Abstract
Human breast cancer is a malignant type of cancer with high prevalence. In the present study, the anticancer effects of alantolactone, a sesquiterpene lactone, on the human breast cancer cell line MF-7 were investigated in vitro. The MCF-7 cell morphology changed from diamond to round subsequent to treatment with alantolactone, and the cell viability reduced significantly compared with that of the control cells. Alantolactone induced apoptosis of MCF-7 cells by regulating the protein expression levels of B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein, p53, caspase-3 and caspase-12, which are associated with the apoptotic pathway, and suppressed colony formation and migration by regulating the protein expression of matrix metalloproteinase (MMP)-2, MMP-7 and MMP-9. Cell signaling pathway analysis confirmed that alantolactone increased the phosphorylation of p38, and decreased the nuclear expression levels of p65 and nuclear factor erythroid 2-related factor 2 (Nrf2), suggesting that the apoptosis-promoting and migration-suppressing effect of alantolactone may partially depend on regulating the p38 MAPK, NF-κB and Nrf2 pathways. These results also suggested that alantolactone may become a potential therapeutic strategy for treating breast cancer.
Keywords: alantolactone, anticancer, metastasis, apoptosis, p38 mitogen-activated protein kinase, nuclear factor-KB
Introduction
Cancer is a global health problem responsible for one in four mortalities worldwide (1,2). According to the World Health Organization, breast cancer accounts for approximately 1/3 of all cancer cases diagnosed in women (3). There are a number of risk factors for breast cancer, including hereditary factors, abnormal hormone levels, smoking history and alcohol consumption (4,5). Breast cancer is typically treated using chemotherapy, biological immune treatments and traditional Chinese medicine, however, there is no definitive therapeutic target for breast cancer treatment (6). The treatment options used currently are accompanied by a number of side effects, and thus a great deal of research has been dedicated to identifying novel drugs with the therapeutic potential in breast cancer in recent years (4). However, the pathogenesis of breast cancer remains unknown (6). Much attention had been given to the medicinal value of natural compounds, and plant-based drugs have been successfully used in the treatment of cancer and other diseases (7-9). Herbal drugs, such as phenolic acids, flavonoids and sesquiterpene, have been demonstrated to be cytotoxic to cancer cells, acting via a range of mechanisms (4).
Inula Helenium L. is a flower of the Compositae herbaceous family, whose roots have historically been used as a medicine (10). This plant has been investigated since the 1970s, and certain studies have reported that it contains >40 different compounds, with the terpenoids being the main components (11-13). Alantolactone is a sesquiterpene lactone extracted from Inula Helenium L. that exhibits a number of biological effects, including anti-inflammatory, antibacterial and anticancer activities (14,15). It has previously been reported that alantolactone induces apoptosis in a number of cancer cell lines, including in the colorectal cancer RKO cell line via the mitogen-activated protein kinase (MAPK) pathway (10), and in the liver cancer HepG2 cell line via regulating the nuclear factor (NF)-κB signaling pathway (15). Alantolactone has also been demonstrated to inhibit cell cycle progression in SK-MES-1 cells (16). This compound may therefore be a promising candidate as a chemotherapeutic drug for cancer therapy (17).
Although the effects of alantolactone have been studied in a variety of human cancer cell lines, the potential anticancer activity of alantolactone in human breast cancer remains unclear. The aim of the present study was to investigate the function and molecular mechanisms of alantolactone in repressing cell proliferation and inducing apoptosis in MCF-7 cells, in order to provide a theoretical basis for its use as a clinical cancer treatment.
Materials and methods
Chemicals and materials
Alantolactone (purity, 99%) was purchased from Chengdu Must Bio-Technology Co., Ltd. (Chengdu, China). Acrylamide, penicillin, streptomycin, phosphate-buffered saline (PBS), Carnoy's solution, Giemsa, BCA kit, enhanced chemiluminescence (ECL) and dimethyl sulfoxide (DMSO) were obtained from Beijing Dingguo Changsheng Biotechnology Co., Ltd. (Beijing, China). Hoechst 33258 staining kit and Annexin V-FITC cell apoptosis assay kits were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Paraformaldehyde (4%), and antibodies against B-cell lymphoma 2 (Bcl-2; cat. no. sc-509), Bcl-2-associated X protein (Bax; cat. no. sc-23959), p53 (cat. no. sc-71820), matrix metalloproteinase (MMP)-2 (cat. no. sc-13594), MMP-7 (cat. no. sc-80205), MMP-9 (cat. no. sc-21733), nuclear factor erythroid 2-related factor 2 (Nrf2; cat. no. sc-518033), proliferating cell nuclear antigen (PCNA; cat. no. sc-25280), caspase-3 (cat. no. sc-271028) and caspase-12 (cat. no. sc-21747) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-p38 (cat. no. sc-271028), anti-phospho-p38 (cat. no. 9215), phospho-inhibitor of NF-κB (p-IκBα; cat. no. 2859), IκBά (cat. no. 9247), anti-NF-κB (p65) antibodies (cat. no. 8242) and β-actin (cat. no. 4970S) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Fetal bovine serum, radioimmunoprecipiation (RIPA) lysis buffer, nuclear protein extraction kit, horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat. no. SE134) and HRP-conjugated goat anti-mouse IgG secondary antibodies (cat. no. SE131) were obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Plates (6 cm in diameter) were obtained from Corning Incorporated (Corning, NY, USA). Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco (Thermo Fisher Scientific. Inc., Waltham, MA, USA).
Cell culture
The human breast cancer cell line MCF-7 was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China), and cultured in Dulbecco's modified Eagle's medium containing 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. MCF-7 cells were maintained at 37°C in an incubator with 5% CO2. Subculture was performed when cells reached 80-90% confluence (18).
Cell proliferation assay
The harvested cells were seeded at a density of 3×103 cells/well in a 96-well plate. MCF-7 cells were treated with various concentrations of alantolactone or with 10 mg/ml fluorouracil (5-Fu) for 24 and 48 h, following which cells were stained with 20 μ1 MTT solution for 4 h at 37°C. The medium was removed from each well, and the purple formazan crystals were dissolved in 150 μ1 DMSO for 10 min using vibration. Absorbance was assessed at 490 nm using a microplate reader (19). 5-Fu was used to determine the inhibitory effect of alantolactone on MCF-7 cells. Cells were observed and images were captured under an Olympus CX22LED microscope (magnification, ×10; Olympus Corporation, Tokyo, Japan) at 24 and 48 h, the adherent cells were counted. All measurements were performed at least in triplicate. MCF-7 cells treated only with cell medium were used as the control.
Apoptosis assay
Annexin V-FITC/PI double staining was used to determine the percentage of apoptotic MCF-7 cells. Briefly, cells were treated with 10, 20 and 30 μM alantolactone for 24 h and washed twice with PBS, then, 5 μ1 Annexin V-FITC and PI were added to the cells for 15 min (20). The percentage of apoptotic MCF-7 cells was assessed using flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA). MCF-7 cells treated only with cell medium were used as the control.
Hoechst 33258 fluorescence staining
MCF-7 cells were seeded at a density of 8×104 cells/well in 12-well plates, then the cells were treated with 10, 20 and 30 μM alantolactone for 24 h. Next, cells were obtained and fixed in 4% paraformaldehyde solution for 30 min. The cells were then washed twice with Buffer A from a Hoechst 33258 staining kit, and stained with 30 μl Hoechst 33258 for 10 min in the dark and observed under a fluorescence microscope (magnification, ×20) (21). MCF-7 cells treated only with cell medium were used as the control.
Wound-healing assay
When the cells reached 80-90% confluence, the cell layer was scratched with a 10 μl sterile pipette tip and the wells were washed twice with PBS. Subsequently, 10, 20 and 30 μM alantolactone was added to the medium, and cell migration was observed under a microscope (magnification, ×10) at 24 and 48 h. MCF-7 cells treated only with cell medium were used as the control.
Colony-forming assay
Cells were seeded at a density of 1×103 cells/plate and cultured with 5 ml medium for 24 h. Next, cells treated with 10, 20 and 30 μM alantolactone were left to cultivate for 15 days until visible colonies formed (22). Following fixation with Carnoy's solution for 30 min and staining with 5 ml Giemsa stain for 10 min, the number of cell colonies was counted. MCF-7 cells treated only with cell medium were used as the control.
Western blot assay
Following treatment with alantolactone for 24 h, cells were harvested and lysed with a RIPA lysis buffer for total protein and the nuclear protein was prepared with nuclear protein extraction kit. Protein concentrations were measured using a BCA protein assay. Equal amounts of protein were separated by 12% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk for 1 h at room temperature, and then incubated with the primary antibodies (1:2,000) overnight at 4°C, followed by incubation with the secondary antibodies for 1 h at room temperature. Finally, bands were observed using an enhanced chemiluminescence kit. The relative level of total protein was normalized to β-actin and the relative level of nuclear protein was normalized to PCNA. The gray intensity was analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA). MCF-7 cells treated only with cell medium were used as control.
Statistical analysis
Data are presented as the mean ± standard deviation of at least three independent experiments. Statistical analyses were performed using one-way analysis of variance with SPSS software, version 20.0 (IBM SPSS, Armonk, NY, USA). Comparisons between groups were assessed using a post hoc Tukey's test and correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of alantolactone on cell morphology and viability
An MTT assay was used to further verify the inhibitory effect of alantolactone on cell proliferation. As shown in Fig. 1A, treatment with different concentrations of alantolactone (5, 10, 20, 30, 40 and 80 μΜ) for 24 and 48 h significantly reduced the cell viability in a concentration- and time-dependent manner compared with the control group and the cell viability at 40 μΜ was similar to the group treated with 5-Fu. The half maximal inhibitory concentration (IC50) was 35.45 μΜ at 24 h and 24.29 μΜ at 48 h. The number of adherent cells decreased with alantolactone treatment in a concentration- and time-dependent manner (Fig. 1B). Therefore, the concentrations of 10, 20 and 30 μΜ and the treatment time of 24 h were selected for subsequent experiments.
Figure 1.
Effects of alantolactone on MCF-7 cell proliferation. (A) Cell viability was determined by an MTT assay. (B) The percentage of adherent cells was expressed as the percentage of the control. Data are expressed as the mean ± standard deviation from three independent experiments. Group with the same letter were not significantly different. Groups with different letters were significantly different, P<0.05. 5Fu, fluorouracil.
Alantolactone induces apoptosis in MCF-7 cells
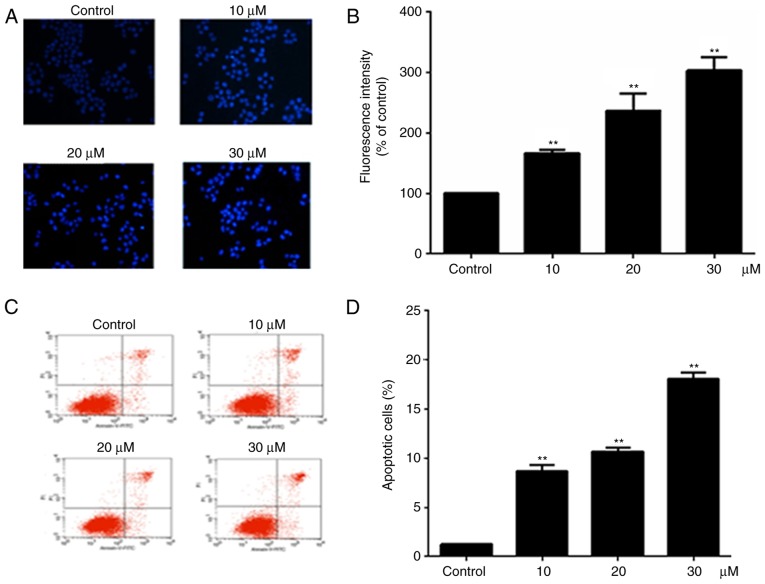
Hoechst 33258 staining was used to examine the cell apoptosis, since the nuclei of apoptotic cells exhibit fluorescent blue staining, whereas live cells have uniformly light blue nuclei. Cells were treated with different concentration of alantolactone (10, 20 and 30 μΜ) for 24 h, and the number of live cells was reduced as the concentration of alantolactone increased. The treated cells exhibited brighter fluorescence in comparison with the control cells, and the fluorescence intensity of treated cells decreased significantly as the concentration of alantolactone increased (Fig. 2B). Furthermore, the percentage of apoptotic cells was determined using the Annexin V-FITC/PI double staining method. It was observed that the percentage of apoptotic cells was increased following treatment with alantolactone for 24 h in a concentration-dependent manner compared with that observed in the control cells (Fig. 2C and D).
Figure 2.
Induction of apoptosis in MCF-7 cells with alantolactone. (A) Fluorescence images and (B) fluorescence intensity of MCF-7 cells subjected to Hoechst 33258 staining of nuclei following treatment with various concentrations of alantolactone for 24 h (magnification, ×20). (C) Flow cytometry and (D) percentage of apoptotic cells examined by Annexin V-FITC and PI assay following treatment with various concentrations of alantolactone for 24 h. Alantolactone induces early/late apoptosis in breast cancer. Data are expressed as the mean ± standard deviation. **P<0.01, vs. control group.
Alantolactone inhibits the migration of MCF-7 cells
The effect of alantolactone on MCF-7 cell migration was analyzed using a wound healing assay. Compared with the control group, alantolactone significantly decreased cell migration in a dose- and time-dependent manner (P<0.05; Fig. 3A and B).
Figure 3.
Inhibition of migration and colony formation ability by alantolactone treated in MCF-7 cells. (A) MCF-7 cells were seeded in 12-well plates, and the cell layer was scratched with a 10 μ1 sterile tip. (B) Cell migration was observed with a microscope (magnification, ×10) at 24 and 48 h. (C) Colony formation and (D) number of colonies in cells were treated with different concentrations (10, 20 and 30 μΜ) of alantolactone and cultivated for 15 days until colonies formed. Data are expressed as the mean ± standard deviation from three independent experiments. Group with the same letter were not significantly different. Groups with different letters were significantly different, P<0.05.
Colony-forming assay
Compared with the control cells, colony formation was significantly lower in cells treated with alantolactone (P<0.01). The colony formation was inhibited by alantolactone in a concentration-dependent manner (Fig. 3C and D).
Expression levels of apoptosis- and migration-associated proteins
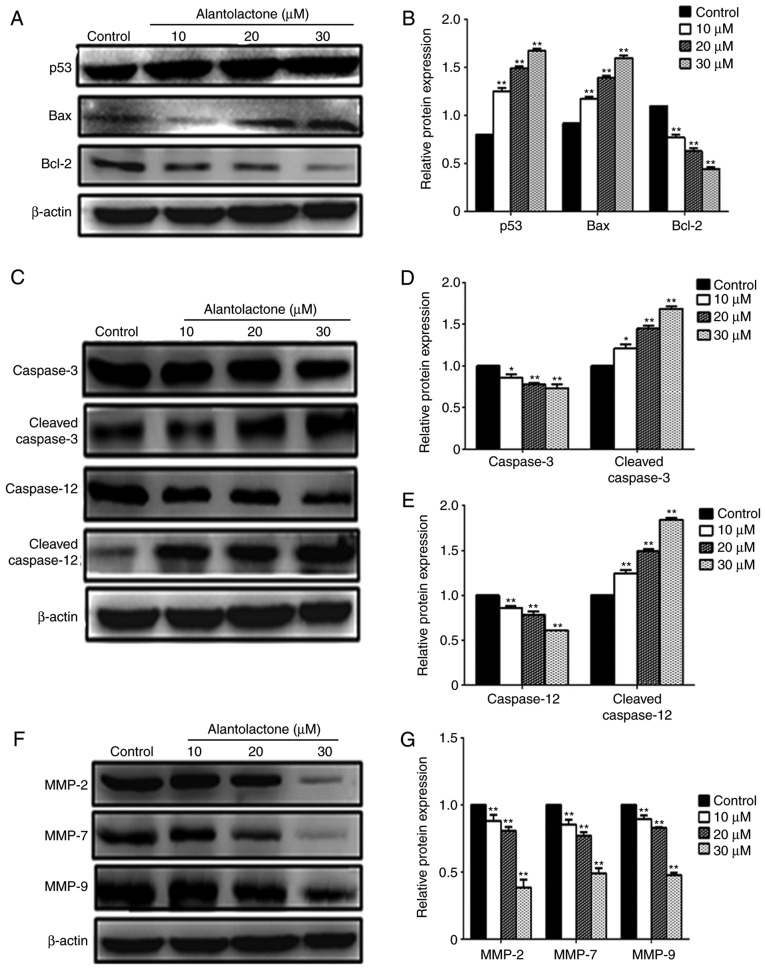
Following treatment with alantolactone, the expression levels of p53, Bax and Bcl-2 protein in MCF-7 cells were measured using western blotting. Treatment with 10, 20 or 30 μΜ alantolactone significantly reduced the expression of Bcl-2 and significantly increased the expression levels of Bax and p53 compared with those in control cells (Fig. 4A and B). Alantolactone also decreased the expression of the caspase precursor and significantly enhanced the expression of cleaved-caspase-3 and cleaved-caspase-12 in a concentration-dependent manner (P<0.05 and P<0.01; Fig. 4C–E). In addition, alantolactone at 10, 20 and 30 μΜ significantly downregulated the expression levels of MMP-2, MMP-7 and MMP-9 protein (Fig. 4F and G).
Figure 4.
Induction of apoptosis in MCF-7 cells by alantolactone treatment. (A) Western blot analyses and (B) quantified protein expression levels of Bcl-2, Bax and p53 in MCF-7 cells treated with different concentrations of alantolactone for 24 h, compared with the control group. (C) Western blot analyses and quantified protein levels of (D) caspase-3 and cleaved caspase-3, and (E) caspase-12 and cleaved caspase-12. (F) Western blots and (G) quantified protein expression levels of MMP-2, MMP-7, MMP-9 and β-actin. Levels are presented relative to β-actin. *P<0.05 and **P<0.01, vs. control group. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; MMP, matrix metalloproteinase.
Effect of alantolactone on the p38 MAPK signaling pathway
To further explore the mechanism by which alantolactone affects MCF-7 cells, the expression levels of p38 and p-p38 protein were measured. The results revealed that the expression of p-p38 was significantly increased by alantolactone in a dose-dependent manner, while alantolactone had no evident effect on the expression of p38 (Fig. 5A and B).
Figure 5.
Protein expression levels in MCF-7 cells treated with alantolactone. (A) Western blot analyses and (B) quantified levels of p38 and p-p38 in MCF-7 cells treated with different concentrations of alantolactone for 24 h. (C) Western blots, and quantified protein levels of (D) total-p65 and nuclear-p65, and (E) ΙκΒα and p-ΙκΒα. (F) Western blots and (G) quantified protein expression levels of total-Nrf2 and nuclear-Nrf2. Data are expressed as the mean ± standard deviation from three independent experiments, and are presented relative to β-actin level. *P<0.05 and **P<0.01 vs. control group. Nrf, nuclear factor erythroid 2-related factor 2.
Effect of alantolactone on the NF-κB signaling pathway
The expression of p65 protein was measured to explore the role of the NF-κB pathway in alantolactone-mediated MCF-7 cell damage. Treatment with 10, 20 and 30 μΜ alantolactone significantly downregulated the nuclear expression of p65 (Fig. 5C and D). However, at these concentrations, alantolactone had no significant effect on the total expression of p65. The expression of p-ΙκΒα was significantly decreased following treatment with 10, 20 and 30 μM alantolactone, whereas no marked effects on ΙκΒα expression were observed (Fig. 5C and E).
Nrf2 signaling pathway serves a role in alantolactone-regulated proliferation
To investigate the role of the Nrf2 pathway in alantolactone-mediated MCF-7 cell damage, the expression of Nrf2 protein was measured. As shown in Fig. 5F and G, treatment with 10, 20 and 30 μM alantolactone significantly upregulated the nuclear expression of Nrf2, while the total Nrf2 expression was significantly downregulated.
Discussion
Breast cancer is known to be one of the most common malignant types of cancer affecting women, and the morbidity of breast cancer has been increasing since the 1970s (14,21). Recently, a great deal of attention has been paid to the potential of natural compounds as novel treatments for cancer (5). Pharmacological agents extracted from plants have been reported to have good therapeutic effects in the treatment and prevention of cancer (23). Alantolactone, a sesquiterpene lactone compound extracted from Inula Helenium L, had been reported to have anticancer activities (24). In the present study, the biological effect of alantolactone on MCF-7 cells was investigated by measuring the levels of apoptosis markers, colony formation and migration.
To investigate whether alantolactone is able to inhibit MCF-7 cell growth, the cell morphology was observed and cell viability was measured. Alantolactone significantly reduced the viability of MCF-7 cells (Fig. 1A), with an IC50 of 35.45 μM at 24 h and 24.29 μM at 48 h, which indicated that it is a promising compound for clinical application. Previously, Kumari et al (25) reported that the IC50 value of coralyne was 76.4±0.92 μM in MCF-7 cells for 24 h. Nikhil et al (26) also reported that the IC50 value of pterostilbene was 65±0.42 μM in MCF-7 cells for 24 h. Furthermore, tangeretin inhibited the proliferation of MCF-7 cells, and the IC50 value of tangeretin was 39.3±1.5 μM (27). Compared with these natural products, alantolactone is more effective as the IC50 value was lower (25-27).
Changes in the balance between cell proliferation and apoptosis serve a role in a number of diseases (28). Three types of cell death occur, including autophagy, apoptosis and cell necrosis (29). Apoptosis serves a vital role in the evolution of organisms, the stability of internal environments and the development of multiple systems, particularly in cancer development (30). Cancer occurs as a result of insufficient apoptosis (31), and thus apoptosis is a common target for a number of anticancer treatments (32). Alantolactone has been reported to induce apoptosis in various cancer cell lines (33). In the present study, Hoechst 33258 and Annexin V/PI staining were used to detect cell apoptosis, and the results demonstrated that alantolactone significantly increased the percentage of apoptotic MCF-7 cells (Fig. 2), suggesting that alantolactone induces apoptosis in human breast cancer cells. Apoptosis occurs via the extrinsic or intrinsic pathways in mammalian cells, and mitochondria serve an important role in the intrinsic apoptotic process (34). The mitochondrial apoptotic pathway is controlled by the Bcl-2 family proteins, including pro-apoptotic and anti-apoptotic proteins, such as Bax and Bcl-2 (35). Alantolactone is able to induce the apoptosis of HepG2 cells via modulating Bcl-2 family proteins (15). A similar trend was observed in the present study. The results shown in Fig. 4A revealed that alantolactone significantly downregulated the expression of Bcl-2 and significantly upregulated the expression of Bax, suggesting that alantolactone induces apoptosis via the mitochondrial apoptotic pathway. In addition, p53 is critical in the evolution from normal cellular function to tumorigenesis and has been identified as a common mutated cancer suppressor in human tumorigenesis (36). In the present study, p53 expression was increased following treatment with alantolactone, suggesting that p53 may serve an important role in alantolactone-induced MCF-7 cell apoptosis via the cellular apoptotic pathway. The cellular apoptotic pathway is mediated by caspase family proteins, including caspase-3 and cleaved-caspase-3, as well as caspase-12 and cleaved-caspase-12. Alantolactone has the ability to induce apoptosis in HepG2 cells via modulating caspase family proteins (37). The current study results demonstrated that alantolactone significantly enhanced the expression levels of cleaved-caspase-3 and cleaved-caspase-12 proteins. However, the effect of alantolactone on the caspase precursor was weak, suggesting that alantolactone induces cell apoptosis via the apoptotic cellular pathway (Fig. 4C).
Chemotherapy is a commonly used clinical treatment for cancer, however, the risk of recurrence and metastasis remains a problem in patients with breast cancer (38). The majority of cancer-associated mortalities occur as a result of metastatic cancer and tumor growth at distant sites (39). Therefore, the migration and invasion inhibiting effects of plant-based drugs may serve an important role in cancer treatment (40). To further evaluate the anticancer effect of alantolactone in MCF-7 cells, colony formation and migration were assessed in the present study. The results revealed that alantolactone significantly inhibited colony formation and migration in breast cancer cells. MMPs, a major proteinase family associated with tumorigenesis, are key kinases in cell migration during invasive and metastatic processes (4). A number of studies have reported that MMP-2, MMP-7 and MMP-9 are able to degrade the basement membrane and extracellular matrix (18). Therefore, to further investigate the inhibitive effect of alantolactone on the migration and invasion of breast cancer cells, the current study measured the expression levels of MMP-2, MMP-7 and MMP-9. The results (Fig. 4F) revealed that alantolactone significantly downregulated MMP-2, MMP-7 and MMP-9 in MCF-7 cells, and blocked cell migration and invasion.
The pathogenic mechanisms of cancer include changes to signal transduction pathways. As such, molecules involved in abnormal signaling pathways may be targets for cancer treatments (2). MAPK is an important signal transduction pathway that regulates a number of physiological processes and serves an important role in the induction of cell damage (40). Three major MAPK signaling pathways have been identified, including extracellular signal-regulated kinase, c-Jun N-terminal kinase and p38 MAPK pathways (41). MAPK activation leads to the phosphorylation of p38, activates transcription factors and promotes apoptosis (22). In addition, p38 MAPK has been identified as the vital signaling pathway that activates Bax subsequent to its translocation to the mitochondria, and p38 MAPK signaling pathways are responsible for cell proliferation (42). In the current study (Fig. 5A), alantolactone significantly upregulated the phosphorylation of p38, suggesting that the anticancer effects of alantolactone in MCF-7 cells may occur partially via regulating the p38 MAPK pathway.
MAPKs are closely associated with a number of other signaling pathways, including NF-κB (p65), which is a downstream target of p38 MAPK. NF-κB is regarded as an important factor in physiological and pathological processes, including proliferation and apoptosis, and a key regulator of oncogenesis (7). Normally, NF-κB is retained in the cytoplasm in its inactive form, and is released and translocated to the nucleus when activated. A number of carcinogens are able to activate the NF-κB signaling pathway, and activated p65 blocks apoptosis and promotes proliferation (43). It also had been reported that NF-κB activation is associated with resistance to various chemotherapeutic agents (44). In the present study, it was demonstrated that alantolactone significantly downregulated nuclear NF-κB in cancer cells and downregulated p-IκBα and its phosphorylation. The results (Fig. 5C) also revealed that alantolactone induces apoptosis in part by blocking the translocation of NF-κB from the cytoplasm into the nucleus and promoting the phosphorylation of IκBα
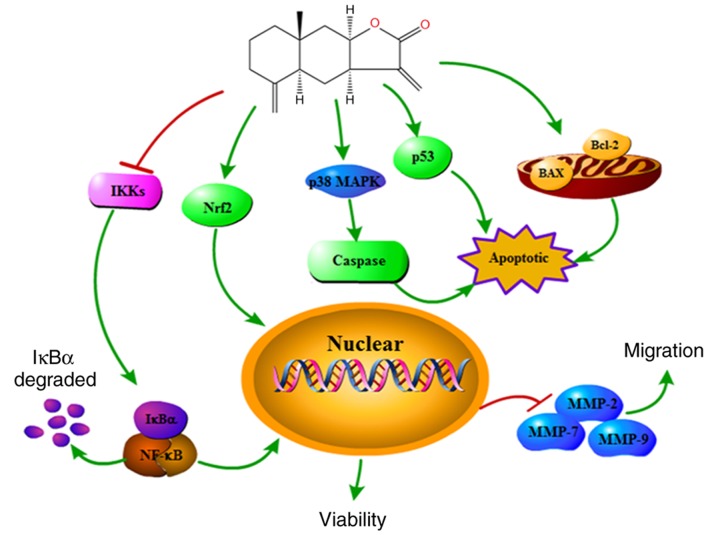
Nrf2 is an essential signaling molecule that serves a role in the biological defense mechanism. As a central transcription factor, Nrf2 is also involved in the suppression of tumorigenesis, and Nrf2 upregulation is associated with treatment resistance in cancer (45). In the present study, the results (Fig. 5F) demonstrated that the expression of Nrf2 was significantly increased in the nucleus. These findings suggest that alantolactone promotes the translocation of Nrf2 from the cytoplasm into the nucleus, and that Nrf2 is a risk factor in breast cancer that may be considered to be a promising target in cancer diagnosis. In addition, the results suggested that alantolactone modulates the NF-κB signaling pathway, resulting in P-IκB downregulation and reduced migration of MCF-7 cells. Cell death is induced via the p38 MAPK and Nrf2 pathways in MCF-7 cells (Fig. 6).
Figure 6.
Alantolactone induced apoptosis and suppressed migration of MCF-7 human breast cancer cells through regulating the p38 MAPK, NF-κB or Nrf2 signaling pathways. MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κΒ; Nrf, nuclear factor erythroid 2-related factor 2.
In conclusion, the present study demonstrated that alantolactone has anticancer effects in human breast cancer cells. The results confirmed that alantolactone is able to inhibit proliferation and apoptosis, as well as to suppress colony formation and cell migration. In addition, the mechanism by which alantolactone acts may involve the p38 MAPK, NF-κB or Nrf2 signaling pathways. Taken together, the results of the present study suggested that alantolactone is an antineoplastic drug, and these findings may be used as a clinical basis for the application of alantolactone as a novel cancer treatment.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of china (grant no. 31770017), the cultivation Plan for Youth Agricultural Science and Technology Innovative Talents of Liaoning Province (grant no. 2015013), the Project Supported by Scientific Research Fund of Liaoning Provincial Education Department (grant no. LQN201714), the Startup Foundation for doctors of Liaoning Province (grant no. 20170520258) and the Innovation Team Project from the Education department of Liaoning Province (grant no. LT2015011).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JLL, MJL and XYC conceived and designed the project and prepared the manuscript. JLL, SW, YH and ZJY conducted the cell experiments. MJL and YPH analyzed the data. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chung TW, Choi H, Lee JM, Ha SH, Kwak CH, Abekura F, Park JY, Chang YC, Ha KT, Cho SH, et al. Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer mcf-7 cells. J Ethnopharmacol. 2017;195:309–317. doi: 10.1016/j.jep.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Li T, Liu RH. 2α-Hydroxyursolic acid inhibited cell proliferation and induced apoptosis in MDA-MB-231 human breast cancer cells through the p38/MAPK signal transduction pathway. J Agric Food Chem. 2016;64:1806–1816. doi: 10.1021/acs.jafc.5b04852. [DOI] [PubMed] [Google Scholar]
- 3.Kreike B, Kouwenhove MV, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Research. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao YF, Rao YK, Tzeng YM. Aqueous extract of Anisomeles indica and its purified compound exerts anti-metastatic activity through inhibition of NF-κB/AP-1-dependent MMP-9 activation in human breast cancer MCF-7 cells. Food Chem Toxicol. 2012;50:2930–2936. doi: 10.1016/j.fct.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 5.Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson CS, Sharmila G, Arunakaran J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215:131–142. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Tan PQ, Zhong YM, Hu ZY, Lou DM. Size distributions, PAHs and inorganic ions of exhaust particles from a heavy duty diesel engine using B20 biodiesel with different exhaust aftertreatments. Energy. 2017;141:898–906. doi: 10.1016/j.energy.2017.09.122. [DOI] [Google Scholar]
- 7.Patel DK, Kumar R, Laloo D, Hemalatha S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac J Trop D. 2012;2:239–250. doi: 10.1016/S2222-1808(12)60054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu T, Geng J, Guo W, Gao J, Zhu X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm Sin B. 2017;7:65–72. doi: 10.1016/j.apsb.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo W, Liu Y, Zhang J, Luo X, Lin C, Guo J. Andrographolide inhibits the activation of NF-κB and MMP-9 activity in H3255 lung cancer cells. Exp Ther Med. 2013;6:743–746. doi: 10.3892/etm.2013.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Bao YL, Wu Y, Yu CL, Huang YX, Sun Y, Zheng LH, Li YX. Alantolactone induces apoptosis in RKO cells through the generation of reactive oxygen species and the mitochondrial pathway. Mol Med Rep. 2013;8:967–972. doi: 10.3892/mmr.2013.1640. [DOI] [PubMed] [Google Scholar]
- 11.Huo Y, Shi HM, Li WW, Wang MY, Li XB. HPLC determination and NMR structural elucidation of sesquiterpene lactones in. Inula Helenium J Pharm Biomed Anal. 2010;51:942–946. doi: 10.1016/j.jpba.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Konishi T, Shimada Y, Nagao T, Okabe H, Konoshima T. Antiproliferative sesquiterpene lactones from the roots of. Inula Helenium Biol Pharm Bull. 2002;25:1370–1372. doi: 10.1248/bpb.25.1370. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Ni ZY, Zhu MC, Dong M, Wang SM, Shi QW, Zhang ML, Wang YF, Huo CH, Kiyota H, Cong B. Antitumour activities of sesquiterpene lactones from Inula Helenium and Inula japonica. Z Naturforsch C. 2012;67:375–380. doi: 10.1515/znc-2012-7-804. [DOI] [PubMed] [Google Scholar]
- 14.Chun J, Kim YS. Alantolactone, a sesquiterpene lactone isolated from Inula Helenium L. selectively suppresses STAT3 activation and exhibits anticancer activity in MDA-MB-231 cells. Planta Med. 2015;81 doi: 10.1055/s-0035-1565388. [DOI] [PubMed] [Google Scholar]
- 15.Khan M, Li T, Ahmad Khan MK, Rasul A, Nawaz F, Sun M, Zheng Y, Ma T. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and Mitochondrial dysfunction. Biomed Res Int. 2013;2013:719858. doi: 10.1155/2013/719858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao P, Pan Z, Luo Y, Zhang L, Li X, Zhang G, Zhang Y, Cui R, Sun M, Zhang X. Alantolactone induces apoptosis and cell cycle arrest on lung squamous cancer SK-MES-1 cells. J Biochem Mol Toxicol. 2015;29:199–206. doi: 10.1002/jbt.21685. [DOI] [PubMed] [Google Scholar]
- 17.Khan M, Yi F, Rasul A, Li T, Wang N, Gao H, Gao R, Ma T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life. 2012;64:783–794. doi: 10.1002/iub.1068. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Liu B, Yang F, Yu Y, Zeng A, Ye T, Yin W, Xie Y, Fu Z, Zhao C. Lobaplatin induces BGC-823 human gastric carcinoma cell apoptosis via ROS-mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed Pharmacother. 2016;83:1239–1246. doi: 10.1016/j.biopha.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 19.Lv J, Cao MQ, Yu JC. Alantolactone pyrazoline analogue inhibits cancer cell proliferation and induces apoptosis in non-small cell lung carcinoma cells. Bangl J Pharmacol. 2015;10:409–415. doi: 10.3329/bjp.v10i2.22619. [DOI] [Google Scholar]
- 20.Del Bino G, Darzynkiewicz Z, Degraef C, Mosselmans R, Fokan D, Galand P. Comparison of methods based on annexin-V binding, DNA content or TUNEL for evaluating cell death in HL-60 and adherent MCF-7 cells. Cell Prolif. 1999;32:25–37. doi: 10.1046/j.1365-2184.1999.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielawski K, Bielawska A, Wołczyήski S. Synthesis, DNA-binding activity and cytotoxicity of carbamate derivatives of Hoechst 33258 in breast cancer MCF-7 cells. Biol Pharm Bull. 2002;25:916–919. doi: 10.1248/bpb.25.916. [DOI] [PubMed] [Google Scholar]
- 22.Ning L, Ma H, Jiang Z, Chen L, Li L, Chen Q, Qi H. Curcumol suppresses breast cancer cell metastasis by inhibiting MMP-9 via JNK1/2 and Akt-dependent NF-κB signaling pathways. Integr Cancer Ther. 2016;15:216–225. doi: 10.1177/1534735416642865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutterbeck CA, Kern DI, Machado ÊL, Kümmerer K. Evaluation of the toxic effects of four anticancer drugs in plant bioassays and its potency for screening in the context of waste water reuse for irrigation. Chemosphere. 2015;135:403–410. doi: 10.1016/j.chemosphere.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Min HY, Park HJ, Chung HJ, Lee JW, Kim MS, Park EJ, Lee SK. Inhibitory effects of alantolactone, a naturally occurring Sesquiterpenoid, on the growth of human colon cancer cells. Cancer Res. 2004;64:1391. [Google Scholar]
- 25.Kumari S, Badana AK, Mohan GM, Shailender Naik G, Malla R. Synergistic effects of coralyne and paclitaxel on cell migration and proliferation of breast cancer cells lines. Biomed Pharmacother. 2017;91:436–445. doi: 10.1016/j.biopha.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Nikhil K, Sharan S, Chakraborty A, Bodipati N, Krishna Peddinti R, Roy P. Role of isothiocyanate conjugate of Pterostilbene on the inhibition of MCF-7 cell proliferation and tumor growth in Ehrlich ascitic cell induced tumor bearing mice. Exp Cell Res. 2014;320:311–328. doi: 10.1016/j.yexcr.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Surichan S, Arroo RR, Tsatsakis AM, Androutsopoulos VP. Tangeretin inhibits the proliferation of human breast cancer cells via cyp1a1/cyp1B1 enzyme induction and cyp1a1/cyp1B1-mediated metabolism to the product 4′ hydroxy tangeretin. Toxicol In Vitro. 2018;50:274–284. doi: 10.1016/j.tiv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 29.Dolka I, Król M, Sapierzynski R. Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: An immunohistochemical and prognostic study. Res Vet Sci. 2016;105:124–133. doi: 10.1016/j.rvsc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhou N, Zhang Y, Zhang X, Lei Z, Hu R, Li H, Mao Y, Wang X, Irwin DM, Niu G, Tan H. Exposure of tumor-associated macrophages to apoptotic MCF-7 cells promotes breast cancer growth and metastasis. Int J Mol Sci. 2015;16:11966–11982. doi: 10.3390/ijms160611966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan W, Zhu F, Zhao Z, Chai Y, Yue W, Shao C, Lu F, Li Q, Wang C. Improved PCR-based subtractive hybridization, a new trategy on cloning differential expression genes in apoptotic MCF-7 cells. Cell Mol Immunol. 2001;17:35–37. In Chinese. [Google Scholar]
- 32.Kalid M, Jahanshiri F, Rahman A, Yusoff K. Gene expression profiling in apoptotic MCF-7 cells infected with newcastle disease virus. Global Vet. 2010;5:334–340. [Google Scholar]
- 33.Rasul A, Di J, Millimouno FM, Malhi M, Tsuji I, Ali M, Li J, Li X. Reactive oxygen species mediate isoalantolactone-induced apoptosis in human prostate cancer cells. Molecules. 2013;18:9382–9396. doi: 10.3390/molecules18089382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei JC, Yu JQ, Yin Y, Liu YW, Zou GL. Alantolactone induces activation of apoptosis in human hepatoma cells. Food Chem Toxicol. 2012;50:3313–3319. doi: 10.1016/j.fct.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Ray S, Reed JC, Ibrado AM, Tang C, Nawabi A, Bhalla K. Estrogen increases intracellular p26Bcl-2 to p21Bax ratios and inhibits taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast Cancer Res Treat. 1997;42:73–81. doi: 10.1023/A:1005777219997. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimoto K, Iwahana H, Fukuda A, Sano T, Saito S, Itakura M. Role of p53 mutations in endocrine tumorigenesis: Mutation detection by polymerase chain reaction-single strand conformation polymorphism. Cancer Res. 1992;52:5061–5064. [PubMed] [Google Scholar]
- 37.Tang D, Lahti JM, Kidd VJ. Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. J Biol Chem. 2000;275:9303–9307. doi: 10.1074/jbc.275.13.9303. [DOI] [PubMed] [Google Scholar]
- 38.Shah N, Mohammad AS, Saralkar P, Sprowls SA, Vickers SD, John D, Tallman RM, Lucke-Wold BP, Jarrell KE, Pinti M, et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res. 2018;132:47–68. doi: 10.1016/j.phrs.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aglund K, Rauvala M, Puistola U, Angström T, Turpeenniemi-Hujanen T, Zackrisson B, Stendahl U. Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to the grade and the stage. Gynecol Oncol. 2004;94:699–704. doi: 10.1016/j.ygyno.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Chun J, Kim YS. Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem Biol Interact. 2013;205:212–221. doi: 10.1016/j.cbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, Lee DU, Kim YS. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating nf-kb, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012;14:375–383. doi: 10.1016/j.intimp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Jiang Y, Tang X, Zhou B, Sun T, Chen H, Zhao X, Wang Y. The ROS-mediated pathway coupled with the MAPK-p38 signalling pathway and antioxidant system plays roles in the responses of Mytilus edulis haemocytes induced by BDE-47. Aquat Toxicol. 2017;187:55–63. doi: 10.1016/j.aquatox.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Wen PH, Zhang XX, Dai Y, He Q. Breviscapine ameliorates CCl4-induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int J Mol Med. 2018;42:755–768. doi: 10.3892/ijmm.2018.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon H, Liu RH. Effect of selected phytochemicals and apple extracts on NF-κB activation in human breast cancer MCF-7 cells. J Agric Food Chem. 2007;55:3167–3173. doi: 10.1021/jf0632379. [DOI] [PubMed] [Google Scholar]
- 45.Park EJ, Kim YM, Park SW, Kim HJ, Lee JH, Lee DU, Chang KC. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula Helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem Toxicol. 2013;55:386–395. doi: 10.1016/j.fct.2012.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.