Abstract
AIM:
Our study aims to make a comparison between the effects of milking of umbilical cord versus delayed cord clamping on Hemoglobin level at 6 weeks from delivery among term neonates and which method is more beneficial for them.
DESIGN:
It was a randomised control study. Participants were randomised into 2 groups; Group 1: 125 women were assigned to delay cord clamping; Group 2: 125 women were assigned to milking of the umbilical cord 5 times before cutting. Student t-test was used to compare between the two groups for quantitative data, for qualitative data chi-square test and the Correlation coefficient was done to test the association between variables.
SETTING:
This study was at El-Galaa Teaching Hospital, labour suite. Cairo, Egypt.
PARTICIPANTS:
A group of 250 pregnant women starting from ≥ 37 weeks’ gestational age.
INTERVENTION:
In this study, we searched if the mechanism of milking or delayed cord clamping could give some of the positive benefits for neonates or not.
RESULTS:
In this study, we found that milking of the umbilical cord five times as in group 1 was associated with higher hemoglobin levels at 6 weeks after birth, at physiological anemia of the fetus and significant but clinically there was no difference between the two groups (10.4 ± 0.5 and 10.6 ± 0.5 respectively, P < 0.001). Also, there was a positive correlation between haemoglobin of the mother and the newborn during the first day and after 6 weeks with r = 0.349 and 0.283 respectively and a P value < 0.001. Furthermore, there was a positive correlation between the haemoglobin of the fetus after the first day and fetus at 6 weeks with r = 0.534 and a P value < 0.001. For most other outcomes (including APGAR score, positive pressure ventilation, poor neonatal outcomes such as respiratory distress syndrome there were no significant differences between the two groups. Our study may recommend the use of umbilical cord milking in term babies when delayed cord clamping is unavailable.
CONCLUSION:
Umbilical cord blood milking after its clamping improves some important haematological parameters for newborns, especially in countries with high incidence of anaemia in newborns and children.
Keywords: Delayed cord clamping, Umbilical cord milking, Neonatal physiologic anaemia, Term newborn
Introduction
The umbilical cord is the essential life-keeping connection between fetus and placenta. It represents a strong connection to the fetomaternal interface while permitting fetal mobility that is essential for general fetal development and neuro-motor development in particular [1]. When a baby is born, the umbilical cord is cut, and there is a stump left which should dry and fall off by the time of 5 to 15 days after birth [2]. Delayed cord clamping, which was adopted by American Academy of Pediatrics in all deliveries, is defined as ligation of the umbilical cord 2-3 minutes after birth or on stoppage of cord pulsations, will lead to a huge amount of blood transfused from the placenta than cord clamping done promptly after delivery [3] [4]. However, delayed cord clamping may not be possible, as it could be forgotten by obstetrician or cord may have to be clamped promptly in case of fetal distress or complications at birth [5]. In such cases, we perform umbilical cord milking to transfer the extra blood to decrease blood transfusions and augment haemoglobin in both preterm and term infants. Both umbilical cord milking and delayed cord clamping have been related to high iron stores in neonates [6], but it may strongly affect the cerebral blood flow dynamics [4]. Delayed cord clamping, in which we clamp the cord after 30 to 180 seconds of birth, permits the transfer of blood from placenta to the newborn, makes the hematological values and iron stores in both preterm [7] and term infants better [8] [9], decreases anemia, decreases the need for blood transfusion, improves cerebral oxygenation in earlier born babies [10] and provides considerable amount of placental stem cells to the baby without causing any adverse effects to the mother [11] [12]. A previous study has shown that there is a transfer of about 80ml of blood from the placenta at 1 minute after birth, reaching about 100 ml at 3 minutes after birth.
These additional amounts of blood can supply extra iron reaching 40–50 mg/kg of body weight. When this extra iron is added to the nearly 75 mg/kg of body iron that a full-term neonate is born with, the total volume of iron can reach 115–125 mg/kg of body weight, which may help to avoid iron deficiency anaemia during the first 12 months of life. Stabilization of the circulatory system of the neonate during the first 24 hours of life occurs if we delay cord clamping for minimum 30 seconds, leading to less need for volume therapy, transfusion and inotropic support, decreases the need for given cell transfusions, decreases the occurrence of intraventricular hemorrhage and improves neuro-developmental outcome [13]. Although apparent benefits in cord clamping after delivery 30-45 seconds, it can prevent neonatal resuscitation. So, the delay in clamping of the cord is not preferred in extremely low birth weight newborns [14]. As a substitutive manner, umbilical cord milking towards the neonate before clamping usually lasts < 5 seconds, therefore, will not prevent resuscitation of the baby. Recent studies have found that umbilical cord milking also leads to significant increase in haemoglobin in both premature [15] [16] and term infants with milking being performed either with clamped [6] or unclamped umbilical cord [17]. At the age of 6 months, high body iron stores were found in delayed-clamped babies in comparison to early-clamped infants by about 27 mg of iron. Delayed cord clamping in newborn found to decrease blood values of lead, because of improved iron status during infancy [18]. Delayed cord clamping was found to be related to some adverse reactions; it may augment rates of hyperbilirubinemia, polycythemia, and transient tachypnea in the neonate but, has never been proven to increase the rate of symptomatic neonatal disease or blood loss in mothers [19]. All healthy newborns show a drop in red blood cells values during the first weeks of life which is due to multiple physiological factors. In sick preterm infants, it occurs due to several additional predisposing causes; the most important of them is phlebotomy which is blood loss for the sake of laboratory testing. The nadir haemoglobin value in healthy term newborns rarely decreases below 10 g/dl at 10 to 12 weeks of age [20]. As this decline in haemoglobin value after birth in term infants is well tolerated and no need for therapy; it is generally known as the “physiological anaemia of infancy.” On the contrary, this drop is immediate, and the blood haemoglobin concentration descends to lower levels in premature infants weighed 1.0 to 1.5 kg at delivery to approximately 8 g/dl in infants, and approximately 7 g/dl in infants weighed < 1 kg at birth.
For this reason, because the marked decrease in haemoglobin concentration that found in many extremely low birth weight neonates is usually seen with abnormal clinical signs and need for allogeneic red blood cell transfusions [21]. Iatrogenic anaemia caused by multiple blood sampling for laboratory investigations is not uncommon and with no symptoms in babies who were born prematurely. Signs and symptoms of hypovolemic shock can become marked and life-threatening to the degree that warrants replacement of blood loss in the newborn baby when losses reach 20% of total blood volume. We should record the amount of the collected blood to prevent any unwanted iatrogenic losing of blood. Chronic losing of blood and moderate haemorrhage usually is asymptomatic in babies except for some pallor. Both term and preterm infants should be sent home on iron supplementation, either as formulas fortified with iron or as a supplementation by the mouth of 2-3 mg/kg per day elemental iron especially infants on breastfeeding. Enteral iron supplementation is available and safe for newborns with birth weight <1301 gram. Early transfusion protocols called for infants to be transfused with “fresh” RBCs (less than 7 days old). The target was to increase the life of cells in vivo and decrease the risk of hyperkalemia and acidosis. At approximately 15 milliliters per kilogram body weight, tiny babies require relatively small volumes of blood per transfusion [22]. Also; an erythropoietin is sometimes needed to limit red blood cell transfusion where the initial r-erythropoietin trials in very low birth weight infants demonstrated that administration of the drug resulted in reticulocytosis with an increase in hematocrit. Furthermore, most r-erythropoietin-exposed infants received fewer and lower volumes of red blood cell transfusion during the study period. This finding was strongest in stable, growing preterm infants, most of whom had received multiple blood transfusions prior to study entry [23].
This randomised control study, from June to December 2017, included 250 pregnant women starting from ≥ 37 weeks’ gestational age attending at El Galaa teaching hospital; to compare the effect of milking of umbilical cord versus delayed cord clamping on infant haemoglobin level at 6 weeks after delivery. The mothers were pregnant women at or above 37 weeks of gestation, with a single baby, free of other medical disorder (Diabetes-hypertension-cardiac problems-renal, hepatic, etc.) and free of other obstetric complications, e.g.: (antepartum haemorrhage, Preeclampsia, etc.). Mothers with twins’ pregnancy, preterm delivery (< 37 weeks, patient should be sure of date and date confirmed by ultrasound, e.g. biparietal diameter and femur length), prolonged rupture of membranes (> 18 hours), fever or foul smelling liquor, antepartum hemorrhage, pregnancy-induced hypertension or diabetes mellitus and history of maternal liver or kidney disease or any other systemic illness were excluded from the study. Written informed consent was obtained from the parents and was included in the medical record. The Ethical Committee of both the National Research Centre and Faculty of Medicine, Ain Shams University have approved the study. Each woman was subjected to the following:
- General examination: pulse, blood pressure and temperature.
- Systemic examination: head and neck, cardiac, chest, abdominal and neurological examination.
- Abdominal: Fundal, umbilical and pelvic grips were done to assess gestational age by fundal height, assess fetal lie, and assess fetal presentation.
- Ultrasound evaluation to confirm fetal presentation, assess fetal growth status and amniotic fluid index. Fetal measurements include biparietal diameter, head circumference and femur length.
- After labour records include a neonatal requirement for resuscitation, admission in a neonatal intensive-care unit (NICU) and Apgar score of the fetus at 1 minutes and 5 minutes.
Neonatal haemoglobin measurement (A sample of cord blood was taken immediately after delivery of haemoglobin) by Easy Touch GCHb device. The measuring range for haemoglobin: 7-26 g/dl (1.1-33.3 mmol/L). Minimal sample volume for haemoglobin analysis: 2.6 µl.
The EasyTouch® GCHb system is made for self-testing of Glucose, Cholesterol and Hemoglobin levels in the blood.
Data were analysed using Statistical Program for Social Science (SPSS) version 20.0. Quantitative data were expressed as the mean± standard deviation (SD). Qualitative data were expressed as frequency and percentage.
Non parametric data was represented by median and range.
Data were analysed to test the statistically significant difference between groups.
For quantitative data (mean ± SD), student t-test was used to compare between 2 groups.
For qualitative data (frequency and proportion), the chi-square test was used.
The correlation coefficient was done to test the association between variables.
P is significant if ≤ 0.05 at a confidence interval of 95%.
Based on the previous study; comparing early versus delayed clamping of the cord and comparing early clamping versus milking of the cord [19], the expected effect size is 0.5. At an alpha level of 0.05 and a power of the study of 0.9, a total sample of 248 cases is required to elicit the difference between groups. Two cases will be added to each group to guard against fallacies in laboratory measurements. Thus, each group will include 125 women delivering a singleton fetus at term vaginally.
Results
The current study was conducted at EL Galaa Teaching Hospital during the period between June and December 2017. A total of 250 pregnant women were included in the current study. The demographic and obstetric characteristics of the study population are shown in (Table 1).
Table 1.
Demographic data of included women
| Group 1 N = 125 | Group 2 N = 125 | t/Z* | P value | |
|---|---|---|---|---|
| Age (years) | 20-37 | 20-38 | -1.195 | 0.233 |
| 25.6 ± 3.2 | 26.2 ± 4.4 | |||
| Parity (years) | 0-4 | 0-4 | 7190.500* | 0.258 |
| 1 ± 1 | 1 ± 1 | |||
| Gestational Age (weeks) | 37-40 | 37.14-40 | -0.516 | 0.606 |
| 38.93 ± 0.90 | 38.99 ± 0.96 | |||
| Gravidity(median) | 3 | 2 | 6833.500* | 0.078 |
| (1-8) | (1-6) | |||
| Hemoglobin of mother | 12.1-8.7 | 11.7-8.5 | 1.855 | 0.065 |
| 10.4 ± 0.6 | 10.3 ± 0.7 |
Group 1: delayed umbilical cord; Group 2: milking umbilical cord; SD standard deviation Data presented as a range, mean ± SD; range, median Z*: Mann-Whitney All mothers’ data taken within one week before delivery. And mother haemoglobin is taken within one week.
Regarding the mode of umbilical cord clamping, there was a significant statistical difference between cases delivered by milking umbilical cord and those delivered by a delayed umbilical cord in haemoglobin after 6weeks, but this deference is not important clinically, regarding the haemoglobin level as shown by (Table 2).
Table 2.
Comparison regarding fetal haemoglobin between the first day and 6 weeks after delivery
| Group 1 (n = 125) | Group 2 (n = 125) | t | P value | |
|---|---|---|---|---|
| HB F1 | 14.3-17.3 | 13.9-18.0 | 1.926 | 0.055 |
| 15.9 ± 0.6 | 15.8 ± 0.7 | |||
| HB F6W | 8.9-11.7 | 9.6-11.9 | -3.804 | 0.001* |
| 10.4 ± 0.5 | 10.6 ± 0.5 |
SD: standard deviation; HBF1: Hemoglobin of fetus during first-day p<0.001*; Hbf6w: haemoglobin of fetus after 6 weeks Data presented as a range, mean ± SD.
There was a significant positive correlation between haemoglobin of mother and haemoglobin of fetus during the first day, and 6 weeks, anaemic mother affected fetus after delivery (Table 3).
Table 3.
Agreement between the mode of delivery of umbilical cord and positive pressure ventilation
| Group (250) | X2 | P-value | ||
|---|---|---|---|---|
| G1 (125) | G2 (125) | |||
| PPv | 9 (7.2%) | 9 (7.2%) | 1.211 | 0.271 |
Ppv: positive pressure ventilation. Chi-square (X2) test was used. Data presented as a probability (p-value).
Figure 1.
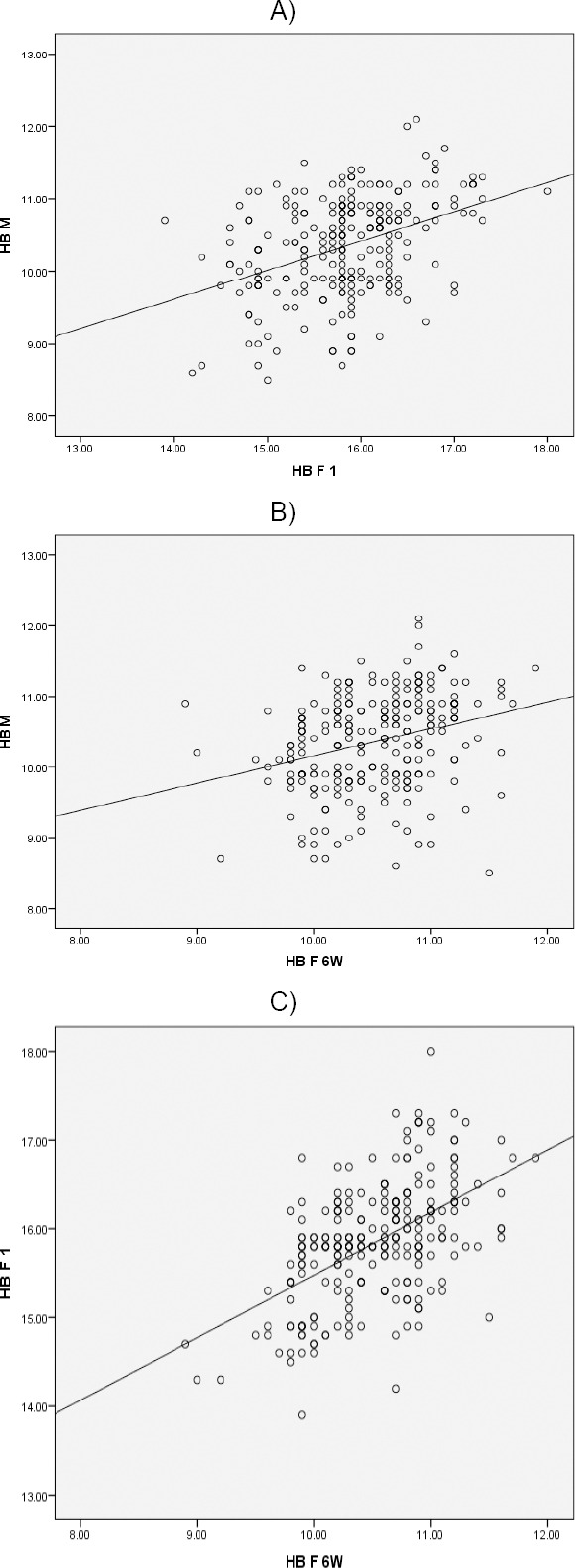
A) Positive correlation and significant between Hb M and Hb F1; P<0.001*; B) Positive correlation and significant between Hb M and Hb F6w; P<0.001*; C) Positive correlation and significant between Hb F1 and Hb F6w; P<0.001*
There was a significant positive correlation between haemoglobin of fetus after first day and haemoglobin of the fetus after 6 weeks (Table 4).
Table 4.
Correlation between haemoglobin of fetus after first day and fetus at 6 weeks
| HB F 1 | ||
|---|---|---|
| HB F 6W | r | 0.534 |
| p | <0.001* | |
Pearson’s correlation coefficient p<0.001*.
Discussion
This study was done at EL Galaa teaching hospital in the period from June till December 2017. Some 250 pregnant women (20-40) years participated in our study. They were divided into two groups to make a comparison between the effects of Milking of umbilical cord versus delayed cord clamping on infant Hemoglobin level at 6 weeks after birth. The first group (delayed umbilical cord clamping) 125 patients aged (20-37) years with (mean ± SD 25.6 ± 3.2) with gestational age (37-40) weeks with (mean±SD 38.93 ± 0.9). The second group (milking umbilical cord) 125 patients aged (20-38) years with (mean ± SD 26.2 ± 4.4) with gestational age (37.14-40) weeks with (mean ± SD 38.99 ± 0.96). According to fetal haemoglobin on the first day, there was no statistical significance between the two groups that agree with other studies [23] [17]. Concerning the fetal haemoglobin at 6 weeks, there was a strongly elevated haemoglobin level with umbilical cord milking rather than delayed cord clamping. Different results were observed in a study had done by Yadav et al., 2015 to estimate hemodynamic parameters between 3 groups. The first group, they adopted umbilical cord milking, the second group delayed cord clamping was performed, the third one delayed cord milking was done, there was no statistical difference between the two groups concerning the haemoglobin levels at 6 weeks. The number of women in each group was 93 in their study, and in our study, each group has 125 participants [24]. Although milking was done 3 times in their study, we milked the cord 5 times in ours. Our study showed that milking of umbilical cord for 5 times improved haemoglobin level at 6 weeks of age in term infants in comparison to delayed cord clamping. However, haemoglobin levels on the first day were the same as in the two other groups. However, no significant change in hemodynamic parameters or clinical adverse effects in the first 24 h of life were found, haemoglobin and hematocrit were markedly elevated in both delayed cord clamping with milking cut cord group in the first 48 h of life [24]. In the process of delayed cord clamping, another 15 to 40 ml/kg of blood is passed to the baby by permitting transfusion through the placenta to be finished [25], which is recommended by a decline in umbilical venous pressure and pulsating contractions of uterus just after birth.
Milking of the cord after finishing of delayed cord clamping may invite extra blood stored in the non-pulsatile cord in addition to the normal placental transfusion and make haematological parameters better [4] [6] [17]. Our findings were similar to other studies as they did not notice a marked difference in the mean haemoglobin in umbilical cord milking and delayed cord clamping group in the period after birth promptly [17] [24]. However, in both groups in our study, we found lower mean haemoglobin levels in comparison to the mean haemoglobin values in a previous study [17]. Different from our results, a study examined 58 preterm infants between 24 and 32 weeks of gestation where clamping of the cord was postponed 30 seconds in delayed cord clamping group, and they milked the cord four times at a similar rate of 10cm/s [17]. In our study, the haemoglobin immediately after birth was lower than those shown in other studies [15] [17]. This can be attributed to non-healthy eating habits and noncompliance with oral iron therapy during the period of pregnancy among mothers in our population in comparison to developed countries. This might explain elevated mean haemoglobin value in umbilical cord milking group in a previous trial [17]. Our study also showed that haemoglobin in the first 48 h was the same as in umbilical cord milking and delayed cord clamping groups in full-term infants [4]. Another study showed that 3min of delayed cord clamping in term infants caused a marked improvement in serum ferritin level at the age of 6 months with no marked change in haemoglobin value that was different from our initial results at the age of 6 weeks in delayed cord clamping group [26].
Although, this outcome of delayed cord clamping did not continue until the age of 12 months [27]. One of the studies showed the place of the baby before clamping of the cord does not cause any effect on the amount of transfusion through the placenta [28]. We performed the umbilical cord milking five times at a speed of 10cm/sec.. However, other studies milked the umbilical cord 4 times at similar speed [17]. In our study, we perform umbilical cord milking before clamping the umbilical cord from the mother’s clitoris to the umbilicus of the baby; similar to other studies where the milking of the cord was done during its attachment to the placental end [29][30]. However, the comparison between milking before and after clamping of the cord was not done, more blood is normally passing if umbilical cord milking is done before clamping. Different from Yadav et al., umbilical cord milking was done after clamping umbilical cord at a distance of 25cm from the neonate end [24]. Concerning the gestational age, there was no statistical difference between the two groups which is similar to Yadav et al., [24]. In this study, we found that no statistical difference between the two groups according to the mean of hemoglobin of the mother that is similar to other studies which showed also no statistical difference between the three groups according to the mean hemoglobin of the mother [14] [24]. Concerning Apgar score at 1 minute and 5 minute, we found no statistical difference between the two groups. The same results were found by other study [4]. There were no marked statistical differences between women of 3 groups regarding neonatal measures of birth weight, 1-min Apgar score and 5-min Apgar score [31]. In our study; concerning maternal blood transfusion, postpartum hemorrhage, and retained placenta after delivery there was no marked statistical difference between the two groups. Also, there was no great difference between the two groups according to positive pressure ventilation of fetus after delivery. The clear strength of our study, that it was a randomized controlled trial with a proper sample size, has few points of weakness and a short duration of follow up. A longer follow up till 6 to 12 months of age is needed to confirm if the primary advantage in hemoglobin remains later during infancy and early childhood. The weakness of our study is that this method may not be suitable for increasing the amount of placental transfusion for newborns with a short cord. We did not measure the real amount of blood transfused in each newborn. Nowadays, there is no direct, correct, easy and rapid way to measure blood volume. We had relied on clinical history and examination to exclude infection at 6 month of age rather than on lab investigation, such as CRP. Another restriction of our study is that we did not measured serum ferritin as it reflects real iron stores of the infant along with hemoglobin.
In conclusion, milking of umbilical cord blood after its clamping improves some important haematological parameters for neonates, particularly in countries characterised by increased rates of neonatal and childhood anaemia.
Recommendations: Umbilical cord milking can be used in term newborns as a routine or in cases when delayed cord clamping is not suitable, future trials with more follow-up are recommended to construct the continuity of haemoglobin benefit and serum ferritin after that in infancy. Other measures as oxygenation of the brain and amount of cerebral blood could also have been more evaluated. Also, superior vena-cava flow values & ECHO can be demonstrated to see the effect of the extra amount of blood passed on the cardiac function of the baby and finally, further studies of other methods of cord clamping perfectly define the future procedures to be done. That will strengthen the correct interpretation of the outcome, supports further meta-analysis, and will lead markedly to the definition of appropriate practice in this new unknown area.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Bosselmann S, Mielke G. Sonographic assessment of the umbilical cord. Geburtshilfe und Frauenheilkunde. 2015;75(8):808. doi: 10.1055/s-0035-1557819. https://doi.org/10.1055/s-0035-1557819 PMid:26366000 PMCid: PMC4554503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlo WA, Ambalavanan N, Kliegman RM, et al. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier;chap 105; 2016. https://archive.org/.../NelsonTextbookOfPediatrics20thEd2016/Nelson%20Textbook%2 . [Google Scholar]
- 3.McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;2(2):CD004074. doi: 10.1002/14651858.CD004074.pub2. https://doi.org/10.1002/14651858.CD004074.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Jaiswal P, Upadhyay A, Gothwal S, Chaudhary H, Tandon A. Comparison of umbilical cord milking and delayed cord clamping on cerebral blood flow in term neonates. The Indian Journal of Pediatrics. 2015;82(10):890–5. doi: 10.1007/s12098-015-1734-2. https://doi.org/10.1007/s12098-015-1734-2 PMid:26008758. [DOI] [PubMed] [Google Scholar]
- 5.Dash MB, Murali R. Effect of delayed cord clamping on hemoglobin level of newborns. The Indian Journal of Pediatrics. 2014;10(81):1113–4. doi: 10.1007/s12098-014-1441-4. https://doi.org/10.1007/s12098-014-1441-4 PMid:24781434. [DOI] [PubMed] [Google Scholar]
- 6.Upadhyay A, Gothwal S, Parihar R, Garg A, Gupta A, Chawla D, Gulati IK. Effect of umbilical cord milking in term and near term infants: randomized control trial. American journal of obstetrics and gynecology. 2013;208(2):120.e1. doi: 10.1016/j.ajog.2012.10.884. https://doi.org/10.1016/j.ajog.2012.10.884 PMid:23123382. [DOI] [PubMed] [Google Scholar]
- 7.Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev. 2004;4(10) doi: 10.1002/14651858.CD003248.pub2. https://doi.org/10.1002/14651858.CD003248.pub2 PMid:15495045. [DOI] [PubMed] [Google Scholar]
- 8.Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. Jama. 2007;297(11):1241–52. doi: 10.1001/jama.297.11.1241. https://doi.org/10.1001/jama.297.11.1241 PMid:17374818. [DOI] [PubMed] [Google Scholar]
- 9.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evidence-Based Child Health: A Cochrane Review Journal. 2014;9(2):303–97. doi: 10.1002/ebch.1971. https://doi.org/10.1002/ebch.1971 PMid:25404605. [DOI] [PubMed] [Google Scholar]
- 10.Baenziger O, Stolkin F, Keel M, von Siebenthal K, Fauchere JC, Kundu SD, Dietz V, Bucher HU, Wolf M. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics. 2007;119(3):455–9. doi: 10.1542/peds.2006-2725. https://doi.org/10.1542/peds.2006-2725 PMid:17332197. [DOI] [PubMed] [Google Scholar]
- 11.Mathew JL. Timing of umbilical cord clamping in term and preterm deliveries and infant and maternal outcomes: a systematic review of randomized controlled trials. Indian pediatrics. 2011;48(2):123–9. doi: 10.1007/s13312-011-0031-z. https://doi.org/10.1007/s13312-011-0031-z PMid:21378422. [DOI] [PubMed] [Google Scholar]
- 12.Ranjit T, Nesargi S, Rao PS, Sahoo JP, Ashok C, Chandrakala BS, Bhat S. Effect of early versus delayed cord clamping on hematological status of preterm infants at 6 wk of age. The Indian Journal of Pediatrics. 2015;82(1):29–34. doi: 10.1007/s12098-013-1329-8. https://doi.org/10.1007/s12098-013-1329-8 PMid:24496587. [DOI] [PubMed] [Google Scholar]
- 13.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane database syst rev. 2012;8(8) doi: 10.1002/14651858.CD003248.pub3. https://doi.org/10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Kilicdag H, Gulcan H, Hanta D, Torer B, Gokmen Z, Ozdemir SI, Antmen BA. Is umbilical cord milking always an advantage? The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(4):615–8. doi: 10.3109/14767058.2015.1012067. https://doi.org/10.3109/14767058.2015.1012067 PMid:25731653. [DOI] [PubMed] [Google Scholar]
- 15.Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, Takahashi S, Harada K. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born less than 29 weeks'gestation: a randomized controlled trial. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2007;2007 doi: 10.1136/adc.2006.108902. [DOI] [PubMed] [Google Scholar]
- 16.March MI, Hacker MR, Parson AW, Modest AM, De Veciana M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. Journal of Perinatology. 2013;33(10):763. doi: 10.1038/jp.2013.70. https://doi.org/10.1038/jp.2013.70 PMid:23867960 PMCid: PMC3916936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabe H, Jewison A, Alvarez RF, Crook D, Stilton D, Bradley R, Holden D. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstetrics & Gynecology. 2011;117(2):205–11. doi: 10.1097/AOG.0b013e3181fe46ff. https://doi.org/10.1097/AOG.0b013e3181fe46ff PMid:21252731. [DOI] [PubMed] [Google Scholar]
- 18.Chaparro CM, Fornes R, Neufeld LM, Alavez GT, Cedillo RE, Dewey KG. Early umbilical cord clamping contributes to elevated blood lead levels among infants with higher lead exposure. The Journal of pediatrics. 2007;151(5):506–12. doi: 10.1016/j.jpeds.2007.04.056. https://doi.org/10.1016/j.jpeds.2007.04.056 PMid:17961694. [DOI] [PubMed] [Google Scholar]
- 19.Andersson O, Hellström-Westas L, Andersson D, Domellöf M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. Bmj. 2011;343:d7157. doi: 10.1136/bmj.d7157. https://doi.org/10.1136/bmj.d7157 PMid:22089242 PMCid: PMC3217058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews. 2008;9(11):e520–5. doi: 10.1542/neo.9-11-e520. https://doi.org/10.1542/neo.9-11-e520 PMid:20463861 PMCid: PMC2867612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss RG. How I transfuse red blood cells and platelets to infants with the anemia and thrombocytopenia of prematurity. Transfusion. 2008;48(2):209–17. doi: 10.1111/j.1537-2995.2007.01592.x. https://doi.org/10.1111/j.1537-2995.2007.01592.x PMid:18194380 PMCid: PMC2874902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New HV, Stanworth SJ, Engelfriet CP, et al. International Forum: Neonatal Transfusion. Vox Sang. 2009;(96):62–85. doi: 10.1111/j.1423-0410.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 23.Vamvakas EC. Purported deleterious effects of “old” versus “fresh” red blood cells: an updated meta-analysis. Transfusion. 2011;51(5):1122–3. doi: 10.1111/j.1537-2995.2010.03017.x. https://doi.org/10.1111/j.1537-2995.2010.03017.x PMid:21545596. [DOI] [PubMed] [Google Scholar]
- 24.Yadav AK, Upadhyay A, Gothwal S, Dubey K, Mandal U, Yadav CP. Comparison of three types of intervention to enhance placental redistribution in term newborns: randomized control trial. Journal of Perinatology. 2015;35(9):720. doi: 10.1038/jp.2015.65. https://doi.org/10.1038/jp.2015.65 PMid:26087318. [DOI] [PubMed] [Google Scholar]
- 25.Blouin B, Penny ME, Maheu-Giroux M, Casapía M, Aguilar E, Silva H, Creed-Kanashiro HM, Joseph SA, Gagnon A, Rahme E, Gyorkos TW. Timing of umbilical cord-clamping and infant anaemia: the role of maternal anaemia. Paediatrics and international child health. 2013;33(2):79–85. doi: 10.1179/2046905512Y.0000000036. https://doi.org/10.1179/2046905512Y.0000000036 PMid:23925280. [DOI] [PubMed] [Google Scholar]
- 26.Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, Lozoff B, Jacobson SW. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;2009 doi: 10.1542/peds.2009-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson O, Domellöf M, Andersson D, Hellström-Westas L. Effect of delayed vs early umbilical cord clamping on iron status and neurodevelopment at age 12 months: a randomized clinical trial. JAMA pediatrics. 2014;168(6):547–54. doi: 10.1001/jamapediatrics.2013.4639. https://doi.org/10.1001/jamapediatrics.2013.4639 PMid:24756128. [DOI] [PubMed] [Google Scholar]
- 28.Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, Prudent LM. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. The Lancet. 2014;384(9939):235–40. doi: 10.1016/S0140-6736(14)60197-5. https://doi.org/10.1016/S0140-6736(14)60197-5. [DOI] [PubMed] [Google Scholar]
- 29.Hosono S, Mugishima H, Fujita H, Hosono A, Okada T, Takahashi S, Masaoka N, Yamamoto T. Blood pressure and urine output during the first 72 hours in infants born less than 29 weeks'gestation related to umbilical cord milking. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2009;2009 doi: 10.1136/adc.2008.142935. [DOI] [PubMed] [Google Scholar]
- 30.Patel S, Clark EA, Rodriguez CE, Metz TD, Abbaszadeh M, Yoder BA. Effect of umbilical cord milking on morbidity and survival in extremely low gestational age neonates. American journal of obstetrics and gynecology. 2014;211(5):519e1. doi: 10.1016/j.ajog.2014.05.037. https://doi.org/10.1016/j.ajog.2014.05.037 PMid:24881823. [DOI] [PubMed] [Google Scholar]
- 31.Jouannelle C, Giansily-Blaizot M, Monpoux F, Casagrande F, Poirée M, Bérard E. Spontaneous umbilical cord haematoma and congenital factor VII deficiency. Haemophilia. 2012;18(1):e24–5. doi: 10.1111/j.1365-2516.2011.02664.x. https://doi.org/10.1111/j.1365-2516.2011.02664.x PMid:21967451. [DOI] [PubMed] [Google Scholar]


