Abstract
Background:
Body burden of mercury has been linked to hypertension in populations exposed to high mercury levels.
Objectives:
We summarized, extracted, and pooled the results of published studies that investigated mercury biomarkers and hypertension or blood pressure (BP) measurements to examine this potential relationship.
Methods:
We searched PubMed, Embase, and TOXLINE and selected studies according to a priori defined inclusion criteria. Study quality was assessed by the Newcastle-Ottawa scale for cohort and case–control studies and the Quality Assessment Tool for cross-sectional studies. Study estimates were pooled using inverse-variance weighted random-effects models. Dose–response meta-analysis was performed with studies reporting hypertension and systolic BP for at least three mercury categories.
Results:
A total of 29 studies were included in the meta-analysis. The pooled odds ratio (OR) for hypertension, comparing the highest and lowest mercury exposure categories, was 1.35 [95% confidence interval (CI): 0.99, 1.83] for populations with hair mercury in comparison with the OR of 1.12 (95% CI: 0.82, 1.52) for populations with hair mercury . Positive associations were also observed for highest versus lowest mercury exposure categories on systolic and diastolic BP. Heterogeneity was observed for mercury species and exposure groups across different studies. Associations estimated using different mercury biomarkers generally agree with each other in the same study. A nonlinear dose–response relationship with an inflection point at was identified, for both hypertension and systolic BP.
Conclusions:
A significant positive association between mercury and hypertension and between mercury and BP was identified. The exposure dose is an important factor in determining the toxic effects of mercury on hypertension. https://doi.org/10.1289/EHP2863
Introduction
Mercury (Hg) is a well-characterized environmental toxicant with known adverse health outcomes worldwide (Ha et al. 2017). Hg is present in three different forms, which include elemental mercury [(), inorganic mercury (mercury salts), and organic mercury (methyl mercury (MeHg)]. The general population is exposed to a relatively low level of inorganic Hg, primarily through dental amalgam; inhalation from anthropogenic sources, such as metal mining and smelting; combustion of fossil fuels; and incineration of municipal wastes (NRC 2000; United Nations Environment Programme 2002). Elevated exposure to inorganic Hg is found among mercury miners, gold miners, dentists, and patients receiving dental amalgams (Lorscheider et al. 1995; Malm 1998). Marine and freshwater fish consumption is the most common route of MeHg exposure for the general population (Driscoll et al. 2013). Many coastal and island dwellers frequently consume locally available marine seafood species that are high in MeHg. Some Indigenous populations also frequently consume traditional wild-caught marine foods, including top predators such as marine mammals. Therefore, coastal or Indigenous populations tend to have elevated exposure (Mergler et al. 2007). Rice is also a significant contributor to MeHg exposure in certain areas when paddy fields are contaminated by Hg (Feng et al. 2008).
Exposure or body burden of Hg is usually estimated by measuring biomarkers. Hg concentrations in tissues, such as hair, urine, blood, and nails, reflect Hg body burden and overall exposure from all sources (Branco et al. 2017; Ha et al. 2017). Hair Hg concentration is an appropriate and noninvasive biomarker to reflect exposure to MeHg (Clarkson and Magos 2006), but not for inorganic , as the in hair is likely to be demethylated from MeHg demethylation or external deposition (Berglund et al. 2005). Blood concentration of total Hg is considered as a useful biomarker of exposure to both MeHg and total Hg (Clarkson and Magos 2006) as MeHg was found to constitute 70–85% of the total Hg in the blood (Health Canada 2010; Mortensen et al. 2014). There is a strong correlation between hair Hg concentrations and whole blood Hg concentration at an average ratio of 250:1 (Bartell et al. 2000; Clarkson and Magos 2006; Liberda et al. 2014). Toenail Hg is also used in biomonitoring studies for the general populations to MeHg (Branco et al. 2017). Urinary Hg concentration is the most common biomarker for inorganic Hg exposure from occupational sources and from dental amalgams (Clarkson and Magos 2006).
High blood pressure (BP) has long been recognized as a leading risk factor for cardiovascular disease. A recent analysis suggests that the burden of high BP has been increasing over the last three decades (Forouzanfar et al. 2017). Besides the traditional risk factors for hypertension, such as high salt intake and overweight/obesity, environmental exposures to heavy metals may also play an important role (Abhyankar et al. 2012; Eum et al. 2008; Houston 2011; Navas-Acien et al. 2007). Although the mechanisms of how Hg induces hypertension are not fully understood, plausible explanations include oxidative stress and inflammation, which promote endothelial and renal dysfunction, and binding of selenium-related enzymes (Houston 2011). MeHg is generally considered to be the most toxic form and a dose–response relationship has been proposed between MeHg and cardiovascular outcomes (Roman et al. 2011). However, hypertension can be induced in experimental animals by acute or chronic administration of both inorganic Hg and MeHg (Carmignani and Boscolo 1984; Perry and Erlanger 1974; Perry et al. 1967; Wakita 1987). Although there is extensive literature supporting differing toxic effects and modes of action of inorganic Hg and MeHg on many human organ systems (e.g., NRC 2000; Clarkson & Magos 2006; etc.), there is no evidence showing a difference in effects on hypertension between inorganic and MeHg. Therefore, this systematic review and meta-analysis will include studies reported for both general and occupational populations exposed to different Hg species. The potential differences will be investigated by sensitivity analysis.
The objectives of this systematic review and meta-analysis were: (1) to evaluate the relationship between mercury exposure and hypertension, systolic BP (SBP), and diastolic BP (DBP), and (2) to explore the heterogeneity in the relationship between mercury exposure and BP outcomes contributed by three main factors: the Hg exposure groups in the studied population; mercury species measured and reported; and the choice of biomarkers. The dose–response relationship between mercury exposure and hypertension and SBP were also explored.
Methods
Search Strategy and Study Selection
Three databases, including PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Embase (https://www.embase.com/home), and TOXLINE (https://toxnet.nlm.nih.gov/newtoxnet/toxline.htm/), were used to find all published epidemiological studies evaluating the association between mercury exposure and hypertension or BP. Free text and Medical Subject Headings (MeSH) terms “mercury” or “methyl mercury” or “Quicksilver” or “dimethylmercury” or “colloidal mercury” AND “hypertension” or “blood pressure” or “cardiovascular disease” or “mortality” or “death” or “myocardial infarction,” “stroke” or terms of other cardiovascular outcomes were used. Detailed searching strategy for PubMed can be found in the Supplementary Materials. We adopted a broad searching strategy for health outcomes to increase the sensitivity because hypertension or blood pressure were likely to be reported in studies with other cardiovascular diseases as the main outcome. The search period was January 1966 through February 2017 with no language restrictions (Figure 1). Reference lists of eligible articles were searched for further pertinent articles. Two studies were identified through this search; however, none of them were included in the quantitative synthesis.
Figure 1.
Study selection flow diagram.
Two investigators (X.F.H. and K.S.) reviewed each paper. Studies that fulfilled the following a priori eligibility criteria were included: (a) original study; (b) cross-sectional, case–control, or a cohort design; and (c) reported Hg exposure and a blood pressure outcome. Our exclusion criteria were: (a) nonoriginal report, an experimental, case report, or a case series, (b) did not include our relationships of interest or no numeric values available (Al-Saleh et al. 2006; Dórea et al. 2005; Johansson et al. 2002), (c) used the same or a population subset of another included study (Choi et al. 2015; Lee and Kim 2011; Valera et al. 2008; Virtanen et al. 2005; Wennberg et al. 2012; Yoshizawa et al. 2002), (d) no adult participants (Grandjean et al. 2004; Kalish et al. 2014; Sørensen et al. 1999), and (e) the exposed group was not comparable with the control group and no adjustment was made for effect size estimate [the body weight of the patients and control group differed by (Oka et al. 2002)]. If more than one paper was published from the same study, the most recent paper or the paper using the best assessment of Hg and/or outcome was included. For studies that reported estimates for more than one biomarker, the estimate for the most appropriate biomarker was preferred. The order of preference was as follows for populations exposed mainly to MeHg: . This order was chosen because the majority of studies measured Hg in hair or blood, and hair Hg most accurately reflects long-term exposure. Urinary Hg was the first choice for studies reporting occupational exposure. We included studies that reported hypertension or BP by Hg exposure categories even if that was not the primary outcome of the study, to ensure all potentially relevant data were considered for meta-analysis.
Data Extraction and Quality Assessment
Two investigators (X.F.H. and K.S.) independently extracted the study data, including study design, study population (location, age, and sex distribution), sample size, Hg exposure matrix and levels, BP and hypertension outcomes, study results (measures of association), and potential confounders accounted for in the statistical analysis. Authors were contacted for information unavailable in the published report (Fillion et al. 2006). For studies with both continuous and categorical definitions of Hg exposure, we extracted the measures of association by Hg category. For studies with multiple levels of adjustment, we extracted the measure of association obtained from the model adjusted for the most covariates. Any discrepancies were resolved by consensus. X.F.H. and K.S. applied the Newcastle-Ottawa Scale (Wells et al. 2009) for case–control and cohort studies to assess quality. The Newcastle-Ottawa Scale includes a series of questions to evaluate the selection of participants into the study, the comparability of groups, and the ascertainment of exposure (for case–control studies) or outcome (for cohort studies), with a maximum score of 9 (Wells et al. 2009). Studies scoring were categorized as high quality. Cross-sectional studies were evaluated with the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Heart, Lung, and Blood Institute 2014). This tool contains 14 questions; however, only those questions that were applicable to cross-sectional studies (7 questions) were selected to evaluate the studies. The overall study rating of poor, fair, or good was based on subjective assessment of the seven questions and agreement among two reviewers. If no adjustments were made for confounding, the cross-sectional study was rated as poor.
Statistical Analysis
For studies that reported hypertension, we extracted or derived odds ratios (ORs), hazard ratios (HRs), and prevalence ratios for hypertension and their standard errors from the published data. For studies with hypertension data but no available measures of association, we estimated the OR and 95% confidence interval (CI) by Hg categories using the number of cases and noncases in the different exposure categories. For studies that reported SBP and DBP levels, we extracted the mean BP levels, corresponding standard errors and sample sizes of the different exposure categories. For studies with missing standard errors for SBP or DBP, an un-weighted average of available standard errors from all included studies was used. For studies that reported OR for hypertension or SBP or DBP change per continuous change of Hg concentration only, OR or SBP or DBP change per interquartile range (IQR) change in Hg concentrations were derived for high vs. low exposure categories.
For summary purposes, we pooled OR estimates for hypertension and weighted mean difference (WMD) comparing the highest and lowest categories of Hg exposure from individual studies using an inverse-variance weighted random-effects model. Studies were categorized into low-to-moderate mercury exposed ( hair Hg or equivalent) and high mercury exposed ( hair Hg or equivalent) by the mean mercury concentrations in the highest exposed group in those studies. Pooled ORs and WMDs were calculated for all studies and separately for studies conducted in populations exposed to low-to-moderate mercury levels and for studies conducted in populations exposed to high mercury levels. Hereafter in the paper, we use the term “high mercury exposure” to refer to mercury levels above which we observe an increased risk of hypertension. The cut-off of hair Hg was a data-driven value based on the results of this study. Heterogeneity was quantified with the statistic (Higgins and Thompson 2002). The relative influence of each study on pooled estimates was estimated by omitting one study at a time. Finally, we assessed publication bias using funnel plots (Figure S1 A/B/C).
Subgroup Analysis
Besides Hg exposure level, three additional sets of subgroup analyses were conducted to explore the contribution of the following three factors to the heterogeneity in the relationship between Hg exposure and BP outcomes: (1) exposure group; (2) Hg species; and (3) Hg biomarkers. By exposure group, studies were categorized into three groups including the general population, coastal and Indigenous populations, and occupationally exposed populations. By Hg species, studies were categorized into MeHg, inorganic Hg, and total Hg exposure. Here, Hg species referred to the Hg speciation measured and/or reported in the study, but not necessarily the main form of Hg the study population was exposed to. It can be assumed that both the general populations, and coastal and Indigenous populations were exposed mainly to MeHg, and occupational exposure was mainly inorganic Hg. By Hg biomarkers, a pairwise comparison was shown for results obtained with two different biomarkers within each study, i.e., hair vs. blood, blood vs. urinary, and hair vs. urinary.
Dose-Response Analysis
For studies that reported hypertension or SBP or DBP results for three or more Hg categories, dose-response meta-analysis was performed. For hypertension, we first plotted the ORs by exposure category from each study (Abhyankar et al. 2012; Liu et al. 2009). Dose-response meta-analyses were then conducted. Besides linear regression (Greenland and Longnecker 1992), a restricted cubic splines regression was also fitted, with knots fixed at percentiles 15%, 50%, and 85% through the distribution (Orsini et al. 2012). For SBP and DBP, we performed dose-response meta-analysis of differences in means (Crippa and Orsini 2016). Harmonized hair Hg equivalent values were assigned to Hg exposure categories of studies eligible for dose-response meta-analysis that reported Hg exposure in another matrix (i.e., blood, toenail, urine). We adopted a conversion ratio of 250 between Hg concentrations in hair (in ) and in the blood (in ) (Clarkson and Magos 2006). Hg concentrations in toenail (in ) and in urine () were converted to hair-Hg (in ) using a regression model developed by Ohno et al. (2007). All statistical analyses were performed using Stata software, version 14.0 (StataCorp, College Station, TX, USA), except for the dose-response meta-analysis of differences in means for SBP, which was conducted using RStudio 1.0.136 (RStudio, Inc.). For reporting, we followed the Meta-analysis of Observational Studies in Epidemiology (Stroup et al. 2000) and the Preferred Reporting Items for Systematic Review and Meta-Analysis (Moher et al. 2009) guidelines.
Results
Study Characteristics
This systematic review covers more than 55,000 participants from 17 countries, including occupational exposures and populations exposed to Hg through diets rich in fish (Table 1). Thirty studies, published between 1990 and 2017 were identified. One is cohort study (Mozaffarian et al. 2012), one is case-control study (Shiue 2014), and all the rest are cross-sectional design. Three studies have certain exposure history data (Choi et al. 2009; Kobal et al. 2004; Yorifuji et al. 2010). One case-control study (Guallar et al. 2002) and three cohort studies (Daneshmand et al. 2016; Virtanen et al. 2012b, 2012a) were considered as cross-sectional as we only used data from controls or the baseline data. Eleven studies were conducted at low to moderate mercury exposure levels (mean Hg concentration of the highest exposure group in hair or equivalent), nine of which were conducted in the United States (Bautista et al. 2009; Goodrich et al. 2013; Mordukhovich et al. 2012; Mozaffarian et al. 2012; Park et al. 2013; Shiue 2014; Siblerud 1990; Vupputuri et al. 2005; Wells et al. 2017), the rests were from Canada (Valera et al. 2011a) and Europe (Guallar et al. 2002). Eighteen studies were conducted at high mercury exposure levels (mean Hg concentration of the highest exposure group in hair or equivalent) (Daneshmand et al. 2016; Eom et al. 2014; Fillion et al. 2006; Hong et al. 2013; Hu et al. 2017; Kobal et al. 2004; Lee and Kim 2013; Nielsen et al. 2012; Park and Choi 2016; Park et al. 2016; Pedersen et al. 2005; Rajaee et al. 2015; Valera et al. 2009, 2011b, 2013, Virtanen et al. 2012b, 2012a; Yorifuji et al. 2010).
Table 1.
Characteristics of studies included in the systematic review and meta-analysis.
| Reference | Population | Exposure group | Mean age or age range (years) | Male (%) | N | Biomarker (unit) | Form | Mercury concentration | Outcome | Matrix available | Definition of HPT | Blood pressure measurement | Variables adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bautista et al. 2009 | Wisconsin, USA | General population | 59.4 | 52.5 | 101 | Hair () | Total | Mean 0.27 (95% CI: 0.23, 0.32) | HPT, BP | H, B | BP medication | Average of 2 measurements in 5 minutes interval using standard mercury sphygmomanometer, after seated for 15 minutes | Age, gender, BMI, fish intake, hypertension |
| Choi et al. 2009 | Whaling men, Faroe Islands | Coastal and Indigenous population | 54.8 | 100 | 42 | Blood () | Methyl | GM 29.5 (range: 5.19, 128.4) | BP | H, B, T | NA | Measured with standard mercury sphygmomanometers in seated position | Age, smoking, alcohol consumption, fish consumption, BMI |
| Daneshmand et al. 2016 | Finland | General population | 42–60 | 100 | 1828 | Hair () | Totala | Mean 1.90 (SD: 1.95) | BP | H | NA | Average of six measurements with zero mercury sphygmomanometer (after a supine rest of 5min, 3 in supine, 1 in standing and 2 in sitting position) | None |
| Eom et al. 2014 | South Korea | General population | 45.5 | 43.5 | 2114 | Blood () | Total | GM 3.90 (geometric SD: 1.88) | HPT, BP | B | , BP medication | Measured according to protocol | Age, gender, smoking, alcohol, residence area, seafood intake () |
| Fillion et al. 2006 | Amazon, Brazil | Coastal and Indigenous population | 35.2 | 53 | 251 | Hair () | Total | Mean 17.8 (range: 0.21, 77.2) | HPT | H | SBP | Measured with standard mercury sphygmomanometers in sitting position | Age, gender, BMI, smoking, community |
| Goodrich et al. 2013 | Dentist, Michigan, USA | Occupational population | 52.3 | 38 | 262 | Hair () | Total | Median 0.28 (IQR: 0.14, 0.55) | BP | H, U | NA | Measured in sitting position with device (Omron HEM 432-C) | Age, gender, BMI, BP medication |
| Guallar et al. 2002 | European & Israel | General population | 53.2 | 100 | 724 | Toenail () | Totala | Mean 0.25 (IQR: 0.15, 0.40) | HPT | T | Self-report | NA | None, age balanced across quintiles |
| Hong et al. 2013 | Smokers, South Korea | General population | 16–75 | 62.7 | 236 | Hair () | Total | Mean 1.41 (SD: 1.1) | BP | H | NA | Measured after 10 min of rest in a sitting position using a model TM-2655P automatic sphygmomanometer | Age |
| Hu et al. 2017 | Inuit, Canada | Coastal and Indigenous population | 18–78 | 38.7 | 2169 | Blood () | Total | Median 7.8 (range: 0.3, 70) | HPT, BP | B | , BP medication, self-report | Average of 3 readings from a BpTRU™ Vital Signs Monitor | Age, sex, smoking status, systolic blood pressure, TC/HDL, BMI, physical inactivity, diabetes, marital status, education, income, red bloodcellomega-3 and omega-6 fatty acids, log transformed blood concentration of lead, cadmium, sum of PCBs and sum of PBDEs |
| Kobal et al. 2004 | Mercury miners, Slovenia | Occupational population | 45 | 100 | 120 | Urine () | Total | Mean 69.3 (range: 26, 158) | HPT, BP | B, U | No detail | None | |
| Lee and Kim 2013 | South Korea | General population | 100 | 3783 | Blood () | Total* | Mean 4.96 (SE: 0.07) | HPT | B | , BP medication | Average of 3 readings using a mercury sphygmomanometer in seated position | Age, BMI, residence area, education level, smoking, drinking status, exercise, AST, ALT, lead, cadmium | |
| Mordukhovich et al. 2012 | Elderly, Boston, USA | General population | 72 | 100 | 639 | Toenail () | Totala | Median 0.22 (IQR: 0.07, 0.38) | HPT, BP | T | , BP medication | Average of both arms with standard mercury sphygmomanometers | Age, smoking, pack-years smoking, season of clinical visit, year of clinical visit, BMI, education, race/ethnicity, alcohol intake, fish intake |
| Mozaffarian et al. 2012 | Health professionals & Nurses, USA | General population | M 60.2 F 53.1 | 26.9 | 6045 | Toenail () | Total | Men Median 0.30 (90% CI: 0.07, 1.31) Women Median 0.21 (90% CI: 0.07, 0.76) | HPT, BP | T | Self-report | Self-reported usual blood pressure in categories | Age, gender, race, month of toenail return, family history of hypertension, smoking status, BMI, diabetes, hypercholesterolemia, future cardiovascular disease status, physical activity, alcohol use, fish consumption, and consumption of whole grains, unprocessed meats, processed meats, fruits, and vegetables |
| Nielsen et al. 2012 | Inuit, Greenland | Coastal and Indigenous population | 46.9 | 43.6 | 1861 | Blood () | Methyl | Men Median 22 (IQR: 11, 41) Women Median 16 (IQR: 8.8, 34.1) | HPT | B | , BP medication | Average of the second and third reading using automatic BP apparatus (Kivex UA 779), after 5 minutes rest | Age, smoking, selenium, n-3/n-6 fatty acids, waist circumference |
| Park et al. 2013 | USA | General population | 46.6 | 48.6 | 6607 | Blood () | Total | GM 1.03 (95% CI: 0.95, 1.11) | HPT, BP | B, U | , BP medication, self-report | Average of up to 3 measurements in 5 minutes interval using standard mercury sphygmomanometer, disregarding the first measurement | Age, gender, race/ethnicity, education, BMI, alcohol, cotinine, omega-3, total caloric intake, BP medication |
| Park and Choi 2016 | South Korea | General population | 49.6 | 8371 | Blood () | Totala | GM 3.90 (geometric SD: 1.80) | BP | B | NA | Average of second and third time measurements in 5 minutes interval using standard mercury sphygmomanometer | Age, gender, smoking status, alcohol consumption, job status, education, residence, diabetes mellitus | |
| Pedersen et al. 2005 | Greenland & Denmark | Coastal and Indigenous population | 20–60 | 43 | 186 | Blood () | Totala | Greenlanders Median 16.2 (range: 0.8, 117.7) Danes Median 2.2 (range: 0.8, 117.7) | BP | B | NA | Twenty-four hour ambulatory BP measurement (every 15 mins during daytime, every 30 mins during night-time) | Age, gender, BMI, residence |
| Rajaee et al. 2015 | Gold miners, Ghana | Occupational population | 30.6 | 60 | 70 | Urine () | Total | Median 4.24 (IQR: 1.24, 11.0) | BP | H, U | NA | Average of 3 readings using a mercury sphygmomanometer in seated position | Age, gender, smoking status |
| Shiue 2014 | USA | General population | 31.2 | 49.6 | 10537 | Urine () | Totala | Normal BP group Mean 0.68 (SD: 1.04) High BP group Mean 0.54 (SD: 0.75) | HPT | U | NA | Age, gender, ethnicity, BMI, urine creatinine | |
| Siblerud 1990 | Dental amalgam, Colorado USA | Occupational population | 23 | 40.6 | 101 | Urine () | Total | Non-amalgam Mean 1.23 (SD: 1.79) Amalgam Mean 3.70 (SD: 3.78) | BP | H, U | Several readings in sitting position by the auscultator method, mostly during evening time | None | |
| Valera et al. 2009 | Inuit, Nunavik, Canada | Coastal and Indigenous population | 34.3 | 43.6 | 732 | Blood () | Totala | Mean 50.2 (95% CI: 46.6, 54.1) | BP | B | NA | Average of the second and third reading using mercury sphygmomanometers, after 5 minutes rest | Age, , gender, EPA, selenium, alcohol consumption, waist circumference |
| Valera et al. 2011a | The Cree, Quebec, Canada | Coastal and Indigenous population | 35 | 53.2 | 791 | Hair () | Totala | Median 0.53 (IQR: 0.15, 1.62) | BP | H, B | NA | Average of the second and third reading using mercury sphygmomanometers, after 5 minutes rest | Age, sex, HDL-cholesterol, LDL-cholesterol, waist circumference, total n-3 PUFAs, triglycerides, fasting glucose, selenium, lead, PCB 153 and smoking. |
| Valera et al. 2011b | French Polynesia | Coastal and Indigenous population | 48.6 | 47.2 | 180 | Blood () | Totala | Median 13.5 (IQR: 8.5, 22) | BP | B | NA | Average of the second and third reading using mercury sphygmomanometers, after 5 minutes rest | Age, sex, waist circumference, fasting glucose, triglycerides, anti-hypertensive treatment, selenium, total n-3 PUFA |
| Valera et al. 2013 | Inuit, Quebec, Canada | Coastal and Indigenous population | 38 | 42.2 | 313 | Blood () | Methyl | Median 17.0 (IQR: 9.0, 28.4) | BP | B | NA | Average of the second and third reading using mercury sphygmomanometers, after 5 minutes rest | Age, sex, waist circumference, , and total PCBs |
| Virtanen et al. 2012a | Finland | General population | 52.8 | 100 | 1857 | Hair () | Methyla | Mean 1.91 (range: 0, 15.67) | HPT | H | No detail | NA | None |
| Virtanen et al. 2012b | Finland | General population | 53–73 | 51.6 | 768 | Hair () | Methyl | Mean 1.42 (SD: 1.54) | BP | H | NA | Average of six measurements with zero mercury sphygmomanometer (after a supine rest of 5min, 3 in supine, 1 in standing and 2 in sitting position) | Age, gender, examination year, hypertension in family, smoking, leisure-time physical activity, alcohol consumption, BMI, education, employment status, 24-h urinary potassium and sodium excretion |
| Vupputuri et al. 2005 | USA | General population | 32.9 | 0 | 1240 | Blood () | Total | Mean 1.8 (range: 0.1, 21.4) | BP | B | NA | Average of up to 3 measurements in 5 minutes interval using standard mercury sphygmomanometer | Age, race, income, body mass index, pregnancy status, and dietary sodium, potassium, and total calories |
| Wells et al. 2017 | Pregnant women, Baltimore, USA | General population | 16.4–36.7 | 0 | 263 | Blood () | Methyl | GM 0.95 (95% CI: 0.87, 1.07) | BP | B | NA | Continuous blood pressure measurements were collected with a General Electric Corometrics model 120 series fetal monitor | Age, race/ethnicity, median neighborhood household income, pregnancy, body mass index, smoking during pregnancy, and selenium. |
| Yorifuji et al. 2010 | Minamata, Japan | Coastal and Indigenous population | 41.7 | 120 | Hair () | Methyl | Low exposure High exposure | HPT, BP | H | Measured using a mercury sphygmomanometer by doctors in lying position | Age, occupation, past history of alcoholism, and past history of diabetes |
Note: BMI, body mass index; BP, blood pressure measurement; HPT, hypertension; GM, geometric mean; IQR, inter-quartile range; SD, standard deviation; SE, standard error; H, hair; B, blood; S, serum; U, urine; T, toenail; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PUFAs, polyunsaturated fatty acids; HDL, high density lipoprotein; TC/HDL, ratio of total cholesterol to high density lipoprotein. NA, outcome was not reported in the study.
Assumed from study.
The studies could be broadly categorized into 3 exposure groups: general population, coastal and Indigenous population, and occupationally exposed population. Within the general population group, US population are generally exposed to low level of Hg. In comparison, the South Korean (Eom et al. 2014; Hong et al. 2013; Lee and Kim 2013; Park and Choi 2016) and Finnish populations (Daneshmand et al. 2016; Virtanen et al. 2012b, 2012a) were exposed to moderate to high level of Hg. The coastal and Indigenous population consists of Inuit living in the Arctic (Hu et al. 2017; Nielsen et al. 2012; Pedersen et al. 2005; Valera et al. 2009, 2013), people living in Faroe Islands and French Polynesia (Choi et al. 2009; Valera et al. 2011b), Brazilian Amazon (Fillion et al. 2006), and Minamata area in Japan (Yorifuji et al. 2010). For occupational exposures, two studies reported exposure related to dental amalgam (Goodrich et al. 2013; Siblerud 1990) and another two studies reported exposure from mining (Kobal et al. 2004; Rajaee et al. 2015). For comparison of Hg species reported, a total of seven studies measured MeHg (Choi et al. 2009; Nielsen et al. 2012; Valera et al. 2013; Virtanen et al. 2012a, 2012b; Wells et al. 2017; Yorifuji et al. 2010) and the other twenty-three studies reported total Hg. Only eight studies reported concentrations of multiple Hg biomarkers in the studied populations within each study, of which three reported Hg in hair and urine (Goodrich et al. 2013; Rajaee et al. 2015; Siblerud 1990), three reported Hg in hair and blood (Bautista et al. 2009; Choi et al. 2009; Valera et al. 2011a), and another two reported in blood and urine (Kobal et al. 2004; Park et al. 2013).
Quality Assessment
Most of the United States and South Korean studies were based on national population bio-monitoring studies like NHANES (CDC & NCHS 2015) and KNHANES (Kweon et al. 2014). Three reports from Finland were all from the Kuopio Ischemic Heart Disease Risk Factor Study (Salonen et al. 2000). Most of the Inuit studies were also based on locally representative bio-monitoring studies (Dewailly et al. 2007; Saudny et al. 2012). All the studies used Hg biomarkers at the individual level to characterize Hg exposure (Table 1). Of the fourteen studies that reported hypertension, four studies defined the outcome based on measured BP only (Fillion et al. 2006; Kobal et al. 2004; Shiue 2014; Yorifuji et al. 2010), two studies defined the outcome based on self-report only (Guallar et al. 2002; Mozaffarian et al. 2012), one study did not report the definition (Virtanen et al. 2012a), and the rests defined the outcome based on measured BP plus either self-report or taking BP medication (Bautista et al. 2009; Eom et al. 2014; Hu et al. 2017; Lee and Kim 2013; Mordukhovich et al. 2012; Nielsen et al. 2012; Park et al. 2013). Of the 23 studies that reported SBP or DBP, most were based on the average of more than two measurements in the sitting position; two studies did not provide any measurement details (Eom et al. 2014; Kobal et al. 2004). Five of the 29 studies did not adjust for potential confounders (Daneshmand et al. 2016; Guallar et al. 2002; Kobal et al. 2004; Siblerud 1990; Virtanen et al. 2012b), one only adjusted for age (Hong et al. 2013), and the rest adjusted for multiple confounders. The quality assessments for the included cross-sectional studies are provided in Table 2. The Newcastle-Ottawa scale score was 7 for the included cohort study (Mozaffarian et al. 2012) and 8 for the case-control study (Shiue 2014).
Table 2.
Quality assessment of cross-sectional studies included in the systematic review and meta-analysis.
| Study | Questions of the Quality Assessment Tool for Cross-Sectional Studiesa | Overall rating | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Bautista et al. 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Fair |
| Choi et al. 2009 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Daneshmand et al. 2016 | Yes | Yes | Yes | Yes | Unclear | No | No | Poor |
| Eom et al. 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Fillion et al. 2006 | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Goodrich et al. 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Guallar et al. 2002 | Yes | No | Yes | Yes | No | No | Yes | Fair |
| Hong et al. 2013 | No | Yes | Yes | Yes | Yes | No | No | Poor |
| Hu et al. 2017 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Lee and Kim 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Fair |
| Mordukhovich et al. 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Nielsen et al. 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Park et al. 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Park and Choi 2016 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Pedersen et al. 2005 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Rajaee et al. 2015 | Unclear | Yes | Yes | Yes | Yes | No | No | Fair |
| Siblerud 1990 | Yes | No | Yes | Yes | Yes | No | No | Poor |
| Valera et al. 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Valera et al. 2011a | No | Yes | Yes | Yes | Yes | No | Yes | Fair |
| Valera et al. 2011b | No | No | Yes | Yes | Yes | No | Yes | Fair |
| Valera et al. 2013 | No | Yes | Yes | Yes | Yes | No | No | Fair |
| Virtanen et al. 2012a | Yes | Yes | Yes | Yes | Unclear | No | No | Poor |
| Virtanen et al. 2012b | No | Yes | Yes | Yes | Yes | No | Yes | Fair |
| Vupputuri et al. 2005 | Yes | Yes | Yes | Yes | Yes | No | Yes | Good |
| Wells et al. 2017 | Yes | Yes | Yes | Yes | No | Yes | No | Fair |
| Yoshizawa et al. 2002 | Yes | Yes | Yes | Yes | Unclear | Yes | No | Poor |
The numbers correspond to the following questions to assess study quality. The overall study rating of poor, fair, or good was based on subjective assessment of the seven questions and agreement among two reviewers. If no adjustments were made for confounding, the cross-sectional study was rated as poor.
1 “Was the participation rate of eligible persons at least 50%?”
2 “Were all subjects selected or recruited from the same or similar populations?”
3 “Did the study examine different levels of the exposure as related to the outcome?”
4 “Were the exposure measures clearly defined, valid, reliable, and implemented consistently across the study participants?”
5 “Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants?”
6 “Were the outcome assessors blinded to the exposure status of participants?”
7 “Were the key potential confounding variables measured and adjusted statistically?”
ORs for Hypertension
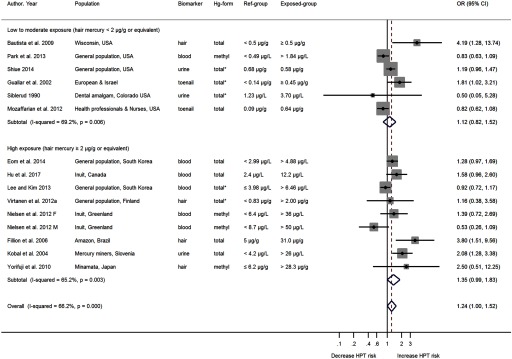
A total of fourteen studies evaluated the association between Hg exposure and hypertension, with one study reporting estimates separately for men and women (Nielsen et al. 2012) (Figure 2). Nine of the fifteen studies reported a positive association (Bautista et al. 2009; Eom et al. 2014; Fillion et al. 2006; Guallar et al. 2002; Hu et al. 2017; Kobal et al. 2004; Shiue 2014; Virtanen et al. 2012a; Yorifuji et al. 2010). Of the six studies conducted at low to moderate mercury exposure level, five were conducted in the United States (Bautista et al. 2009; Mozaffarian et al. 2012; Park et al. 2013; Shiue 2014; Siblerud 1990), and one included participants from Europe and Israel (Guallar et al. 2002). There was only one cohort study included in the review and that study reported a negative association comparing the highest with the lowest exposure group with an OR 0.82 (95% CI: 0.62,1.08) (Mozaffarian et al. 2012). Guallar et al. (2002) reported a positive association with an OR 1.81 (95% CI: 1.02, 3.21) based on 724 participants from eight European countries and Israel. Nine studies evaluated the association between Hg exposure and hypertension at high exposure level. Only two studies in the high exposure category showed negative associations (Lee and Kim 2013; Nielsen et al. 2012). Nielsen et al. (2012) identified a positive association in females, where as a negative association in males. Studies were sorted in ascending order according to the converted hair Hg concentration in the highest exposure categories.
Figure 2.
ORs of hypertension by mercury exposure levels. The area of each square is proportional to the inverse of the variance of the estimated log OR. Black diamonds represent point estimates of OR and horizontal lines represent 95% confidence intervals (CIs). The open diamonds represent the combined OR for each subgroup and the overall OR for all studies. The solid line represents . The dash line represents the point estimate of overall OR for all studies. The “metan” package in Stata only outputs p value up to 3-digit numbers for the heterogeneity tests. We reported in the text “” when the figures showed “”. Note: CI, confidence interval; HPT, hypertension; OR, odds ratio. * indicates mercury form was not described in the Methods section and was assumed by authors of the present review.
The pooled OR (Figure 2) for the six studies conducted at low to moderate mercury exposure level was 1.12 (95% CI: 0.82, 1.52; p value for ; ), with the study by Bautista et al. (2009) as moderately influential. The OR estimate excluding this study was 1.02 (95% CI: 0.78, 1.34; p value for ; ). The corresponding pooled OR for the nine studies conducted at high mercury exposure level was 1.35 (95% CI: 0.99, 1.83; p value for ; ). The overall pooled OR for hypertension was 1.24 (95% CI: 1.00, 1.52; p value for ; ).
SBP and DBP
Twenty-three studies (10 at low-to-moderate mercury-exposure level, 13 at high mercury-exposure level) investigated the association between Hg exposure and SBP or DBP (Figure S2, S3). The pooled mean difference in SBP between the highest and lowest Hg exposure categories for studies conducted at low-to-moderate exposure levels, and at high exposure levels, and for all studies were (95% CI: , 1.13; p-value for ; ), (95% CI: 0.90, 3.49; p-value for ; ), and (95% CI: 0.03, 2.60; p-value for ; ), respectively (Figure S2). The corresponding mean difference estimates for DBP were (95% CI: , 1.56; p-value for ; ), (95% CI: , 2.51; p-value for ; ), and (95% CI: 0.08, 1.85; p-value for ; ), respectively (Figure S3).
Subgroup Analysis
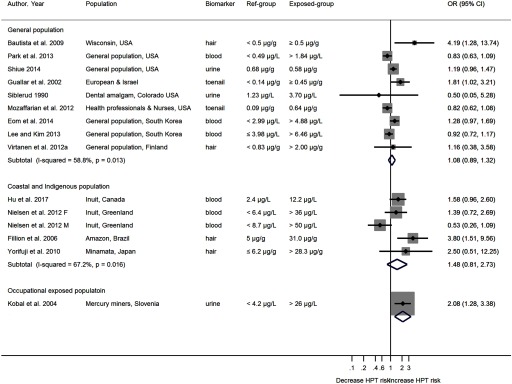
The heterogeneity in the relationship between Hg exposure and BP outcomes by exposure group, and by Hg species were explored by population subgroups. The pooled OR for hypertension in general population, coastal and Indigenous population, and occupational exposed population were 1.08 (95% CI: 0.89, 1.32), 1.48 (95% CI: 0.81, 2.73), and 2.08 (95% CI: 1.28, 3.38) respectively (Figure 3). Similar to hypertension, the pooled mean differences in SBP and DBP were larger for the occupationally exposed population [ (95% CI: 0.41, 7.42) in SBP and (95% CI: , 5.74) in DBP], followed by the coastal and Indigenous population and then the general population [ (95% CI: , 3.84) in SBP and (95% CI: , 2.40) in DBP] (Figure S4, S5). Within each population subgroup, there was a general trend towards larger differences in SBP and DBP with elevated Hg exposure levels. No differences in SBP or DBP were observed in studies that measured MeHg; marginal differences were observed in studies measured total Hg [( (95% CI: , 2.71) in SBP and (95% CI: , 2.14) in DBP], and significant differences were observed in studies measured inorganic Hg (mainly in inorganic form) [ (95% CI: 1.01, 9.14) in SBP and (95% CI: , 7.63) in DBP] (Figure S6, S7). The impact of the choice of biomarkers on the relation between Hg exposure and blood pressure outcomes was also investigated (Figure S8, S9). Within each study, the direction and the effect size were similar between Hg biomarkers, except for one study (Kobal et al. 2004). In this study, the blood MeHg concentration (which is not a good biomarker for inorganic exposure from mining) is higher in the control group than among the miners. In contrast, the miners had higher urine total Hg concentrations than subjects in the control group had.
Figure 3.
Odds ratios (ORs) of hypertension by mercury exposure groups. The area of each square is proportional to the inverse of the variance of the estimated log OR. Black diamonds represent point estimates of OR and horizontal lines represent 95% CIs. The open diamonds represent the combined OR for each subgroup. The solid line represents . Note: CI, confidence interval; HPT, hypertension; OR, odds ratio.
Dose–response Analysis for Hypertension
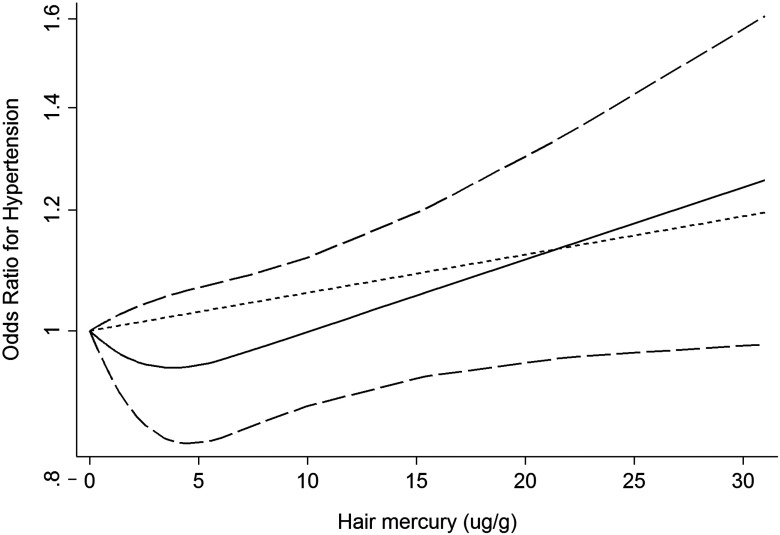
Eleven entries from nine studies reported more than three Hg exposure categories (Table 3), two of which reported men and women separately (Lee and Kim 2010; Nielsen et al. 2012). A nonlinear dose–response curve was fitted from the dose–response meta-analysis (Figure 4). The OR of hypertension decreased with hair Hg concentration until , then began to increase steadily. The slope of the curve after the hair Hg concentration of might not be as accurate because there are only 9 data points (20%) available (Table 3). Of the nine studies, six showed a positive dose–response relationship (Eom et al. 2014; Fillion et al. 2006; Guallar et al. 2002; Hu et al. 2017; Virtanen et al. 2012a; Yorifuji et al. 2010), two studies conducted in the United States showed a negative relationship (Mozaffarian et al. 2012; Park et al. 2013), and one study showed opposite trend for male and female (Nielsen et al. 2012) (Figure S10). The dose–response relationship between mercury exposure and SBP was also explored. The trend was similar to that of hypertension, the mean difference in systolic blood pressure decreased first and then started to increase at hair Hg concentration . However, this figure needs to be interpreted with caution because the estimates for CIs were not reliable due to lack of data (Figure S11).
Table 3.
Studies eligible for dose–response analysis.
| Reference | Original biomarker | Exposure category | Assigned hair mercury concentration ()a | cases | N | OR (95% CI) |
|---|---|---|---|---|---|---|
| Eom et al. 2014 | Blood Hg () | 0 | 199 | 705 | –b | |
| 2.99–4.88 | 1.1 | 258 | 705 | 1.12 (0.86, 1.46) | ||
| 2.74 | 335 | 705 | 1.28 (0.97, 1.69) | |||
| Fillion et al. 2006c | Hair Hg () | 5.6 | 0 | 11 | 80 | – |
| 15.4 | 15.4 | 17 | 87 | 2.11 (0.84, 5.36) | ||
| 31.0 | 31.0 | 28 | 92 | 3.80 (1.50, 9.50) | ||
| Guallar et al. 2002 | Toenail Hg () | 0.11 | 0 | 15 | 145 | – |
| 0.17 | 0.49 | 19 | 145 | 1.27 (0.62, 2.59) | ||
| 0.24 | 0.66 | 17 | 145 | 1.13 (0.55, 2.36) | ||
| 0.36 | 0.96 | 21 | 145 | 1.40 (0.69, 2.82) | ||
| 0.66 | 1.69 | 25 | 145 | 1.66 (0.84, 3.29) | ||
| Mozaffarian et al. 2012 M | Toenail Hg () | 0.08 | 0 | 144 | 324 | – |
| 0.18 | 0.52 | 152 | 325 | 1.02 (0.81, 1.30) | ||
| 0.30 | 0.81 | 155 | 325 | 1.03 (0.81, 1.32) | ||
| 0.46 | 1.2 | 149 | 325 | 0.97 (0.75, 1.25) | ||
| 1.00 | 2.52 | 138 | 325 | 0.82 (0.62, 1.08) | ||
| Mozaffarian et al. 2012 F | Toenail Hg () | 0.08 | 0 | 578 | 884 | – |
| 0.15 | 0.44 | 558 | 884 | 0.99 (0.88, 1.12) | ||
| 0.21 | 0.59 | 561 | 884 | 1.01 (0.90, 1.14) | ||
| 0.31 | 0.83 | 553 | 884 | 1.01 (0.89, 1.14) | ||
| 0.64 | 1.64 | 552 | 884 | 0.96 (0.84, 1.09) | ||
| Nielsen et al. 2012 M | Blood Hg () | 4.78 | 0 | 82 | 161 | – |
| 12.75 | 3.57 | 85 | 173 | 1.04 (0.62, 1.73) | ||
| 21.65 | 6.06 | 53 | 151 | 0.65 (0.37, 1.15) | ||
| 35.43 | 9.92 | 69 | 159 | 0.84 (0.45, 1.57) | ||
| 81.07 | 22.7 | 43 | 161 | 0.53 (0.26, 1.10) | ||
| Nielsen et al. 2012 F | Blood Hg () | 3.33 | 0 | 51 | 209 | – |
| 9.20 | 2.58 | 66 | 213 | 1.29 (0.77, 2.18) | ||
| 15.81 | 4.43 | 56 | 204 | 1.09 (0.63, 1.89) | ||
| 26.54 | 7.43 | 78 | 206 | 1.51 (0.85, 2.69) | ||
| 54.63 | 15.3 | 71 | 208 | 1.39 (0.72, 2.70) | ||
| Park et al. 2013 | Blood Hg () | 0.10–0.49 | 0 | 168 | 611 | – |
| 0.50–0.95 | 0.2 | 212 | 612 | 1.13 (0.95, 1.33) | ||
| 0.96–1.83 | 0.39 | 212 | 612 | 0.98 (0.75, 1.27) | ||
| 1.84–32.8 | 0.52 | 195 | 612 | 0.83 (0.63, 1.09) | ||
| Virtanen et al. 2012a | Hair Hg () | 0 | 249 | 624 | – | |
| 0.84–1.99 | 1.42 | 256 | 625 | 1.03 (0.84, 1.26) | ||
| 4.06 | 272 | 622 | 1.09 (0.89, 1.34) | |||
| Yorifuji et al. 2010 | Hair Hg () | 2.1 | 0 | 160 | 755 | – |
| 21.5 | 21.5 | 217 | 1450 | 0.60 (0.50, 0.80) | ||
| 30.0 | 30 | 215 | 833 | 1.60 (1.20, 2.10) | ||
| Hu et al. 2017 | Blood Hg () | 2.4 | 0 | 183 | 916 | – |
| 5.2 | 1.46 | 26 | 166 | 0.48 (0.26, 0.88) | ||
| 12.2 | 3.42 | 57 | 168 | 1.58 (0.96, 2.60) | ||
| 19.6 | 5.45 | 266 | 919 | 1.04 (0.71, 1.52) |
Note: Studies reporting at least three mercury exposure categories were eligible for dose–response meta-analysis.
Mercury (Hg) concentrations in blood (in ) were converted to Hg concentration in hair (in ) with a ratio of 280 based on our own unpublished meta-analysis. Hg concentrations in toenail (in ) were converted to hair mercury (in ) using regression model developed by Ohno et al. (2007). No conversion factor available between serum Hg and hair-Hg.
Reference group.
From personal communication from M. Fillion.
Figure 4.
Dose–response relationship between mercury exposure and odds ratio of hypertension (). Data were modeled with fixed-effects restricted cubic spline models with 3 knots (at 15th, 50th, and 85th percentile) using the Greenland and Longnecker method to estimate the covariances of multivariable-adjusted odds ratios. Lines with long dashes represent the pointwise 95% confidence intervals for the fitted nonlinear trend (solid line). Short dash line represents the linear trend.
Discussion
The association of Hg exposure and hypertension has been controversial. Differences in study populations and exposure levels, different exposure groups, different Hg species, different Hg biomarkers used to assess the exposure, and confounding effects (e.g., fish consumption), all contribute to the discrepancies observed in the published studies. To our knowledge, this review is the first meta-analysis to summarize the association of human Hg exposure with hypertension, SBP, and DBP reported in the literature thus far. The estimates of association are based on more than 55,000 participants from 17 countries, including occupational exposures and populations exposed to Hg through diets rich in fish. The most noteworthy finding of this meta-analysis is the discrepancies in the association between Hg and blood pressure outcomes by exposure level: no or weak association from studies of populations with low-to-moderate mercury exposure and positive association among populations with high mercury exposures. The current evidence suggests a hair Hg concentration of as the threshold of Hg’s toxic effect on blood pressure outcomes, although further study is required to confirm this value. Hair Hg concentration higher than is associated with a 59% increase in OR for hypertension, an increase of and in SBP and DBP, respectively.
As a generic method to integrate results from multiple studies, traditional meta-analysis calculates a summary estimate of the association (Egger et al. 2001). When a simple summary of association is not appropriate, subgroup meta-analysis provides useful information about patterns of the associations and their relations to study characteristics, such as the Hg species and biomarkers (Sterne et al. 2002). Dose–response meta-analysis characterizes the pattern of the exposure–response relations from multiple original studies (Orsini et al. 2012). In this study, we focused on the discrepancies in the association of Hg and hypertension at different exposure levels and also explored the contributions of three main factors including exposure groups, Hg species and use of biomarkers, to the heterogeneity of the association.
Apparent discrepancies in the association of hypertension with Hg exposure were observed between populations exposed to low-to-moderate and high Hg. One potential concern is that the studies reporting high exposure and the studies reporting low-to-moderate exposure were conducted in different populations (Figure 2, S2, S3). Some inherent differences among these populations, e.g., lifestyle and social economic status, background prevalence of hypertension and mean BP levels, and genetic adaptation to Hg exposure may confound the association between Hg and BP. Such confounding cannot be fully ruled out, although most of the included studies adjust for these characteristics within each population to some extent. Another noteworthy phenomenon is that negative associations were reported from multiple studies conducted at low-to-moderate exposure level (Mordukhovich et al. 2012; Mozaffarian et al. 2012; Park et al. 2013; Vupputuri et al. 2005). This phenomenon raises three questions: (1) should we adopt a linear nonthreshold model, or a nonlinear threshold model to assess the effects of Hg on hypertension? (2) what dose should be proposed as the threshold if we adopt the latter? and (3) what is the best approach to identify the threshold? A nonlinear threshold model seems more plausible to characterize the dose–response relationship between Hg and hypertension, based on both traditional forest plots (Figure 2, S2, S3) and the dose–response curve (Figure 4, S10, S11). We propose hair Hg concentration at as a possible threshold for the positive association because (1) there was an apparent difference between the observed associations in populations exposed below and above it and (2) cohort studies found similar threshold for other cardiovascular outcomes, e.g., heart rate variability, carotid intima-media thickness, and myocardial infarction (Choi et al. 2009; Virtanen et al. 2005; Wennberg et al. 2012). The third question is beyond the scope of this review. Future research on new effect size combining methods, for example, modifying the cumulative meta-analysis process or dose–response meta-analysis with different reference dose group, may improve the dose–response analysis and refine the threshold.
Beyond the dose, the toxic effects of Hg also depend on the exposure group and its chemical form (Clarkson and Magos 2006). In terms of exposure group, the included studies can be classified broadly into general populations (mostly from the United States, South Korea, and multiple European countries), coastal and Indigenous population exposed to MeHg from fish and marine mammal consumption (e.g., Inuit people living in the Arctic), and occupationally exposed populations (Figure 3, S4, S5). No particular pattern was observed for the general population. Mixed results were observed in the United States general population. In contrast, relatively consistent and significant effects were observed in the studied populations in South Korea (Eom et al. 2014; Park and Choi 2016), Finland (Daneshmand et al. 2016), and other European countries (Guallar et al. 2002), in which the fish and seafood consumption were higher in comparison with seafood consumption in the U.S. population. Effects observed within the exposure subgroups (i.e., coastal and Indigenous population and occupationally exposed population) were dependent on Hg exposure level (Figure S4, S5). These results suggest that exposure dose, rather than the exposure group, is a more important factor for the association.
For Hg speciation, our subgroup analyses results showed no association for the MeHg subgroup, the marginal association for the total Hg subgroup, and significant association for the inorganic Hg subgroup (Figure S6, S7). However, these results should also be interpreted very carefully because studies were categorized by the Hg species measured, but not by the major form of Hg exposed to. The participants in many of the studies that reported only total Hg were in fact exposed mainly to MeHg. Another noteworthy finding was that the association between Hg and SBP and between Hg and DBP tended to be stronger with elevated Hg levels within each subgroup. This result again suggests that Hg exposure level is the main determinant for BP, regardless of which Hg species was measured or reported.
The use of different biomarkers reported in different studies adds additional uncertainty. We investigated the influence of biomarkers on the association by comparing the results using different biomarkers in the same study (Figure S8, S9). For studies that measured Hg in both hair and blood, the results agreed with each other well because they both reflect MeHg exposure (Bautista et al. 2009; Choi et al. 2009; Valera et al. 2011a). These results suggest that the intrastudy variability of the impact of the choice of biomarkers on the association between Hg and blood pressure is relatively small. However, it is more challenging to estimate the interstudy variability. For example, three studies used toenail-Hg as the only exposure biomarker, and they all fell into the low-to-moderate exposure level. The two studies conducted in the United States reported a negative association (Mordukhovich et al. 2012; Mozaffarian et al. 2012), and the one conducted in Europe reported a positive association (Guallar et al. 2002). Moreover, in the dose–response meta-analysis, we converted blood and toenail-Hg concentration to hair Hg concentration. The conversion of different biomarkers to hair Hg introduces an uncertainty in the dose–response meta-analysis and can affect the shape of the curve. Nevertheless, the overall trend of the relationship should remain the same.
Our subgroup analysis showed that the studies on occupationally exposed populations, reporting results on inorganic Hg and urinary Hg concentrations, had higher OR than did the other groups. These results suggest that miners exposed to elevated Hg may be at the highest risk of Hg’s effects on hypertension. This result is the opposite of the results reported in two U.S. studies. Wells et al. (2017) reported a positive association between hypertension and total Hg and MeHg concentrations but a negative association with inorganic Hg. Another similar study reported that SBP was positively associated with hair-Hg (which is a biomarker of MeHg exposure) and negatively associated with urinary Hg (a biomarker of inorganic Hg exposure) (Goodrich et al. 2013). Because both studies were conducted on the general populations with low Hg exposure, this discrepancy suggests that the effects of inorganic Hg may be determined by the exposure dose, i.e., negative effect at a low dose and positive effect at the high dose. Another possibility is that miners exposed to high Hg from occupational exposure may be more at risk to the effects of Hg on hypertension.
Fish consumption and the nutrients from fish, e.g., omega-3 fatty acids and selenium, may partially or completely offset Hg’s toxic effect on cardiovascular outcomes (Chan and Egeland 2004; Mozaffarian and Rimm 2006). This possibility poses an additional challenge to estimating the effect of Hg exposure on hypertension, especially when study participants are exposed to mercury through a diet rich in fish. We adopted different strategies to ensure the most unbiased estimates were extracted. The three examples hereafter help to illustrate our strategies. In Example 1, SBP was reported to be positively associated with Hg exposure among nonfish consumers; however, SBP was negatively associated with Hg among fish consumers in the U.S. population (Vupputuri et al. 2005). The estimates from nonfish consumers were extracted for the pooling of effect size estimates. In Example 2, OR of hypertension in the highest exposure category is smaller than that in the second highest exposure category (Hu et al. 2017; Mozaffarian et al. 2012; Nielsen et al. 2012; Park et al. 2013). We extracted data from highest exposure category, except the study from our own group (Hu et al. 2017), as the fish consumption and blood selenium concentration in the highest exposure category was not comparable to the reference category. This factor also reflects the general challenge to estimate the effect of Hg exposure alone in high fish-consuming populations, because intakes of nutrients and contaminants are highly correlated (Laird et al. 2013). In Example 3, if there were apparent discrepancies between the unadjusted measures of association and measures adjusted for fish consumption or omega-3 fatty acids (Guallar et al. 2002; Valera et al. 2011a), we extracted the adjusted measure of association.
Fish-consumption advisories have been issued for pregnant women and women of childbearing age to avoid Hg’s neurotoxic effects on the fetus (U.S. EPA and U.S. FDA 2017). Moreover, women might need to choose the type of fish more wisely in comparison with men’s need to choose types of fish as several studies have shown that women are more vulnerable to the effects of Hg exposure (Choi et al. 2015; Nielsen et al. 2012; Yorifuji et al. 2010). The ORs of hypertension were higher among women exposed to similar Hg levels than men were exposed to in all three studies that reported estimates for men and women separately (Choi et al. 2015; Nielsen et al. 2012; Yorifuji et al. 2010). This finding is more suggestive than conclusive. Further research is needed to better examine whether a sex difference exists in Hg’s toxic effect on blood pressure outcomes. Hg also reduces the effectiveness of metalloenzymes by binding to metallothionein and substitutes for zinc, copper, and other trace metals (Carmignani et al. 1983). In experimental studies, female rats were also reported more susceptible to MeHg than were males (Magos et al. 1981; Tamashiro et al. 1986; Thomas et al. 1982).
As a meta-analysis of observational studies, there are also some inherent limitations. First, most of the studies were of cross-sectional design. Hence, we cannot rule out the possibility that the positive association observed between Hg exposure and hypertension reflects the dietary or behavior changes due to the diagnosis of hypertension (i.e., reverse causation). Second, the outcome of hypertension was not consistently defined across studies and the methods of BP measurement varied across studies. Although this meta-analysis supports a positive relationship between Hg and hypertension, the substantial heterogeneity among studies might reflect outcome misclassification and measurement discrepancies. Third, the use of multiple biomarkers across studies may introduce uncertainty to assess Hg exposure, especially for the dose–response meta-analysis. Data manipulation during the data extraction stage and biomarker conversion in the data analysis stage may introduce additional uncertainty to the pooled estimates. The lack of studies with moderate and high Hg exposure also poses a challenge to identifying the threshold. Fourth, a meta-analysis is not able to solve potential problems with confounding that could be inherent in the original studies. Inadequate adjustment for confounders could have resulted in over- or underestimation of the true association between Hg exposure and hypertension. Finally, we should acknowledge that the included studies are generally of North American, European and Asian populations; the lack of studies from other regions, especially developing countries, represents a major gap in the literature. Conducting relevant studies in these regions will be a future research priority.
Conclusions
The association between Hg exposure and the prevalence of hypertension was nonlinear, with no association in populations exposed to low-to-moderate mercury (hair Hg ) and evident association in populations exposed to high mercury (hair Hg ). However, the interpretation of causal association of Hg exposure and hypertension is limited by the cross-sectional design of original studies. Current evidence suggests that hair Hg concentration of might be considered as the threshold of Hg’s toxic effect on hypertension. Heterogeneity was observed for Hg species and exposure groups across different studies. Associations estimated using different Hg biomarkers generally agree with each other in the same study. Prospective cohort studies in populations exposed to moderate and high Hg and studies in populations not yet covered by this review are needed to better characterize the relationship between Hg exposure and hypertension. Systematic review and dose–response meta-analysis of Hg exposure and other cardiovascular outcomes would add further evidence about Hg’s toxic effect.
Supplemental Material
Acknowledgments
Funding support from the Northern Contaminant Program of the Government of Canada and the Canada Research Chair Program is acknowledged. We thank the very useful feedback from the two anonymous external reviewers.
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. 2012. Arsenic exposure and hypertension: a systematic review. Environ Health Perspect 120(4):494–500, PMID: 22138666, 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saleh I, Shinwari N, Mashhour A, Mohamed GE-D, Ghosh MA, Shammasi Z, et al. 2006. Cadmium and mercury levels in Saudi women and its possible relationship with hypertension. Biol Trace Elem Res 112(1):13–29, PMID: 16943613, 10.1385/BTER:112:1:13. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Ponce R. A, Sanga RN, Faustman EM. 2000. Human variability in mercury toxicokinetics and steady state biomarker ratios. Environ Res 84(2):127–132, PMID: 11068925, 10.1006/enrs.2000.4104. [DOI] [PubMed] [Google Scholar]
- Bautista LE, Stein JH, Morgan BJ, Stanton N, Young T, Nieto FJ. 2009. Association of blood and hair mercury with blood pressure and vascular reactivity. WMJ 108(5):250–252, PMID: 19743756, 10.1038/nbt.3121.ChIP-nexus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund M, Lind B, Björnberg KA, Palm B, Einarsson Ö, Vahter M. 2005. Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health 4(1):20, PMID: 16202128, 10.1186/1476-069X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco V, Caito S, Farina M, Teixeira da Rocha J, Aschner M, Carvalho C. 2017. Biomarkers of mercury toxicity: past, present, and future trends. J Toxicol Environ Health B Crit Rev 20(3):119–154, PMID: 28379072, 10.1080/10937404.2017.1289834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignani M, Boscolo P. 1984. Cardiovascular homeostasis in rats chronically exposed to mercuric chloride. Arch Toxicol Suppl 7:383–388, PMID: 6596006. [DOI] [PubMed] [Google Scholar]
- Carmignani M, Finelli V, Boscolo P. 1983. Mechanisms in cardiovascular regulation following chronic exposure of male rats to inorganic mercury. Toxicol Appl Pharmacol 69(3):442–450, PMID: 6879611. [DOI] [PubMed] [Google Scholar]
- CDC & NCHS (Center for Disease Control & National Center for Health Statistics). 2015. National Health and Nutrition Examination Survey Data.
- Chan HM, Egeland G. 2004. Fish consumption, mercury exposure, and heart diseases. Nutr Rev 62(2):68–72, PMID: 15080369, 10.1301/nr.2004.janr.68. [DOI] [PubMed] [Google Scholar]
- Choi AL, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Salonen JT, Tuomainen TP, et al. 2009. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect 117(3):367–372, PMID: 19337510, 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Yeum K-J, Park S-J, Kim K-N, Joo N-S. 2015. Elevated serum ferritin and mercury concentrations are associated with hypertension; analysis of the fourth and fifth Korea national health and nutrition examination survey (KNHANES IV-2, 3, 2008–2009 and V-1, 2010). Environ Toxicol 30(1):101–108, PMID: 23929718, 10.1002/tox. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. 2006. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36(8):609–662, PMID: 16973445, 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Crippa A, Orsini N. 2016. Dose-response meta-analysis of differences in means. BMC Med Res Methodol 16:91, PMID: 27485429, 10.1186/s12874-016-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshmand R, Kurl S, Tuomainen T-P, Virtanen JK. 2016. Associations of serum n-3 and n-6 PUFA and hair mercury with the risk of incident stroke in men: the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). Br J Nutr 115(10):1851–1859, PMID: 26991769, 10.1017/S0007114516000982. [DOI] [PubMed] [Google Scholar]
- Dewailly É, Chateau-Degat M-L, Ékoé J-M, Ladouceur R, Rochette L. 2007. Qanuippitaa? How are we? Status of Cardiovascular Disease and Diabetes in Nunavik. Report 20. Institut national de santé publique du Québec and Nunavik Regional Board of Health and Social Services; https://www.inspq.qc.ca [accessed 13 July 2018]. [Google Scholar]
- Dórea JG, De Souza JR, Rodrigues P, Ferrari Í, Barbosa AC. 2005. Hair mercury (signature of fish consumption) and cardiovascular risk in Munduruku and Kayabi Indians of Amazonia. Environ Res 97(2):209–219, PMID: 15533337, 10.1016/j.envres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. 2013. Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47(10):4967–4983, PMID: 23590191, 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Sterne JAC. 2001. Uses and abuses of meta-analysis. Clin Med (Lond) 1(6):478–484, 10.7861/clinmedicine.1-6-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom S-Y, Choi S-H, Ahn S-J, Kim D-K, Kim D-W, Lim J-A, et al. 2014. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health 87(5):501–513, PMID: 23824410, 10.1007/s00420-013-0891-8. [DOI] [PubMed] [Google Scholar]
- Eum KD, Lee MS, Paek D. 2008. Cadmium in blood and hypertension. Sci Total Environ 407(1):147–153, PMID: 18845316, 10.1016/j.scitotenv.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Feng X, Li P, Qiu G, Wang S, Li G, Meng B, et al. 2008. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ Sci Technol 42(1):326–332, PMID: 18350916, 10.1021/es071948x. [DOI] [PubMed] [Google Scholar]
- Fillion M, Mergler D, Passos CJS, Larribe F, Lemire M, Guimarães JRD. 2006. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ Health 5:29, PMID: 17032453, 10.1186/1476-069X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. 2017. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 317(2):165–838, PMID: 28097354, 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. 2013. Methylmercury and elemental mercury differentially associate with blood pressure among dental professionals. Int J Hyg Environ Health 216(2):195–201, PMID: 22494934, 10.1016/j.ijheh.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Murata K, Budtz-Jørgensen E, Weihe P. 2004. Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J Pediatr 144(2):169–176, PMID: 14760255, 10.1016/j.jpeds.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Greenland S, Longnecker MP. 1992. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135(11):1301–1309, PMID: 1626547, 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van't Veer P, Bode P, Aro A, Gómez-Aracena J, et al. 2002. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 347(22):1747–1754, PMID: 12456850, 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Ha E, Basu N, Bose-O'Reilly S, Dórea JG, McSorley E, Sakamoto M, et al. 2017. Current progress on understanding the impact of mercury on human health. Environ Res 152:419–433, PMID: 27444821, 10.1016/j.envres.2016.06.042. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2010. Report on human biomonitoring of environmental chemicals in Canada–results of the Canadian Health Measures Survey Cycle 1 (2007–2009). Ottawa, ON: Health Canada. [Google Scholar]
- Higgins JPT, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558, PMID: 12111919, 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hong D, Cho SH, Park SJ, Kim SY, Park SB. 2013. Hair mercury level in smokers and its influence on blood pressure and lipid metabolism. Environ Toxicol Pharmacol 36(1):103–107, PMID: 23603462, 10.1016/j.etap.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Houston MC. 2011. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich) 13(8):621–627, PMID: 21806773, 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XF, Eccles K, Chan HM. 2017. High selenium exposure lowers the odds ratios for hypertension, stroke, and myocardial infarction associated with mercury exposure among Inuit in Canada. Environ Int 102:200–206, 10.1016/j.envint.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Johansson N, Basun H, Winblad B, Nordberg M. 2002. Relationship between mercury concentration in blood, cognitive performance, and blood pressure, in an elderly urban population. Biometals 15:189–195, PMID: 12046928. [DOI] [PubMed] [Google Scholar]
- Kalish BT, Rifas-Shiman SL, Wright RO, Amarasiriwardena CJ, Jayawardene I, Gillman MW, et al. 2014. Associations of prenatal maternal blood mercury concentrations with early and mid-childhood blood pressure: a prospective study. Environ Res 133:327–333, PMID: 25019468, 10.1016/j.envres.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal AB, Horvat M, Prezelj M, Briški AS, Krsnik M, Dizdarevič T, et al. 2004. The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J Trace Elem Med Biol 17(4):261–274, PMID: 15139389, 10.1016/S0946-672X(04)80028-2. [DOI] [PubMed] [Google Scholar]
- Kweon S, Kim Y, Jang M, Kim Y, Kim K, Choi S, et al. 2014. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 43(1):69–77, PMID: 24585853, 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird BD, Goncharov AB, Egeland GM, Chan HM. 2013. Dietary advice on Inuit traditional food use needs to balance benefits and risks of mercury, selenium, and n3 fatty acids 1–3. J Nutr 143(6):923–930, PMID: 23616502, 10.3945/jn.112.173351.923. [DOI] [PubMed] [Google Scholar]
- Lee B-K, Kim Y. 2013. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005–2010 Korean National Health and Nutrition Examination Survey. Am J Ind Med 56(6):682–692, PMID: 22911659, 10.1002/ajim.22107. [DOI] [PubMed] [Google Scholar]
- Lee B-K, Kim Y. 2011. Relationship between blood manganese and blood pressure in the Korean general population according to KNHANES 2008. Environ Res 111(6):797–803, PMID: 21601843, 10.1016/j.envres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim J. 2010. Vegetable intake in Korea: data from the Korean National Health and Nutrition Examination Survey 1998, 2001 and 2005. Br J Nutr 103(10):1499–1506, PMID: 20128936, 10.1017/S0007114509993527. [DOI] [PubMed] [Google Scholar]
- Liberda EN, Tsuji LJ, Martin ID, Ayotte P, Dewailly E, Nieboer E. 2014. The complexity of hair/blood mercury concentration ratios and its implications. Environ Res 134:286–294, PMID: 25194499, 10.1016/j.envres.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Cook NR, Bergström A, Hsieh CC. 2009. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Comput Stat Data Anal 53(12):4157–4167, 10.1016/j.csda.2009.05.001. [DOI] [Google Scholar]
- Lorscheider FL, Vimy MJ, Summers AO. 1995. Mercury exposure from “silver” tooth fillings: emerging evidence questions a traditional dental paradigm. FASEB J 9(7):504–508, PMID: 7737458. [PubMed] [Google Scholar]
- Magos L, Peristianis GC, Clarkson TW, Brown A, Preston S, Snowden RT. 1981. Comparative study of the sensitivity of male and female rats to methylmercury. Arch Toxicol 48(1):11–20, PMID: 7283745. [DOI] [PubMed] [Google Scholar]
- Malm O. 1998. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res 77(2):73–78, PMID: 9600798, 10.1006/enrs.1998.3828. [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, et al. 2007. Methylmercury exposure and health effects in humans: a worldwide concern. AMBIO 36(1):3–11, PMID: 17408186, 10.1579/0044-7447(2007)36[3:MEAHEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, et al. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097, PMID: 19621072, 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. 2012. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the Normative Aging Study. Environ Health Perspect 120(1):98–104, PMID: 21878420, 10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen ME, Caudill SP, Caldwell KL, Ward CD, Jones RL. 2014. Total and methyl mercury in whole blood measured for the first time in the U.S. population: NHANES 2011–2012. Environ Res 134:257–264, PMID: 25173092, 10.1016/j.envres.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Rimm EB. 2006. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296(15):1885–1900, PMID: 17047219, 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Shi P, Morris JS, Grandjean P, Siscovick DS, Spiegelman D, et al. 2012. Mercury exposure and risk of hypertension in US men and women in 2 prospective cohorts. Hypertension 60(3):645–652, PMID: 22868395, 10.1161/HYPERTENSIONAHA.112.196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. 2014. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort [accessed 15 April 2017].
- NRC (National Research Council). 2000. Toxicological Effects of Methylmercury. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. 2007. Lead exposure and cardiovascular disease - a systematic review. Environ Health Perspect 115(3):472–482, PMID: 17431501, 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen ABS, Davidsen M, Bjerregaard P. 2012. The association between blood pressure and whole blood methylmercury in a cross-sectional study among Inuit in Greenland. Environ Health 11(1):44, PMID: 22747793, 10.1186/1476-069X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Sakamoto M, Kurosawa T, Dakeishi M, Iwata T, Murata K. 2007. Total mercury levels in hair, toenail, and urine among women free from occupational exposure and their relations to renal tubular function. Environ Res 103(2):191–197, PMID: 16890218, 10.1016/j.envres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Oka T, Matsukura M, Okamoto M, Harada N, Kitano T, Miike T, et al. 2002. Autonomic nervous functions in fetal type Minamata disease patients: assessment of heart rate variability. Tohoku J Exp Med 198(4):215–221, PMID: 12630553, 10.1620/tjem.198.215. [DOI] [PubMed] [Google Scholar]
- Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. 2012. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175(1):66–73, PMID: 22135359, 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Choi H-S, Bae J-H. 2016. Instant noodles, processed food intake, and dietary pattern are associated with atopic dermatitis in an adult population (KNHANES 2009-2011.). Asia Pac J Clin Nutr 25:602–613, PMID: 27440696, 10.6133/apjcn.092015.23. [DOI] [PubMed] [Google Scholar]
- Park S, Choi N-K. 2016. Associations of blood heavy metal levels with intraocular pressure. Ann Epidemiol 26(8):546–550, PMID: 27497680, 10.1016/j.annepidem.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Park SK, Lee S, Basu N, Franzblau A. 2013. Associations of blood and urinary mercury with hypertension in U.S. adults: the NHANES 2003-2006. Environ Res 123:25–32, PMID: 23472608, 10.1016/j.envres.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen EB, Jorgensen ME, Pedersen MB, Siggaard C, Sorensen TB, Mulvad G, et al. 2005. Relationship between mercury in blood and 24-h ambulatory blood pressure in Greenlanders and Danes. Am J Hypertens 18(5 Pt 1):612–618, PMID: 15882543, 10.1016/j.amjhyper.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Perry HMJ, Erlanger MW. 1974. Metal-induced hypertension following chronic feeding of low doses of cadmium and mercury. J Lab Clin Med 83(4):541–547, PMID: 4817779. [PubMed] [Google Scholar]
- Perry HM Jr, Erlanger M, Yunice A, Perry EF. 1967. Mechanisms of the acute hypertensive effect of intra-arterial cadmium and mercury in anesthetized rats. J Lab Clin Med 70(6):963–972, PMID: 6059406, 10.5555/uri:pii:002221436790159X. [DOI] [PubMed] [Google Scholar]
- Rajaee M, Sánchez BN, Renne EP, Basu N. 2015. An investigation of organic and inorganic mercury exposure and blood pressure in a small-scale gold mining community in Ghana. Int J Environ Res Public Health 12(8):10020–10038, PMID: 26308023, 10.3390/ijerph120810020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman HA, Walsh TL, Coull BA, Dewailly É, Guallar E, Hattis D, et al. 2011. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose–response function for regulatory benefits analysis. Environ Health Perspect 119(5):607–614, PMID: 21220222, 10.1289/ehp.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Seppanen K, Lakka TA, Salonen R, Kaplan GA. 2000. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 148(2):265–273, PMID: 10657561. [DOI] [PubMed] [Google Scholar]
- Saudny H, Leggee D, Egeland G. 2012. Design and methods of the Adult Inuit Health Survey 2007–2008. Int J Circumpolar Health 71(1):19752–19759, PMID: 23166895, 10.3402/ijch.v71i0.19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue I. 2014. Higher urinary heavy metal, arsenic, and phthalate concentrations in people with high blood pressure: US NHANES, 2009–2010. Blood Press 23(6):363–369, PMID: 24945898, 10.3109/08037051.2014.925228. [DOI] [PubMed] [Google Scholar]
- Siblerud RL. 1990. The relationship between mercury from dental amalgam and the cardiovascular system. Sci Total Environ 99(1–2):23–35, PMID: 2270468. [DOI] [PubMed] [Google Scholar]
- Sørensen N, Murata K, Budtz-Jørgensen E, Weihe P, Grandjean P. 1999. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 10(4):370–375, PMID: 10401870. [PubMed] [Google Scholar]
- Sterne JAC, Jüni P, Schulz KF, Altman DG, Bartlett C, Egger M. 2002. Statistical methods for assessing the influence of study characteristics on treatment effects in “meta-epidemiological” research. Stat Med 21(11):1513–1524, PMID: 12111917, 10.1002/sim.1184. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. 2000. Meta-analysis of observational studies in epidemiology - a proposal for reporting. JAMA 283(15):2008–2012, PMID: 10789670. [DOI] [PubMed] [Google Scholar]
- Tamashiro H, Arakaki M, Akagi H, Hirayama K, Murao K, Smolensky MH. 1986. Sex differential of methylmercury toxicity in spontaneously hypertensive rats (SHR). Bull Environ Contam Toxicol 37(6):916–924, PMID: 3790765, 10.1007/BF01607858. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Fisher HL, Hall LL, Mushak P. 1982. Effects of age and sex on retention of mercury by methyl mercury-treated rats. Toxicol Appl Pharmacol 62(3):445–454, PMID: 7071859, 10.1016/0041-008X(82)90145-4. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme. 2002. Global Mercury Assessment.
- U.S. EPA (U.S. Environmental Protection Agency), U.S. FDA (U.S. Food and Drug Administration). 2017. “Advice About Eating Fish: What Pregnant Women & Parents Should Know.” https://www.fda.gov/Food/ResourcesForYou/Consumers/ucm393070.htm [accessed 13 July 2018].
- Valera B, Dewailly E, Poirier P. 2008. Cardiac autonomic activity and blood pressure among Nunavik Inuit adults exposed to environmental mercury: a cross-sectional study. Environ Health 7(1):29, 10.1186/1476-069X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera B, Dewailly E, Poirier P. 2011a. Impact of mercury exposure on blood pressure and cardiac autonomic activity among Cree adults (James Bay, Quebec, Canada). Environ Res 111(8):1265–1270, PMID: 21962568, 10.1016/j.envres.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Valera B, Dewailly É, Poirier P. 2013. Association between methylmercury and cardiovascular risk factors in a native population of Quebec (Canada): a retrospective evaluation. Environ Res 120:102–108, PMID: 22959488, 10.1016/j.envres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Valera B, Dewailly E, Poirier P. 2009. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension 54(5):981–986, PMID: 19805642, 10.1161/HYPERTENSIONAHA.109.135046. [DOI] [PubMed] [Google Scholar]
- Valera B, Dewailly E, Poirier P, Counil E, Suhas E. 2011b. Influence of mercury exposure on blood pressure, resting heart rate and heart rate variability in French Polynesians: a cross-sectional study. Environ Health 10(1):99, PMID: 22078280, 10.1186/1476-069X-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen JK, Laukkanen JA, Mursu J, Voutilainen S, Tuomainen T-P. 2012a. Serum long-chain n-3 polyunsaturated fatty acids, mercury, and risk of sudden cardiac death in men: a prospective population-based study. PLoS One 7(7):e41046, PMID: 22815906, 10.1371/journal.pone.0041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen JK, Nyantika AN, Kauhanen J, Voutilainen S, Tuomainen T-P. 2012b. Serum long-chain n-3 polyunsaturated fatty acids, methylmercury and blood pressure in an older population. Hypertens Res 35(10):1000–1004, PMID: 22673531, 10.1038/hr.2012.80. [DOI] [PubMed] [Google Scholar]
- Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen T-P, Korhonen MJ, et al. 2005. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol 25(1):228–233, PMID: 15539625, 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. 2005. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999-2000. Environ Res 97(2):195–200, PMID: 15533335, 10.1016/j.envres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wakita Y. 1987. Hypertension induced by methyl mercury in rats. Toxicol Appl Pharmacol 89(1):144–147, PMID: 3590186. [DOI] [PubMed] [Google Scholar]
- Wells EM, Herbstman JB, Hong Y, Hibbeln JR, Halden RU, Witter FR, et al. 2017. Methyl mercury, but not inorganic mercury, associated with higher blood pressure during pregnancy. Environ. Res 154:247–252, PMID: 28110211, 10.1016/j.envres.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. 2009. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 16 August 2017].
- Wennberg M, Strömberg U, Bergdahl IA, Jansson JH, Kauhanen J, Norberg M, et al. 2012. Myocardial infarction in relation to mercury and fatty acids from fish: a risk-benefit analysis based on pooled Finnish and Swedish data in men. Am J Clin Nutr 96(4):706–713, PMID: 22894940, 10.3945/ajcn.111.033795.706. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Tsuda T, Kashima S, Takao S, Harada M. 2010. Long-term exposure to methylmercury and its effects on hypertension in Minamata. Environ Res 110(1):40–46, PMID: 19922910, 10.1016/j.envres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Rimm EB, Morris JS, Spate VL, Hsieh C-C, Spiegelman D, et al. 2002. Mercury and the risk of coronary heart disease in men. N Engl J Med 347(22):1755–1760, 10.1056/NEJMoa021437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.