Abstract
BACKGROUND:
The use of laser therapy in the biostimulation of bone repair has been growing steadily.
AIM:
This study aimed to evaluate the radio-densitometric effect of low-intensity laser therapy on the osseointegration of immediately loaded dental implants in patients under vitamin C, omega-3 and calcium therapy.
PATIENTS AND METHODS:
A single implant was placed in the mandibular first molar region of twenty patients which were equally divided into two groups. In the non-laser group, the healing phase was left to progress spontaneously without any intervention, while in the laser group it was augmented with low-level laser therapy of wavelength 904 nm in contact mode, continuous wave, 20 mW output power and exposure time 30 sec with a dose 4.7 J/cm2. Patients in both groups were given vitamin C, calcium and omega-3 starting one month preoperatively. Postoperative digital panoramas were taken immediately after surgery, 1.5 months and 6 months postoperatively. Changes in bone density along the bone-implant interface at the mesial, distal and apical sides were assessed using the Digora software.
RESULTS:
Independent student t-test was used to compare means of variables between the laser and the non-laser group while repeated measures ANOVA was used to compare bone densities at different times for the same group. Significant increased differences were observed at the mesial, distal and apical sides surrounding the implants of both groups per time. However, the rate of increase was significantly higher in the laser group. The mean difference at the mesial side after 6 months was 21.99 ± 5.48 in the laser group and 14.21 ± 4.95 in the non-laser group, while it read 21.74 ± 3.56 in the laser group and 10.78 ± 3.90 in non-laser group at the distal side and was 18.90 ± 5.91 in the laser group and 10.39 ± 3.49 in non-laser group at the apical side. Significance was recorded at P = 0.004, P = 0.0001, and 0.001 at the mesial, distal and apical sides respectively.
CONCLUSION:
The low-intensity laser irradiation significantly promoted bone healing and speeded up the osseointegration process emphasising the laser’s biostimulatory effect.
Keywords: Calcium, Immediately loaded implant, Laser therapy, Omega-3, Osseointegration, Vitamin C
Introduction
The success of the endosseous dental implants depends mainly on the successful osseointegration of the implant with bone, and many attempts have tried to enhance this process, one of which was the use of low-level laser therapy (LLLT) [1].
The effect of LLLT on bone regeneration has become a focus of recent research, as it improves vascularisation, enhances collagen synthesis and concerning the bone, it modulates inflammation, accelerates cell proliferation and enhances healing [2] [3] [4]. In several studies, it was demonstrated that LLLT stimulates stem cells of the bone and accelerates its repair process [5] [6].
Recently, immediate implant loading has become more common and is better accepted by many patients as it negates the need for second surgery where provisionalization is simplified by the immediate loading of the implant after surgery [7] [8].
Vitamin C is an essential water-soluble vitamin for humans, as it is a powerful reducing agent and is important for proper wound healing as it leads to fibroblast differentiation and collagen synthesis [9, 10]. Furthermore, vitamin C has immune-modulating functions influencing the susceptibility of a host to infectious disease, playing a role in bone formation due to hydroxylation of proline and lysine, and protecting the tissue from harmful free radicals [11] [12].
Fish oils are rich sources of the omega-3 polyunsaturated fatty acids (PUFA) eicosapentaenoic (EPA) acid and dexahexaenoic acid (DHA) that are distributed in almost all body cells, thus impacting cell functions, cell communication, production of various biomolecules and antioxidant activities [13]. Furthermore, decreased bone resorption or enhanced bone formation may be a consequence of the dual bone-sparing effect of omega-3 [14].
Calcium (Ca) is one of the most important minerals for bone health. The size of calcium reserve was affected by dietary calcium through the mobilisation necessary to maintain a normal blood calcium level. However, it never impairs those cellular functions. The current suggested daily intake of calcium is 1200 mg for adults [15]. It has been established that the osseointegration of dental implants was improved by the local delivery of calcium, in the form of hydroxyapatite [16]. A beneficial interaction between calcium and omega 3 FAs is plausible based on work done mainly in animal and in vitro models suggesting up-regulation of duodenal calcium absorption and decreased calcium excretion with the treatment of omega 3 FAs [17] [18].
The present study attempted to evaluate the radio-densitometric effect of LLLT on the osseointegration of immediately loaded dental implants in patients under vitamin C, omega-3 and calcium therapy.
Subjects and Methods
Sample size calculation was determined to detect an expected difference between laser and non-laser group of bone density changes from baseline to 6 months about 7 ± 3.2 [19]. Using power 95% and 5% significance level, 7 patients were required in each group. Recruitment of 10 participants per group was done to account for possible losses. Sample size calculation was achieved using PS: Power and Sample Size Calculation software Version 3.1.2 (Vanderbilt University, Nashville, Tennessee, USA).
The present study was conducted on twenty patients with age ranging from 30-40 years old, who were selected from the outpatient clinic of the National Research Centre, Cairo, Egypt in the period between 2017 and 2018. The study has been carried out by The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All patients signed an informed consent before enrollment, and the Ethical Committee of the National Research Centre approved the protocol. Inclusion criteria were patients with good oral hygiene having a missing tooth in the mandibular first molar region with the adjacent teeth free from peri-apical pathology, and sufficient bone volume in the receptor site to accommodate for implant length and diameter. Patients who were smokers, alcoholic or drug abusers, suffering from bruxism, having a history of jaw irradiation or exhibited signs and symptoms of any systemic diseases that could influence the outcome of the therapy, were excluded.
A complete medical and dental history together with a preoperative panoramic X-ray was taken for each patient. A detailed oral and general examinations and thorough scaling and root planing were done for all selected patients. Patients were instructed to use Hexitol (Chlorhexidine HCL, The Arab Drug Company, A.R.E) mouthwash twice daily and take Augmentin (Amoxicillin 875 mg and clavulanic acid 125 mg, GlaxoSmithKline, A.R.E.) 1 gm tablet one hour preoperatively. All patients were instructed to take 500 mg tablet of vitamin C (C-Retard 500 mg, Hikma pharma S.A.E., 6th of October City - Egypt) once daily, 500 mg tablet of calcium (Bone-Cal, Amoun Pharmaceutical Co. S.A.E El-Obour City, Cairo, Egypt.) twice daily and 1000 mg tablet of omega 3 (Omega-300, The Arab Co. For Gelatin and Pharmaceutical Products for MONTANA PHARMACEUTICAL) once daily starting one month preoperatively.
Endosseous root form dental implants (Dentium, made in Korea) were used in the present study. All implants used were of length ranged from 10-12 mm, and diameter ranged from 4-4.5 mm. One implant was placed in each patient. Patients were divided into two groups, in the non-laser group; the healing phase was left to progress spontaneously without any intervention, while in the laser group healing phase was augmented with LLLT.
A gingival incision was performed through interdental papillae of the teeth on both sides of the edentulous area and connected by a crestal incision deep into the alveolar bone. The flap was then elevated buccally and slightly lingual, and a trephine bur was used to penetrate the alveolar crest.
Drilling was accompanied with copious saline irrigation, and enlarged sequentially by a series of gradually increasing drills, to a dimension just smaller than the implant diameter. The implant was then inserted in the bone by hand driven screw tightened with a ratchet wrench. Before wound closure, a temporary abutment, adjusted to the desired height, was secured onto the implant.
The surgical wound was irrigated with sterile saline then the flap was repositioned back and sutured. A temporary crown was made, trimmed, smoothened, polished and temporarily cemented to the secured abutment. The temporary crown was adjusted to be out of occlusion with the opposing maxillary teeth. All immediately loaded implants had implant insertion torque of 35 N-cm.
After removing the temporary crowns and freeing the abutments, the final abutment was screwed, and the prosthetic part (porcelain-fused-to-metal) was fabricated (five months postoperatively).
Figure 1.
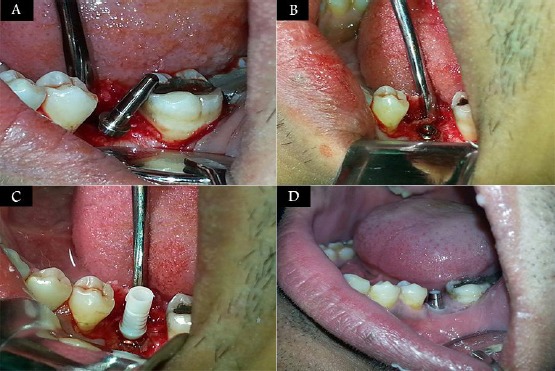
A) A photograph showing flap elevation and the parallel pin inside the implant bed; B) A photograph showing complete seating of the implant at the alveolar crest; C) A photograph showing seating the temporary abutment into the implant; D) A photograph showing screwing the final abutment into the implant
A 904 nm Gallium-Arsenide diode laser device (OPTODAN, Saratovskaja provinces, Saratov, Russia) was used in the present study. Implant site in the laser group was irradiated using contact mode, continuous wave, 20 mW output power, spot diameter 4mm and exposure time 30 sec [20] with a dose 4.7 J/cm2 [21].
Figure 2.
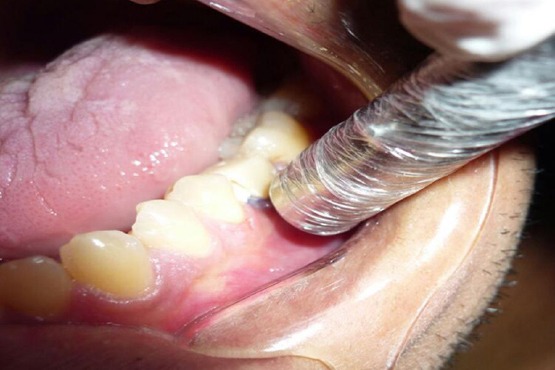
A photograph showing laser application at the implant site
The laser probe was directed towards the implant site, gently touching the tissues, and moving in a continuous slow circular motion to assure full exposure of the target surface to the laser beam. The patients were subjected to 9 sessions during the first week postoperatively (on 2nd, 4th and 6th days), three sessions per day with (1-hour) rest period in-between each session.
Baseline digital panoramic radiographs were taken postoperatively in the same day of surgery, 1.5 months postoperatively and the final digital panoramic radiographs were taken 6 months postoperatively.
Radio-densitometric evaluations were done using the Digora software system, around the mesial, distal and apical surfaces of the implant in both groups. The bone density was measured using grayscale value.
The peri-implant densitometric measurements were performed as follows: Three lines were drawn mesial, distal and apical to the implant. The first line extended mesially from the first thread of the implant to the apex of the implant passing just tangential to the threads, the second line extended distally from the first thread of the implant to the apex of the implant passing just tangential to the threads and the third line extended apically from the mesial aspect of the implant to the distal aspect of the implant.
Figure 3.
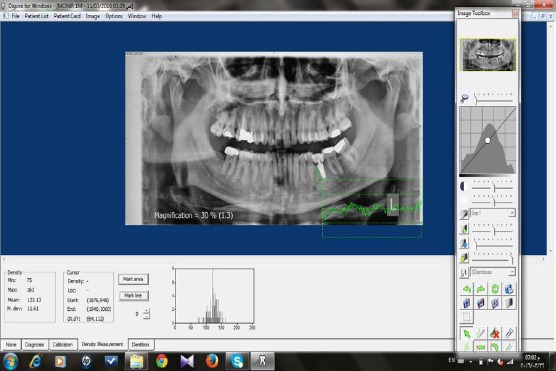
Measurement of bone density using Digora software
Numerical data were presented as mean and standard deviation (SD) values. Data were explored for normality using the Kolmogorov-Smirnov test of normality, variables were found to be normally distributed. Independent student t-test was used to compare means of variables between the laser and the non-laser group. Repeated measures ANOVA was used to compare bone densities at different times for the same group. Least significant difference (LSD) test was used as a post-hoc test to detect the follow-up time responsible for significance. The significance level was set at P ≤ 0.05. Statistical analysis was performed with IBM SPSS software 18.0, Chicago, IL, USA [22].
Results
As regards the changes of the mean bone density per time, the calculation of the repeated measures ANOVA revealed statistically significant values with time. This increase was seen in all three zones and both laser and non-laser groups.
Immediate post-operatively, the mean and standard deviation values of bone density in the laser group were 131.85 ± 13.95 and were 131.24 ± 10.99 in the non-laser group. While after 1.5 months, the mean and standard deviation values of bone density in the laser group were 143.45 ± 16.68 and were 138.18 ± 10.26 in the non-laser group. Moreover, after 6 months the mean and standard deviation values of bone density were 153.84 ± 16.41 in the laser group and 145.45 ± 11.44 in the non-laser group.
Immediate post-operatively the mean and standard deviation values of bone density in the laser group were 129.02 ± 9.29 and were 127.08 ± 18.60 in the non-laser group. While after 1.5 months, the mean and standard deviation values of bone density in the laser group were 139.53 ± 11.43 and 132.06 ± 19.57 in the non-laser group. Moreover, after 6 months the mean and standard deviation values of bone were 150.76 ± 9.86 in the laser group and 137.86 ± 18.81 in the non-laser group.
Immediate post-operatively the mean and standard deviation values of bone density in the laser group were 130.19 ± 6.58 and were 129.01 ± 18.09 in the non-laser group. After 1.5 months, the mean and standard deviation values of bone density in the laser group were 142.76 ± 5.96 and were 134.67 ± 17.91 in the non-laser group. Moreover, after 6 months the mean and standard deviation values of bone 149.09 ± 5.27 in the laser group and were 139.40 ± 18.83 in the non-laser group.
Results of Independent student t-test comparing the mean differences of both groups revealed statistically significant differences at the three zones during all the follow-up periods (being higher in the laser group).
There were significant differences in the mean bone density values at the mesial, distal and apical sides when the baseline mean bone density values were compared to values of the first follow-up (after 1.5 months), where at the mesial side the mean difference was 11.59 ± 4.87 in the laser group and was 6.94 ± 3.99 in the non-laser group, while it read 10.51 ± 4.33 in the laser group and 4.98 ± 4.67 in non-laser group at the distal side, and it was 12.57 ± 6.23 in the laser group and was 5.66 ± 2.87 in non-laser group at the apical side. Significance was recorded at P = 0.031, 0.013, and 0.007 at the mesial, distal and apical sides respectively.
There were significant differences in the mean bone density values at the mesial, distal and apical sides when the first follow-up (after 1.5 months) mean bone density values were compared to the values of the second follow-up (after 6 months), where at the mesial side the mean difference was 10.39 ± 1.86 in the laser group and was 7.27 ± 2.49 in the non-laser group, while it read 11.23 ± 4.37 in the laser group and 5.80 ± 4.34 in non-laser group at the distal side and was 6.33 ± 1.67 in the laser group and 4.74 ± 1.58 in non-laser group at the apical side. Significance was recorded at P = 0.005, 0.012, and 0.042 at the mesial, distal and apical sides respectively.
There were significant differences in the mean bone density values at the mesial, distal and apical sides when the baseline mean bone density values were compared to the values of the second follow-up (after 6 months), where at the mesial side the mean difference was 21.99 ± 5.48 in the laser group and 14.21 ± 4.95 in the non-laser group, while it read 21.74 ± 3.56 in the laser group and 10.78 ± 3.90 in non-laser group at the distal side and was 18.90 ± 5.91 in the laser group and 10.39±3.49 in non-laser group at the apical side. Significance was recorded at P = 0.004, P = 0.0001, and 0.001 at the mesial, distal and apical sides respectively.
Discussion
The replacement of missing teeth using implants over classic prosthetic solutions has gained wide attraction because of superior functional and aesthetic acceptance and the fact that implants stimulate the alveolar bone and induce an increased density in response to functional loading [23].
Increased failure rates with implants placed in type IV bone have been reported. The mandibular first molar region that was used in the present study as a standard site for implant insertion is known to have a relatively lower bone quality and a higher failure rate compared to the anterior region. Its bone type is type IV and offers little cortex and minimal internal strength [24][25]. On the other hand, when immediate and early implant loading regimes are applied, higher failure rates seem to be present, as the early occlusal loading during healing may affect the potential of the newly formed bone to repair the zone of damaged bone at the implant-bone interface [26].
Because the present study aimed to evaluate the effect of low-level laser on osseointegration and to decrease the failure possibilities of immediately loaded implants in bone type IV, both laser and non-laser groups were given omega-3, vitamin C and calcium.
In the current study, low-intensity gallium arsenide laser with wavelength 904nm was used as a regenerative approach to enhance osseointegration and increase the density of bone surrounding the implants. Although many researchers investigated the effect of LLLT in bone tissue in various branches of medicine and dentistry, with wavelengths varying from 670 to 1,064 nm, there are few studies on the use of 904 nm laser on bone tissue. The frequently used lasers are 670, 690, 780, 830, and 1,064 nm [27] [28].
In previous studies, the authors recommended the application of the 904 nm infrared laser on bone tissue. The wavelength 904 nm, which is emitted in the near-infrared region, had a low absorption coefficient and hence, better penetration potential into the tissue, thus raising the resistance and improving bone mineralisation [29] [30].
Gallium arsenide laser of wavelength 904 nm in a continuous mode with adjusted power of 0.02 W and a 30 second exposure time in nine sessions on three alternate days starting from the second postoperative day was used in the present study which was in accordance to a previous study observing the strongest bio modulatory effects at exposure time ranging from 30 to 120 seconds [20].
The energy density used in the current study was 4.7 J/cm2 based on previous research where the authors evaluated the action of laser therapy (830 nm) on the repair of bone defects in rat models histologically. The results concluded a more enhanced repair in the irradiated group with the improved bone formation and collagen fibres around the graft inside the cavity from the 15th day after surgery [21].
It was reported that laser irradiation of bone stimulates the proliferation of fibroblastic, osteoblastic and mesenchymal cells in their early phase. Immediately after injury, the bone repair process starts in the vascularized regions in tissue anoxia and is accelerated by the stimulatory effect of laser on bone matrix [29]. It was demonstrated that the duration of the positive effect of LLLT is not longer than 1 week postoperatively, which is in agreement with the results of the present study [31].
The three bony zones surrounding the implants revealed a statistically significant cumulative effect in bone density of both groups per time (Table 1 and 2). This effect is assumed to be as a result of the cumulative effect of the drugs that started one month preoperatively, providing a circulating reservoir of micronutrients and minerals essential for the bone integrity and health and this was in accordance to previous studies that established the benefits of these drugs on the bone around implants [16].
Table 1.
Repeated measures ANOVA comparing mean bone densities at different times for the non-laser group
| Time | P-value | |||
|---|---|---|---|---|
| Immediate | 1.5 months | 6 months | ||
| Bone densities(mesial) | 131.24 ± 10.99a | 138.18 ± 10.26b | 145.45 ± 11.44c | 0.0001* |
| Bone densities (distal) | 127.08 ± 18.60a | 132.06 ± 19.57b | 137.86 ± 18.81c | 0.0001* |
| Bone densities (apical) | 129.01 ± 18.09a | 134.67 ± 17.91b | 139.40 ± 18.83c | 0.0001* |
Statistically significant difference, p-value ≤0.05.
Different small letters indicate significant differences between the two follow-up times.
Table 2.
Repeated measures ANOVA comparing bone densities at different times for the laser group
| Time | P-value | |||
|---|---|---|---|---|
| Immediate | 1.5 months | 6 months | ||
| Bone densities(mesial) | 131.85 ± 13.95a | 143.45 ± 16.68b | 153.84 ± 16.41c | 0.0001* |
| Bone densities (distal) | 129.02 ± 9.29a | 139.53 ± 11.43b | 150.76 ± 9.86c | 0.0001* |
| Bone densities (apical) | 130.19 ± 6.58a | 142.76 ± 5.96b | 149.09 ± 5.27c | 0.0001* |
Statistically significant difference, p-value ≤0.05.
Different small letters indicate significant differences between the two follow-up times.
Moreover, this was in line with a study performed on white New Zealand rabbits using implants coated with eicosapentaenoic acid that was shown to enhance osteoconduction and anchorage of the implant to the surrounding bone [14]. Also, it was in line with a study performed by Park et al., 2007, where the results concluded that Ca and vitamin D supplementation promoted bone healing around dental implants [32].
Although both laser and non-laser groups revealed a statistically significant increase in mean bone density of the three zones during all the follow-up periods, the rate of increase was significantly higher in the laser group where this increase started earlier and was sustained in the three zones in the laser group when compared to the slower and more delayed increase in bone density in the non-laser group (Table 3). This effect might be due to laser provided angiogenesis, improved vascularisation and perfusion that facilitated the presence of high levels of such micronutrients and minerals in the wounded area, with a subsequent increase in mineral deposition and bone density during a relatively short period.
Table 3.
Comparison of the mean difference in bone density between times at different sites between the 2 groups (change by time in bone densities)
| Time change | Group | p-value | |
|---|---|---|---|
| Laser Mean ± SD | Non-laser Mean ± SD | ||
| Mesial | 11.59 ± 4.87 | 6.94 ± 3.99 | 0.031* |
| Distal | 10.51 ± 4.33 | 4.98 ± 4.67 | 0.013* |
| Apical | 12.57 ± 6.23 | 5.66 ± 2.87 | 0.007* |
| Mesial | 21.99 ± 5.48 | 14.21 ± 4.95 | 0.004* |
| Distal | 21.74 ± 3.56 | 10.78 ± 3.90 | 0.0001* |
| Apical | 18.90 ± 5.91 | 10.39 ± 3.49 | 0.001* |
| Mesial | 10.39 ± 1.86 | 7.27 ± 2.49 | 0.005* |
| Distal | 11.23 ± 4.37 | 5.80 ± 4.34 | 0.012* |
| Apical | 6.33 ± 1.67 | 4.74 ± 1.58 | 0.042* |
Statistically significant difference, P-value ≤ 0.05.
These findings are in line with a previous study who reported that bone formation and maturation around the implants were enhanced by the use of low-level laser [33]. Furthermore, it was concluded that cellular proliferation, bone nodule formation and alkaline phosphatase (ALP) activity were improved by the application of LLLT [34] [35]. LLLT was proven to enhance the functional attachment of titanium implants to bone and improves bone healing and mineralisation [36] [37] [38].
In conclusion, the low-intensity laser irradiation significantly promoted bone healing and speeded up the osseointegration process surrounding immediately loaded titanium implants emphasising the laser’s bio stimulatory effect.
Acknowledgements
The study was done for partial fulfilment of the requirements of PhD and was partly funded by the National Research Centre. All authors have made a substantive contribution to this study, and all have reviewed the final paper before its submission.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Petri Ad, Teixeira LN, Crippa GE, Belotib MM, Oliveira PT, Rosa AL. Effects of low –level laser therapy on human osteoblastic cells grown on titanium. Braz Dent J. 2010;21(6):1–9. doi: 10.1590/s0103-64402010000600003. https://doi.org/10.1590/S0103-64402010000600003. [DOI] [PubMed] [Google Scholar]
- 2.De Vasconcellos R, Barbara M, Deco P, Junqueira C. Healing of normal and osteopenic bone with titanium implant and low-level laser therapy (GaAlAs):a histomorphometric study in rats. Lasers Med Sci. 2014;29(2):575–80. doi: 10.1007/s10103-013-1326-1. https://doi.org/10.1007/s10103-013-1326-1 PMid:23624654. [DOI] [PubMed] [Google Scholar]
- 3.Torricelli P, Giavaresi G, Fini GA, et al. Laser biostimulation of cartilage:in vitro evaluation. Biomed Pharmacother. 2001;55:117–20. doi: 10.1016/s0753-3322(00)00025-1. https://doi.org/10.1016/S0753-3322(00)00025-1. [DOI] [PubMed] [Google Scholar]
- 4.El-Maghraby EM, El-Rouby DH, Saafan AM. Assessment of the effect of low-energy diode laser irradiation on gamma irradiated rats'mandibles. Arch Oral Biol. 2013;58(7):796–805. doi: 10.1016/j.archoralbio.2012.10.003. https://doi.org/10.1016/j.archoralbio.2012.10.003 PMid:23102551. [DOI] [PubMed] [Google Scholar]
- 5.Dortbudak O, Haas R, Mallath-Pokorny G. Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res. 2000;11(6):540–5. doi: 10.1034/j.1600-0501.2000.011006540.x. https://doi.org/10.1034/j.1600-0501.2000.011006540.x PMid:11168247. [DOI] [PubMed] [Google Scholar]
- 6.Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, da Cruz-Hofling MA. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B. 2003;70(2):81–9. doi: 10.1016/s1011-1344(03)00058-7. https://doi.org/10.1016/S1011-1344(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 7.Misch CE, Wang HL, Misch CM, Sharawy M, Lemons J, Judy KW. Rationale for the application of immediate load in implant dentistry:Part I. Implant Dent. 2004;13:207–17. doi: 10.1097/01.id.0000140461.25451.31. https://doi.org/10.1097/01.id.0000140461.25451.31 PMid:15359155. [DOI] [PubMed] [Google Scholar]
- 8.Misch CE, Wang HL, Misch CM, Sharawy M, Lemons J, Judy KW. Rationale for the application of immediate load in implant dentistry:part II. Implant Dent. 2004;13:310–21. doi: 10.1097/01.id.0000148556.73137.24. https://doi.org/10.1097/01.id.0000148556.73137.24 PMid:15591992. [DOI] [PubMed] [Google Scholar]
- 9.Schulte W. Implant and periodontium. Int. Dent. J. 1995;45:16. PMid:7607740. [PubMed] [Google Scholar]
- 10.Sugiura K, and Sugiura M. Vitamin C and Skin. J Clin Exp Dermatol Res. 2018;9(2):444. https://doi.org/10.4172/2155-9554.1000444. [Google Scholar]
- 11.Naidu KA. Vitamin C in human health and disease is still a mystery? An Overview. J Nutr. 2003;2:7. doi: 10.1186/1475-2891-2-7. https://doi.org/10.1186/1475-2891-2-7 PMid:14498993 PMCid:PMC201008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooshki A, and Golafrooz M. Nutrient Intakes Affecting Bone Formation Compared with Dietary Reference Intake (DRI) in Sabzevar Elderly Subjects. J Nutr. 2009;8(3):218–21. https://doi.org/10.3923/pjn.2009.218.221. [Google Scholar]
- 13.Chandrasekar B, and Fernandes G. Decreased pro-inflammmatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994;200:893–98. doi: 10.1006/bbrc.1994.1534. https://doi.org/10.1006/bbrc.1994.1534 PMid:8179624. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa A, Lung CY, Mustafa N, et al. EPA-coated titanium implants promote osteoconduction in white New Zealand rabbits. Clin. Oral Impl. Res. 2014;0:1–7. doi: 10.1111/clr.12525. [DOI] [PubMed] [Google Scholar]
- 15.Bousten Y, Jamart J, Esselinckx W, Devogelear JP. Primary prevention of glucocorticoid-induced osteoporosis with intravenous pamidronate and calcium. A prospective controlled 1-year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. J. Bone. Miner. Res. 2001;16:104–12. doi: 10.1359/jbmr.2001.16.1.104. https://doi.org/10.1359/jbmr.2001.16.1.104 PMid:11149473. [DOI] [PubMed] [Google Scholar]
- 16.Najeeb S, Zafar MS, Khurshid Z, Siddiqui F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016;60:12–19. doi: 10.1016/j.jpor.2015.10.001. https://doi.org/10.1016/j.jpor.2015.10.001 PMid:26520679. [DOI] [PubMed] [Google Scholar]
- 17.Haag M, Kruger MC. Upregulation of duodenal calcium absorption by poly-unsaturated fatty acids:events at the basolateral membrane. Med. hypotheses. 2001;56(5):637–40. doi: 10.1054/mehy.2000.1182. https://doi.org/10.1054/mehy.2000.1182 PMid:11388782. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Tamaki H, Ishimaru T, et al. Inhibition of osteoporosis due to restricted food intake by the fish oils DHA and EPA and perilla oil in the rat. Biosci Biotechnol Biochem. 2004;68(12):2613–15. doi: 10.1271/bbb.68.2613. https://doi.org/10.1271/bbb.68.2613 PMid:15618634. [DOI] [PubMed] [Google Scholar]
- 19.Awad SME, Mounir RM, Dine E, Salah M, Nasry SA. Effect of Laser Irradiation on Bony Implant Sites in Diabetic Patients:A Preliminary Study. Res J Pharm Biol Chem Sci. 2017;8(2):1484–95. [Google Scholar]
- 20.Anwer AG, Gosnell ME, Perinchery SM, Inglis DW, Goldys EM. Visible 532 nm laser irradiation of human adipose tissue-derived stem cells:effect on proliferation rates, mitochondria membrane potential and autofluorescence. Lasers Surg Med. 2012;44(9):769–78. doi: 10.1002/lsm.22083. https://doi.org/10.1002/lsm.22083 PMid:23047589. [DOI] [PubMed] [Google Scholar]
- 21.Pinheiro ALB, Limeira FA Jr, Gerbi MEMM, Ramalho LMP, Marzola C, Ponzi EAC. Effect of low-level therapy on the repair of bone defects grafted with inorganic bovine bone. Braz Dent J. 2003;14:177–81. doi: 10.1590/s0103-64402003000300007. https://doi.org/10.1590/S0103-64402003000300007 PMid:15057393. [DOI] [PubMed] [Google Scholar]
- 22.Chicago, IL, USA: SPSS:Statistical Package Software Inc; [Google Scholar]
- 23.Barone A, Covani U, Cornelini R, Gherlone E. Radiographic bone density around immediately loaded oral implants:A case series. Clin. Oral Implants Res. 2003;14:610–15. doi: 10.1034/j.1600-0501.2003.00878.x. https://doi.org/10.1034/j.1600-0501.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 24.Jaffin RA, Berman CL. The excessive loss of Branemark fixtures in type IV bone:a 5 year analysis. J Periodontol. 1991;62:2–4. doi: 10.1902/jop.1991.62.1.2. https://doi.org/10.1902/jop.1991.62.1.2 PMid:2002427. [DOI] [PubMed] [Google Scholar]
- 25.Stanford C, Brand R. Toward understanding of implant occlusion and strain adaptive bone modelling and remodelling. J Prosthet Dent. 1999;81:553–61. doi: 10.1016/s0022-3913(99)70209-x. https://doi.org/10.1016/S0022-3913(99)70209-X. [DOI] [PubMed] [Google Scholar]
- 26.Branemark P-I. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. https://doi.org/10.1016/S0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 27.Diniz JS, et al. Effect of low-power gallium-aluminum arsenium laser therapy (830 nm) in combination with bisphosph bisphosphonate treatment on osteopenic bone structure:an experimental animal study. Lasers Med Sci. 2009;24(3):347–52. doi: 10.1007/s10103-008-0568-9. https://doi.org/10.1007/s10103-008-0568-9 PMid:1⇈870. [DOI] [PubMed] [Google Scholar]
- 28.Renno ACM, et al. The effects of laser irradiation on the osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg. 2007;25:275–80. doi: 10.1089/pho.2007.2055. https://doi.org/10.1089/pho.2007.2055 PMid:17803384. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro ALB, Gerbi MEMM. Photoengineering of bone repair processes. Photomed Laser Surg. 2006;24(2):169–78. doi: 10.1089/pho.2006.24.169. https://doi.org/10.1089/pho.2006.24.169 PMid:16706695. [DOI] [PubMed] [Google Scholar]
- 30.Ninomiya T, et al. Increase of bone volume by a nanosecond pulsed laser irradiation is caused by a decreased osteoclast number and an activated osteoblasts. Bone. 2007;40:140–48. doi: 10.1016/j.bone.2006.07.026. https://doi.org/10.1016/j.bone.2006.07.026 PMid:16978938. [DOI] [PubMed] [Google Scholar]
- 31.Garavello-Freitas I, Baranauskas V, Joazeiro P, Padovani CR, Dal Pai-Silva M, Cruz-Hofling MA. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B. 2003;70:81–89. doi: 10.1016/s1011-1344(03)00058-7. https://doi.org/10.1016/S1011-1344(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 32.Park KI, Lee JY, Hwang UK, Kim YD, Kim GC, Shin SH, et al. Effect of calcium and vitamin D supplementation on bone formation around titanium implant. J Korean Assoc Oral Maxillofac Surg. 2007;33(2):131–138. [Korean] [Google Scholar]
- 33.Lopes CB, Pinheiro AL, Sathaiah S, Duarte J, Cristinamartins M. Infrared laser light reduces loading time of dental implants:a Raman spectroscopic study. Photomed Laser Surg. 2005;23:27–31. doi: 10.1089/pho.2005.23.27. https://doi.org/10.1089/pho.2005.23.27 PMid:15782028. [DOI] [PubMed] [Google Scholar]
- 34.Ozawa Y, Shimizu N, Mishima H, Kariya G, Yamaguchi M, Takiguchi H, Iwasawa T, Abiko Y. Stimulatory effects of low-power laser irradiation on bone formation in vitro. InAdvanced Laser Dentistry. 1995 Apr 17;1984:281–289. International Society for Optics and Photonics. PMCid:PMC179986. [Google Scholar]
- 35.Shimizu N, Mayahara K, Kiyosaki T, Yamaguchi A, Ozawa Y, Abiko Y. Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg Med. 2007;39(6):551–9. doi: 10.1002/lsm.20521. https://doi.org/10.1002/lsm.20521 PMid:17659585. [DOI] [PubMed] [Google Scholar]
- 36.Khadra M, Rønold HJ, Lyngstadaas SP, Ellingsen JE, Haanæs HR. Low?level laser therapy stimulates bone–implant interaction:an experimental study in rabbits. Clinical oral implants research. 2004 Jun;15(3):325–32. doi: 10.1111/j.1600-0501.2004.00994.x. https://doi.org/10.1111/j.1600-0501.2004.00994.x PMid:15142095. [DOI] [PubMed] [Google Scholar]
- 37.Ueda Y, Shimizu N. Pulse irradiation of low-power laser stimulates bone nodule formation. Journal of oral science. 2001;43(1):55–60. doi: 10.2334/josnusd.43.55. https://doi.org/10.2334/josnusd.43.55 PMid:11383637. [DOI] [PubMed] [Google Scholar]
- 38.Silva Júnior AN, Pinheiro AL, Oliveira MG, Weismann R, Pedreira Ramalho LM, Amadei Nicolau R. Computerized morphometric assessment of the effect of low-level laser therapy on bone repair:an experimental animal study. Journal of clinical laser medicine & surgery. 2002 Apr 1;20(2):83–7. doi: 10.1089/104454702753768061. https://doi.org/10.1089/104454702753768061 PMid:12017432. [DOI] [PubMed] [Google Scholar]


