Abstract
Background.
This study examined whether lifetime heroin-use consequences mediate the relationship between trait impulsivity and three current mood outcomes: depression symptoms, stress levels, and perception of life events.
Method.
Regular heroin users (N = 163) were assessed using the Barratt Impulsiveness Scale (BIS-11) to measure trait impulsivity; a standardized Drug History and Use Questionnaire to measure lifetime adverse consequences of heroin use; Beck Depression Inventory II to measure current depression symptoms; Stress subscale of the Depression Anxiety Stress scale; and Hassles and Uplifts scale to measure perception of life events.
Results.
BIS-11 Attentional and Motor impulsivity were positively related to number of adverse heroin-use consequences, depression symptoms, and stress level, and negatively associated with positive perception of events. A greater number of heroin-use consequences was related to more depression symptoms, higher stress, more negative perception of events, injection heroin use, and earlier ages of first and regular heroin use. In six mediation models, lifetime heroin-use consequences partially mediated relationships between two trait impulsivity domains (Attentional, Motor) and current mood measures (depression symptoms, stress, perception of events).
Conclusions.
The present findings suggest that current negative mood can be a response to the accumulated burden of heroin-use consequences, particularly in the presence of high trait impulsivity.
Keywords: substance use, depression, stressors, negative perception, mediation, impulsivity
Introduction
Rates of heroin use in the United States more than doubled between 2002 and 2012 (Substance Abuse and Mental Health Services Administration 2013). Globally, about 17.7 million individuals used heroin and other opioids during the past year (UNODC 2017). Regular heroin users experience adverse heroin-use consequences, including overdose, mental (e.g., depression) and physical health (e.g. HIV, hepatitis C, pain) problems, and functional impairment (Degenhardt et al. 2011; Ross et al. 2005). Heroin users with current mood problems exhibit more difficulty abstaining from heroin use, higher rates of injection drug use, and greater vulnerability to overdose death relative to heroin users without mood symptoms (Hasin et al. 2002; Havard et al. 2006; Tobin and Latkin 2003). Due to these problems, improving understanding of pathways among trait attributes (e.g. impulsivity), heroin use and its consequences, and mood variables, could be useful for clinical practice.
Trait impulsivity is a stable personality attribute reflecting the tendency to approach situations or potential rewards without fully considering consequences (Zuckerman and Kuhlman 2001). High impulsivity has been consistently linked to increased responsiveness to immediate reward and reduced sensitivity to negative consequences (e.g. Martin and Potts 2010), particularly regarding substance use (de Wit 2009; Kreek et al. 2005). For instance, regular heroin users exhibit steeper delay discounting (more impulsive decision making) than healthy volunteers (Kirby et al. 1999; Madden et al. 1997), especially during acute opioid withdrawal (Stoltman et al. 2015) and during continued abstinence (Li et al. 2013). Trait impulsivity is associated with more frequent heroin use, injection (vs. non-injection) heroin use, greater heroin consumption and withdrawal severity, and lower likelihood of treatment seeking (Dissabandara et al. 2014; Nielsen et al. 2012); all factors associated with increased adverse consequences and dysfunction (de Wit 2009).
Both high trait impulsivity and regular heroin use are related to co-occurring mood disorders. Attentional impulsivity (i.e. lack of focus, thought insertions, and intrusive and racing thoughts during decision making; Patton et al. 1985) is positively associated with depressive episodes, hopelessness, anhedonia, and suicidality (Peluso et al. 2007; Swann et al. 2008). Regular heroin use is also related to depression, anxiety, and experienced stress (Kreek et al. 2002; Wisniewski et al. 2005). Among heroin users entering treatment for substance abuse, 38–54% report lifetime depression and 25% report current depression (Darke et al. 2009; Sordo et al. 2012). Although the temporal relationship between mood symptoms and heroin-use sequelae is poorly understood, evidence suggests major depressive disorder influences relapse, remission, and substance use (Hasin et al. 2002; Samet et al. 2013). Specifically, depression that precedes substance use, and substance-induced depression at treatment entry, predict substance use following discharge from psychiatric treatment. We previously found temporal relationships between high trait impulsivity, cocaine-use consequences, and later depression symptoms among cocaine users (Lister et al. 2015).
Above, we described relationships among trait impulsivity, substance use, adverse drug-use consequences, and depression. Another important factor in these relationships is stress. Regular heroin users report more psychosocial stressors than non-users (Preston and Epstein 2011; Williams and Latkin 2007). Stress has been dually identified as motivation for and outcome of substance use. Neurobiological and psychosocial literature has emphasized initiation and continued use of substances as a response to experienced stress (e.g. Sinha 2008), congruent with a self-medication hypothesis. Chronic substance use is associated with elevated baseline stress hormone levels and acute reactivity to stressors (e.g. Fox et al. 2008). Thus, pathways among impulsivity, drug-use consequences and stress levels remain unknown. Improved understanding of associations among personality attributes, substance use and its consequences, and current stress levels would be clinically beneficial, as stressors can potentiate substance use, and predict relapse and treatment attrition (Greenwald et al. 2013; Sinha 2007). This information is critical in advancing scientific knowledge about antecedents and correlates of chronic and regular heroin use.
The present study investigated lifetime heroin-use adverse consequences as a mediator of the relationship between subdomains of trait impulsivity (predictors) and three current mood outcomes: (1) depression symptoms, (2) stress level, and (3) perception of life events. We followed guidelines for mediation analyses that assess mediational pathways in observational data (Baron and Kenny 1986; MacKinnon and Luecken 2008; Preacher and Hayes 2004; Shrout and Bolger 2002). By these guidelines, the independent variable (trait impulsivity) must temporally precede and predict the mediator (lifetime heroin-use consequences) that, in turn, precedes and predicts current outcomes of depression symptoms, stress level, and perception of life events. MacKinnon and Luecken (2008) also note, “Ideally, repeated measures of the mediator and dependent variable are available to investigate temporal relations, but often these causal relations must be inferred based on theory or prior research” (p. 2). Thus, mediation analyses of cross-sectional data can provide valuable insight regarding empirical pathways among key variables identified through theory or literature, insofar as these examinations order pathways using temporal measurement of the variables. Last, mediational analyses have been widely used in cross-sectional data to help explain pathways among substance use and mental health outcomes (e.g. Asberg and Renk 2012; Klanecky et al. 2012; Lister et al. 2015). Our current use of mediation is consistent with assumptions outlined for cross-sectional data (MacKinnon and Luecken 2008).
Though literature shows that mood disorders often precede substance dependence (Martins et al. 2009; O’Neil et al. 2011), there also is evidence that substance dependence may precede mood disorder onset (e.g. Brook et al. 2002; Hasin et al. 2002; Samet et al. 2013). Based on this literature, and our work using mediational models to identify connections among impulsivity, mood, and cocaine-use consequences (Lister et al. 2015), we hypothesized that the number of lifetime heroin-use consequences would mediate relationships of trait impulsivity with current depression symptoms, stress level, and life-event perception. Specifically, we expected chronic heroin users with higher (vs. lower) trait impulsivity would experience more lifetime heroin-use consequences, and therefore report higher levels of current depression and stress symptoms, and more negative perception of current life events.
Methods
Participant selection
The local Institutional Review Board approved all study procedures, conducted in accordance with the Declaration of Helsinki (1964). Regular heroin users (aged 18–55 years), who reported no current interest in treatment for their substance use, were recruited via newspaper advertisements and word-of-mouth referrals in the Detroit/metropolitan area for participation in one of two experimental laboratory-based studies (Greenwald et al. 2013; Greenwald et al. in preparation). Certificates of Confidentiality were obtained from the National Institutes of Health.
Following an initial telephone-screening interview, eligible and interested participants underwent a comprehensive battery of in-person screening procedures. Complete inclusion and exclusion criteria are published elsewhere (Woodcock et al. 2015b). During the screening visit, participants provided monitored urine (CLIA Waived, Inc., San Diego, CA) and breath (Alco-Sensor III Breathalyzer, Intoximeter Inc., St. Louis, MO) samples for testing recent substance use, and completed self-report measures of substance use history, impulsivity, and current mood state.
Measures
Demographics and urine toxicology.
After verifying sobriety (breath-alcohol samples < .02%) and adequate cognitive function (estimated IQ score > 80 on Shipley Institute of Living Scale [Zachary 1991]), participants provided written informed consent to screening procedures. An opioid-positive urine sample (> 300 ng/ml) was required for inclusion in this analysis. Demographic information including gender, race, age, and education were collected during the screening visit.
Heroin-use characteristics and consequences.
Substance use characteristics were assessed via self-report using the comprehensive Drug History and Use Questionnaire (DHUQ) routinely used in our laboratory studies. Characteristics assessed included lifetime and current heroin use, and cumulative lifetime heroin-use consequences. Past-month heroin-use frequency was defined as past-week number of daily uses multiplied by the number of past-month heroin-use days. Lifetime heroin-use consequences were operationalized as the total number of heroin-related use consequences endorsed (ever/never) on a 21-item checklist (range: 0 – 21; e.g. overdose, emergency room visits, inability to stop using, arrests/legal problems, job losses, family problems; Woodcock et al. 2015a). Other self-reported heroin use variables included: age at heroin use onset (initial and regular [≥ 3 uses/week]), duration of lifetime heroin use (difference score: age at screening minus age at initial use), lifetime heroin injection use (i.e. never vs. ever), and lifetime number of attempts to quit using heroin (range: 0 – 100).
Trait impulsivity.
The Barratt Impulsiveness Scale (BIS-11) is a self-report measure of trait impulsivity that uses a 4-point Likert response scale (Barratt and Patton 1983; Patton et al. 1995). The instrument has 3 second-order subscales: Attentional (impulsive decision making), Motor (engagement in spontaneous behavior), and Non-Planning (lack of concern for consequences of future actions). The BIS-11 measures the predispositional, stable trait of impulsivity rather than situational responses (Moeller et al. 2001), and demonstrates reliability over time (Stanford et al. 2009).
Current depression symptoms.
Current depression symptoms were assessed using the Beck Depression Inventory, Second Edition (BDI-II), a self-report measure of past 14-day neurovegetative depression symptoms (Beck et al. 1988). Items are summed for analyses and scores range from 0 to 63. The threshold for clinical depression is > 14, and a score of > 29 is the threshold for severe depression.
Current stress level.
Current stress level was assessed using the Depression Anxiety Stress Scales (DASS), a past-week self-report measure with three 7-item scales: Depression (dysphoria), Anxiety (physiological arousal, fear), and Stress (tension, difficulty relaxing, irritability) (Lovibond and Lovibond 1995a). Only the Stress scale was included in analyses to avoid overlap among these inter-correlated scales (Antony et al. 1998; Lovibond and Lovibond 1995b).
Perception of current life events.
Perception of current life events was assessed using the Hassles and Uplifts scale, a self-report, multi-domain measure of the extent to which these events affected the respondent during the past day (DeLongis et al. 1988). For each item (e.g. health or well-being of a family member, intimacy, job security, neighborhood, legal matters), the respondent indicated how much the event was a Hassle and/or Uplift. Respondents endorsed a score of 0 (none/not applicable) to 3 (a great deal) for both Hassle and Uplift (i.e. an item may be endorsed as both hassle and uplift). A difference score was calculated (total Uplifts score minus total Hassles score) that reflects the degree to which an individual has a relatively negative or positive perception of recent life events. Negative difference scores reflect more negative perception, whereas positive difference scores reflect more positive perception of current life events.
Data analysis
Participants with complete data on all measures were included in the current analysis. Statistical analyses were conducted using SPSS v.22 (IBM Corporation, Armonk, NY). Raw data were screened for statistical outliers and non-normal distributions were corrected (log10 transformation) prior to outcome analyses.
Bivariate Pearson correlations were used to assess statistical relationships among trait impulsivity subscales (BIS-11; predictor), number of lifetime heroin-use consequences (DHUQ; mediator), and current mood outcome variables (depression symptoms (BDI-II), stress level (DASS), and perception of current life events [Uplifts minus Hassles]). Significant correlations between the independent variables (BIS-11 subscales), mediator (heroin-use consequences), or dependent variables (current mood variables: depression, stress, or life-event perception) were required for inclusion in mediation models. The non-planning impulsivity subscale (BIS-11) failed to meet this inclusion criterion, and was excluded from subsequent mediation models. BIS-11 subscales (rather than total score) were analyzed to evaluate effects of dimensional aspects of impulsivity on heroin-use consequences and mood outcome variables.
Primary analyses were six separate mediation models based on previous recommendations (Baron and Kenny 1986; MacKinnon and Luecken 2008; Preacher and Hayes 2004; Shrout and Bolger 2002). Mediation analyses assessed the influence of lifetime heroin-use consequences on links between trait impulsivity and current mood outcomes: depression, stress level, and perception of life events. Therefore, we examined two sets (impulsivity dimension: Attentional and Motor) of three mediation models (current mood outcome: depression symptoms, stress level, and perception of life events).
This approach facilitated investigation of pathways between the independent variables (trait Attentional or Motor impulsivity) that we hypothesized to precede the mediator (lifetime heroin-use consequences) and predict the criterion variables (current depression symptoms, stress level, and life-event perception). We used Preacher and Hayes’ (2004) bootstrapping technique to estimate direct and indirect effects of the predictor and mediator variables on each outcome. In addition, effect sizes (R2) were estimated for each mediation model. This technique is appropriate for the sample size of our study (Preacher and Hayes 2004).
Results
Participant characteristics
Table 1 presents descriptive statistics. The overall sample (N = 163) was mostly male and about equally Caucasian and African American, with the remainder identifying as Hispanic, Asian, or multiethnic. On average, participants were in the early 40s and most (77.6%) reported at least high-school education.
Table 1.
Participant characteristics
| Sociodemographic variables | Mean (SD) or percentage |
|---|---|
| Gender | |
| Male | 74.2% |
| Female | 25.8% |
| Ethnicity | |
| Caucasian | 50.9% |
| African-American | 45.4% |
| Hispanic, Asian, or multiethnic | 3.7% |
| Age (years) | 42.1 (11.1) |
| Education | 12.2 ( 1.6) |
| Heroin use characteristics | |
| Age of initial heroin use (years) | 25.4 ( 8.3) |
| Age of regular heroin use (years) | 26.9 ( 8.6) |
| Lifetime duration of regular heroin use (years) | 14.9 (11.1) |
| Past-month heroin use (days) | 28.1 ( 4.9) |
| Lifetime attempts to quit heroin use | 9.8 (19.5) |
| Lifetime injection use (% yes) | 67.5% |
| Lifetime heroin-use consequences | 7.8 ( 4.4) |
| Study variables | |
| BIS-11 Attentional impulsivity | 15.6 ( 3.3) |
| BIS-11 Motor impulsivity | 23.8 ( 4.0) |
| BIS-11 Non-planning impulsivity | 28.8 ( 4.9) |
| BDI-II depression | 15.3 (10.2) |
| DASS Stress | 5.1 ( 4.0) |
| Perception of life events | 18.1 (34.9) |
Participants reported initial heroin use in their mid-20s, with regular heroin use starting about 1.5 years later. Average lifetime duration of regular heroin use was about 15 years. All participants reported regular heroin use (≥ 3 uses/week) at screening, with nearly daily heroin use (28.1 ± 4.9 days during the past 30 days). On average, participants had tried to quit heroin about 10 times. Lifetime heroin injection was endorsed by 2/3 of the sample. Thirty-one percent of the sample (n = 50) reported only non-injection heroin use (primarily insufflation/“snorting”). Participants reported experiencing about 8 different types of lifetime heroin use-related consequences.
Relative to general population means for BIS-11 trait impulsivity scores (Stanford et al. 2009), BIS-11 scores in this heroin-using sample were similar for Attentional (15.6 ± 3.3 vs. 16.7 ± 4.1), and Motor (23.8 ± 4.0 vs. 22 ± 4), whereas Non-planning scores were somewhat higher (28.8 ± 4.9 vs. 23.6 ± 4.9). The mean BDI-II depression symptom total score was 15.3 ± 10.2. In this sample, 47.2% of participants endorsed sub-clinical depression symptoms (scores of 0–13; n = 76), 42.5% of participants reported mild to moderate depression symptoms (scores of 14–28; n = 68), and 10.2% reported severe depression symptoms (scores > 29; n = 17). The mean DASS Stress score was 5.1 ± 4.0, which is in the upper range of normal (scores of 0–7) (Lovibond and Lovibond 1995a). The mean perception of life-events score was 18.07 ± 34.9 (range, −60 to 148). Thus, although the sample reported an optimistic bias in life-event perception (mean positive difference score), the confidence interval in this measure included negative values (negative perception).
Bivariate relationships
Table 2 presents correlations among all major variables. Current depression symptoms (BDI-II) were positively correlated with lifetime heroin-use consequences (DHUQ), Attentional and Motor impulsivity (BIS-11), stress level (DASS), and negatively correlated with positive perception of events (Uplifts–Hassles). Higher stress-level scores correlated with more lifetime heroin-use consequences, higher Attentional and Motor impulsivity scores, and more negative perception of life events. More negative scores for perception of events correlated with greater lifetime heroin-use consequences, and higher Attentional and Motor impulsivity scores. Correlations between perception of events and primary study variables indicate that as negative mood, impulsivity, and consequences of heroin use scores increase, negative perception of life events also increases.
Table 2.
Bivariate correlations among impulsivity, heroin use consequences, and mood variables
| Mediation variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1. BIS-11 Attentional | .49*** | .28*** | .54*** | .57*** | −.22** | .08 | .02 | .02 | .09 | |
| 2. BIS-11 Motor | .21** | .37*** | .34*** | −.22** | .18* | .06 | .10 | −.12 | ||
| 3. Heroin consequences | .27*** | .37*** | −.22** | .35*** | .14 | −.10 | −.30*** | |||
| 4. BDI-II total score | .58*** | −.39*** | .08 | .02 | .03 | −.12 | ||||
| 5. DASS Stress score | −.28*** | .05 | .08 | −.04 | −.10 | |||||
| 6. Uplifts – Hassles score | −.24** | .06 | .18 | .13 | ||||||
| Heroin use characteristics | ||||||||||
| 7. Route of heroin use | .17 | −.09 | −.15 | |||||||
| 8. Number of heroin quit attempts | .01 | .06 | ||||||||
| 9. Past-month heroin use frequency | −.04 | |||||||||
| 10. Age of first regular heroin use | ||||||||||
p < .05
p < .01
p < .001
(1) Barratt Impulsiveness Scale (BIS-11) Attentional subscale score; (2) BIS-11 Motor subscale score; (3) Drug History and Use Questionnaire (DHUQ) lifetime heroin-use consequences total score; (4) Beck Depression Inventory-II total score; (5) Depression Anxiety Stress (DASS) Stress subscale score; (6) Uplifts – Hassles difference score; (7) DHUQ route of heroin administration (0=no lifetime injection use, 1=lifetime injection use); (8) DHUQ lifetime number of heroin quit attempts; (9) DHUQ past-month heroin use frequency; (10) DHUQ age of first regular heroin use.
Mediating effect of heroin-use consequences through attentional impulsivity
The first set of three mediation tests investigated relationships between Attentional impulsivity (independent variable), lifetime heroin-use consequences (mediator), separately for each current mood outcome (depression, stress, and perception of life events; Figure 1). In addition, we provide beta and standard error values for the mediation model’s direct effect (c’ paths) and the unmediated model’s total effect (c paths), and the 95% confidence interval range for each mediation to demonstrate the indirect effect differed from zero and represented partial mediation.
Figure 1.
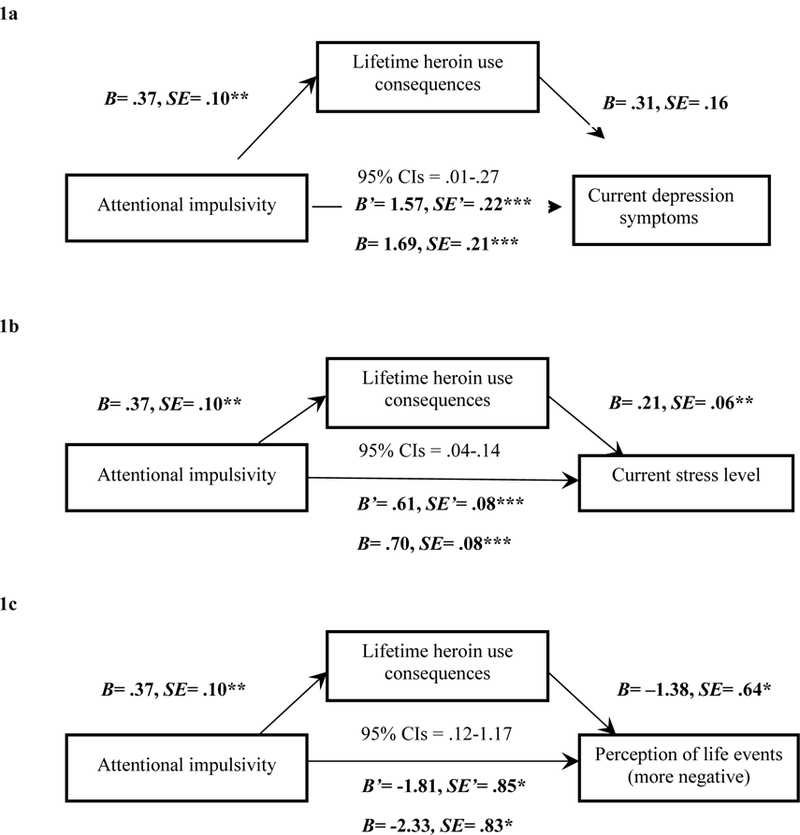
Mediation models with Attentional impulsivity (X), lifetime heroin-use consequences (M), and the parallel outcome measures (Y) of current depression symptoms (1a), stress level (1b), and perception of life events (1c). Note. *p<.05; **p<.01; ***p<.001. The unstandardized coefficients and standard errors shown account for the mediator in the equation. For each respective model, we list beta and standard error values for the mediation model’s direct effect (c’ path) and the unmediated model’s total effect (c path), as well as the 95% confidence interval range for the mediation model to demonstrate the indirect effect was different from zero and represented partial mediaiton.
Current depression symptoms.
There was a significant direct effect of Attentional impulsivity on current depression symptoms (Figure 1a). The indirect effect through lifetime heroin-use consequences was also significant, and was estimated to range (95% confidence interval) between .01 and .27. These results indicate that experiencing more lifetime heroin-use consequences partially mediated the relationship between higher Attentional impulsivity and more current depression symptoms (R2 = .31).
Current stress level.
The direct effect of Attentional impulsivity on stress was significant (Figure 1b). The indirect effect through lifetime heroin-use consequences was also significant, and was estimated to range between .04 and .14. These results indicate that more lifetime heroin-use consequences partially mediated the relationship between higher Attentional impulsivity and higher current stress level (R2 = .38).
Perception of current life events.
The direct effect of Attentional impulsivity on perception of life events was significant (Figure 1c). The indirect effect through lifetime heroin-use consequences was also significant, and was estimated to range between .12 and 1.17. These results indicate that more lifetime heroin-use consequences partially mediated the relationship between higher Attentional impulsivity and relatively more negative perception of life events (R2 = .07).
Mediating effect of heroin-use consequences through motor impulsivity
The second set of three mediation tests was identical to the first but included Motor impulsivity as the independent variable (Figure 2). Again, we provide beta and standard error values for the mediation model’s direct effect (c’ paths) and the unmediated model’s total effect (c paths), and the 95% confidence interval range for each mediation to demonstrate the indirect effect differed from zero and represented partial mediation.
Figure 2.
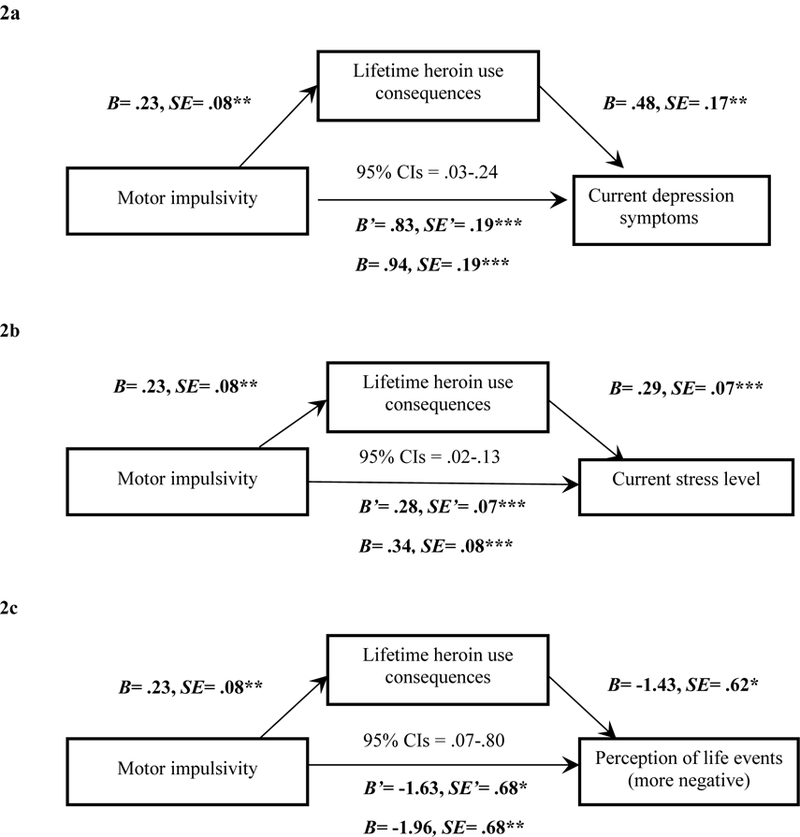
Mediation models with Motor impulsivity (X), lifetime heroin-use consequences (M), and the parallel outcome measures (Y) of current depression symptoms (2a), stress level (2b), and perception of life events (2c). Note. *p<.05; **p<.01; ***p<.001. The unstandardized coefficients and standard errors shown account for the mediator in the equation. For each respective model, we list beta and standard error values for the mediation model’s direct effect (c’ path) and the unmediated model’s total effect (c path), as well as the 95% confidence interval range for the mediation model to demonstrate the indirect effect was different from zero and represented partial mediaiton.
Current depression symptoms.
There was a significant direct effect of Motor impulsivity on current depression symptoms (Figure 2a). The indirect effect through lifetime heroin-use consequences was also significant, and estimated to range between .03 and .24. These results indicate that more lifetime heroin-use consequences partially mediated the relationship between higher Motor impulsivity and more current depression symptoms (R2 = .18).
Current stress level.
The direct effect of Motor impulsivity on stress was significant (Figure 2b). The indirect effect through lifetime heroin-use consequences was also significant, and was estimated to range between .02 and .13. These results indicate that more lifetime heroin-use consequences partially mediated the relationship between higher Motor impulsivity and higher current stress level (R2 = .21).
Perception of current life events.
The direct effect of Motor impulsivity on perception of life events was significant (Figure 2c). The indirect effect through lifetime heroin-use consequences was also significant, and was estimated to range between .07 and .80. These results indicate that more lifetime heroin-use consequences partially mediated the relationship between higher Motor impulsivity and more negative perception of events (R2 = .08).
Discussion
The present study examined pathways among trait impulsivity dimensions, heroin use-related consequences, and current mood indices (depression symptoms, stress levels, and perception of life events). Results indicated that experiencing a wider range of lifetime heroin-use consequences mediated relationships between two trait-impulsivity domains (Attentional and Motor) and all current mood measures. In particular, high Attentional and Motor trait impulsivity predicted increased heroin-use consequences, which related to elevated levels of depression, stress, and negative perception of life events. These findings may suggest an alternative to the self-medication hypothesis of heroin use and negative mood states, wherein heroin is used in response to stress and/or negative affect. Results from this study indicate that specific domains of trait impulsivity predict current mood outcomes, via heroin-use consequences. Non-planning impulsivity was not significantly related to heroin-use consequences. Further, mediation models fit better for Attentional than Motor impulsivity. These findings build on previous studies demonstrating that the BIS-11 Attentional subscale, which assesses task focus, thought insertions, and intrusive and racing thoughts in the context of decision making (Patton et al. 1995) specifically relate to prefrontal cortex-mediated executive function (Cheung et al. 2004; Whitney et al. 2004) and mood symptoms such as depression, hopelessness, and anhedonia (Swann et al. 2008). It is also likely that impulsive decision making (Barratt et al. 1985; Patton et al. 1995), urgency, and lack of pre-meditation (Whiteside et al. 2001) as measured by BIS-11 Attentional and Motor subscales are central to relationships among these features of trait impulsivity and the negative consequences of heroin use.
The present findings echo our results with regular cocaine users (Lister et al. 2015), which indicated that lifetime cocaine use-related adverse consequences (measured with the DHUQ) mediated relations between trait impulsivity (BIS-11) and depression symptoms (BDI-II). The present study findings replicate and extend our initial study of mediational relationships to another drug class (heroin), underscoring the robustness of the proposed mediational pathway of drug-related consequences. In our earlier study, cocaine-use consequences significantly mediated relationships among all three BIS-11 subdomains of impulsivity and current depression symptoms (Lister et al. 2015). The present study identified significant mediating effects of heroin-use consequences on relationships between trait impulsivity and all three outcome variables. However, relative to the outcomes of depression symptoms and stress levels, we found small effect sizes in analyses of perception of life events. These data suggest heroin-use consequences may yield a more robust effect on current mood than on appraisals of current life events. Taken together, results of our two studies suggest that relationships among drug-use consequences, dimensions of trait impulsivity, and current mood differ somewhat across drug classes. Future research is needed to clarify paths through which distinct dimensions of trait impulsivity differentially influence substance use and mood outcomes.
Prior studies have pointed to stress as a causal factor in heroin use and associated outcomes; yet, the present findings suggest an alternative pathway. Namely, greater depression symptoms, stress levels, and more negative perception of life events may also occur in response to accumulated heroin-use consequences (e.g. medical and job-related problems). These findings highlight important clinical characteristics among chronic heroin users. First, this population experiences high rates of depression (53% reported clinically relevant depression symptoms), partly due to associated lifetime heroin-use consequences. The high levels of depressive symptoms reported in the current sample is consistent with clinical characteristics observed in longitudinal, international studies (e.g. VEdeTTE, NTORS, ATOS) of heroin use and treatment outcomes (Bargagli et al. 2009; Gossop et al. 2003; Ross et al. 2005). We urge mental health providers to assess heroin use and depression before making treatment recommendations for heroin users (e.g. through emergency services, psychiatric outreach, and treatment intake). Second, although our participants were not seeking treatment at the time of screening, they reported numerous failed lifetime heroin-quit attempts, and most had previously sought treatment. Taken alongside our mediation findings, this suggests chronic heroin users’ ability to quit is complicated by ostensibly unaddressed adverse heroin-use consequences that contribute to depressive symptoms, both of which are unlikely to change without formal treatment. In addition, higher levels of impulsivity may impair cognitive skills (e.g. executive function, goal direction; Paulus 2007) required to gain mastery over, or maintain positive affect following, the experience of heroin-related stressors. Future research should attempt to replicate our findings among a heroin treatment sample to determine how to improve intervention for patients, particularly those with high impulsivity and co-occurring mood problems.
These results should be interpreted in the context of study limitations. First, these data are cross-sectional and not intended to directly assess causal relationships or temporality. They instead aim to establish pathways using prior literature and temporality of study variables (i.e. trait preceding lifetime; lifetime preceding current) to inform clinical assessment. Longitudinal research is needed to examine whether these pathways are causal (e.g. through use of repeated measures).
Second, subject characteristics could have influenced our findings, as the sample was drawn from a community rather than a clinical population. In addition, use of self-reported mood, trait, and heroin-use measures did not enable objective assessment of stressors, mood symptoms, and behavior. However, accuracy of endorsement has been demonstrated for self-reported drug use history, impulsivity, and mood symptoms (e.g. Hjorthoj et al. 2012; Zaldivar Basurto et al. 2009).
Our study also did not evaluate differential effects of specific subtypes of heroin-use consequences (e.g. legal, financial, medical; Moses et al. 2017) on mood outcomes, which may be useful to consider given present findings about the association among consequences of use, depression, stressors, and perception of life events. It is also unclear whether these findings would generalize to non-heroin opioids and, thus, whether non-medical opioid use and related consequences would similarly influence relationships between impulsivity and mood state.
Conclusion
This study examined pathways among trait impulsivity, lifetime heroin-use consequences, and current depression symptoms, stress levels, and perceptions of life events. Our findings improve conceptualization of pathways linking trait-level function, heroin-use consequences, and mood, which may inform clinical practice regarding chronic heroin use. Continued investigation into mechanisms underlying these relationships could enhance clinical and theoretical understanding of personality and mood variables central to heroin-use experiences.
Acknowledgements
The authors thank Ken Bates for participant recruitment, and Elorie Eggleston, Debra Kish, Katherine Mattison, Lisa Sulkowski and Melissa Williams for assistance with data collection and management. We also acknowledge Justin McManus for his contribution to the interpretation of mediation analyses.
Sources of funding
NIH 2 R01 DA015462 from the National Institute on Drug Abuse (to MKG), a postdoctoral research fellowship award (to JJL) from the Wayne State University Office of the Vice President, a grant (Joe Young Sr./Helene Lycaki Funds) from the State of Michigan, a predoctoral fellowship from the National Institute on Drug Abuse (awarded to EAW; NIH F31 DA040369), and funds from the Detroit Wayne Mental Health Authority supported this research. Funding sources had no role in the design, conduct, or analysis of these data, nor the decision to submit this manuscript. Data for this analysis were obtained under registered NIH clinical trials NCT00684840 and NCT01536925.
Footnotes
Disclosure of interest
All authors declare no conflict of interest with respect to the conduct or content of this work.
References
- al’Absi M. 2006. Hypothalamic–pituitary–adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol 59(3): 218–227. http://dx.doi:10.1016/j.ijpsycho.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Antony M, Bieling PJ, Cox BJ, Enns MW, Swinson RP. 1998. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and community a sample. Psychol Assess 10(2): 176–181. 10.1037//1040-3590.10.2.176 [DOI] [Google Scholar]
- Asberg K, Renk K. 2012. Substance use coping as a mediator of the relationship between trauma symptoms and substance use consequences among incarcerated females with childhood sexual abuse histories. Substance Use Misuse 47(7): 799–808. 10.3109/10826084.2012.669446 [DOI] [PubMed] [Google Scholar]
- Bargagli AM, Faggiano F, Amato L, Salamina G, Davoli M, Mathis F. 2009. VEdeTTE, a longitudinal study on effectiveness of treatments for heroin addiction in Italy: study protocol and characteristics of study population. Substance Use Misuse 41(14): 1861–1879. 10.1080/10826080601025482 [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51(6): 1173–1182. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Barratt ES, Patton JH. 1983. Impulsivity: cognitive, behavioral, and psychophysiological correlates. Zuckerman M, editor, Biological Bases of Sensation Seeking, Impulsivity, and Anxiety Hillsdale (NJ): Erlbaum; p. 77–116. [Google Scholar]
- Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory-II San Antonio, TX: Psychological Corporation. [Google Scholar]
- Brook DW, Brook JS, Zhang C. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry 59(11): 1039–1044. 10.1001/archpsyc.59.11.1039 [DOI] [PubMed] [Google Scholar]
- Cheung AM, Mitsis EM, Halperin JM. 2004. The relationship of behavioral inhibition to executive functions in young adults. J Clin Exp Neuropsychol 26(3): 393–404. 10.1080/13803390490510103 [DOI] [PubMed] [Google Scholar]
- de Wit H. 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14(1): 22–31. 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Mills K, Teesson M, Ross J, Williamson A, Havard A. 2009. Patterns of major depression and drug-related problems amongst heroin users across 36 months. Psychiatry Res 166(1): 7–14. 10.1016/j.psychres.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Dissabandara LO, Loxton NJ, Dias SR, Dodd PR, Daglish M, Stadlin A. 2014. Dependent heroin use and associated risky behavior: the role of rash impulsiveness and reward sensitivity. Addict Behav 39(1): 71–76. 10.1016/j.addbeh.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. 2011. Mortality among regularly or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 106: 32–51. 10.1111/j.1360-0443.2010.03140.x [DOI] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, Lazarus RS. 1988. The impact of daily stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol 54(3): 486–495. 10.1037//0022-3514.54.3.486 [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. 2008. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addict Behav 33(2): 388–394. http://dx.doi:10.1016/j.addbeh.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, Kidd T. 2003. The National Treatment Outcome Study (NTORS): 4–5 year follow-up results. Addiction 98(3): 291–303. 10.1046/j.1360-0443.2003.00296.x [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. 2013. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology 225(4): 811–824. 10.1007/s00213-012-2868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. 2002. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiatry 59(4): 375–380. 10.1001/archpsyc.59.4.375 [DOI] [PubMed] [Google Scholar]
- Havard A, Teesson M, Darke S, Ross J. 2006. Depression among heroin users: 12-month outcomes from the Australian Treatment Outcome Study (ATOS). J Subst Abuse Treat 30: 355–362. https://doi-org/10.1016/j.jsat.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Hjorthoj CR, Hjorthoj AR, Nordentoft M. 2012. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances—systematic review and meta-analysis. Addict Behav 37(3): 225–233. 10.1016/j.addbeh.2011.11.025 [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. 1999. Heroin addicts discount delayed rewards at higher rates than non-drug using controls. J Exp Psychol Gen 128(1): 78–87. 10.1037//0096-3445.128.1.78 [DOI] [PubMed] [Google Scholar]
- Klanecky A, McChargue D, Bruggeman L. 2012. Desire to dissociate: implications for problematic drinking in college students with childhood or adolescent sexual abuse exposure. Am J Addict 21(3): 250–256. 10.1111/j.1521-0391.2012.00228.x [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. 2002. Pharmacotherapy of addictions. Nature Reviews Drug Discovery, 1(9): 710 10.1038/nrd897 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. 2005. Genetic influences on impulsivity, risk-taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 8(11): 1450–1457. 10.1038/nn1583 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang F, Zhou Y, Zhang M, Wang X, Shen M. 2013. Decision-making deficits are still present in heroin abusers after short- to long-term abstinence. Drug Alcohol Depend 130(1–3): 61–67. 10.1016/j.drugalcdep.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Lister JJ, Ledgerwood DM, Lundahl LH, Greenwald MK. 2015. Examining causal pathways between impulsivity, cocaine use consequences, and depression. Addict Behav 41: 1–6. 10.1016/j.addbeh.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. 1995a. Manual for the Depression Anxiety Stress Scales Sydney: Psychology Foundation. [Google Scholar]
- Lovibond PF, Lovibond SH. 1995b. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 33(3): 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Luecken LJ. 2008. How and for whom? Mediation and moderation in health psychology. Health Psychol 27(2): 99 10.1037/0278-6133.27.2(Suppl.).S99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. 1997. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5(3): 256–263. 10.1037/1064-1297.5.3.256 [DOI] [PubMed] [Google Scholar]
- Martin LE, Potts GF. 2010. Impulsivity in decision-making: an event-related potential investigation. Pers Individ Diff 46(3): 303–313. 10.1016/j.paid.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Keyes KM, Storr CL, Zhu H, Chilcoat HD. 2009. Pathways between nonmedical opioid use/dependence and psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 103(1–2): 16–24. 10.1016/j.drugalcdep.2009.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. 2001. Psychiatric aspects of impulsivity. Am J Psychiatry 158: 1783–1793. 10.1176/appi.ajp.158.11.1783 [DOI] [PubMed] [Google Scholar]
- Moses TEH, Woodcock EA, Lister JJ, Lundahl LH, Greenwald MK. 2017. Developing a scale of domains of negative consequences of chronic heroin use. Addict Behav 77: 260–266. 10.1016/j.addbeh.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Ho A, Bahl A, Varma P, Kellogg S, Borg L, Kreek MJ. 2012. Former heroin addicts with or without a history of cocaine dependence are more impulsive than controls. Drug Alcohol Depend 124(1–2): 113–120. 10.1016/j.drugalcdep.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil KA, Conner BT, Kendall PC. 2011. Internalizing disorders and substance use disorders in youth: Comorbidity, risk, temporal order, and implications for intervention. Clin Psych Review 31(1): 104–221. 10.1016/j.cpr.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. 1995. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 51(6): 768–774. [DOI] [PubMed] [Google Scholar]
- Paulus MP. 2007. Decision-making dysfunctions in psychiatry—altered homeostatic processing? Science 318(5850): 602–606. 10.1126/science.1142997 [DOI] [PubMed] [Google Scholar]
- Peluso MA, Hatch JP, Glahn DC, Monkul ES, Sanches M, Najt P, Bowden CL, Barratt ES, Soares JC. 2007. Trait impulsivity in patients with mood disorders. J Affect Disord 100(1–3): 227–231. 10.1016/j.jad.2006.09.037 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. 2004. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instr Comput 36(4): 717–731. 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. 2011. Stress in the daily lives of cocaine and heroin users: Relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology 218(1): 29–37. 10.1007/s00213-011-2183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Teesson M, Darke S, Lynskey M, Ali R, Ritter A, Cooke R. 2005. The characteristics of heroin users entering treatment: findings for the Australian Treatment Outcome Study (ATOS). Drug Alcohol Depend 24: 411–418. 10.1037/adb0000090 [DOI] [PubMed] [Google Scholar]
- Samet S, Fenton MC, Nunes E, Greenstein E, Aharonovich E, Hasin D. 2013. Effects of independent and substance-induced major depressive disorder on remission and relapse of alcohol, cocaine and heroin dependence. Addiction 108(1): 115–123. 10.1111/j.1360-0443.2012.04010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. 2002. Mediation in experimental and nonexperimental studies: New procedures and recommendation. Psychol Methods 7(4): 422–445. 10.1037//1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Sinha R. 2007. The role of stress in addiction relapse. Curr Psychiatry Rep 9(5): 388–395. [DOI] [PubMed] [Google Scholar]
- Sinha R. 2008. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141: 105–130. 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Chahua M, Bravo MJ, Barrio G, Brugal MT, Domingo-Salvany A, Molist G, De la Fuente L; ITINERE Project Group. 2012. Depression among regular heroin users: the influence of gender. Addict Behav 37(1): 148–152. 10.1016/j.addbeh.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. 2009. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Individ Diff 47(5): 385–395. 10.1016/j.paid.2009.04.008 [DOI] [Google Scholar]
- Stoltman JJK, Woodcock EA, Lister JJ, Lundahl LH, Greenwald MK. 2015. Heroin delay discounting: modulation by pharmacological state, drug-use impulsivity and intelligence. Exp Clin Psychopharmacol 23: 455–463. 10.1037/pha0000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2013. Results from the 2012 National Survey on Drug Use and Health: summary of national findings. NSDUH Series H-46, HHS Publication No. (SMA) 13–4795 Rockville, MD: Substance Abuse and Mental Health Services Administration; Retrieved from: http://www.samhsa.gov/data/sites/default/files/NSDUHresults2012/NSDUHresults2012.pdf [Google Scholar]
- Swann AC, Steinberg JL, Lijffijt M, Moeller FG. 2008. Impulsivity: differential relationship to depression and mania in bipolar disorder. J Affect Disord 106(3): 241–248. 10.1016/j.jad.2007.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin KE, Latkin CA. 2003. The relationship between depressive symptoms and nonfatal overdose among a sample of drug users in Baltimore, Maryland. J Urban Health 80: 220–229. 10.1093/jurban/jtg025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, World Drug Report 2017 (ISBN: 978-92-1-148291-1, eISBN: 978-92-1-060623-3, United Nations publication, Sales No. E.17.XI.6). [Google Scholar]
- Whiteside SP, Lynam DR. 2001. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Diff 30(4): 669–689. 10.1016/S0191-8869(00)00064-7 [DOI] [Google Scholar]
- Whitney P, Jameson TL, Hinson JM. 2004. Impulsiveness and executive control of working memory. Pers Individ Diff 37(2): 417–428. 10.1016/j.paid.2003.09.013 [DOI] [Google Scholar]
- Williams CT, Latkin CA. 2007. Neighborhood socioeconomic status, personal network attributes, and use of heroin and cocaine. Am J Prev Med 32(6): 203–210. 10.1016/j.amepre.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski AB, Brown TT, John M, Cofranceso J, Golub ET, Ricketts EP, Wand G, Dobs AS. 2005. Cortisol levels and depression in men and women using heroin and cocaine. Psychoneuroendocrinology 31(2): 250–255. 10.1016/j.psyneuen.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Lundahl LH, Burmeister M, Greenwald MK. 2015a. Functional mu opioid receptor polymorphism (OPRM1 A118G) associated with heroin use outcomes in Caucasian males: a pilot study. Am J Addict 24(4): 329–335. 10.1111/ajad.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Lundahl LH, Stoltman JJK, Greenwald MK. 2015b. Progression to regular heroin use: examination of patterns, predictors, and consequences. Addict Behav 45: 287–293. https://doi-org.proxy.lib.wayne.edu/10.1016/j.addbeh.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA. 1991. The Manual of the Shipley Institute of Living Scale Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Zaldivar Basurto F, Garcia Montes JM, Flores Cubos P, Sanchez Santed F, Lopez Rios F, Molina Moreno A. 2009. Validity of the self-report on drug use by university students: correspondence between self-reported use and use detected in urine. Psicothema 21(2): 213–219. [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. 2001. Personality and risk-taking: common biosocial factors. J Personality 68(6): 999–1029. 10.1111/1467-6494.00124 [DOI] [PubMed] [Google Scholar]


