Abstract
Background:
Pesticides have been associated with reproductive disorders, but there is limited research on pesticide exposures and human fertility.
Objective:
We aimed to investigate the effects of preconception exposure to pesticides on time to pregnancy (TTP) and on infertility in a general population of couples planning to become pregnant in Shanghai, China.
Methods:
A total of 615 women who were planning a pregnancy were enrolled before conception and were prospectively followed for 1 y to observe their TTP. Preconception pesticide exposures were assessed by measuring urinary metabolites of organophosphates (OPs) and pyrethroids (PYRs). Fecundability odds ratios (FORs) and odds ratios (ORs) of infertility were estimated using Cox and logistic regression models, respectively. All analyses were repeated after restricting the sample to nulliparous women ().
Results:
After adjusting for age, prepregnancy BMI, current smoking, education, annual household income, age at menarche, and two items from the Perceived Stress Scale (PSS-10), women in the highest quartile of diethylthiophosphate (DETP; an OP metabolite) had significantly longer TTP [adjusted (95% CI: 0.51, 0.92)] and increased infertility [adjusted (95% CI: 1.19, 3.93)] compared with women in the lowest quartile. The highest versus lowest quartile of 3-phenoxybenzoic acid (3PBA; a PYR metabolite) was associated with longer TTP and infertility, with significant associations in nulliparous women [adjusted (95% CI: 0.53, 0.98); adjusted OR for (95% CI: 1.10, 3.74)].
Conclusion:
Our study provides some of the first evidence that preconception OP and PYR exposures are associated with decreased fertility in Chinese couples. Given that OPs and PYRs are rapidly metabolized in humans, more studies are needed to confirm our findings. https://doi.org/10.1289/EHP2987
Introduction
Growing evidence suggests that human fertility rates are declining in both developed and developing countries (Clementi et al. 2008). This reduced fertility has been assumed to be associated with socioeconomic changes and adverse lifestyle factors (Den Hond et al. 2015; Snijder et al. 2012). However, environmental contaminants such as pesticides have attracted international attention and have recently come to be regarded as possible contributors to reduced human fertility (Mehrpour et al. 2014; Smarr et al. 2016).
China is one of the largest agricultural countries in the world, with of agricultural pesticides used annually (Shu et al. 2016). Organophosphates (OPs) and pyrethroids (PYRs) are the most widely used groups of pesticides in agricultural and residential areas in China (Tan et al. 2006; Ye et al. 2015), with 70,000 tons of OPs used in the year of 2015 and 4,000 tons of PYRs used in the year of 2013, respectively (Shu et al. 2014, 2016). Environmental exposure to OPs and PYRs is thought to occur primarily via consumption of food contaminated with pesticide residues and via inhalation or ingestion of contaminated household dust after the application of pesticides indoors (Ding et al. 2015; Ji et al. 2011; Wang et al. 2012). Human exposure to OPs and PYRs is now widespread in many countries and has become a global health issue (Babina et al. 2012; CDC 2017; Imai et al. 2014; Martenies and Perry 2013; Mehrpour et al. 2014; Wang et al. 2012). Epidemiological studies conducted in China have demonstrated relatively high levels of exposure to OPs and PYRs in women, raising concerns about chronic exposures and potential effects on reproductive health (Ding et al. 2015; Ji et al. 2011; Liu et al. 2016; Wang et al. 2012; Xia et al. 2008).
Animal studies have found adverse effects of OP and PYR exposures on female reproductive functions, including inhibited steroid hormones, disordered estrous cycles, and restrained follicle cells, which might ultimately lead to decreased fertility (Fei et al. 2010; Geng et al. 2015; Guerra et al. 2011; Li et al. 2013; Okamura et al. 2009; Rao and Kaliwal 2002; Rastogi et al. 2014). Only a few cross-sectional studies have estimated associations between fertility and occupational pesticide exposures in women, and results have been inconsistent (Bretveld et al. 2006; Greenlee et al. 2003; Idrovo et al. 2005; Lauria et al. 2006). In a study of female flower workers in Colombia, self-reported occupational pesticide exposure was associated with a longer time to pregnancy (TTP) (Idrovo et al. 2005), but no significant association was found in a multicenter study in Italy based on a questionnaire survey (Lauria et al. 2006). None of these previous epidemiological studies assessed pesticide exposures by measuring pesticide metabolites in urine, and to our knowledge, no cohort study of pesticide exposures and infertility has yet been conducted. Therefore, we aimed to prospectively evaluate the associations between preconception pesticide exposure and couple fertility in Shanghai, China, where OPs and PYRs are the most widely used pesticides.
Methods
Study Population
To improve birth outcomes for couples who plan to become pregnant, the Chinese government promotes preconception care at designated clinics that provide health education and physical examinations (Zhang et al. 2016; Yang et al. 2015). The Shanghai Birth Cohort recruited women from two preconception care clinics in Shanghai, China. Detailed information on study recruitment has been described previously (Zhou et al. 2017). Briefly, women were eligible if they were registered Shanghai residents who were not planning to move in the next two years, were of age, had stopped using contraception recently, and planned to conceive without assisted reproductive technology and to give birth in one of the hospitals participating in the Shanghai Birth Cohort. Women were not eligible for enrollment if they were part of a couple diagnosed with infertility, had tried and failed to conceive spontaneously for , or had sought reproductive assistance. After recruitment, telephone follow-up was performed every 2 mo for to collect information on TTP as described below.
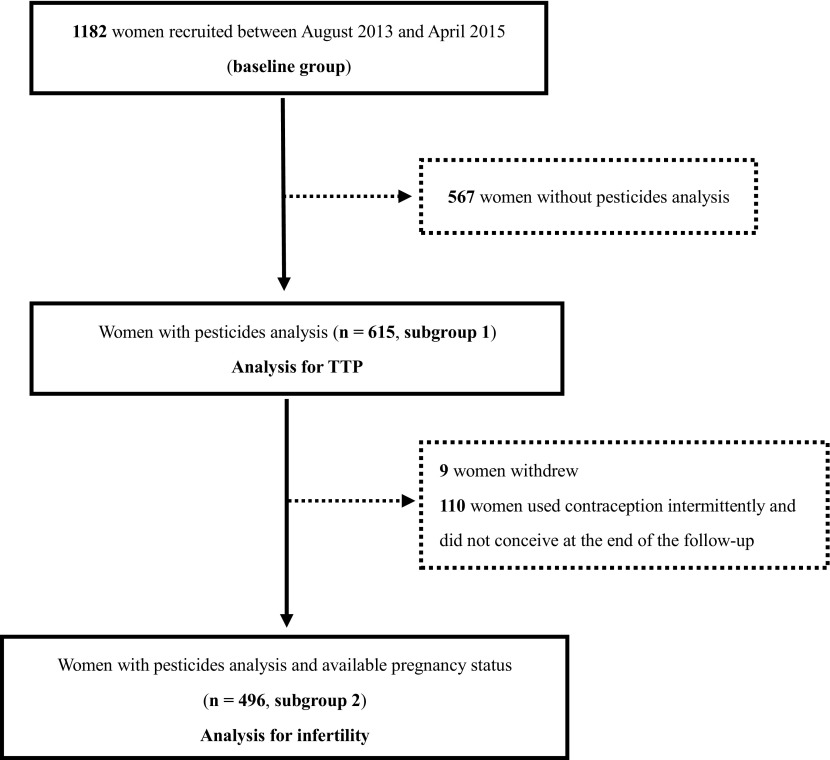
Between August 2013 and April 2015, 1,182 eligible women (referred to as the baseline group) were recruited at two preconception care clinics in Shanghai, China (Figure 1). To conserve biological samples for future research, OP and PYR metabolites were measured only in urine samples with a volume , which were available for 615 women and were included in the TTP analysis (subgroup 1). Of these 615 women, nine women withdrew, and 110 women who used contraception intermittently and did not conceive before the end of the follow-up period were excluded, resulting in 496 women for the analysis of infertility (subgroup 2).
Figure 1.
Flowchart for participant selection.
The protocol was approved by the Medical Ethics Committee of Shanghai Xinhua Hospital, Shanghai Jiao Tong University School of Medicine. All study participants provided written informed consent.
Questionnaires
Trained research staff interviewed the couples separately using a standardized questionnaire, which included demographic characteristics, lifestyle characteristics, perceived stress, occupational pesticide exposure, and reproductive and medical histories. For occupational pesticide exposure, subjects were asked “What is your occupation (clerk, accountant, salesman, worker, farmer…)?” and “Do you produce or use pesticides at work? (Yes or no)”. For reproductive history, women were asked about their history of pregnancies and live births, and their spouses were asked about diseases of the genitourinary system (such as cryptorchidism, hypospadias, and varicocele). Perceived stress has been reported to be a risk factor for infertility (Louis et al. 2011; Lynch et al. 2014); therefore, women were asked to complete a self-administered Perceived Stress Scale (PSS-10) after enrollment. The validity and reliability of the PSS-10 have been reported elsewhere (Wang et al. 2011) (see Table S1 for questions included in the PSS-10).
Outcomes
TTP was defined as the number of months it took for a couple to conceive (Abell et al. 2000; Snijder et al. 2012) and was assessed during the bimonthly telephone follow-ups using the following question: “Have you become pregnant since the last telephone follow-up (or since the recruitment)?”. If the answer was yes, then “When was the first day of your last menstrual period (LMP)?”. If the answer was no, then “Did you take any contraceptive measures since the last telephone follow-up (or since the recruitment)?” and “Do you still plan to become pregnant?”. The couples were prospectively followed until they achieved pregnancy or for a maximum of 12 mo. In our study, TTP was determined based on the number of months of attempted pregnancy before enrollment (self-reported during a face-to-face interview) and the duration of telephone follow-up from enrollment to the LMP (or the end of follow-up). For women who withdrew from the study, TTP was censored upon dropout; for women who used contraception intermittently during follow-up, only the number of months without contraception were counted. In our study, self-reported pregnancies were all confirmed in the Shanghai Birth Cohort–participating hospitals.
Infertility was defined as having a TTP (Vélez et al. 2015a). Based on the data from 12 mo of telephone follow-up, participants were divided into a pregnancy group and an infertility group.
OP and PYR Exposure
Preconception urine samples (spot urine) were collected from each participant at enrollment and then aliquoted to polypropylene tubes and stored at until further analysis. Six dialkylphosphate (DAP) metabolites of OPs [dimethylphosphate (DMP), dimethylthiophosphate (DMTP), diethylphosphate (DEP), diethylthiophosphate (DETP), dimethyldithiophosphate (DMDTP) and diethyldithiophosphate (DEDTP)] and three metabolites of PYRs [3-phenoxybenzoic acid (3PBA), trans/cis-3-(2,2-dichlorovinyl)-1-methylcyclopropane-1,2-dicarboxylic acid (TDCCA and CDCCA, respectively)] were examined using gas chromatography–mass spectrometry (GC-MS; OP metabolites were analyzed using a PE Clarus 600s/MSD, and PYR metabolites were analyzed using an Agilent 7890B/5977A GC/MSD) based on the modified method reported by Kühn et al. (1996) and Ueyama et al. (2010).
The limits of detection (LODs) of OP metabolites were for DEP and DETP, for DEDTP, for DMP, and for DMTP and DMDTP, respectively. The LODs of PYR metabolites were for 3PBA, for TDCCA, and for CDCCA. Metabolite levels below the LOD were assigned a value equivalent to the (Hornung and Reed 1990). The molar concentrations of DMP, DMTP, DEP, and DETP were summed to derive total DAPs as a summary measure of environmental OP exposures (Arcury et al. 2006).
Quality control samples prepared by pooling eight urine samples from healthy female adult volunteers were randomly assayed along with the study samples to ensure the quality of the analytical methods. Urine creatinine concentrations were measured using an automated chemistry analyzer (Hitachi 7100).
Statistical Analysis
Descriptive statistical analyses were performed for characteristics of the study population and concentrations of pesticide urinary metabolites. Demographic characteristics were compared between the baseline population and the study population and between women with and without detected pesticides using Student’s t-tests or analyses of variance (ANOVAs) for continuous variables and tests for categorical variables. Spearman correlations (for continuous variables) and rank sum tests (for categorical variables) were used to examine associations between demographic characteristics and TTP; Student’s t-tests (for continuous variables) and tests (for categorical variables) were used to explore associations between demographic characteristics and infertility. Urine concentrations of pesticide metabolites were adjusted for creatinine (to account for dilution) and were modeled as continuous variables or categorized into quartiles, using the lowest quartile as the reference. Statistical analysis was limited to metabolites detected in of the population. Metabolites detected in of the population (DMDTP, 13.5%; DEDTP, 29.2%; TDCCA, 60.9%; and CDCCA, 14.1%) were excluded from further analyses.
Fecundability odds ratios (FORs) were estimated using Cox models, where reflect a longer TTP or reduced fertility. In addition, we used logistic regression models to estimate odds ratios (ORs) for infertility (Vélez et al. 2015a).
Potential confounders were identified from previous studies of environmental pollutants on TTP or infertility (Greenlee et al. 2003; Lynch et al. 2014; Vélez et al. 2015b), and the following were included as covariates in all Cox and logistic regression models: maternal age (continuous), prepregnancy body mass index (BMI; continuous), current smoking [no (nonsmoking and no smoker in the home), live with smoker (nonsmoking but live with a smoker), yes (current smoking)], education ( or ), annual household income (, 150,000–300,000, or CNY), and age at menarche (continuous). In addition, we adjusted for two variables based on questions from the PSS-10 that were associated with TTP or infertility (): “Have you found that you could not cope with all the things that you had to do?” and “How often have you been able to control irritations in your life?”, which were categorized as never or almost never, sometimes or fairly often, or very often. All covariates were defined based on information provided before conception.
We did not adjust for parity because of the potential for overadjustment bias (Vélez et al. 2015a). However, because parity is influenced by female fertility and is proof of former fertility (Bach et al. 2015; Vélez et al. 2015a, 2015b), we conducted sensitivity analyses restricted to nulliparous women only. In addition, Wald tests were performed to test for trends across quartiles of OP and PYR metabolite concentrations. All statistical analyses were performed using SPSS v.19 (IBM); (two-tailed) was considered statistically significant.
Results
In general, demographic characteristics were similar among the 1,182 women in the baseline group and women in subgroup 1 (analysis for TTP, ) and subgroup 2 (analysis for infertility, ) (see Table S2). Demographic characteristics were also similar between women with and without urine pesticide metabolite measurements (see Table S3).
Mean values [ (SD)] for maternal age, prepregnancy BMI, and age at menarche were , and , respectively (Table 1). The majority of women (92.5%) were nulliparous, 81.0% had a bachelor’s degree or above, and 70.7% reported an annual household income CNY. None of the couples in our study reported producing or using pesticides at work. Only nine women (1.5%) were current smokers at enrollment, but 24.9% lived with smokers.
Table 1.
Characteristics of the study population at enrollment and their associations with time to pregnancy, pregnancy, and infertility.
| Characteristic | Study population () (range) or (%) | TTP (months) () Median (IQR) | p-Valuea | Pregnancy () or (%) | Infertility () or (%) | p-Valueb |
|---|---|---|---|---|---|---|
| Maternal age (years) | (24.00–44.00) | — | 0.023 | 0.271 | ||
| Prepregnancy BMI () | (14.45–37.65) | — | 0.094 | 0.017 | ||
| Age at menarche (years) | (10.00–18.00) | — | 0.323 | 0.660 | ||
| Current smoking | ||||||
| No | 453 (73.5) | 4.5 (2.0–10.0) | 0.035 | 293 (80.3) | 96 (73.3) | 0.204 |
| Live with smoker | 153 (24.9) | 5.5 (2.0–12.0) | 68 (18.6) | 32 (24.4) | ||
| Yes | 9 (1.5) | 10.0 (6.5–12.0) | 4 (1.1) | 3 (2.3) | ||
| Education | ||||||
| 117 (19.0) | 5 (2.0–9.5) | 0.988 | 62 (17.0) | 20 (15.3) | 0.650 | |
| 498 (81.0) | 5 (2.0–10.0) | 303 (83.0) | 111 (84.7) | |||
| Annual household income (CNY) | ||||||
| 140 (22.8) | 4.0 (2.0–10.0) | 0.363 | 84 (23.0) | 29 (22.1) | 0.496 | |
| 150,000–300,000 | 329 (53.5) | 4.5 (1.5–10.0) | 201 (55.1) | 65 (49.6) | ||
| 106 (17.2) | 6.0 (2.0–12.0) | 59 (16.1) | 27 (20.6) | |||
| Refused to answer | 40 (6.5) | 6.0 (2.0–12.0) | 21 (5.8) | 10 (7.6) | ||
| Parity | ||||||
| 0 | 569 (92.5) | 5.0 (2.0–10.0) | 0.062 | 334 (91.5) | 121 (92.4) | 0.759 |
| 46 (7.5) | 4.0 (2.0–7.0) | 31 (8.5) | 10 (7.6) | |||
| Have you found that you could not cope with all the things that you had to do?c | ||||||
| Never or almost never | 267 (43.4) | 5.0 (2.0–9.0) | 0.239 | 175 (48.0) | 43 (32.8) | 0.013 |
| Sometimes | 333 (54.2) | 5.5 (2.0–12.0) | 183 (50.1) | 84 (64.1) | ||
| Fairly often or very often | 15 (2.4) | 3.0 (1.0–12.0) | 7 (1.9) | 4 (3.1) | ||
| How often have you been able to control irritations in your life?c | ||||||
| Never or almost never | 27 (4.4) | 4.0 (2.0–8.0) | 0.078 | 17 (4.7) | 5 (3.8) | 0.023 |
| Sometimes | 168 (27.3) | 4.0 (1.5–8.0) | 104 (28.5) | 22 (16.8) | ||
| Fairly often or very often | 420 (68.3) | 5.5 (2.0–12.0) | 244 (66.8) | 104 (79.4) | ||
Note: —, data not available; BMI, body mass index; IQR, interquartile range; SD, standard deviation; TTP, time to pregnancy.
p-Values for difference in time to pregnancy according to characteristics at enrollment; Spearman correlation for continuous variables, rank sum test for categorical variables.
p-Values for differences in characteristics at enrollment according to pregnancy or infertile status at the end of follow-up; t test for continuous variables, chi-square test for categorical variables.
Questions from the PSS-10 questionnaire.
At the end of follow-up, 131 (26.4%) women who did not get pregnant within a year were classified as infertile, and 365 (73.6%) conceived spontaneously during follow-up, with an average TTP of 4.5 mo. Maternal age and current smoking were significant predictors of TTP, and prepregnancy BMI was inversely associated with infertility (Table 1). In addition, compared with fertile women, infertile women were less likely respond “never or almost never” to “Have you found that you could not cope with all the things that you had to do?” and less likely to respond “fairly often or very often” to “How often have you been able to control irritations in your life?”.
Individual OP metabolites were detected in of samples, with the exceptions of DMDTP (14.1%) and DEDTP (28.0%) (Table 2). Detection rates for PYR metabolites varied from 99.0% for 3PBA to 13.8% for CDCCA. Creatinine-adjusted median concentrations of urinary DMP, DMTP, DEP, DETP, 3PBA, and TDCCA were 6.99, 2.79, 10.25, 3.47, 0.73, and creatinine, respectively. Median concentrations of DMDTP, DEDTP, and CDCCA were not calculated owing to their low detection rates.
Table 2.
Urinary concentrations of organophosphate (OP) and pyrethroid (PYR) metabolites ().
| Pesticides | Metabolites | Detection rate | Adjustment | Percentile | |||
|---|---|---|---|---|---|---|---|
| (%) | 25th | 50th | 75th | 95th | |||
| OPs | DMP | 579 (94.1) | Unadjusteda | 2.03 | 4.83 | 11.31 | 45.43 |
| Adjustedb | 2.99 | 6.99 | 16.14 | 53.83 | |||
| DMTP | 572 (93.0) | Unadjusteda | 0.75 | 1.77 | 4.67 | 21.47 | |
| Adjustedb | 1.25 | 2.79 | 5.97 | 22.14 | |||
| DEP | 614 (99.8) | Unadjusteda | 3.99 | 7.22 | 13.60 | 31.54 | |
| Adjustedb | 5.86 | 10.25 | 16.63 | 35.59 | |||
| DETP | 615 (100) | Unadjusteda | 1.25 | 2.54 | 5.13 | 16.34 | |
| Adjustedb | 2.16 | 3.47 | 6.43 | 15.48 | |||
| DMDTP | 87 (14.1) | Unadjusteda | 0.60 | ||||
| Adjustedb | 1.18 | ||||||
| DEDTP | 172 (28.0) | Unadjusteda | 0.28 | 0.65 | |||
| Adjustedb | 0.31 | 1.40 | |||||
| Total DAPc | 108.50 | 198.43 | 335.51 | 766.22 | |||
| PYRs | 3PBA | 609 (99.0) | Unadjusteda | 0.29 | 0.51 | 1.04 | 3.14 |
| Adjustedb | 0.44 | 0.73 | 1.31 | 3.99 | |||
| TDCCA | 368 (59.8) | Unadjusteda | 0.29 | 0.59 | 2.22 | ||
| Adjustedb | 0.44 | 0.82 | 3.35 | ||||
| CDCCA | 85 (13.8) | Unadjusteda | 0.94 | ||||
| Adjustedb | 1.82 | ||||||
Note: 3PBA, 3-phenoxybenzoic acid; CDCCA, cis-3-(2,2-dichlorovinyl)-1-methylcyclopropane-1,2-dicarboxylic acid; DEDTP, diethyldithiophosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DMDTP, dimethyldithiophosphate; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; LOD, limit of detection; OP, organophosphate; PYR, pyrethroid; TDCCA, trans-3-(2,2-dichlorovinyl)-1-methylcyclopropane-1,2-dicarboxylic acid.
Not adjusted for creatinine ().
Adjusted for creatinine ( creatinine).
Total DAP was the summary of molar concentrations of DMP, DMTP, DEP, and DETP ( creatinine).
Compared with women in the lowest quartile of DETP, women in the highest quartile of DETP had significantly lower odds of pregnancy [adjusted (95% CI: 0.51, 0.92); ], with a similar estimate when limited to nulliparous women [adjusted (95% CI: 0.50, 0.91); ] (Table 3). TTP was not significantly associated with other urinary OP metabolites. Women in the highest quartile of 3PBA had lower odds of pregnancy than women in the lowest quartile [adjusted (95% CI: 0.57, 1.03); ], with a slightly stronger association when restricted to nulliparous women [adjusted (95% CI: 0.53, 0.98); ].
Table 3.
Fecundability odds ratios and 95% confidence intervals for preconception organophosphate and pyrethroid exposure ().
| Exposure ( creatinine) | FOR (95% CI) | |||
|---|---|---|---|---|
| All women () | Nulliparous women () | |||
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |
| OPs | ||||
| DMP | ||||
| Continuousa | 0.97 (0.82, 1.15) | 0.97 (0.82, 1.15) | 0.97 (0.82, 1.15) | 0.99 (0.83, 1.17) |
| Q1 () | Reference | Reference | Reference | Reference |
| Q2 (2.99–6.99) | 1.04 (0.78, 1.38) | 1.03 (0.77, 1.37) | 1.04 (0.78, 1.40) | 1.02 (0.76, 1.38) |
| Q3 (6.99–16.14) | 0.98 (0.73, 1.30) | 0.93 (0.69, 1.24) | 0.97 (0.72, 1.30) | 0.91 (0.68, 1.24) |
| Q4 () | 0.88 (0.66, 1.18) | 0.85 (0.63, 1.15) | 0.90 (0.66, 1.29) | 0.86 (0.64, 1.17) |
| p for trend | 0.955 | 0.883 | 0.958 | 0.836 |
| DMTP | ||||
| Continuousa | 1.09 (0.89, 1.33) | 1.06 (0.86, 1.29) | 1.08 (0.88, 1.33) | 1.07 (0.97, 1.32) |
| Q1 () | Reference | Reference | Reference | Reference |
| Q2 (1.25–2.79) | 0.87 (0.65, 1.17) | 0.91 (0.67, 1.23) | 0.88 (0.65, 1.20) | 0.90 (0.66, 1.23) |
| Q3 (2.79–5.97) | 0.97 (0.73, 1.30) | 0.97 (0.73, 1.30) | 0.98 (0.72, 1.32) | 0.97 (0.71, 1.31) |
| Q4 () | 1.12 (0.85, 1.49) | 1.15 (0.86, 1.53) | 1.17 (0.87, 1.32) | 1.19 (0.89, 1.60) |
| p for trend | 0.589 | 0.734 | 0.670 | 0.717 |
| DEP | ||||
| Continuousa | 0.90 (0.68, 1.19) | 0.90 (0.67, 1.20) | 0.89 (0.67, 1.20) | 0.91 (0.67, 1.23) |
| Q1 () | Reference | Reference | Reference | Reference |
| Q2 (5.86–10.25) | 1.04 (0.78, 1.40) | 1.05 (0.78, 1.41) | 1.06 (0.78, 1.43) | 1.02 (0.75, 1.38) |
| Q3 (10.25–16.63) | 1.11 (0.83, 1.47) | 1.12 (0.84, 1.50) | 1.08 (0.80, 1.46) | 1.08 (0.80, 1.47) |
| Q4 () | 1.05 (0.78, 1.40) | 1.08 (0.81, 1.45) | 1.03 (0.76, 1.39) | 1.04 (0.77, 1.41) |
| p for trend | 0.635 | 0.601 | 0.997 | 0.778 |
| DETP | ||||
| Continuousa | 1.07 (0.81, 1.41) | 1.09 (0.82, 1.45) | 1.07 (0.80, 1.43) | 1.10 (0.82, 1.48) |
| Q1 () | Reference | Reference | Reference | Reference |
| Q2 (2.16–3.47) | 0.88 (0.66, 1.16) | 0.85 (0.64, 1.13) | 0.88 (0.66, 1.17) | 0.83 (0.61, 1.11) |
| Q3 (3.47–6.43) | 0.82 (0.62, 1.10) | 0.84 (0.63, 1.12) | 0.77 (0.57, 1.04) | 0.78 (0.58, 1.06) |
| Q4 () | 0.71 (0.53, 0.95)* | 0.68 (0.51, 0.92)* | 0.71 (0.52, 0.96)* | 0.67 (0.50, 0.91)* |
| p for trend | 0.235 | 0.153 | 0.231 | 0.143 |
| Total DAP | ||||
| Continuousa | 0.93 (0.69, 1.27) | 0.94 (0.70, 1.26) | 0.92 (0.68, 1.25) | 0.95 (0.70, 1.28) |
| Q1 () | Reference | Reference | Reference | Reference |
| Q2 (108.5–198.43) | 1.06 (0.79, 1.42) | 1.07 (0.80, 1.43) | 1.06 (0.78, 1.43) | 1.04 (0.77, 1.41) |
| Q3 (198.43–335.51) | 0.96 (0.72, 1.29) | 0.79 (0.58, 1.08) | 0.94 (0.70, 1.28) | 0.87 (0.64, 1.19) |
| Q4 () | 1.08 (0.81, 1.44) | 0.90 (0.67, 1.21) | 1.07 (0.79, 1.44) | 1.03 (0.76, 1.39) |
| p for trend | 0.874 | 0.988 | 0.914 | 0.873 |
| PYR | ||||
| 3PBA | ||||
| Continuousa | 1.14 (0.88, 1.48) | 1.19 (0.91, 1.54) | 1.19 (0.91, 1.56) | 1.23 (0.93, 1.61) |
| Q1 () | Reference | Reference | Reference | Reference |
| Q2 (0.44–0.73) | 0.86 (0.65, 1.14) | 0.83 (0.62, 1.11) | 0.82 (0.61, 1.11) | 0.80 (0.60, 1.08) |
| Q3 (0.73–1.31) | 0.88 (0.66, 1.17) | 0.85 (0.63, 1.13) | 0.84 (0.63, 1.12) | 0.83 (0.62, 1.11) |
| Q4 () | 0.75 (0.56, 1.00) | 0.77 (0.57, 1.03) | 0.74 (0.54, 0.99)* | 0.72 (0.53, 0.98)* |
| p for trend | 0.216 | 0.160 | 0.121 | 0.089 |
Note: 3PBA, 3-phenoxybenzoic acid; CI, confidence interval; DAP, dialkyl phosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; FOR, fecundability odds ratio; OP, organophosphate; PYR, pyrethroid; Q1–4, quartiles 1–4.
.
Urine concentrations of pesticide metabolites were adjusted for creatinine then .
Adjusted for age, prepregnancy body mass index (BMI), age at menarche, current smoking, education, annual household income, “Have you found that you could not cope with all the things that you had to do?”, and “How often have you been able to control irritations in your life?” in Cox models.
When compared with women in the lowest quartile of DETP, women in the highest quartile of DETP had higher odds of infertility [adjusted (95% CI: 1.19, 3.93); ], with a slightly stronger association in nulliparous women [adjusted (95% CI: 1.23, 4.30); ] (Table 4). Other urinary OP metabolites were not clearly associated with infertility. The OR for infertility was positive but nonsignificant for women in the highest versus lowest quartile of 3PBA [adjusted (95% CI: 0.86, 2.79); ]; however, the association was stronger and significant when limited to nulliparous women [adjusted (95% CI: 1.10, 3.74); ].
Table 4.
Odds ratios and 95% confidence intervals for preconception organophosphate and pyrethroid exposure ().
| Exposure ( creatinine) | OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| All women () | Nulliparous women () | |||||||
| Pregnancy | Infertility | Unadjusted | Adjustedc | Pregnancy | Infertility | Unadjusted | Adjustedc | |
| OPs | ||||||||
| DMP | ||||||||
| Continuousa | 1.04 (0.75, 1.44) | 1.03 (0.74, 1.44) | 1.02 (0.72, 1.43) | 0.98 (0.69, 1.38) | ||||
| Q1 ()b | 96 | 29 | Reference | Reference | 89 | 27 | Reference | Reference |
| Q2 (2.99–6.74)b | 84 | 39 | 0.92 (0.51, 1.64) | 0.94 (0.51, 1.72) | 72 | 36 | 0.90 (0.50, 1.64) | 0.97 (0.52, 1.81) |
| Q3 (6.74–16.17)b | 91 | 32 | 1.41 (0.81, 2.46) | 1.60 (0.90, 2.84) | 84 | 28 | 1.48 (0.83, 2.64) | 1.74 (0.96, 3.17) |
| Q4 () b | 94 | 31 | 1.07 (0.60, 1.89) | 1.13 (0.63, 2.05) | 89 | 30 | 0.99 (0.55, 1.79) | 1.07 (0.58, 1.98) |
| p for trend | 0.710 | 0.483 | 0.706 | 0.415 | ||||
| DMTP | ||||||||
| Continuousa | 0.81 (0.54, 1.23) | 0.83 (0.54, 1.26) | 0.77 (0.50, 1.19) | 0.79 (0.51, 1.24) | ||||
| Q1 ()b | 89 | 34 | Reference | Reference | 77 | 32 | Reference | Reference |
| Q2 (1.31–2.85)b | 86 | 38 | 1.28 (0.73, 2.28) | 1.30 (0.72, 2.35) | 82 | 35 | 1.40 (0.76, 2.56) | 1.42 (0.76, 2.66) |
| Q3 (2.85–5.92)b | 95 | 29 | 1.29 (0.74, 2.26) | 1.32 (0.74, 2.34) | 91 | 29 | 1.43 (0.79, 2.60) | 1.49 (0.81, 2.77) |
| Q4 ()b | 95 | 30 | 0.95 (0.53, 1.70) | 0.91 (0.49, 1.66) | 84 | 25 | 1.07 (0.58, 1.97) | 1.07 (0.57, 2.03) |
| p for trend | 0.347 | 0.332 | 0.217 | 0.198 | ||||
| DEP | ||||||||
| Continuousa | 1.00 (0.56, 1.77) | 0.94 (0.52, 1.68) | 0.98 (0.53, 1.78) | 0.91 (0.49, 1.68) | ||||
| Q1 ()b | 90 | 33 | Reference | Reference | 84 | 32 | Reference | Reference |
| Q2 (5.86–10.12)b | 92 | 33 | 1.01 (0.58, 1.78) | 1.10 (0.61, 1.97) | 85 | 30 | 1.09 (0.61, 1.95) | 1.21 (0.66, 2.22) |
| Q3 (10.12–16.15)b | 91 | 32 | 0.98 (0.56, 1.72) | 1.00 (0.56, 1.77) | 79 | 29 | 1.01 (0.56, 1.82) | 1.04 (0.57, 1.91) |
| Q4 () b | 92 | 33 | 1.01 (0.58, 1.79) | 1.02 (0.57, 1.82) | 86 | 30 | 1.05 (0.58, 1.91) | 1.07 (0.58, 1.98) |
| p for trend | 0.996 | 0.778 | 0.815 | 0.590 | ||||
| DETP | ||||||||
| Continuousa | 0.74 (0.41, 1.32) | 0.68 (0.37, 1.26) | 0.72 (0.39, 1.32) | 0.66 (0.35, 1.26) | ||||
| Q1 ()b | 91 | 33 | Reference | Reference | 84 | 32 | Reference | Reference |
| Q2 (2.14–3.49)b | 90 | 33 | 1.32 (0.73, 2.39) | 1.44 (0.77, 2.66) | 81 | 30 | 1.38 (0.75, 2.55) | 1.52 (0.80, 2.88) |
| Q3 (3.49–6.28)b | 91 | 32 | 1.53 (0.85, 2.77) | 1.56 (0.84, 2.87) | 83 | 29 | 1.37 (0.73, 2.56) | 1.40 (0.73, 2.68) |
| Q4 ()b | 93 | 33 | 2.07 (1.17, 3.68)* | 2.17 (1.19, 3.93)* | 86 | 30 | 2.11 (1.16, 3.85)* | 2.30 (1.23, 4.30)* |
| p for trend | 0.231 | 0.146 | 0.274 | 0.180 | ||||
| Total DAP | ||||||||
| Continuousa | 1.00 (0.55, 1.79) | 0.91 (0.50, 1.66) | 0.96 (0.52, 1.78) | 0.85 (0.45, 1.61) | ||||
| Q1 ()b | 94 | 29 | Reference | Reference | 90 | 28 | Reference | Reference |
| Q2 (113.42–200.42)b | 83 | 40 | 0.82 (0.46, 1.45) | 0.88 (0.49, 1.59) | 71 | 39 | 0.79 (0.44, 1.42) | 0.90 (0.49, 1.66) |
| Q3 (200.42–332.46)b | 96 | 28 | 1.31 (0.76, 2.25) | 1.64 (0.93, 2.91) | 89 | 21 | 1.40 (0.80, 2.45) | 1.89 (1.04, 3.43)* |
| Q4 ()b | 92 | 34 | 0.77 (0.43, 1.38) | 0.86 (0.48, 1.56) | 84 | 33 | 0.60 (0.32, 1.12) | 0.68 (0.36, 1.30) |
| p for trend | 0.957 | 0.628 | 0.988 | 0.526 | ||||
| PYR | ||||||||
| 3PBA | ||||||||
| Continuousa | 0.78 (0.46, 1.32) | 0.81 (0.47, 1.39) | 0.81 (0.47, 1.40) | 0.84 (0.48, 1.46) | ||||
| Q1 ()b | 92 | 34 | Reference | Reference | 86 | 28 | Reference | Reference |
| Q2 (0.44–0.73)b | 92 | 32 | 1.29 (0.73, 2.30) | 1.22 (0.68, 2.21) | 80 | 30 | 1.22 (0.66, 2.23) | 1.17 (0.63, 2.19) |
| Q3 (0.73–1.38)b | 86 | 37 | 1.27 (0.71, 2.27) | 1.26 (0.69, 2.29) | 76 | 37 | 1.40 (0.77, 2.56) | 1.39 (0.75, 2.60) |
| Q4 ()b | 95 | 28 | 1.56 (0.88, 2.76) | 1.55 (0.86, 2.79) | 92 | 26 | 1.94 (1.08, 3.50)* | 2.03 (1.10, 3.74)* |
| p for trend | 0.306 | 0.403 | 0.287 | 0.349 | ||||
Note: 3PBA, 3-phenoxybenzoic acid; CI, confidence interval; DAP, dialkyl phosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; OP, organophosphate; OR, odds ratio; PYR, pyrethroid; Q1–4, quartiles 1–4.
.
Urine concentrations of pesticide metabolites were adjusted for creatinine then .
The cut point of OP and PYR metabolites was determined in a sample size of 496 in the analysis for infertility.
Adjusted for age, prepregnancy BMI, age at menarche, current smoking, education, annual household income, “Have you found that you could not cope with all the things that you had to do?”, and “How often have you been able to control irritations in your life?” in logistic regression models.
Discussion
Exposures to OPs and PYRs were associated with both TTP and infertility in our study population. Women in the highest quartiles of DETP (one of the major OP urinary metabolites) and 3PBA (the major PYR urinary metabolite) had longer TTP and increased odds of infertility compared with women in the lowest quartile of each exposure.
Our findings are supported by previous epidemiological studies conducted in Colombia (Idrovo et al. 2005), Denmark (Abell et al. 2000), Finland (Sallmén et al. 2003), Canada (Curtis et al. 1999), and France (Thonneau et al. 1999), suggesting that pesticide exposures have adverse effects on fertility. For example, in a cross-sectional study of 492 female flower greenhouse workers in Denmark, women who handled pesticide-treated cultures without wearing gloves had significantly lower fertility [ (95% CI: 0.46, 0.98)] than women who always used gloves (Abell et al. 2000). Idrovo et al. (2005) studied 2,085 nulliparous agricultural workers in Colombia and reported that women who had been employed in the flower production industry for had a significantly longer TTP [ (95% CI: 0.63, 0.84)] than other workers. However, two cross-sectional studies conducted in the Netherlands (Bretveld et al. 2006) and Italy (Lauria et al. 2006) found no associations between pesticide exposures and TTP. It is noteworthy that published studies of the association between pesticide exposures and TTP or fertility have primarily focused on women with occupational exposures or women living in agricultural regions and have classified exposures based on self-reported responses to postal questionnaires or telephone interviews (Abell et al. 2000; Bretveld et al. 2006; Curtis et al. 1999; Idrovo et al. 2005; Lauria et al. 2006; Sallmén et al. 2003; Thonneau et al. 1999). In contrast with previous studies, we measured pesticide metabolites in urine to quantitatively estimate environmental pesticide exposures in women before conception.
In the present study, we found that both DETP and 3PBA were significantly associated with prolonged TTP and infertility, suggesting that exposures to OPs and PYRs have adverse effects on fertility. In addition, when the analysis was limited to nulliparous women, associations for DETP and 3PBA with prolonged TTP and infertility were somewhat stronger, which suggests that parity may have modified the associations between pesticide exposures and fertility, a finding that should be confirmed in future studies.
The prevalence of infertility in our sample was 26.4%, which is higher than the infertility rate in China [15% in 2009 (Ma 2017)]. Several factors may contribute to these differences. First, according to the committee opinion expressed by the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine (ASRM), women’s fertility decreases gradually but significantly beginning at approximately 32 y of age (American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee 2014), and 25% of the women in our study were old. Hou (2011) explored potential risk factors for female infertility in a cross-sectional study of 1,606 women registered with an infertility clinic in Shandong province and reported that the OR for infertility was 2.14 (95% CI: 1.72, 2.65) for women with a bachelor’s degree or higher compared with women who had a middle school education or less, and the OR for infertility was 1.31 (95% CI: 1.04, 1.65) for urban versus rural populations. Because our study population was composed of better-educated urban women, the infertility rate is expected to be higher than the population average. In the Longitudinal Investigation of Fertility and the Environment (LIFE) study of 501 U.S. couples without diagnosed infertility who were seeking to become pregnant, which also enrolled couples before conception and followed up for 12 menstrual cycles, 31% did not achieve pregnancy during the follow-up period (Buck Louis et al. 2014). Women in the LIFE study were highly educated (95% with a college or technical education) and had a mean age of 30 y () at enrollment.
Potential biological mechanisms that might explain associations of lower fertility with OP and PYR exposures are unclear. Findings from several animal studies suggest that OP exposures could cause disturbances in estrous cycles, resulting in a decrease in the number of estrous cycles and in the duration of proestrus, estrus, and metestrous with a concomitant prolonged diestrous phase, which may ultimately result in reduced fertility (Nanda and Kaliwal 2003; Rao and Kaliwal 2002; Tello et al. 2013). Another possible mechanism might be through effects on follicular development (Ghodageri and Katti 2013; Mahadevaswami and Kaliwal 2002; Nair et al. 2014; Katti et al. 2012). Animal studies have revealed that PYR exposures can inhibit steroid hormones, restrain the growth of follicles, and damage ovarian corpus luteum cells, which might further contribute to decreased fertility (Fei et al. 2010; Guerra et al. 2011; He et al. 2006; Sangha et al. 2013). However, although findings from animal studies provide supportive evidence that exposure to OPs and PYRs may affect fertility in humans, more research is warranted.
Our study has several strengths. To our knowledge, it is the first prospective study of preconceptional pesticide exposures and TTP and couple fertility in China with quantitative measures of exposures to the most widely used groups of pesticides, OPs and PYRs. In addition, China is one of the few countries to offer free preconception care to any couple who wants to become pregnant, which enabled us to recruit women before conception (Zhang et al. 2016). Unlike women enrolled from infertility clinics, women in our study were recruited from the general population, and none reported occupational exposure to pesticides. Thus, our findings may be more generalizable than those of previous studies. Furthermore, we prospectively ascertained TTP through telephone follow-up, which avoided recall bias, a concern for retrospective studies. Moreover, although many previous studies enrolled pregnant women, we reduced the potential for selection bias by enrolling women from the general population before conception (Tingen et al. 2004). Finally, instead of classifying pesticide exposure based on self-report (Abell et al. 2000; Idrovo et al. 2005; Lauria et al. 2006), we measured urinary concentrations of OPs and PYRs.
Several limitations also need to be acknowledged. First, we did not measure pesticide exposures in the women’s male partners; thus, we were unable to estimate quantitative associations between spousal pesticide exposures and fertility. None of the male participants reported a history of diseases affecting the reproductive system (such as syphilis, cryptorchidism, hypospadias, or varicocele), which should reduce the potential contribution of the male factor to infertility, although misclassification, particularly underreporting of disease history, remains a concern. Second, compared with a large cross-sectional investigation of a general population sample of women in Shanghai () (Xie et al. 2014), where 68% had an annual household income CNY and 60% had a bachelor’s degree or above, our study population had higher annual household income (87% had annual household income CNY) and higher educational backgrounds (81% had a bachelor’s degree or above). Third, OP and PYR metabolites that were measured in a single spot urine sample collected upon enrollment might not represent exposure levels during the entire preconception period (Koureas et al. 2012).
Conclusion
In summary, findings from our prospective cohort study suggest that preconceptional exposures to environmental pesticides may adversely affect TTP and couple fertility in Shanghai, China. OP and PYR exposures that were quantified based on urinary DETP and 3PBA concentrations were associated with longer TTP and increased odds of infertility. As pesticide use becomes more widespread in China and in other parts of the world, adverse health effects have emerged as a major public health concern. Further studies are needed to confirm associations between pesticide exposures and couple infertility in other populations and to evaluate potential mechanisms that might be responsible for associations between infertility and the widely used OP and PYR pesticides.
Supplemental Material
Acknowledgments
This study was funded by the National Key Research and Development Program of China (grants 2017YFC1600500 and 2016YFC1000203), the National Basic Research Program of China (973 Program 2014CB943300), the National Natural Science Foundation of China (grants 81630085, 81602823, and 81773387), the scientific research program of Shanghai Municipal Commission of Health and Family Planning (grant 201640174), and the Science and Technology Commission of Shanghai Municipality (grant 17ZR1415800), and was supported by Xinhua Hospital Biobank.
References
- Abell A, Juul S, Bonde JP. 2000. Time to pregnancy among female greenhouse workers. Scand J Work Environ Health 26(2):131–136, PMID: 10817378, 10.5271/sjweh.522. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. 2014. Female age-related fertility decline. Committee opinion no. 589. Fertil Steril 101(3):633–634, PMID: 24559617, 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Arcury TA, Grzywacz JG, Davis SW, Barr DB, Quandt SA. 2006. Organophosphorus pesticide urinary metabolite levels of children in farmworker households in eastern North Carolina. Am J Ind Med 49(9):751–760, PMID: 16804908, 10.1002/ajim.20354. [DOI] [PubMed] [Google Scholar]
- Babina K, Dollard M, Pilotto L, Edwards JW. 2012. Environmental exposure to organophosphorus and pyrethroid pesticides in South Australian preschool children: a cross sectional study. Environ Int 48:109–120, PMID: 22892382, 10.1016/j.envint.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Bach CC, Liew Z, Bech BH, Nohr EA, Fei C, Bonefeld-Jorgensen EC. 2015. Perfluoroalkyl acids and time to pregnancy revisited: an update from the Danish National Birth Cohort. Environ Health 14:59, PMID: 26148742, 10.1186/s12940-015-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretveld R, Zielhuis GA, Roeleveld N. 2006. Time to pregnancy among female greenhouse workers. Scand J Work Environ Health 32(5):359–367, PMID: 17091203, 10.5271/sjweh.1031. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. 2014. Urinary bisphenol A, phthalates, and couple fecundity, the Longitudinal Investigation of Fertility and the Environment (LIFE Study). Fertil Steril 101(5):1359–1366, PMID: 24534276, 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. “Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables, January 2017, Volume One.” Atlanta, GA:CDC; https://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf [accessed 20 April 2017]. [Google Scholar]
- Clementi M, Tiboni GM, Causin R, La Rocca C, Maranghi F, Raffagnato F, et al. 2008. Pesticides and fertility: an epidemiological study in Northeast Italy and review of the literature. Reprod Toxicol 26(1):13–18, PMID: 18599266, 10.1016/j.reprotox.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Curtis KM, Savitz DA, Weinberg CR, Arbuckle TE. 1999. The effect of pesticide exposure on time to pregnancy. Epidemiology 10(2):112–117, PMID: 10069244, 10.1097/00001648-199903000-00005. [DOI] [PubMed] [Google Scholar]
- Den Hond E, Tournaye H, De Sutter P, Ombelet W, Baeyens W, Covaci A, et al. 2015. Human exposure to endocrine disrupting chemicals and fertility: a case-control study in male subfertility patients. Environ Int 84:154–160, PMID: 26292060, 10.1016/j.envint.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Ding G, Cui C, Chen L, Gao Y, Zhou Y, Shi R, et al. 2015. Prenatal exposure to pyrethroid insecticides and birth outcomes in rural northern China. J Expo Sci Environ Epidemiol 25(3):264–270, PMID: 25515377, 10.1038/jes.2014.86. [DOI] [PubMed] [Google Scholar]
- Fei J, Qu JH, Ding XL, Xue K, Lu CC, Chen JF, et al. 2010. Fenvalerate inhibits the growth of primary cultured rat preantral ovarian follicles. Toxicology 267(1–3):1–6, PMID: 19892000, 10.1016/j.tox.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Geng X, Bo C, Han G, Shao H. 2015. Effects of malathion on testicular spermatogenic function in rats [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 33(3):180–185, PMID: 25916442. [PubMed] [Google Scholar]
- Ghodageri MG, Katti P. 2013. In vitro induction/inhibition of germinal vesicle breakdown (GVBD) in frog (Euphlyctis cyanophlyctis) oocytes by endocrine active compounds. Drug Chem Toxicol 36(2):217–223, PMID: 22946474, 10.3109/01480545.2012.710623. [DOI] [PubMed] [Google Scholar]
- Greenlee AR, Arbuckle TE, Chyou PH. 2003. Risk factors for female infertility in an agricultural region. Epidemiology 14(4):429–436, PMID: 12843768, 10.1097/01.EDE.0000071407.15670.aa. [DOI] [PubMed] [Google Scholar]
- Guerra MT, de Toledo FC, Kempinas Wde G. 2011. In utero and lactational exposure to fenvalerate disrupts reproductive function in female rats. Reprod Toxicol 32(3):298–303, PMID: 21889588, 10.1016/j.reprotox.2011.08.002. [DOI] [PubMed] [Google Scholar]
- He J, Chen JF, Liu R, Song L, Chang HC, Wang XR. 2006. Fenvalerate-induced alterations in calcium homeostasis in rat ovary. Biomed Environ Sci 19(1):15–20, PMID: 16673813. [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Hou L. 2011. An epidemiological study on the infertility in three provinces in China [in Chinese; Dissertation]. Beijing, China:Peking Union Medical College. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1985844 [accessed 8 February 2017].
- Idrovo AJ, Sanìn LH, Cole D, Chavarro J, Cáceres H, Narváez J, et al. 2005. Time to first pregnancy among women working in agricultural production. Int Arch Occup Environ Health 78(6):493–500, PMID: 15918035, 10.1007/s00420-005-0615-9. [DOI] [PubMed] [Google Scholar]
- Imai K, Yoshinaga J, Yoshikane M, Shiraishi H, Mieno MN, Yoshiike M, et al. 2014. Pyrethroid insecticide exposure and semen quality of young Japanese men. Reprod Toxicol 43:38–44, PMID: 24189267, 10.1016/j.reprotox.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Ji G, Xia Y, Gu A, Shi X, Long Y, Song L, et al. 2011. Effects of non-occupational environmental exposure to pyrethroids on semen quality and sperm DNA integrity in Chinese men. Reprod Toxicol 31(2):171–176, PMID: 20955780, 10.1016/j.reprotox.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Katti P, Ghadage A, Ghodageri MG. 2012. Induction of germinal vesicle break down (GVBD) in vitro by metabolic hormones in frog (Euphlyctis cyanophlyctis) oocytes. Int J Curr Res 4:500–504. http://www.journalcra.com/sites/default/files/2876.pdf. [Google Scholar]
- Koureas M, Tsakalof A, Tsatsakis A, Hadjichristodoulou C. 2012. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol Lett 210(2):155–168, PMID: 22020228, 10.1016/j.toxlet.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Kühn KH, Leng G, Bucholski KA, Dunemann L, Idel H. 1996. Determination of pyrethroid metabolites in human urine by capillary gas chromatography-mass spectrometry. Chromatographia 43(5–6):285–292, 10.1007/BF02270996. [DOI] [Google Scholar]
- Lauria L, Settimi L, Spinelli A, Figà-Talamanca I. 2006. Exposure to pesticides and time to pregnancy among female greenhouse workers. Reprod Toxicol 22(3):425–430, PMID: 16483739, 10.1016/j.reprotox.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Li YF, Pan C, Hu JX, Li J, Xu LC. 2013. Effects of cypermethrin on male reproductive system in adult rats. Biomed Environ Sci 26(3):201–208, PMID: 23425803, 10.3967/0895-3988.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Liu P, Wu C, Chang X, Qi X, Zheng M, Zhou Z. 2016. Adverse associations of both prenatal and postnatal exposure to organophosphorous pesticides with infant neurodevelopment in an agricultural area of Jiangsu Province, China. Environ Health Perspect 124(10):1637–1643, PMID: 27153333, 10.1289/EHP196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, et al. 2011. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril 95(7):2184–2189, PMID: 20688324, 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Sundaram R, Maisog JM, Sweeney AM, Buck Louis GM. 2014. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study-the LIFE Study. Hum Reprod 29(5):1067–1075, PMID: 24664130, 10.1093/humrep/deu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. 2017. The new developments in assisted reproductive technology [in Chinese]. Chin J Family Planning Gynecotokol 9(1):4–7. [Google Scholar]
- Mahadevaswami MP, Kaliwal BB. 2002. Effect of dimethoate administration schedules on compensatory ovarian hypertrophy, follicular dynamics, and estrous cycle in hemicastrated mice. J Basic Clin Physiol Pharmacol 13(3):225–248, PMID: 12670031, 10.1515/JBCPP.2002.13.3.225. [DOI] [PubMed] [Google Scholar]
- Martenies SE, Perry MJ. 2013. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology 307:66–73, PMID: 23438386, 10.1016/j.tox.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. 2014. Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett 230(2):146–156, PMID: 24487096, 10.1016/j.toxlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Nair R, Singh VJ, Salian SR, Kalthur SG, D’Souza AS, Shetty PK, et al. 2014. Methyl parathion inhibits the nuclear maturation, decreases the cytoplasmic quality in oocytes and alters the developmental potential of embryos of Swiss albino mice. Toxicol Appl Pharmacol 279(3):338–350, PMID: 25038315, 10.1016/j.taap.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Nanda N, Kaliwal BB. 2003. Effect of edifenphos on compensatory ovarian hypertrophy, follicular kinetics and estrous cycle in hemicastrated rats. J Basic Clin Physiol Pharmacol 14(4):373–386, PMID: 15198308, 10.1515/JBCPP.2003.14.4.373. [DOI] [PubMed] [Google Scholar]
- Okamura A, Kamijima M, Ohtani K, Yamanoshita O, Nakamura D, Ito Y, et al. 2009. Broken sperm, cytoplasmic droplets and reduced sperm motility are principal markers of decreased sperm quality due to organophosphorus pesticides in rats. J Occup Health 51(6):478–487, PMID: 19779279, 10.1539/joh.L9019. [DOI] [PubMed] [Google Scholar]
- Rao RP, Kaliwal BB. 2002. Monocrotophos induced dysfunction on estrous cycle and follicular development in mice. Ind Health 40(3):237–244, PMID: 12141371. [DOI] [PubMed] [Google Scholar]
- Rastogi D, Narayan R, Saxena DK, Chowdhuri DK. 2014. Endosulfan induced cell death in Sertoli-germ cells of male Wistar rat follows intrinsic mode of cell death. Chemosphere 94:104–115, PMID: 24125708, 10.1016/j.chemosphere.2013.09.029. [DOI] [PubMed] [Google Scholar]
- Sallmén M, Liesivuori J, Taskinen H, Lindbohm ML, Anttila A, Aalto L, et al. 2003. Time to pregnancy among the wives of Finnish greenhouse workers. Scand J Work Environ Health 29(2):85–93, PMID: 12718493, 10.5271/sjweh.709. [DOI] [PubMed] [Google Scholar]
- Sangha GK, Kaur K, Khera KS. 2013. Cypermethrin induced pathological and biochemical changes in reproductive organs of female rats. J Environ Biol 34(1):99–105, PMID: 24006814. [PubMed] [Google Scholar]
- Shu F, Wang Q, Han M. 2014. The production and usage of pesticides of China in 2013 [in Chinese]. Pesticide Science and Administration 34(12):49–52. [Google Scholar]
- Shu F, Xiong YK, Han M. 2016. The production and usage of pesticides of China in 2015 [in Chinese]. Pesticide Science and Administration 37:1–6. [Google Scholar]
- Smarr MM, Grantz KL, Sundaram R, Maisog JM, Honda M, Kannan K, et al. 2016. Urinary paracetamol and time-to-pregnancy. Hum Reprod 31(9):2119–2127, PMID: 27412248, 10.1093/humrep/dew172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder CA, te Velde E, Roeleveld N, Burdorf A. 2012. Occupational exposure to chemical substances and time to pregnancy: a systematic review. Hum Reprod Update 18(3):284–300, PMID: 22431564, 10.1093/humupd/dms005. [DOI] [PubMed] [Google Scholar]
- Tan L, Wang S, Ji J, Sun X, Li Y, Wang Q, et al. 2006. Effects of fenvalerate exposure on semen quality among occupational workers. Contraception 73(1):92–96, PMID: 16371303, 10.1016/j.contraception.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Tello JA, Kohout T, Pineda R, Maki RA, Scott Struthers R, Millar RP. 2013. Reproductive physiology of a humanized GnRH receptor mouse model: application in evaluation of human-specific analogs. Am J Physiol Endocrinol Metab 305(1):E67–E77, PMID: 23632635, 10.1152/ajpendo.00624.2012. [DOI] [PubMed] [Google Scholar]
- Thonneau P, Abell A, Larsen SB, Bonde JP, Joffe M, Clavert A, et al. 1999. Effects of pesticide exposure on time to pregnancy: results of a multicenter study in France and Denmark. Am J Epidemiol 150(2):157–163, PMID: 10412960, 10.1093/oxfordjournals.aje.a009975. [DOI] [PubMed] [Google Scholar]
- Tingen C, Stanford JB, Dunson DB. 2004. Methodologic and statistical approaches to studying human fertility and environmental exposure. Environ Health Perspect 112(1):87–93, PMID: 14698936, 10.1289/ehp.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama J, Kamijima M, Kondo T, Takagi K, Shibata E, Hasegawa T, et al. 2010. Revised method for routine determination of urinary dialkyl phosphates using gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878(17–18):1257–1263, PMID: 20207202, 10.1016/j.jchromb.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, Fraser WD. 2015a. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC Study. Hum Reprod 30(3):701–709, PMID: 25567616, 10.1093/humrep/deu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, Fraser WD. 2015b. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril 103(4):1011–1020.e2, PMID: 25681860, 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Wang P, Tian Y, Wang XJ, Gao Y, Shi R, Wang GQ, et al. 2012. Organophosphate pesticide exposure and perinatal outcomes in Shanghai, China. Environ Int 42:100–104, PMID: 21601922, 10.1016/j.envint.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen J, Boyd JE, Zhang H, Jia X, Qiu J, et al. 2011. Psychometric properties of the Chinese version of the perceived stress scale in policewomen. PLoS One 6(12):e28610, PMID: 22164311, 10.1371/journal.pone.0028610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Han Y, Wu B, Wang S, Gu A, Lu N, et al. 2008. The relation between urinary metabolite of pyrethroid insecticides and semen quality in humans. Fertil Steril 89(6):1743–1750, PMID: 17765231, 10.1016/j.fertnstert.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Xie F, Li XT, Yang YL, Yan D. 2014. Survey on implementation of pre pregnancy health care in Shanghai [in Chinese]. Chin J Perinat Med 17(6):410–414. [Google Scholar]
- Yang Y, He Y, Li Q, Wang Y, Peng Z, Xu J, et al. 2015. Preconception blood pressure and risk of preterm birth: a large historical cohort study in a Chinese rural population. Fertil Steril 104(1):124–130, 10.1016/j.fertnstert.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Ye M, Beach J, Martin JW, Senthilselvan A. 2015. Associations between dietary factors and urinary concentrations of organophosphate and pyrethroid metabolites in a Canadian general population. Int J Hyg Environ Health 218(7):616–626, PMID: 26141242, 10.1016/j.ijheh.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tian Y, Wang W, Huang H, Shen X, Sun K. 2016. Toward a national birth cohort study in China. Am J Public Health 106(12):2111–2112, PMID: 27831791, 10.2105/AJPH.2016.303484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhang L, Tong C, Fang F, Zhao S, Tian Y, et al. 2017. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ Health Perspect 125(6):067012, PMID: 28657892, 10.1289/EHP1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.