Abstract
In this article we summarize the birth of the field of nuclear receptors, the discovery of untransformed and transformed isoforms of ligand-binding macromolecules, the discovery of the three-domain structure of the receptors, and the properties of the Hsp90-based heterocomplex responsible for the overall structure of the oligomeric receptor and many aspects of the biological effects. The discovery and properties of the subfamily of receptors called orphan receptors is also outlined. Novel molecular aspects of the mechanism of action of nuclear receptors and challenges to resolve in the near future are discussed.
Keywords: Nuclear receptor, Steroid receptor, Chaperones, Heat-shock proteins, Hsp90, Transcriptional regulation
1. Introduction
The largest group of transcription factors in eukaryotes is grouped in a superfamily of structurally related proteins referred to as the nuclear receptor superfamily. These receptors were first understood as ligand-regulated DNA-binding transcription factors, which regulate biological programs involved in a broad spectrum of physiological phenomena. With time, the so called ‘orphan’ receptors (receptors with no ligand discovered to date) were also characterized and included into this superfamily based on the structural domain properties of these transcription factors. It is currently accepted that in humans, the nuclear receptor superfamily comprises 48 members.
Nuclear receptors play several roles in the normal physiology of the cells, including metabolism, electrolyte balance, cell proliferation, immune response, enzyme activity, development, and reproduction, as well as in many pathological processes, such as cancer, neurologic and psychiatric syndromes, immunosuppression, diabetes, rheumatoid arthritis, asthma, hormone-resistance syndromes, cardiovascular diseases, metabolic syndrome, premature ageing, etc. [1–6]. Therefore, despite their already long history, these factors are still of great interest in modern biomedical research and drug discovery.
Even do the biological processes commanded by nuclear receptors is extremely vast, these proteins share remarkable structural similarity. Before the first genes encoding nuclear receptors were cloned, it was already known that they are modular proteins [7]. The greatest homology is preserved in the amino acid sequence of the DNA-binding domain (DBD) and the ligand-binding domain (LBD) [8, 9], in that order (Figure 1). As their names indicate, these domains are responsible for the association of the transcription factor with specific DNA sequences, the DBD, and the binding of small ligands, usually of lipophilic nature, the LBD. There is a third, non-conserved N-terminal domain named the transactivation domain (TD), which was first thought to be the most selective region of the protein due to interactions with other nuclear factors, consequently being responsible for the specificity of the biological effect (see [10–12] for comprehensive reviews). The TD, also named domain AF-1, shows variable length and sequence in the different family members and is recognized by coactivators and/or other transcription factors. Because of that variability, in the past it was also named the ‘immunoreactive domain’. The ability of the LBDs to activate transcription is controlled by the C-terminal helix 12, also termed AF-2, such that ligand-binding triggers a mechanism that transforms AF-2 into a transcriptionally competent domain. Nonetheless, today we know that those protein-protein contacts responsible for receptor specificity are not restricted to the TD only.
Figure 1: Domain structure of nuclear receptors (A-F).

The DNA binding domain (DBD) or C region consists of two zinc-binding motifs (or Zn-fingers), that often includes a hinge domain or D region. A nuclear localization signal 1 (NL1) is usually located at the C-terminal end of this region. The ligand binding domain (LBD) or E/F region binds the cognate ligand. Ligands of the orphan receptor subfamily are not known, and it is thought that they could be endogenous compounds, possibly metabolic intermediates, or even environmental factors, which may explain some of their apparently constitutive transactivation activity and the difficulty encountered to identify their ligands. The AF1 and AF2 activation function 1 and 2 contact co-regulatory molecules, but AF-1 is typically a variable ligand-independent (first named transactivation domain. TD) while the AF-2 of the E/F region is ligand-dependent.
In the 1950s, the model for steroid hormone action told us that the steroid entered the cells by simple diffusion through the plasma membrane, after which a series of metabolic oxidations and reductions took place thus providing the ‘needed energy’ for growth stimulation and other specific actions. Subsequently, Elwood Jensen entirely overturned that elemental notion [13] when he proved that tritiated estrogens do not undergo chemical changes, but they rather bind to a protein within the cell. Then, this hormone-receptor complex must translocate to the cell nucleus and regulates the expression of specific genes. At that time, this idea was almost a heresy. “That really got him into some hot water,” recalled Gene DeSombre [14], Professor Emeritus in the Ben May Institute for Cancer Research, who worked with Jensen as a post-doctoral fellow and then as his colleague. “Jensen struggled quite a lot,“ echoed Shutsung Liao, another Ben May Institute colleague, who subsequently reported a similar mechanism for testosterone action. When Dr. Jensen first presented his data at the IV International Congress of Biochemistry in Vienna (1958), only five people attended to that session, three of whom were the other speakers. There were more than 1,000 attended registered to the meeting, but they attended to other simultaneous symposium on the metabolic processing of estrogen. Nonetheless, with this ‘trivial’ and totally unnoticed report, the concept of hormone receptor, as we understand it today, was born.
2. The Nuclear Receptor Superfamily
Before the genes encoding these receptors were cloned, the first member of the family to be identified biochemically was the estrogen receptor (ER) [15]. About two decades after, the cDNA for the human glucocorticoid receptor (GR) was the first to be elucidated [16]. followed by the ER [17] and the mineralocorticoid receptor (MR) [18]. Since then, nuclear receptors have become recognized as a superfamily of transcription factors, and steroid receptors were grouped as a subfamily within the former. Then, the nuclear receptor research field has undergone very rapid development and covers areas ranging from structural and functional analyses to the molecular mechanisms of transcription regulation. As soon as the founder members of the superfamily were characterized, a small group of non-steroidal receptors was also added to the family, i.e. the thyroid hormone receptors (TRs), the retinoic-acid receptors (RARs), and a relatively small legion of receptors whose endogenous ligands were unknown and were consequently grouped as the orphan receptor subfamily [8, 19].
Nuclear receptor genes are encoded and expressed even in the simplest organisms of the animal kingdom. It is accepted that more than 900 nuclear receptors genes have been identified in all animals examined [20], from the simplest to the most complex (NureXbase, http://nurexbase.prabi.fr/). The simplest metazoans belong to the phylum Porifera, for example sponges, which show primitive bodies with pores and channels allowing water to circulate through them. They have two genes encoding for nuclear receptors, whereas Trichoplax adherens (a flat organism of one millimeter in diameter, lacking any organs or internal structures), encodes for four receptors [20]. When the morphological and functional complexity of the organisms becomes more multifaceted, the number of receptors increases, reaching forty-nine members in mammals (the total number may vary if splicing variants are also counted). Nonetheless, it is pertinent to point out that nuclear receptors are absent in fungi, plants and also in the closest known relatives of metazoans, the free-living unicellular and colonial eukaryotes of the Choanoflagellatea class [21]. Hence, this evolutionary tree is telling us that these receptors should have arrived on the scene of evolution about 635 million years ago, i.e. when metazoans first appeared in the fossil records. Importantly, it seems that nuclear receptors play a cardinal role during the Cambrian explosion of life forms nearly 540 million years ago. Researchers have discussed for decades over what ignited that evolutionary burst. Some of them have postulated that a steep rise in oxygen sparked the change, whereas others say that it sprang from the development of some key, already still uncertain, evolutionary innovation. In this sense, it is interesting to pose the fact that the rising of the nuclear receptor family occurred during that time οCause or consequence? The precise reason has remained elusive and probably will be forever, mainly because so little is known about the physical and chemical environment of the planet at that time [22].
Regarding the origin of the family, one possible hypothesis could be the notion that an ancestral nuclear receptor promoted ligands evolved independently in many lineages (Figure 2). An alternative theoretical possibility is that the ancestral receptor was indeed a protein designed for other biological purposes, but it gained specific functions upon ligand-binding in fortuitous event where preexisting small molecules, also designed for other purposes, began to activate these proteins in a typical gain-of-function event. It is difficult to affirm with absolute certainty what was first, the egg or the chicken. However, it is interesting to highlight that the same ligands used by these ‘novel’ receptors had appeared in nature well before than the proteins themselves. For example, the presence of progesterone in plants was first reported in the 60’s [23], and later detected in a wide range of plant species from 50 families [24]. Plant progesterone in turn, become a substrate precursor of corticosteroids, androstanes and estranes in this very same kingdom (see [25] for recent review). On the other hand, it is well known that there are mechanisms able to activate nuclear receptors in the absence of the cognate ligand [26–28], suggesting the possibility that ligands could have not been required during the evolution of this family.
Figure 2: Evolutionary tree of the nuclear receptor family.

The tree branches of the scheme show nodes well supported at bootstrap values >90%. Roman numbers group each subfamily of the superfamily. Note that the consensus phylogenetic position of the nuclear receptors is not correlated with the chemical nature of the cognate ligand.
Sequence alignment and phylogenetic tree construction resulted in a classification of the human nuclear receptor superfamily into six evolutionary groups of unequal size [29]: (I)- This large group contains the receptors TRs, RARs, VDR, all PPARs (peroxisome proliferator-activated receptors), and orphan receptors such as the Rev-Erb receptor, RORs (receptor tyrosine kinase-like orphan receptors), CAR (constitutive androstane receptor), PXR (pregnane X receptor), LXRs (liver X receptors), and others. (II)- This group includes RXRs (retinoid X receptor), COUP-TFII (COUP transcription factor II receptor), and HNF-4 (hepatocyte nuclear factor-4 receptor). (Ill)- This subfamily includes the steroid receptors and the ERRs (estrogen-related receptors). (IV)- This group contains the nerve growth factor induced clone B group of orphan receptors (NGFI-B, NURR1, and NOR1). (V)- This small group that includes the steroidogenic factor 1 (NR5A1) and receptors related to the Drosophila FTZ-F1. (VI)- This subfamily contains only the germ cell nuclear factor-1 (GCNF1) receptor, which does not fit into any other subfamily.
2.1. Steroid receptor subfamily
As it was commented before, the early beginning of the nuclear receptor superfamily is traced back to the late 50’s when tritiated-estrogens were used by the first time in studies on the biochemistry of steroid receptors [13, 30]. By using this ‘novel tool’, it was demonstrated the selective accumulation and retention of [3H]-labeled steroid in the reproductive organs of immature female animals when physiological amounts of hormone were injected. Therefore, it was postulated that the retention of [3H]-estradiol in the uterus and vagina reflected binding to putative receptors located in the cells [15], this being the first arguable evidence for binding of a hormone to a receptor. With time, several members of the steroid receptor family were cloned during the ‘80s, such as the glucocorticoid receptor [26], the estrogen receptor [27], the thyroid hormone receptor [28], and the mineralocorticoid receptor [29].
Studies on maximum likelihood sequences of ancestral receptors and branch lengths were reconstructed on the most parsimonious phylogeny [31]. The hypothesis by Joseph Thornton was based on the fact that an ancestral protein is likely to have been most similar in sequence and therefore in function to the descendant gene that diverged more slowly after the duplication event. The observed sequence divergence rate suggested that the ancestral steroid receptor was a functional estrogen receptor. Therefore, it is currently thought that ER was the first receptor, followed by the PR. Similarly, the youngest members of this subfamily are GR and MR, in that order [31].
Also, the appearance of puffs on the polytene chromosomes of insect salivary glands incubated with 20-hydroxyecdysone provided the first evidence that steroids may act directly at the gene transcriptional level to bring about subsequent cellular changes [32, 33], In line with these findings, early studies also showed that estrogen can selectively activate the genes encoding egg-white and yolk proteins. The first attempts to characterize those [3H]estradiol-bound proteins in native tissues were performed during the middle ‘60s when Toft and Gorski [34] prepared cytosolic fractions from uteri of rats injected with [3H]estradiol and resolved its components through a gradient of 5–20% sucrose. The estradiol radioactivity sedimented in a symmetrical peak at 9.5S, which disappeared after protease treatment. Shortly thereafter, it was shown [35] that the direct addition of [3H] steroid to cytosol extracts obtained from uteri of untreated rats also yielded identical 9.5S complexes. This was the first direct demonstration of steroid-binding to a receptor in a cell-free system, and set the basis to establish the so called “untransformed”, 9.5S isoform of the receptor versus the “transformed” isoform evidenced at 4-5S at higher temperatures than 2-3°C or by increasing the ionic strength of the buffer. The term “transformation” began to be employed to identify the conversion of the 9.5S, non-DNA-binding isoform of the receptor, to the 4S, DNA-binding form, which is demonstrated after the dissociation of the associated chaperone heterocomplex. On the other hand, the term “activation” was used to specifically refer to the conversion of receptors from a form that does not bind steroid to a steroid-binding form.
In spite of the overwhelming evidences related to the existence and roles of steroid receptors, as late as in 1968 the use of the term “receptor” was still questioned by some researchers, as it can be read in articles published in prestigious journals, where it was even argued that the steroid-binding macromolecules “may be without physiologic significance” [32]. Curiously, the concept of steroid receptor evolved in parallel with the discovery of close-associated proteins that are essential for receptor folding and function—the molecular chaperones. Notably, the biological relevance of these proteins was also questioned by that time.
2.2. Molecular chaperones
From the etymologic perspective, the term chaperone refers to a person (usually a matron) who used to accompany young unmarried women in public to supervise them at a social gathering. Therefore, a chaperone person was a ‘social protector’ who safeguarded the proper conduct of teen-agers. Analogously, proteins that assist others in their proper folding are also referred to as ‘chaperones’, i.e. Hsp90, Hsp70, CyPA, TRiC/CCT, Grp94, GroEL/GroES, etc. The term ‘molecular chaperone’ was first used to describe the ability of nucleoplasmin to prevent the aggregation of histones with DNA during the assembly of nucleosomes, and then, it was extended to other proteins that mediate the post-translational assembly of protein complexes (see an appealing background in [36]). Moreover, those molecular chaperones induced by heat-stress are also named heat-shock proteins (HSPs). Nonetheless, temperature is not the only stimulus able to trigger such a biological response, but also UV radiation, chemicals, toxic compounds, metals, inappropriate pH or osmotic pressure, nutrient starvation, oxidants, fever, cancer, infections, neurodegenerative diseases, etc. [37, 38]. Also, the heat-shock response is useful even in the absence of stress during the normal growth cycle of the cells even without the existence of stressors in the medium. Importantly, while all HSPs are molecular chaperones, not all chaperones are always induced by heat-shock or other types of stress. Therefore, the level of expression for the latter group is quite stable. On the other hand, the term ‘co-chaperone‘ refers to proteins that are associated to chaperones assisting them in their properties to modulate client-proteins properties (for example, TPR-domain immunophilins, p23, Ahal, CDC37/p50, Hop/p60, SGT, etc.). This does not imply that a co-chaperone cannot show chaperone properties, which depends on the client protein and the abundance of other factors.
The biological importance of HSPs can be traced back to the same period the concept of receptor was born, the early 1960s. The Italian scientist Ferruccio Ritossa was studying the synthesis of nucleic acid in puffs of Drosophila salivary glands. A colleague accidentally changed the temperature of the cell incubator and something unexpected was noticed—an incredible transcriptional activity of new chromosomal puffs. New RNAs were detected as soon as to 2-3 nun after increasing the temperature. The importance of this fortuitous observation was immediately grasped—cells react in response to elevated temperature through the synthesis of unknown factors [39]. Today, we know that these factors are the HSPs, and this finding remains to date as the clearest demonstration of environmentally induced changes in gene expression. As it often happens, this unanticipated concept was very difficult to accept at the time of discovery. Ritossa’s fortuitous but clever observation was systematically rejected from top journals with the argument that, again, “the finding is interesting, but it lacks biological relevance” [40]. Today anyone could even think about the possibility to question the biological relevance of chaperones in protein folding, client protein stability and biological function. Moreover, the further evolution of knowledge of both the steroid receptor field and the chaperone field became close-related one another. In the particular case of the chaperone heterocomplex associated to steroid receptors, it is not only essential to favor steroid binding and to prevent receptor transcriptional activity, but also for the stabilization of the receptor conformation preventing its degradation by the proteasome, as well as it is also a critical component of the molecular mechanism of transport and subnuclear redistribution of these receptors [41–43], as well as other factors [44–47].
3. Receptor Transformation
Initially, laboratories focused their studies on the capability of transformed cytosolic receptors to bind to nuclei. Several assays for receptor transformation were available, such as the finding that treatment of nuclei with DNAse released transformed receptors, and it was shown that transformed receptors are able to bind polyanions in general. Researchers took advantage of these observations and assays of receptor transformation were developed based on binding to phosphocellulose, ATP-Sepharose, and carboxymethyl-Sephadex.
The Gustafsson laboratory [48] was the first to combine steroid- and temperature-dependent transformation of the GR to the DNA-binding state as a first step to enrich preparations to purify receptor. This allowed to define by the first time by limited proteolysis the ligand binding domain (LBD) and the DNA-binding domain (DBD) [49], well determined years after when the receptor was cloned. The delimitation of these domains was useful in later studies localizing the Hsp90-binding region on molybdate-stabilized untransformed receptor [50]. By that time, it was well known that Hsp90 was a highly conserved and wide distributed chaperone in all organisms, located mostly in the cytoplasm, and phosphorylated mostly on serine residues. A 90-kDa protein with the same characteristics had been reported bound to the untransformed receptor [51]. Three independent studies reported by 1985 proved that that 90-kDa protein was the chaperone Hsp90 [52–54]. By co-immunoadsorption assays, it was demonstrated that Hsp90 dissociated from the GR when cytosolic receptors were transformed, a phenomenon that was prevented by molybdate [55]. Subsequent to these first studies where PR and GR oligomeric complexes were characterized, Hsp90 was demonstrated to be present in all other untransformed members of the steroid receptor subfamily, ER, AR, MR, and VDR (vitamin D receptor), as well as in other members of the nuclear receptor superfamily such as Rev-Erb, AhR, PPARγ, CAR, etc., but not in TR, RAR, RXR, and some members of the orphan receptor subfamily.
Hsp90 is a highly conserved HSP expressed ubiquitously in all organisms. It is the most abundant constitutive HSP accounting for ~1-3% of the total cytosolic proteins of the cell. Two genes encode Hsp90 in mammalian cells, where the Hsp90α form shows 86% identity with respect to Hsp90β [56]. Moreover, there is extensive homology with lower species; thus, the human Hsp90β shows 78% identity with the Drosophila 83-kDa HSP, and 61% identity with yeast Hsp90 [57]. In Escherichia coli, the homologous HSP is a 63-kDa protein 42% identical to human Hsp90 [58]. In the mouse, there is a clear difference in the electrophoretic migration of both forms of the protein on denaturing gels, and the α and β forms are often called Hsp86 and Hsp84 respectively, with Hsp84 being more abundant than Hsp86. By coimmunoadsorption, it was demonstrated that Hsp86•Hsp84 heterodimers exist as native complexes [59]. In addition to being expressed at a high level in normal cells, Hsp90 is heavily phosphorylated even in the absence of stress and it migrates as several species on two-dimensional gels [60].
As it is expected for any chaperone, Hsp90 facilitates the proper folding of proteins, but it also provides biological activity to client proteins that still preserve an intact tertiary structure acting like a biological switch, becoming essential for various cellular processes, such heterocomplexes assembly, protein degradation, signal transduction cascades, and morphological evolution [61–63]. Hsp90 is commonly located in the cytoplasm, but a small fraction of Hsp90 is also present in the nucleus, in particular when cells are exposed to stress. Stability of various oncogenic factors is almost entirely dependent on Hsp90 binding, such that cancer cells require this chaperone to survive in the demanding milieu generated by oncogenic transformation. Consequently, Hsp90 has become an attractive antineoplastic drug target, and several Hsp90 inhibitors are being currently tested in various stages of clinical trials [64–66].
During the early ‘90s, it was shown that Hsp90-based heterocomplexes could be assembled in vitro with client factors by incubating immunopurihed receptor or protein kinase with rabbit reticulocyte lysate and a source of ATP [67, 68]. Most of the advances in this regard were achieved by similar studies performed in parallel with the GR and PR. These reconstitutions could also be achieved by using purified proteins [67, 69, 70], such that the 9S, untransformed complex, could be rebuilt. These types of studies permitted the elucidation of the sequential assembly cycle of the Hsp90 heterocomplex with the GR, one of the best characterized complexes. Thus, it was demonstrated that receptor complexes are assembled in a specific order and dynamic manner, the first step being the formation of an (Hsp90)2•Hop•Hsp70•Hsp40 oligomer, which is stabilized by the presence of the Hsp90-binding co-chaperone p23. Today we know that the stabilizing action of p23 mimicked that action first assigned to the addition of molybdate to buffer preparations, which in turn restricts the nuclear accumulation of GR.
The TPR-domain co-chaperone Hop is finally released from Hsp90, and its site is occupied by a TPR-domain co-chaperone, usually FKBP51, FKBP52, PP5, or Cyp40, which dynamically exchange on Hsp90 dimers bound to untransformed, 9S receptors [71–73]. Studies of saturation binding of Hop to Hsp90 dimer [74] and cross-linking of Hsp90•FKBP52 complexes [75] are consistent with one TPR acceptor site per Hsp90 dimer, so the relative expression of a given TPR protein in the cell may be proportional to the extent of such protein present in the untransformed complexes [44,45,64,76]. Even so, the role of the steroid hormone is also quite relevant. Recent evidence showed that aldosterone-binding to MR favors the exchange of FKBP51 for FKBP52, whereas the synthetic agonist 11,19-oxidoprogesterone favors the recruitment of PP5 [71]. From the perspective of receptor trafficking, both FKBP52 and PP5 are equally effective for MR retrotransport because they associate dynein in similar extent [77]. The qualitative composition of the untransformed form of the receptor may vary according to the nature of the receptor. Thus, some immunophilins such as CyP40 shows selective preference for the ER rather than the GR or MR, whereas FKBP52 shows preference for GR and PR, and not for AhR, which in turn recruits exclusively XAP2 and not the other TPR-domain immunophilins.
4. The Subfamily of Orphan Nuclear Receptors
During the decade 1988 to 1998, there was an increased research focus on the nuclear receptor superfamily. By using known and naturally occurring ligands, receptors such as ER, AR, GR, TR and VDR became what are now nuclear receptors, whose function and ligands were well identified [78–80]. Insight into structural similarities and conserved domains amongst steroid hormone receptors paved the way for the discovery and addition of new members into the nuclear receptor superfamily [80]. In contrast to the historically named “classic receptors”, the newly introduced family members lacked an established partnership to endogenous ligands, and thus were designated as “orphans”.
With the advent of molecular techniques and the generation of cDNA libraries, members of the superfamily were discovered to share a conserved domain arrangement consisting of the modulator region (A/B) with an activation function 1 (AF1) domain, a DBD, a hinge region, a LBD, and, within this domain, an activation function 2 (AF2) region [78, 79, 81, 82]. The information derived from the sequences and conserved regions of known nuclear receptors enabled the development of molecular probes that could be used to screen cDNA libraries from various tissues, and led to the identification of additional orphan receptors. This time period came to be known as “the genomic era” [83].
In 1988, using the sequence of the DNA-binding domain from the ER, the first two orphan nuclear receptors were discovered, which included Estrogen Related Receptor α (ERRα) and Estrogen Related Receptor β (ERRβ) [84]. Likewise, following a screen using a mouse liver cDNA library, and with the belief that chemicals known as peroxisome proliferators (PP) may act via nuclear receptors similar to that of the steroid hormones. Peroxisome Proliferator-activated receptors (PPARs) were added to the family in the early 1990’s [85, 86].
With the continuous discovery of orphan receptor superfamily members, research goals steered to the search for potential receptor ligands, which was achieved with the use of “reverse endocrinology”. Reverse endocrinology uses the receptor itself to screen for ligands, as opposed to using known ligands to find receptors [87]. This methodology not only allowed for the screening of potential endogenous ligands, but also any additional compounds, natural or synthetic, acting as ligand partners for the orphan receptor in question. When an endogenous ligand for an orphan receptor is identified, the receptor is no longer an orphan, and it is placed into the “adopted” orphan receptor family.
The first endogenous ligand established for one of the orphans was 9-cis-retinoic acid, moving the retinoid X receptor (RXR) from the orphan family to the adopted family. By the end of 1998, ligands had been discovered for 13 orphans, including but not limited to PPAR, LXR, and PXR [88–91]. Moreover, once a ligand has been identified for a given orphan receptor, that information can be used to assist in determining an orphan receptor’s physiological function(s), and discerning new physiological pathways in which a specific receptor plays a role. In light of the fact that all NRs play significant roles in the regulation of human physiology, establishing the ligands that activate orphan receptors can open the door to the development of novel therapeutic strategies for diseases [78, 79]. Even though identification of endogenous ligands for orphan receptors is crucial, adoption of orphan receptors is not completely achieved until said ligands have been validated under physiological conditions. To date, there is a lack of consensus for the possible ligands of most members of the orphan NR superfamily. Adopted or true orphan NRs, such as PPAR, LXR, PXR, and Nurr1, have shown potential as therapeutic targets in the treatment of diabetes, obesity, and neurodegenerative diseases.
Of the previously mentioned orphan nuclear receptors PPAR, LXR, FXR, and PXR have been adopted, yet controversy still remains due to the relatively low affinity binding of their identified interacting ligands (micromolar range), as well as the promiscuous interactions of these receptors. However, the low affinity binding was also the first sign that not all nuclear receptors could need high binding-affinities for their ligands. Today we know that even for the case of formerly named classic receptors (ER, RAR, VDR, AR, etc.), our first understanding of their interaction with cognate ligands could have been an oversimplification since some receptors are activated only after physiological levels of their ligands have been exceeded. Moreover, local concentrations in some tissues (particularly, in the nervous system) hormone levels could largely exceed the values of an “acceptable” Ka value. Moreover, ligands first thought to be side metabolites of the normal metabolism of agonists such as allopregnanolone (or tetrahydroprogesterone) became biologically active in several ways; thus, in addition to be a known GABAA receptor agonist, allopregnanolone has recently been demonstrated to activate PXR-dependent pathways in the μM range [92]. Thus, certain bile acids (e.g., lithocholic acid) have been shown to directly activate PXR at concentrations between 10 μM to 100 μM [93], Moreover, even though three bile acid precursors (7α-hydroxy-4-cholesten-3-one, 5β-cholestan-3α, 7α,12α-triol, and 4-cholesten-3-one) activate mouse PXR in the low μM range, the same ligands are less potent activators of its human ortholog [94]. This type of differences for ligand specificity also extends to other xenobiotic ligands [95].
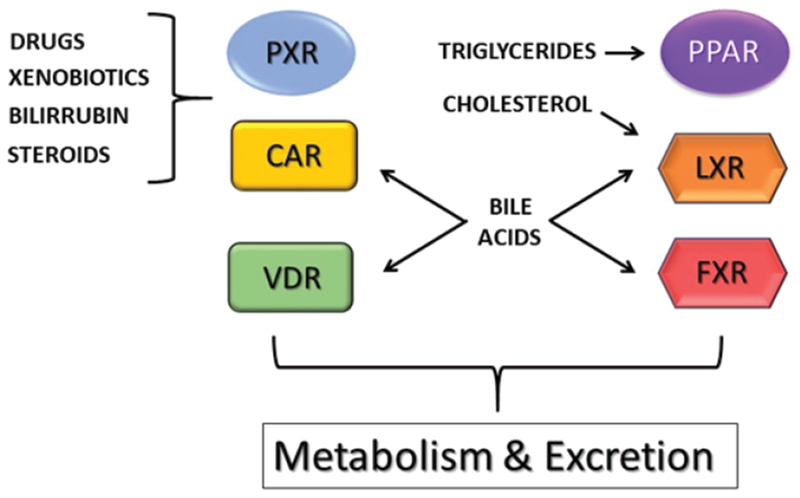
Other nuclear receptors, which were once orphans and are now adopted, are the xenobiotic sensors CAR and PXR. These receptors participate in the response against xenobiotics, and are known to facilitate the excretion of toxic metabolites from both endogenous and exogenous sources [98]. CAR and PXR were initially identified as xenosensors that regulate the expression of phase I and II xenobiotic metabolizing enzymes, and are known to be activated by common ligands such as ethinyl estradiol, diethylhexyl phthalate, and clotrimazole [86]. Remarkably, CAR and PXR have structurally distinct properties from those of other members of the family. PXR has the ability to bind ligands with varying sizes due to its flexible ligand binding domain; while CAR is constitutively active partly due to its rigid ligand domain, which might reduce the ability of some ligands to activate the receptor [99]. Taken together, PXR and CAR demonstrate the ability to influence drug metabolism, and the potential to shape current treatments owing to recently discovered knowledge on environmental and dietary chemicals that alter their activity.
Originally, ligands of the PXR and CAR receptors were exogenous drugs and xenobiotics, 1α,25-dihydroxyvitamin D3 for VDR, oxysterols for LXR, and bile acids for FXR (see Figure 2). However, later findings showed that a number of endogenous compounds are also able to influence PXR and CAR activity and that these xenosensors share an overlapping ligand pattern with other members of the subfamily (see Figure 3). The overlap of endogenous lipids to activate CAR, PXR, FXR, and LXR indicates a functional connection between these receptors in liver physiology [96].
Figure 3: Endogenous and xenobiotic lipophilic ligands.
Various xenobiotics and endogenous lipids are able to activate several nuclear receptors, which in turn control have the physiological role of controlling the intrahepatic and extrahepatic levels of these compounds. Thus, these receptors regulate the metabolism and excretion of these compounds. Note that a relatively high redundancy exists for several substance classes to bind to multiple receptors.
On the other hand, PPAR was initially named after a lipid-lowering drug that causes peroxisome proliferation was able to activate the receptor. The three known PPAR subtypes are PPARα, PPARβ, and PPARδ, which help support fatty acid oxidation, and PPARγ, which aids in maintaining glucose homeostasis by increasing insulin sensitivity [97]. These receptors have been regarded as promising therapeutic targets in the treatment for obesity and diabetes. Similarly, LXRs have been shown to play a significant role in glucose homeostasis and metabolism by upregulating glucokinase and promoting glycogen synthesis [97]. Furthermore, LXRs respond to elevated levels of sterols to minimize buildup of cholesterol in the liver [80]. LXR and PPAR demonstrate the importance in human disease progression of establishing endogenous ligands to orphan receptors. Ultimately, the use of specific ligands for LXR and PPAR also offer an interesting and novel approach to treat atherosclerosis and type II diabetes, respectively [83, 91].
Much like CAR, Nurr1, from the NR4A subfamily of nuclear receptors, is structurally different from the other members of the superfamily. Although the ligand binding domain of Nurr1 is noticeably similar to that in any other nuclear receptor, it does not contain a cavity for ligand binding [100]. Therefore, Nurr1 cannot rely on a cognate ligand for its regulation, but rather by alternative mechanisms, leaving Nurr1 as an orphan NR. Nurr1 is expressed primarily in the central nervous system and is known to be essential for the development, survival, and migration of dopaminergic neurons [83, 101]. Because Parkinson’s disease (PD) results from the loss of dopaminergic neurons, Nurr1 is suggested to play a role in pathogenesis of PD [83]. Not surprisingly, Nurr1 mutations have been linked to PD and dysregulated Nurr1 expression has been observed in PD midbrains [102]. Despite a lack of well-defined regulatory partners and the challenge this poses for disease treatment, Nurr1 demonstrates great potential as a therapeutic target for PD treatment options.
Identifying biological functions and physiological ligands that activate orphan receptors has broadened our knowledge about a variety of diseases implicated in activation of these receptors. Further dissecting signaling networks and the presence or absence of physiological ligands is of utmost importance to promote our understanding of disease progression involving orphan receptors.
Based upon phylogenetic analysis, the unified nomenclature system divides the nuclear receptor superfamily into six structural and distinct groups [86, 103]. The chronological discovery of orphan receptors in order of the discovery date of the first orphan in each family, the discoverer and adopted ligand determinations are summarized in Table 1.
Table 1:
The Orphan Receptor Subfamily.
| Unified Nomenclature | Common Abbreviations | Date Identified | Discovered By | Ligand |
|---|---|---|---|---|
| NR3B | Nreb1/ERRα | 1988 | [84] | |
| Nr3b2/Errβ | 1988 | [84] | ||
| Nr3b3/Errγ | 1998 | [109] | ||
| NR4A | Nr4a1/Nur77 (Ngfi-b, Tr3, N10, Nak-1, St-59), | 1988 | [110, 111] | |
| Nr4a2/Nurr1 (Rnr-1, Not, Tinur) | 1992 | [112] | ||
| Nr4a3/Nor1 (Tec, Minor, Chn) | 1995 | [113] | ||
| NR2F | Nr2f2/Coup-tfII | 1988 | [114] | |
| Nr2f6/Ear2 | 1988 | [114] | ||
| Nr2f1/Coup-tfI | 1989 | [115] | ||
| NR2C | Nr2c1/Tr2 | 1988 | [116] | |
| Nr2c2/Tr4 | 1994 | [117] | ||
| NR1D | Nr1d1/Rev-erbα | 1989 | [118] | Heme [119–122] |
| Nr1d2/Rev-erbβ | 1994 | [123] | ||
| NR2B | Nr2b1/Rxrα | 1990 | [90] | 9-CIS retinoic acid as well as interacting with other NR [89, 124–127] |
| Nr2b2/Rxrβ | 1991 | [128] | ||
| Nr2b3/Rxrγ | 1991 | [129] | ||
| NR2E | Nr2e1/T1x | 1990 | [130] | |
| Nr2e3/Pnr | 1999 | [131, 132] | ||
| NR1C | Nr1c1/Pparα | 1990 | [85] | Fatty acids [133, 134] |
| Nr1c2/Pparβ or δ | 1993 | [135] | ||
| Nr1c3/Pparγ | 1993 | [135] | ||
| NR5A | Nr5a/Sf1 | 1992 | [136] | Phospholipids [137] |
| Nr5a2/Lrh1 | 1992 | [138] | ||
| NR1F | Nr1f1/Rorα | 1993 | [139] | Sterols ? |
| Nr1f2/Rorβ | 1993 | [139] | ||
| Nr1f3/Rorγ | 1996 | [140]. | ||
| NR2A | Nr2a1/Hnfα | 1994 | [141] | Fatty acids ? [142] |
| Nr2a2/Hnfγ | 1996 | [143] | ||
| NR1I | Nr1i3/Car | 1994 | [144] | Xenobiotics and endobiotic [79] |
| Nr1i2/Pxr | 1998 | [145–148] | ||
| NR6A | Nr6a1/Gcnf | 1994 | [149] | |
| NR0B | Nr0b1/Dax-1 | 1994 | [150] | |
| Nr0b2/Shp | 1996 | [151] | ||
| NR1H | Nr1h2/Lxrβ | 1995 | [152] | Oxysterols and bile salts [78, 79] |
| Nr1h4/Fxrα | 1995 | [153] | ||
| Nr1h3/Lxrα | 1995 | [152] | ||
5. Future Challenges
Given the wide variety of processes controlled by nuclear receptors, their dysregulation usually contribute to the development and/or progression of numerous diseases, including cancer, immunosuppression, diabetes, infertility, pharmacologic intolerance, etc. Because most of these receptors bind small molecules able to regulate their biological activity, these receptors also represent promising therapeutic targets for which selective agonists and antagonists can be engineered [104]. In view of the fact that nuclear receptors regulate many genes in several tissues, novel synthetic ligands usually show beneficial therapeutic effects and unwanted side effects that limit clinical use. Major goals in the nuclear receptor field therefore include attaining a better understanding of the mechanisms underlying their actions in specific cell types and the ways in which these receptors selectively modulate their activities.
Selective nuclear receptor modulators also show partial agonist-antagonist activity. Thus, ligands with such particular features have been developed for a number of NRs, such as ER, AR or PPARs [105]. These properties are associated with differential recruitment of coactivators and corepressors and the tissue-selective expression profiles of these coregulators. A similar proposal has been made for the regulatory Hsp90-binding immunophilins that regulate steroid receptor and NF-kB function [45], Interestingly, there are cases where subtype selectivity and response element selectivity overlap in quite complex manners. A good example is the case of the ER ligand raloxifene, which acts as an antagonist of estradiol at a simple estrogen response element for both subtypes of the estrogen receptors, ERα and ERβ. However, at an AP1 response element the same ER ligand behaves like an agonist with the ERβ subtype, estradiol being an antagonist [106]. Binding studies evidence no difference in the affinity constant of raloxifene to either receptor subtype, leading to the conclusion that the differences must involve something subtler at the transactivating surfaces of the two ER subtypes at an AP-1 element.
Drugs that target nuclear receptors are to date among the most widely used and commercially successful. For example, bexarotene and alitretinoin (RXRs), fibrates (PPARα), and thiazolidinediones (PPARγ) are drugs approved for treating cancer, hyperlipidemia, and type 2 diabetes, respectively [107]. Looking out on the event horizon of small ligand discovery for nuclear receptors, it is noteworthy that LXR and FXR agonists are in development for treating non-alcoholic steatohepatitis and preventing atherosclerosis [108]. Perhaps just as importantly, PXR is now used routinely in the pharmaceutical industry to screen all new drug candidates for potentially dangerous drug-drug interactions. In line with this, the RXRs and similar receptor partners will become the next generation of relevant therapeutic targets.
From an evolutionary perspective, remarkable advances have been made in the field of nuclear receptors and their cognate ligands sheading light in our understanding of the evolutionary origins of life. This allowed a new view of nuclear receptors and their ligands to emerge. Clearly, the endocrine system is an issue of evolution that has prompted today’s biochemists to revise the old hypothesis that the hormone and its receptor could have been preexisting structures, the interaction of their corner stones being necessarily the result of evolution itself. Indeed, the information for hormonal regulation is written not only in the hormone structure, but also in the receptor, so that both components function as a unit. In higher organisms, the nuclear receptor superfamily bears a close resemblance to its primordial predecessor. On the other hand, signaling molecules seem to have acquired their present role in a long evolutionary process, which may well sharp the separation between close nuclear receptors partners such as GR and MR. This view of the modern day endocrine nuclear receptors evolving from more ancient receptors that originally sensed their environment or ligands that were first used for other purposes is not only consistent with the early events in the history of life in the Earth, but also with the prevalence of environmental signals that may act as endocrine disrupters via nuclear receptors. Similarly, these ancient properties could also be used to take advantage for the design of novel ligands that resemble those lost or attenuated biological activities.
Acknowledgements
The authors acknowledge the financial support of the following agencies: M.D.G. from ANPCyT (PICT 2016–0545), UBACYT and Instituto Nacional del Cáncer. G.I.M from ANPCyT (PICT 2016–2607). M.B.C., J.C.S and O.B.S. are partially supported by the Research Centers in Minority Institutions (RCMI) program, grant 2G12MD007592 to the Border Biomedical Research Center (BBRC) at UTEP, from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the NIH. M.B.C is also supported by the Department of Defense (DOD) Prostate Cancer Research Program (PCRP) through grant number W81XWH-17-1-0435.
Footnotes
Competing Interests
The authors declare no competing interests.
Editor: Alejandro De Nicola
References
- [1].Hoffmann JM and Partridge L, “Nuclear hormone receptors: Roles of xenobiotic detoxification and sterol homeostasis in healthy aging,” Critical Reviews in Biochemistry and Molecular Biology, vol. 50, no. 5, pp. 380–392. 2015. [DOI] [PubMed] [Google Scholar]
- [2].Ranhotra HS, “The orphan nuclear receptors in cancer and diabetes.” Journal of Receptors and Signal Transduction, vol. 33 no. 4, pp. 207–212. 2013. [DOI] [PubMed] [Google Scholar]
- [3].Lonai’d DM and O’Malley BW. “Nuclear receptor coregulators: Modulators of pathology and therapeutic targets.” Nature Reviews Endocrinology, vol. 8 no. 10, pp. 598–604. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Malek G and Lad EM. “Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration.” Cellular and Molecular Life Sciences, vol. 71 no. 23, pp. 4617–4636. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kadmiel M and Cidlowski JA, “Glucocorticoid receptor signaling in health and disease.” Trends in Pharmacological Sciences, vol. 34 no. 9, pp. 518–530. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oyekan A, “PPARs and their effects on the cardiovascular system.” Clinical and Experimental Hypertension, vol. 33 no. 5, pp. 287–293. 2011. [DOI] [PubMed] [Google Scholar]
- [7].Wrange O and Gustafsson JA, “Separation of the hormone- and DNA-binding sites of the hepatic glucocorticoid receptor by means of proteolysis.” The Journal of Biological Chemistry, vol. 253 no. 3, pp. 856–865. 1978. [PubMed] [Google Scholar]
- [8].Evans RM. “The steroid and thyroid hormone receptor superfamily.” Science, vol. 240 no. 4854, pp. 889–895. 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Escriva H, Bertrand S, and Laudet V. “The evolution of the nuclear receptor superfamily.” Essays in Biochemistry, vol. 40 pp. 11–26. 2004. [DOI] [PubMed] [Google Scholar]
- [10].Rastinejad F. Huang P Chandra V, and Khorasanizadeh S, “Understanding nuclear receptor form and function using structural biology.” Molecular Endocrinology, vol. 51 no. 3, pp. T1–T21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gustafsson J-A. “Historical overview of nuclear receptors.” The Journal of Steroid Biochemistry and Molecular Biology, vol. 157 pp. 3–6. 2016. [DOI] [PubMed] [Google Scholar]
- [12].Kumar R and McEwan IJ. “Allosteric modulators of steroid hormone receptors: Structural dynamics and gene regulation.” Endocrine Reviews, vol. 33 no. 2, pp. 271–299. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jensen EV. “Studies of growth phenomena using tritium labeled steroids.” in Proceedings of the Proc 4th International Congress Biochem, vol. 15 p. 119 1958. [Google Scholar]
- [14].Easton J. “Jensen wins Lasker for research on estrogen receptors.” The University of Chicago Chronicle, p. 24 2004. [Google Scholar]
- [15].Jensen EV. “On the Mechanism of Estrogen Action.” Perspectives in Biology and Medicine, vol. 6 no. 1, pp. 47–60. 1962. [DOI] [PubMed] [Google Scholar]
- [16].Hollenberg SM, Weinberger C, Ong ES et al. , “Primary structure and expression of a functional human glucocorticoid receptor cDNA,” Nature, vol. 318 no. 6047, pp. 635–641. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Green S. Walter P. Kumar V et al. , “Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A.” Nature, vol. 320 no. 6058, pp. 134–139. 1986. [DOI] [PubMed] [Google Scholar]
- [18].Arriza JL, Weinberger C, Cerelli G et al. , “Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor,” Science, vol. 237, no. 4812, pp. 268–275, 1987. [DOI] [PubMed] [Google Scholar]
- [19].Petkovich M, Brand NJ, Rrust A, and Chambon P, “A human retinoic acid receptor which belongs to the family of nuclear receptors,” Nature, vol. 330, no. 6147, pp. 444–450, 1987. [DOI] [PubMed] [Google Scholar]
- [20].Sladek FM, “What are nuclear receptor ligands?” Molecular and Cellular Endocrinology, vol. 334, no. 1–2, pp. 3–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].King N and et al. ,, “The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans,” Nature, vol. 451 (7180), pp. 783–788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fox D, “What sparked the Cambrian explosion?” Nature, vol. 530, no. 7590, pp. 268–270, 2016. [DOI] [PubMed] [Google Scholar]
- [23].Gawienowski AM and Gibbs CC, “Identification of cholesterol and progesterone in apple seeds,” Steroids, vol. 12, no. 4, pp. 545–550, 1968. [DOI] [PubMed] [Google Scholar]
- [24].Simons RG and Grinwich DL, “Immunoreactive detection of four mammalian steroids in plants,” Botany, vol. 67, no. 2, pp. 288–296, 1989. [Google Scholar]
- [25].Lindemann P, “Steroidogenesis in plants - Biosynthesis and conversions of progesterone and other pregnane derivatives,” Steroids, vol. 103, pp. 145–152, 2015. [DOI] [PubMed] [Google Scholar]
- [26].Weigel NL and Zhang Y, “Ligand-independent activation of steroid hormone receptors,” Journal of Molecular Medicine, vol. 76, no. 7, pp. 469–479, 1998. [DOI] [PubMed] [Google Scholar]
- [27].Zwijsen RML, Buckle RS, Hijmans EM, Loomans CJM, and Bernards R, “Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin Dl,” Genes & Development, vol. 12, no. 22, pp. 3488–3498, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bennesch MA and Picard D, “Minireview: Tipping the balance: ligand-independent activation of steroid receptors,” Molecular Endocrinology, vol. 29, no. 3, pp. 349–363, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nuclear Receptors Nomenclature C, “A unified nomenclature system for the nuclear receptor superfamily,” Cell, vol. 97, no. 2, pp. 161–163, 1999. [DOI] [PubMed] [Google Scholar]
- [30].Glascock RF and Hoekstra WG, “Selective accumulation of tritium-labelled hexoestrol by the reproductive organs of immature female goats and sheep.,” Biochemical Journal, vol. 72, pp. 673–682, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thornton JW, “Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 98, no. 10, pp. 5671–5676, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Becker HJ, “[The puffs of salivary gland chromosomes of Drosophilia melanogaster. Part 1. Observations on the behavior of a typical puff in the normal strain and in two mutants, giant and lethal giant larvae],” Chromosoma, vol. 10, pp. 654–78, 1959. [DOI] [PubMed] [Google Scholar]
- [33].Clever U and Karlson P, “[Induction of puff changes in the salivary gland chromosomes of Chironomus tentans by ecdysone],” Exp Cell Res, vol. 20, p. 623, 1960. [DOI] [PubMed] [Google Scholar]
- [34].Toft D and Gorski J, “A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization.,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 55, no. 6, pp. 1574–1581, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Toft D, Shyamala G, and Gorski J, “A receptor molecule for estrogens: studies using a cell-free system.,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 57, no. 6, pp. 1740–1743, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ellis RJ, “Discovery of molecular chaperones.,” Cell stress & chaperones, vol. 1, no. 3, pp. 155–160, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Quinta HR, Galigniana NM, Erlejman AG, Lagadari M, Piwien-Pilipuk G, and Galigniana MD, “Management of cytoskeleton architecture by molecular chaperones and immunophilins,” Cellular Signalling, vol. 23, no. 12, pp. 1907–1920, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Galigniana M, “Editorial (Thematic Issue: The Biology of Molecular Chaperones - Very Complex Activities for Quite Simple Proteins),” Current Protein & Peptide Science, vol. 15, no. 3, pp. 169–170, 2014. [DOI] [PubMed] [Google Scholar]
- [39].Ritossa FA, “A new puffing pattern induced by temperature shock and DNP in drosophila,” Experientia, vol. 18, no. 12, pp. 571–573, 1962. [Google Scholar]
- [40].De Maio A, Gabriella Santoro M, Tanguay RM, and Hightower LE, “Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: A new view of biology, a new society, and a new journal,” Cell Stress and Chaperones, vol. 17, no. 2, pp. 139–143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Galigniana MD, Echeverria PC, Erlejman AG, and Piwien-Pilipuk G, “Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore,” Nucleus, vol. 1, no. 4, pp. 299–308, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Echeverria PC and Picard Didier D, “Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility,” Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, vol. 1803, no. 6, pp. 641–649, 2010. [DOI] [PubMed] [Google Scholar]
- [43].Pratt WB, Galigniana MD, Harrell JM, and DeFranco DB, “Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement,” Cellular Signalling, vol. 16, no. 8, pp. 857–872, 2004. [DOI] [PubMed] [Google Scholar]
- [44].Erlejman AG, De Leo SA, Mazaira GI et al. , “NF-κΒ transcriptional activity is modulated by FK506-Binding proteins FKBP51 and FKBP52:A role for peptidyl-prolyl isomerase activity,” The Journal of Biological Chemistry, vol. 289, no. 38, pp. 26263–26276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lagadari M, De Leo SA, Camisay MF, Galigniana MD, and Erlejman AG, “Regulation of NF-kB signalling cascade by immunophilins,” Current Molecular Pharmacology, vol. 9, no. 2, pp. 99–108, 2015. [DOI] [PubMed] [Google Scholar]
- [46].Schuster M, Schnell L, Feigl P et al. , “The Hsp90 machinery facilitates the transport of diphtheria toxin into human cells,” Scientific Reports, vol. 7, no. 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vafopoulou X and Steel CGH, “Cytoplasmic travels of the ecdysteroid receptor in target cells: Pathways for both genomic and non-genomic actions,” Frontiers in Endocrinology, vol. 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wrange O, Carlstedt-Duke J, and Gustafsson JA, “Purification of the glucocorticoid receptor from rat liver cytosol,” The Journal of Biological Chemistry, vol. 254, no. 18, pp. 9284–9290, 1979. [PubMed] [Google Scholar]
- [49].Payvar F, Wrange O, Carlstedt-Duke J, Okret S, Gustafsson JA, and Yamamoto KR, “Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo.,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 78, no. 11, pp. 6628–6632, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Denis M, Gustafsson J-A, and Wikstrom A-C, “Interaction of the M(r) = 90,000 heat shock protein with the steroid-binding domain of the glucocorticoid receptor,” The Journal of Biological Chemistry, vol. 263, no. 34, pp. 18520–18523, 1988. [PubMed] [Google Scholar]
- [51].Renoir J-M, Buchou T, and Baulieu E-E, “Ihvolvement of a Non-Hormone-Binding 90-Kilodalton Protein in the Nontransformed 8S Form of the Rabbit Uterus Progesterone Receptor,” Biochemistry, vol. 25, no. 21, pp. 6405–6413, 1986. [DOI] [PubMed] [Google Scholar]
- [52].Catelli MG et al. , “The common 90-kd protein component of non-transformed ‘8S’ steroid receptors is a heat-shock protein,” EMBO J, vol. 4, no. 12, p. 3131, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schuh S, Yonemoto W, Brugge J et al. , “A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60(v-src),” The Journal of Biological Chemistry, vol. 260, no. 26, pp. 14292–14296, 1985. [PubMed] [Google Scholar]
- [54].Sanchez et ah ER, “Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein,” J Biol Chem, vol. 260, no. 23, pp. 12398–401, 1985. [PubMed] [Google Scholar]
- [55].Mendel DB et al. , “Molybdate-stabilized nonactivated glucocorticoid-receptor complexes contain a 90-kDa non-steroid-binding phosphoprotein that is lost on activation,” J Biol Chem, vol. 261, no. 8, pp. 3758–63, 1986. [PubMed] [Google Scholar]
- [56].Hickey E, Brandon SE, Smale G, Lloyd D, and Weber LA, “Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein,” Molecular and Cellular Biology, vol. 9, no. 6, pp. 2615–2626, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rebbe NF, Ware J, Bertina RM, Modrich R, and Stafford DW, “Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family,” Gene, vol. 53, no. 2–3, pp. 235–245, 1987. [DOI] [PubMed] [Google Scholar]
- [58].Bardwell JC and Craig EA, “Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli.,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 84, no. 15, pp. 5177–5181, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Perdew GH, Hord N, Hollenback CE, and Welsh MJ, “Localization and Characterization of the 86- and 84-kDa Heat Shock Proteins in Hepa lclc7 Cells,” Experimental Cell Research, vol. 209, no. 2, pp. 350–356, 1993. [DOI] [PubMed] [Google Scholar]
- [60].Welch WJ et al. , “Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins,” J Biol Chem, vol. 258, no. 11, pp. 7102–11, 1983. [PubMed] [Google Scholar]
- [61].Erlejman AG, Lagadari M, Toneatto J, Piwien-Pilipuk G, and Galigniana MD, “Regulatory role of the 90-kDa-heat-shockprotein (Hsp90) and associated factors on gene expression,” Biochimica et Biophysica Acta, vol. 1839, no. 2, pp. 71–87, 2014. [DOI] [PubMed] [Google Scholar]
- [62].Schopf FH, Biebl ΜM, and Buchner J, “The HSP90 chaperone machinery,” Nature Reviews Molecular Cell Biology, vol. 18, no. 6, pp. 345–360, 2017. [DOI] [PubMed] [Google Scholar]
- [63].Fares MA, “Survival and innovation: The role of mutational robustness in evolution,” Biochimie, vol. 119, pp. 254–261, 2015. [DOI] [PubMed] [Google Scholar]
- [64].Mazaira GI, Camisay MF, De Leo S, Erlejman AG, and Galigniana MD, “Biological relevance of Hsp90-binding immunophilins in cancer development and treatment,” International Journal of Cancer, vol. 138, no. 4, pp. 797–808, 2016. [DOI] [PubMed] [Google Scholar]
- [65].Taldone T, Ochiana SO, Patel RD, and Chiosis G, “Selective targeting of the stress chaperome as a therapeutic strategy,” Trends in Pharmacological Sciences, vol. 35, no. 11, pp. 592–603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kim T, Keum G, and Pae AN, “Discovery and development of heat shock protein 90 inhibitors as anticancer agents: A review of patented potent geldanamycin derivatives,” Expert Opinion on Therapeutic Patents, vol. 23, no. 8, pp. 919–943, 2013. [DOI] [PubMed] [Google Scholar]
- [67].Smith DF, Schowalter DB, Kost SL, and Toft DO, “Reconstitution of progesterone receptor with heat shock proteins,” Molecular Endocrinology, vol. 4, no. 11, pp. 1704–1711, 1990. [DOI] [PubMed] [Google Scholar]
- [68].Hutchison KA, Brott BK, De Leon JH, Perdew GH, Jove R, and Pratt WB, “Reconstitution of the multiprotein complex of pp60src, hsp90, and p50 in a cell-free system,” The Journal of Biological Chemistry, vol. 267, no. 5, pp. 2902–2908, 1992. [PubMed] [Google Scholar]
- [69].Dittmar KD, Banach M, Galigniana MD, and Pratt WB, “The role of DnaJ-like proteins in glucocorticoid receptor-hsp90 Heterocomplex assembly by the reconstituted hsp90-p60-hsp70 Foldosome complex,” The Journal of Biological Chemistry, vol. 273, no. 13, pp. 7358–7366, 1998. [DOI] [PubMed] [Google Scholar]
- [70].Smith DF, Sullivan WP, Marion TN et al. , “Identification of a 60-Kilodalton Stress-Related Protein, p60. Which Interacts with hsp90 and hsp70,” Molecular and Cellular Biology, vol. 13, no. 2, pp. 869–876, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gallo LI, Ghini AA, Pilipuk GP, and Galigniana MD, “Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity,” Biochemistry, vol. 46, no. 49, pp. 14044–14057, 2007. [DOI] [PubMed] [Google Scholar]
- [72].Sivils JC, Storer CL, Galigniana MD, and Cox ΜB, “Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52),” Current Opinion in Pharmacology, vol. 11, no. 4, pp. 314–319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Storer CL, Dickey CA, Galigniana MD, Rein T, and Cox ΜB, “FKBP51 and FKBP52 in signaling and disease,” Trends in Endocrinology & Metabolism, vol. 22, no. 12, pp. 481–490, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Young JC, Obermann WMJ, and Hartl FU, “Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90,” The Journal of Biological Chemistry, vol. 273, no. 29, pp. 18007–18010, 1998. [DOI] [PubMed] [Google Scholar]
- [75].Silverstein AM, Galigniana MD, Kanelakis KC, Radanyi C, Renoir J-M’, and Pratt WB, “Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein,” The Journal of Biological Chemistry, vol. 274, no. 52, pp. 36980–36986, 1999. [DOI] [PubMed] [Google Scholar]
- [76].Galigniana NM, Ballmer LT, Toneatto J, Erlejman AG, Lagadari M, and Galigniana MD, “Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51,” Journal of Neurochemistry, vol. 122, no. 1, pp. 4–18, 2012. [DOI] [PubMed] [Google Scholar]
- [77].Galigniana MD, Harrell JM, Murphy PJM et al. , “Binding of hsp90-associated immunophilins to cytoplasmic dynein: Direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain,” Biochemistry, vol. 41, no. 46, pp. 13602–13610, 2002. [DOI] [PubMed] [Google Scholar]
- [78].Aranda A and Pascual A, “Nuclear hormone receptors and gene expression,” Physiological Reviews, vol. 81, no. 3, pp. 1269–1304, 2001. [DOI] [PubMed] [Google Scholar]
- [79].Evans RM and Mangelsdorf DJ, “Nuclear receptors, RXR, and the big bang,” Cell, vol. 157, no. 1, pp. 255–266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mullican SE, DiSpirito JR, and Lazar MA, “The orphan nuclear receptors at their’ 25-year reunion,” Molecular Endocrinology, vol. 51, no. 3, pp. T115–T140, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Giguere V, Hollenberg SM, Rosenfeld MG, and Evans RM, “Functional domains of the human glucocorticoid receptor,” Cell, vol. 46, no. 5, pp. 645–652, 1986. [DOI] [PubMed] [Google Scholar]
- [82].Mangelsdorf DJ, Thummel C, Beato M et al. , “The nuclear receptor super-family: the second decade,” Cell, vol. 83, no. 6, pp. 835–839, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Willson TM and Moore JT, “Genomics versus orphan nuclear receptors - A half-time report,” Molecular Endocrinology, vol. 16, no. 6, pp. 1135–1144, 2002. [DOI] [PubMed] [Google Scholar]
- [84].Giguere V, Yang N, Segui R, and Evans RM, “Identification of a new class of steroid hormone receptors,” Nature, vol. 331, no. 6151, pp. 91–94, 1988. [DOI] [PubMed] [Google Scholar]
- [85].Issemann I and Green S, “Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators,” Nature, vol. 347, no. 6294, pp. 645–650, 1990. [DOI] [PubMed] [Google Scholar]
- [86].Roberts-Thomson SJ, “Peroxisome proliferator-activated receptors in tumorigenesis: targets of tumour promotion and treatment,” Immunology & Cell Biology, vol. 78, no. 4, pp. 436–441, 2000. [DOI] [PubMed] [Google Scholar]
- [87].Kliewer SA, Lehmann JM, and Willson TM, “Orphan nuclear receptors: Shifting endocrinology into reverse,” Science, vol. 284, no. 5415, pp. 757–760, 1999. [DOI] [PubMed] [Google Scholar]
- [88].Heyman RA, Mangelsdorf DJ, Dyck JA et al. , “9-cis retinoic acid is a high affinity ligand for the retinoid X receptor,” Cell, vol. 68, no. 2, pp. 397–406, 1992. [DOI] [PubMed] [Google Scholar]
- [89].Mangelsdorf DJ, Borgmeyer U, Heyman RA et al. , “Characterization of three RXR genes that mediate the action of 9-cis retinoic acid,” Genes & Development, vol. 6, no. 3, pp. 329–344, 1992. [DOI] [PubMed] [Google Scholar]
- [90].Mangelsdorf DJ, Ong ES, Dyck JA, and Evans RM, “Nuclear receptor that identifies a novel retinoic acid response pathway,” Nature, vol. 345, no. 6272, pp. 224–229, 1990. [DOI] [PubMed] [Google Scholar]
- [91].Shi Y, “Orphan nuclear receptors in drug discovery,” Drug Discovery Therapy, vol. 12, no. 11–12, pp. 440–445, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Langmade SJ, Gale SE, Frolov A et al. , “Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 103, no. 37, pp. 13807–13812, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xie W, Radominska-Pandya A, Shi Y et al. , “An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 98, no. 6, pp. 3375–3380, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Goodwin B, Gauthier KC, Umetani M et al. , “Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 100, no. 1, pp. 223–228, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Jones SA, Moore LB, Shenk JL et al. , “The pregnane X receptor: A promiscuous xenobiotic receptor that has diverged during evolution,” Molecular Endocrinology, vol. 14, no. 1, pp. 27–39, 2000. [DOI] [PubMed] [Google Scholar]
- [96].Handschin C and Meyer UA, “Regulatory network of lipid-sensing nuclear receptors: Roles for CAR, PXR, LXR, and FXR,” Archives of Biochemistry and Biophysics, vol. 433, no. 2, pp. 387–396, 2005. [DOI] [PubMed] [Google Scholar]
- [97].Nanduri R, Bhutani I, Somavarapu AK, Mahajan S, Parkesh R, and Gupta P, “ONRLDB - Manually curated database of experimentally validated ligands for orphan nuclear receptors: Insights into new drug discovery,” Database, vol. 2015, no. 1, Article ID 112015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].di Masi A, Marinis ED, Ascenzi P, and Marino M, “Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects,” Molecular Aspects of Medicine, vol. 30, no. 5, pp. 297–343, 2009. [DOI] [PubMed] [Google Scholar]
- [99].Timsit YE and Negishi M, “CAR and PXR: The xenobiotic-sensing receptors,” Steroids, vol. 72, no. 3, pp. 231–246, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Germain P, Staels B, Dacquet C, Spedding M, and Laudet V, “Overview of nomenclature of nuclear receptors,” Pharmacological Reviews, vol. 58, no. 4, pp. 685–704, 2006. [DOI] [PubMed] [Google Scholar]
- [101].Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, and Perlmann T, “Dopamine neuron agenesis in Nurrl-deficient mice,” Science, vol. 276, no. 5310, pp. 248–250, 1997. [DOI] [PubMed] [Google Scholar]
- [102].Jankovic J, Chen S, and Le WD, “The role of Nurrl in the development of dopaminergic neurons and Parkinson’s disease,” Progress in Neurobiology, vol. 77, no. 1–2, pp. 128–138, 2005. [DOI] [PubMed] [Google Scholar]
- [103].Laudet V, “Evolution of the nuclear receptor superfamily: Early diversification from an ancestral orphan receptor,” Molecular Endocrinology, vol. 19, no. 3, pp. 207–226, 1997. [DOI] [PubMed] [Google Scholar]
- [104].Burris TP, Busby SA, and Griffin PR, “Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity,” Chemistry & Biology, vol. 19, no. 1, pp. 51–59, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Smith CL and O’Malley BW, “Coregulator Function: A Key to Understanding Tissue Specificity of Selective Receptor Modulators,” Endocrine Reviews, vol. 25, no. 1, pp. 45–71, 2004. [DOI] [PubMed] [Google Scholar]
- [106].Paech K, Webb P, Kuiper GGJM et al. , “Differential ligand activation of estrogen receptors ERα and ERrβ at API sites,” Science, vol. 277, no. 5331, pp. 1508–1510, 1997. [DOI] [PubMed] [Google Scholar]
- [107].Moore JT, Collins JL, and Pearce KH, “The nuclear receptor superfamily and drug discovery,” ChemMedChem, vol. 1, no. 5, pp. 504–523, 2006. [DOI] [PubMed] [Google Scholar]
- [108].Calkin AC and Tontonoz P, “Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR,” Nature Reviews Molecular Cell Biology, vol. 13, no. 4, pp. 213–224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Eudy JD, Yao S, Weston MD et al. , “Isolation of a gene encoding a novel member of the nuclear receptor superfamily from the critical region of usher syndrome type IIa at 1q41,” Genomics, vol. 50, no. 3, pp. 382–384, 1998. [DOI] [PubMed] [Google Scholar]
- [110].Hazel TG, Nathans D, and Lau LF, “A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 85, no. 22, pp. 8444–8448, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Milbrandt J, “Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene,” Neuron, vol. 1, no. 3, pp. 183–188, 1988. [DOI] [PubMed] [Google Scholar]
- [112].Law SW, Conneely ΟM, DeMayo FJ, and O’Malley BW, “Identification of a new brain-specific transcription factor, nurr1,” Molecular Endocrinology, vol. 6, no. 12, pp. 2129–2135, 1992. [DOI] [PubMed] [Google Scholar]
- [113].Hedvat CV and Ewing SG, “The isolation and characterization of MINOR, a novel mitogen-inducible nuclear orphan receptor,” Molecular Endocrinology, vol. 9, no. 12, pp. 1692–1700, 1995. [DOI] [PubMed] [Google Scholar]
- [114].Miyajtma N, Kandowaki Y, Fukushige S-I et al. , “Identification of two novel members of erbA superfamily by molecular cloning: The gene products of the two are highly related to each other,” Nucleic Acids Research, vol. 16, no. 23, pp. 11057–11074, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang L-H, Tsai SY, Cook RG, Beattie WG, Tsai M-J, and O’Malley BW, “COUP transcription factor is a member of the steroid receptor superfamily,” Nature, vol. 340, no. 6229, pp. 163–166, 1989. [DOI] [PubMed] [Google Scholar]
- [116].Chang C and Kokontis J, “Identification of a new member of the steroid receptor super-family by cloning and sequence analysis,” Biochemical and Biophysical Research Communications, vol. 155, no. 2, pp. 971–977,1988. [DOI] [PubMed] [Google Scholar]
- [117].Chang C, Da Silva SL, Ideta R, Lee Y, Yeh S, and Burbach JPH, “Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 91, no. 13, pp. 6040–6044, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lazar MA, Hodin RA, Darling DS, and Chin WW, “A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit.,” Molecular and Cellular Biology, vol. 9, no. 3, pp. 1128–1136, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Burris TΡ, “Nuclear hormone receptors for heme: REV-ERBα and REV-ERBβ are ligand-regulated components of the mammalian clock,” Molecular Endocrinology, vol. 22, no. 7, pp. 1509–1520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Raghuram S, Stayrook KR, Huang P et al. , “Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ,”Nature Structural & Molecular Biology, vol. 14, no. 12, pp. 1207–1213, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Reinking J, “The Nuclear Receptor E75 Contains Heme and Is Gas Responsive,” in The Drosophila Nuclear Receptor E75 Contains Heme and Is Gas Responsive, vol. 122, pp. 195–207, Cell, The Nuclear Receptor E75 Contains Heme and Is Gas Responsive, 2005. [DOI] [PubMed] [Google Scholar]
- [122].Yin L, Wu N, Curtin JC et al. , “Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways,” Science, vol. 318, no. 5857, pp. 1786–1789, 2007. [DOI] [PubMed] [Google Scholar]
- [123].Dumas B, Harding HP, Choi H-S et al. , “A new orphan member of the nuclear hormone receptor superfamily closely related to rev-Erb,” Molecular Endocrinology, vol. 8, no. 8, pp. 996–1005, 1994. [DOI] [PubMed] [Google Scholar]
- [124].Kliewer SA, Umesono K, Mangelsdorf DJ, and Evans RM, “Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling,” Nature, vol. 355, no. 6359, pp. 446–449, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Leid M, Kastner P, Lyons R et al. , “Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently,” Cell, vol. 68, no. 2, pp. 377–395, 1992. [DOI] [PubMed] [Google Scholar]
- [126].Xanthoudakis S and Curran T, “Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity,” EMBO Journal, vol. 11, no. 2, pp. 653–665, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yu VC, Delsert C, Andersen B et al. , “RXRβ: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements,” Cell, vol. 67, no. 6, pp. 1251–1266,1991. [DOI] [PubMed] [Google Scholar]
- [128].Rowe A, Eager NSC, and Brickell PM, “A member of the RXR nuclear receptor family is expressed in neural-crest-derived cells of the developing chick peripheral nervous system,” Development, vol. 111, no. 3, pp. 771–778, 1991. [DOI] [PubMed] [Google Scholar]
- [129].Yu VC, RXR β: A coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements, vol. 67, Cell, and vitamin D receptors to their’ cognate response elements, 1991. [DOI] [PubMed] [Google Scholar]
- [130].Pignoni F, Baldarelli RM, Steingrimsson E et al. , “The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily,” Cell, vol. 62, no. 1, pp. 151–163, 1990. [DOI] [PubMed] [Google Scholar]
- [131].Chen F, Figueroa DJ, Marmorstein AD et al. , “Retina-specific nuclear receptor: A potential regulator of cellular retinaldehyde-binding protein expressed in retinal pigment epithelium and Muller glial cells,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 96, no. 26, pp. 15149–15154, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kobayashi M, Takezawa S-I, Hara K et al. , “Identification of a photoreceptor cell-specific nuclear receptor,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 96, no. 9, pp. 4814–4819, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nolte RT, Wisely GB, Westin S et al. , “Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ,” Nature, vol. 395, no. 6698, pp. 137–143, 1998. [DOI] [PubMed] [Google Scholar]
- [134].Uppenberg J, Svensson C, Jaki M, Bertilsson G, Jendeberg L, and Berkenstam A, “Crystal structure of the ligand binding domain of the human nuclear receptor PPARγ,” The Journal of Biological Chemistry, vol. 273, no. 47, pp. 31108–31112, 1998. [DOI] [PubMed] [Google Scholar]
- [135].Dreyer C, Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors, vol. 68, Cell, oxidation pathway by a novel family of nuclear hormone receptors, 1993. [DOI] [PubMed] [Google Scholar]
- [136].Lala DS, Rice DA, and Parker KL, “Steroidogenic factor i, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor i,” Molecular Endocrinology, vol. 6, no. 8, pp. 1249–1258, 1992. [DOI] [PubMed] [Google Scholar]
- [137].Lee JM et al. , “Antidiabetic actions of a phosphatidylcholine ligand for nuclear receptor LRH-1,” Nature, vol. 474, no. 7352, pp. 506–510, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tsukiyama T, Ueda H, Hirose S, and Niwa O, “Embryonal Long Terminal Repeat-Binding Protein Is a Murine Homolog of FTZ-F1, a Member of the Steroid Receptor Superfamily,” Molecular and Cellular Biology, vol. 12, no. 3, pp. 1286–1291, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Becker-André M, André E, and DeLamarter JF, “Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences,” Biochemical and Biophysical Research Communications, vol. 194, no. 3, pp. 1371–1379, 1993. [DOI] [PubMed] [Google Scholar]
- [140].Medvedev A, Yan Z-H, Hirose T, Giguère V, and Jetten AM, “Cloning of a cDNA encoding the murine orphan receptor RZR/RORγ and characterization of its response element,” Gene, vol. 181, no. 1–2, pp. 199–206, 1996. [DOI] [PubMed] [Google Scholar]
- [141].Chartier FL, Bossu J-P, Laudet V, Fruchart J-C, and Laine B, “Cloning and sequencing of cDNAs encoding the human hepatocyte nuclear factor 4 indicate the presence of two isoforms in human liver,” Gene, vol. 147, no. 2, pp. 269–272, 1994. [DOI] [PubMed] [Google Scholar]
- [142].Dhe-Paganon S, Duda K, Iwamoto M, Chi Y-I, and Shoelson SE, “Crystal structure of the HNF4α ligand binding domain in complex with endogenous fatty acid ligand,” The Journal of Biological Chemistry, vol. 277, no. 41, pp. 37973–37976, 2002. [DOI] [PubMed] [Google Scholar]
- [143].Drewes T, Senkel S, Holewa B, and Ryffel GU, “Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes,” Molecular and Cellular Biology, vol. 16, no. 3, pp. 925–931, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Baes M, Gulick T, Choi H-S, Martinolu MG, Simha D, and Moore DD, “A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements,” Molecular and Cellular Biology, vol. 14, no. 3, pp. 1544–1552, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Bertilsson G, Heidrich J, Svensson K et al. , “Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction,” Proceedings of the National Acadamy of Sciences of the United States of America, vol. 95, no. 21, pp. 12208–12213, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Blumberg B and Evans RM, “Orphan nuclear receptors - New ligands and new possibilities,” Genes & Development, vol. 12, no. 20, pp. 3149–3155, 1998. [DOI] [PubMed] [Google Scholar]
- [147].Kliewer SA, Moore JT, Wade L et al. , “An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway,” Cell, vol. 92, no. 1, pp. 73–82, 1998. [DOI] [PubMed] [Google Scholar]
- [148].Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, and Kliewer SA, “The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions,” The Journal of Clinical Investigation, vol. 102, no. 5, pp. 1016–1023, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chen F, Cooney AJ, Wang Y, Law SW, and O’Malley BW, “Cloning of a novel orphan receptor (gcnf) expressed during germ cell development,” Molecular Endocrinology, vol. 8, no. 10, pp. 1434–1444, 1994. [DOI] [PubMed] [Google Scholar]
- [150].Zanaria E, Muscatelli F, Bardoni B et al. , “An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita,” Nature, vol. 372, no. 6507, pp. 635–641, 1994. [DOI] [PubMed] [Google Scholar]
- [151].Seol W, Choi H-S, and Moore DD, “An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors,” Science, vol. 272, no. 5266, pp. 1336–1339, 1996. [DOI] [PubMed] [Google Scholar]
- [152].Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, and Mangelsdorf DJ, “LXR, a nuclear receptor that defines a distinct retinoid response pathway,” Genes & Development, vol. 9, no. 9, pp. 1033–1045, 1995. [DOI] [PubMed] [Google Scholar]
- [153].Forman BM, Goode E, Chen J et al. , “Identification of a nuclear receptor that is activated by farnesol metabolites,” Cell, vol. 81, no. 5, pp. 687–693, 1995. [DOI] [PubMed] [Google Scholar]


