Abstract
Purpose
Na,K-ATPase activity in lens epithelium is subject to control by Src family tyrosine kinases (SFKs). Previously we showed hyposmotic solution causes an SFK-dependent increase in Na,K-ATPase activity in the epithelium. Here we explored the role of cAMP in the signaling mechanism responsible for the SFK and Na,K-ATPase response.
Methods
Intact porcine lenses were exposed to hyposmotic Krebs solution (200 mOsm) then the epithelium was assayed for cAMP, SFK phosphorylation (activation) or Na,K-ATPase activity.
Results
An increase of cAMP was observed in the epithelium of lenses exposed to hyposmotic solution. In lenses exposed to hyposmotic solution SFK phosphorylation in the epithelium approximately doubled as did Na,K-ATPase activity and both responses were prevented by H89, a protein kinase A inhibitor. The magnitude of the SFK response to hyposmotic solution was reduced by a TRPV4 antagonist HC067047 added to prevent TRPV4-mediated calcium entry, and by a cytoplasmic Ca2+ chelator BAPTA-AM. The Na,K-ATPase activity response in the epithelium of lenses exposed to hyposmotic solution was abolished by BAPTA-AM. As a direct test of cAMP-dependent SFK activation, intact lenses were exposed to 8-pCPT-cAMP, a cell-permeable cAMP analog. 8-pCPT-cAMP caused robust SFK activation. Using Western blot, two calcium-activated adenylyl cyclases, ADCY3 and ADCY8, were detected in lens epithelium.
Conclusions
Calcium-activated adenylyl cyclases are expressed in the lens epithelium and SFK activation is linked to a rise of cAMP that occurs upon hyposmotic challenge. The findings point to cAMP as a link between TRPV4 channel-mediated calcium entry, SFK activation, and a subsequent increase of Na,K-ATPase activity.
Keywords: calcium-activated adenylyl cyclase; cAMP; lens; Na,K-ATPase; Src family kinase
Lens ion and water homeostasis is of paramount importance to maintenance of its transparency and hence normal vision. Active ion transport by Na,K-ATPase in the monolayer of lens epithelium plays a critically important role1 because the mature lens fiber cells, which make up the bulk of the lens, have little or no Na,K-ATPase activity.2 There is still much to be learned about how the epithelium matches its Na,K-ATPase activity to the needs of the fiber mass. In studies on the signaling mechanisms that modulate Na,K-ATPase activity in the epithelium, we discovered that a Src family tyrosine kinase (SFK)-dependent signaling mechanism is responsible for an increase in Na,K-ATPase activity in the epithelium that occurs when the intact lens is subjected to hyposmotic stress.3 We found evidence that the SFK phosphorylation and Na,K-ATPase activity responses were dependent on TRPV4 channel activation and hemichannel opening in the epithelium of lenses exposed to hyposmotic solution.3 In cultured lens epithelium, we showed that TRPV4 activation and opening of connexin hemichannels caused a sustained increase in cytoplasmic calcium concentration.4
An increase in cytoplasmic calcium concentration is a common and versatile step in many cellular signaling cascades.5 One of the distinguishing features of calcium signaling is crosstalk with other signaling pathways. For example, increased cytoplasmic calcium can act directly or through Ca2+/calmodulin or Protein Kinase C (PKC) to regulate certain members of adenylate cyclase family, the so-called calcium-sensitive isoforms6–8 or Ca2+/calmodulin-sensitive phosphodiesterase PDE1.9 This has the consequence of changing cytoplasmic cAMP concentrations. Because SFK activation is known in some tissues to be cAMP-dependent, and protein kinase A (PKA)-dependent,10,11 we examined the contribution of cAMP/PKA-dependent SFK activation to the signaling response in lens epithelium. The purpose of the present study was to explore cAMP in the context of the signaling mechanism responsible for the activation of SFK and Na,K-ATPase activity in the epithelium of intact porcine lenses subjected to a hyposmotic challenge. We show that the lens epithelium expresses two different calcium-dependent adenylyl cylases and provides several lines of evidence that point to cAMP/PKA-dependent SFK activation.
Materials and Methods
Ouabain, HC067047, ODQ, IBMX, H89, alamethicin, Dowex 50W x4-400 mesh cation exchange resin, and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). BAPTA-AM was purchased from Invitrogen (Carlsbad, CA, USA). Other chemicals including those for preparing the Krebs solution were purchased from Sigma-Aldrich Corp. Rabbit polyclonal antiphospho-Src-family (Tyr 416) kinase antibody and rabbit polyclonal anti-β-actin antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal antiadenylate cyclase 1 (ADCY1), antiadenylate cyclase 3 (ADCY3), and antiadenylate cyclase 8 (ADCY8) were purchased from Biorbyt (San Francisco, CA, USA). Goat antirabbit secondary antibody conjugated with IRDye 680 and goat antimouse secondary antibody conjugated with IR Dye 800 were obtained from LI-COR Biosciences (Lincoln, NE, USA). A biotinylated protein ladder along with antibiotin antibody was purchased from Cell Signaling Technology. Goat antirabbit IgG-HRP-conjugated secondary antibody was purchased from Santa Cruz Biotechnology, Inc. (Fort Worth, TX, USA). Super Signal west Pico Chemiluminescent substrate was obtained from Thermo Scientific (Waltham, MA, USA) and Amersham Hyper Film ECL was purchased from GE Health Care Ltd. (Pittsburgh, PA, USA). Adenosine 3′5′-cyclic phosphate, [5′,8-3H] (cAMP,[5′,8-3H]) and a cAMP radioimmunoassay kit (cAMP [125I] RIA Kit) were purchased from Perkin Elmer (Waltham, MA, USA). The Micro Bicinchoninic Acid (BCA) Protein Assay Kit was purchased from Thermo Scientific Pierce.
Krebs Solution Composition
Intact lenses were incubated at 37°C in control (300 mOsm) Krebs solution that contained (in mM) 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose. Prior to use, the solution was equilibrated with 5% CO2 for 40 minutes and adjusted to pH 7.4. In specified experiments, lenses were exposed to hyposmotic Krebs solution (200 mOsm) in which the NaCl concentration was reduced to 69 mM.
Intact Lenses
Pig eyes were obtained from the University of Arizona Meat Science Laboratory or from West Valley Processing Meat processors (Buckeye, AZ, USA), or Hatfield Quality Meats (Philadelphia, PA, USA). Human donor eyes were purchased from National Disease Research Interchange (Philadelphia, PA, USA), and the use was approved by the Institutional Review Board of the University of Arizona. The use of porcine tissue was approved by the University of Arizona Institutional Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The posterior of the eye was dissected open and the zonules were cut, allowing the intact lens to be removed and transferred to Krebs solution. Prior to use, lenses were allowed to equilibrate in Krebs solution for 3 hours at 37°C and 5% CO2 in a humidified incubator.
Na,K-ATPase Activity
The capsule-epithelium was removed from each lens and homogenized in 350 μL ice-cold double-strength ATPase assay buffer that contained (mM): L-Histidine, 80; NaCl 200; KCl, 10; MgCl2, 6.0; EGTA, 2.0 (pH 7.4) plus 10 μL/mL Protease Inhibitor Cocktail (Thermo Scientific). The homogenization protocol, which was carried out on ice, was four cycles of 15 seconds at 5-second intervals using a Misonix S3000 sonicator at a 6 W power setting (Misonix, Farmingdale, NY, USA). The homogenate was centrifuged at 13,000g for 30 minutes at 4°C to remove nuclei, mitochondria, and unbroken debris. Then the supernatant was frozen in liquid nitrogen and used at a later date to measure Na,K-ATPase activity. Protein in the supernatant was measured by the BCA assay12 (Pierce Biotechnology, Rockford, IL, USA), using bovine serum albumin as a standard.
Na,K-ATPase activity was measured using a modification of a method described earlier.13 In brief, 150 μL supernatant was placed in duplicate tubes containing 50 μL double-strength ice-cold Na,K-ATPase buffer. To ensure access of ions and ATP to membrane vesicles, alamethicin (5 μL) was added to give a final approximate concentration of 0.1 mg alamethicin per mg of protein.14 Either ouabain (10 μL) (final concentration 300 μM) or an equivalent volume of distilled water was added to each tube. Ouabain is a potent and selective inhibitor of Na,K-ATPase,15 and prior studies confirmed 300 μM ouabain is sufficient to cause maximal inhibition of porcine lens epithelium Na,K-ATPase activity. The remainder of the tubes received an additional 145 μL distilled water. The tubes were preincubated at 37°C for 5 minutes then concentrated ATP solution (40 μL) was added to each tube (final ATP concentration of 2 mM), bringing the total volume of the assay mixture to 400 μL and the concentration of the double-strength Na,K-ATPase buffer to single strength. The assay mixture was incubated in the dark at 37°C for 30 minutes with mild shaking. At the end of the incubation period, the ATP hydrolysis reaction was stopped by the addition of 150 μL 15% ice-cold trichloroacetic acid, and the tubes were placed on ice for 30 minutes with occasional mixing by shaking to encourage protein precipitation.
ATP hydrolysis was determined by measuring the amount of inorganic phosphate released in each reaction tube. The tubes were placed in a centrifuge at 3000 rpm for 15 minutes at 4°C then 400 μL supernatant was removed and mixed with 400 μL 4.0% FeSO4 solution in ammonium molybdate (1.25 g of ammonium molybdate in 100 mL 2.5N sulphuric acid). Standard solutions, which contained NaH2PO4 and were equivalent to 0, 10, 62.5, 125, 250, and 500 nmoles of PO4−3, were treated similarly. The mixture was allowed to sit for 5 minutes at room temperature, and then 200 μL of each standard or sample was transferred to a 96-well plate and absorbance was measured at 750 nm using a Perkin Elmer plate reader (Victor V1420-040, Perkin Elmer). Na,K-ATPase activity was calculated as the difference between ATP hydrolysis in the presence and absence of ouabain, and values are presented as nmoles ATP hydrolyzed per milligram protein per 30 minutes. Because Na,K-ATPase activity was variable between batches of eyes, data from different experiments were not pooled.
Western Blot
Infrared Detection System
The capsule-epithelium was isolated from each lens and placed in an Eppendorf tube containing ice-cold lysis buffer that contained (in mM) 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, as well as a protease inhibitor cocktail (Thermo Fisher Scientific) and phosphatase inhibitor cocktails 1 and 2 (EMD Millipore, Billerica, MA, USA) at manufacturer's recommended concentration (pH of 7.5). Following homogenization for 1 minute (four strokes of 15 seconds at 5-second intervals) using a Misonix S3000 sonicator at 6 W power setting (Misonix), the mixture was centrifuged at 13,000g for 30 minutes at 4°C to remove nuclei, mitochondria, and unbroken debris. The supernatant was frozen in liquid nitrogen and used at a later date when it was thawed and then subjected to Western blot analysis. The protein content in the sample was measured by the BCA assay.12 For Western blot, proteins were separated by 7.5% SDS-PAGE electrophoresis and transferred to nitrocellulose membrane. The membrane was kept overnight at 4°C in blocking buffer (AquaBlock/EIA/WB, East Coast Bio, Inc., North Berwick, ME, USA) then incubated overnight at 4°C with mouse monoclonal antiphospho-Src-family (Tyr 416) antibody (1:1000) and rabbit polyclonal anti-β-actin antibody (1:5000). After three 5-minute washes with a mixture of Tris-buffered saline and 0.1% (v/v) Tween 20 (TTBS), the nitrocellulose membrane was incubated at room temperature for 60 to 90 minutes with goat antirabbit secondary antibody conjugated with IRDye 680 or goat antimouse secondary antibody conjugated with IR Dye 800 (1:20,000) (LI-COR Biosciences). Then the membrane was washed three times with a mixture of Tris-buffered saline and Tween 20 and three times with PBS. Immunoreactive bands were detected and quantified using an Odyssey infrared scanner (LI-COR Biosciences). By using two secondary antibodies that are detectable at different infra-red wavelengths, β-actin could be measured simultaneously with pY416 SFK. pY416 SFK band density was normalized to β-actin band density.
Chemiluminescence Detection System
The procedure was similar to that described above for the Infrared Detection System except that the blocking buffer was replaced with 5% nonfat dry milk in TTBS, and a biotinylated protein ladder and a HRP-conjugated secondary antibody were used. Then, after transfer of proteins, the nitrocellulose membrane was cut into three pieces, one with lanes for the target proteins for primary antibody incubation, another with lanes for target proteins for no primary antibody incubation (-ive control) and the third with the lane for the biotinylated protein ladder. This separation of lanes was necessary because the antibiotin HRP-linked antibody used to visualize biotinylated protein ladder also interacted with endogenously biotinylated proteins, such as carboxylases, and produced overlapping bands with adenylyl cyclases. The primary, secondary and antibiotin antibodies were dissolved in 5% nonfat dry milk in TTBS. One piece of the membrane with the target proteins was incubated with respective primary antibodies, that is, rabbit polyclonal anti-ADCY1 (1:1000), anti-ADCY3 (1:500), or anti-ADCY8 (1:1000) at 4°C for 24 hours. The second piece with the target protein (-ive control) and the third piece with the protein ladder were incubated with the blocking buffer at 4°C for 24 hours. The membranes were washed three times (5 minutes each) with TTBS. The membrane pieces with the target protein were then incubated with the secondary antibody solution (goat antirabbit IgG-HRP; 1:10,000), and the piece with the biotinylated protein ladder was incubated with the antibiotin HRP-linked antibody (1:1000), both for 1 hour at room temperature with shaking. The membranes were washed three times (5 minutes each) with TTBS, then excess liquid was removed by absorbent towel and the membranes were placed in clear plastic wrap. Freshly prepared working substrate (Super Signal West Pico Chemiluminescent substrate, Thermo Fisher Scientific) was then added in sufficient amount just to cover the membrane. Working in a dark room with a safe light, the covered membrane was placed in a film cassette with the protein side facing up. Amersham Hyper Film TM ECL (GE Health Care Ltd.) was placed on top of the membrane, exposed for 15 minutes, and then developed using SRX-101A Medical Film Processor (Konica Minolta Medical and Graphic, Inc., Shanghai, China).
cAMP
The capsule-epithelium was removed from each lens and placed in 0.5 mL 10% trichloroacetic acid (TCA) in an Eppendorf tube that was frozen in liquid nitrogen and stored at −80°C until further processing. Cyclic AMP was extracted and measured according to a modification of a method described previously.16,17 Frozen samples in the Eppendorf tubes were subjected to three cycles of freeze-thaw then sonicated using a Misonix S3000 sonicator (6 W power setting, four strokes of 15 seconds each with a 5-second interval). After sonication, ∼4000 cpm of 3H-cAMP was added to each tube to determine recovery efficiency. At a specific activity of approximately 37 Ci/mmol and at a 50% counting efficiency, this represents approximately 0.1 pmol (100 fmol) of cyclic AMP. This amount was taken into account when calculating cAMP content of a sample. The samples were centrifuged at 13,000g for 30 minutes, and each supernatant was transferred to new Eppendorf tubes and designated an SN tube. Each pellet was washed with 200 μL 10% TCA, briefly sonicated, centrifuged again at 13,000g for 30 minutes, and the supernatant was transferred to its respective SN tube. The supernatant samples were used for cAMP assay, and pellet samples were used for protein measurement.
Cyclic AMP was extracted from the supernatant using ion exchange chromatography. Cation exchange columns (5 cm) were prepared using washed and preswollen (overnight) Dowex 50W x4-400 mesh cation exchange resin (H+-form) loaded into glass Pasteur pipettes (the narrow ends were plugged with glass wool). The columns were washed 10 times (1 mL each) with double distilled water and three times (1 mL each) with 0.02N HCl. Each supernatant sample (∼0.7 mL) was applied to a chromatography column that was first washed with 0.5 mL of water and the eluted material discarded. The next four washes (1 mL each) were collected and pooled in glass tubes. The eluted samples were dried under a stream of air at 40°C. Dried samples were reconstituted in 400 μL cAMP assay buffer (Na-acetate buffer). A 100-μL aliquot of each reconstituted sample was mixed with 7 mL liquid scintillation fluid in a scintillation vial and counted using a beta counter to calculate recovery percentage. Recovery efficiency was calculated from the total and blank counts. Blank-count tubes contained only the assay buffer, and the total-count tubes contained 4000 cpm of 3H cAMP. Another 100 μL reconstituted cAMP solution from each sample was subjected to radio-immunoassay using a cAMP assay kit (Cat. # NEK033001KT, Perkin Elmer) following the acetylated cAMP assay procedure recommended by the manufacturer. According to this protocol, each 100 μL (out of a total of 400 μL reconstituted cAMP solution) was acetylated with 5.0 μL acetylation reagent and then diluted to 1.0 mL with double distilled water giving an effective dilution of the assayed samples to 40 fold. Thus, cAMP measured in 100 μL was multiplied by 40 to calculate the total cAMP content in each sample. Results were expressed as pmoles of cAMP/mg protein. Protein was measured in the pellet by BCA assay.
Statistical Analysis
Results are expressed as the mean ± SEM of data from a specified number of independent experiments. Statistical comparisons were made by 1-way analysis of variance (ANOVA) followed by the Bonferroni post hoc multiple comparison test. To validate the homogeneity of variance assumption, we performed Levene's test where possible. A probability (P) value of < 0.05 was considered significant.
Results
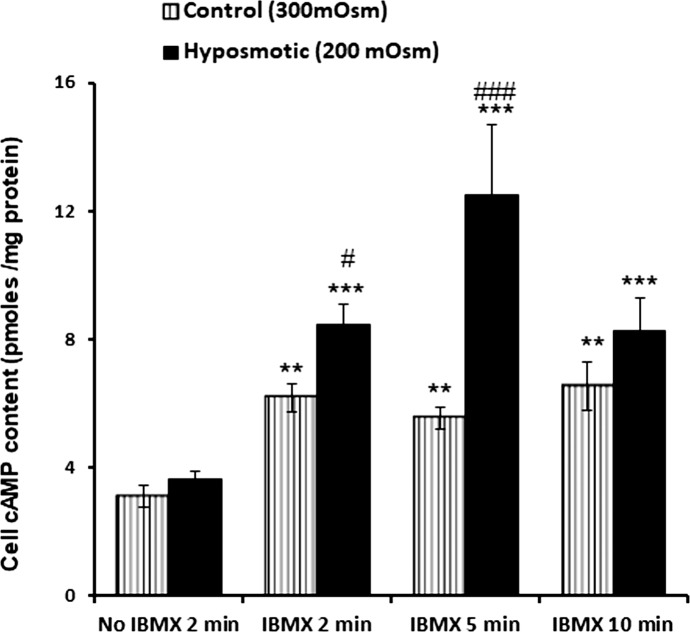
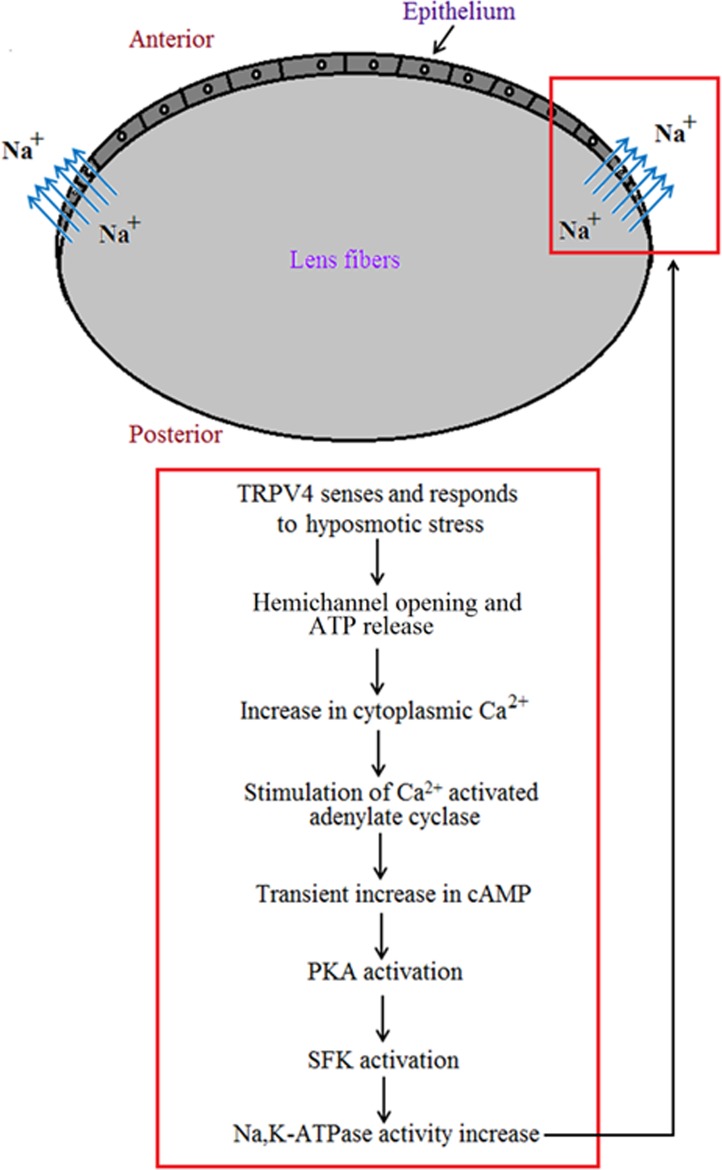
Intact lenses were exposed to hyposmotic solution (200 mOsm) in the presence of IBMX (500 μM), added to inhibit phosphodiesterase activity. Under these conditions, a transient increase of cAMP was detected in the epithelium (Fig. 1) The cAMP increase was detectable at 2 minutes and peaked at 5 minutes. In separate experiments, intact lenses were exposed to hyposmotic solution (200 mOsm) for 2 minutes and then the epithelium was removed and probed for SFK phosphorylation (activation) or Na,K-ATPase activity. Significant increase in SFK phosphorylation was observed in the epithelium of lenses that had been exposed to hyposmotic solution. Importantly, the hyposmotic solution-induced SFK activation in the epithelium was not observed when the lenses were exposed to hyposmotic solution in the presence of H89 (10 μM), an inhibitor of the cAMP-activated protein kinase, PKA (Fig. 2A). Na,K-ATPase activity was almost doubled in epithelium removed from lenses exposed to hyposmotic solution. In contrast, Na,K-ATPase activity did not increase in the epithelium of lenses exposed to hyposmotic solution in the presence of H89 (Fig. 2B).
Figure 1.
Increased cAMP content detected by radioimmunoassay in the epithelium of intact lenses exposed to hyposmotic solution. Lenses were incubated for 2, 5, or 10 minutes in control (isosmotic; 300 mOsm) or hyposmotic (200 mOsm) Krebs solution either in the absence or in the presence of 500 μM IBMX, a phosphodiesterase inhibitor added to prevent cAMP breakdown. Values are mean ± SEM of results from five to eight lenses. ** and *** indicate a significant difference (P < 0.01 and P < 0.001) from control in the absence of IBMX. # and ### indicate significant difference (P < 0.05 and P < 0.001) from the respective time-matched control in the presence of IBMX.
Figure 2.
The effect of PKA inhibitor H89 (10 μM) on the SFK phosphorylation and Na,K-ATPase activity responses to hyposmotic solution. Intact lenses were exposed to hyposmotic Krebs solution (200 mOsm) for 2 minutes in the presence or absence (control; Con) of H89 added 20 minutes beforehand. Then the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416 or used to determine Na-K-ATPase activity. β actin was probed as a loading control in Western blot analysis. (A) A representative SFK Western blot (upper panel) and pooled data (lower panel) on phospho-SFK (pY416 SFK) band density (mean ± SEM of results from six independent experiments). (B) Na,K-ATPase activity as mean ± SEM of results from four to six lenses. ** or *** indicates a significant difference from control (P < 0.01 or P < 0.001), and ## indicates a significant difference from group subjected to hyposmotic solution alone (P < 0.01).
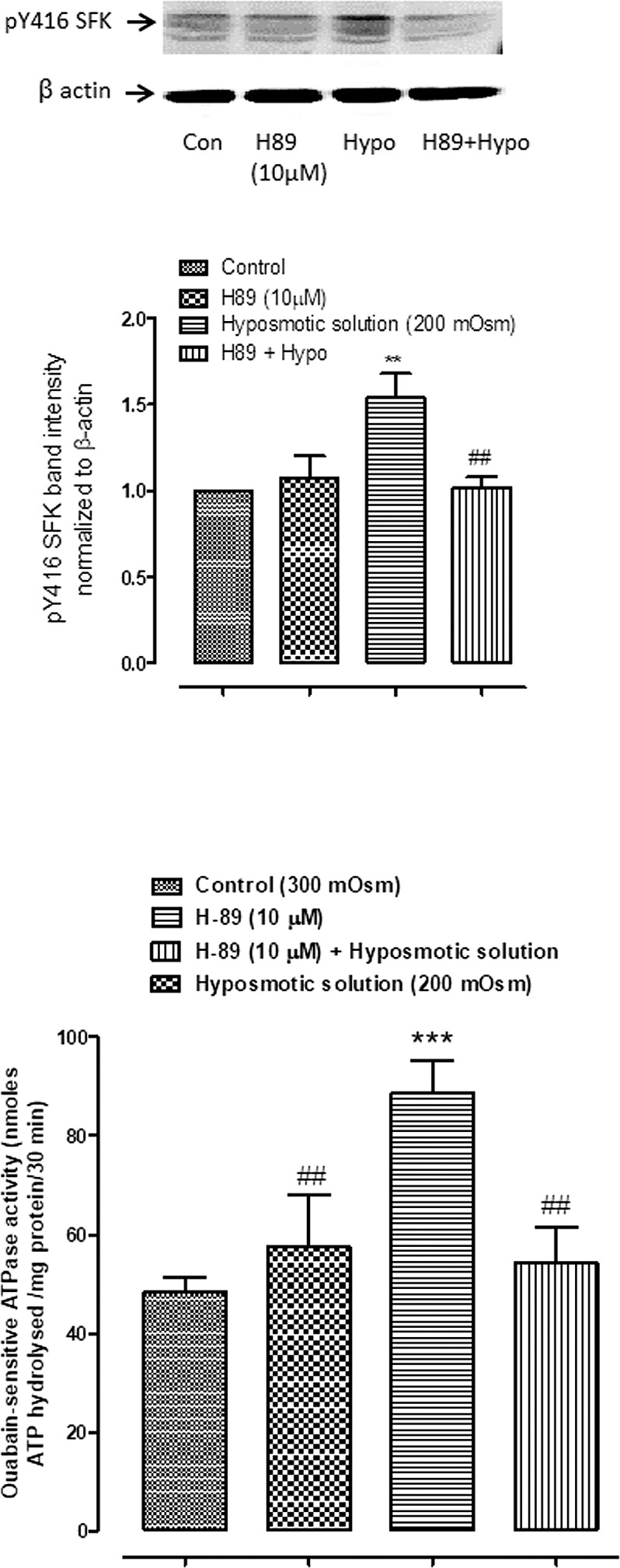
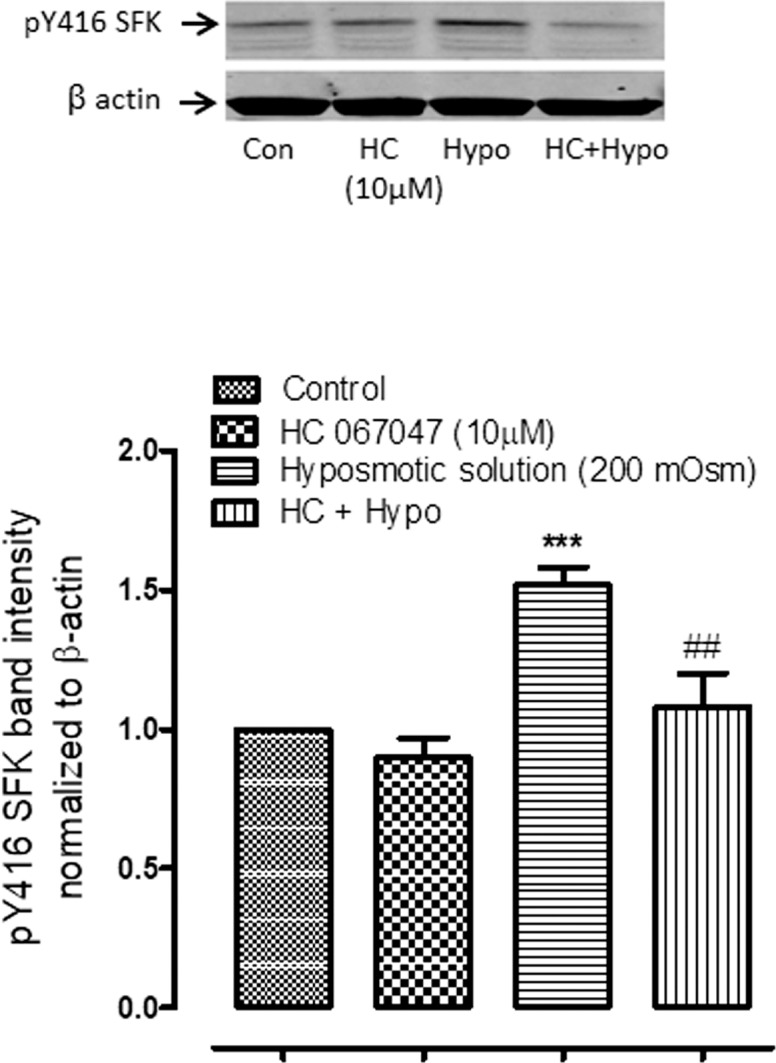
To examine calcium-dependence of the responses, intact lenses were exposed to hyposmotic solution in the presence or absence of HC067047 (10 μM) an inhibitor of TRPV4 channel-mediated calcium entry (Fig. 3). Under these conditions SFK activation was not increased as seen in the case of hyposmotic stress alone. To confirm calcium dependence of SFK activation, a group of lenses was preincubated with a cytosolic calcium chelating agent, BAPTA-AM (10 μM) for 1 hour and then exposed to hyposmotic solution. BAPTA-AM treatment was found to abolish the increase of SFK phosphorylation that occurs in hyposmotic solution (Fig. 4A). SFK activation has previously been reported as a critical step that precedes the Na,K-ATPase activity response in the epithelium of lenses exposed to hyposmotic solution. Consistent with this notion, BAPTA-AM treatment was found to abolish the increase of Na,K-ATPase activity in the epithelium of lenses exposed to hyposmotic solution (Fig. 4B). SFK phosphorylation in the epithelium also was absent when lenses were exposed to hyposmotic solution in the presence of an inhibitor of SFK activation, PP2 (10 μM) (Fig. 5).
Figure 3.
The effect of TRPV4 antagonist HC067047 (10 μM) on the SFK phosphorylation response to hyposmotic solution (Hypo). Intact lenses were exposed to hyposmotic Krebs solution (200 mOsm) for 2 minutes in the presence or absence (control; Con) of HC067047 (HC) added 20 minutes beforehand. Then the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416. β actin was probed as a loading control. Upper panel: Representative Western blot. Lower panel: Pooled data (mean ± SEM of results from five independent experiments) showing phospho-SFK (pY416 SFK) band density (relative to control). *** indicates a significant difference from control (P < 0.001). ## indicates a significant difference from group subjected to hyposmotic solution alone (P < 0.01).
Figure 4.
The effect of cytoplasmic calcium chelator BAPTA-AM on the SFK phosphorylation and on the Na,K-ATPase activity responses to hyposmotic solution (Hypo). Intact lenses were exposed to hyposmotic Krebs solution (200 mOsm) for 2 minutes in the presence or absence (control; Con) of either BAPTA-AM (10 μM) added 60 minutes beforehand. Then the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416 (A) or Na,K-ATPase activity measurement (B). β actin was probed as a loading control for Western blot analysis. The upper panel of (A) shows representative Western blots, and the lower panel shows pooled data (mean ± SEM of results from six independent experiments) on phospho-SFK (pY416 SFK) band density (relative to control). ** and *** indicate a significant difference from control (P < 0.01 and P < 0.001). ## indicates a significant difference from group subjected to hyposmotic solution alone (P < 0.01).
Figure 5.
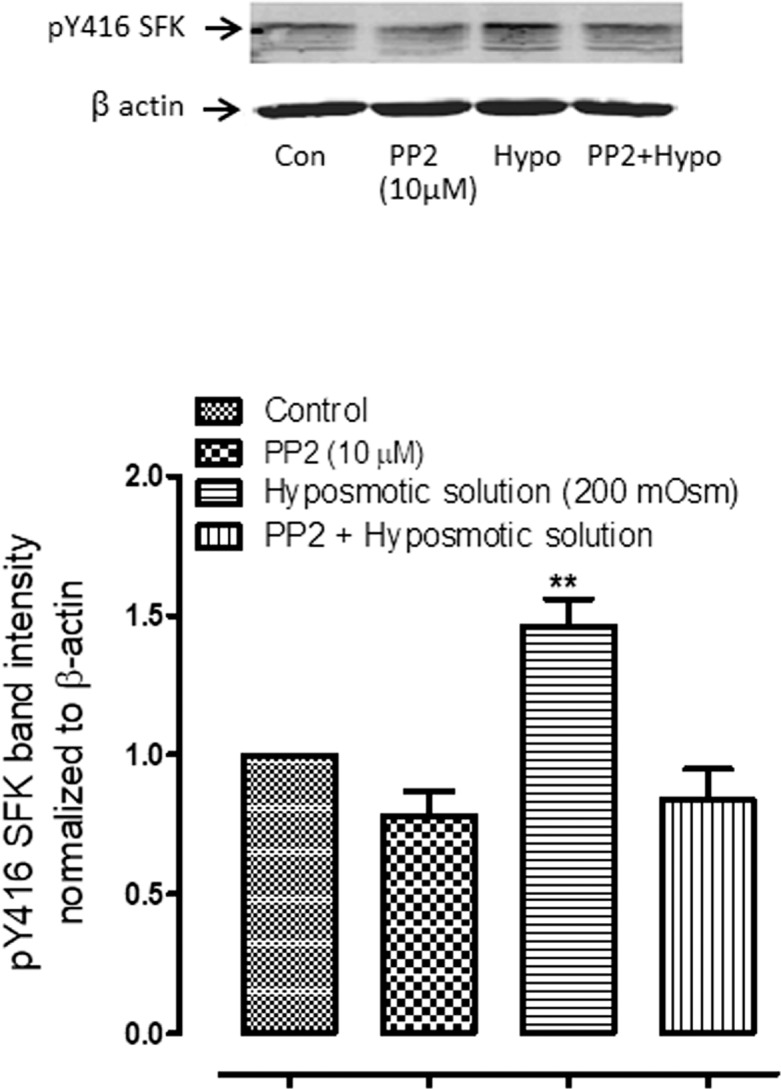
The effect of PP2, a specific SFK inhibitor, on the SFK phosphorylation and Na,K-ATPase activity responses to hyposmotic solution (Hypo). Intact lenses were exposed to hyposmotic Krebs solution (200 mOsm) for 2 minutes in the presence or absence (Control) of 10 μM PP2, added 45 minutes beforehand. Then the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416. Values are mean ± SEM of results from six independent experiments. ** indicates a significant difference (P < 0.001) from control.
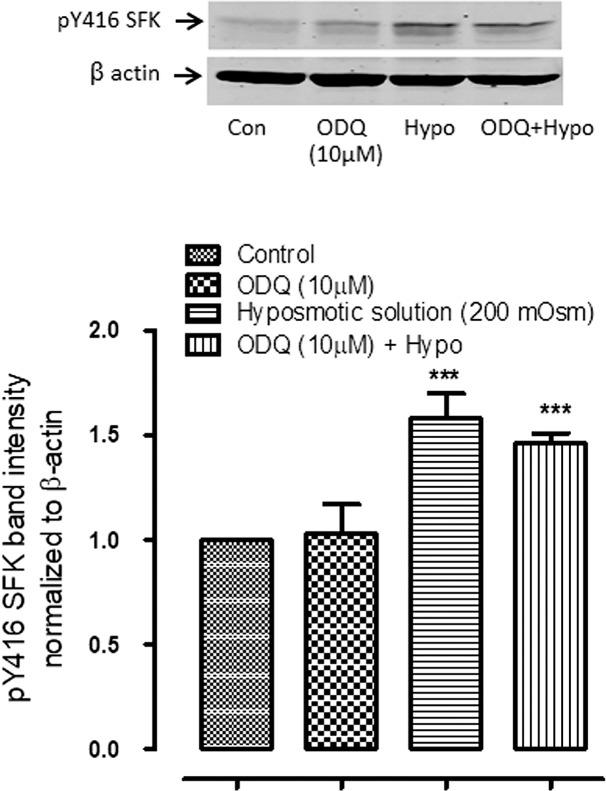
Earlier we reported that the NO-sGC-cGMP pathway influences SFK activation and Na,K-ATPase activity in the nonpigmented ciliary epithelium.18 Since an increase in cytoplasmic calcium concentration has the potential to activate nitric oxide synthase (NOS), which would generate nitric oxide that in turn activate soluble guanylate cyclase (sGC) to produce cGMP, we tested whether the sGC inhibitor ODQ alters the SFK activation response to hyposmotic solution. ODQ (10 μM) did not alter the magnitude of hyposmotic solution-induced SFK phosphorylation (Fig. 6).
Figure 6.
The effect of sGC inhibitor ODQ (10 μM) on the SFK phosphorylation response to hyposmotic solution (Hypo). Intact lenses were exposed to hyposmotic Krebs solution (200 mOsm) for 2 minutes in the presence or absence (control; Con) of ODQ added 20 minutes beforehand. Then the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416. β actin was probed as a loading control. Upper panel: Representative Western blot. Lower panel: Pooled data (mean ± SEM of results from six independent experiments) showing phospho-SFK (pY416 SFK) band density. *** indicates a significant difference from control (P < 0.001).
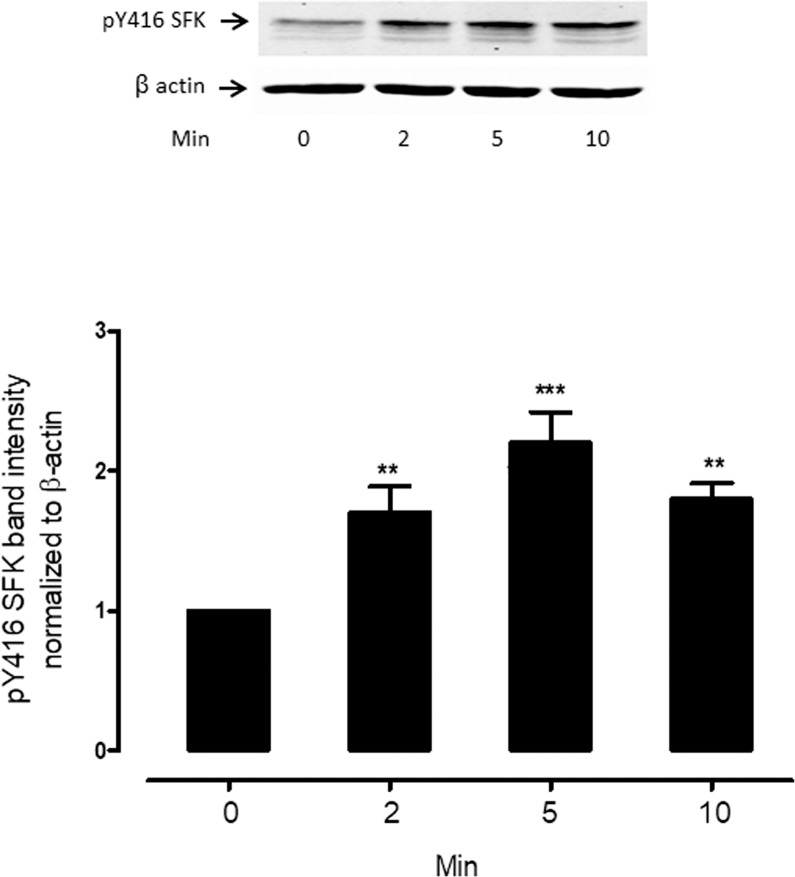
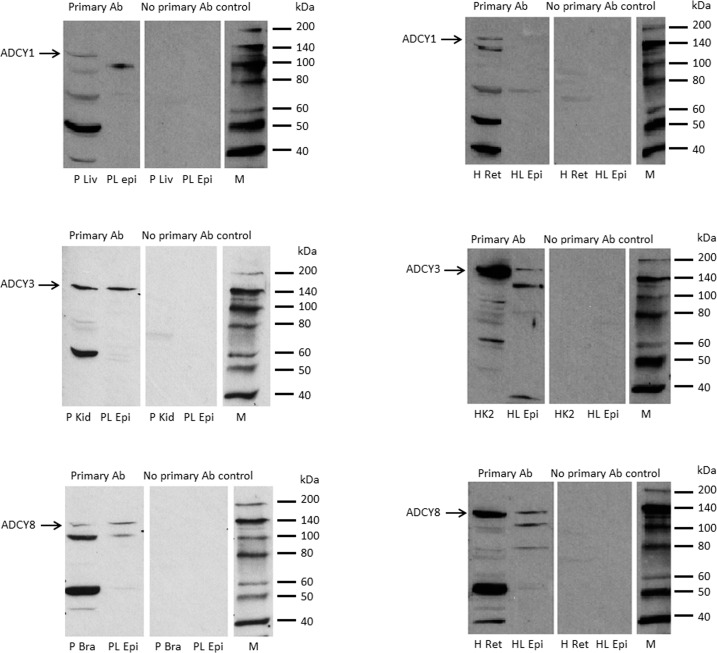
Because hyposmotic solution was found to cause cAMP to increase, and because H89 prevented SFK activation, we considered the possibility that SFK activation is cAMP-dependent. To test this notion, intact lenses were exposed to 8pCPT-cAMP, a cell-permeable cAMP analog. 8pCPT-cAMP caused robust and transient SFK activation (Fig. 7). Because the findings are consistent with calcium activation of adenylyl cyclase, Western blot analysis was used to examine expression of the three known calcium-stimulated adenylyl cyclases in the epithelium removed from freshly isolated porcine lenses. Immunoreactive bands were detected for ADCY3 and ADCY8 in both porcine (Fig. 8A) and human (Fig. 8B) lens epithelium. ADCY1 was not detected. To the best of our knowledge, this is the first demonstration of calcium-activated adenylyl cyclases in porcine and human lens epithelium.
Figure 7.
The time course of the 8-pCPT-cAMP effect on SFK phosphorylation. Intact lenses were exposed to 10 μM 8-pCPT-cAMP for 0 (control), 2 or 5 or 10 minutes. Then the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416. β actin was probed as a loading control. Upper panel: Representative Western blot. Lower panel: Pooled data (mean ± SEM of results from three independent experiments) showing phospho-SFK (pY416 SFK) band density (relative to control). ** and *** indicate significant differences from control (P < 0.01 and P < 0.001).
Figure 8.
Expression of calcium-activated adenylyl cyclase proteins in porcine (A) and human (B) lens epithelium. Using Western blot, epithelium samples isolated from fresh porcine or human lenses were probed for ADCY1, ADCY3, or ADCY8. Pig liver (PL), pig kidney cortex (PK), or pig brain (PB) and human retina (H Ret), human kidney cells (HK2), or human retina were used as positive control for ADCY1, ADCY3, or ADCY8, respectively. One hundred micrograms of protein was loaded per lane for positive controls, and 150 μg protein was loaded for adenylyl cyclases. Note that ADCY3 and ADCY8 were present in both porcine and human lens epithelium. ADCY1 was not detectable in either species.
Discussion
Previous studies have shown the importance of SFKs in the lens. Zhou and Menko19 reported conditions in which simultaneous activation of Src and p38 precedes cataract formation. There have been reports that Src and Fyn influence lens cell proliferation and differentiation20,21 and our own laboratory has reported SFK-dependent regulation of lens Na,K-ATPase activity.3,22 Here, we present findings that link SFK activation in the porcine lens epithelium to cAMP. Previous studies have shown SFK activation occurs shortly after exposure of the intact lens to hyposmotic solution. Under these conditions we observed a significant increase of cAMP in the epithelium and SFK activation was prevented by H89, an inhibitor of PKA. We reasoned that if SFK activation is cAMP-dependent, exposure of the lens to a cell-permeable cAMP analog, 8-pCPT-cAMP, should cause SFK activation and this was the case.
The response of the lens to hyposmotic stress has been studied in some detail and found to be associated with the opening of TRPV4 ion channels, an event that leads to opening of connexin and pannexin hemichannels.3 These channels and hemichannels provide a means of entry of extracellular calcium to the epithelium, and hyposmotic stress was observed to cause a sustained, but physiologically relevant, increase of cytoplasmic calcium concentration in cultured lens epithelium.4 Calcium is a ubiquitous and extremely versatile second messenger that plays numerous roles in cellular signaling cascades,5 and crosstalk with other second messenger signaling pathways is common. In some cases, a calcium rise has the ability to change the concentrations of cAMP or cGMP by modulating Ca2+/calmodulin-sensitive phosphodiesterases.9,23–26 Changes in cGMP concentration can also be the result of calcium-dependent stimulation of NOS-sGC pathways.27 The NOS-cGMP-sGC pathway did not appear to play a significant role here because ODQ, a highly selective and potent sGC inhibitor, failed to prevent hyposmotic solution-induced SFK activation in the lens epithelium. Instead, the evidence seems to fit with the ability of calcium to regulate certain members of adenylyl cyclase family, the so-called calcium-sensitive isoforms.6–8
Western blot detection data show presence of ADCY3 and ADCY8 in porcine as well as in human lens epithelium. To the best our knowledge, this is the first demonstration of calcium-activated adenylyl cyclases in lens epithelium. Interestingly, ADCY1 was absent both in the porcine and in the human. This shows strikingly similar expression pattern of calcium-activated adenylyl cyclases in the two species. The implication of this similarity is that results of studies in porcine lenses could be extrapolated to human. In mammals, nine particulate (designated as ADCY1-9) and one soluble adenylyl cyclase isoforms (designated as ADCY10 or sAC) have been reported so far. Five of the particulate ACs are reported to be directly sensitive to calcium, and these Ca2+-sensitive adenylyl cyclases are considered to be critical integrators of cell signaling.8 It has been shown that ADCY1, ADCY3,28,29 and ADCY830 are stimulated by calcium. In contrast, an increase of cytoplasmic calcium causes inhibition of ADCY5 and ADCY6.31–33 In the present investigation, the detection of two Ca2+-sensitive adenylyl cyclases, ADCY3 and ADCY8, in the lens epithelium is consistent with the notion that calcium entry links to stimulated production of cAMP. It should be made clear that we have no direct evidence to link any particular adenylyl cyclase or combination of adenylate cyclases to the observed cAMP generation in the lens. It has been suggested that calcium-dependent adenylyl cyclase responses may vary according the source of the calcium rise, perhaps reflecting the transient nature of an increase caused by release from intracellular stores compared to different modes of calcium entry.34 In an earlier study on lens epithelium, we observed such a pattern of sustained doubling of calcium concentration in response to hyposmotic solution,3,4 and it is of particular interest to note that ADCY8 is sensitive to sustained calcium elevation.35,36 On this basis, the conditions appear feasible for ADCY8 to contribute to cAMP production in the epithelium of lenses exposed to hyposmotic solution. In pancreatic β cells, calcium influx has been shown to activate ADCY8, which in turn regulates insulin release by the combined influence of calcium and cAMP/PKA signaling.35 This is just one of several instances where increased cytoplasmic calcium concentration stimulates adenylate cyclase activity and increases cAMP.28,30,37–39
The epithelium of lenses exposed to hyposmotic solution showed a peak cAMP level at 5 minutes followed by a decay at 10 minutes, although the cAMP level at 10 minutes was still significantly higher than that in control lenses. Generally, the cAMP level, and thus the strength of cAMP-mediated signaling, reflects a dynamic balance between the influence of adenylate cyclases and phosphodiesterases (PDEs). In mammalian cells, there are several families of phosphodiesterases.40,41 Although it is beyond the scope of this manuscript to identify the enzymes responsible for the phosphodiesterase activity that reduces cAMP levels after the initial rise, we speculate that one or more of the Ca2+-dependent phosphodiesterases (such as PDE1 isoforms) might contribute to the decrease. Coordinated control of adenylate cyclase and phosphodiesterase activity by Ca2+ is a common occurrence.42,43
To further examine calcium-dependence of the response, lenses were exposed to either HC067047 or BAPTA. The strategy was as follows: HC067047 inhibits the initiating step in the response, TRPV4-mediated calcium entry. BAPTA is a cytoplasmic calcium chelator that suppresses an increase of cytoplasmic calcium even though calcium entry occurs. Importantly, SFK activation was inhibited by BAPTA, HC067047, and a PKA inhibitor H89. Taken together, the findings point to cAMP as a link between TRPV4 channel-mediated calcium entry, activation of calcium-dependent adenylyl cyclases, a rise of cAMP and SFK activation. In accordance with this proposed role of calcium-dependent adenylyl cyclases, and the dependence of Na,K-ATPase activity on SFK activation,3 the doubling of Na,K-ATPase activity in the epithelium of lenses exposed to hyposmotic solution was prevented both by H89 and by BAPTA. In an earlier report, phosphorylation of Src has been linked to PKA in the bovine sperm.44 As a direct test of cAMP-dependent SFK activation, intact lenses were exposed to 8-pCPT-cAMP, a cell permeable stable analog of cAMP, and SFK phosphorylation was confirmed to occur. In summary, our results point to the following sequence of events in lenses exposed to hyposmotic solution: TRPV4 activation and calcium entry followed by calcium-mediated production of cAMP, PKA-dependent SFK activation, and a subsequent increase of Na,K-ATPase activity (Fig. 9). Regulation of Na,K-ATPase activity in the lens epithelium has significance because the lens is made up almost entirely of differentiated fiber cells that have little or no Na,K-ATPase activity. The fiber cells, however, are well coupled, and as a consequence active transport by Na,K-ATPase in relatively few cells in the epithelium is able to regulate cytoplasmic sodium and potassium levels in the entire structure. If sodium and potassium levels are not properly controlled, osmotic forces cause the cells to swell and this impairs lens transparency. The findings here are consistent with our earlier proposal that TRPV4 enables the epithelium to sense swelling and adjust Na,K-ATPase activity accordingly.3 The discovery of SFK-mediated regulation of Na,K-ATPase through the integration of TRPV4-mediated calcium entry and cAMP signaling illustrates a complex regulatory mechanism. The complexity may be greater still because different SFKs may have different effects on Na,K-ATPase activity.45 Earlier we showed that at least four SFK members (Src, Fyn, Yes, Hck) are expressed in the porcine lens epithelium and that endothelin-1 (ET-1) activated Fyn but inhibited Src and Hck, and had no effect on Yes.45 In regard to pathology, maintenance of Na,K-ATPase activity is significant in relation to cataract, the most common cause of age-related vision loss. In the human lens, a progressive increase of sodium and water content and a decrease potassium is associated with cortical cataract formation.46 In other words, cataract is associated with failure of Na,K-ATPase-mediated active transport to keep pace with ion leakage.
Figure 9.
Schematic diagram showing steps in the response mechanism that links TRPV4 activation to an increase of Na,K-ATPase activity when the lens is exposed to hyposmotic solution.
Acknowledgments
The authors thank the University of Arizona Meat Science Laboratory, West Valley Processing Meat Processors (Buckeye, AZ, USA) and Hatfield Quality Meats (Philadelphia, PA, USA) for the supply of porcine eyes. The authors are thankful to Dhruva Bhattacharya of the Department of Ophthalmology and Vision Science, University of Arizona, for the technical help in developing X-ray film using SRX-101A Medical Film Processor.
Supported by a grant from the National Institutes of Health (EY 006915).
Disclosure: M. Shahidullah, None; A. Mandal, None; N.A. Delamere, None
References
- 1.Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. J Membr Biol. 2000;178:89–101. doi: 10.1007/s002320010017. [DOI] [PubMed] [Google Scholar]
- 2.Delamere NA, Dean WL. Distribution of lens sodium-potassium-adenosine triphosphatase. Invest Ophthalmol Vis Sci. 1993;34:2159–2163. [PubMed] [Google Scholar]
- 3.Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol. 2012;302:C1751–C1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandal A, Shahidullah M, Delamere NA. Calcium entry via connexin hemichannels in lens epithelium. Exp Eye Res. 2015;132:52–58. doi: 10.1016/j.exer.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Antoni FA. Calcium regulation of adenylyl cyclase relevance for endocrine control. Trends Endocrinol Metab. 1997;8:7–14. doi: 10.1016/s1043-2760(96)00206-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Zhang M. The role of Ca(2)(+)-stimulated adenylyl cyclases in bidirectional synaptic plasticity and brain function. Rev Neurosci. 2012;23:67–78. doi: 10.1515/revneuro-2011-0063. [DOI] [PubMed] [Google Scholar]
- 8.Mons N, Decorte L, Jaffard R, Cooper DM. Ca2+sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sci. 1998;62:1647–1652. doi: 10.1016/s0024-3205(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 9.Kakkar R, Raju RV, Sharma RK. Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1) Cell Mol Life Sci. 1999;55:1164–1186. doi: 10.1007/s000180050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol Cell. 2002;9:85–94. doi: 10.1016/s1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- 11.Obara Y, Labudda K, Dillon TJ, Stork PJ. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci. 2004;117:6085–6094. doi: 10.1242/jcs.01527. [DOI] [PubMed] [Google Scholar]
- 12.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 13.Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie ZJ, Wang YH, Ganjeizadeh M, McGee R, Jr, Askari A. Determination of total (Na+ + K+)-ATPase activity of isolated or cultured cells. Anal Biochem. 1989;183:215–219. doi: 10.1016/0003-2697(89)90470-3. [DOI] [PubMed] [Google Scholar]
- 15.Wallick ET, Schwartz A. Interaction of cardiac glycosides with Na+K+ATPase. Method Enzymol. 1988;156:201–213. doi: 10.1016/0076-6879(88)56022-6. [DOI] [PubMed] [Google Scholar]
- 16.Shahidullah M, Mandal A, Wei G, Levin LR, Buck J, Delamere NA. Nonpigmented ciliary epithelial cells respond to acetazolamide by a soluble adenylyl cyclase mechanism. Invest Ophthalmol Vis Sci. 2014;55:187–197. doi: 10.1167/iovs.13-12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahidullah M, Wilson WS, Millar C. Effects of timolol, terbutaline and forskolin on IOP, aqueous humour formation and ciliary cyclic AMP levels in the bovine eye. Curr Eye Res. 1995;14:519–528. doi: 10.3109/02713689508998398. [DOI] [PubMed] [Google Scholar]
- 18.Shahidullah M, Mandal A, Wei G, Delamere NA. Nitric oxide regulation of Na, K-ATPase activity in ocular ciliary epithelium involves Src family kinase. J Cell Physiol. 2014;229:343–352. doi: 10.1002/jcp.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Menko AS. The role of Src family kinases in cortical cataract formation. Invest Ophthalmol Vis Sci. 2002;43:2293–2300. [PubMed] [Google Scholar]
- 20.Leonard M, Zhang L, Bleaken BM, Menko AS. Distinct roles for N-Cadherin linked c-Src and fyn kinases in lens development. Dev Dyn. 2013;242:469–484. doi: 10.1002/dvdy.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng C, Ansari MM, Cooper JA, Gong X. EphA2 and Src regulate equatorial cell morphogenesis during lens development. Development. 2013;140:4237–4245. doi: 10.1242/dev.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahidullah M, Mandal A, Delamere NA. Damage to lens fiber cells causes TRPV4-dependent Src family kinase activation in the epithelium. Exp Eye Res. 2015;140:85–93. doi: 10.1016/j.exer.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni Q, Ganesan A, Aye-Han N-N, et al. Signaling diversity of PKA achieved via a Ca(2+)-cAMP-PKA oscillatory circuit. Nat Chem Biol. 2011;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Goas F, Amiel C, Friedlander G. Protein kinase C modulates cAMP content in proximal tubular cells: role of phosphodiesterase inhibition. Am J Physiol. 1991;261:F587–F592. doi: 10.1152/ajprenal.1991.261.4.F587. [DOI] [PubMed] [Google Scholar]
- 25.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 26.Goraya TA, Masada N, Ciruela A, Cooper DM. Sustained entry of Ca2+ is required to activate Ca2+-calmodulin-dependent phosphodiesterase 1A. J Biol Chem. 2004;279:40494–40504. doi: 10.1074/jbc.M313441200. [DOI] [PubMed] [Google Scholar]
- 27.Fleming I, Bauersachs J, Busse R. Calcium-dependent and calcium-independent activation of the endothelial NO synthase. J Vasc Res. 1997;34:165–174. doi: 10.1159/000159220. [DOI] [PubMed] [Google Scholar]
- 28.Choi EJ, Xia Z, Storm DR. Stimulation of the type III olfactory adenylyl cyclase by calcium and calmodulin. Biochemistry. 1992;31:6492–6498. doi: 10.1021/bi00143a019. [DOI] [PubMed] [Google Scholar]
- 29.Kitaguchi T, Oya M, Wada Y, Tsuboi T, Miyawaki A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem J. 2013;450:365–373. doi: 10.1042/BJ20121022. [DOI] [PubMed] [Google Scholar]
- 30.Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994;269:12190–12195. [PubMed] [Google Scholar]
- 31.Werthmann RC, von Hayn K, Nikolaev VO, Lohse MJ, Bünemann M. Real-time monitoring of cAMP levels in living endothelial cells: thrombin transiently inhibits adenylyl cyclase 6. J Physiol. 2009;587:4091–4104. doi: 10.1113/jphysiol.2009.172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chetham PM, Guldemeester HA, Mons N, et al. Ca(2+)-inhibitable adenylyl cyclase and pulmonary microvascular permeability. Am J Physiol. 1997;273:L22–L30. doi: 10.1152/ajplung.1997.273.1.L22. [DOI] [PubMed] [Google Scholar]
- 33.Katsushika S, Chen L, Kawabe J, et al. Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci U S A. 1992;89:8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dou H, Wang C, Wu X, et al. Calcium influx activates adenylyl cyclase 8 for sustained insulin secretion in rat pancreatic beta cells. Diabetologia. 2015;58:324–333. doi: 10.1007/s00125-014-3437-z. [DOI] [PubMed] [Google Scholar]
- 36.Everett KL, Cooper DM. An improved targeted cAMP sensor to study the regulation of adenylyl cyclase 8 by Ca2+ entry through voltage-gated channels. PLoS One. 2013;8:e75942. doi: 10.1371/journal.pone.0075942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louiset E, Duparc C, Lenglet S, Gomez-Sanchez CE, Lefebvre H. Role of cAMP/PKA pathway and T-type calcium channels in the mechanism of action of serotonin in human adrenocortical cells. Mol Cell Endocrinol. 2017;441:99–107. doi: 10.1016/j.mce.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Hungen K, Roberts S. Catecholamine and Ca 2+ activation of adenylate cyclase systems in synaptosomal fractions from rat cerebral cortex. Nat New Biol. 1973;242:58–60. doi: 10.1038/newbio242058a0. [DOI] [PubMed] [Google Scholar]
- 39.Lefkowitz RJ, Roth J, Pastan IRA. Effects of calcium on ACTH stimulation of the adrenal: separation of hormone binding from adenyl cyclase activation. Nature. 1970;228:864–866. doi: 10.1038/228864a0. [DOI] [PubMed] [Google Scholar]
- 40.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 41.Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci. 1997;22:217–224. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- 42.Piascik MT, Wisler PL, Johnson CL, Potter JD. Ca2+dependent regulation of guinea pig brain adenylate cyclase. J Biol Chem. 1980;255:4176–4181. [PubMed] [Google Scholar]
- 43.Borisy FF, Ronnett GV, Cunningham AM, Juilfs D, Beavo J, Snyder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotfeld H, Hillman P, Ickowicz D, Breitbart H. PKA. and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction. 2014;147:347–356. doi: 10.1530/REP-13-0560. [DOI] [PubMed] [Google Scholar]
- 45.Mandal A, Shahidullah M, Beimgraben C, Delamere NA. The effect of endothelin-1 on Src-family tyrosine kinases and Na,K-ATPase activity in porcine lens epithelium. J Cell Physiol. 2011;226:2555–2561. doi: 10.1002/jcp.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan G, Bushell AR. Ion analyses of human cataractous lenses. Exp Eye Res. 1975;20:223–230. doi: 10.1016/0014-4835(75)90136-0. [DOI] [PubMed] [Google Scholar]