Abstract
Background:
Traffic-related air pollution is emerging as a risk factor for Alzheimer's disease (AD) and impaired brain development. Individual differences in vulnerability to air pollution may involve the allele of Apolipoprotein E (APOE) gene, the primary genetic risk factor for AD.
Objective:
We analyzed whether the association between traffic air pollution and neurodevelopmental outcomes is modified by APOE status in children.
Methods:
Data on parent-reported behavior problems (total difficulties scores, Strengths and Difficulties Questionnaire), teacher-reported attention-deficit hyperactivity disorder (ADHD) symptom scores, cognitive performance trajectories (computerized tests of inattentiveness and working memory repeated 2–4 times during January 2012–March 2013), and APOE genotypes were obtained for 1,667 children age 7–11 y attending 39 schools in or near Barcelona. Basal ganglia volume (putamen, caudate, and globus pallidum) was measured in 163 of the children by MRI (October 2012–April 2014.) Average annual outdoor polycyclic aromatic hydrocarbons (PAHs), elemental carbon (EC), and nitrogen dioxide () concentrations were estimated based on measurements at each school (two 1-wk campaigns conducted 6 months apart in 2012).
Results:
APOE allele carriers had significantly higher behavior problem scores than noncarriers, and adverse associations with PAHs and were stronger or limited to carriers for behavior problem scores (P-interaction 0.03 and 0.04), caudate volume (P-interaction 0.04 and 0.03), and inattentiveness trajectories (P-interaction 0.15 and 0.08, respectively). Patterns of associations with the same outcomes were similar for EC.
Conclusion:
PAHs, EC, and were associated with higher behavior problem scores, smaller reductions in inattentiveness over time, and smaller caudate volume in APOE allele carriers in our study population, and corresponding associations were weak or absent among noncarriers. These findings support a potential role of APOE in biological mechanisms that may contribute to associations between air pollution and neurobehavioral outcomes in children. https://doi.org/10.1289/EHP2246
Introduction
There is growing evidence that exposure to traffic-related air pollution (TRAP) has a detrimental effect on cognitive and behavioral developmental outcomes in children. In the BREATHE project ( schoolchildren), conducted in the Barcelona metropolitan area, we found that outdoor and indoor estimated concentrations of elemental carbon (EC, equivalent to black carbon) and nitrogen dioxide (), two pollutants highly correlated with road traffic emissions (Amato et al. 2014; Reche et al. 2014; Rivas et al. 2014), were associated with slower improvements in cognitive function over time (Sunyer et al. 2015) and higher scores on parent-reported behavior problems (Forns et al. 2016). Using a weighted average estimate of EC and levels, brain changes of a functional but not structural nature were associated with air pollution exposure in children drawn from the BREATHE project who underwent MRI scan () (Pujol et al. 2016). Specifically, higher air pollution levels were associated with lower functional integration and segregation in key brain networks (Pujol et al. 2016). Another study based on BREATHE cohort () found that exposure to polycyclic aromatic hydrocarbons (PAHs) was associated with smaller caudate volumes (Mortamais et al. 2017).
These findings are consistent with other studies reporting associations between TRAP and neurobehavioral outcomes in children. For instance, prenatal exposure to PAHs has been associated with cognitive developmental delays at age 3 (Perera et al. 2006) and decreased intelligence quotient at age 5 (Perera et al. 2009). Prenatal exposure to PAHs, characterized by air monitoring and specific biomarkers (DNA adducts in maternal and cord blood), has been associated with symptoms of anxiety and depression, and attention problems at age 6–7 (Perera et al. 2012). Furthermore, postnatal PAHs exposure has been shown to contribute to disturbances in prefrontal white matter development in brain at ages 7–9 (Peterson et al. 2015). Other studies have related exposure at school with neurobehavioral function indicating worse cognitive performance among those exposed to higher air pollution levels (van Kempen et al. 2012; Wang et al. 2009). However, mechanisms through which TRAP might adversely affect neural development remain largely unknown (Calderón-Garcidueñas and Torres-Jardón 2015b).
Systemic inflammation and oxidative stress are among the most well-established mechanisms underlying the health effects of air pollution (Block and Calderón-Garcidueñas 2009; Brockmeyer and D’Angiulli 2016). Interestingly, these pathogenic pathways are also involved in neurodegenerative diseases, such as dementia, which is characterized by progressively impaired cognitive function (Reynolds et al. 2007; Rodrigues et al. 2012). In line with this, air pollution has been associated with cognitive impairment in elderly people (Ranft et al. 2009; Wellenius et al. 2012). More recently, a large population-based cohort showed that living near roads with heavy traffic was associated with a higher risk of dementia, supporting a potential link between cognitive impairment, neurodegeneration and exposure to air pollution (Chen et al. 2017). Similarly, Alzheimer’s Disease (AD)-type pathology has been observed in autopsy samples of the frontal cortex from children living in highly polluted areas of Mexico (Calderón-Garcidueñas et al. 2013). Because Apolipoprotein E (APOE) epsilon 4 () allele is the strongest known genetic risk factor for AD (Liu et al. 2013), Calderón-Garcidueñas et al. (2012) evaluated whether the AD-related pathological processes that are associated with air pollution were more pronounced in children carrying the APOE allele. They found that carriers had more hyperphosphorylated tau protein and diffuse () plaques in comparison with carriers (Calderón-Garcidueñas et al. 2012). Furthermore, carriers presented metabolic alterations in frontal white matter and poor cognitive performance affecting their attention and memory functions (Calderón-Garcidueñas et al. 2015a). These results suggest that APOE genotypes could modify responses to air pollution on brain and cognitive function. Cognitive function develops steadily from childhood to early adulthood; later in life, this function can remain stable or decline with age, depending on several factors, including genetics and environmental exposures (Craik and Bialystok 2006). Together, air pollution exposure and APOE allele status may inhibit children’s ability to achieve and consolidate cognitive and behavioral function and may impair these capacities in elderly people. To the best of our knowledge, studies evaluating APOE status as a modifier of the association between air pollution and cognition have been conducted only in elderly women. The first study found that exposure to air pollution, including road traffic emissions, was associated with poorer performance in neuropsychological tasks among carriers, but not among noncarriers (Schikowski et al. 2015). More recently, female carriers were shown to have stronger associations between air pollution exposure and dementia risk and between air pollution exposure and global cognitive decline, than female carriers (Cacciottolo et al. 2017).
We sought to extend these findings by examining whether APOE status modifies associations between TRAP and neurodevelopmental outcomes in children, including measures of behavior, cognitive function, and brain morphology. We hypothesized that associations between the outcomes and TRAP exposure, indexed by outdoor measurements of PAHs, EC and , would be more pronounced among carriers than among noncarriers.
Materials and Methods
Study Population and Setting
Participants were drawn from the BREATHE project (European Commission: FP7-ERC-2010-AdG, ID 268,479), a population-based cohort of primary schoolchildren designed to analyze the association between air pollution and behavior, cognitive function and brain morphology. We used modeled levels to select 39 primary schools so as to maximize the contrast in TRAP levels at schools (Sunyer et al. 2015). Thirty-eight schools were located in Barcelona, and one school was in an adjacent municipality, Sant Cugat del Vallés. The socioeconomic vulnerability index and levels estimated for the participating schools were similar to those for the remaining 380 schools in Barcelona city (Socioeconomic vulnerability index: 0.66 vs. 0.62, ; levels: 51.5 vs. , ). All families of children without special needs who were enrolled in second, third, and fourth grades at the selected schools were invited to participate in the study (2012). A total of 2,897 children ages 7 to 11 y accepted the invitation and participated in the project. Genotype data were available for 1,667 children of European ethnic origin. Among these, MRI data was available for 163 children, who were scanned between October 2012 and April 2014. Further details on recruitment of the MRI subsample is available elsewhere (Pujol et al. 2016). Briefly, with the aim of including children from all participating schools in BREATHE project, a document asking whether they were interested in further information regarding the MRI study was given to all children. From the initial sample (), the document was returned by 1,564 families, of whom 810 indicated that they were interested in participating in the MRI study. From those, parents of 491 children were successfully contacted. Twenty-one children were excluded because of dental braces, consent to participate was not obtained in 165 cases, and 27 children were lost before the assessment. From these children, 263 completed the imaging protocol. Further exclusions included poor-quality brain scans (), and no genetic data available (), leaving a final sample of 163 children with MRI and genetic data available.
All children had been in the school for more than 6 months before the beginning of the study, and 98% for more than 1 y. A full description of the project is available elsewhere (Sunyer et al. 2015). All parents or legal guardians gave written informed consent, and the study was approved by the IMIM-Parc de Salut Mar Research Ethics Committee (No. 2010/41,221/I), Barcelona, Spain; and the FP7-ERC-2010-AdG Ethics Review Committee (268,479-22,022,011).
Exposure
We investigated exposure to outdoor PAHs, EC, and measured at each school as surrogates for TRAP exposure at each school. We analyzed EC and because exposures to these pollutants at schools have been shown to be (i) highly associated with vehicle exhaust levels in Barcelona (Amato et al. 2014); (ii) associated with parent-reported behavior problem scores and teacher-reported ADHD symptom scores (Forns et al. 2016); and (iii) involving the pollutants most consistently associated with working memory, superior working memory, and inattentiveness in this sample (Basagaña et al. 2016; Forns et al. 2017). Similarly, in a subset of BREATHE project participants, PAHs levels at schools were associated with differences such as smaller basal ganglia volumes (Mortamais et al. 2017). The levels of these pollutants in the schoolyards were measured twice during two 1-wk periods separated by 6 months, in the warm and cold periods of the year 2012. The average of these two 1-wk measurements was used to estimate yearly outdoor air pollution levels at the schools. Not all participating schools were monitored simultaneously, so to eliminate the effects of temporal fluctuation in background air pollution levels, the levels of each pollutant were adjusted for the weekly average level of that pollutant (during the corresponding sampling week for each school), as measured by a background monitoring station in Barcelona (Rivas et al. 2014).
Samples of ambient air particulate matter () were collected for 8 h (school time, from 09:00 to 17:00 h) at each school using a high-volume Sampler (MCV SA) with quartz filters (Pall, ). Further details of the measurement campaigns and analysis of filter chemicals to determine the concentration of several pollutants (including EC and PAHs) are described elsewhere (Alier et al. 2013; Amato et al. 2014; Rivas et al. 2014). Weekly average concentrations were estimated using passive samplers ( diffusion tube, Gradko International Ltd.). PAHs included the total sum of benz[a]anthracene (BAAN), chrysene (CHR), (BFL), benzo[e]pyrene (BEP), benzo[a]pyrene (BAP), indeno(1,2,3-c,d)pyrene (IP) and benzo[g,h,i]perylene (BGP), which were the compounds that showed detectable levels in all samples. To reduce temporal fluctuations when comparing PAHs levels between schools, data were seasonalized after adjusting for the mean daily BAP level measured at three urban monitoring stations in Barcelona. BAP is the only PAH assessed in this study that is also monitored in Barcelona. The urban monitoring stations were exposed to traffic, and BAP was continuously measured during one day at one or more of these sites during the study period. To obtain seasonalized levels, daily concentrations at each school were multiplied by the ratio of the yearly average to the same day concentration at the three fixed air quality background monitoring stations (Rivas et al. 2014).
Neurodevelopmental Outcomes
Behavioral Outcomes.
Behavioral outcomes included scores on behavior problems and attention deficit-hyperactivity disorder (ADHD) symptoms. These measures were obtained at the beginning of cognitive data collection (visit 1) during the first trimester of 2012. Behavioral problems were characterized using the Strengths and Difficulties Questionnaire (SDQ; Goodman 1997), which was rated by parents. The SDQ is a brief behavioral screening questionnaire of 25 items, for which raters are asked to indicate on a 3-point response scale (ranging from not true to certainly true) how well each item described the behavior of the child. The questionnaire consists of four difficulty subscales (emotional problems, peer problems, conduct problems, and hyperactivity), and one strength subscale (prosocial behavior), each including five items. A SDQ total difficulties score, ranging from 0 to 40, is calculated by summing the four difficulties subscales. Higher SDQ total difficulties scores indicate more behavioral problems. ADHD symptoms were assessed using a questionnaire based on the ADHD diagnostic criteria described in Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV; American Psychiatric Association, 2002), which was completed by teachers. The ADHD-DSM-IV questionnaire consists of a list of 18 symptoms, assessing two separate symptom groups: inattention (nine symptoms) and hyperactivity/impulsivity (nine symptoms). Each ADHD symptom is rated on a 4-point frequency scale from never or rarely (0) to very often (3). The questionnaire can be found in Methods S1. The ADHD symptom score ranges from 0 to 54, with higher scores indicating more numerous symptoms.
Cognitive Function.
Cognitive function included inattentiveness and working memory trajectories. These data were collected between January 2012 and March 2013. During this period, BREATHE participants completed computerized tests assessing inattentiveness and working memory; participants completed this test four times (every 3 months) over one year. These four repeated measurements allowed us to model the 1-y trajectories of inattentiveness and working memory. Because only children assessed at least twice were included in the analyses, the modeled 1-y trajectory may include 2 to 4 repeated measures of inattentiveness or working memory based on available data. Inattentiveness was assessed using the computerized Attentional Network Test (ANT; Rueda et al. 2004). Reaction times (i.e., time between introducing a stimulus and the participant’s reaction to that stimulus) were used to calculate the different outcomes that can be obtained using the ANT. The inattentiveness outcome analyzed in this study is the standard error of reaction time for correct responses [standard error of hit reaction time (HRT-SE)], a measure of intra-individual variability reflecting response speed and consistency throughout the test. We chose HRT-SE because it has previously been associated with exposure to air pollution (Sunyer et al. 2015) and, as a measure of intra-individual variability, it can be considered a good indicator of central nervous system integrity (Hedden and Gabrieli 2004; MacDonald et al. 2006). We refer to HRT-SE as inattentiveness because higher HRT-SE scores are related to reduced executive and attentional resources, and are characteristic of the performance of patients with ADHD (Bellgrove et al. 2004, MacDonald et al. 2006). Inattentiveness was analyzed as a continuous variable representing the 1-y trajectory, taking into account the four repeated measures. Further details are available elsewhere (Forns et al. 2014).
Working memory was assessed using the computerized n-back task (Anderson 2002; Nelson et al. 2000; Vuontela et al. 2003). In this task, the subject is required to monitor a series of stimuli presented in the centre of the screen and to respond whenever a stimulus is presented that matches the one presented in n trials previously (, 2, or 3), which are known as loads (one-back, two-back, and three-back). Higher loads imply higher demands on working memory. Participants complete three blocks (1-, 2-, and 3-back) for each stimulus. Stimuli included colors, letters, numbers, and words. At the two-back level, the target was any stimulus that matched the one presented two trials previously. Here, we used numbers and words as stimuli in the two-back level. We choose this load because it predicts general mental abilities (Shelton et al. 2010) and previous research in this sample showed limited improvement in the trajectories of three-back compared to two-back tasks, which may be due to immaturity of the brain areas supporting processes involved in this higher-demand task, such as storage, processing, and executive-control functions (López-Vicente et al. 2016). We obtained various measures for each trail, including accuracy measures (hits, correct rejections, false alarms, and misses) and hit reaction time (HRT), recorded when the participant correctly identified a target. Usually, the outcome analyzed is a combination of these measures. In this regard, a widely used outcome for assessing working memory is the d prime (d′), which is derived from signal detection theory and allows us to distinguish between signal and noise (Haatveit et al. 2010; Wickens 2002); d′ is computed as z (hit rate) − z (false alarm rate), with higher d′ indicating better signal detection and more accurate performance. Because d′ incorporates more information about working memory capacity, it has been suggested as a better measure of interindividual variability than HRT (Forns et al., 2014). Therefore, we used two-back numbers d′ and two-back words d′ in our analyses. These measurements were analyzed as continuous variables representing the 1-y trajectory, taking into account the four repeated measures.
Brain Structure.
Among the brain-structure measurements, we focused on basal ganglia volumes (including caudate, putamen, and globus pallidum), based on previous findings in the BREATHE sample (Mortamais et al. 2017) and the key role of basal ganglia on attention function (McKenna et al. 2013; Riccio et al. 2002), which has also been associated with TRAP in this sample (Sunyer et al. 2015). We performed MRI of brain anatomy using a 1.5 Tesla Signa Excite system (General Electric, Inc.) equipped with an eight-channel phased-array head coil and single-shot echo planar imaging (EPI) software. High-resolution 3D anatomical images were obtained using an axial T1-weighted, three dimensional fast spoiled gradient inversion recovery-prepared sequence. A total of 134 contiguous slices were acquired with repetition time of , echo time of , flip angle 15°, field of view of , pixel matrix, and slice thickness . To avoid including poor-quality images, all images were visually inspected by a trained researcher before and after the preprocessing steps. Cases were excluded based on expert subjective criteria if the raw images showed obvious motion artifacts (ghost and blurring of the image), ringing or truncation artifacts, and susceptibility phenomena. After preprocessing, cases were excluded if the images showed deformation of the three-dimensional brain anatomy and large truncated brain areas, nonoptimal removal of nonbrain tissue, and obvious tissue (gray and white matter) misclassification. Cortical reconstruction and volumetric segmentation were carried out using the FreeSurfer tool (http://surfer.nmr.mgh.harvard.edu/). In total, 71 brain measurements were generated using FreeSurfer (version 5.3; FreeSurfer analysis suite). Processing steps included removal of nonbrain tissue, automated Talairach transformation, and segmentation of the subcortical white matter and deep gray matter volumetric structures. Additional details are available in (Pujol et al. 2016; Vilor-Tejedor et al. 2016).
APOE Genotypes.
The major APOE allelic variants , , and can be obtained from allelic combinations of the rs429358 and rs7412 polymorphisms. Briefly, the allele is the combination of the C allele at both sites (Radmanesh et al. 2014). According to the genotypes of these polymorphisms, children were classified as carriers (with at least one allele) and noncarriers. Genotype frequencies for the rs429358 and rs7412 polymorphisms were obtained from genome-wide genotyping data for 1,667 participants in the BREATHE project.
A full description of the genotyping and quality-control procedures is available elsewhere (Alemany et al. 2016). Briefly, from the 2,897 children participating in the original BREATHE cohort, 2,492 (86%) accepted to provide saliva for DNA genotyping. Saliva samples were collected using the Oragene DNA OG-500kit (DNA Genotek). From these children with available saliva samples, a final subset of 1,778 (61%) children was selected for genome-wide genotyping after applying a filtering criterion. Filtering criteria included low quality DNA ( exclusions), adopted children ( exclusions), siblings or twins ( exclusions), being born outside Europe or having parents born outside Europe ( exclusions), and no data available on residential address ( exclusions).
Genome-wide genotyping was performed using the HumanCore BeadChipWG-330-1,101 (Illumina). Genotypes were called using the GeneTrain2.0 algorithm (with a default threshold of 0.15) based on HapMap clusters implemented in the GenomeStudio software. PLINK was used to perform genotyping quality control (Purcell et al. 2007); we included samples with a minimum of 97% call rate ( exclusions) and a maximum of 4 SD heterozygosity ( exclusions), gender discordance excluding mismatch information ( exclusions) and relatedness ( exclusions). Five subjects were excluded due to mental disability. Thus, a total of 111 subjects were excluded, leaving 1,667 individuals in the analysis.
The polymorphisms analyzed for this study were imputed using IMPUTE2 (version 2), taking the 1,000 Genomes Project Phase I integrated variant set (http:/www.1000genomes.org/) as a reference haplotype panel. For rs429358, the minor allele frequency (MAF) and quality of imputation were 0.95 and 0.122, respectively, and for rs7412, were 0.97 and 0.062, respectively. Hardy–Weinberg equilibrium was verified for both polymorphisms (rs429358: ; ; rs7412 ; ).
Covariates.
Sociodemographic data were collected by the BREATHE baseline questionnaire, completed by the parents (Sunyer et al. 2015), including child age and sex, maternal educational level (no or primary school/secondary school/university), maternal smoking during pregnancy (yes/no), and exposure to environmental tobacco smoke at home (no smoking at home/smoking outside home (e.g., terrace)/smoking inside). We also obtained data on residential neighborhood socioeconomic status (SES) vulnerability index (based on level of education, unemployment, and occupation in each census tract, the finest spatial census unit, with median area of ) (Ministerio de Fomento 2012). Air pollution at home was characterized by and levels at time of the study, estimated at the geocoded postal address of each participant using land use regression (LUR) models developed in the context of ESCAPE project as described in Supplementary Material (see Methods S2).
Statistical Analysis
The final analysis included 1,667 children with data available for genetic polymorphisms, behavior problem scores (), ADHD symptom scores (), inattentiveness (5,999 observations for 1,488 participants), working memory (6,058 observations for 1,591 participants), and basal ganglia volume () (Figure S1). Associations between predictors and behavior problem scores and ADHD symptom scores (modeled as continuous variables) were estimated using negative binomial mixed effects models with school included as a nested random effect to account for the multilevel nature of the data (i.e., children within schools). Exponentiated regression coefficients from the negative binomial models (mean ratios, or MR) represent the relative difference in the outcome score with a one-unit increase in the predictor. Associations with basal ganglia volumes (modeled as continuous variables) were estimated using linear mixed-effects models with random effects for school. Associations with changes in inattentiveness (HRT-SE) and working memory test scores over time (trajectories) were estimated for children with at least two outcome measurements using linear mixed-effects models, with individual children nested within schools as random effects. The modeled 1-y trajectory may include two to four repeated measurements of inattentiveness or working memory per individual based on available data. We analyzed trajectories because previous findings in BREATHE project showed statistically significant associations between TRAP levels (EC and ) and inattentiveness and working memory scores at follow-up and not at baseline (Sunyer et al. 2015). A possible explanation may be the fact that the baseline estimates were related to cumulative TRAP exposures that took place prior to the study period, whereas we estimated air pollution levels at the schools where participants were attending during the study period. This estimate could have resulted in exposure misclassification for the baseline associations. For this reason, we focused on trajectories for those outcomes with repeated measurements (i.e., inattentiveness and working memory). We fit separate models to estimate associations of each outcome with PAHs, EC, and , respectively, and report effect estimates for an interquartile range (IQR) increase in each pollutant. All models were adjusted for age, sex, maternal educational level, and residential neighborhood SES. These covariates were selected based on previous associations reported between air pollution exposures and these outcomes using the BREATHE sample (Forns et al. 2016; Mortamais et al. 2017; Sunyer et al. 2015). Models of basal ganglia volumes were additionally adjusted for intracranial volume to minimize confounding due to interindividual variation in cranial volume (Malone et al. 2015).
To assess effect modification by APOE status, we included two-way interaction terms between APOE status and air pollution levels in mixed-effects models for behavior-problem scores, ADHD-symptom scores, and basal ganglia volumes. For models of inattentiveness and working memory trajectories, we included three-way interaction terms between age (centered at visit 1), air pollution exposure, and APOE status.
To represent interaction effects graphically, we used R statistical software package (version 3.3.1; R Development Core Team).
In addition, where we detected interaction effects on the brain volumes studied, we performed ad hoc analyses to facilitate interpretation of the results, testing whether larger or smaller volumes were associated with more behavior problem and ADHD symptom scores, or poorer cognitive function.
In the sensitivity analyses, we further adjusted the models for maternal smoking during pregnancy, and exposure to environmental tobacco smoke and air pollution at home. We also used inverse probability weighting (IPW) to account for possible selection biases when excluding individuals with no genetic data () or missing outcome or covariate data. We use the entire BREATHE sample as a target sample. To predict the probability of being a complete case, we considered adjustment covariates in addition to other sociodemographic variables, such as school performance (low or regular vs. normal or excellent), siblings (0, ), duration of breastfeeding (never, , 1 wk months, 3–6, 6–12, ) and birthweight (, 2.5–4, ). We used the inverse of these probabilities as weights in the complete case analysis so that results would be representative of the whole population. Weights were unstabilized. Because extreme weight values may have undue influence in the results, we repeated the adjusted analyses by IPW, replacing the extreme 1% of weight by the weight at the first and 99th percentiles. We report areas under the receiver operator characteristic curve (AUC) as a measure of goodness-of-fit of the IPW models.
Statistical significance was set at , and all statistical analyses were carried out using Stata 12.1 (Stata Corporation).
Results
Descriptive Results
In the sample included in the present analysis (), mean age was , 47.7% were female, and 23% were APOE carriers () (Table 1). In comparison with BREATHE participants who were excluded (), children who were included were significantly more likely to be male and have mothers with higher education (Table S1). In addition, children included in the present analysis have completed more follow-up visits of the inattentiveness and working memory tests, had lower parent-reported behavior problem scores, had lower teacher-reported ADHD symptom scores, had better baseline values for computerized measures of inattentiveness and working memory; and had lower estimated PAHs, EC, and exposures at their schools. When children included in the present analysis were compared according to status, carriers () had significantly lower estimated home exposures, and significantly worse behavior problem scores and two-back words performance than noncarriers (), but there were no significant differences in other individual characteristics, exposures, or outcomes between carriers and noncarriers (Table 1).
Table 1.
Characteristics of the study population according to APOE status.
| Characteristic | Study population or n (%) () | APOE carriers or n (%)() | Non-carriers or n (%)() | P-valuea |
|---|---|---|---|---|
| Assessed individually () | ||||
| Age (years) | 0.39 | |||
| Sex (female), n (%) | 795 (47.7%) | 172 (45.2%) | 610 (48.2%) | 0.34b |
| Maternal education, n (%) | ||||
| Primary or less | 159 (9.8%) | 36 (9.7%) | 123 (9.9%) | 0.88b |
| Secondary | 434 (26.8%) | 100 (26.8%) | 334 (26.9%) | |
| University | 1024 (63.3%) | 237 (63.5%) | 786 (63.2%) | |
| Residential neighbourhood SESc | 0.44 (0.20) | 0.43 (0.20) | 0.44 (0.20) | 0.26 |
| Smoking during pregnancy, n (%) | ||||
| No | 1449 (89.4%) | 334 (89.5%) | 1114 (89.4%) | 0.94b |
| Yes | 171 (10.6%) | 39 (10.5%) | 132 (10.6%) | |
| Tobacco exposure at home, n (%) | ||||
| No smoking at home | 1008 (62.8%) | 229 (61.9%) | 778 (63%) | 0.93b |
| Smoking outside (e.g. terrace) | 395 (24.6%) | 93 (25.1%) | 302 (24.5%) | |
| Smoking inside | 203 (12.6%) | 48 (13%) | 155 (12.5%) | |
| Behavior problem scores | 8.06 (5.06) | 8.69 (5.48) | 7.87 (4.91) | 0.04 |
| ADHD symptoms scores | 7.67 (9.41) | 8.03 (9.65) | 7.55 (9.34) | 0.26 |
| Baseline HRT-SE (Inattentiveness) | 0.72 | |||
| Number of follow-up visits | 0.06 | |||
| Baseline working memory | ||||
| Number of follow-up visits | 0.51 | |||
| Two-back numbers d′ | 0.41 | |||
| Two-back words d′ | ||||
| Assessed individually in MRI subsample () | ||||
| Intracranial Volume () | 0.41 | |||
| Caudate () | 0.15 | |||
| Putamen () | 0.52 | |||
| Globus pallidum () | 0.45 | |||
| Assessed at home address () | ||||
| () | 0.02 | |||
| () | 0.07 | |||
| Assessed at each school (schools) | ||||
| PAHs Outdoor ()d | 0.43 | |||
| EC Outdoor ()d | 0.20 | |||
| Outdoor ()d | 0.15 | |||
Note: Parent-reported SDQ Total Difficulties score (range 0–40), higher scores suggest more behavior problems. Teacher questionnaire based on DSM-IV diagnostic criteria for ADHD [sum of points assigned on a scale from 0 (never) to 3 (very often) for 18 individual criteria, range 0–54]; higher scores suggest more ADHD symptoms. Standard error of hit reaction time (HRT-SE) from the Attentional Network Test, higher scores suggest more inattentiveness (range 76.5–528.5). d′ values from the n-back task, where (hit rate) – z (false alarm rate) for two-back number and two-back word tasks, respectively. Higher values suggest better working memory, range and for two-back numbers d′ and two-back words d′, respectively.
, nitrogen dioxide; PM, Particulate Matter; ADHD, Attention Deficit Hyperactivity Disorder; PAHs, Polycyclic aromatic hydrocarbons; EC, Elemental Carbon.
Kruskal-Wallis P-value unless otherwise indicated.
P-value.
Vulnerability index based on education, unemployment, and occupation in each census tract; higher values indicate lower neighborhood SES.
Annual average.
In the MRI subsample (), children were old on average, 49.7% were female, and 22.7% were APOE carriers () (Table S2). In comparison with noncarriers included in the MRI subsample (), carriers had significantly lower estimated and exposures at home, and significantly higher behavior problem scores, but there were no significant differences with regard to other characteristics, outcomes, or exposures.
The frequencies of APOE genotypes for the whole sample and the MRI subsample are shown in Table S3. Less frequent APOE genotypes (, and ) were present only in the whole study population and not in the MRI subsample. However, the proportion of APOE allele carriers and noncarriers was very similar in the whole and MRI subsample (29.9% and 22.7%, respectively).
Association Results
APOE status was associated with higher behavior problem scores (; 95% confidence interval (CI): 1.02, 1.17) and a similar but nonsignificant difference in ADHD symptom scores (; 95% CI: 0.97, 1.23) (Table 2). No significant associations were observed between APOE status and inattentiveness test trajectories, though the positive estimate suggests that the reduction in HRT-SE over 12 months (i.e., the reduction in HRT-SE with age, an indication of less inattentiveness) was smaller in APOE carriers than in noncarriers. APOE status was inversely (though not significantly) associated with working-memory test score trajectories (two-back numbers d′, ; 95% CI: , 0.07; two-back words d′, ; 95% CI: , 0.09), indicating less improvement with age among carriers in comparison with noncarriers. APOE status was not significantly associated with brain volume measures, though caudate volumes were smaller, on average, in carriers in comparison with noncarriers (; 95% CI: , 83).
Table 2.
Estimated effects of APOE status ( allele carrier vs. noncarrier) and IQR increases in annual average PAHs, EC, and concentrations at each child′s school on behavioral problem scores and ADHD symptom scores [mean ratios (95% CI), and for behavior problem and ADHD symptom scores, respectively]; 12-month trajectories for HRT-SE (inattentiveness) tests and tests of working memory [ (95% CI), and for inattentiveness and working memory scores, respectively]; and MRI-based measures of caudate, putamen, and globus pallidus volumes [ (95% CI), ].
| Outcome | APOE status | PAHs | EC | |
|---|---|---|---|---|
| Estimate (CI 95%) | Estimate (CI 95%) | Estimate (CI 95%) | Estimate (CI 95%) | |
| Behavioral problem scoresa | 1.09 (1.02, 1.17) | 1.05 (0.99, 1.10) | 1.06 (1.01, 1.11) | 1.06 (0.99, 1.13) |
| ADHD symptom scorea | 1.09 (0.97, 1.23) | 1.00 (0.92, 1.09) | 0.97 (0.90, 1.04) | 1.05 (0.95, 1.16) |
| Inattentiveness trajectoryb | 2.05 (, 8.75) | 4.44 (0.48, 8.40) | 2.48 (, 6.05) | 2.22 (, 7.12) |
| Working Memoryb | ||||
| Two-back numbers d′ | (, 0.07) | (, ) | (, 0.01) | (, ) |
| Two-back words d′ | (, 0.09) | (, 0.04) | 0.002 (, 0.05) | (, 0.04) |
| Caudatec | (, 83.2) | (, ) | (, 30.6) | (, ) |
| Putamenc | (, 294) | 9.1 (, 133.5) | (204.7, 235.1) | 93.4 (, 177.5) |
| Globus Pallidusc | ( 70.6) | 1.1 (, 39.5) | (, 57.1) | (, 62.7) |
Note: Parent-reported SDQ Total Difficulties score (range 0–40), higher scores suggest more behavior problems. Teacher questionnaire based on DSM-IV diagnostic criteria for ADHD [sum of points assigned on a scale from 0 (never) to 3 (very often) for 18 individual criteria, range 0–54], higher scores suggest more ADHD symptoms. Changes over 12 months in the standard error of hit reaction time (HRT-SE) from the Attentional Network Test, higher scores suggest smaller average reductions in inattentiveness over 12 months relative to the reference group (range 60.6–571.6). Changes over 12 months in d′ values from n-back test, where (hit rate) – z (false alarm rate) for two-back number and two-back word tasks, respectively. Higher scores suggest better working memory, range and for two-back numbers d′ and two-back words d′, respectively.
IQR for PAHs, EC and are , , and , respectively.
Negative binomial mixed effects models with schools as random effects, adjusted for gender, age at baseline, maternal education, and residential neighborhood SES. Model coefficients are exponentiated to derive Mean Ratios (MR) representing the difference in the mean score according to APOE e4 status or with an IQR increase in TRAP.
Coefficients from linear mixed-effects models with children nested within schools as random effects, adjusted for gender, age at baseline, maternal education, and residential neighborhood SES.
Coefficients from linear mixed-effects models with schools as random effects, adjusted for gender, age at MRI examination, maternal education, residential neighborhood SES, and intracranial volume.
IQR increases in school exposures to all three air pollutants were associated with higher behavior problem scores (significant for EC, ; 95% CI: 1.01, 1.11) and worse performance in inattentiveness (significant for PAHs, ; 95% CI: 0.48, 8.40) and two-back numbers d′ values (working memory) over time (significant for PAHs: ; 95% CI: , and for , ; 95% CI: , ) (Table 2). In addition, IQR increases in PAHs and were associated with significantly smaller caudate volumes (; 95%CI: , and ; 95% CI: , , respectively.) Associations with school TRAP exposures were close to the null for other outcomes.
Effect Modification
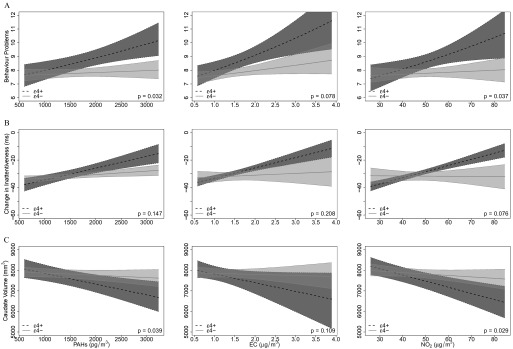
School TRAP exposures were associated with significantly worse behavior problem scores among APOE carriers, whereas associations with the same exposures were close to the null for noncarriers, with interaction P-values of 0.03, 0.08, and 0.04 for PAHs, EC, and , respectively (Table 3 and Figure 1).
Table 3.
Estimated effects of IQR increases in annual average PAHs, EC, and concentrations at each child′s school on behavioral problem scores and ADHD symptom scores [mean ratios (95% CI)]; 12-month trajectories for HRT-SE (inattentiveness) tests and tests of working memory [ (95% CI)]; and MRI-based measures of caudate, putamen, and globus pallidum volumes [ (95% CI)], stratified by APOE status.
| Outcome | PAHs | EC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-valued | Estimate (95% CI) | P-valued | Estimate (95% CI) | P-valued | |||||
| Behavior problem scoresa | ||||||||||
| carriers () | 1.13 (1.04, 1.22) | 0.03 | 1.11 (1.04, 1.19) | 0.08 | 1.14 (1.04, 1.26) | 0.04 | ||||
| non-carriers () | 1.01 (0.95, 1.08) | 1.04 (0.99, 1.10) | 1.02 (0.95, 1.10) | |||||||
| ADHD symptom scoresa | ||||||||||
| carriers () | 0.93 (0.73, 1.18) | 0.56 | 0.88 (0.71, 1.09) | 0.59 | 0.88 (0.65, 1.19) | 0.22 | ||||
| non-carriers () | 0.97 (0.85, 1.11) | 0.90 (0.79, 1.01) | 1.08 (0.96, 1.21) | |||||||
| Inattentiveness trajectoriesb | ||||||||||
| carriers () | 10.04 (2.29, 17.81) | 0.15 | 6.32 (, 13.20) | 0.21 | 10.17 (0.52, 19.83) | 0.08 | ||||
| non-carriers () | 2.81 (, 7.38) | 1.31 (, 5.46) | (, 5.33) | |||||||
| Working memory trajectoriesb | ||||||||||
| Two-back numbers | ||||||||||
| carriers () | (, 0.08) | 0.74 | (, 0.06) | 0.77 | (, 0.05) | 0.86 | ||||
| non-carriers () | (, 0.01) | (, 0.03) | (, 0.01) | |||||||
| Two-back words | ||||||||||
| carriers () | (, 0.07) | 0.06 | (, ) | 0.27 | (, 0.07) | 0.06 | ||||
| non-carriers () | ( , 0.05) | (, 0.06) | (, 0.06) | |||||||
| Caudatec | ||||||||||
| carriers () | (, ) | 0.04 | (, 26.88) | 0.11 | (, ) | 0.03 | ||||
| non-carriers () | ( , 47.84) | (, 137.10) | (, 73.62) | |||||||
| Putamenc | ||||||||||
| carriers () | (, 245.27) | 0.90 | (, 382.25) | 0.55 | (, 458.81) | 0.74 | ||||
| non-carriers () | 19.45 (, 495.30) | 52.89 (, 312.08) | (, 240.54) | |||||||
| Globus Pallidusc | ||||||||||
| carriers () | (, 126.75) | 0.40 | (, 140.16) | 0.97 | (, 138.60) | 0.79 | ||||
| non-carriers () | (, 70.88) | (, 49.83) | (, 50.35) | |||||||
Note: Parent-reported SDQ Total Difficulties score (range 0–40), higher scores suggest more behavior problems. Teacher questionnaire based on DSM-IV diagnostic criteria for ADHD [sum of points assigned on a scale from 0 (never) to 3 (very often) for 18 individual criteria, range 0–54], higher scores suggest more ADHD symptoms. Changes over 12 months in the standard error of hit reaction time (HRT-SE) from the Attentional Network Test, higher scores suggest smaller average reductions in inattentiveness over 12 months relative to the reference group (range 60.6-571.6). Changes over 12 months in d′ values, where (hit rate) – z(false alarm rate) for two-back number and two-back word tasks, respectively. Higher scores suggest better working memory, range and for two-back numbers d′ and two-back words d′, respectively.
IQR for PAHs, EC and are , , and , respectively.
Negative binomial mixed effects models with schools as random effects, adjusted for gender, age at baseline, maternal education, and residential neighborhood SES. Model coefficients are exponentiated to derive Mean Ratios (MR) representing the difference in the mean score according to APOE status or with an IQR increase in TRAP.
Coefficients from linear mixed-effects models with children nested within schools as random effects, adjusted for gender, age at baseline, maternal education, and residential neighborhood SES.
Coefficients from linear mixed-effects models with schools as random effects, adjusted for gender, age at MRI examination, maternal education, residential neighborhood SES, and intracranial volume.
Interaction P-values for behavior problem scores, ADHD scores, and basal ganglia volumes correspond to P-values for two-way interaction terms between APOE status and each TRAP. Interaction P-values for inattentiveness and working memory trajectories correspond to P-values for three-way interaction terms between APOE status, TRAP, and age (centered at the baseline visit).
Figure 1.
Differences according to APOE status in associations between annual average PAHs, EC, and concentrations at schools and selected outcomes. (A) Mean ratios [with 95% confidence interval (CI) bands] for associations with baseline parent-reported SDQ total difficulties scores (a measure of behavior problems) from negative binomial mixed effects models with schools as random effects, adjusted for gender, age at baseline, maternal education, and residential neighborhood SES. A positive slope indicates that increasing TRAP concentrations are associated with higher scores for behavior problems. Interaction P-values are for two-way interaction terms between APOE status and air pollutants. carriers , noncarriers . (B) Coefficients from linear mixed-effects models for average changes in HRT-SE (a measure of inattentiveness) over 12 months (2–4 follow-up visits), with children nested within schools as random effects, adjusted for gender, age at baseline, maternal education, and residential neighborhood SES. A positive slope indicates that higher TRAP concentrations are associated with smaller average decreases in HRT-SE (ms) over time (suggesting less improvement in attention with age). Interaction P-values are for three-way interaction terms between APOE status, air pollutants, and age (centered at baseline). carriers , noncarriers . (C) Coefficients from linear mixed-effects models with schools as random effects, adjusted for gender, age at MRI examination, maternal education, residential neighborhood SES, and intracranial volume. A negative slope indicates that increasing TRAP concentrations are associated with smaller average caudate volumes (mm3). P-values are for two-way interaction terms between APOE status and air pollutants. carriers , noncarriers .
APOE status did not appear to modify associations between TRAP and ADHD symptom scores, which generally were close to the null in both carriers and noncarriers (Table 3).
School TRAP exposures were positively associated with inattentiveness score trajectories among APOE carriers (significant for PAHs and ), indicating a slower average rate of decline in inattentiveness (HRT-SE) with age, whereas associations were close to the null for noncarriers (interaction P-values of 0.15, 0.21, and 0.08 for PAHs, EC, and , respectively) (Table 3 and Figure 1).
APOE status did not appear to modify associations between TRAP and changes in two-back numbers d′ values over time (interaction P-values ), with negative model coefficients indicating that an IQR increase in TRAP exposure was associated with less improvement in this measure of working memory in carriers and noncarriers (Table 3). In contrast, negative associations between TRAP and changes in two-back word d′ values were limited to or stronger in carriers, with interaction P values of 0.06, 0.27, and 0.06 for PAHs, EC, and , respectively (Table 3).
Negative associations between school TRAP exposures and caudate volumes were stronger among APOE carriers than noncarriers, resulting in interaction P-values of 0.04, 0.11, and 0.03 for PAHs, EC, and , respectively (Table 3 and Figure 1). There were no statistically significant associations between caudate volume and behavior problem scores, ADHD symptom scores, or in inattentiveness and working memory test trajectories (Table S4). APOE status did not appear to modify associations between school TRAP exposures and putamen or globus pallidus volumes, which were consistent with the null for both carriers and noncarriers (Table 3).
Sensitivity Analyses
Further adjustment of analyses by air pollution levels at home ( and ), exposure to environmental tobacco smoke at home, and maternal smoking did not change meaningfully our main findings on behavior problem scores, inattentiveness trajectories and caudate volume (Table S5).
Patterns of associations according to APOE status were consistent with the primary models for all TRAP exposures and outcomes when IPW adjustment was used (Table S6). However, differences in associations with behavior problem scores were less pronounced than in the primary models (interaction P-values 0.11–0.41), whereas differences in HRT-SE trajectories (reductions in inattentiveness) were more pronounced (interaction P-values 0.001–0.03).
Mean weights ranged from 2.02 to 19.30 depending on the exposure and outcome being modeled, with minimum and maximum values of 1.20 and 67.7, respectively. Replacing weights below the first percentile and above the 99th percentile with the values at the first and 99th percentiles, respectively, had little influence on model estimates (Table S7).
The AUC ranged from 65.1% to 68.9%.
Discussion
To our knowledge, this study is the first in children to investigate effect modification by APOE status of associations between air pollution and behavior (parent-reported SDQ total difficulties scores and teacher-reported ADHD symptom scores), cognitive function trajectories (changes over 1 y in HRT-SE, a measure of inattentiveness, and in two-back numbers and two-back words d′ values as measures of working memory), and brain morphology (MRI-based measures of basal ganglia volumes). Annual average PAHs, EC, and concentrations at the children’s schools were associated with smaller caudate volumes, higher behavioral problem scores, and less improvement in HRT-SE over time in children carrying the APOE allele than in other children, which suggests that carriers may be more vulnerable to adverse neurobiological effects of TRAP exposure than noncarriers. Although differences between APOE carriers and noncarriers were not statistically significant in all cases, the patterns and magnitudes of differences according to status were robust to additional adjustment and IPW analyses.
As previously found in the BREATHE entire cohort () from which this current study sample was selected, EC and exposures were associated with more behavioral problems (Forns et al. 2016) and worse performance in 1-y trajectories of inattentiveness and two-back numbers d′ scores as a measure of working memory (Sunyer et al. 2015). In agreement with the findings by Mortamais et al. (2017) using a larger sample of BREATHE participants undergoing MRI scan (), exposure to PAHs was associated with smaller caudate volume.
When we studied the effects of TRAP stratifying by APOE status, we found that PAHs, EC, and were consistently associated only with behavior-problem scores among carriers. Interaction tests indicated that the association with PAHs and was significantly different between carriers and noncarriers. However, after adjusting by IPW, these modification effects were no longer statistically significant, suggesting that the results for behavior problem scores may be subject to selection bias. Thus, further research is needed to confirm the findings on behavior problem scores that should be interpreted with caution.
APOE carriers exposed to an IQR increase in TRAP had a smaller reduction in inattentiveness (HRT-SE) over time than noncarriers, with significant differences in associations by status for all three exposures based on the IPW model, whereas interaction P-values for the primary model ranged from 0.08 to 0.21. Among APOE carriers, associations with inattentiveness were significant for IQR increases in all three air pollutants based on the IPW model, and for PAHs and based on the primary model. Previous research suggests that prenatal or childhood exposure to TRAP may affect neurodevelopment, though findings have not been consistent among study populations (Suades-González et al. 2015). There have been conflicting results on the role of APOE status in cognitive function early in life, with several studies reporting cognitive benefits in carriers that were not confirmed in a meta-analysis (Ihle et al. 2012). In our study population, associations between air pollution and smaller reductions in inattentiveness (HRT-SE) over time appeared to be limited to children who were carriers. Stronger associations with air pollution among carriers vs. noncarriers have also been reported for studies of cognitive decline in the elderly (Cacciottolo et al. 2017; Schikowski et al. 2015).
Regarding caudate volumes, PAs and were associated with significantly smaller volumes in carriers in comparison with noncarriers based on both the primary and IPW adjusted models, with a similar pattern for EC. Although in our sample we did not detect significant associations between behavioral and cognitive outcomes and smaller caudate volumes, the estimates were in the expected direction, with smaller caudate volumes being associated with higher behavior problem scores, and with worse 2-back numbers d′ trajectories. Previous research have reported reduced caudate volumes in adolescents and young adults diagnosed with either oppositional defiant disorder or conduct disorder, phenotypes that constitute the clinical expression of behavior problems (Fairchild et al. 2013a, 2013b). Connectivity between cortex and striatum (caudate and putamen nuclei) is extremely relevant for the development of appropriate goal-directed behaviors, which involve motivation and cognitive functions (Haber 2016). Of note, the striatum (caudate and putamen nuclei) has been shown to be particularly affected by oxidative stress (Cardozo-Pelaez et al. 1999), which can be induced by exposure to air pollution (Block and Calderón-Garcidueñas 2009). Thus, it is relevant to establish whether TRAP exposure could disrupt the optimal development of the striatum and to what extent the potential vulnerability of this region to TRAP exposure is more pronounced among APOE carriers.
APOE, the protein encoded by the human APOE gene on chromosome 19, is the major lipid-transporting protein expressed in the brain (Liu et al. 2013) and is involved in mechanisms of neurogenesis, plasticity, and neural repair (Liu et al. 2013). It has been suggested that carriers (approximately 25% of the population) are less efficient than noncarriers in delivering cholesterol and lipids, which are essential for maintaining synaptic integrity and plasticity (Liu et al. 2013). Among AD patients, carriers have enhanced AD pathology, including aggregates of toxic amyloid-beta (), accelerated age-dependent cognitive decline, and poorer memory performance than noncarriers have (Liu et al. 2013). Air pollution may contribute to these neurodegenerative processes through neuroinflammation and oxidative stress. It has been suggested that APOE has pro-inflammatory and/or reduced anti-inflammatory functions (Jofre-Monseny et al. 2008; Ringman et al. 2012). Similarly, exposure to air pollution is related to oxidative stress (Block and Calderón-Garcidueñas 2009), and APOE is considered a poorer antioxidant than the and variants (Dose et al. 2016). Thus, it is possible that carriers are more vulnerable to neuroinflammatory and oxidative stress induced by air pollution exposure.
We found that APOE status did not modify the associations between TRAP and ADHD symptom scores or working memory, only borderline significant modifying effects were observed for PAHs and on two-back words d′ trajectories. A meta-analysis representing 40,942 cognitively healthy adults concluded that the performance in memory tests was worse among APOE carriers in comparison with that of noncarriers (Wisdom et al. 2011). Deficits in both attention and short-term memory were found carriers compared to carriers children exposed to air pollution (Calderón-Garcidueñas et al. 2015a). In this regard, we do not have any particular explanation for the lack of consistent associations between TRAP exposures and changes over time in the two selected measures of working memory.
The strengths of our study include the extensive and robust characterization of neurodevelopmental outcomes, including several behavioral, cognitive, and brain structural outcomes using a combination of methods encompassing questionnaires, computerized tests, and neuroimaging. The cognitive function outcomes were assessed repeatedly using computerized tests, which have a number of advantages, such as increased efficiency and sensitivity and reduced examiner bias (Forns et al. 2014). Our results should be interpreted considering the following limitations. First, no replication sample was available. Second, TRAP levels were not assessed at the same time as the outcome assessments, but rather correspond to a yearly average obtained from two 1-wk measurement campaigns during the same period, which may not fully characterize the temporal variation in TRAP levels to which children are exposed at school. However, temporally adjusted annual concentrations of EC and at each school were highly correlated with the annual estimate of BC at each school from LUR (Spearman correlation estimates were 0.73 and 0.74, respectively), indicating that average concentrations of these pollutants were well captured at the schools. Similarly, we used BAP levels from monitoring stations to obtain seasonalized levels and found that these remained quite constant between 2008 and 2011 (http://qualitatdelaire.cat/contaminant/cerca/14/one-fourth.html, further discussed in Mortamais et al. 2017), and were highly correlated with our analyzed total outdoor PAHs measurements (Spearman correlation estimate 0.64). Therefore, our TRAP measures are likely to be reasonably accurate estimates of chronic exposures experienced by the children when attending school. Third, our analytical strategy resulted in a total of 24 comparisons. Instead of adjusting for multiple comparisons (Feise 2002; Perneger 1998; Rothman 1990), we emphasized on the consistency of results between associations observed across our evaluated exposure-outcome pairs and how they corroborated with our hypothesized pathways. Fourth, because none of the behavioral instruments used in this study were completed by both teachers and parents, we are unable to test whether the lack of consistency between our results for behavior problem scores (assessed using the SDQ and reported by parents) and those for ADHD symptoms (assessed using the ADHD-DSM-IV questionnaire and reported by teachers) may be due to differences in the construct assessed or by rater biases.
To conclude, associations between TRAP and some neurodevelopmental outcomes were stronger among APOE carriers than among noncarriers, consistent with differences in susceptibility according to APOE status. Specifically, we detected evidence of interactions between APOE status and TRAP levels on total difficulties scores for the SDQ, an indication of behavioral problems, and on caudate volume. Although support was weaker for interactions between APOE status and TRAP on reductions in inattentiveness over time, associations between TRAP and 1-y trajectories were stronger or limited to carriers in comparison with noncarriers. Evidence of a role of APOE in neural maintenance and repair, and in the acceleration of neurodegenerative processes in AD (Liu et al. 2013), supports the biological plausibility of an effect of the APOE allele on children’s vulnerability to effects of PAHs, EC, and on neurodevelopmental outcomes. Because traffic, one of the main sources of PAHs, EC, and , can be regulated, our findings, if replicated, indicate that regulations aimed at reducing traffic would contribute to protecting the environmental conditions needed for optimal neurodevelopment during childhood, which in turn would have consequences for health later in life.
Further studies are warranted to replicate these findings in independent samples and investigate other behavior, cognitive, or brain MRI measurements during different developmental periods, such as the prenatal and early postnatal periods.
Supplemental Material
Acknowledgments
We are grateful to all of the children and families who participated in this study for their altruism, and particularly to the following schools: Antoni Brusi, Baloo, Betània – Patmos, Centre d′estudis Montseny, Col•legi Shalom, Costa i Llobera, El sagrer, Els Llorers, Escola Pia de Sarrià, Escola Pia Balmes, Escola concertada Ramon Llull, Escola Lourdes, Escola Tècnica Professional del Clot, Ferran i Clua, Francesc Macià, Frederic Mistral, Infant Jesús, Joan Maragall, Jovellanos, La Llacuna del Poblenou, Lloret, Menéndez Pidal, Nuestra Señora del Rosario, Miralletes, Ramon Llull, Rius i Taulet, Pau Vila, Pere Vila, Pi d'en Xandri, Projecte, Prosperitat, Sant Ramon Nonat – Sagrat Cor, Santa Anna, Sant Gregori, Sagrat Cor Diputació, Tres Pins, Tomàs Moro, Torrent d'en Melis, and Virolai. We also thank C. Persavento, J. González, L. Bouso, M. López and Pere Figueras for their contribution to the field work.
The research leading to these results has received funding from the European Research Council under the ERC Grant Agreement number 268479 – the BREATHE project. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. We thank the La Caixa Foundation for their financial support in the PAHs analyses. S. Alemany is funded by a Sara Borrell postdoctoral grant (CD14/00214) from the Instituto de Salud Carlos III. N. Vilor-Tejedor is funded by a pre-doctoral grant from the Agència de Gestió d′Ajuts Universitaris i de Recerca (2015 FI_B 00636) Generalitat de Catalunya – Fons Social Europeu (FSE). P. Dadvand is funded by a Ramón y Cajal fellowship (RYC-2012-10995) awarded by the Spanish Ministry of Economy and Finance.
References
- Alemany S, Vilor-Tejedor N, Bustamante M, Pujol J, Macià D, Martinez-Vilavella G. 2016. A genome-wide association study of attention function in a population-based sample of children. PLoS One 11(9):e0163048, PMID: 27656889, 10.1371/journal.pone.0163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alier M, van Drooge BL, Dall'Osto M, Querol X, Grimalt JO, Tauler R. 2013. Source apportionment of submicron organic aerosol at an urban background and a road site in Barcelona (Spain) during SAPUSS. Atmos Chem Phys 13(20):10353–10371, 10.5194/acp-13-10353-2013. [DOI] [Google Scholar]
- Amato F, Rivas I, Viana M, Moreno T, Bouso L, Reche C, et al. 2014. Sources of indoor and outdoor PM2.5 concentrations in primary schools. Sci Total Environ 490:757–765, PMID: 24907610, 10.1016/j.scitotenv.2014.05.051. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2002. Manual diagnóstico y estadístico de los trastornos mentales [in Spanish]. Barcelona, Spain: Masson. [Google Scholar]
- Anderson P. 2002. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8(2):71–82, PMID: 12638061, 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Esnaola M, Rivas I, Amato F, Álvarez-Pedrerol M, Forns J. 2016. Neurodevelopmental deceleration by urban fine particles from different emission sources: a longitudinal observational study. Environ Health Perspect 124:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. 2004. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia 42(14):1910–1916, PMID: 15381021, 10.1016/j.neuropsychologia2004.05.007. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: 19716187, 10.1016/j.tins.2009.05.009.Air. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer S, D'Angiulli A. 2016. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci 7(1):24–30, PMID: 28123818, 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, et al. 2017. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7(1):e1022, PMID: 28140404, 10.1038/tp.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Franco-Lira M, Mora-Tiscareño A, Medina-Cortina H, Torres-Jardón R, Kavanaugh M. 2013. Early alzheimer’s and parkinson’s disease pathology in urban children: Friend versus foe responses - It is time to face the evidence. Biomed Res Int 2013:1–16, PMID: 23509683, 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Kavanaugh M, Block M, D'Angiulli A, Delgado-Chávez R, Torres-Jardón R, et al. 2012. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis 28(1):93–107, PMID: 21955814, 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Franco-Lira M, Zhu H, Lu Z, Solorio E, et al. 2015a. Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein ε4 children exposed to air pollution. J Alzheimers Dis 45(3):757–770, PMID: 25633678, 10.3233/JAD-142685. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Torres-Jardón R. 2015b. The impact of air pollutants on the brain. JAMA Psychiatry 59812:5–6, 10.1001/jamapsychiatry.2015.57.2. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Song S, Parthasarathy A, Hazzi C, Naidu K, Sanchez-Ramos J. 1999. Oxidative DNA damage in the aging mouse brain. Mov Disord 14(6):972–980, PMID: 10584672, . [DOI] [PubMed] [Google Scholar]
- Chen H, Rey J, Kwong C, Copes R, Tu K, Villeneuve PJ, et al. 2017. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 6736:1–9, 10.1016/S0140-6736(16)32399-6. [DOI] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. 2006. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci (Regul Ed) 10(3):131–138, PMID: 16460992, S1364-6613(06)00022-2 [pii] 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Dose J, Huebbe P, Nebel A, Rimbach G. 2016. APOE genotype and stress response - a mini review. Lipids Health Dis 15:121, PMID: 27457486, 10.1186/s12944-016-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. 2013a. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry 54(1):86–95, 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Calder AJ, Goodyer IM. 2013b. Research review: evaluating and reformulating the developmental taxonomic theory of antisocial behaviour. J Child Psychol Psychiatry 54(9):924–940, PMID: 23826820, 10.1111/jcpp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feise RJ. 2002. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2:8, PMID: 12069695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Esnaola M, Álvarez-Pedrerol M, López-Vicente M, Garcia-Esteban R, et al. 2017. Longitudinal association between air pollution exposure at school and cognitive development in school children over a period of 3.5 years. Env. Res 159:416–421, [pii] 10.1016/j.envres.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Foraster M, Álvarez-Pedrerol M, Rivas I, López-Vicente M, et al. 2016. Traffic-related air pollution, noise at school, and behavioral problems in barcelona schoolchildren: a cross-sectional study. Environ Health Perspect 124(4):529–535, PMID: 26241036, 10.1289/ehp.1409449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Esnaola M, López M, Suades E, Álvarez M, Julvez J, et al. 2014. The n-back test and the Attentional Network Task as measures of child neuropsychological development in epidemiological studies. Neuropsychology 28(4):519–529, PMID: 24819069, 10.1037/neu0000085. [DOI] [PubMed] [Google Scholar]
- Goodman R. 1997. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 38(5):581–586, PMID: 9255702, 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Haatveit BC, Sundet K, Hugdahl K, Ueland T, Melle I, Andreassen OA. 2010. The validity of d prime as a working memory index: results from the “Bergen n-back task”. J Clin Exp Neuropsychol 32(8):871–880, PMID: 20383801, 10.1080/13803391003596421. [DOI] [PubMed] [Google Scholar]
- Haber S. 2016. Corticostriatal circuitry. Dialogues Clin Neurosci 18(1):7–21, PMID: 27069376, 10.1007/978-3-642-01310-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. 2004. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 5(2):87–96, PMID: 14735112, 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M. 2012. APOE ε4 and cognitive function in early life: a meta-analysis. Neuropsychology 26(3):267–277, PMID: 22329498, 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- Jofre-Monseny L, Minihane A-M, Rimbach G. 2008. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res 52(1):131–145, PMID: 18203129, 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G. 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9(2):106–118, PMID: 23296339, 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vicente M, Forns J, Suades-González E, Esnaola M, García-Esteban R, Álvarez-Pedrerol M, et al. 2016. Developmental trajectories in primary schoolchildren using n-back task. Front Psychol 7:716, PMID: 27242625, 10.3389/fpsyg.2016.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, et al. 2015. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 104:366–372, PMID: 25255942, 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Backman L. 2006. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci 29(8):474–480, 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- McKenna BS, Young JW, Dawes SE, Asgaard GL, Eyler LT. 2013. Bridging the bench to bedside gap: validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res 212(3):183–191, PMID: 23570915, 10.1016/j.pscychresns.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Ministerio de Fomento. 2012. Atlas of urban vulnerability in Spain. Methodology and Contents. Madrid, Spain: Ministerio de Fomento de España. http://www.fomento.es/NR/rdonlyres/8E15B071-12B5-4390-A913-E7349266E9FA/111284/20120125_METODOLOGIA_ATLAS.pdf [accessed 5 July 2018].
- Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, et al. 2017. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ Int 105:12–19, PMID: 28482185, 10.1016/j.envint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. 2000. Functional neuroanatomy of spatial working memory in children. Dev Psychol 36(1):109–116, PMID: 10645748. [DOI] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, et al. 2009. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 Years. Pediatrics 124(2):e195–e113, 10.1542/peds.2008-3506.Prenatal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114(8):1287–1292, 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, et al. 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect 120(6):921–926, PMID: 22440811, 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger TV. 1998. What’s wrong with Bonferroni adjustments. BMJ 316(7139):1236–1238, PMID: 9553006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, et al. 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA psychiatry 72(6):531–540, PMID: 25807066, 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Martínez-Vilavella G, Macià D, Fenoll R, Álvarez-Pedrerol M, Rivas I, et al. 2016. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage 129:175–184, PMID: 26825441, 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575, PMID: 17701901, 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh F, Devan WJ, Anderson CD, Rosand J, Falcone GJ. 2014. Accuracy of imputation to infer unobserved APOE epsilon alleles in genome-wide genotyping data. Eur J Hum Genet 22(10):1239–1242, 10.1038/ejhg.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. 2009. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res 109(8):1004–1011, PMID: 19733348, 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Reche C, Viana M, Rivas I, Bouso L, Álvarez-Pedrerol M, Alastuey A, et al. 2014. Outdoor and indoor UFP in primary schools across Barcelona. Sci Total Environ 493:943–953, PMID: 25003584, 10.1016/j.scitotenv.2014.06.072. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Lee Mosley R, Gendelman HE. 2007. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol 82:297–325. PMID: 17678968, 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, Moore JJ. 2002. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol 17(3):235–272, PMID: 14589726, 10.1016/S0887-6177(01)00111-1. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Elashoff D, Geschwind DH, Welsh BT, Gylys KH, Lee C, et al. 2012. Plasma signaling proteins in persons at genetic risk for Alzheimer disease: influence of APOE genotype. Arch Neurol 69(6):757–764, PMID: 22689192, 10.1001/archneurol.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas I, Viana M, Moreno T, Pandolfi M, Amato F, Reche C, et al. 2014. Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environ Int 69:200–212, PMID: 24875803, 10.1016/j.envint.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Rodrigues R, Smith MA, Wang X, Perry G, Lee HG, Zhu X, et al. 2012. Molecular neuropathogenesis of Alzheimer’s disease: an interaction model stressing the central role of oxidative stress. Future Neurol 7(3):287–305, PMID: 23086377, 10.2217/fnl.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. 1990. No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46, PMID: 2081237. [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. 2004. Development of attentional networks in childhood. Neuropsychologia 42:1029–1040, 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Schikowski T, Vossoughi M, Vierkötter A, Schulte T, Teichert T, Sugiri D, et al. 2015. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res 142:10–16, PMID: 26092807, 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Shelton JT, Elliott EM, Matthews RA, Hill BD, Gouvier WD. 2010. The relationships of working memory, secondary memory, and general fluid intelligence: working memory is special. J Exp Psychol Learn Mem Cogn 36(3):813–820, 10.1037/a0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suades-González E, Gascon M, Guxens M, Sunyer J. 2015. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology 156(10):3473–3482, PMID: 26241071, 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, Esnaola M, Álvarez-Pedrerol M, Forns J, Rivas I, López-Vicente M, et al. 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med 12(3):e1001792, 10.1371/journal.pmed.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen E, Fischer P, Janssen N, Houthuijs D, van Kamp I, Stansfeld S, et al. 2012. Neurobehavioral effects of exposure to traffic-related air pollution and transportation noise in primary schoolchildren. Environ Res 115:18–25, PMID: 22483436, 10.1016/j.envres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Vilor-Tejedor N, Alemany S, Forns J, Cáceres A, Murcia M, Macià D, et al. 2016. Assessment of susceptibility risk factors for ADHD in imaging genetic studies. J Atten Disord, 10.1177/1087054716664408. [DOI] [PubMed] [Google Scholar]
- Vuontela V, Steenari MR, Carlson S, Koivisto J, Fjällberg M, Aronen ET. 2003. Audiospatial and visuospatial working memory in 6-13 year old school children. Learn Mem 10(1):74–81, PMID: 12551966, 10.1101/lm.53503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang J, Zeng X, Zeng Y, Wang S, Chen S. 2009. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ Health Perspect 117(10):1612–1618, PMID: 20019914, 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Boyle LD, Coull BA, Milberg WP, Gryparis A, Schwartz J, et al. 2012. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc 60 (11):2075–2080, 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens TD. 2002. Elementary signal detection theory. New York, NY: Oxford UP. [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. 2011. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 32(1):63–74, PMID: 19285755, 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.