Abstract
BACKGROUND:
The composition of the root canal filling materials together with the apical limit of the root canal obturation affect the complete periapical healing after root canal therapy.
AIM:
This study was performed to evaluate and compare the periapical healing in response to calcium-silicate (iRoot SP) and calcium-hydroxide (Apexit) based-sealers.
MATERIAL AND METHODS:
Seventy-two upper premolars root canals of six dogs were used. The teeth were randomly assigned to four groups: Group one: roots were obturated using gutta-percha and Apexit-sealer; Group two: roots were obturated using gutta-percha&iRoot SP-sealer; Group three: the teeth were left open without obturation; Group four: where healthy teeth were used as a negative control. Teeth were evaluated after one, two and three months. The newly formed mineralised apical tissue and the periapical inflammatory infiltrate of the obtained photomicrographs were evaluated, and scorings were statistically-analysed.
RESULTS:
The mean percentage of the periapical inflammatory infiltrates and mineralisation scoring after one, two and three months evaluation period were not significantly different among the four groups (P > 0.05).
CONCLUSIONS:
Regardless of the sealer used, iRoot SP and Apexit promote healing of periapical tissues. IRoot SP sealer showed early insignificant more partial and almost full healing after two and three months.
Keywords: Bioceramic sealer, Calcium hydroxide, Root canal sealer
Introduction
The main objective of endodontic therapy is to eliminate microorganisms, their products, and sub-products [1] [2]. Complete periapical healing after root canal therapy may be influenced by the apical limit of the root canal obturation and the composition of the filling material [3]. Root canal sealers used for the treatment of teeth with periapical lesions should be biologically compatible and allow for periapical healing due to their close contact with living periapical tissues over a long period [4]. Their biocompatibility may be responsible for completing the histologic repair of periapical tissues after root canal treatment, without triggering any adverse reactions, including, inflammation, carcinogenicity, toxicity or allergy [5].
Sealers currently being used in clinical practice include resin-based, zinc oxide-eugenol-based, glass ionomer-based, calcium hydroxide-based, and silicone-based endodontic sealers [6] [7]. iRoot SP (Innovative BioCreamix Inc, Vancouver, Canada) is a ready-to-use injectable white hydraulic cement paste developed for permanent root canal filling and sealing applications.
According to the manufacturer, iRoot SP is an aluminium-free, calcium silicate and resin-based material similar in composition to white MTA and is hydrophilic that utilises the water inherent in the dentinal tubules to drive the hydration reaction. The iRoot SP chemical composition includes zirconium oxide, calcium silicates, calcium phosphate, calcium hydroxide, filler, and thickening agents. It has both excellent physical properties and antimicrobial activity [8]. The calcium silicates content in the powder hydrate to produce a calcium silicate hydrate gel C-H-C and calcium hydroxide CH. The calcium hydroxide reacts with the phosphate ions in the dentinal fluid and precipitates hydroxyapatite and water [9].
On the other hand; according to the manufacturer, Apexit (Ivoclar Vivadent, Schaan, Lichtenstein) is a two-component (base and activator) calcium hydroxide-based sealer. The base is formed mainly of calcium hydroxide/calcium oxide, hydrated collophonium, fillers and other auxiliary materials. The activator contains disalicylate, bismuth hydroxide/bismuth carbonate, fillers and other auxiliary materials. The material sets by complex formation. Apexit promotes hard tissue formation but tends to dissolve over time and may thus compromise the endodontic seal [10].
The aim of the present study was to in vivo to evaluate and compare the periapical healing in response to a calcium silicate based sealers (iRoot SP) and a calcium hydroxide-based sealer (Apexit).
Materials and Methods
All animal procedures in this study were performed according to the protocols reviewed and approved by the Ethical Committee of National Research Centre, Giza, Egypt in compliance with the applicable ethical guidelines and regulations of the international guiding principles for biomedical research involving animals.
Seventy-two root canals from the upper premolars of six dogs (ages 12-18 months and weight 8-15 kg) were used during this study. The animals were anaesthetized intravenously with sodium thiopental (30 mg/kg body weight, Thiopental eipico, Eipico, Egypt). Standardized periapical radiographs were taken for later comparison with that obtained after root canal treatment. The dental arch was isolated using a rubber dam and 2% chlorhexidine gluconate was used as an antiseptic agent. Access cavity was prepared, and pulp was removed.
The apical cementum layer characteristic of dogs’ teeth was then perforated with the sequential use of size #15 to #30 K-files, thus creating standardised apical openings [11]. Root canals were left exposed to the oral cavity for 7 days to allow microbial contamination in the experimental groups. Access openings were then sealed using zinc oxide-eugenol-based temporary filling (Coltosol-ldent). After 45 days, the development of apical periodontitis was radiographically confirmed.
The root canals were instrumented to the working length up to a size #60 K-file. A size #30 K-file was taken to the total root length to ensure apical patency. After final irrigation with saline solution, the root canals were dried and then filled with 14.3% buffered EDTA, pH 7.4 (Meta BioMed Co., Chungbuk, Korea), for 3 minutes then rinsed using saline solution and dried.
The teeth were then randomly assigned to four groups as follows: group 1: (n = 24); roots were obturated using gutta-percha and Apexit sealer with lateral condensation technique; group 2: (n = 24) roots were obturated using gutta-percha and I-Root SP sealer, Group 3: (n = 12) the teeth were left open without obturation or coronal restoration (+ve control group) and Group 4: (n = 12) where healthy teeth were used as a -ve control samples for comparison with the findings obtained from the experimental groups. Amalgam restorative material was used for coronal restorations for all teeth. The dogs were fed a soft diet for 3 to 5 days after dental procedures.
Dogs were then divided into three groups (n = 2) according to the post evaluation periods (1 month, 2 months and 3 months). At the end of each evaluation period, standardised radiographs were taken to detect the presence or absences of periapical radiolucency then 2 dogs were sacrificed by the use of overdose of sodium thiopental. The mandibles were removed immediately by dissection of the surrounding soft tissues. The experimental teeth with the surrounding bone were sectioned using the electrical surgical saw. Block sections were fixed in 10% formalin and decalcified in EDTA.
Three sections from each block were cut 4-6 µm thick and stained with hematoxylin-eosin. The sections were evaluated by two blind evaluators at different magnifications using a CX21 Olympus microscope (Tokyo-Japan). The following parameters were evaluated and scored as follows: (a) newly formed mineralized apical tissue: absent (0), partial (1), almost complete (2) and complete (3); and (b) periapical inflammatory infiltrate: absent (0), mild (1), moderate (2), and severe (3).
A non-parametric one-way ANOVA (Kruskal–Wallis) test followed by paired group comparisons using Mann–Whitney U tests at a 5% significance level were used to analyse the effect of the two materials on the bone deposition (the mineralisation process) and inflammation together with the effect of time on the inflammation and mineralisation. Statistical analysis was performed with IBM® SPSS® Version 22 for Windows (SPSS Inc., IBM Corporation, NY, USA).
Results
Percentages of periapical inflammatory infiltrate and mineralisation scoring after 1, 2, 3 months evaluation period for the tested groups are listed in Table 1 and 2.
Table 1.
Percentages of periapical inflammatory infiltrate scorings after 1, 2 and 3 months evaluation period for the tested groups
| Post-operative evaluation periods | p-value | ||||
|---|---|---|---|---|---|
| 1 Month | 2 Months | 3 Months | |||
| Scoring % | |||||
| -ve control | 0 | 100.0% | 100.0% | 100.0% | 1.00 NS |
| 1 | 0.0% | 0.0% | 0.0% | ||
| 2 | 0.0% | 0.0% | 0.0% | ||
| 3 | 0.0% | 0.0% | 0.0% | ||
| a | a | a | |||
| +ve control | 0 | 0.0% | 0.0% | 0.0% | 1.00 NS |
| 1 | 0.0% | 0.0% | 0.0% | ||
| 2 | 100.0% | 0.0% | 0.0% | ||
| 3 | 0.0% | 100.0% | 100.0% | ||
| c | c | c | |||
| iRoot SP | 0 | 60.0% | 60.0% | 60.0% | 0.459 NS |
| 1 | 40.0% | 40.0% | 20.0% | ||
| 2 | 0.0% | 0.0% | 20.0% | ||
| 3 | 0.0% | 0.0% | 0.0% | ||
| b | b | b | |||
| Apexit | 0 | 20.0% | 80.0% | 40.0% | 0.308 NS |
| 1 | 60.0% | 20.0% | 60.0% | ||
| 2 | 20.0% | 0.0% | 0.0% | ||
| 3 | 0.0% | 0.0% | 0.0% | ||
| b | b | b | |||
| p- value | 0.011* | 0.006* | 0.008* | ||
Mean scoring % with different lower case letters in the same column indicate statistically significant difference.
significant (P < 0.05); ns, non-significant (p > 0.05); NS = Non-Significant.
Table 2.
Percentages of mineralisation scoring after 1, 2, 3 months evaluation period for the tested groups
| Post-operative evaluation periods | p-value | ||||
|---|---|---|---|---|---|
| 1 Month | 2 Months | 3 Months | |||
| Scoring % | |||||
| -ve control | 0 | 0.0% | 0.0% | 0.0% | 1.00 NS |
| 1 | 0.0% | 0.0% | 0.0% | ||
| 2 | 0.0% | 0.0% | 0.0% | ||
| 3 | 100.0% | 100.0% | 100.0% | ||
| a | a | a | |||
| +ve control | 0 | 100.0% | 100.0% | 100.0% | 1.00 NS |
| 1 | 0.0% | 0.0% | 0.0% | ||
| 2 | 0.0% | 0.0% | 0.0% | ||
| 3 | 0.0% | 0.0% | 0.0% | ||
| c | c | c | |||
| iRoot SP | 0 | 60.0% | 40.0% | 40.0% | 0.545 NS |
| 1 | 40.0% | 40.0% | 20.0% | ||
| 2 | 0.0% | 20.0% | 40.0% | ||
| 3 | 0.0% | 0.0% | 0.0% | ||
| b | b | b | |||
| Apexit | 0 | 40.0% | 60.0% | 60.0% | 0.779 NS |
| 1 | 60.0% | 40.0% | 40.0% | ||
| 2 | 0.0% | 0.0% | 0.0% | ||
| 3 | 0.0% | 0.0% | 0.0% | ||
| b | b | b | |||
| p- value | 0.003* | 0.014* | 0.006* | ||
Mean scoring % with different lower case letters in the same column indicate statistically significant difference.
significant (P < 0.05); ns, non-significant (P > 0.05); NS = Non-Significant.
Although a statistically significant difference was revealed between the control groups and the iRoot SP and Apexit sealer groups; no statistical differences were found between the two sealers regarding inflammatory response and mineralisation. However, results showed early signs of mild and moderate inflammatory infiltrate in Apexit sealer when compared with iRoot SP. After two and three months; iRoot SP sealers showed insignificant more partial and almost complete mineralisation scorings than Apexit type.
Representative photomicrography of iRoot SP, Apexit and the control groups’ specimens after 1, 2 and 3 months evaluation periods are shown in Figures 1 and 2. For iRoot SP sealed specimen; one month evaluation period showed healing with connective tissue (C.T.) with no signs of bone formation (Figure 1A); scattered osteocytes appeared after 2 months evaluation period (Figure 1B); while new bone formation foci appeared after 3 months (Figure 1C).
Figure 1.
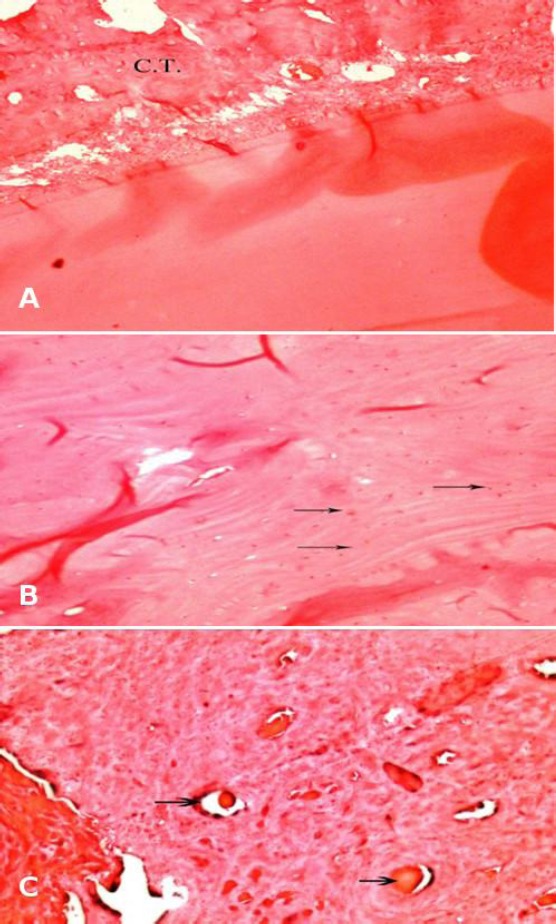
Photomicrograph of representative HE-stained microscopic sections in specimen filled with iRoot SP sealer after: (A) one month, showing healing with connective tissue (C.T.) with no signs of bone formation (x100); (B) two months showing bone formation appeared as scattered osteocytes (arrows) (x100) and (C) three months, showing new bone formation foci with no signs of inflammation (x200)
Figure 2.
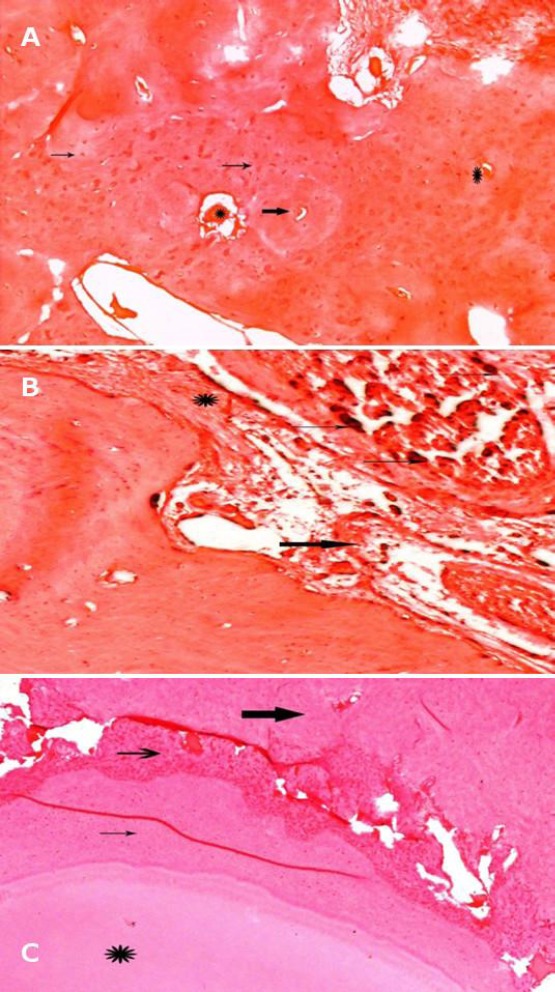
Photomicrograph of representative HE-stained microscopic sections in specimen filled with Apexit sealer after: (A) one month showing moderate inflammatory areas (thin arrows), moderate angiogenesis (thick arrows) with some calcified foci (* ) (x100 ); (B) two months showing new blood vessels formation with mild mineralization and mild inflammation (*) (x100) and (C) three months showing the root tip (*) with periodontal ligament regeneration (thin arrow ) new bone formation (thick arrow ) (x100)
On the other hand, the photomicrographs of Apexit sealed specimen showed moderate inflammatory areas and moderate angiogenesis with some calcified foci after one month evaluation period (Figure 2A). New blood vessels formation with mild mineralisation and mild inflammation appeared after 2 months (Figure 2B); while new bone formation and periodontal ligament regeneration appeared after 3 months (Figure 2C).
Discussion
Sealers are responsible for preventing reinfection and filling of irregularities in the prepared canal system. Root canal sealer should support and accelerate the repair and the regenerative processes of the injured periradicular tissues [12].
The experimental model of lesion induction used in this study was based on previously established criteria [12]. In this study, the apical cementum barrier of roots was perforated to obtain a patent foramen which is considered as an important step in foraminal debridement [14] [16].
Alkalinity and the ability of a material to release calcium ions were suggested to be responsible for stimulation of repair by deposition of mineralised tissue through the activation of alkaline phosphatase. Additionally, the released calcium ions extracellularly have been reported to induce BMP-2 expression. Calcium ions would also react with the carbonate ions present in the periapical tissue, leading to precipitation of calcite granules, which would trigger the process of deposition of mineralised tissue [17].
Calcium hydroxide-based sealers, are known for their remineralisation effect and antibacterial properties due to the release of hydroxyl ions. Some drawbacks such as poor cohesive strength, greater solubility, marginal leakage and concerns regarding weakening of roots; led to the search for newer calcium silicate based sealers with properties similar to MTA but with lower cost, shorter setting time and proper handling characteristics [18].
iRoot SP has been introduced for use as a bioactive, alkaline, injectable root canal sealer with certain antibacterial properties, of high toxicity when tested in cell culture study on L929 cells but with nontoxic extract [19]. The effect of iRoot SP on the viability of RAW 264.7 macrophages was also tested and proved to be non-toxic [20]. This study was directed to evaluate and compare the periapical healing in response to a calcium silicate based sealers (iRoot SP) with that of a calcium hydroxide-based sealer (Apexit).
It has been suggested that calcium oxide in calcium silicate based sealers reacts with the tissue fluids eventually producing calcium hydroxide. The produced calcium hydroxide would then dissociate into hydroxide and calcium ions causing an increase in the pH of the medium.
The insignificant difference between the tested sealers regarding the presence of inflammatory infiltrate and mineralisation of apical tissue; is probably due to the ability of both materials to release calcium and hydroxyl ions. However early signs of mild and moderate inflammatory infiltrate in case of Apexit sealer was detected. This may indicate the early release of hydroxyl ions; increasing alkalinity and stimulating inflammation in the surrounding tissue. In deeper areas of tissues, calcium hydroxide acts as a mild irritant, stimulating hard tissue formation [17]. Such finding was confirmed by the photomicrographs of Apexit where moderate inflammatory areas, moderate angiogenesis and some calcified foci were detected as early as one month evaluation period. Calcium hydroxide based formulations are known for their initial degenerative response followed by rapid mineralisation and ossification [21] [22].
The early and insignificant more partial and almost complete healing after two and three months revealed by iRoot SP than Apexit type may be the result of the hydrophilicity of the iRoot SP and the presence of nanoparticles providing a homogenous mixture, high solubility, and good flow characteristics. Diffusion of calcium and hydroxyl ions from a material depends on such characteristics. Such finding is in agreement with Chetna Dudeja et al., [23], where teeth filled with iRoot SP showed higher pH and calcium ion release when compared with a calcium hydroxide based sealer (Ultracal).
Further studies comparing cohesive strength, marginal leakage and roots weakening the effect of both materials are recommended to conclude the clinical efficiency of both materials.
In conclusion, based on the results above, it could be concluded that the investigated sealers promote healing of periapical tissues. IRoot SP sealer showed early insignificant more partial and almost complete healing after two and three months.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Wu MK, Tigos E, Wesselink PR. An l8-month longitudinal study on a new silicon-based sealer, RSA RoekoSeal:A leakage study in vitro. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2002;94(4):499–502. doi: 10.1067/moe.2002.124859. https://doi.org/10.1067/moe.2002.124859. [DOI] [PubMed] [Google Scholar]
- 2.Schäfer E, Zandbiglari T. Solubility of root?canal sealers in water and artificial saliva. International endodontic journal. 2003;36(10):660–9. doi: 10.1046/j.1365-2591.2003.00705.x. https://doi.org/10.1046/j.1365-2591.2003.00705.x PMid:14511222. [DOI] [PubMed] [Google Scholar]
- 3.Leonardo MR, Utrilla LS, Assed S, Ether SS. Calcium hydroxide root canal Sealers—Histopathologic evaluation of apical and peripaical repair after endodontic treatment. Journal of endodontics. 1997;23(7):428–32. doi: 10.1016/s0099-2399(97)80296-8. https://doi.org/10.1016/S0099-2399(97)80296-8. [DOI] [PubMed] [Google Scholar]
- 4.Al-Awadhi S, Spears R, Gutmann JL, Opperman LA. Cultured primary osteoblast viability and apoptosis in the presence of root canal sealers. J Endod. 2004;30:527–33. doi: 10.1097/00004770-200407000-00016. https://doi.org/10.1097/00004770-200407000-00016 PMid:15220652. [DOI] [PubMed] [Google Scholar]
- 5.Al-Haddad A, Ab Aziz C, Zeti A. Bioceramic-based root canal sealers:a review. International journal of biomaterials. 2016;2016 doi: 10.1155/2016/9753210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanomaru Filho M, Leonardo MR, Silva LA, Utrilla LS. Effect of different root canal sealers on periapical repair of teeth with chronic periradicular periodontitis. Int Endod J. 1998;31:85–9. doi: 10.1046/j.1365-2591.1998.00134.x. https://doi.org/10.1046/j.1365-2591.1998.00134.x PMid:9868933. [DOI] [PubMed] [Google Scholar]
- 7.Leonardo MR, Almeida WA, Da Silva LA, Utrilla LS. Histological evaluation of the response of apical tissues to glass ionomer and zinc oxide?eugenol based sealers in dog teeth after root canal treatment. Dental Traumatology. 1998;14(6):257–61. doi: 10.1111/j.1600-9657.1998.tb00849.x. https://doi.org/10.1111/j.1600-9657.1998.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. Journal of endodontics. 2009;35(7):1051–5. doi: 10.1016/j.joen.2009.04.022. https://doi.org/10.1016/j.joen.2009.04.022 PMid:19567333. [DOI] [PubMed] [Google Scholar]
- 9.Koch K, Brave D. Bioceramic technology-the game changer in endodontics. Endodontic Practice US. 2009;12:7–11. [Google Scholar]
- 10.Huang F-M, Tai K-W, Chou M-Y, Chang Y-C. Cytotoxicity of resin-, zinc oxide–eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002;35:153–8. doi: 10.1046/j.1365-2591.2002.00459.x. https://doi.org/10.1046/j.1365-2591.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 11.de Paula-Silva FW, Júnior MS, Leonardo MR, Consolaro A, da Silva LA. Cone-beam computerized tomographic, radiographic, and histologic evaluation of periapical repair in dogs'post-endodontic treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2009;108(5):796–805. doi: 10.1016/j.tripleo.2009.06.016. https://doi.org/10.1016/j.tripleo.2009.06.016 PMid:19734073. [DOI] [PubMed] [Google Scholar]
- 12.Brasil DS, Soares JA, Horta MC, Ferreira CL, Nunes E, Chaves GG, Silveira FF. Periapical repair in dog teeth:root canal adhesive filling by using the Resilon system. Journal of endodontics. 2010;36(3):482–8. doi: 10.1016/j.joen.2009.11.020. https://doi.org/10.1016/j.joen.2009.11.020 PMid:20171367. [DOI] [PubMed] [Google Scholar]
- 13.Holland R, Otoboni-Filho JA, Souza V, Nery MJ, Bernabé PF, Dezan E., Jr A comparison of one versus two appointment endodontic therapy in dogs'teeth with apical periodontitis. J Endod. 2003;29:121–4. doi: 10.1097/00004770-200302000-00009. https://doi.org/10.1097/00004770-200302000-00009 PMid:12597712. [DOI] [PubMed] [Google Scholar]
- 14.Holland R, Sant'anna Júnior A, Souza VD, Dezan Junior E, Otoboni Filho JA, Bernabé PF, Nery MJ, Murata SS. Influence of apical patency and filling material on healing process of dogs'teeth with vital pulp after root canal therapy. Brazilian dental journal. 2005;16(1):9–16. doi: 10.1590/s0103-64402005000100002. https://doi.org/10.1590/S0103-64402005000100002 PMid:16113927. [DOI] [PubMed] [Google Scholar]
- 15.Leonardo MR, Flores DS, de Toledo Leonardo R, da Silva LA. A comparison study of periapical repair in dogs'teeth using RoekoSeal and AH plus root canal sealers:a histopathological evaluation. Journal of endodontics. 2008;34(7):822–5. doi: 10.1016/j.joen.2008.03.029. https://doi.org/10.1016/j.joen.2008.03.029 PMid:18570987. [DOI] [PubMed] [Google Scholar]
- 16.Peters OA, Peters CI. Cleaning and shaping of the root canal system. Pathways of the Pulp. 2006;9:290–357. [Google Scholar]
- 17.de Vasconcelos BC, Bernardes RA, Luna Cruz SM, Húngaro Duarte MA, de Magalhães Padilha P, Bernardineli N, Garcia RB, Bramante CM, de Moraes IG. Ceará, Brazil Evaluation of pH and calcium ion release of new root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:135–9. doi: 10.1016/j.tripleo.2009.02.026. https://doi.org/10.1016/j.tripleo.2009.02.026 PMid:19451009. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi Z, Dummer P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. https://doi.org/10.1111/j.1365-2591.2011.01886.x PMid:21535021. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Li Z, and Peng B. Assessment of a new root canal sealer's apical sealing ability. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2009;107:e79–e82. doi: 10.1016/j.tripleo.2009.02.024. https://doi.org/10.1016/j.tripleo.2009.02.024 PMid:19464650. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Yuan Z, Yan P, Li Y, Jiang H, Huang S. Effect of iRoot SP and mineral trioxide aggregate (MTA) on the viability and polarization of macrophages. Arch Oral Biol. 2017;80:27–33. doi: 10.1016/j.archoralbio.2017.03.010. https://doi.org/10.1016/j.archoralbio.2017.03.010 PMid:28364673. [DOI] [PubMed] [Google Scholar]
- 21.Desai S, Chandler N. Calcium hydroxide–based root canal sealers:a review. Journal of endodontics. 2009;35(4):475–80. doi: 10.1016/j.joen.2008.11.026. https://doi.org/10.1016/j.joen.2008.11.026 PMid:19345790. [DOI] [PubMed] [Google Scholar]
- 22.Kumaravadivel MS, Pradeep S. Recent advancements of endodontic sealers-a review. International Journal of Pharmacy and Technology. 2016;8:4060–75. [Google Scholar]
- 23.Dudeja C, Taneja S, Kumari M, Singh N. An in vitro comparison of effect on fracture strength, pH and calcium ion diffusion from various biomimetic materials when used for repair of simulated root resorption defects. Journal of conservative dentistry:JCD. 2015;18(4):279. doi: 10.4103/0972-0707.159720. https://doi.org/10.4103/0972-0707.159720 PMid:26180410 PMCid:PMC4502121. [DOI] [PMC free article] [PubMed] [Google Scholar]


