Abstract
BACKGROUND:
Obesity is a multifactorial disease, associated with metabolic disorders and chronic low-grade inflammation. Procalcitonin (PCT) is well known as a biomarker of infection, and systemic inflammation. Recently, it has potential as a marker for chronic low-grade inflammation.
AIM:
This study aims to evaluate the role of serum PCT as an inflammatory biomarker in the diagnosis of obesity-related low-grade inflammation.
METHOD:
In this case-control study, 50 obese and 35 normal weight children and adolescents aged 5–15 years were enrolled. Anthropometric parameters were measured in all subjects. Blood samples were collected for measurement of lipid profile, blood glucose, insulin, high sensitivity-CRP (Hs-CRP) and serum procalcitonin. Serum (PCT) levels were assessed using enzyme-linked immunosorbent assay.
RESULTS:
Obese participants had higher concentrations of serum PCT, total cholesterol, triglycerides, LDL-c, glucose and Hs-CRP than control group. On correlation analysis, procalcitonin had significant positive correlation with (BMI) z-score (P = 0.02), insulin (P = 0.00), insulin resistance (HOMA-IR) (P = 0.006), Hs-CRP (P = 0.02), total cholesterol (P = 0.04) and triglycerides (P = 0.00) in obese group.
CONCLUSION:
The increased serum procalcitonin concentrations were closely related to measures of adiposity, Hs-CRP and insulin resistance, suggesting that PCT may be an excellent biomarker for obesity-related chronic low-grade inflammation in children and adolescents.
Keywords: Procalcitonin, Inflammatory Marker, Egyptian Children, Obesity
Introduction
Childhood obesity is one of the most important public health problems with increasing prevalence worldwide in this century. Childhood obesity is more prevalent in low and middle-income countries, especially in urban areas. In 2016, the number of overweight children below 5 years old, worldwide, is approximately 41 million. About fifty per cent of overweight children below five years are from Asia, and twenty-five per cent are from Africa [1]. The aetiology of obesity is complicated, a range of factors are suggested to play a role, including factors related to the lifestyle preferences, genetic, neuroendocrine, metabolic, immunologic, environmental, social and cultural factors [2] Obesity is associated with the chronic low-grade inflammatory reaction. This type of inflammation can be differentiated from normal inflammation by the absence of ordinary signs of inflammatory reactions. However, it shares the same diseases caused by typical inflammatory mediators and signalling pathway [2] [3].
Procalcitonin is the precursor to the hormone calcitonin, which is produced by all tissues throughout the body [4]. Production of procalcitonin occurs mainly in response to bacterial toxins and some inflammatory mediators. On the contrary, the downregulation of procalcitonin occurs in the course of viral infection. The definite physiological role of procalcitonin is not yet completely recognised [5]. Procalcitonin level can be detected in serum after about 3-6 hours after the onset of inflammation and stay raised for 12-36 hours after recovery [6].
Procalcitonin has been identified as a marker of infections and significant systemic inflammatory states [7]. Previously, research proved the ability of adipose tissue to express and produce procalcitonin [8]. This provides evidence for the relation between inflammation and obesity, considering procalcitonin a potential marker for it [9].
The objective of this study is to investigate the role of procalcitonin as a marker of inflammation in childhood obesity and its relationship with markers of obesity and other metabolic indices.
Subjects and Method
The present study included fifty children with simple obesity. Their age ranged from 5 to 15 years, with mean age 10.1 ± 2.5 years and 35 non-obese healthy children were enrolled as a control group with a mean age of 9.3 ± 2.1 years. Children with a diagnosis of obesity were recruited from the child health clinic in Medical and Scientific Centre of Excellence, National Research Centre. Obesity is defined as BMI greater than the 95th percentile on the growth charts from the National Center of Health and Statistics (NCHS). Exclusion criteria included genetic and endocrinal causes of obesity, children with chronic debilitating diseases, mental retardation, and use of drugs that affect blood pressure, lipid profile, or glucose level. Informed consents were obtained from the parents of the children studied, and the study was approved by the medical ethical committee of the National Research Centre, Cairo, Egypt.
A full history was taken from all participants. Also, thorough clinical examination and anthropometric measurements were done. A calibrated Seca scale was used to weigh children to the nearest 0.1 kg (Seca, Hamburg, Germany), whereas a Seca 225 stadiometer was used to measure height to the nearest 0.1 cm, with the children dressed in minimal clothes and without shoes [10]. Each measurement was taken as the mean of three consecutive readings following the recommendations of the International Biological Program [11]. BMI for age was recorded according to WHO standards using AnthroPlus software for personal computers [12]. Weight for age, height for age and BMI Z-score were determined using the new WHO reference [13]. Measurements of waist circumference, hip circumference, W/H ratio and blood pressure were done.
Morning venous blood sample (3 ml) was withdrawn after 12 hours overnight fasting into a plain tube and left to clot. The serum was separated by centrifugation for 10 minutes at 5000 rpm and stored at-20 until assays done. Fasting serum glucose, fasting serum insulin, cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-c) were measured by calorimetric method.
Serum LDL-C levels were calculated using the Friedewald formula [LDL-C=Total cholesterol-HDL-C- (Triglyceride/5)] [14].
C-reactive protein was determined using a latex agglutination technique [15]. Procalcitonin (Human) ELISA Kit was used for the quantitative measurement of human Procalcitonin in serum (Bioassay Technology Laboratory). The detection range of this kit was 5 pg/ml - 20000 pg/ml [16].
Data entry was carried out in excel sheet, and statistical analysis was done using SPSS software program version 20.0, the measurement data presented as a mean ± standard deviation. A t-test was done for comparison between two means. Simple linear correlation (Pearson correlation) for quantitative data was also done. P value was considered statistically significant when P was <0.05 and considered statistically highly significant when its value was < 0.001.
Results
Comparisons between mean ± SD values of studied parameters in obese and non-obese groups are shown in (Table 1 & 2). The study comprised fifty obese children (34 females and 16 males) with mean age 10.1 ± 2.5 years and 35 non-obese healthy children (18 females and 17 males) with mean age 9.3 ± 2.1 years, considered as control group, there were highly significant statistical differences between them as regard weight z-score, body mass index z-score, waist circumference, hip circumference, mid-arm circumference, fasting blood glucose, cholesterol, triglycerides, LDL, hs-CRP, and PCT. Also significant statistical differences between them as regard waist/hip ratio, insulin and HOMA-IR. The comparison between males and females as regards the mean PCT level revealed a non-significant difference.
Table 1.
Characteristics of the study group
| Characteristics | Obese N = 50 Mean ± SD | Non Obese N = 35 Mean±SD | t | p- value |
|---|---|---|---|---|
| Age (years) | 10.1 ± 2.5 | 9.3 ± 2.1 | 1.34 | 0.185 |
| Weight z-score | 2.54 ± 1.07 | 0.96 ± 0.6 | 7.02 | 0.000** |
| Height z-score | -0.91 ± 0.79 | -0.70 ± 0.9 | 0.79 | 0.4 |
| Z score-BMI | 2.77 ± 0.6 | 1.7 ± 0.46 | 7.72 | 0.00** |
| Waist circumference | 100.7 ± 18.6 | 69.9 ± 9.8 | 7.23 | 0.000** |
| Hip circumference | 109.6 ± 17.8 | 79.4 ± 14.8 | 7.84 | 0.000** |
| Waist/hip ratio (WHR) | 0.96 ± 0.34 | 0.79 ± 0.41 | 2.74 | 0.04* |
| Mid arm circumference (MAC) | 33.4 ± 7.5 | 18.4 ± 5.4 | 9.75 | 0.000** |
SD: standard deviation, BMI: body mass index.
if p≤0.05, then the relation is statistically significant.
if p≤0.001, then the relation is statistically highly significant.
Table 2.
Laboratory characteristics of the study group
| Characteristics | Obese N = 50 Mean ± SD | Non Obese N = 35 Mean ± SD | t | P- value |
|---|---|---|---|---|
| Insulin (mg/dl) | 14.80 ± 4.5 | 9.50 ± 3.4 | 2.208 | 0.03* |
| HOMA IR | 3.87 ± 1.12 | 1.75 ± 0.93 | 3.243 | 0.00** |
| FBG (mg/dl) | 97.43 ± 15.5 | 72.64 ± 11.78 | 7.323 | 0.00** |
| Cholesterol (mg/dl) | 201.46 ± 39.84 | 89.81 ± 17.5 | 13.244 | 0.0** |
| LDL (mg/dl) | 119.8 ± 40.63 | 40.74 ± 9.53 | 12.069 | 0.00** |
| HDL (mg/dl) | 47.39 ± 10.3 | 61.47 ± 9.4 | -3.864 | 0.00** |
| TG (mg/dl) | 121.74 ± 39.76 | 79.89 ± 19.74 | 3.644 | 0.00** |
| Hs-CRP (mg/dl) | 4.89 ± 2.2 | 1.02 ± 0.66 | 9.462 | 0.00** |
| Procalcitonin (pg/ml) | 388.68 ± 114.19 | 122.38 ±21.48 | 12.815 | 0.000** |
Hs-CRP: high sensitive C-reactive protein, FBG: fasting blood glucose; HOMA-IR: homeostasis model assessment of insulin resistance, LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglycerides.
if P ≤ 0.05, then the relation is statistically significant.
if P ≤ 0.001, then the relation is statistically highly significant.
Results of the correlations of the various parameters with procalcitonin in obese children showed that there was strong significant positive association between procalcitonin and Weight z-score (r = 0.34; P = 0.01), BMI z-score (r = 0.31; P = 0.02) as shown in Fig. 1, insulin (r = 0.4; P = 0.00), HOMA-IR (r = 0.37; P = 0.006) as shown in Fig. 2, Hs-CRP (P = 0.02), cholesterol (r = 0.3; P = 0.04), and triglycerides (r = 0.41; P = 0.00) as shown in Fig. 3, while there were no significant correlations with age, height z-score, waist circumference, fasting blood glucose, LDL-cholesterol and HDL-cholesterol.
Figure 1.
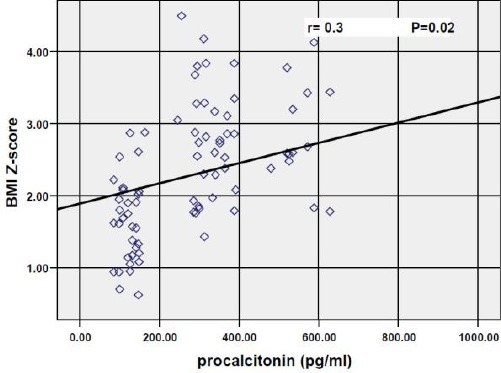
Correlation between procalcitonin and BMI z-score
Figure 2.
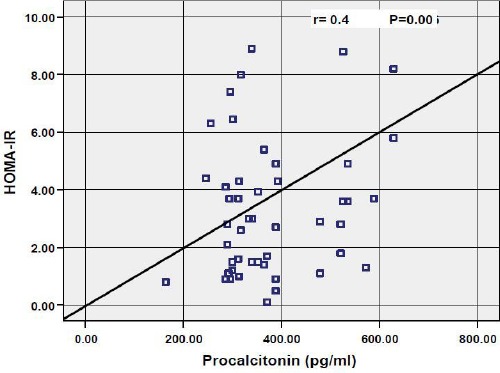
Correlation between procalcitonin and HOMA-IR
Figure 3.
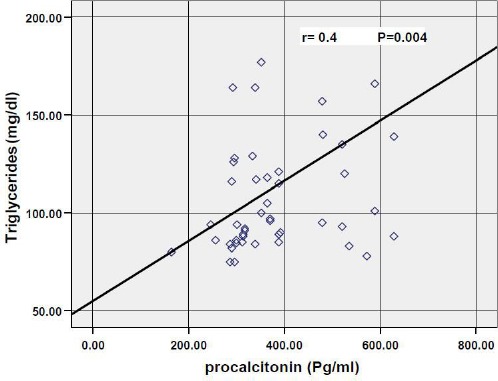
Correlation between procalcitonin and triglycerides
Discussion
The research for the pathogenesis of obesity during the last decades had shown a strong link between excessive nutrient intake and activating the innate immune response in many organs relevant to energy homeostasis [17] [18].
Strong correlations were reported between plasma concentrations of procalcitonin and the degree of inflammatory responses [19]. Procalcitonin is indicated mainly for diagnosing bacterial infections that precipitate systemic inflammatory responses. It shows high degrees of stability, with prolonged half-life and easy method of determination, making it perfect for clinical application [20].
Moreover, it was suggested that plasma PCT could be a marker of inflammation without the manifestations of systemic infection or sepsis [21] [22]. Adipose tissues have been considered as an endocrine organ, expressing calcitonin mRNA [23]. Also, it was found that adipocyte excretion of procalcitonin in vitro was triggered by activated macrophages [24], and the existence of those macrophages in adipose tissue has been reported to be associated proportionately to the extent of obesity [25].
The objective of this study is to investigate procalcitonin serum concentrations in a group of obese children in comparison with non-obese children, and studying the correlation of procalcitonin with some metabolic indices and anthropometric measurements.
The study findings showed a significant difference in procalcitonin serum concentration between obese and non-obese children and a significant positive association between procalcitonin and BMI z-score. In accordance, it was reported that, in obesity-related inflammation, there is a proportional association between the amount of adipose tissue and the increased generation of inflammatory mediators [26]. Also, our results showed a positive correlation between procalcitonin and weight z-score, insulin and HOMA-IR.
In agreement with our results, Abbasi and colleagues who conducted a cross-sectional study on a general population, reported a higher procalcitonin level in more obese subjects, our results were also matching regarding the association of plasma procalcitonin with insulin resistance [9]. A recent study in Egypt, investigated procalcitonin level in type 2 diabetic patients and assessed its relation with obesity, the authors reported, significantly higher concentrations of procalcitonin, hs-CRP and HOMA-IR in obese compared to non-obese patients [27]. Moreover, Boursier et al., [28] found high plasma procalcitonin levels of their subjects associated with the degree of obesity, but in contrast to our results, they found no association between procalcitonin and insulin resistance. However, it is well known that increased adiposity is one of the major predisposing factors in developing insulin resistance [29]. Moreover, several studies suggest that inflammatory reactions that occur as a result of obesity may be implicated in the generation of insulin resistance, deficient insulin production, and disrupted energy homeostasis [30]. In accordance, Chen and his colleagues found a significant positive correlation between inflammatory markers and insulin resistance [31]. Also, Indulekha and his colleagues suggested that the relation between inflammatory reactions and insulin resistance indicates a continued cytokine- generated acute phase reactions [32].
On the other hand, the group of obese children in this study presented a state of disturbed lipid profile, and there was a significant correlation between procalcitonin, total cholesterol and triglycerides levels. These findings are supported by the accumulating evidence that reveals the association of systemic-obesity-related inflammation with the risk of developing cardiovascular disease (CVD). Hence, several obesity-associated factors including dyslipidemia are involved in CVD risk. In this regard, pro-inflammatory cytokines, are suggested to affect the liver, leading to alterations in the release of lipoproteins and inflammatory mediators [33] [34]. Particularly, they cause an elevation of very low-density lipoprotein, apolipoprotein B, and triglyceride levels [35]. C-reactive protein is a highly sensitive inflammatory marker, it is produced from the liver, and its production is controlled mostly by IL-6 [36]. Previous research has evidenced that concentrations of C-reactive protein have a positive relationship with BMI in healthy subjects [37].
Moreover, several studies have shown that CRP is associated with most obesity markers [38]. Our results showed significantly higher hs-CRP levels in obese children compared to non- obese. In accordance, Ahmed et al., [39], evaluated the role of some inflammatory mediators and adipokines in obese Egyptian children; they reported that the mean level of CRP was significantly elevated in obese children compared with controls.
In conclusion, the findings of this study revealed the significance of serum procalcitonin as a marker of obesity-related low-grade inflammation in obese children.
Footnotes
Funding: This work was supported and funded by the National Research Centre, Egypt
Competing Interests: The authors have declared that no competing interests exist
References
- 1.World Health Organization (WHO) Childhood overweight and obesity. Global Strategy on Diet, Physical Activity and Health. 2018. [Last accessed 11/6/2018]. Available at http://www.who.int/dietphysicalactivity/childhood/en/
- 2.Castro AM, Macedo-de la Concha LE, Pantoja-Meléndez CA. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev Med Hosp Gen Méx. 2017;80(2):101–105. https://doi.org/10.1016/j.hgmx.2016.06.011. [Google Scholar]
- 3.Castro AM, Toledo Rojas A, Macedo de la Concha LE, et al. Laobesidad infantil, un problema de salud multisistémico. Rev Med Hosp Gen Mex. 2001;75:37–40. [Google Scholar]
- 4.Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. British journal of pharmacology. 2010;159(2):253–64. doi: 10.1111/j.1476-5381.2009.00433.x. https://doi.org/10.1111/j.1476-5381.2009.00433.x PMid:20002097 PMCid: PMC2825349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Wakeel MA, Nassar MS, El Batal WH, Amer AF, Darwish MK, Aziz AA. Evaluation of procalcitonin as a biomarker for bacterial and nonbacterial community-acquired pneumonia in children. J Arab Soc Med Res. 2017;12:68–72. https://doi.org/10.4103/jasmr.jasmr_19_17. [Google Scholar]
- 6.Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiological Research. 2000;49:S57–62. PMid:10984072. [PubMed] [Google Scholar]
- 7.Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, Périat P, Bucher HC, Christ-Crain M. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Archives of internal medicine. 2008;168(18):2000–7. doi: 10.1001/archinte.168.18.2000. https://doi.org/10.1001/archinte.168.18.2000 PMid:18852401. [DOI] [PubMed] [Google Scholar]
- 8.Linscheid P, Seboek D, Zulewski H, Keller U, Muller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146(6):2699–708. doi: 10.1210/en.2004-1424. https://doi.org/10.1210/en.2004-1424 PMid:15761041. [DOI] [PubMed] [Google Scholar]
- 9.Abbasi A, Corpeleijn E, Postmus D, Gansevoort RT, De Jong PE, Gans RO, Struck J, Hillege HL, Stolk RP, Navis G, Bakker SJ. Plasma procalcitonin is associated with obesity, insulin resistance, and the metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism. 2010;95(9):E26–31. doi: 10.1210/jc.2010-0305. https://doi.org/10.1210/jc.2010-0305 PMid:20534760. [DOI] [PubMed] [Google Scholar]
- 10.Lohman TG, Roche AF. In: Anthropometric standardization reference manual. Martorell R, editor. Champaign: Human kinetics books; 1988. PMCid: PMC279682. [Google Scholar]
- 11.Tanner JM. Growth and physique studies. Human biology: a guide to field methods. 1969 [Google Scholar]
- 12.World Health Organization. WHO AnthroPlus for personal computers Manual: Software for assessing growth of the world's children and adolescents. Geneva: WHO; 2009. [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18(6):499–502. PMid:4337382. [PubMed] [Google Scholar]
- 14.World Health Organization. WHO child growth standards: length/height for age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age, methods and development. World Health Organization; 2006. [Google Scholar]
- 15.Wadsworth C, Wadsworth E. Efficacy of latex agglutination and quantification methods for determination of C-reactive protein (CRP) in pediatric sera. Clin Chim Acta. 1984;138(3):309–18. doi: 10.1016/0009-8981(84)90138-4. https://doi.org/10.1016/0009-8981(84)90138-4. [DOI] [PubMed] [Google Scholar]
- 16.Arkader R, Troster EJ, Lopes MR, Júnior RR, Carcillo JA, Leone C, Okay TS. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Archives of disease in childhood. 2006;91(2):117–20. doi: 10.1136/adc.2005.077446. https://doi.org/10.1136/adc.2005.077446 PMid:16326799 PMCid: PMC2082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. https://doi.org/10.1172/JCI57132 PMid:21633179 PMCid: PMC3104776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. https://doi.org/10.1038/nrendo.2015.189 PMid:26553134. [DOI] [PubMed] [Google Scholar]
- 19.Ghanem AI, Khalid M. Association of Serum Procalcitonin (PCT) and High-Sensitivity C-Reactive Protein (hs-CRP) Levels with Insulin Resistance and Obesity in Type 2 Egyptian Diabetic Patients. Acta Medica Mediterranea. 2012;28(2):95. [Google Scholar]
- 20.Hatzistilianou M, Hitoglou S, Gougoustamou D, Rekliti A, Tzouvelekis G, Nanas C, Catriu D, Kotsis A. Serum procalcitonin, adenosine deaminase and its isoenzymes in the aetiological diagnosis of pneumonia in children. International journal of immunopathology and pharmacology. 2002;15(2):119–27. doi: 10.1177/039463200201500207. https://doi.org/10.1177/039463200201500207 PMid:12590874. [DOI] [PubMed] [Google Scholar]
- 21.Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Muller B. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. The Journal of Clinical Endocrinology & Metabolism. 2005;90(11):6014–21. doi: 10.1210/jc.2005-1002. https://doi.org/10.1210/jc.2005-1002 PMid:16105965. [DOI] [PubMed] [Google Scholar]
- 22.van Ree RM, de Vries AP, Oterdoom LH, Seelen MA, Gansevoort RT, Schouten JP, Struck J, Navis G, Gans RO, van der Heide JJ, van Son WJ, Bakker SJ. Plasma procalcitonin is an independent predictor of graft failure late after renal transplantation. Transplantation. 2009;88:279–287. doi: 10.1097/TP.0b013e3181ac9ea0. https://doi.org/10.1097/TP.0b013e3181ac9ea0 PMid:19623026. [DOI] [PubMed] [Google Scholar]
- 23.Linscheid P, Seboek D, Nylen ES, Langer I, Schlatter M, Becker KL, Keller U, Muller B. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144(12):5578–84. doi: 10.1210/en.2003-0854. https://doi.org/10.1210/en.2003-0854 PMid:12960010. [DOI] [PubMed] [Google Scholar]
- 24.Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Müller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Critical care medicine. 2004;32(8):1715–21. doi: 10.1097/01.ccm.0000134404.63292.71. https://doi.org/10.1097/01.CCM.0000134404.63292.71 PMid:15286549. [DOI] [PubMed] [Google Scholar]
- 25.Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Prac Res Clin Endocrinol Metab. 2013;27:163–177. doi: 10.1016/j.beem.2013.02.005. https://doi.org/10.1016/j.beem.2013.02.005 PMid:23731879. [DOI] [PubMed] [Google Scholar]
- 26.Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875. doi: 10.1038/nature05487. https://doi.org/10.1038/nature05487 PMid:17167476. [DOI] [PubMed] [Google Scholar]
- 27.Ghanem AI, Khalid M. Association of Serum Procalcitonin (PCT) and High-Sensitivity C-Reactive Protein (hs-CRP) Levels with Insulin Resistance and Obesity in Type 2 Egyptian Diabetic Patients. Med J Cairo Univ. 2016;84(1):1165–1171. [Google Scholar]
- 28.Boursier G, Avignon A, Kuster N, Boegner C, Leprieur E, Picandet M, Bargnoux AS, Badiou S, Dupuy AM, Cristol JP, Sultan A. Procalcitonin, an Independent Marker of Abdominal Fat Accumulation in Obese Patients. Clinical laboratory. 2016;62(3):435–41. doi: 10.7754/clin.lab.2015.150736. https://doi.org/10.7754/Clin.Lab.2015.150736. [DOI] [PubMed] [Google Scholar]
- 29.Wilding JPH. Obesity and nutritional factors in the pathogenesis of type 2 diabetes mellitus. In: Pickup JC, editor. Text book of Diabetes. 3rd ed. Oxford: U.K., Black-well Science Ltd; 2003. pp. 21–21. [Google Scholar]
- 30.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. The Journal of clinical investigation. 2017;127(1):1–4. doi: 10.1172/JCI92035. https://doi.org/10.1172/JCI92035 PMid:28045402 PMCid: PMC5199709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Wildman RP, Hamm LL, Muntner P, Reynolds K, Whelton PK, He J. Association between inflammation and insulin resistance in US nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes care. 2004;27(12):2960–5. doi: 10.2337/diacare.27.12.2960. https://doi.org/10.2337/diacare.27.12.2960 PMid:15562214. [DOI] [PubMed] [Google Scholar]
- 32.Indulekha K, Suren-Dar J, Moha V. High Sensitivity C-Reactive Protein, Tumor Necrosis Factor-a, Interleukin-6, and Vascular Cell Adhesion Molecule-1 Levels in Asian Indians with Metabolic Syndrome and Insulin Resistance (CURES-105) Journal of Diabetes Science and Technology. 2011;5(4):982–988. doi: 10.1177/193229681100500421. https://doi.org/10.1177/193229681100500421 PMid:21880241 PMCid: PMC3192605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabro P, Golia E, Maddaloni V, Malvezzi M, Casillo B, Marotta C, Calabro R, Golino P. Adipose tissue-mediated inflammation: the missing link between obesity and cardiovascular disease? Internal and emergency medicine. 2009;4(1):25–34. doi: 10.1007/s11739-008-0207-2. https://doi.org/10.1007/s11739-008-0207-2 PMid:19052701. [DOI] [PubMed] [Google Scholar]
- 34.Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53(4):577–84. doi: 10.1161/HYPERTENSIONAHA.108.110320. https://doi.org/10.1161/HYPERTENSIONAHA.108.110320 PMid:19237685. [DOI] [PubMed] [Google Scholar]
- 35.Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circulation research. 2005;96(10):1042–52. doi: 10.1161/01.RES.0000165803.47776.38. https://doi.org/10.1161/01.RES.0000165803.47776.38 PMid:15920027. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. International journal of endocrinology. 2013;2013 doi: 10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford ES. The metabolic syndrome and C-reactive protein, fibrinogen, and leukocyte count: findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003;168(2):351–8. doi: 10.1016/s0021-9150(03)00134-5. https://doi.org/10.1016/S0021-9150(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 38.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-αand IL-6. Diabetes research and clinical practice. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. https://doi.org/10.1016/j.diabres.2004.11.007 PMid:15955385. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed HH, Abdel Hameed ER, Shehata MA, El Wakeel MA, Elsawy DH, Elshafie AI. Relation between afamin level and some inflammatory markers in obese children. Medical Research Journal. 2015;14(1):1–6. https://doi.org/10.1097/01.MJX.0000464329.16129.c0. [Google Scholar]


