Abstract
AIM:
To evaluate the association between VEGF-634G>C gene polymorphism with premalignant gastric lesions as well as the level of VEGF.
METHODS:
This cross-sectional study included patients with H. pylori gastritis at Haji Adam Malik General Hospital, Permata Bunda General Hospital, and Universitas Sumatera Utara Hospital, Medan, Indonesia. Detection of H. pylori infection was made using positive results of 14C-UBT, rapid urease test, and/or immunohistochemistry. Gastric premalignant lesion diagnosis was made when one or more of the following were present: chronic atrophic gastritis, intestinal metaplasia, or dysplasia. Real-time polymerase chain reaction (RT-PCR) was used to examine VEGF-634G>C gene polymorphism. Additionally, serum samples of patients with H. pylori gastritis were obtained to determine the level of circulating VEGF. Data were analysed using SPSS version 22.
RESULTS:
A total number of 87 patients with H. pylori gastritis were included in this study. Of all participants, 26 patients (29.9%) showed gastric premalignancy. There was a significant association between GG+GC genotype of VEGF-634G>C and gastric premalignant lesions (P = 0.003; OR (CI 95%) = 6.07 (1.88-41.71)). VEGF-634 G>C polymorphism also showed an association with VEGF serum levels (P = 0.005). Patients with the GG+GC genotype would be at risk of 3.16 times to have high VEGF levels compared to CC genotypes.
CONCLUSION:
VEGF-634G>C polymorphism, in particular, GG+GC genotype was associated with an increased risk of gastric premalignant transformation as well as having high VEGF levels in patients with H.pylori gastritis.
Keywords: Gastric premalignant lesion, Helicobacter pylori, VEGF, polymorphism
Introduction
Gastric cancer remains to be the second leading cause of all cancer mortality, causing approximately 700.000 death worldwide [1]. Gastric carcinogenesis is a continuous process commonly initiated with atrophic gastritis [2]. Chronic atrophic gastritis is considered the first step in a sequence of mucosal changes in the stomach leading to cancer. The current model for stomach carcinogenesis begins with gastritis, proceeding to chronic atrophic gastritis, then to intestinal metaplasia, dysplasia and, finally, carcinoma [3]. Risk factors of gastric cancer are Helicobacter pylori (H. pylori) infection, salt intake, smoking, alcohol, family history of gastric cancer, and presence of premalignant gastric lesions such as atrophic gastritis, intestinal metaplasia, and dysplasia. Gastric premalignant lesions are well-known risk factors for the development of gastric cancer [4] [5] [6] [7] [8] [9] [10] [11] [12].
H. pylori infection has been well studied as the main aetiology of chronic gastritis. Subsequently, atrophic gastritis, intestinal metaplasia, dysplasia, and gastric cancer may follow. Not all patients with H. pylori infection would develop such progression. One other factor that may contribute to the malignant progression is genetic [13].
Previous evidence suggests the Vascular Endothelial Growth Factor (VEGF) may play a role in gastric carcinogenesis, as VEGF promotes angiogenesis that is required for tumour survival and metastasis [14]. Patients with premalignant lesions such as atrophic gastritis, intestinal metaplasia, and dysplasia also show overexpression of VEGF that may contribute in the early step of carcinogenesis [15].
The VEGF gene, located at 6p21.3 chromosome, composed of 8 exons separated by 7 introns, is a very polymorphic gene (about 140 variants) [16] [17]. Previous studies show VEGF-634G>C polymorphism to be associated with an increased risk of gastric cancer as well as elevated VEGF levels. This polymorphism is influenced by ethnicity to previous studies showed different results [18] [19] [20] [21]. There were no similar studies in Southeast Asia after searching on PubMed. To the authors’ knowledge, no published studies evaluating the association between VEGF-634G>C polymorphism with gastric premalignant lesions and serum VEGF levels in H. pylori gastritis patients are available. This study was conducted to determine the association between VEGF-634G>C polymorphism with premalignant gastric lesions and serum level of VEGF in H. pylori gastritis patients.
Methods
Patients Selection
This study was a cross-sectional study on 87 consecutive H. pylori gastritis patients that were admitted to the Endoscopy Unit at Haji Adam Malik General Hospital, Permata Bunda General Hospital, and Universitas Sumatera Utara Hospital, Medan, Indonesia between October and December 2017. Inclusion criteria include gastritis patients diagnosed based on histopathological examination, at least 18 years old, and willing to take part in the study. Patients with one of the following criteria were excluded: a history of H. pylori eradication treatment in the last 6 months or currently on antibiotics therapy commonly used in H. pylori eradication; history of proton pump inhibitor, H2 receptor antagonist use within the past 1 month; patients with systemic disease, malignancy or currently pregnant. This study was approved by the Institutional Review Board of Universitas Sumatera Utara.
Endoscopy was conducted to evaluate gastric mucosae such as the presence of oedema, erythema (spotted, patchy, linear), exudate, bleeding, erosion; as well as to take a tissue sample for the rapid urease test, immunohistochemistry test for H. pylori and histopathology. Tissue biopsy was performed within the greater and lesser curvature of the distal antrum, the lesser curvature at incisura angular, the anterior and posterior wall of the proximal corpus. An additional biopsy was also done in suspicious regions that were not included in the areas mentioned previously.
Diagnosis of Gastric Premalignant Lesion
Microscopic evaluation was done to diagnose premalignant gastric lesions such as chronic atrophic gastritis, intestinal metaplasia, and dysplasia. The presence of one or more findings is positive for a premalignant gastric lesion. Histopathologic examination was done by two pathologists blindly at Universitas Sumatera Utara Medan. If there were differences in the results of the examination of both experts, then a third pathologist was required to perform the histopathological examination.
Helicobacter pylori detection
The diagnosis of H. pylori infection was made using positive results of 14C-UBT, rapid urease test, and/ or immunohistochemistry. Before the 14C-UBT examination, the subjects fasted for at least 6 h, usually overnight. Patients swallowed 37 kBq (1 μCi) of encapsulated 14 C urea/citric acid composition in 25 ml water. Breath samples of patients were collected into Heliprobe Breath Cards (Noster system) 10 min after administration of 14C urea. Patients exhaled into the breath card until its colour indicator changed from orange to yellow. The breath samples were measured using the Heliprobe analyser (Noster system), and the activity was counted for 250 s. Results were expressed as counts per minute (cpm) and counts < 25 cpm were defined as Heliprobe 0 = not infected, counts between 25 cpm and 50 cpm as Heliprobe 1 = equivocal and counts > 50 cpm as Heliprobe 2 = infected [22].
The rapid urease test (Pronto Dry®, France) was also used to establish the diagnosis of H. pylori infection. The positive result is indicated by the colour changing of the indicator from amber to pink-red at room temperature within 24 hours. The yellow indicator colour is considered to be negative [23].
Immunohistochemical (IHC) staining for evaluation of H. pylori status carried-out with the procedure as follows [24]. Tissue sections were deparaffinized, rehydrated, and pretreated with Proteinase K for 8 min and incubated with ChemMate peroxidase blocking solution at room temperature for 10 min. The slides were subsequently incubated with the polyclonal rabbit anti- H. pylori primary antibody (B0471: Dako Corporation, Glostrup, Denmark) with a dilution of 1:50 was conducted at room temperature for 1 hour. After samples had been washed 3 times with phosphate-buffered saline, the Dako EnVision Dual Link System–HRP (K4065: Dako Corporation) was applied for 30 minutes. Finally, sections were incubated in diaminobenzidine for 10 minutes, followed by hematoxylin counterstaining and mounting. H. pylori-infected gastric mucosa from chronic gastritis patients served as positive controls. Negative controls were obtained by replacing the primary antibody with phosphate-buffered saline. H. pylori infection in the tissue sections was confirmed when short, curved or spiral bacilli resting on the epithelial surface, in the mucus layer, or deep in the gastric pits could be observed by light microscopy.
Serum Levels of VEGF
Venous blood samples were drawn into serum separator tubes and allowed to clot for 30-45 minutes at room temperature, before being centrifuged for 15 minutes at approximately 1,000 g. Serum was immediately stored frozen in aliquots at -20°C until assay for VEGF was performed. Circulating VEGF levels were examined in serum using the Quantikine Human VEGF-ELISA (Quantikine, R&D Systems, Inc., Minneapolis).
VEGF-634 G>C Polymorphism
Genomic DNA was extracted and purified from peripheral blood using the High Pure PCR Template Preparation Kit (Roche Applied Science) and stored until processed for genotyping. Analysis of the VEGF SNP -634G>C was performed using real-time polymerase chain reaction (RT-PCR). The PCR primers used for the –634G>C polymorphisms were 5’-CGACGGCTTGGGGAGATTGC-3’ (forward) and 5’-GGGCGGTGTCTGTCTGTCTG-3’ (reverse). The PCR cycle conditions consisted of an initial denaturation step at 94°C for 5 min, followed by 35 cycles of 30 s at 94°C, 30 s at 62°C, 30 s at 72°C and a final elongation at 72°C for 10 min.
Statistical Methods
Data analysis was performed through univariate and bivariate (Chi-Square test) analyses using SPSS 22nd version (SPSS Inc., Chicago). A value of P < 0.05 with a 95% confidence interval was considered statistically significant.
Results
Baseline and clinical characteristics of subjects
A total number of 87 H. pylori gastritis patients were included in this study. The diagnosis of H. pylori infection was made using positive results of 14C-UBT, rapid urease test, and/ or IHC. Figure 1 shows H. pylori in the gaster tissues by IHC.
Figure 1.
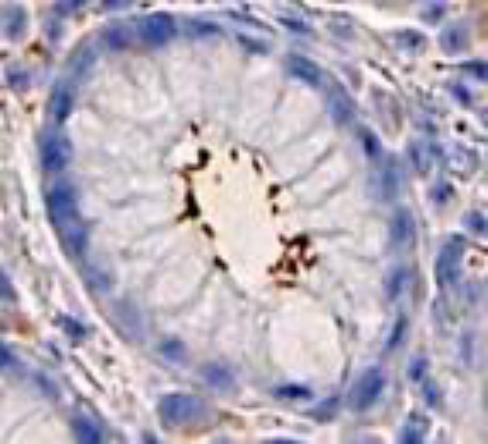
H. pylori in the gaster tissues by immunohistochemistry (IHC)
Patients’ characteristics show a majority of males (63.2%) with a mean age of 50.8 years, with mostly Batak ethnicity (52.9%). There were 46 patients (52.9%) having VEGF-634G>C polymorphism GC genotype, followed by 24 patients (27.6%) GG genotype and 17 patients (19.5%) CC genotype. The median value of VEGF levels was 441.7 pg/mL with a minimum level of 80.7 pg/mL and a maximum level of 2185.2 pg/mL. We grouped VEGF levels into 2 categories based on the median value, > 442 and <442. As many as 48.3% of patients had VEGF >442 pg/mL, 51.7% of patients had VEGF <442 pg/mL. There were 26 patients (29.9%) with premalignant gastric lesions (Table 1).
Table 1.
Baseline and clinical characteristics of subjects
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 55 (63.2%) |
| Female | 32 (36.8%) |
| Age, mean ± SD (years) | 50.8 ± 12.2 |
| ≥51 years | 58 (66.7%) |
| <51 years | 29 (33.3%) |
| Ethnicity | |
| Batak | 46 (52.9%) |
| Javanese | 20 (23%) |
| Aceh | 12 (13.8%) |
| Malay | 9 (10.3%) |
| Education | |
| Elementary school | 7 (8%) |
| Middle school | 12 (13.8%) |
| High school | 57 (65.5%) |
| University | 11 (12.6%) |
| BMI, mean ± SD (kg/m2) | 22.2 ± 3.8 |
| Overweight | 39 (44.8%) |
| Not overweight | 48 (55.2%) |
| Gastric Premalignant Lesion | |
| Yes | 26 (29.9%) |
| No | 61 (70.1%) |
| VEGF-634 G>C polymorphism | |
| GG genotype | 24 (27.6%) |
| GC genotype | 46 (52.9%) |
| CC genotype | 17 (19.5%) |
| VEGF level, median (min-max) (pg/ml) | 441.7 (80.7 – 2185.2) |
| High (≥ 442) | 42 (48.3%) |
| Low (< 442) | 45 (51.7%) |
Association between patient’s characteristics and VEGF levels with premalignant gastric lesions
There was a significant association between age and gastric premalignant lesions (P = 0.020), patients > 51 years were at risk of 2.75 times to get premalignant gastric lesions compared to age < 51 years. There was a significant association between ethnic group and gastric premalignant lesions (P = 0.016), where the Batak ethnic group had a 2.97 times risk of having premalignant gastric lesions compared to non-Bataks. There was a significant association between VEGF levels and premalignant gastric lesions (P <0.001), where patients with high VEGF levels had a 12.86 times risk of having gastric premalignant lesions compared to patients with low VEGF levels. There were no associations between gender, education, and overweight with premalignant gastric lesions (P > 0.05) (Table 2).
Table 2.
Association between patients’ characteristic and VEGF level with premalignant gastric lesions
| Variables | Gastric premalignant lesions | Total | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Present | Absent | ||||
| Gender | |||||
| Male | 14 (25.5%) | 41 (74.5%) | 55 (100%) | 0.237 | 0.68 (0.36-1.28) |
| Female | 12 (37.5%) | 20 (62.5%) | 32 (100%) | ||
| Age | |||||
| ≥51 years | 22 (37.9%) | 36 (62.1%) | 58 (100%) | 0.020* | 2.75 (1.05-7.24) |
| <51 years | 4 (13.8%) | 25 (86.2%) | 29 (100%) | ||
| Education | |||||
| Low level | 7 (36.8%) | 12 (63.2%) | 19 (100%) | 0.454 | 1.32 (0.65-2.66) |
| High level | 19 (27.9%) | 49 (72.1%) | 68 (100%) | ||
| Ethnic group | |||||
| Batak | 20 (43.5%) | 26 (56.5%) | 46 (100%) | 0.016* | 2.97 (1.32-6.67) |
| Non-Batak | 6 (14.6%) | 35 (85.4%) | 41 (100%) | ||
| Overweight | |||||
| Present | 10 (2.6%) | 29 (74.4%) | 39 (100%) | 0.436 | 0.77 (0.4-1.5) |
| Absent | 16 (33.3%) | 32 (66.7%) | 48 (100%) | ||
| VEGF level | |||||
| High | 24 (57.1%) | 18 (42.9%) | 42 (100%) | <0.001* | 12.86 (3.24-51.1) |
| Low | 2 (4.4%) | 43 (95.6%) | 45 (100%) | ||
P < 0.05; Low level of education : elementary school + middle school; High level of education: high school + university.
Association between VEGF-634G>C polymorphism and premalignant gastric lesions
There was a significant association between VEGF-634G>C polymorphism and premalignant gastric lesions. GG genotype increased the risk of 7.08 times for premalignant gastric lesions compared to CC genotype (P = 0.014). GC genotype increased the risk of 5.54 times for premalignant gastric lesions compared to CC genotype (P = 0.048). GG + GC genotype was 6.07 times more likely to have premalignant gastric lesions than patients with CC genotypes (P = 0.003). Patients with the G allele were at risk of 1.75 times for premalignant gastric lesions compared to C allele (p = 0.022) (Table 3).
Table 3.
Association between VEGF-634G>C polymorphism and premalignant gastric lesions
| VEGF-634G>C Polymorphism | Gastric premalignant lesion | Total | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Present | Absent | ||||
| GG | 10 (41.7%) | 14 (58.3%) | 24 (100%) | 0.014* | 7.08 (1.2-50.26) |
| GC | 15 (32.6%) | 31 (67.4%) | 46 (100%) | 0.048* | 5.54 (1.79-38.82) |
| CC | 1 (5.9%) | 16 (94.1%) | 17 (100%) | 1 (ref.) | |
| GG+GC | 25 (35.7%) | 45 (64.3%) | 70 (100%) | 0.003* | 6.07 (1.88-41.71) |
| CC | 1 (5.9%) | 16 (94.1%) | 17 (100%) | 1 (ref.) | |
| Allele G | 35 (37.2%) | 59 (62.8%) | 94 (100%) | 0.022* | 1.75 (1.07 – 2.88) |
| Allele C | 17 (21.3%) | 63 (78.8%) | 80 (100%) | 1 (ref.) | |
P <0.05.
Association between VEGF-634G>C polymorphism and serum VEGF levels
Further analysis was done to evaluate the association between VEGF-634G>C polymorphism and serum levels of VEGF. GG genotype increased the risk of 3.54 times to have high VEGF levels compared to CC genotype (P = 0.004). GC genotype increased the risk of 2.96 times to have high VEGF levels compared to CC genotype (P = 0.014). GG + GC genotype was 3.16 times more likely to have high VEGF levels than patients with CC genotypes (P = 0.005). Patients with G allele were at risk 1.53 times to have high VEGF levels compared to C allele (P = 0.009) (Table 4).
Table 4.
Association between VEGF -634 G>C polymorphism and the level of serum VEGF
| VEGF-634G>C Polymorphism | VEGF Level | Total | P | OR (95% CI) | |
|---|---|---|---|---|---|
| High | Low | ||||
| GG | 15 (62.5%) | 9 (37.5%) | 24 (100%) | 0.004* | 3.54 (1.21-10.35) |
| GC | 24 (52.2%) | 22 (47.8%) | 46 (100%) | 0.014* | 2.96 (1.02-8.56) |
| CC | 3 (17.6%) | 14 (82.4%) | 17 (100%) | 1 (ref.) | |
| GG+GC | 39 (55.7%) | 31 (44.3%) | 70 (100%) | 0.005* | 3.16 (1.11-9) |
| CC | 3 (17.6%) | 14 (82.4%) | 17 (100%) | 1 (ref.) | |
| Allele G | 54 (57.4%) | 40 (42.6%) | 94 (100%) | 0.009* | 1.53 (1.1 – 2.13) |
| Allele C | 30 (37.5%) | 50 (62.5%) | 80 (100%) | 1 (ref.) | |
P < 0.05.
Discussion
H. pylori is a type 1 carcinogen according to the International Agency for Research on Cancer (IARC). It promotes malignant changes through triggering an inflammatory cascade, including neutrophil and monocyte recruitment and upregulation of proinflammatory cytokines destroying the gastric mucosa. Epidemiological studies have shown an association between chronic inflammation and malignant transformation. H. pylori may also play a role in angiogenesis, essential for malignant cells survival. Among angiogenic factors, VEGF is known to be the most potent stimulus for neoangiogenesis [3]. This VEGF has a mitogenic effect on endothelial cells, promotes tumour cell growth, stimulates cell migration, and metastasis from the primary site [25].
The previous meta-analysis by Chen et al. reported higher VEGF expression in Asian patients with gastric cancer compared to controls (OR= 112.41, 95% CI= 64.12 – 197.06). High expression of VEGF also showed poor 5-year survival rates (RR= 2.45, P = 0.000), indicating its potential use as a marker for gastric cancer prognosis [26]. In gastric adenocarcinoma, a higher level of VEGF was also observed along with the increased density of intratumour small vessels. Moreover, higher expression of VEGF was also seen in gastric premalignant lesions such as chronic atrophic gastritis, intestinal metaplasia, and gastric dysplasia; as well as gastric carcinoma [15] [27] [28]. Raica et al also reported there was an association between increased levels of VEGF expression with gastric carcinogenesis [28]. These results are consistent with this study, that there was a significant association between high VEGF levels and premalignant gastric lesions (P <0.001).
Several SNP on the VEGF gene is thought to affect its expression. Certain allele variation may lead to overexpression of the transcription factor that will bind to the promoter site, which serves as the initial RNA polymerase binding site that will initiate transcription [29]. A previous study by Oh et al. evaluated 190 gastric cancer patients in Korea reported that the GG genotype of VEGF-634G>C polymorphism was associated with higher VEGF serum levels as well as poor outcome in patients with advanced stage [21]. Similar with that study, this present study also found a significant association between VEGF-634G>C polymorphism and premalignant gastric lesions, in particular, those with the GG+GC genotype had an increased risk of 3.16 fold to have high VEGF levels compared to those with the CC genotype.
A gene polymorphism was not only related to an increase or decrease in serum levels, but also with susceptibility to certain diseases [16] [30]. A study in Texas by Guan et al. included 171 gastric cancer patients (vs 353 controls) found that VEGF-634CG+CC showed an increased risk of gastric cancer compared to -634GG genotype [18]. Patients with VEGF heterozygous -634GC showed a poorer 1-year survival compared to patients with VEGF-634GG genotype [31]. Similar findings were reported by Tzanakis et al. (2006) in Greece that evaluated 100 gastric cancer patients. Tzanakis et al. found the VEGF-634CC genotype was associated with increased risk of gastric cancer and lower survival rates [19]. Meanwhile, a study by Chae et al. (2006) in Korea on 413 gastric cancer patients and 413 subjects as controls showed that VEGF-634CC was significantly associated with a lower risk of gastric cancer. VEGF-634 C allele was associated with a significant reduction in gastric cancer susceptibility (OR 0.686; 95% CI 0.564-0.834) [20]. Another study in Oman by Al-Moundhri (2009) found a significant association between VEGF-634 CC genotype with poor tumour differentiation and lymph nodes metastasis [32]. There were still limited studies that evaluate the relationship of VEGF polymorphism with premalignant gastric lesions. Tahara et al. reported that 1612G>A polymorphism of the VEGF gene was associated with premalignant gastric lesions in older individuals. The 1612 GA genotype showed a significantly higher incidence of intestinal metaplasia in H. pylori-positive individuals whose age was more than 65 years old [13]. This current study showed that VEGF-634G>C polymorphism was associated with premalignant gastric lesions, where -634GG+GC genotype increased the risk of 6.07 times for premalignant gastric lesions, presumably due to elevated serum VEGF levels in those genotypes.
These study indicated that older age was associated with an increased risk of premalignant gastric lesions. Previous studies reported a similar result [33] [34]. Benberin et al. showed the prevalence of premalignant gastric lesions increased with age. This condition was rarely seen in individuals under the age of 40 years [35]. The majority ethnic group in this study were Bataks (52,9%) because Bataks inhabit most of the North Sumatera region. There was a significant association between ethnicity and premalignant gastric lesions. Batak ethnic group increased the risk of 2.97 times for premalignant gastric lesions (P = 0.016). Further study is required to evaluate the high prevalence of premalignant gastric lesions in Bataks. Bataks have a habit of consuming alcohol both in a traditional ceremony and daily life [36]. Background of genetic factors, nutritional factors or lifestyle, immune responses to H. pylori infection may be considered. Ethnic differences may influence the risk of premalignant gastric lesions, which may be due to genetic variation [37].
In conclusion, the polymorphism of VEGF-634 G>C, in particular, GG+GC genotype, was associated with an increased risk of premalignant gastric lesions and high serum levels of VEGF in patients with H. pylori gastritis in Medan, Indonesia. There was a significant association between age, ethnic, and VEGF levels with premalignant gastric lesions.
Acknowledgements
We would like to express our gratitude to Lidya Imelda Laksmi who has conducted the histopathological analysis in this study, as well as to Khairani, Sulasmi, Tiolan, Tiambun who have assisted the endoscopy procedure in all patients.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. https://doi.org/10.3322/canjclin.55.2.74 PMid:15761078. [DOI] [PubMed] [Google Scholar]
- 2.Yanaoka K, Oka M, Mukoubayashi C, Yoshimura N, Enomoto S, Iguchi M, et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17(4):838–45. doi: 10.1158/1055-9965.EPI-07-2762. https://doi.org/10.1158/1055-9965.EPI-07-2762 PMid:18398025. [DOI] [PubMed] [Google Scholar]
- 3.Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109(1):138–43. doi: 10.1002/ijc.11680. https://doi.org/10.1002/ijc.11680 PMid:14735480. [DOI] [PubMed] [Google Scholar]
- 4.Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20(1):25–40. doi: 10.15430/JCP.2015.20.1.25. https://doi.org/10.15430/JCP.2015.20.1.25 PMid:25853101 PMCid: PMC4384712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zullo A, Hassan C, Romiti A, Giusto M, Guerriero C, Lorenzetti R, et al. Follow-up intestinal metaplasia in the stomach: when, how, and why. World J Gastrointest Oncol. 2012;4(3):30–6. doi: 10.4251/wjgo.v4.i3.30. https://doi.org/10.4251/wjgo.v4.i3.30 PMid:22468181 PMCid: PMC3312926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lage J, Uedo N, Dinis-Ribeiro M, Yao K. Surveillance of patients with gastric precancerous conditions. Best Pract Res Clin Gastroenterol. 2016;30(6):913–22. doi: 10.1016/j.bpg.2016.09.004. https://doi.org/10.1016/j.bpg.2016.09.004 PMid:27938786. [DOI] [PubMed] [Google Scholar]
- 7.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. https://doi.org/10.3748/wjg.v12.i3.354 PMCid: PMC4066052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H, Ekheden IG, Zheng Z, Ericsson J, Nyren O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:1–7. doi: 10.1136/bmj.h3867. https://doi.org/10.1136/bmj.h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almouradi T, Hiatt T, Attar B. Gastric intestinal metaplasia in an underserved population in the USA: prevalence, epidemiologic, and clinical features. Gastroenterol Res Pract. 2013;2013:856256. doi: 10.1155/2013/856256. https://doi.org/10.1155/2013/856256 PMid:24235966 PMCid: PMC3819766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XY, Zhang PY, Aboul-Soud MAM. From inflammation to gastric cancer: role of Helicobacter pylori. Oncol Lett. 2017;13(2):543–8. doi: 10.3892/ol.2016.5506. https://doi.org/10.3892/ol.2016.5506 PMid:28356927 PMCid: PMC5351277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh LY, Raj M, Hassan S, Aziz SA, Othman NH, Mutum SS, et al. Chronic atrophic antral gastritis and risk of metaplasia and dysplasia in an area with low prevalence of Helicobacter pylori. Indian J Gastroenterol. 2009;28(2):49–52. doi: 10.1007/s12664-009-0017-0. https://doi.org/10.1007/s12664-009-0017-0 PMid:19696988. [DOI] [PubMed] [Google Scholar]
- 12.de Vries AC, van Grieken NC, Looman CW, Casparie MK, Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: A Nationwide Cohort Study in the Netherlands. Gastroenterology. 2008;134(4):945–52. doi: 10.1053/j.gastro.2008.01.071. https://doi.org/10.1053/j.gastro.2008.01.071 PMid:18395075. [DOI] [PubMed] [Google Scholar]
- 13.Tahara T, Arisawa T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, et al. Effect of polymorphisms in the 3'-untranslated region (3'-UTR) of VEGF gene on gastric premalignant condition. Anticancer Res. 2009;29(2):458–90. [PubMed] [Google Scholar]
- 14.Chekhonin VP, Shein SA, Korchagina AA, Gurina OI. VEGF in tumor progression and targeted therapy. Curr Cancer Drug Targets. 2013;13(4):423–43. doi: 10.2174/15680096113139990074. https://doi.org/10.2174/15680096113139990074 PMid:23167597. [DOI] [PubMed] [Google Scholar]
- 15.Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: correlation with clinical features. BMC Cancer. 2002;2:8. doi: 10.1186/1471-2407-2-8. https://doi.org/10.1186/1471-2407-2-8 PMid:11978184 PMCid: PMC113262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng L, Liu G. VEGF pathway polymorphisms as prognostic and pharmacogenetics factors in cancer: a 2013 update. Pharmacogenomics. 2013;14(13):1659–67. doi: 10.2217/pgs.13.165. https://doi.org/10.2217/pgs.13.165 PMid:24088136. [DOI] [PubMed] [Google Scholar]
- 17.Eng L, Azad AK, Habbous S, Pang V, Xu W, Maitland-van der Zee AH, et al. Vascular endothelial growth factor pathway polymorphisms as prognostic and pharmacogenetic factors in cancer: a systematic review and meta-analysis. Clin Cancer Res. 2012;18(17):4526–37. doi: 10.1158/1078-0432.CCR-12-1315. https://doi.org/10.1158/1078-0432.CCR-12-1315 PMid:22733538. [DOI] [PubMed] [Google Scholar]
- 18.Guan X, Zhao H, Niu J, Tang D, Ajani JA, Wei Q. The VEGF -634G>C promoter polymorphism is associated with risk of gastric cancer. BMC Gastroenterol. 2009;9:77. doi: 10.1186/1471-230X-9-77. https://doi.org/10.1186/1471-230X-9-77 PMid:19835575 PMCid: PMC2771032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzanakis N, Gazouli M, Rallis G, Giannopulous G, Papaconstantinou I, Theodoropoulos G, et al. Vascular endothelial growth factor polymorphisms in gastric cancer development, prognosis, and survival. J Surg Oncol. 2006;94(7):624–30. doi: 10.1002/jso.20619. https://doi.org/10.1002/jso.20619 PMid:17111394. [DOI] [PubMed] [Google Scholar]
- 20.Chae YS, Kim JG, Sohn SK. Investigation of vascular endothelial growth factor gene polymorphisms and its association with clinicopathologic characteristics in gastric cancer. Oncology. 2006;71:266–72. doi: 10.1159/000106788. https://doi.org/10.1159/000106788 PMid:17671399. [DOI] [PubMed] [Google Scholar]
- 21.Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Hwang JA, et al. The relationship of Vascular endothelial growth factor gene polymorphisms and clinical outcome in advanced gastric cancer patients treated with FOLFOX: VEGF polymorphism in gastric cancer. BMC Cancer. 2013;13:43. doi: 10.1186/1471-2407-13-43. https://doi.org/10.1186/1471-2407-13-43 PMid:23374220 PMCid: PMC3573956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour-Ghanaei F, Sanaei O, Joukar F. Clinical Validation of an Office-Based 14C-UBT (Heliprobe) for H. pylori Diagnosis in Iranian Dyspeptic Patients. Gastroenterol Res Pract. 2011;2011:930941. doi: 10.1155/2011/930941. https://doi.org/10.1155/2011/930941 PMid:21760778 PMCid: PMC3132501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojborwonwitaya J, Vijitjunykul N. Comparison of the accuracy of two commercial rapid urase tests, CLOtest®, and Pronto Dry®, in detecting Helicobacter pylori Infection. Thai J Gastroenterol. 2005;6(2):55–60. [Google Scholar]
- 24.Wang F, Sun GP, Zou YF, Zhong F, Ma T, Li XQ, et al. Helicobacter pylori infection predicts favorable outcome in patients with gastric cancer. Curr Oncol. 2013;20(5):e388–95. doi: 10.3747/co.20.1417. https://doi.org/10.3747/co.20.1417 PMid:24155636 PMCid: PMC3805408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarnawski AS, Chai J, Jones MK. Esophageal and gastrointestinal microcirculation: essential for mucosal protection, a target for injury, and a critical component of injury and ulcer healing. In: Ishii H, Suematsu M, Tanishita K, editors. Organ microcirculation: a gateway to diagnostic and therapeutic intervention. Tokyo: Springer; 2005. pp. 49–61. https://doi.org/10.1007/4-431-27174-0_7. [Google Scholar]
- 26.Chen J, Li T, Wu Y, He L, Zhang L, Shi T, et al. Prognostic significance of vascular endothelial growth factor expression in gastric carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2011;137(12):1799–812. doi: 10.1007/s00432-011-1057-2. https://doi.org/10.1007/s00432-011-1057-2 PMid:21918901. [DOI] [PubMed] [Google Scholar]
- 27.Tuccillo C, Cuorno A, Rocco A, Martinelli E, Staibano S, Mascolo M, et al. Vascular endothelial growth factor and neo-angiogenesis in H. pylori gastritis in humans. J Pathol. 2005;207(3):277–84. doi: 10.1002/path.1844. https://doi.org/10.1002/path.1844 PMid:16184519. [DOI] [PubMed] [Google Scholar]
- 28.Raica M, Mogoant˘a L, Cimpean AM, Alexa A, Ioanovici S, M˘arg˘aritescu C. Immunohistochemical expression of vascular endothelial growth factor (VEGF) in intestinal type gastric carcinoma. Rom J Morphol Embryol. 2008;49(1):37–42. PMid:18273500. [PubMed] [Google Scholar]
- 29.Corvalan AH, Carrasco G, Saavedra K. The genetic and epigenetic bases of gastritis. In: Mozsik G, editor. Current Topics in Gastritis. London: In Tech; 2012. pp. 79–95. [Google Scholar]
- 30.Sripichai O, Fucharoen S. Genetic polymorphisms, and implication for human diseases. J Med Assoc Thai. 2007;90(2):394–8. PMid:17375650. [PubMed] [Google Scholar]
- 31.Guan X, Zhao H, Niu J, Tan D, Ajani JA, Wei Q. Polymorphisms of TGFβ1 and VEGF genes and survival of patients with gastric cancer. J Exp Clin Cancer Res. 2009;28(1):94. doi: 10.1186/1756-9966-28-94. https://doi.org/10.1186/1756-9966-28-94 PMid:19566948 PMCid: PMC2717936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Moundhri MS, Al-Nabhani M, Burney IA, Al-Farsi A, Al-Bahrani B. Gastric cancer risk predisposition and prognostic significance of vascular endothelial growth factor (VEGF) gene polymorphisms –a case-control study in an Omani population. Mol Carcinog. 2009;48(12):1170–6. doi: 10.1002/mc.20572. https://doi.org/10.1002/mc.20572 PMid:19676106. [DOI] [PubMed] [Google Scholar]
- 33.Kim N, Park YS, Cho SI, Lee HS, Choe G, Kim IW, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastro-duodenal disease. Helicobacter. 2008;13:245–55. doi: 10.1111/j.1523-5378.2008.00604.x. https://doi.org/10.1111/j.1523-5378.2008.00604.x PMid:18665932. [DOI] [PubMed] [Google Scholar]
- 34.den Hoed CM, van Eijck BC, Capelle LG, van Dekken H, Biermann K, Siersema PD, et al. The prevalence of premalignant gastric lesions in asymptomatic patients: predicting the future incidence of gastric cancer. Eur J Cancer. 2011;47:1211–8. doi: 10.1016/j.ejca.2010.12.012. https://doi.org/10.1016/j.ejca.2010.12.012 PMid:21239166. [DOI] [PubMed] [Google Scholar]
- 35.Benberin V, Bektayeva R, Karabayeva R, Lebedev A, Akemeyeva K, Paloheimo L, et al. Prevalence of H. pylori Infection and Atrophic Gastritis Among Symptomatic and Dyspeptic Adults in Kazakhstan. A Hospital based Screening Study Using a Panel of Serum Biomarkers. Anticancer Res. 2013;33:4595–6. PMid:24123036. [PubMed] [Google Scholar]
- 36.Gaol NL, Husin Dilema pemberantasan minuman keras terhadap pelestarian budaya masyarakat Batak Toba (studi kasus di Desa Ria-Ria Kecamatan Pollung Kabupaten Humbang Hasundutan) Citizenship. 2013;1:101–21. [Google Scholar]
- 37.Maran S, Lee YY, Xu S, Rajab N, Hasan N, Aziz SH, et al. Gastric precancerous lesions are associated with gene variants in Helicobacter pylori-susceptible ethnic Malays. World J Gastroenterol. 2013;19:3615–22. doi: 10.3748/wjg.v19.i23.3615. https://doi.org/10.3748/wjg.v19.i23.3615 PMid:23801863 PMCid: PMC3691040. [DOI] [PMC free article] [PubMed] [Google Scholar]


