Abstract
BACKGROUND:
Hydroxyurea (HU) is an antimetabolite agent that interferes with the S-phase of cellular replication and inhibits DNA synthesis, with little or no effect on RNA or protein synthesis. It is used in the treatment of many myeloproliferative disorders (MD) and is particularly a first line treatment drug for intermediate to high-risk essential thrombocythemia. Although safe and very well tolerated by the patients suffering from MD, there have been numerous reports of a broad palette of cutaneous side effects associated with prolonged intake of the medication. These may include classical symptoms such as xerosis, diffuse hyperpigmentation, brown-nail discolouration, stomatitis and scaling of the face, hands, and feet or more serious side effects such as actinic keratosis lesions, leg ulcers and multiple skin carcinomas.
CASE REPORT:
We report a case of a 52-year-old man, on long-term therapy with HU for essential thrombocytosis, with several concurrent skin lesions. Despite the perennial use of HU, the cutaneous changes were neglected. The local dermatological examination revealed oval perimalleolar ulcer on the right leg, with dimensions 6 x 4 cm, clearly demarcated from the surroundings with regular margins, periulcerous erythema, with very deep and highly fibrinous bed of the ulcer, positive for bacterial infection. The ulcer was treated with topical wound therapy with alginate and parenteral antibiotics. The extended dermatological screening also showed two nummular lesions in the right brachial region, presenting as erythematous papules with sharp margins from the surrounding skin, gritty desquamation and dotted hyperpigmentations inside the lesion. Further dermoscopy and biopsy investigations confirmed a diagnosis of basal cell carcinoma. Nasal actinic keratosis was also noted. The patient was advised for discontinuing or substituting the HU therapy.
CONCLUSION:
We present this case to draw attention to the various cutaneous side effects that occur with perennial HU use and suggest an obligatory reference to a dermatological consult.
Keywords: Hydroxyurea therapy, Cutaneous side effects, Leg ulcer, Basal cell carcinoma
Introduction
Hydroxyurea (HU), a hydroxylated molecule of urea, is an antimetabolite drug that interferes with the synthesis of DNA, with little or no effect on RNA or protein synthesis [1][2]. It is used in the treatment of many myeloproliferative disorders (MD) such as chronic myeloid leukaemia, polycythemia vera or management of other diseases like sickle cell anaemia or thalassemia [3]. It is particularly a drug of choice as a first-line treatment for intermediate to high-risk essential thrombocythemia [4]. Although safe and very well tolerated by the patients suffering from MD with primary thrombocytosis [5], there have been numerous reports of cutaneous side-effects associated with prolonged intake of the medication [6]. These include major skin changes as actinic keratosis lesions, leg ulcers and multiple skin carcinomas, [6][7] with leg ulcers affecting as many as 9% of HU-treated patients [8].
The objective of our report is to raise awareness of the possible cutaneous lesions induced by long-term use of hydroxyurea.
Case report
A 52 years old Caucasian male patient, affected by essential thrombocytosis on perennial therapy of more than 10 years with hydroxyurea, presented to the Department of Dermatology with painful leg ulceration.
The patient has experienced two myocardial infarctions in the past (2001/2013) which was both treated with angioplasty through stent placement. He also had an episode of bleeding gastric erosion. Moreover, the patient suffers from hypertension. The chronic therapy that the patient receives is as follows: Furosemide, Carvedilol, Acetylsalicylic acid and Lisinopril. After being diagnosed with essential thrombocytosis, the patient is on a perennial therapy with hydroxyurea (1.5 g/day).
Eight months before the examination, the patient reports an oval skin defect in the perimalleolar region of the right leg, which he does not associate with a mechanical injury. He was never referred to a regular check-up. Owing to the neglected nature of the problem, the lesion progressed with ulceration and became very painful. The patient treated the lesion himself, with dough, sugar and alcohol-unsuccessfully, after which he presented himself to the Department of Dermatology. The local dermatological examination revealed oval perimalleolar ulcer on the right leg, with dimensions 6 x 4 cm, clearly demarcated from the surroundings with regular margins and definite limits, periulcerous erythema and hyperalgesia over the lesion, all the way down to the foot. The bed of the ulcer appeared very deep, involving cutaneous and subcutaneous tissues, highly fibrinous and showed signs of bacterial infection. The microbiological smear was performed and came back positive with a Staphylococcus aureus, Streptococcus β haemolyticus and Enterococcus contamination. The patient was treated with parenteral antibiotics by antibiogram (Clindamycin), as well as topical wound therapy with alginate. Doppler sonography and photoplethysmography were also commenced, by which vascular etiology of the ulcer was excluded. A consultation with a hematologist was advised right away for discontinuing or substituting the HU therapy. A consultation with a hematologist was advised right away for discontinuing HU therapy and substituting it with recommended second-line therapy with pegylated IFN-α. Treatment with barotherapy was introduced and shaving and meshed graft transplantation planned after cleaning the bed ulcer.
The patient was then examined for other cutaneous side effects. The dermatological screening revealed two nummular lesions in the right brachial region, presenting as erythematous papules with sharp margins from the surrounding skin, gritty desquamation and dotted hyperpigmentations inside the lesions.
Figure 1.
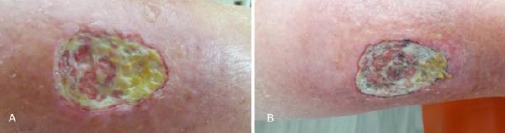
a), b) Oval perimalleolar ulcer on the right leg, with dimensions 6 x 4 cm, clearly demarcated from the surroundings with regular margins and definite limits, periulcerous erythema. The bed of the ulcer appears very deep and highly fibrinous. The a and b figures are respectively time framed to cover 5 months, from initial examination (Fig. 1a) to last control (Fig. 1b). We see no significant change in the course of treatment
When asked, the patient reported that he detected them several years ago and recorded discreet bleeding with mechanical trauma and crust formation over time, but the changes were also neglected, and he did not report them to a doctor before. A dermoscopy was initiated, which supported the initial suspicion of a cancer lesion.
Figure 2.
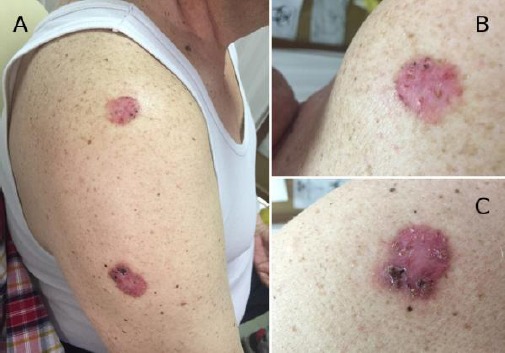
a), b) and c) Two nummular lesions (Fig. 2b upper lesion, Fig. 2c lower lesion) in the right brachial region, presenting as erythematous papules with sharp margins from the surrounding skin, gritty desquamation and dotted hyperpigmentations inside the lesion
Per protocol, biopsy followed, and the histopathological findings sustained basal cell carcinoma (BCC). The patient was advised for surgical treatment of the lesions.
Figure 3.
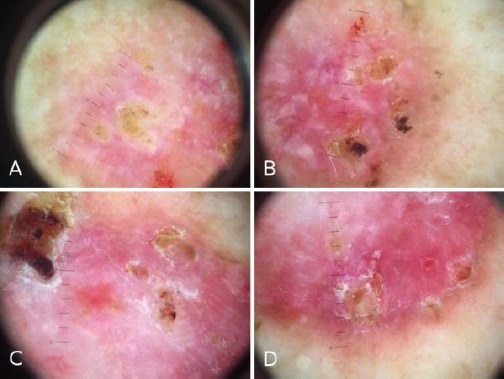
a), b) (upper lesion), c), d) (lower lesion) Dermoscopic findings of cancer like lesions: shiny white to red areas, short fine telangiectasias, leaf-like areas and small surface erosions
Furthermore, an actinic keratosis lesion in the nasal region was to be noted, which was dermoscopically verified. The treatment of choice was cryotherapy, with a successful outcome.
Figure 4.
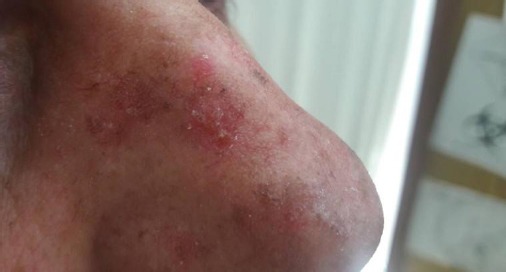
Nasal actinic keratosis lesion
Discussion
Essential thrombocythemia is a myeloproliferative disorder, characterised by stem cell-derived clonal myeloproliferative. The therapeutic approach is directly influenced by the risk grade of the disease, which is determined by the extensity of thrombocytosis, history and advanced age [3]. Thus, our patient is classified as an intermediate to high-risk thrombocytosis.
As presented by Tefferi et al., (2018) the current treatment algorithm for intermediate and high-risk ET is hydroxyurea as a first line drug of choice and a low dose of Aspirin [4], guidelines, which were correspondingly followed for the disorder management in our case.
HU is an antimetabolite agent, which acts upon the S-phase of cellular replication and inhibits DNA synthesis by barring the ribonucleotide reductase but does not affect RNA synthesis [1] [2]. As expected by an antineoplastic medication, Guillot et al., (2004) illustrate a broad palette of skin-related side effects associated with the long-term utilisation of the drug [7]. These may include classical symptoms such as xerosis, diffuse hyperpigmentation, brown-nail discoloration, stomatitis, erythema, and scaling of the face, hands, and feet, but three cutaneous lesions, in particular, are more specific to this drug: leg ulcers, actinic keratosis and increased frequency of skin carcinomas [2] [7] [9]. As in numerous other cases, our patient underwent cutaneous changes which are believed to be closely related to the long-term use of 1.5 g daily dose of HU. Most strikingly observant is the leg ulcer.
Stahl and Silber (1985) were the first one to report an HU-induced leg ulcer in 1985 [10]. Quattrone et al., (2013) appraised a pathophysiologic theory of three pathogenic factors in the formation of HU-induced skin ulcers- minor external traumas, direct HU toxicity on basal cells and hypoxia due to HU-induced macrocytosis [9]. Multiple publications show similarities to our case regarding the proposed aetiology of the leg ulcer. Consistently, dosage and duration of HU administration, as well as the location of the ulcer and the progression of the lesion: long-term use of HU (> 5 years), (1 or 1.5 mg per day), very painful perimalleolar ulcers, with no prior trauma [5] [11] [12] [13] [14]. Notwithstanding the standard wound therapy, treatment of choice in HU-induced leg ulcers is discontinuation of the drug or substitution therapy [8]. In contrast, there were few cases, where only standard debridement therapy, without discontinuation of HU, was enough to achieve successful partial or complete remission of the ulcers [6] [11] [13]. Correspondingly to some publications [6] [13] [14], our case showed refraction to standard conservative therapy. Hence a consultation with a haematologist for discontinuation and substituting it with recommended second-line therapy with pegylated IFN-α was commenced [4].
We also report of two carcinoma-like lesions, which were thoroughly examined according to protocol. As the physical examination and dermoscopy gave ambiguous findings, a biopsy was indicated. The result was a histopathological image of BCC. The significance of reporting these lesions is by other publications of similar lesions, with similar dermatological status, but histopathologically proven SCC [6]. Consequently, we advise an in-depth examination of all cancer-like lesions, due to the broad spectrum of different possible pathohistological findings.
The actinic keratosis lesion is reported within this paper, not only in association with HU [6] but because of its pre-malignant nature and high rate of malignisation, so a detailed follow-up is always advised.
In conclusion, our case study highlights the importance of understanding the cutaneous side effects of long-term HU use. Haematologists and patients should be drawn to attention to cutaneous lesions induced by prolonged HU intake. If there are skin changes already present, substitute therapy should always be advised if possible. Dermatological consult ought to be mandatory for all patients on perennial HU use.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Best PJ, Petitt RM. Multiple skin cancers associated with hydroxyurea therapy. Mayo Clin Proc. 1998;73(10):961–3. doi: 10.4065/73.10.961. https://doi.org/10.4065/73.10.961 PMid:97≆6. [DOI] [PubMed] [Google Scholar]
- 2.Sirieix ME, Debure C, Baudot N, et al. Leg ulcers and hydroxyurea:forty-one cases. Arch Dermatol. 1999;135(7):818–820. doi: 10.1001/archderm.135.7.818. https://doi.org/10.1001/archderm.135.7.818 PMid:10411157. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia:2015 update on diagnosis, risk?stratification and management. Am J Hematol. 90:162–173. doi: 10.1002/ajh.23895. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera treatment algorithm 2018. Blood Cancer Journal. 2018;8(1):3. doi: 10.1038/s41408-017-0042-7. https://doi.org/10.1038/s41408-017-0042-7 PMid:29321547 PMCid:PMC5802495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randi ML, Ruzzon E, Tezza F, et al. Toxicity and side effects of hydroxyurea used for primary thrombocythemia. Platelets. 2005;16(3-4):181–4. doi: 10.1080/09537100400020179. https://doi.org/10.1080/09537100400020179 PMid:16011962. [DOI] [PubMed] [Google Scholar]
- 6.Antar A, Ishak RS, Otrock ZK, et al. Successful treatment of hydroxyurea-associated chronic leg ulcers associated with squamous cell carcinoma. Hematol Oncol Stem Cell Ther. 2014;7(4):166–9. doi: 10.1016/j.hemonc.2014.09.008. https://doi.org/10.1016/j.hemonc.2014.09.008 PMid:25467031. [DOI] [PubMed] [Google Scholar]
- 7.Guillot B, Bessis D, Dereure O. Mucocutaneous side effects of antineoplastic chemotherapy. Expert Opin Drug Saf. 2004;3(6):579–87. doi: 10.1517/14740338.3.6.579. https://doi.org/10.1517/14740338.3.6.579 PMid:15500416. [DOI] [PubMed] [Google Scholar]
- 8.Hwang S-W, Hong S-K, Kim S-H, Seo J-K, Lee D, Sung H-S. A Hydroxyurea-induced Leg Ulcer. Annals of Dermatology. 2009;21(1):39–41. doi: 10.5021/ad.2009.21.1.39. https://doi.org/10.5021/ad.2009.21.1.39 PMid:20548853 PMCid:PMC2883366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravandi-Kashani F, Cortes J, Cohen P, et al. Cutaneous ulcers associated with hydroxyurea therapy in myeloproliferative disorders. Leuk Lymphoma. 1999;35(1-2):109–18. doi: 10.3109/10428199909145710. https://doi.org/10.3109/10428199909145710 PMid:10512168. [DOI] [PubMed] [Google Scholar]
- 10.Stahl LR, Silber R. Vasculitic leg ulcers in chronic myelogenous leukemia. Am J Hematol. 1985;78:869–72. doi: 10.1016/0002-9343(85)90297-9. https://doi.org/10.1016/0002-9343(85)90297-9. [DOI] [PubMed] [Google Scholar]
- 11.Fioramonti P1, Fino P, Parisi P, et al. A case of hydroxyurea-induced leg ulcer after definitive treatment suspension in a patient affected by thrombocythemia:effectiveness of a new collagenase. In Vivo. 2012;26(6):1053–6. [PubMed] [Google Scholar]
- 12.Hoff NP, Akanay-Diesel S, Pippirs U, et al. Cutaneous side effects of hydroxyurea treatment for polycythemia vera. Hautarzt. 2009;60(10):783–7. doi: 10.1007/s00105-009-1844-8. https://doi.org/10.1007/s00105-009-1844-8 PMid:19756436. [DOI] [PubMed] [Google Scholar]
- 13.Boneberger S, Rupec RA, Ruzicka T. Ulcers following therapy with hydroxyurea. Three case reports and review of the literature. Hautarzt. 2010;61(7):598–602. doi: 10.1007/s00105-009-1794-1. https://doi.org/10.1007/s00105-009-1794-1 PMid:19763519. [DOI] [PubMed] [Google Scholar]
- 14.Saravu K, Velappan P, Lakshmi N, Shastry BA, Thomas J. Hydroxyurea Induced Perimalleolar Ulcers. Journal of Korean Medical Science. 2006;21(1):177–179. doi: 10.3346/jkms.2006.21.1.177. https://doi.org/10.3346/jkms.2006.21.1.177 PMid:16479088 PMCid:PMC2733971. [DOI] [PMC free article] [PubMed] [Google Scholar]


