemitzi A, Petalas C, Tsihrintzis VA, Pisinaras V. 2006. Assessment of groundwater vulnerability to pollution: a combination of GIS, fuzzy logic and decision making techniques. Environ Geol 49(5):653–673, 10.1007/s00254-005-0104-1. [DOI] [Google Scholar]
- Giesy JP, Kannan K. 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35(7):1339–1342, PMID: 11348064, 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Gordon SC. 2011. Toxicological evaluation of ammonium 4,8-dioxa-3H-perfluorononanoate, a new emulsifier to replace ammonium perfluorooctanoate in fluoropolymer manufacturing. Regul Toxicol Pharmacol 59(1):64–80, PMID: 20875479, 10.1016/j.yrtph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Guelfo JL, Higgins CP. 2013. Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environ Sci Technol 47(9):4164–4171, PMID: 23566120, 10.1021/es3048043. [DOI] [PubMed] [Google Scholar]
- Guelfo J, Adamson D. 2018. Evaluation of a national data set for insights into sources, composition, and concentrations of per- and polyfluoroalkyl substances (PFASs) in U.S. drinking water. Environ Pollut 236:505–513, PMID: 29427949, 10.1016/j.envpol.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding-Marjanovic KC, Houtz EF, Yi S, Field JA, Sedlak DL, Alvarez-Cohen L. 2015. Aerobic biotransformation of fluorotelomer thioether amido sulfonate (Lodyne) in AFFF-amended microcosms. Environ Sci Technol 49(13):7666–7674, PMID: 26042823, 10.1021/acs.est.5b01219. [DOI] [PubMed] [Google Scholar]
- Higgins CP, Luthy RG. 2006. Sorption of perfluorinated surfactants on sediments. Environ Sci Technol 40(23):7251–7256, PMID: 17180974, 10.1021/es061000n. [DOI] [PubMed] [Google Scholar]
- Horsley Witten Group, Inc., 2016. Response to August 4, 2016 Request for Information on Barnstable Municipal Airport Hyannis, Massachusetts. http://public.dep.state.ma.us/fileviewer/Default.aspx?formdataid=0&documentid=398894 [accessed 11 May 2017].
- Houtz EF, Higgins CP, Field JA, Sedlak DL. 2013. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ Sci Technol 47(15):8187–8195, PMID: 23886337, 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Sedlak DL. 2012. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ Sci Technol 46(17):9342–9349, PMID: 22900587, 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- Howard PH, Muir DCG. 2010. Identifying new persistent and bioaccumulative organics among chemicals in commerce. Environ Sci Technol 44(7):2277–2285, PMID: 20163179, 10.1021/es903383a. [DOI] [PubMed] [Google Scholar]
- Howard PH, Muir DCG. 2011. Identifying new persistent and bioaccumulative organics among chemicals in commerce II: pharmaceuticals. Environ Sci Technol 45(16):6938–6946, PMID: 21740030, 10.1021/es201196x. [DOI] [PubMed] [Google Scholar]
- Howard PH, Muir DCG. 2013. Identifying new persistent and bioaccumulative organics among chemicals in commerce. III: byproducts, impurities, and transformation products. Environ Sci Technol 47(10):5259–5266, PMID: 23594256, 10.1021/es4004075. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett, PMID: 27752509, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R, Barber LB, LeBlanc DR, Weber AK, Vecitis CD. 2017. Poly- and perfluoalkyl substances in contaminated groundwater, Cape Cod, Massachusetts, 2014–2016. https://www.sciencebase.gov/catalog/item/580e746be4b0f497e794b882 [accessed 11 May 2017].
- Keawmanee S, Boontanon SK, Boontanon N. 2015. Method development and initial results of testing for perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in waterproof sunscreens. Environ Eng Res 20(2):127–132, 10.4491/eer.2014.S1.006. [DOI] [Google Scholar]
- Kissa E. 2001. Fluorinated Surfactants and Repellents, 2nd Edition Revised and Expanded. Hubbard A.T., ed. New York, NY:Marcel Dekker, Inc. [Google Scholar]
- Kucharzyk KH, Darlington R, Benotti M, Deeb R, Hawley E. 2017. Novel treatment technologies for PFAS compounds: a critical review. J Environ Manage 204(Pt 2):757–764, PMID: 28818342, 10.1016/j.jenvman.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Kumar A, Xagoraraki I. 2010. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: a proposed ranking system. Sci Total Environ 408(23):5972–5989, PMID: 20869754, 10.1016/j.scitotenv.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90(2):510–518, PMID: 16415327, 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieder PH, Chang S-C, York RG, Butenhoff JL. 2009a. Toxicological evaluation of potassium perfluorobutanesulfonate in a 90-day oral gavage study with Sprague-Dawley rats. Toxicology 255(1–2):45–52, PMID: 18992301, 10.1016/j.tox.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Lieder PH, York RG, Hakes DC, Chang S-C, Butenhoff JL. 2009b. A two-generation oral gavage reproduction study with potassium perfluorobutanesulfonate (K+PFBS) in Sprague Dawley rats. Toxicology 259(1–2):33–45, PMID: 19428941, 10.1016/j.tox.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Delinsky AD, Nakayama SF, McMillan L, Libelo EL, et al. 2011. Application of WWTP biosolids and resulting perfluorinated compound contamination of surface and well water in Decatur, Alabama, USA. Environ Sci Technol 45(19):8015–8021, PMID: 21513287, 10.1021/es1039425. [DOI] [PubMed] [Google Scholar]
- Liu J, Lee LS. 2005. Solubility and sorption by soils of 8:2 fluorotelomer alcohol in water and cosolvent systems. Environ Sci Technol 39(19):7535–7540, PMID: 16245825, 10.1021/es051125c. [DOI] [PubMed] [Google Scholar]
- Liu JX, Lee LS. 2007. Effect of fluorotelomer alcohol chain length on aqueous solubility and sorption by soils. Environ Sci Technol 41(15):5357–5362, PMID: 17822102, 10.1021/es070228n. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Mondal D, Armstrong B, Bloom MS, Fletcher T. 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect 120(7):1036–1041, PMID: 22453676, 10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless SE, Finlay C, Everds NE, Frame SR, Gillies PJ, O'Connor JC, et al. 2006. Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO). Toxicology 220(2–3):203–217, PMID: 16448737, 10.1016/j.tox.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Loveless SE, Slezak B, Serex T, Lewis J, Mukerji P, O'Connor JC, et al. 2009. Toxicological evaluation of sodium perfluorohexanoate. Toxicology 264(1–2):32–44, PMID: 19632293, 10.1016/j.tox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Mahmoud MAM, Kärrman A, Oono S, Harada KH, Koizumi A. 2009. Polyfluorinated telomers in precipitation and surface water in an urban area of Japan. Chemosphere 74(3):467–472, PMID: 19081600, 10.1016/j.chemosphere.2008.08.029. [DOI] [PubMed] [Google Scholar]
- MassDEP (Massachusetts Department of Environmental Protection). 2017. DRAFT Fact Sheet, Guidance on Sampling and Analysis for PFAS at Disposal Sites Regulated under the Massachusetts Contingency Plan. http://www.mass.gov/eea/docs/dep/cleanup/draft-guidance-on-sampling-for-pfcs-2017-01-17.pdf [accessed 21 May 2017].
- McGuire ME, Schaefer C, Richards T, Backe WJ, Field JA, Houtz E, et al. 2014. Evidence of remediation-induced alteration of subsurface poly- and perfluoroalkyl substance distribution at a former firefighter training area. Environ Sci Technol 48(12):6644–6652, PMID: 24866261, 10.1021/es5006187. [DOI] [PubMed] [Google Scholar]
- McKenzie ER, Siegrist RL, McCray JE, Higgins CP. 2015. Effects of chemical oxidants on perfluoroalkyl acid transport in one-dimensional porous media columns. Environ Sci Technol 49(3):1681–1689, PMID: 25621878, 10.1021/es503676p. [DOI] [PubMed] [Google Scholar]
- McKenzie ER, Siegrist RL, McCray JE, Higgins CP. 2016. The influence of a non-aqueous phase liquid (NAPL) and chemical oxidant application on perfluoroalkyl acid (PFAA) fate and transport. Water Res 92:199–207, PMID: 26854608, 10.1016/j.watres.2016.01.025. [DOI] [PubMed] [Google Scholar]
- MDHHS (Michigan Department of Health & Human Services). 2016. Perfluorinated Chemicals (PFCs) in Drinking Water Wells Near the Former Wurth smith Air Force Base. https://www.michigan.gov/documents/mdhhs/PFCs_in_Drinking_Water_Wells_532618_7.pdf [accessed 11 May 2017].
- MDOH (Minnesota Department of Health). 2012. Perfluorochemical Contamination in Southern Washington County, Northern Dakota County, and Southeastern Ramsey County, Minnesota. http://www.health.state.mn.us/divs/eh/hazardous/topics/pfcs/pha/woodbury/pha3m0112.pdf [accessed 12 May 2016].
- Mejia Avendaño S, Liu J. 2015. Production of PFOS from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere 119:1084–1090, PMID: 25460746, 10.1016/j.chemosphere.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Moody CA, Field JA. 1999. Determination of perfluorocarboxylates in groundwater impacted by fire-fighting activity. Env. Sci Technol 33:2800–2806, 10.1021/es981355+. [DOI] [Google Scholar]
- Moody CA, Hebert GN, Strauss SH, Field JA. 2003. Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J Environ Monitor 5(2):341–345, 10.1039/b212497a. [DOI] [PubMed] [Google Scholar]
- Moody CA, Kwan WC, Martin JW, Muir DCG, Mabury SA. 2001. Determination of perfluorinated surfactants in surface water samples by two independent analytical techniques: liquid chromatography/tandem mass spectrometry and F-19 NMR. Anal Chem 73(10):2200–2206, 10.1021/ac0100648. [DOI] [PubMed] [Google Scholar]
- Negri E, Metruccio F, Guercio V, Tosti L, Benfenati E, Bonzi R, et al. 2017. Exposure to PFOA and PFOS and fetal growth: a critical merging of toxicological and epidemiological data. Crit Rev Toxicol 47(6):489–515, PMID: 28617200, 10.1080/10408444.2016.1271972. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118(2):197–202, PMID: 20123614, 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell C, McLeod K, Gonzales J.R. 1996. BIOSCREEN Natural Attenuation Decision Support System User’s Manual Version 1.3. [Google Scholar]
- NH DHHS (New Hampshire Department of Health and Human Services). 2016. Investigation into Contaminant Found in Pease Tradeport Water System. http://www.dhhs.nh.gov/dphs/investigation-pease.htm [accessed 10 July 2016].
- NHDES (New Hampshire Department of Environmental Services). 2017a. NHDES PFAS Sampling. Concord, NH. https://www.des.nh.gov/organization/divisions/waste/hwrb/documents/hwrb_master_qapp.pdf [accessed 21 May 2017].
- NHDES. 2017b. Master Quality Assurance Project Plan of the Hazardous Waste Remediation Bureau Waste Management Division New Hampshire Department of Environmental Services. https://www.des.nh.gov/organization/divisions/waste/hwrb/documents/hwrb_master_qapp.pdf [accessed 21 May 2017].
- NJDWQI (New Jersey Drinking Water Quality Institute). 2016. Health-based Maximum Contaminant Level Support Document: Perfluorooctanoic Acid (PFOA). http://www.nj.gov/dep/watersupply/pdf/pfoa-hb-talk.pdf [accessed 9 June 2017].
- NYDEC (New York State Department of Environmental Conservation). 2016. New York State Department of Environmental Conservation is Installing Water Filtration Systems for Hoosick Falls Residents. https://www.health.ny.gov/environmental/investigations/hoosick/docs/factsheet_install.pdf [accessed 9 July 2016].
- Oliaei F, Kriens D, Weber R, Watson A. 2013. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut Res Int 20(4):1977–1992, PMID: 23128989, 10.1007/s11356-012-1275-4. [DOI] [PubMed] [Google Scholar]
- Oliaei F, Kriens D, Kessler K. 2006. Investigation of Perfluorochemical (PFC) Contamination in Minnesota Phase One, Report to Senate Environment Committee. https://www.leg.state.mn.us/archive/leg/minutes/database/84-s-1261-0-20060227-a.pdf [accessed 26 July 2017].
- Ololade IA, Zhou Q, Pan G. 2016. Influence of oxic/anoxic condition on sorption behavior of PFOS in sediment. Chemosphere 150:798–803, PMID: 26350897, 10.1016/j.chemosphere.2015.08.068. [DOI] [PubMed] [Google Scholar]
- Park S, Lee LS, Medina VF, Zull A, Waisner S. 2016. Heat-activated persulfate oxidation of PFOA, 6:2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere 145:376–383, PMID: 26692515, 10.1016/j.chemosphere.2015.11.097. [DOI] [PubMed] [Google Scholar]
- Post GB, Gleason JA, Cooper KR. 2017. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: contaminants of emerging concern. PLoS Biol 15(12):e2002855, 10.1371/journal.pbio.2002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. 2006. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40(1):32–44, PMID: 16433330, 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Rahman A. 2008. A GIS based DRASTIC model for assessing groundwater vulnerability in shallow aquifer in Aligarh, India. Appl Geogr 28(1):32–53, 10.1016/j.apgeog.2007.07.008. [DOI] [Google Scholar]
- Rahman MF, Peldszus S, Anderson WB. 2014. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res 50:318–340, PMID: 24216232, 10.1016/j.watres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- RIDEM (Rhode Island Department of Environmental Management). 2012. Groundwater Quality Standard. http://www.rigis.org [accessed 11 July 2017].
- Ritter EE, Dickinson ME, Harron JP, Lunderberg DM, DeYoung PA, Robel AE, et al. 2017. PIGE as a screening tool for Per- and polyfluorinated substances in papers and textiles. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At 407:47–54, 10.1016/j.nimb.2017.05.052. [DOI] [Google Scholar]
- Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, et al. 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246, PMID: 27179585, 10.1016/j.envres.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin H-M, et al. 2012a. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 23(3):386–392, PMID: 22370857, 10.1097/EDE.0b013e31824cb93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Stein CR, Elston B, Wellenius GA, Bartell SM, Shin H-M, et al. 2012b. Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the mid-Ohio Valley. Environ Health Perspect 120(8):1201–1207, PMID: 22450153, 10.1289/ehp.1104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider LA, Ackerman JM, Rudel RA. 2016. Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Sci Total Environ 547:470–481, PMID: 26822473, 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Barofsky DF, Field JA. 2004. Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ Sci Technol 38(6):1828–1835, PMID: 15074696, 10.1021/es035031j. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, Field JA. 2006. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ Sci Technol 40(23):7350–7357, PMID: 17180988, 10.1021/es061025m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, Vieira VM, Ryan PB, Detwiler R, Sanders B, Steenland K, et al. 2011. Environmental fate and transport modeling for perfluorooctanoic acid emitted from the Washington Works Facility in West Virginia. Environ Sci Technol 45(4):1435–1442, PMID: 21226527, 10.1021/es102769t. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Wilford BH, Jones KC, Zhu J. 2005. Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: occurrence, partitioning, and human exposure. Environ Sci Technol 39(17):6599–6606, PMID: 16190217, 10.1021/es048340y. [DOI] [PubMed] [Google Scholar]
- Squillace PJ, Pankow JF, Korte NE, Zogorski JS. 1997. Review of the environmental behavior and fate of methyl tert-butyl ether. Environ Toxicol Chem 16(9):1836–1844, 10.1002/etc.5620160911. [DOI] [Google Scholar]
- Squillace PJ, Zogorski JS, Wilber WG, Price CV. 1996. Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993–1994. Environ Sci Technol 30(5):1721–1730, 10.1021/es9507170. [DOI] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 118(8):1100–1108, PMID: 20423814, 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Winquist A, Parks C. 2013. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio Valley. Environ Health Perspect 121(8):900–905, PMID: 23735465, 10.1289/ehp.1206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton GR, Dai F, Bellona CL, Holsen TM, Dickenson ERV, Mededovic Thagard S. 2017. Plasma-based water treatment: efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ Sci Technol 51(3):1643–1648, PMID: 28080043, 10.1021/acs.est.6b04215. [DOI] [PubMed] [Google Scholar]
- Strempel S, Scheringer M, Ng CA, Hungerbühler K. 2012. Screening for PBT chemicals among the “Existing” and “New” chemicals of the EU. Environ Sci Technol 46:5680–5687, PMID: 22494215, 10.1021/es3002713. [DOI] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ Sci Technol 49(19):11622–11630, PMID: 26392038, 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Tang CY, Fu QS, Gao DW, Criddle CS, Leckie JO. 2010. Effect of solution chemistry on the adsorption of perfluorooctane sulfonate onto mineral surfaces. Water Res 44(8):2654–2662, PMID: 20172580, 10.1016/j.watres.2010.01.038. [DOI] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE. 2015. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol 54:26–36, PMID: 25499722, 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicorn Mgmt Consultants. 2016. Remedial Investigation Work Plan Phase I. http://dec.vermont.gov/sites/dec/files/co/pfoa/documents/General-Cable-Remedial-Investigation-Work-Plan-FINAL-20160601.PDF [accessed 20 July 2017].
- U.S. EPA (U.S. Environmental Protection Agency). 1989. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf [accessed 23 April 2018].
- U.S. EPA. 2012. The Third Unregulated Contaminant Monitoring Rule. http://water.epa.gov/scitech/datait/databases/drink/ncod/databases-index.cfm [accessed 22 June 2017].
- U.S. EPA. 2016a. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). https://www.epa.gov/sites/production/files/2016-05/documents/pfos_health_advisory_final_508.pdf [accessed 10 June 2016].
- U.S. EPA. 2016b. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_health_advisory_final_508.pdf [accessed 10 June 2016].
- U.S. EPA. 2016c. HRS Documentation Record Saint-Gobain Performance Plastics. https://semspub.epa.gov/work/02/363676.pdf [accessed 22 July 2017].
- U.S. EPA. 2016d. Methyl Tertiary Butyl Ether Overview. https://archive.epa.gov/mtbe/web/html/faq.html#background [accessed 10 May 2017].
- U.S. EPA. 2016e. The Third Unregulated Contaminant Monitoring Rule. Available: http://water.epa.gov/scitech/datait/databases/drink/ncod/databases-index.cfm [accessed 20 June 2017].
- USGS (U.S. Geological Survey). 2016. Water Questions & Answers: What is most of the freshwater in the U.S. used for? http://water.usgs.gov/edu/qa-usage-freshwater.html [accessed 6 September 2016].
- VTDEC (Vermont Department of Environment Conservation). 2016. Vermont PFOA Contamination Response, Information for Impacted Communities. Vermont Department of Environmental Conservation:Montpelier, VT. http://dec.vermont.gov/commissioners-office/pfoa/communities [accessed 20 July 2017].
- VTDOH (Vermont Department of Health). 2016. Perfluorooctanoic acid (PFOA) and Perfluorooctanesulfonic acid (PFOS) Vermont Drinking Water Health Advisory. https://anrweb.vt.gov/PubDocs/DEC/PFOA/PFOA%20-%20PFOS%20Health%20Advisories/Vermont/PFOA_PFOS_HealthAdvisory_June_22_2016.pdf [accessed 11 April 2018].
- Wagner A, Raue B, Brauch H-J, Worch E, Lange FT. 2013. Determination of adsorbable organic fluorine from aqueous environmental samples by adsorption to polystyrene-divinylbenzene based activated carbon and combustion ion chromatography. J Chromatogr A 1295:82–89, PMID: 23683893, 10.1016/j.chroma.2013.04.051. [DOI] [PubMed] [Google Scholar]
- Wang J, He J, Chen H. 2012. Assessment of groundwater contamination risk using hazard quantification, a modified DRASTIC model and groundwater value, Beijing Plain, China. Sci Total Environ 432:216–226, PMID: 22750168, 10.1016/j.scitotenv.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518, PMID: 28224793, 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Weber AK, Barber LB, LeBlanc DR, Sunderland EM, Vecitis CD. 2017. Geochemical and hydrologic factors controlling subsurface transport of poly- and perfluoroalkyl substances, Cape Cod, Massachusetts. Environ Sci Technol 51(8):4269–4279, PMID: 28285525, 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- Weiner B, Yeung LWY, Marchington EB, D'Agostino LA, Mabury SA. 2013. Organic fluorine content in aqueous film forming foams (AFFFs) and biodegradation of the foam component 6:2 fluorotelomermercaptoalkylamido sulfonate (6:2 FTSAS). Environ Chem 10(6):486, 10.1071/EN13128. [DOI] [Google Scholar]
- Weston & Sampson. 2016. Pease Pilot Testing Program. http://cityofportsmouth.com/publicworks/PeaseTradeportWaterSystemWellTreatmentPilotReportFinal.pdf [accessed 24 July 2017].
- Weston Solutions. 2009. Remedial Design/Response Action Plan Cottage Grove Site. https://www.pca.state.mn.us/sites/default/files/c-pfc3-07.pdf [accessed 20 July 2017].
- Weston Solutions. 2016. Groundwater, Surface Water, and Influent/Effluent Sampling Activities at the Burgess Brothers Landfill, Bennington/Woodford, Bennington County, Vermont. http://dec.vermont.gov/sites/dec/files/co/pfoa/documents/0099_Site-File-Memo_Burgess-Brothers-Landfill_Final-20160610.pdf [accessed 20 July 2017].
- Wolf CJ, Rider CV, Lau C, Abbott BD. 2014. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology 316:43–54, PMID: 24374136, 10.1016/j.tox.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, et al. 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 106:135–143, PMID: 28645013, 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Ulrich BA, Chen B, Higgins CP. 2017. Sorption of poly- and perfluoroalkyl substances (PFASs) relevant to aqueous film-forming foam (AFFF)-impacted groundwater by biochars and activated carbon. Environ Sci Technol 51(11):6342–6351, PMID: 28582977, 10.1021/acs.est.7b00970. [DOI] [PubMed] [Google Scholar]
- Yingling V. 2016. Poly- and Perfluoroalkyl Substances (PFAS) in Minnesota: An Update on the Chemicals Formerly Known as PFCs. http://www.mgwa.org/meetings/2016_fall/Yingling.pdf [accessed 11 May 2017].
- Yu Q, Zhang R, Deng S, Huang J, Yu G. 2009. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: kinetic and isotherm study. Water Res 43(4):1150–1158, PMID: 19095279, 10.1016/j.watres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Zaggia A, Conte L, Falletti L, Fant M, Chiorboli A. 2016. Use of strong anion exchange resins for the removal of perfluoroalkylated substances from contaminated drinking water in batch and continuous pilot plants. Water Res 91:137–146, PMID: 26774262, 10.1016/j.watres.2015.12.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.