Abstract
Background:
Prenatal exposure to fine particulate matter air pollution with aerodynamic diameter () has been associated with preterm delivery and low birth weight (LBW), but few studies have examined possible effect modification by oxidative potential.
Objectives:
The aim of this study was to evaluate if regional differences in the oxidative potential of modify the relationship between and adverse birth outcomes.
Methods:
A retrospective cohort study was conducted using 196,171 singleton births that occurred in 31 cities in the province of Ontario, Canada, from 2006 to 2012. Daily air pollution data were collected from ground monitors, and city-level oxidative potential was measured. We used random-effects meta-analysis to combine the estimates of effect from regression models across cities on preterm birth, term LBW, and term birth weight and used meta-regression to evaluate the modifying effect of oxidative potential.
Results:
An interquartile increase () in first-trimester was positively associated with term LBW among women in the highest quartile of glutathione (GSH)-related oxidative potential [; 95% confidence interval (CI): 1.10, 1.48], but not the lowest quartile (; 95% CI: 0.87, 1.14; ). on the day of delivery also was associated with preterm birth among women in the highest quartile of GSH-related oxidative potential [; 95% CI: 1.01, 1.04], but not the lowest quartile [; 95% CI: 0.95, 1.00; ]. Between-city differences in ascorbate (AA)-related oxidative potential did not significantly modify associations with .
Conclusions:
Between-city differences in GSH-related oxidative potential may modify the impact of on the risk of term LBW and preterm birth. https://doi.org/10.1289/EHP2535
Introduction
A large number of studies have suggested associations between ambient fine particulate matter with aerodynamic diameter () and adverse birth outcomes, with the strongest evidence for preterm delivery and term low birth weight (LBW) (Dadvand et al. 2013; Li et al. 2017; Shah et al. 2011; Stieb et al. 2012). While the underlying mechanism(s) explaining the impact of maternal exposure to on adverse birth outcomes have yet to be fully elucidated, evidence suggests that oxidative stress plays an important role in the health impacts of during pregnancy (Kannan et al. 2006; Nagiah et al. 2015; Schlesinger et al. 2006). Recently, it has been demonstrated that the oxidative potential of may modify the cardiovascular and respiratory health effects of these pollutants (Weichenthal et al. 2016a, 2016b, 2016c), but it is not clear if oxidative potential may also modify the relationship between maternal exposure to and adverse birth outcomes.
The antioxidants glutathione (GSH) and ascorbate (AA) are important in the body since they act as a first line of defense against inhaled pollutants (Kelly 2003). During pregnancy, these antioxidants are of importance to protect both the mother and the developing fetus against undue cellular damage from free radicals (Nagiah et al. 2015). In fact, pregnancy itself leads to a state of susceptibility to oxidative stress due to increased energy expenditure and altered physiological processes (Nagiah et al. 2015; Risom et al. 2005). Recent evidence also suggests that pregnant women exposed to higher air pollutant levels display increased markers for oxidative stress and lower systemic antioxidant concentrations, including reduced GSH, compared to those exposed to low levels of ambient air pollution (Nagiah et al. 2015).
Studies have shown that the oxidative potential of varies both between (Künzli et al. 2005; Weichenthal et al. 2016a, 2016b, 2016c) and within regions of the Netherlands, Belgium, and the United Kingdom (Godri et al. 2010; Janssen et al. 2014; Yang et al. 2015; Yanosky et al. 2012). Regional differences in the oxidative potential of are attributable to compositional differences including transition metals, polycyclic aromatic hydrocarbons, and/or quinones (Ayres et al. 2008; Godri et al. 2010; Janssen et al. 2014; Künzli et al. 2005). A growing number of studies suggests that exposure to fine particulate matter with high oxidative potential is associated with respiratory and cardiovascular outcomes (Delfino et al. 2013; Maikawa et al. 2016; Weichenthal et al. 2016b, 2016c; Zhang et al. 2016). Nevertheless, no studies to date have evaluated whether between-city differences in oxidative potential modify the association between and adverse birth outcomes. Although current evidence supports the use of mass concentrations as the most appropriate indicator of exposure, there are potential regional differences in particle composition and biological activity, with limited evidence to indicate the role of oxidative potential. In addition, current evidence supports the use of mass concentrations as equally toxic despite potential differences in particle composition or biological activity. Our hypothesis is that for the same level of mass concentrations, we expect that higher levels of oxidative potential would be associated with a greater risk of preterm birth, greater risk of LBW, and greater decrease in term birth weight than lower levels of oxidative potential.
In this study, we evaluated the impact of between-city differences in oxidative potential on the relationship between and the risk of adverse birth outcomes in the province of Ontario, Canada. To our knowledge, this is the first study to examine how between-city differences in oxidative potential may modify associations between and adverse birth outcomes at the population level.
Methods
Study Population
The study population included a retrospective cohort of pregnant women giving birth to live born, nonplanned C-sections, nonlabor-induced, singleton infants in 31 cities across Ontario, Canada, between 1 January 2006 and 31 March 2012. Birth data were obtained from the Better Outcomes Registry & Network (BORN) Ontario, a province-wide birth registry that captures maternal health, obstetric, intrapartum, and neonatal information in and around the perinatal period of pregnancy (Dunn et al. 2011). This birth registry captures hospital births and home births in the province of Ontario, Canada. Between 2006 and 2012, data capture for all births across the province of Ontario improved from an estimated 82% to about 100% of births. In fact, a quality assurance project conducted in 2008 showed that 96% of all births in Ontario were captured in the birth registry (Dunn et al. 2011). Missing births were due to a small number of hospitals and midwifery practices, spread across the province, that were not participating in the birth registry in the early years.
Birth outcomes examined in this study and for which information was extracted from the birth registry included preterm birth (gestational age ) and term LBW (gestational age and birth weight ) (Kramer et al. 2001). Gestational age was determined by first trimester ultrasound dating and the mother's last menstrual period. Only births with estimated conception dates ranging between 20 wk (i.e., shortest pregnancy) before the study started and 44 wk (i.e., longest pregnancy) before it ended were included in order to account for the fixed cohort bias (Strand et al. 2011).
We limited study participants to those identified in BORN who, at the time of delivery, were living within of a ground monitoring site where oxidative potential was measured (described below) (Weichenthal et al. 2016b, 2016c). This criterion was used to reduce potential exposure measurement error for oxidative potential. Sites in the following cities were included in this study: Barrie, Belleville, Brantford, Chatham, Cornwall, Dorset, Guelph, Hamilton, Kingston, Kitchener, Mississauga, Morrisburg, North Bay, Oakville, Oshawa, Parry Sound, Petawawa, Peterborough, Port Stanley, Sarnia, Sault Ste. Marie, Simcoe, St. Catherine’s, Sudbury, Thunder Bay, and Toronto. Each city had one monitoring site with the exception of Hamilton (two sites) and Toronto (five sites).
Birth data were linked with the Registered Persons Database, a registry of all Ontario residents currently or previously possessing a health insurance number, in order to identify each mother's residential six-digit postal code during pregnancy (Lavigne et al. 2016b; Lavigne et al. 2017). This information allowed us to identify mothers living within of a ground monitoring site and to subsequently link environmental exposures. The encrypted unique identifier (also referred to the IKN) was used to link health administrative data at the Institute for Clinical Evaluative Sciences in Ontario, Canada. Information on maternal residential location(s) based on residential postal code(s) was geocoded using the Postal Code Conversion File Plus (PCCF version 6A) program from Statistics Canada. In urban areas, the six-digit postal code typically represents one side of a city block or a large apartment complex, while it usually represents a larger area in rural areas. In this study, most postal codes were located in urban areas. Study participants with postal codes of residence outside Ontario (i.e., ) of all pregnancies and those that moved outside of the radius during pregnancy were excluded from the analysis. Subjects without a valid health card number for data linkage or missing date of birth and six-digit postal code value and/or exposure estimates were also excluded from the study. We excluded 11.1% of subjects (24,494 out of 220,665 eligible subjects) based on the exclusion criteria. Ethics approval for this study was granted by the Health Canada and the Public Health Agency of Canada’s Research Ethics Board, the Children’s Hospital of Eastern Ontario, and the Ottawa Health Science Network Research Ethics Board.
Exposure Assessment to Ambient Air Pollutants
Daily average concentrations of ambient , , and were collected from ground monitoring stations in Ontario, which are part of Canada’s National Air Pollution Surveillance (NAPS) network. Daily mean temperature and relative humidity data were also collected from the closest Environment Canada weather station where the NAPS station was located. Data for were available for the study period at each site ( y; range: 5–7 y). All data were collected using tapered element oscillating microbalance (TEOM), TEOM™, Series 1400ab monitors (Thermo Scientific) at the ground monitoring sites and were adjusted for potential bias during winter months (owing to the loss of volatile components) using data provided by Environment Canada. All participants residing within of a given monitoring site during pregnancy were assigned the daily environmental data corresponding to the different periods of their pregnancy (i.e., daily exposures in the last 4 wk before delivery, weekly exposures throughout pregnancy, each trimester and whole-pregnancy exposures), and levels were averaged over these specific periods for analyses investigating term LBW, term birth weight, and preterm birth as outcomes. Exposure periods were based on gestational age at birth and were defined as the following: first trimester was defined as day 1 to 90 of pregnancy, second trimester as day 91 to 180 of pregnancy, third trimester as day 181 of pregnancy to birth, and whole pregnancy as day 1 of pregnancy to birth.
City-Level Estimates of Oxidative Potential
The laboratory methods used in this study are the same as those applied in recently published papers by our research team examining regional differences in oxidative potential and long-term mortality risk as well as emergency room visits for myocardial infarction and respiratory diseases (Weichenthal et al. 2016a, 2016b, 2016c). Briefly, regional (i.e., city-level) samples were collected between 2012 and 2013 from 31 sites in 26 cities across Ontario. Sites were located mainly in urban areas, although some rural sites were also included. City-level estimates of oxidative potential were based on a mean duration of 103 sampling days (range: 12–311 d). These estimates were based on multiple filter samples per site (range: 1–7); oxidative potential values were estimated using a time-weighted average over the entire monitoring period at each site. The sampling period at each site depended on the number of filters available, the start date of sample collection, and how often TEOM filters were changed by station managers throughout the year, which is typically every 4 to 6 wk.
An in vitro assay based on a synthetic respiratory tract lining fluid was used to quantify GSH- () and AA-related () oxidative potential as previously described (Godri et al. 2011; Weichenthal et al. 2016a). Briefly, regional filter extracts were incubated with a synthetic human respiratory tract lining fluid for 4 h at 37°C. This fluid was a composite solution of physiologically relevant antioxidants including AA and GSH. In this study, the term “oxidative potential” (reflecting long-term city-level oxidative potential) is used to describe the ability of filter extracts of given concentration () to deplete GSH and AA in the simulated respiratory tract lining fluid (units: % ). The term “oxidative burden” is used to describe the product of mass concentrations () and estimates of city-level oxidative potential. While the oxidative burden measure rescales mass concentrations according to oxidative potential, it can also be viewed as a different exposure measure in units of percentage as opposed to .
Covariates
A number of potential confounders available from the birth registry were evaluated in this study. These confounders, as categorized in Table 1, included maternal age at delivery, maternal cigarette smoking anytime during pregnancy, infant sex, parity, previous preterm delivery, previous caesarean section delivery, presence of maternal comorbidities (i.e., asthma, hypertension, preeclampsia, type 1 and 2 diabetes mellitus, gestational diabetes, and heart disease), month of birth, and year of birth. Maternal comorbidities were identified from health administrative databases based on validated algorithms (Lavigne et al. 2016b) for conditions being present prior to pregnancy (i.e., asthma, hypertension, type 1 and 2 diabetes mellitus, and heart disease) or that occurred during the gestational period (i.e., preeclampsia and gestational diabetes). We also evaluated gestational age, measured in weeks from conception to delivery, as a confounder in term LBW and term birth weight models. In order to evaluate confounding by socioeconomic status (SES), we abstracted three SES variables from the 2006 Canadian census at the dissemination-area (DA) level: median family income, proportion of population in the DA who are visible minority, and percentage of the adult female population aged 25–64 y who completed postsecondary education. All of Canada is divided into DAs, which are a small geographical unit composed of one or more neighboring dissemination blocks, with a population of 400 to 700 persons. These area-based variables have been shown to be reasonable measures of neighborhood-level SES (Subramanian et al. 2006). These area-based SES variables were assigned to study subjects based on their postal codes at the time of delivery or using a weighted SES based on time at each residence during pregnancy for those who moved during pregnancy. Median family income in the DA, proportion of population in the DA who are visible minority, and percentage of the adult female population aged 25–64 y who completed postsecondary education (i.e., collegiate degree or higher) were categorized in quartiles.
Table 1.
Selected characteristics of the study population, Ontario, Canada (2006–2012).
| Characteristics | Total (%) | Preterm birth (%) | Term low birth weight (%) |
|---|---|---|---|
| Sex | |||
| Male | 100,585 (51.3) | 8,401 (54.6) | 1,646 (40.9) |
| Female | 95,586 (48.7) | 6,977 (45.4) | 2,377 (59.1) |
| Maternal age | |||
| 7,792 (4.0) | 649 (4.2) | 201 (5.0) | |
| 20–34 | 148,042 (75.5) | 10,982 (71.4) | 2,938 (73.0) |
| 40,337 (20.6) | 3,747 (24.4) | 884 (22.0) | |
| Parity | |||
| 0 | 90,541 (45.2) | 7,148 (46.5) | 2,080 (51.7) |
| 1 | 67,504 (34.4) | 4,912 (31.9) | 1,190 (29.6) |
| 38,092 (19.4) | 3,313 (21.5) | 751 (18.7) | |
| Missing | 34 (0.02) | 5 (0.03) | 2 (0.05) |
| Smoking during pregnancy | |||
| Yes | 15,890 (8.1) | 1,276 (8.3) | 342 (8.5) |
| No | 158,898 (81.0) | 12,425 (80.8) | 3,247 (80.7) |
| Missing | 21,383 (10.9) | 1,676 (10.9) | 434 (10.8) |
| Previous caesarean section | |||
| Yes | 26,483 (13.5) | 2,153 (14.0) | 539 (13.4) |
| No | 167,726 (85.5) | 13,071 (85.0) | 3,484 (86.6) |
| Missing | 1,962 (1.0) | 154 (1.0) | 44 (1.1) |
| Maternal preexisting asthma | |||
| Yes | 11,770 (6.0) | 938 (6.1) | 233 (5.8) |
| No | 184,400 (94.0) | 14,440 (93.9) | (94.2) |
| Maternal preexisting hypertension | |||
| Yes | 4,708 (2.4) | 384 (2.5) | 105 (2.6) |
| No | 176,554 (90.0) | 13,825 (89.9) | 3613 (89.8) |
| Missing | 14,909 (7.6) | 1,169 (7.6) | 306 (7.6) |
| Gestational hypertension | |||
| Yes | 12,555 (6.4) | 984 (6.4) | 237 (5.9) |
| No | 170,669 (87.0) | 13,379 (87.0) | 3,520 (87.5) |
| Missing | 12,947 (6.6) | 1,015 (6.6) | 266 (6.6) |
| Maternal preexisting heart disease | |||
| Yes | 1,177 (0.6) | 77 (0.5) | 12 (0.3) |
| No | 180,085 (91.8) | 14,132 (91.9) | 3,705 (92.1) |
| Missing | 14,909 (7.6) | 1,169 (7.6) | 306 (7.6) |
| Maternal preexisting type 1 or type 2 diabetes | |||
| Yes | 6,278 (3.2) | 523 (3.4) | 129 (3.2) |
| No | 174,985 (89.2) | 13,748 (89.4) | 3,589 (89.2) |
| Missing | 14,909 (7.6) | 1,169 (7.6) | 306 (7.6) |
| Gestational diabetes | |||
| Yes | 15,498 (7.9) | 1,276 (8.3) | 323 (8.2) |
| No | 180,674 (92.1) | 14,102 (91.7) | 3,693 (91.8) |
| Missing | 12,947 (6.6) | 1,015 (6.6) | 266 (6.6) |
| Dissemination-area median family income | |||
| Quartile 1 | 49,368 (25.2) | 4,034 (26.2) | 1,150 (28.6) |
| Quartile 2 | 48,911 (24.9) | 3,878 (25.2) | 1,058 (26.3) |
| Quartile 3 | 49,312 (25.1) | 3,801 (24.7) | 973 (24.2) |
| Quartile 4 | 47,424 (24.2) | 3,568 (23.2) | 820 (20.4) |
| Missing | 1,156 (0.6) | 97 (0.6) | 22 (0.6) |
| Dissemination-area percent of females who completed postsecondary education | |||
| Quartile 1 | 48,420 (24.7) | 3,953 (25.7) | 1,030 (25.6) |
| Quartile 2 | 50,658 (25.8) | 4,036 (26.3) | 1,083 (26.9) |
| Quartile 3 | 47,236 (24.1) | 3,741 (24.3) | 946 (23.5) |
| Quartile 4 | 48,701 (24.8) | 3,551 (23.1) | 942 (23.4) |
| Missing | 1,156 (0.6) | 97 (0.6) | 22 (0.6) |
| Dissemination-area percent of visible minority | |||
| Quartile 1 | 46,287 (23.6) | 3,633 (23.6) | 781 (19.4) |
| Quartile 2 | 48,421 (24.7) | 3,826 (24.9) | 875 (21.8) |
| Quartile 3 | 49,284 (25.1) | 3,864 (25.1) | 1,017 (25.3) |
| Quartile 4 | 50,843 (25.9) | 3,938 (25.6) | 1,326 (33.0) |
| Missing | 1,336 (0.7) | 117 (0.8) | 24 (0.6) |
| Total | 196,171 (100) | 15,378 (100) | 4,023 (100) |
Statistical analysis
A two-stage approach was used for analysis. In the first stage, multivariate regression models were applied separately in each city to estimate the associations between exposure to mass concentrations and oxidative burden metrics during specific periods of pregnancy (i.e., daily exposures in the last 4 wk before delivery, weekly exposures throughout pregnancy, each trimester and whole-pregnancy exposures), and adverse birth outcomes. Specifically, we used logistic regression models when investigating term LBW and preterm birth as outcomes and linear regression models when using term birth weight (i.e., continuous measurement) as an outcome in relationship with chronic exposure periods (i.e., weekly exposures throughout pregnancy, each trimester and whole-pregnancy exposures) to air pollution estimates. Similar to a previous paper estimating the effect of air pollution during pregnancy on the risk of preterm delivery (Hao et al. 2016), a discrete time survival model with logistic link (Chang et al. 2012) was used to estimate associations between preterm delivery and exposure to mass concentrations and oxidative burden metrics during the third trimester and the total pregnancy. This was done in order to account for the varying lengths of the third trimester among the births (Chang et al. 2013). In all analyses using logistic regression models, city-specific odds ratios (ORs) with their 95% confidence intervals (CIs) were obtained to estimate the effect of exposure to during pregnancy on the risk of term LBW and preterm delivery. City-specific beta coefficients were extracted when using linear regression models. We also used separate Cox proportional hazards models with gestational age (in days) as the time scale to estimate city-specific hazard ratios (HRs) and 95% CIs for preterm delivery in association with acute short-term exposures to air pollution (i.e., daily exposures in the week before delivery and the 4 wk before delivery). City-specific HRs with their 95% CIs were obtained to estimate the effect of short-term exposure to air pollution measures during pregnancy on the risk of preterm delivery. This approach has been previously used for acute exposures on the risk of preterm delivery when taking into account fetuses at risk (Darrow et al. 2009; Lavigne et al. 2016a; Strand et al. 2012).
oxidative burden metrics were generated for GSH () and AA () by multiplying period-specific ambient mass concentrations () by city-level estimates of oxidative potential. These parameters reflect a reweighting of mass concentrations according to city-level oxidative potential and were treated as separate exposure variables in the analysis. We report effect estimates and 95% CI for interquartile range (IQR) increases in mass concentrations or oxidative burden metrics.
In the second stage, we pooled city-specific estimates of and birth outcomes associations using a multivariate meta-analytical model (Gasparrini et al. 2012; Gasparrini and Armstrong 2013). Random-effects multivariate meta-regression models were used to test potential effect modification by between-site differences in oxidative potential (Borenstein et al. 2009). The outcome variables in the meta-regression models in this study were the pooled estimates (i.e., ORs, HRs, beta coefficients), and the explanatory variables (i.e., potential effect modifiers) were the oxidative potential measures at the site level. Effect modification was considered statistically significant if the effect modifier’s p-values (i.e., meta-regression model p-values when using oxidative potential measures at the site level as a meta-predictor) were . Tests for residual heterogeneity using Cochran Q test and statistic (Gasparrini et al. 2012; Higgins and Thompson 2002) were also conducted for each of the meta-regression models.
We also generated concentration–response curves for exposures during different time periods (overall and stratified by oxidative potential measures) using natural cubic splines with 3 degrees of freedom (df). In the current paper, we presented concentration–response curves for exposure periods and outcomes where a statistically significant association was observed. The remaining concentration–response curves were provided as supplemental material. Models were also evaluated with 2 or 4 df; however, the Akaike information criterion (AIC), a measure of model fit based on splines with 2 or 4 df, was always larger than the AIC for models with 3 df (data not shown). We also generated distributed lag curves to investigate the possibility of sensitive windows of exposure during the entire pregnancy (modeled using B-splines) and during the last 4 wk before delivery (modeled using natural cubic splines with 3 df).
Potential confounders were evaluated in the multivariable models using covariates previously mentioned using a backward deletion approach (Rothman et al. 2008). This was accomplished by adjusting for all potential confounders and then removing the covariate with the largest p-value one by one in a stepwise manner as long as the total proportional change in the estimate compared with the fully adjusted model was . In all models, we used a missing data indicator approach, whereby missing values for covariates were categorized as “missing” or “unknown” so that all observations were retained in the models. Analyses were performed with the R software, (version 3.1.3; R Development Core Team), using packages dlnm, version 2.1.4, and mvmeta, version 0.4.5.
A number of sensitivity analyses were conducted. First, we additionally adjusted models for , , the combined oxidant capacity () of , and calculated as their sum (Williams et al. 2014) as well as their redox-weighted oxidant capacity () calculated as a weighted average using redox potentials (Bratsch 1989) as the weights, i.e., . The redox-weighted measure accounts for the fact that is a stronger oxidant than . These air pollution metrics have been previously used by our research team when investigating impacts on cardiovascular and respiratory outcomes (Weichenthal et al. 2016b, 2016c). We also controlled for ambient temperature and humidity by including mean temperature and humidity for the gestational periods evaluated as covariates in the model. Lastly, we estimated associations with on the different outcomes according to quartiles of regional oxidative potential.
Results
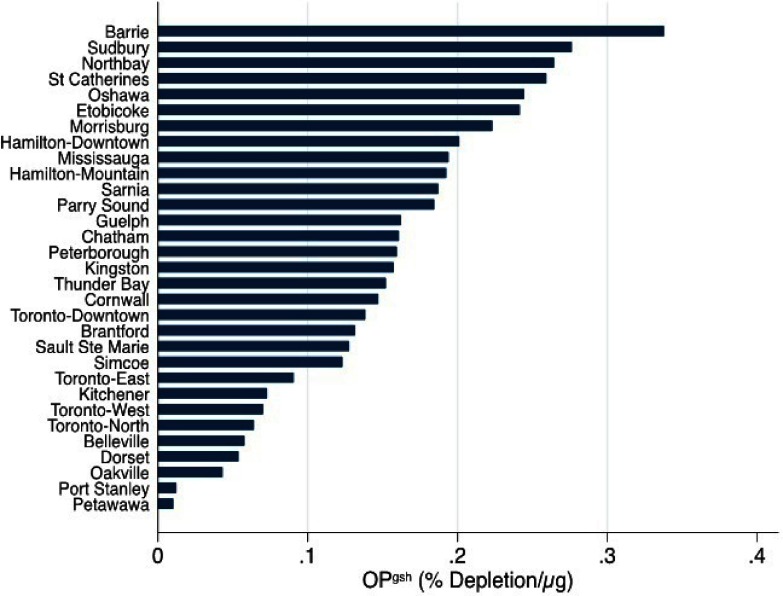
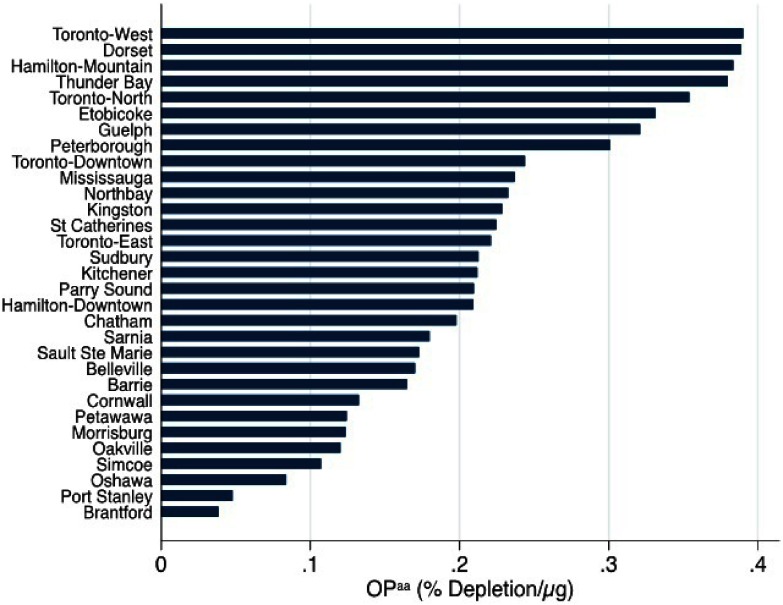
There were a total of 196,171 births during the study period, including 15,378 preterm births (7.8% of 196,171 births) and 4,023 (2.0% of 180,793 term births) term LBW cases (Table 1). Birth outcomes under investigation were more prevalent across specific infant, maternal, and neighborhood characteristics. For instance, both preterm delivery and term LBW were more prevalent among pregnant women who delivered at of age, those that were nulliparous, those who smoked during pregnancy, among women with specific health problems (i.e., asthma, hypertension, preeclampsia, type 1 and 2 diabetes mellitus, gestational diabetes, and heart disease) and in the lowest quartiles of neighborhood median family income indicator. Both outcomes were also more prevalent in neighborhoods with a higher proportion of individuals of visible minorities. Average exposure to mass concentrations over the entire pregnancy was with an IQR of (Table 2). The IQR for mass concentrations measured on a daily basis in the last 4 wk before delivery was . Average exposures and IQRs for mass concentrations were similar across the different trimesters. Moderate correlations were observed between exposures in different trimesters () (Table S1). mass concentrations were not correlated with city-level oxidative potential measures () (i.e., cities with higher oxidative potential did not necessarily have higher mass concentrations). GSH- and AA-related oxidative potential measures were weakly correlated with each other (), and levels varied substantially between cities (Figures 1 and 2).
Table 2.
Summary of exposure to ambient air pollutants and weather variables among study participants in Ontario, Canada (2006–2012).
| Air pollutants and weather variables | Mean (SD) | Median | IQR | Range |
|---|---|---|---|---|
| () | ||||
| First trimester | 9.0 (2.3) | 8.9 | 2.9 | 2.8–19.0 |
| Second trimester | 9.1 (2.3) | 8.8 | 2.9 | 2.8–19.0 |
| Third trimester | 9.0 (2.4) | 8.8 | 3.0 | 1.5–28.7 |
| Overall pregnancy | 9.0 (2.0) | 9.0 | 2.6 | 3.7–17.2 |
| Last 4 wk of pregnancy | 9.0 (5.6) | 7.6 | 7.1 | 0.1–67.9 |
| (ppb) | 14.7 (5.8) | 14.4 | 9.3 | 2.0–30.7 |
| (ppb) | 24.9 (4.2) | 25.0 | 6.0 | 12.9–39.4 |
| (ppb) | 39.4 (4.2) | 39.3 | 5.5 | 26.0–55.8 |
| (ppb) | 21.3 (2.0) | 21.2 | 2.9 | 15.0–33.4 |
| Oxidative potential metrics | ||||
| (% ) | 0.15 (0.08) | 0.16 | 0.12 | 0.01–0.34 |
| (% ) | 0.22 (0.10) | 0.21 | 0.12 | 0.04–0.39 |
| Oxidative burden metrics | ||||
| (% ) | 1.29 (0.76) | 1.00 | 1.16 | 0.04–3.78 |
| (% ) | 2.37 (1.03) | 2.19 | 1.42 | 0.24–5.21 |
| Temperature (°C) | 8.2 (4.13) | 8.4 | 7.2 | |
| Relative humidity (%) | 73.1 (4.51) | 73.1 | 6.6 | 58.8–93.1 |
Note: IQR, interquartile range; ascorbate-related oxidative potential; , glutathione-related oxidative potential; , fine particulate matter air pollution with aerodynamic diameter ; , ascorbate-related oxidative burden; , glutathione-related oxidative burden; SD, standard deviation.
Figure 1.
City-level estimates of glutathione-related oxidative potential () for locations studied across Ontario, Canada (2012–2013).
Figure 2.
City-level estimates of ascorbate-related oxidative potential () for locations studied across Ontario, Canada (2012–2013).
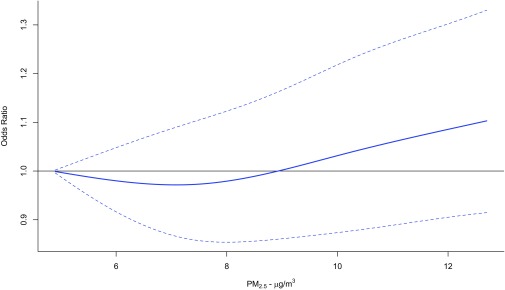
concentrations during the first trimester were positively associated with term LBW (; 95% CI: 1.01, 1.15 for a IQR increase), while associations with both preterm birth and term LBW were close to the null or inverse (and not significant) for exposures during other time periods (Table 3). The concentration–response curve for during the first trimester and term LBW appeared to be linear above (Figure 3). The association between term LBW and an IQR increase in GSH-related oxidative burden () during the first trimester (; 95% CI: 1.07, 1.61) was stronger than the corresponding association with an IQR increase in mass concentration (Table 3). Associations of first trimester and with term LBW remained positive but were closer to the null after adjusting for , , , and and the antioxidant capacity and redox-weighted oxidant capacity of and combined (Table S2). In general, associations of and GSH- and AA-related oxidative burden metrics with term LBW, preterm birth, and term birth weight were not statistically significant for exposures during any time period (Tables S2–S7).
Table 3.
Associations between exposure to and oxidative burden over different periods of exposure during pregnancy and birth outcomes in Ontario, Canada (2006–2012).
| Birth outcome and exposure time period | |||
|---|---|---|---|
| Term low birth weight OR (95% CI) | |||
| First trimester | 1.08 (1.01, 1.15) | 1.31 (1.07, 1.61) | 1.12 (0.95, 1.32) |
| Second trimester | 0.96 (0.91, 1.02) | 0.94 (0.80, 1.10) | 0.92 (0.82, 1.04) |
| Third trimester | 0.95 (0.90, 1.00) | 0.92 (0.79, 1.07) | 0.89 (0.79, 1.00) |
| Whole pregnancy | 0.96 (0.86, 1.07) | 1.15 (0.84, 1.56) | 0.90 (0.73, 1.11) |
| Preterm birth | |||
| Chronic exposure OR (95% CI) | — | — | — |
| First trimester | 0.95 (0.77, 1.18) | 0.70 (0.36, 1.36) | 0.90 (0.53, 1.54) |
| Second trimester | 1.00 (0.91, 1.10) | 0.99 (0.74, 1.33) | 1.03 (0.82, 1.30) |
| Third trimester | 0.98 (0.94, 1.03) | 0.96 (0.85, 1.09) | 0.97 (0.87, 1.07) |
| Whole pregnancy | 0.94 (0.84, 1.04) | 0.79 (0.55, 1.12) | 0.90 (0.67, 1.20) |
| Acute exposure HR (95% CI) | — | — | — |
| Last 4 wk of pregnancy | 0.94 (0.89, 0.99) | 0.93 (0.86, 1.08) | 0.96 (0.88, 1.09) |
| Same day of delivery | 0.99 (0.98, 1.00) | 1.01 (0.99, 1.02) | 0.99 (0.99, 1.00) |
| Term birth weight (95% CI) | |||
| First trimester | (, 3.83) | (, 7.76) | (, 7.63) |
| Second trimester | 0.88 (, 5.45) | (, 8.96) | 3.26 (, 11.23) |
| Third trimester | 6.60 (, 12.98) | 6.69 (, 17.63) | 10.62 (2.89, 18.35) |
| Whole pregnancy | 5.49 (, 12.33) | 1.50 (, 15.98) | 9.19 (, 20.32) |
Note: Estimates of association are for IQR increases in exposure to (), (1.16% ), or (1.42% ). Models represent pooled city-specific estimates derived using two-stage random-effects meta-analysis and logistic regression [term low birth weight (LBW) and ORs for preterm birth in association with chronic exposures], Cox proportional hazard models (HRs for preterm birth in association with IQR increases in exposure to () during the last 4 wk of pregnancy or on the day of delivery), or linear regression (term birth weight). Models were adjusted for maternal age at delivery, marital status, maternal cigarette smoking during pregnancy, infant sex, parity, previous caesarean section delivery, maternal comorbidities (i.e. asthma, hypertension, type 1 and 2 diabetes mellitus, preeclampsia, and gestational diabetes), year of birth, month of birth, census dissemination-area (DA) median family income, census DA proportion of population who are visible minority, and census DA proportion of the adult female population aged 25–64 y old who completed postsecondary education; gestational age was also included in term LBW and term birth weight models; mean temperature and mean relative humidity were also included in preterm birth acute exposure models. —, data not available; CI, confidence interval; HR, hazard ratio; OR, odds ratio; , fine particulate matter air pollution with aerodynamic diameter ; , ascorbate-related oxidative burden; , glutathione-related oxidative burden; , beta coefficient.
Figure 3.
Concentration–response curve using natural cubic splines with 3 degrees of freedom for the association between exposure to fine particulate matter air pollution with aerodynamic diameter () during the first trimester and term low birth weight in Ontario, Canada (2006–2012). Solid line reflects point estimates, and dotted lines reflect 95% confidence intervals. Models were adjusted for maternal age at delivery, marital status, maternal cigarette smoking during pregnancy, infant sex, parity, previous caesarean section delivery, maternal comorbidities (i.e., asthma, hypertension, type 1 and 2 diabetes mellitus, preeclampsia, and gestational diabetes), year of birth, month of birth, census dissemination-area (DA) median family income, census DA proportion of population who are visible minority, census DA proportion of the adult female population aged 25–64 y old who completed postsecondary education, and gestational age.
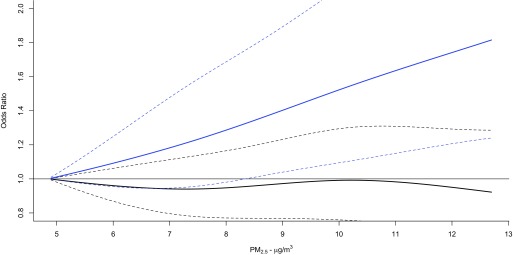
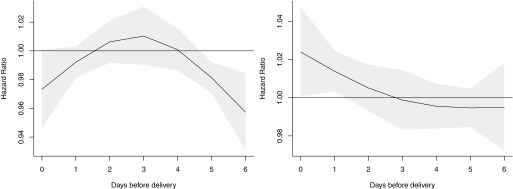
When associations between IQR increases in and the birth outcomes were stratified by quartiles of (Table 4) and (Table S7), there was little evidence of effect modification overall. However, associations between term LBW and mass concentrations during the first trimester increased with increasing quartiles of (), while associations with during the entire pregnancy changed from inverse to positive with increasing of () (Table 4). The concentration–response curves for the associations between during the first trimester and term LBW below 25th percentile of and above the 75th percentile of supported this finding (Figure 4). In addition, associations between on the day of delivery and preterm birth changed from inverse to positive with increasing (; 95% CI: 1.01, 1.05 for the highest quartile of ; ) (Table 4). This finding was supported by concentration–response curves for associations between during the week before delivery and preterm birth among those with below the 25th percentile and above the 75th percentile (Figure 5). and did not significantly modify associations between term LBW and according to week of gestation (Figures S1 and S2, ), term birth weight and according to week of gestation (Figures S3 and S4, ), or preterm and during the last 4 wk of gestation (Figure S5 and Table 4, Figure S6 and Table S7, respectively), and did not appear to modify the association between preterm and during the week before delivery (Figure S7, ).
Table 4.
Associations between exposure to across quartiles of glutathione-related oxidative potential () over different periods of exposure during pregnancy and birth outcomes in Ontario, Canada (2006–2012).
| Birth outcome and exposure time period | Percentile of | p-Value for effect modification | |||
|---|---|---|---|---|---|
| 25–50th | 50–75th | ||||
| Term low birth weight OR (95% CI) | |||||
| First trimester | 0.99 (0.87, 1.14) | 1.00 (0.85, 1.18) | 1.06 (0.91, 1.23) | 1.28 (1.10, 1.48) | 0.03 |
| Second trimester | 0.95 (0.86, 1.04) | 0.98 (0.85, 1.11) | 1.01 (0.87, 1.17) | 0.94 (0.83, 1.06) | 0.93 |
| Third trimester | 0.92 (0.83, 1.03) | 0.90 (0.78, 1.04) | 0.96 (0.78, 1.19) | 0.97 (0.85, 1.12) | 0.43 |
| Whole pregnancy | 0.85 (0.72, 1.00) | 0.90 (0.69, 1.17) | 1.04 (0.82, 1.33) | 1.22 (0.94, 1.57) | 0.01 |
| Preterm birth | |||||
| Chronic exposure OR (95% CI) | — | — | — | — | — |
| First trimester | 0.87 (0.82, 0.92) | 0.65 (0.30, 1.41) | 0.94 (0.87, 1.02) | 1.00 (0.88, 1.13) | 0.72 |
| Second trimester | 0.95 (0.85, 1.06) | 1.11 (0.81, 1.52) | 0.96 (0.87, 1.06) | 1.00 (0.86, 1.15) | 0.89 |
| Third trimester | 0.98 (0.93, 1.02) | 0.97 (0.90, 1.03) | 1.03 (0.94, 1.11) | 0.97 (0.87, 1.09) | 0.29 |
| Whole pregnancy | 0.91 (0.69, 1.19) | 1.01 (0.61, 1.90) | 0.94 (0.84, 1.06) | 0.98 (0.79, 1.21) | 0.39 |
| Acute exposure HR (95% CI) | — | — | — | — | — |
| Last 4 wk of pregnancy | 0.96 (0.92, 1.00) | 0.97 (0.90, 1.05) | 0.85 (0.73, 1.00) | 0.96 (0.86, 1.08) | 0.65 |
| Same day of delivery | 0.97 (0.95, 1.00) | 0.99 (0.96, 1.02) | 1.01(0.99, 1.03) | 1.02 (1.01, 1.04) | 0.04 |
| Term birth weight (95% CI) | |||||
| First trimester | 5.25 (, 17.13) | (, 7.19) | (, 5.12) | 4.48 (, 14.22) | 0.55 |
| Second trimester | 2.71 (, 12.90) | 3.35 (, 10.84) | 2.99 (, 14.29) | (, 8.44) | 0.95 |
| Third trimester | 5.88 (, 15.37) | 8.50 (1.26, 15.74) | 9.42 (, 21.03) | 8.23 (, 17.04) | 0.62 |
| Whole pregnancy | 10.31 (, 25.90) | 8.94 (, 20.89) | 6.04 (, 22.41) | 10.46 (, 24.39) | 0.71 |
Note: Estimates of association are for IQR increases in exposure to (). Models represent pooled city-specific estimates derived using two-stage random-effects meta-analysis and logistic regression [term low birth weight (LBW) and ORs for preterm birth in association with chronic exposures], Cox proportional hazard models (HRs for preterm birth in association with IQR increases in exposure to () during the last 4 wk of pregnancy or on the day of delivery), or linear regression (term birth weight). Models were adjusted for maternal age at delivery, marital status, maternal cigarette smoking during pregnancy, infant sex, parity, previous caesarean section delivery, maternal comorbidities (i.e. asthma, hypertension, type 1 and 2 diabetes mellitus, preeclampsia, and gestational diabetes), year of birth, month of birth, census dissemination-area (DA) median family income, census DA proportion of population who are visible minority, and census DA proportion of the adult female population aged 25–64 y old who completed postsecondary education; gestational age was also included in term LBW and term birth weight models; mean temperature and mean relative humidity were also included in preterm birth acute exposure models. Random-effects multivariate meta-regression models were used to test potential effect modification by between-city differences in . The outcome variables in the meta-regression models in this study were the pooled estimates, and the explanatory variable (i.e. potential effect modifier) was the categorical variable of at the city level. Effect modification was considered statistically significant if the effect modifier’s p-value was less than 0.05. —, data not available; CI, confidence interval; HR, hazard ratio; OR, odds ratio; , fine particulate matter air pollution with aerodynamic diameter ; , ascorbate-related oxidative burden; , glutathione-related oxidative burden; , beta coefficient.
Figure 4.
Concentration–response curves using natural cubic splines with 3 degrees of freedom for the association between exposure to fine particulate matter air pollution with aerodynamic diameter () during the first trimester and term low birth weight, stratified according to below 25th percentile of glutathione-related oxidative potential () (solid line) and above the 75th percentile of (dashed line) in Ontario, Canada (2006–2012). Dotted lines reflect 95% confidence intervals. Models were adjusted for maternal age at delivery, marital status, maternal cigarette smoking during pregnancy, infant sex, parity, previous caesarean section delivery, maternal comorbidities (i.e., asthma, hypertension, type 1 and 2 diabetes mellitus, preeclampsia, and gestational diabetes), year of birth, month of birth, census dissemination-area (DA) median family income, census DA proportion of population who are visible minority, census DA proportion of the adult female population aged 25–64 y old who completed postsecondary education, and gestational age.
Figure 5.
Associations between daily fine particulate matter air pollution with aerodynamic diameter () levels (per interquartile range increase) over the last week of pregnancy and preterm birth, stratified according to below 25th percentile of glutathione-related oxidative potential () (left) and above the 75th percentile of (right) in Ontario, Canada (2006–2012). Solid lines reflect point estimates, and gray areas reflect 95% confidence intervals. Models were adjusted for maternal age at delivery, marital status, maternal cigarette smoking during pregnancy, infant sex, parity, previous caesarean section delivery, maternal comorbidities (i.e., asthma, hypertension, type 1 and 2 diabetes mellitus, preeclampsia, and gestational diabetes), year of birth, month of birth, census dissemination-area (DA) median family income, census DA proportion of population who are visible minority, and census DA proportion of the adult female population aged 25–64 y old who completed postsecondary education, mean temperature, and mean relative humidity.
Results from the analysis of effect modification by regional oxidative potential measures are also illustrated in Table S8, with a comparison of statistics from the simple multivariate random- effects meta-analysis (i.e., no meta-predictor) and multivariate random-effects meta-regressions with a single meta-predictor (i.e., and ) each time. Tests for effect modification by regional oxidative potential were statistically significant for exposures during the first trimester and the whole-pregnancy periods () when evaluating associations between mass concentrations and term LBW. As well, regional oxidative potential modified the association between exposure to mass concentrations on the same day of delivery and risk of preterm birth. Cochran Q test results did not provide evidence for heterogeneity in models for term LBW, term birth weight, and acute exposures on preterm birth (p-value for Q tests ). AA-related oxidative potential was not an effect modifier in models for term LBW and preterm birth. In sensitivity analyses, additional adjustment for temperature and humidity in birth weight models did not change estimates, and therefore, these variables were not included in models investigating these outcomes (Table S9).
Discussion
In this study, exposure to ambient was positively associated with LBW among term births in Ontario during 2006–2012, consistent with previous studies (Dadvand et al. 2013; Li et al. 2017; Shah et al. 2011; Stieb et al. 2012). In addition, our findings suggest that between-city differences in the GSH-related oxidative potential of may modify associations of ambient with term LBW and preterm birth. To our knowledge, this is the first epidemiological study to examine how between-city differences in oxidative potential may modify associations between exposure to ambient mass concentrations during pregnancy and adverse birth outcomes.
Prenatal exposure to ambient particulate air pollution has been previously associated with term LBW in many studies, while the evidence for an association with preterm birth is more inconsistent (Stieb et al. 2012). For example, in a previous study in Ontario, we found that an IQR increase () in assigned through satellite measurements over the entire pregnancy was associated with a 4% (95% CI: 2.4, 5.6) increased odds of preterm birth, but not LBW (Lavigne et al. 2016b), while in a national study, was associated with reduced term birth weight but not preterm birth (Stieb et al. 2016). While the exact mechanism(s) linking ambient air pollution and adverse birth outcomes has not been clearly elucidated, evidence suggests that oxidative stress may have an important influence (Duhig et al. 2016). Effects of oxidative stress on enzymatic antioxidants may contribute to adverse birth outcomes such as infertility, miscarriage, preeclampsia, intrauterine growth restriction, and preterm delivery (Duhig et al. 2016; Poston and Raijmakers 2004). In this study, an IQR increase in GSH-related oxidative burden was more strongly associated with term LBW than an IQR increase in mass concentration alone. This suggests that regional differences in GSH-related oxidative potential may play an important role in explaining between-city differences in the effects of exposure to ambient during pregnancy on adverse birth outcomes.
During normal pregnancy, increased energy expenditure and altered physiological processes are associated with increased levels of oxidative stress (Nagiah et al. 2015; Risom et al. 2005). However, several antioxidants will also increase in concentration during gestation in order to protect the mother and the fetus from free radicals. GSH is an important antioxidant for the developing fetus and the mother. In particular, GSH has an important role in detoxifying pollutants in the placenta before they reach the developing child (Mistry and Williams 2011). Maternal serum and placental levels of GSH have been inversely associated with miscarriage, preterm labor, birth defects, and other pregnancy complications (Agarwal et al. 2012; Poston and Raijmakers 2004). GSH concentrations were lower in peripheral lymphocytes collected during the third trimester from 50 pregnant women attending antenatal clinics in an industrialized area than in samples from 50 pregnant women attending clinics in a less industrialized area, which suggests that exposure to air pollution during pregnancy may increase oxidative stress (Nagiah et al. 2015). Therefore, findings from our study are supported by the importance of the biological function of GSH during pregnancy.
We also observed that regional differences in GSH-related oxidative potential may modify the effect of exposure to ambient on the same day of delivery on the risk of preterm birth. Previous studies have found associations between short-term exposure to ambient air pollution and risk of preterm delivery (Li et al. 2016; Zhao et al. 2011). The potential mechanism could involve oxidative stress, which is reflected with the effect modification by observed in this study.
AA is also an important antioxidant in plasma during pregnancy, and reduced concentrations have been associated with adverse pregnancy outcomes (Mistry and Williams 2011). However, between-city differences in AA-related oxidative potential did not modify the relationship between and adverse birth outcomes investigated. Reasons for this are not entirely clear, but the low correlation between and suggests that these metrics are capturing different sources. It could also be related to maternal dietary intake or supplementation of vitamin C that counteracts the oxidative stress associated with exposure to fine particulate matter. In fact, one study found that an adequate dietary intake of vitamin C during pregnancy attenuated levels of oxidative stress associated with exposure to ambient polycyclic aromatic hydrocarbons (Kim et al. 2011). Alternatively, depletion of AA concentrations in the lung lining fluid following the inhalation of air pollutants may not translate into lower plasma AA concentration, which is a recognized marker for overall antioxidant capacity during pregnancy (Richter et al. 2012). Future studies should aim to clarify this issue.
A number of limitations need to be acknowledged in this study. First, while we included several important confounding factors, we cannot rule out potential residual confounding by unmeasured individual risk factors such as maternal body mass index, alcohol consumption, ethnicity, income, and education. Secondly, the oxidative potential data were collected after the study period and assumed to have remained constant, which may increase the likelihood of exposure measurement error and may impact our assessment of between-city differences in oxidative potential. Also, the fact that city-level oxidative potential data were based on a relatively short time period () likely also contributed to this uncertainty. Another limitation is related to the fact that city-level estimates of oxidative potential were based on measurements collected from fixed-site monitors, and thus, our analyses do not account for spatial differences in or in oxidative potential within cities, although we did restrict our analysis to mothers living within of monitoring sites, and is generally homogenous over this spatial scale (Bari and Kindzierski 2017). In fact, recent evidence suggests that spatial variations in oxidative potential are greater than for mass concentrations (Yang et al. 2015). This may hinder direct comparisons in effect estimates per IQR increases in pollutant concentrations for mass concentrations and oxidative burden. We were also not able to account for temporal (i.e., day-to-day) changes in oxidative potential, which may be particularly relevant for the investigation of preterm birth as an outcome. The loss of volatile components from TEOM filters likely underestimated estimates of city-level oxidative potential, in particular in regions with a higher proportion of volatile components in ; this effect would more likely reduce rather than increase variability in oxidative burden between sites. In fact, the losses of volatile and semivolatile species in the heated TEOM inlet and the impact of these species on PM OP were previously raised when oxidative potential (OP) measurements were conducted on TEOM filters by the King’s College London group as part of study funded by the Health Effects Institute (Kelly et al. 2011). It should be noted that similar to our study, the King’s College London group also used the respiratory tract-lining fluid (RTLF) assay to measure PM OP. To address these concerns, the King’s College London group measured the OP of filter samples collected using a TEOM and a filter dynamics measurement system (FDMS) that were operated in parallel. The FDMS microbalance uses a Nafion dryer, (Ion Power Inc.) to remove water from the inlet air stream. The FDMS alternates between two sampling modes every 6 min; air is first collected on a microbalance maintained at 30°C to determine a base concentration and then directed through a filter held at 4°C prior to the microbalance to measure the purge concentration. OP was measured for FDMS filters used to measure the base and purge concentrations to explore the contribution of volatile and semivolatile species to the measured OP of PM. No differences were found between AA- or GSH-related OP measured across the 4°C (FDMS), 30°C (FDMS), and 50°C (TEOM) collected filters. This suggests volatile and semivolatile species do not strongly contribute to the PM OP measured using the RTLF assay. We acknowledge that the contribution of liable reactive oxygen species and other transient radicals to the measured OP is likely to be minimal in archived filter samples. We opportunistically measured oxidative potential using PM extracted from TEOM filters. These PM-loaded filters were stored at room temperature following removal from the TEOM. As the TEOM collected PM from a heated air stream (50°C), we rationalized that most volatile and semivolatile species would have already been lost. The remaining oxidative activity of PM constituents, primarily metals, would be stable over the storage period. It should be noted that PM samples were extracted and suspended to a mass concentration based on the mass measured pre- and postextraction. Moreover, the TEOM filter samples collected for this study were not analyzed for metal concentration, given the limited availability of PM material, which restricted this additional chemical analysis. We have incorporated metals analysis in our expanded analysis of TEOM filters collected from locations across Canada. Also, it is plausible that the OP of is sensitive to interactions with ambient , , and PM collected on the filter over the sampling periods, but this issue was not investigated in this study. Future studies should capture both spatial and temporal differences in oxidative potential and should capture the specific components and sources of fine particulate air pollution that are mostly associated with oxidative stress. Notable strengths of this study include the large sample size, the ability to estimate effects at comparatively low levels of exposure, and detailed city-level oxidative potential data over a broad geographic area.
Conclusions
In this multicity study in the most populated province of Canada, we found initial evidence that between-city differences in GSH-related oxidative potential may modify the impact of on the risk of term LBW and preterm birth. This finding may be helpful in prioritizing risk management activities aimed at reducing the public health impacts of particulate air pollution.
Supplemental Material
Acknowledgments
We would like to thank F. Mansoor for conducting the oxidative burden analyses and C. McMann, T. Shin, and H. You for their help in compiling oxidative burden data. We thank L. White (Environment Canada) and the Ontario Ministry of the Environment and Climate Change for their help in collecting filters for oxidative burden analyses. We also thank B. Jessiman and K. Van Ryswyk for reviewing a previous version of this paper.
This study was funded by Health Canada.
References
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. 2012. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:49, PMID: 22748101, 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, et al. 2008. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential–a workshop report and consensus statement. Inhal Toxicol 20(1):75–99, PMID: 18236225, 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Bari MA, Kindzierski WB. 2017. Characterization of air quality and sources of fine particulate matter (PM2.5) in the city of Calgary, Canada. Atmos Pollut Res 9(3):534–543, 10.1016/j.apr.2017.11.014. [DOI] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to Meta-Analysis. Chichester, UK:John Wiley & Sons, Ltd. [Google Scholar]
- Bratsch SG. 1989. Standard electrode potentials and temperature coefficients in water at 298.15 K. J Phys Chem Ref Data 18(1):1, 10.1063/1.555839. [DOI] [Google Scholar]
- Chang HH, Reich BJ, Miranda ML. 2012. Time-to-event analysis of fine particle air pollution and preterm birth: results from North Carolina, 2001–2005. Am J Epidemiol 175(2):91–98, PMID: 22167746, 10.1093/aje/kwr403. [DOI] [PubMed] [Google Scholar]
- Chang HH, Reich BJ, Miranda ML. 2013. A spatial time-to-event approach for estimating associations between air pollution and preterm birth. J R Stat Soc Ser C Appl Stat 62(2):167, PMID: 24353351, 10.1111/j.1467-9876.2012.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. 2013. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect 121(3):267–373, PMID: 23384584, 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Strickland MJ, Klein M, Waller LA, Flanders WD, Correa A, et al. 2009. Seasonality of birth and implications for temporal studies of preterm birth. Epidemiology 20(5):699–706, PMID: 19535987, 10.1097/EDE.0b013e3181a66e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM. 2013. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J Expo Sci Environ Epidemiol 23(5):466–473, PMID: 23673461, 10.1038/jes.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhig K, Chappell LC, Shennan AH. 2016. Oxidative stress in pregnancy and reproduction. Obstet Med 9(3):113–116, PMID: 27630746, 10.1177/1753495X16648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S, Bottomley J, Ali A, Walker M. 2011. 2008 Niday Perinatal database quality audit: report of a quality assurance project. Chronic Dis Inj Can 32(1):32–42, PMID: 22153174. [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B. 2013. Reducing and meta-analysing estimates from distributed lag non-linear models. BMC Med Res Methodol 13:1, PMID: 23297754, 10.1186/1471-2288-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2012. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med 31(29):3821–3839, PMID: 22807043, 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godri KJ, Duggan ST, Fuller GW, Baker T, Green D, Kelly FJ, et al. 2010. Particulate matter oxidative potential from waste transfer station activity. Environ Health Perspect 118(4):493–498, PMID: 20368130, 10.1289/ehp.0901303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godri KJ, Harrison RM, Evans T, Baker T, Dunster C, Mudway IS, et al. 2011. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and urban background schools sites in London. PLoS One 6(7):e21961, 10.1371/journal.pone.0021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Chang HH, Holmes HA, Mulholland JA, Klein M, Darrow LA, et al. 2016. Air pollution and preterm birth in the U.S. state of Georgia (2002–2006): associations with concentrations of 11 ambient air pollutants estimated by combining Community Multiscale Air Quality Model (CMAQ) simulations with stationary monitor measurements. Environ Health Perspect 124(6):875–880, PMID: 26485731, 10.1289/ehp.1409651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558, PMID: 12111919, 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Janssen NA, Yang A, Strak M, Steenhof M, Hellack B, Gerlofs-Nijland ME, et al. 2014. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci Total Environ 472:572–581, PMID: 24317165, 10.1016/j.scitotenv.2013.11.099. [DOI] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A. 2006. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect 114(11):1636–1642, PMID: 17107846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ. 2003. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60(8):612–616, PMID: 12883027, 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F, Anderson HR, Armstrong B, Atkinson R, Barratt B, Beevers S, et al. 2011. The impact of the congestion charging scheme on air quality in London. Part 2. Analysis of the oxidative potential of particulate matter. Res Rep Health Eff Inst 155:73–144, PMID: 21830497. [PubMed] [Google Scholar]
- Kim H, Hwang JY, Ha EH, Park H, Ha M, Lee SH, et al. 2011. Fruit and vegetable intake influences the association between exposure to polycyclic aromatic hydrocarbons and a marker of oxidative stress in pregnant women. Eur J Clin Nutr 65(10):1118–1125, PMID: 21587280, 10.1038/ejcn.2011.77. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. 2001. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108(2):E35, PMID: 11483845, 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- Künzli N, Mudway IS, Götschi T, Shi T, Kelly FJ, Cook S, et al. 2005. Comparison of oxidative properties, light absorbance, and total and elemental mass concentration of ambient PM2.5 collected at 20 European sites. Environ Health Perspect 114(5):684–690, PMID: 16675421, 10.1289/ehp.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Gasparrini A, Stieb DM, Chen H, Yasseen AS 3rd, Crighton E, et al. 2016a. Maternal exposure to aeroallergens and the risk of early delivery. Epidemiology 28(1):107–115, PMID: 27748684, 10.1097/EDE.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Yasseen AS 3rd, Stieb DM, Hystad P, van Donkelaar A, Martin RV, et al. 2016b. Ambient air pollution and adverse birth outcomes: differences by maternal comorbidities. Environ Res 148:457–466, PMID: 27136671, 10.1016/j.envres.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Lavigne É, Bélair MA, Do MT, Stieb DM, Hystad P, van Donkelaar A, et al. 2017. Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario, Canada. Environ Int 100:139–147, PMID: 28108116, 10.1016/j.envint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Li S, Guo Y, Williams G. 2016. Acute impact of hourly ambient air pollution on preterm birth. Environ Health Perspect 124(10):1623–1629, PMID: 27128028, 10.1289/EHP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, et al. 2017. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut 227:596–605, PMID: 28457735, 10.1016/j.envpol.2017.03.055. [DOI] [PubMed] [Google Scholar]
- Maikawa CL, Weichenthal S, Wheeler AJ, Dobbin NA, Smargiassi A, Evans G, et al. 2016. Particulate oxidative burden as a predictor of exhaled nitric oxide in children with asthma. Environ Health Perspect 124(10):1616–1622, PMID: 27152705, 10.1289/EHP175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry HD, Williams PJ. 2011. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev 2011:841749, PMID: 21918714, 10.1155/2011/841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah S, Phulukdaree A, Naidoo D, Ramcharan K, Naidoo RN, Moodley D, et al. 2015. Oxidative stress and air pollution exposure during pregnancy: a molecular assessment. Hum Exp Toxicol 34(8):838–847, PMID: 25403174, 10.1177/0960327114559992. [DOI] [PubMed] [Google Scholar]
- Poston L, Raijmakers MT. 2004. Trophoblast oxidative stress, antioxidants and pregnancy outcome–a review. Placenta 25 (Suppl A):S72–S78, PMID: 15033311, 10.1016/j.placenta.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Richter HG, Camm EJ, Modi BN, Naeem F, Cross CM, Cindrova-Davies T, et al. 2012. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol 590(6):1377–1387, PMID: 22289909, 10.1113/jphysiol.2011.226340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risom L, Møller P, Loft S. 2005. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592(1–2):119–137, PMID: 16085126, 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. 2008. Modern Epidemiology. Philadelphia, PA:Lippincott, Williams & Wilkins, 262–263. [Google Scholar]
- Schlesinger RB, Kunzli N, Hidy GM, Gotschi T, Jerrett M. 2006. The health relevance of ambient particulate matter characteristics: Coherence of toxicological and epidemiological inferences. Inhal Toxicol 18(2):95–125, PMID: 16393926, 10.1080/08958370500306016. [DOI] [PubMed] [Google Scholar]
- Shah PS, Balkhair T, Knowledge Synthesis Group on Determinants of Preterm/LBW births. 2011. Air pollution and birth outcomes: a systematic review. Environ Int 37(2):498–516, PMID: 21112090, 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Beckerman BS, Jerrett M, Crouse DL, Omariba DW, et al. 2016. Associations of pregnancy outcomes and PM2.5 in a national Canadian study. Environ Health Perspect 124(2):243–249, PMID: 26090691, 10.1289/ehp.1408995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. 2012. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 117:100–111, PMID: 22726801, 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S. 2011. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol 11:49, PMID: 21501523, 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S. 2012. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol 175(2):99–107, PMID: 22167749, 10.1093/aje/kwr404. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. 2006. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: a multilevel analysis of Massachusetts births, 1989-1991. Am J Epidemiol 164(9):823–834, PMID: 16968866, 10.1016/j.envres.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Crouse DL, Pinault L, Godri-Pollitt K, Lavigne E, Evans G, et al. 2016a. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Res 146:92–99, PMID: 26745732, 10.1016/j.envres.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Weichenthal SA, Lavigne E, Evans GJ, Godri Pollitt KJ, Burnett RT. 2016c. Fine particulate matter and emergency room visits for respiratory illness: effect modification by oxidative potential. Am J Respir Crit Care Med 194(5):577–586, PMID: 26963193, 10.1164/rccm.201512-2434OC. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT. 2016b. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environ Health 15:46, PMID: 27012244, 10.1186/s12940-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Atkinson RW, Anderson HR, Kelly FJ. 2014. Associations between daily mortality in London and combined oxidant capacity, ozone and nitrogen dioxide. Air Qual Atmos Health 7(4):407–414, PMID: 25431629, 10.1007/s11869-014-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Wang M, Eeftens M, Beelen R, Dons E, Leseman DL, et al. 2015. Spatial variation and land use regression modeling of the oxidative potential of fine particles. Environ Health Perspect 123(11):1187–1192, PMID: 25840153, 10.1289/ehp.1408916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanosky JD, Tonne CC, Beevers SD, Wilkinson P, Kelly FJ. 2012. Modeling exposures to the oxidative potential of PM10. Environ Sci Technol 46(14):7612–7620, PMID: 22731499, 10.1021/es3010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Staimer N, Gillen DL, Tjoa T, Schauer JJ, Shafer MM, et al. 2016. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ Res 150:306–319, PMID: 27336235, 10.1016/j.envres.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Liang Z, Tao S, Zhu J, Du Y. 2011. Effects of air pollution on neonatal prematurity in Guangzhou of China: a time-series study. Environ Health 10:2, PMID: 21214958, 10.1186/1476-069X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.