Abstract
Primary damage or dysfunction of the nervous system may cause or initiate neuropathic pain. However, it has been difficult to establish an effective treatment for neuropathic pain, as the mechanisms responsible for its pathology remain largely unknown. Autophagy is closely associated with the pathological process of neurodegenerative diseases, neuropathic injury and cancer, among others. The aim of the present study was to examine the changes in the autophagy-lysosomal pathway and discuss the effects of autophagy on allodynia, hyperalgesia and astrocyte activation in neuropathic pain. A neuropathic pain model was induced by chronic constriction injury (CCI) in rats. Inducers and inhibitors of autophagy and lysosomes were used to assess autophagy, allodynia, hyperalgesia and astrocyte activity. Neuropathic pain was found to induce an increase in the levels of the autophagy-related proteins, LC3II and Beclin 1 and, and in those of the lysosomal proteins, lysosomal-associated membrane protein type 2 (LAMP2) and Ras-related protein Rab-7a (RAB7), whereas p62 levels were found to decrease from day 1 to 14 following CCI. The autophagy inducer, rapamycin, further increased the LC3II, Beclin 1, lysosomal-associated membrane protein 2 (LAMP2) and Ras-related protein Rab-7a (RAB7) expression levels, and decreased the p62 expression levels, which were accompanied by alleviation of allodynia, hyperalgesia and astrocyte activation in the rats subjected to CCI; the autophagy inhibitor, 3-methyladenine, reversed these effects. The use of the lysosomal inhibitors, bafilomycin and chloroquine, resulted in the accumulation of LC3II and Beclin 1, a decrease in the levels of LAMP2 and RAB7, and the exacerbation of allodynia, hyperalgesia and astrocyte activation in rats with neuropathic pain. On the whole, the findings of this study indicate that neuropathic pain activates autophagy, which alleviates mechanical and thermal hyperalgesia and suppresses astrocyte activity. Therefore, neuropathic pain induced by CCI in rats appears to be mediated via the autophagy-lysosomal pathway.
Keywords: neuropathic pain, autophagy, astrocyte, allodynia, hyperalgesia
Introduction
Neuropathic pain is a relatively common occurrence, observed in ~0.6–1.5% of the US population, and it is a chronic severely adynamic state initiated by a primary injury or the dysfunction of the nervous system (1). There are several similarities between pain and other neurobiological processes, such as learning and memory (2). Two particularly aggravating and prominent symptoms in different types of neuropathic pain, caused by abnormal sensory perception of pain or independent stimulating or persistent pain, are hyperalgesia and allodynia (3). However, the underlying potential cellular and molecular mechanisms are relatively obscure, and current pain treatment remains unsatisfactory. Previous studies have demonstrated that astrocytes are activated during persistent neuropathic pain; in particular, the late participation of astrocytic activation has been correlated with nociception maintenance (4-7). With rapid and sensitive response to various noxious or physiological stimuli, astrocytes can increase the expression of glial fibrillary acidic protein (GFAP) (8). Kim et al observed that the lack of GFAP resulted in brief persistent allodynia following nerve ligation (9). These findings indicate that peripheral nerve damage-induced GFAP activity not only serves as a marker for astrocyte hypertrophy, but also plays an important role during the process of neuropathic pain. Moreover, GFAP plays a key role in regulating astrocyte motility (10) and may be involved in synaptic modulation in the central nervous system (CNS) (11-13).
Autophagy, which is a process of self-digestion, is a dynamic regulated process consisting of autophagosomal induction, formation and autophagosome-lysosome fusion, for the recycling of damaged or malfunctioning proteins and organelles, such as injured mitochondria (14). Following exposure to various stressors, the double-membrane vesicle encircles the degraded cytoplasm and organelles and fuses with the lysosomal membrane to form an autolysosome. The autophagosomes are then degraded and recycled to remodel new protein or organelles for cell survival. Failure of the autophagosome-lysosome fusion may lead to the accumulation of autophagosomes (15). Therefore, a simple assessment of autophagy may be misleading, due to the increased autophagosomal formation. It is also necessary to investigate the mechanisms underlying autophagosomal induction, formation and autophagosome-lysosome fusion (16). A number of studies have clearly demonstrated high levels of autophagy-related proteins and/or numbers of autophagosomes in a model of CNS injury due to hypoxia/ischemia or trauma (17 and refs. therein). Accumulating evidence indicates a close association between autophagy and neuropathic pain (18-20). Piao et al (21) reported that lack of the p62 autophagic protein plays a key role in the pathophysiology of neuropathic pain. Autophagy inducers have been shown to protect neurons during traumatic brain injury in mice and to also relieved brain damage in a model of neonatal hypoxia/ischemia (22,23). Furthermore, autophagy regulates the expression GFAP in astrocytes and has been associated with allodynia and hyperalgesia in different diseases (24,25).
However, whether the autophagic-lysosomal pathway is activated and whether autophagy regulates GFAP activity, allodynia and hyperalgesia in neuropathic pain induced by chronic constriction injury (CCI), remains unknown. Thus, the aim of the present study was to discuss the entire autophagy process, from autophagosomal formation to autophagosome-lysosome fusion, in the setting of neuropathic pain. In addition, autophagy inhibitors and inducers were used to examine the effects of autophagy on GFAP activity, allodynia and hyperalgesia.
Materials and methods
Animals
A total of 192 Adult male Sprague-Dawley rats (8–10 weeks old, weighing 200–250 g) were used in all the experiments. The rats were housed in a temperature-controlled (25°C) room with an alternating 12-h light/dark cycle under specific pathogen-free conditions; water and chow were provided ad libitum until the commencement of the experiments. The rats were obtained from the Laboratory Animal Center of the Military Medical Science Academy of the Chinese People's Liberation Army. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University and were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
Neuropathic pain induced by CCI
Neuropathic pain was induced by CCI of the sciatic nerve according to the model previously described by Bennett and Xie (26). The rats were anesthetized with sevoflurane (induction with 3.0%; maintenance with 1.5%) mixed with air through a nose mask under sterile conditions. The left sciatic nerve was exposed and 3 loose ligatures with 5-0 silk suture thread were made on the nerve at 1.0–1.5 mm intervals. The muscle and skin were sutured after complete hemostasis. The rats were divided into 8 groups according to the ligation/medicinal intervention as follows: The control (Con) (n=40), CCI (n=48), CCI + 3-methyladenine (3-MA) (n=16), CCI + rapamycin (Rap) (n=24), CCI + chloroquine (CQ) (n=16), CCI + bafilomycin (Baf) (n=16), CCI + Rap + CQ (n=16) and CCI + Rap + Baf (n=16) groups in all the experiments. In the Con group, the sciatic nerve was exposed but without ligation; the rats in all the other groups underwent both exposure and ligation of the left sciatic nerve. At the end of the experiments, the rats were euthanized by CO2 exposure (CO2 displacement rate equivalent to 20% of the chamber volume/min) and cervical dislocation.
Experimental protocol
The experimental protocol was as follows:
Experiment 1: Autophagy-related protein expression in rats with CCI-induced neuropathic pain
Neuropathic pain was induced by CCI of the sciatic nerve and the L4-L6 spinal cord was collected for further detection of autophagy-related proteins. Part of the spinal cord was used to test for LC3II, Beclin 1 and p62 by western blot analysis prior to (Con; Fig. 1) and at 1, 3, 7 and 14 days after CCI. In addition, the expression of GFAP, another autophagy-related protein, was detected by western blot analysis (as described below) at 1, 3, 7 and 14 days after CCI (n=8 for Con and each point time). The remaining part of the L4-L6 spinal cord was immediately stored in 4% paraformaldehyde for the measurement of GFAP expression by immunofluorescence assay (as described below) at 1, 3 and 7 days after CCI.
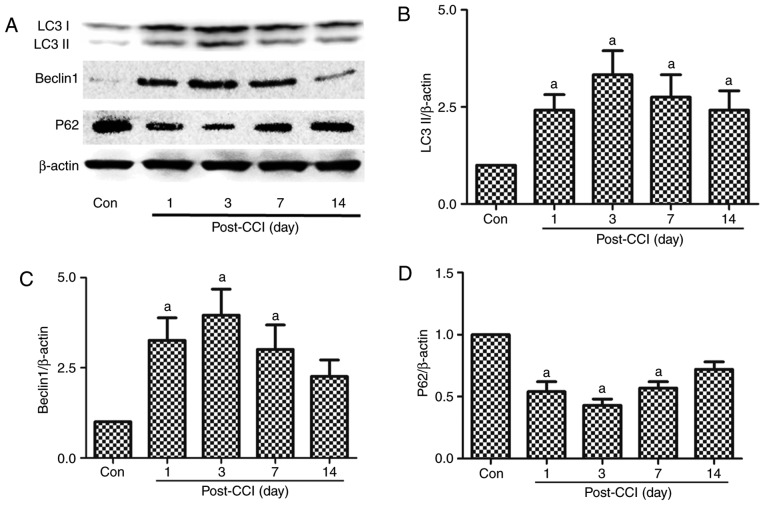
Figure 1.
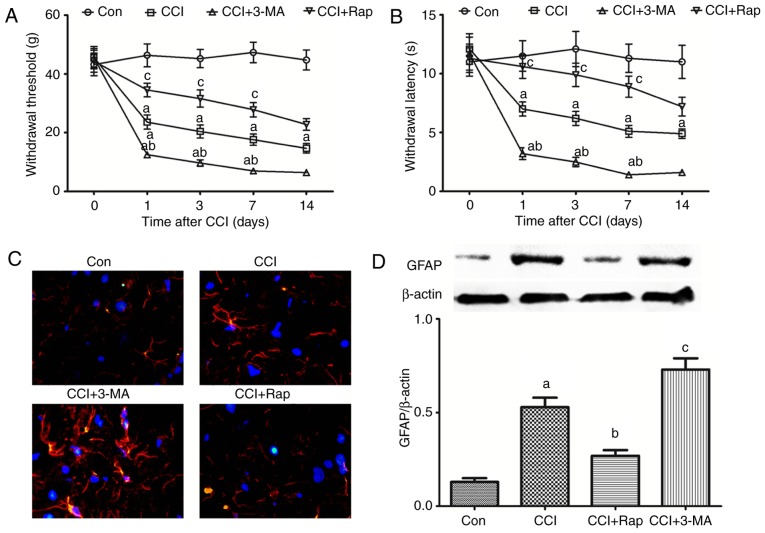
Expression of the autophagy-related proteins, LC3II, Beclin 1 and p62, in the spinal dorsal horn of rats with neuropathic pain. A part of the L4-L5 spinal cord was collected for the detection of the autophagy-related proteins (A and B) LC3II, (A and C) Beclin 1, and (A and D) p62 by western blot analysis prior to and at 1, 3, 7 and 14 days after CCI. The value in the Con group was set as 1, and other relative values in the CCI groups were calculated by comparison. The values are expressed as the means ± standard deviation (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. Con, control; CCI, chronic constriction injury.
Experiment 2: Effect of autophagy induction and inhibition on the expression of the autophagy-related proteins, LC3II, Beclin 1 and p62, in rats with CCI-induced neuropathic pain
The rats were randomly divided into 4 groups, namely the Con (n=8 from Fig. 1), CCI (n=8 from Fig. 1), 3-MA (n=8) and Rap (n=8) groups. According to previous studies (14,22,23), 3-MA and Rap (Bio Vision, Mountain View, CA, USA) were administered by intraperitoneal injection at a dose of 15 and 10 mg/kg, respectively, 1 h prior to the CCI operation. At 7 days after CCI, the autophagy-related protein expression of LC3II, Beclin 1 and p62 in the spinal cord was detected by western blot analysis in all 4 groups.
Experiment 3: Evaluation of the effects of autophagy inhibition and induction on allodynia, hyperalgesia and GFAP expression in CCI-induced neuropathic pain by western blot analysis and immunofluorescence
Grouping was performed as described above in Experiment 2. Mechanical allodynia and thermal hyperalgesia were tested prior to (0 days) and at 1, 3, 7 and 14 days after CCI. At 7 days after CCI, GFAP expression in the spinal cord was detected by western blot analysis and immunofluorescence assay in all 4 groups (n=8 from Fig. 1).
Experiment 4: Lysosome-related protein expression in rats with CCI-induced neuropathic pain
Neuropathic pain was induced by CCI (n=8) of the sciatic nerve. A part of the L4-L6 spinal cord was collected for the detection of lysosome-related proteins, lysosomal-associated membrane protein type 2 (LAMP2) and Ras-related protein Rab-7a (RAB7), by western blot analysis prior to (Con; Fig. 5) and at 1, 3, 7 and 14 days after CCI (n=8 from Fig. 1).
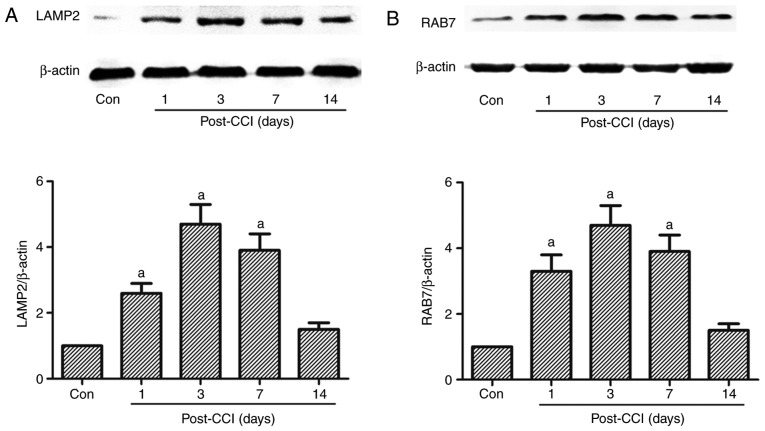
Figure 5.
Expression of lysosome-related proteins in rats with CCI-induced neuropathic pain. A part of L4-L5 spinal cord was collected for the detection of autophagy-related proteins (A) LAMP and (B) RAB7 by western blot analysis prior to and at 1, 3, 7 and 14 days after CCI. The value in the Con group was set as 1 and other relative values in the CCI groups were calculated by comparison. The values are expressed as the means ± standard deviation (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. Con, control; CCI, chronic constriction injury; LAMP2, lysosomal-associated membrane protein type 2; RAB7, Ras-related protein Rab-7a.
Experiment 5: Effect of autophagy inhibition and induction on lysosome-related protein expression, allodynia and hyperalgesia in rats with CCI-induced neuropathic pain
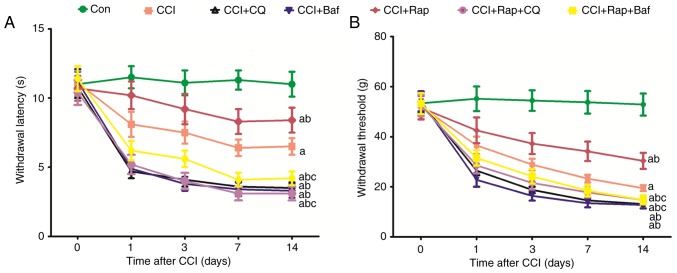
The rats were randomly divided into 7 groups as follows: The Con (n=8 from Fig. 1), CCI (n=8 from Fig. 1), CCI + chloroquine (CQ) (n=8), CCI + bafilomycin (Baf) (n=8), CCI + Rap (n=8 from Fig. 3), CCI + Rap + CQ (n=8) and CCI + Rap + Baf (n=8) groups. According to previous studies (15,27,28), Rap (Bio Vision), Baf (LC Laboratories, Woburn, MA, USA) and CQ (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were administered by intraperitoneal injection at a dose of 10, 1 and 60 mg/kg, respectively, 1 h prior to the CCI operation. At 7 days after CCI, the expression levels of the autophagy-related proteins, LC3II and Beclin 1, and those of the lysosome-related proteins, LAMP2 and RAB7 in the spinal cord were detected by western blot analysis in all 7 groups. Mechanical allodynia and thermal hyperalgesia were tested before and at 1, 3, 7 and 14 days after CCI.
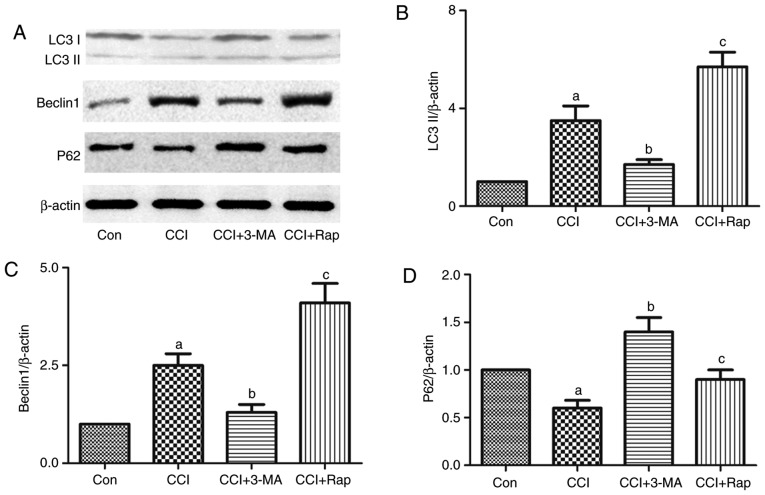
Figure 3.
Effect of the autophagic inducer, rapamycin, and inhibitor, 3-MA, on the expression of the autophagy-related proteins, LC3II, Beclin 1 and p62, in the spinal dorsal horn of rats with neuropathic pain. 3-MA and rapamycin were administered by intraperitoneal injection at a dose of 15 and 10 mg/kg body weight, respectively, 1 h prior to the CCI operation. L4-L5 spinal dorsal horn tissue was collected for the detection of (A and B) LC3II, (A and C) Beclin 1 and (A and D) p62 7 days after CCI operation by western blot analysis. The value in the Con group was set as 1 and other relative values in the CCI groups were calculated by comparison. The values are expressed as the means ± standard deviation (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. bP<0.05 vs. CCI group. cP<0.05 vs. CCI group and CCI + 3-MA group. Con, control; CCI, chronic constriction injury; 3-MA, 3-methyladenine.
Nociceptive behavioral assessment
To evaluate mechanical hyperalgesia, Von Frey filaments were used to assess the presence of mechanical hypersensitivity by measuring paw withdrawal threshold (BSEVF3, Harvard Apparatus Co., Holliston, MA, USA). The procedure was as follows: The rats were placed individually in transparent Perspex boxes with wire mesh walls and floor for 30 min of habituation time prior to behavioral tests. The filaments were individually applied vertically to the plantar side of the right hind paw in ascending order and repeated 3 times at 10-min intervals at each time point per paw. A positive response was defined as the minimal force that caused at least 2 withdrawals observed out of 3 consecutive trials (29). A maximal cut-off value of 60 g was used to prevent tissue damage.
Thermal hyperalgesia was measured as previously described by Bianchi et al (30). The rats were placed on a hot plate (a round heated surface surrounded by Plexiglas) to acclimate to the device (YLS-6B, Zhenghua Biological Instrument Equipment Co., Huaibei, China). The temperature was increased by a heat source under the plantar surface of the hind paw. Clear paw withdrawal, shaking and/or licking were considered nociceptive-like responses. The nociceptive threshold was recorded in sec and repeated 3 times at 10-min intervals at each time point. A cut-off time of 30 sec was used to avoid tissue damage (4).
Western blot analysis
The L4-L6 spinal cord segments were rapidly removed and homogenized in ice-cold sodium dodecyl sulfate (SDS) sample buffer containing protease inhibitors (Sigma-Aldrich; Merck KGaA). The lysate was centrifuged and the supernatant was removed as the total protein. Total protein homogenates were separated on a 10% SDS-polyacrylamide gel and blotted onto polyvinylidene difluoride membranes by semidry electrophoretic transfer at 15 V for 60 min. The membranes were blocked with Tris-buffered saline containing 5% non-fat milk in Tris-Tween buffer saline for 1 h at room temperature, and were first incubated overnight at 4°C with primary antibodies against rat LC3II (dilution 1:1,000; Cat. no. ab48394, Abcam, Cambridge, UK), Beclin 1 (dilution 1:1,000; Cat. no. ab62557, Abcam), p62 (dilution 1:500; Cat. no. ab91526, Abcam), GFAP (dilution 1:2,000; Cat. no. ab7260, Abcam), RAB7 (dilution 1:1,000; Cat. no. ab229647, Abcam) and LAMP2 (dilution 1:1,000; Cat. no. ab203224, Abcam). Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (anti-mouse, sc-2005; 1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA and anti-rabbit, sc-2357, 1:5,000; Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. The bands were visualized by exposing the blots to Kodak Biomax MR Films and quantified with the Bio-Rad Gel Doc 2000 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemistry
The spinal cord was post-fixed and infiltrated, and subsequently sliced into 10-µm-thick sections, permeabilized, blocked and incubated with GFAP primary antibodies (dilution 1:500, Abcam). The following day, the sections were rewarmed to room temperature, washed and incubated with the secondary antibody (dilution 1:500; Cat. no. ab150075, Abcam), counterstained with DAPI (D-21490, Thermo Fisher Scientific, Inc.), and washed for 30 min with PBS. Fluorescent images were captured using a fluorescence microscope (TCS SP2; Leica, Wetzlar, Germany).
Statistical analysis
All the results were analyzed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) and Prism® version 5.0 (GraphPad Software, San Diego, CA, USA) software. Statistical differences between more than 2 groups were analyzed with one-way analysis of variance, followed by Tukey's post hoc test. All data are expressed as the means ± standard deviation. Statistical significance was set at P<0.05.
Results
Activation of autophagy in the spinal dorsal horn of rats with neuropathic pain induced by CCI
LC3 is a mammalian autophagic protein that localizes to the autophagosome membrane in the cytosol. After autophagy is activated, LC3I from the cytosol is converted into LC3II in the autophagosome membrane. Beclin 1 is distributed in the plasma membrane, cytoplasm and nucleus, and is vital for the localization of autophagic proteins to a pre-autophagosomal structure (PAS), depending on the interaction with the class III type phosphoinositide 3-kinase (PI3KC3)/Vps34 (31). p62/SQSTM1 (p62) is a selective autophagy receptor and is degraded by autophagy; thus, increased levels of p62 reflect the inhibition of autophagy (32). In this experiment, spinal dorsal horn tissues were collected for the detection of autophagy-regulated proteins at 1, 3, 7 and 14 days after CCI. The expression levels of LC3II and Beclin 1 increased significantly from day 1 to 7 after CCI compared with the Con group, being the highest on day 3 after CCI; no significant differences were observed in these levels compared to the Con group at 14 days after CCI (Fig. 1A–C). By contrast, p62 exhibited an opposite trend in the neuropathic pain model compared with LC3II and Beclin 1. The p62 levels decreased from day 1 to 14, with the lowest expression observed at 3 days after CCI (Fig. 1A and D). Autophagy was thus activated after CCI, and there was a negative association between LC3II, Beclin 1 and p62.
Astrocyte activation at different time points in the model of neuropathic pain
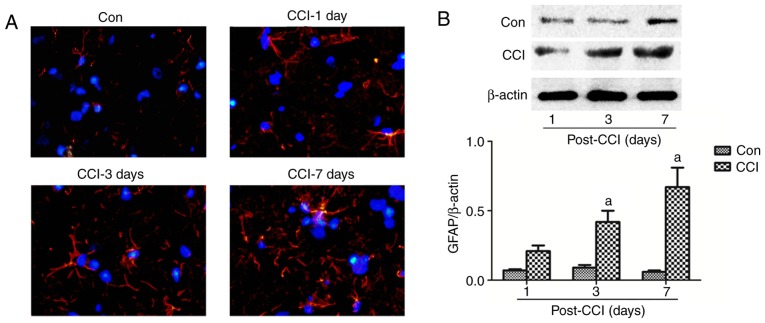
The activation of astrocytes, which are characterized by hyperplasia and hypertrophy with increased GFAP expression, has been previously described in this pain model (33,34). To specifically detect the regulatory effect of neuropathic pain on astrocyte function, the expression of GFAP was tested at various time points after CCI by immunohistochemistry and western blot analysis. Compared with GFAP staining and expression in the Con group, there was a significant increase from day 1 to 7 after CCI (Fig. 2). Therefore, pain appeared to activate astrocytes.
Figure 2.
Astrocyte changes in the spinal dorsal horn of rats with neuropathic pain. (A) A part of the L4-L6 spinal cord was obtained for the measurement of GFAP by immunofluorescence assay at 1, 3 and 7 days after CCI. Red, GFAP; blue, DAPI; magnification, ×40). (B) The L4-L5 spinal cord was collected for the detection of GFAP expression by western blot analysis at 1, 3, 7 and 14 days after CCI. The values are expressed as the means ± standard deviation (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. Con, control; CCI, chronic constriction injury; GFAP, glial fibrillary acidic protein; DAPI, 4′,6-diamidino-2-phenylindole.
Effect of autophagy induction and inhibition on LC3, Beclin 1 and p62 expression in the spinal dorsal horn of rats with neuropathic pain
CCI induced the expression of the autophagy markers, LC3 and Beclin 1, and inhibited the expression of the autophagy adaptor, p62 (Figs. 1 and 3). Compared with the CCI group, the use of the autophagy inhibitor, 3-MA, reduced the expression of LC3 and Beclin 1, and increased the expression of p62 (Fig. 3), whereas the use of the autophagy inducer, Rap, increased the expression of LC3 and Beclin 1, and decreased the expression of p62 in this neuropathic pain model (Fig. 3).
Effect of autophagy on allodynia, hyperalgesia and GFAP expression in a rat model of neuropathic pain
To determine whether autophagy regulates allodynia, hyperalgesia and GFAP expression in a rat model of neuropathic pain induced by CCI of the sciatic nerve, the rats were treated with the autophagy inducer, Rap, and the autophagy inhibitor, 3-MA. Neuropathic pain induced a rapid decrease in the threshold of mechanical allodynia and thermal hyperalgesia compared with the Con group (Fig. 4A and B). The threshold of mechanical allodynia and thermal hyperalgesia markedly decreased 1 day after CCI and remained stable until at least day 14 (Fig. 4A and B). Compared with the CCI group, the use of the autophagy inhibitor, 3-MA, significantly aggravated the decline of the threshold of mechanical and thermal hyperalgesia in the CCI + 3-MA group (Fig. 4A and B). By contrast, the threshold of mechanical and thermal hyperalgesia was markedly increased following treatment with the autophagy inducer, Rap, in the CCI + Rap group compared with the CCI group (Fig. 4A and B).
Figure 4.
Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in CCI-induced neuropathic pain. 3-MA and rapamycin were administered by intraperitoneal injection at a dose of 15 and 10 mg/kg body weight, respectively, 1 h prior to the CCI operation. (A) Mechanical allodynia and (B) thermal hyperalgesia were examined prior to and at 1, 3, 7 and 14 days after CCI. At 7 days after CCI, the spinal cord was collected to detect the expression of GFAP by (C) immunofluorescence and (D) western blot analysis. The values are expressed as the means ± standard error of the mean (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. bP<0.05 vs. CCI group. cP<0.05 vs. CCI group and CCI + 3-MA group. Con, control; CCI, chronic constriction injury; 3-MA, 3-methyladenine; GFAP, GFAP, glial fibrillary acidic protein.
Compared with the Con group, CCI induced GFAP expression, and GFAP expression exhibited an increase at 7 days after CCI surgery. Following treatment with 3-MA, GFAP expression further increased in the CCI + 3-MA group compared with the CCI group (Fig. 4C and D). By contrast, the autophagy inducer, Rap, significantly inhibited the increase in GFAP expression in the CCI + Rap group (Fig. 4C and D). These results indicate that autophagy markedly inhibits mechanical allodynia, thermal hyperalgesia and GFAP expression induced by CCI, whereas 3-MA reverses the inhibitory effects of autophagy on mechanical allodynia, thermal hyperalgesia and GFAP expression.
Expression of the lysosome-related proteins, LAMP2 and RAB7, in a rat model of neuropathic pain induced by CCI of the sciatic nerve
Autophagy is the dynamic process of sequestering cytoplasmic proteins or organelles into the lytic component; this process requires the fusion of autophagosomes and lysosomes (14,16,17). If the fusion of autophagosomes and lysosomes is blocked, damaged or malfunctioning proteins or organelles cannot be recycled. LAMP2 and RAB7 are required for the fusion of autophagosomes with lysosomes (14). LAMP2, a heavily glycosylated protein, constitutes the majority of all membrane proteins in the lysosome. RAB7 is a member of the Rab family involved in transport to late endosomes and in the biogenesis of the perinuclear lysosome compartment (14). In the present study, the expression of LAMP2 and RAB7 increased from day 1 to 14 after CCI, and this increase was significant on days 1, 3 and 7 compared with the Con group, peaking at 3 days after CCI; however, no significant increase was observed 14 days after CCI (Fig. 5).
Effect of lysosomal inhibitors, Baf and CQ, on the expression of the autophagy-related proteins, LC3II and Beclin 1, and that of the lysosome-related proteins, LAMP2 and RAB7, in a rat model of CCI
Autophagic activation does not only depend on the increased synthesis or lipidation of LC3 and Beclin 1, but also on a series of regulatory proteins orchestrating different autophagic steps, from autophagosome formation to fusion with the lysosome and subsequent release of the breakdown products (35). On the one hand, the increase in the levels of the autophagy markers, LC3 or Beclin 1, was attributed to the potent autophagic activation; on the other hand, the increase was due to the inhibition of the fusion of autophagosomes and lysosomes when autophagic activity was not profound (35). To better elucidate the mechanisms responsible for the increase in the levels of the autophagy-related proteins, LC3 and Beclin 1, in neuropathic pain, the lysosomal inhibitors, Baf A1 and CQ, were used to block the fusion process of autophagosomes and lysosomes.
Compared with the Con group, CCI induced an increase in the expression levels of LC3II and Beclin 1 (Figs. 1 and 3). The autophagy agonist, Rap, and the lysosomal inhibitors, Baf A1 and CQ, markedly increased the expression levels of LC3II and Beclin 1 compared with the CCI group (Fig. 6A–C). LC3II and Beclin 1 expression further increased by treatment with Rap and CQ, or Rap and Baf A1 compared with the CCI + CQ, CCI + Baf, or CCI + Rap groups (Fig. 6A–C).
Figure 6.
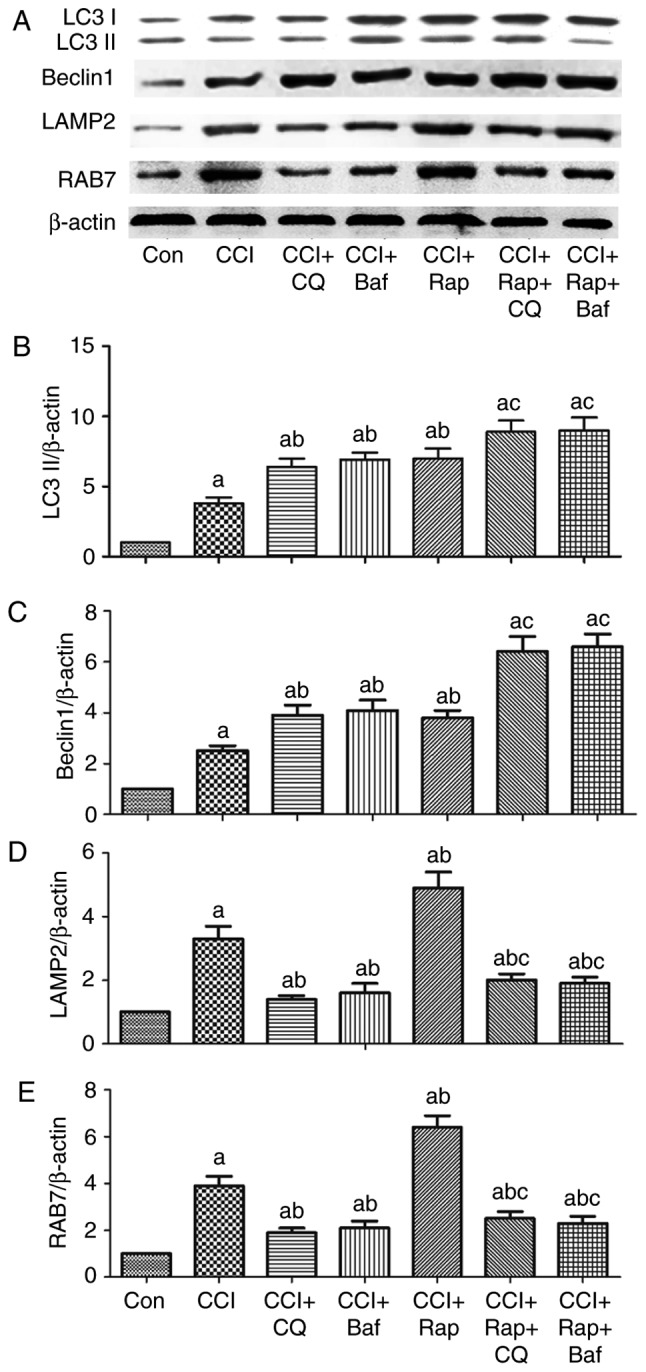
Effect of autophagy inhibition and induction on the expression of the autophagy-related proteins, LC3II and Beclin 1, and on the lysosomal-related proteins, LAMP2 and RAB7 in rats with CCI-induced neuropathic pain. L4-L5 spinal cord was collected for the detection of the autophagy-related proteins (A and B) LC3, (A and C) Beclin 1, and the lysosomal-related proteins (A and D) LAMP2 and (A and E) RAB7 by western blot analysis at 7 days after CCI. The value in the Con group was set as 1 and other relative values in the CCI groups were calculated by comparison. The values are expressed as the means ± standard deviation (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. bP<0.05 vs. CCI group. cP<0.05 vs. CCI + Rap group. Con, control; CCI, chronic constriction injury; LAMP2, lysosomal-associated membrane protein type 2; RAB7, Ras-related protein Rab-7a.
Compared with the Con group, CCI induced an increase in LAMP2 and RAB7 expression (Figs. 5, and 6A, D and E). Compared with the CCI group, the autophagy inducer, Rap, increased the expression of LAMP2 and RAB7 (Fig. 6A, D and E), whereas the lysosomal inhibitors, Baf A1 and CQ, markedly inhibited LAMP2 and RAB7 expression. Therefore, the expression of LAMP2 and RAB7 decreased significantly following treatment with Rap and CQ, or Rap and Baf A1 compared with the CCI + Rap group (Fig. 6A, D and E).
Effect of the lysosomal inhibitors, Baf A1 and CQ, on allodynia and hyperalgesia in a rat model of CCI
CCI induced a rapid decrease in the threshold of mechanical allodynia and thermal hyperalgesia compared with the Con group (Figs. 4A and B; and 7A and B). The threshold of mechanical allodynia and thermal hyperalgesia markedly decreased at 1, 3, 7 and 14 days after CCI (Figs. 4A and B; and 7). The lysosomal inhibitors, Baf A1 CCI group (Fig. 7). These results indicated that inhibiting the fusion of autophagosomes and lysosomes aggravates the decline of the threshold of mechanical and thermal hyperalgesia.
Figure 7.
Effect of autophagy inhibition and induction on allodynia and hyperalgesia in CCI-induced neuropathic pain. The grouping method was the same as that for Fig. 6. (A) Thermal hyperalgesia and (B) mechanical allodynia were examined prior to and at 1, 3, 7 and 14 days after CCI. The values are expressed as the means ± standard deviation (n=8 per group). Statistical analysis was performed with one-way analysis of variance followed by Tukey's test. aP<0.05 vs. Con group. bP<0.05 vs. CCI group. cP<0.05 vs. CCI + Rap group. Con, control; CCI, chronic constriction injury.
Discussion
Neuropathic pain is a chronic adynamic condition, which is caused by a primary injury or dysfunction in the nervous system and is primarily characterized by abnormal sensory perception of pain persistent pain, such as allodynia and hyperalgesia (1). Neuropathic pain caused by peripheral nerve injury is associated with pathological changes in the damaged peripheral nerves, dorsal root ganglion (DRG) and the activation of astrocytes, which play a critical role in the maintenance of a persistent pain state following peripheral nerve injury. The findings of the present study were as follows: i) Neuropathic pain induced an increase in the levels of the autophagy-related proteins, LC3II and Beclin 1, and the lysosome-related proteins, LAMP2 and RAB7, at different time points in the rats with CCI-induced neuropathic pain; ii) the effect of the autophagy inducer, Rap, and inhibitor, 3-MA, alleviated and exacerbated allodynia, hyperalgesia and GFAP expression in rat model of neuropathic pain, respectively; iii) the autophagy inducer, Rap, increased the expression of LC3II, Beclin 1, LAMP2 and RAB7, whereas the lysosomal inhibitors, Baf and CQ, inhibited the fusion of autophagosomes and lysosomes, increased the expression of LC3II and Beclin 1, and attenuated the expression of LAMP2 and RAB7 in rats with neuropathic pain; iv) the lysosomal inhibitors, Baf and CQ, also aggravated allodynia and hyperalgesia in rats with neuropathic pain. On the whole, these results demonstrate that peripheral nerve injury activates autophagy, which is involved in the renewal and regeneration of the injured peripheral nerves.
Peripheral nerve injury may lead to a state of chronic neuropathic pain, characterized by dysesthesia, hyperalgesia and allodynia (36). As the etiology and mechanisms underlying neuropathic pain remain unclear, the currently available treatments, including anticonvulsant agents, local anesthetics and opioids, are often unsatisfactory. Therefore, it is imperative to elucidate the mechanisms associated with the occurrence of neuropathic pain in order to develop more effective therapies. CCI has been frequently used in a rat model to induce signs of neuropathic pain (37,38), which manifests with decreased thermal and mechanical nociceptive thresholds following CCI of the sciatic nerve. Of the several experimental animal models available, signs of mechanical allodynia and thermal hyperalgesia are most evident in the neuropathic pain of the nerve ligation model. In the present study, we also examined the changes in mechanical allodynia and thermal hyperalgesia following CCI of the sciatic nerve. Consistent with previous findings (37,38), neuropathic pain induced by CCI generated allodynia and hyperalgesia, manifesting as a markedly decreased threshold of mechanical allodynia and thermal hyperalgesia 1 day after CCI, remaining constant for at least 14 days.
Astrocytes are abundant, constituting 40–50% of all glial cells (39). Under physiological conditions, astrocytes are relatively static (40); however, following injury or under disease conditions, they may become activated and participate in the pathogenesis of neurological disorders (41). Astrocyte activation is responsible for the maintenance of chronic pain, which has important implications for the development of therapeutics. Astrocytes are closely associated with neurons and blood vessels, and are crucial for the nutrition, support and protection of neurons. It is estimated that a single astrocyte enwraps 4–6 neuronal somata and is in contact with 300–600 neuronal dendrites (41). The intermediate filament protein, GFAP, is not only a marker of astrocytes in the CNS, but also facilitates the maintenance of pain. Intrathecal GFAP antisense oligonucleotide treatment has been reported to alleviate neuropathic pain behaviors in animals with nerve injury (9). The present study also focused on astrocyte activation and GFAP expression in neuropathic pain. It was demonstrated that neuropathic pain induced by CCI could activate astrocytes, which exhibited a higher GFAP expression until day 7 after CCI. Astrocyte activation participated in maintaining the pain state.
Cell death is a complex and well controlled process, including apoptosis and autophagy. Apoptosis, the main mechanism and pathogenesis of programmed cell death (type I cell death), has been extensively investigated and well verified. However, autophagy, type II cell death, participates in the initial and developmental process of disease, which is evoked in response to various stresses that finally lead to apoptosis (43); however, its pathogenesis remains obscure. As is known, autophagy is an evolutionarily conserved regulated process and gate-keeping mechanism through which damaged cytoplasmic macromolecules, organelles and redundant proteins are degraded via lysosomes to stabilize intracellular homeostasis and metabolism and to recycle cellular nutrients (44,45). Autophagic stress is responsible for Parkinson's (46,47), Huntington's (48) and Alzheimer's disease (49,50), stroke (51), and other neuropathies (52). In addition, changes in autophagy regulation have been observed in neuropathic pain (35,53) and traumatic brain injury (54). LC3 and Beclin 1 are two crucial markers of autophagy, which are closely associated with the autophagic process, particularly in its early stages (55). LC3 is associated with the formation of autophagosomes. After becoming conjugated to the lipid phosphatidylethanolamine, LC3I is cleaved to form LC3II and localizes to the autophagosome membrane. Beclin 1, a key autophagic protein localized to the PAS, forms a complex by interacting with PI3KC3/Vps34 to control autophagic nucleation and promote autophagy in mammals; the suppression of Beclin 1 expression impairs autophagy (31,55,56). p62, which is used as an autophagy marker, is the first protein reported to have such an adaptor function in autophagy, and is degraded by autophagy activation. Therefore, there is a reported association between the inhibition of autophagy and increased levels of p62 (32,57,58).
Our team always focuses on the autophagy in different model and focus on the different mechanism in neuropathic pain (59-62). In the present study, neuropathic pain activated autophagy, manifesting as an increase in LC3II and Beclin 1 expression from day 1 to 3 after CCI by sciatic nerve ligation, and then gradually declined by day 14 of the experiment, although it remained higher compared with that in the Con group. The accumulation of p62 or increased p62 levels are frequently used as signs of autophagy impairment (57,63). p62 is a major LC3-interacting protein, which possesses a C-terminal ubiquitin-binding domain and a short LC3-interacting region sequence (63,64). The present study also examined the changes in the levels of p62. p62 expression was decreased in rats with neuropathic pain, with the lowest expression being observed 3 days after CCI, and increasing again until day 14 of the experiment. Treatment with Rap, a well-known inducer of autophagy, further activated autophagy by increasing autophagosome formation and autophagosome-lysosome fusion. 3-MA is generally accepted as a specific inhibitor of autophagy. In the present study, Rap and 3-MA were used to induce and inhibit autophagy, respectively. Treatment with 3-MA and Rap notably inhibited and induced the expression of LC3II and Beclin 1, respectively, and resulted in p62 accumulation and degradation, respectively, in rats subjected to CCI. 3-MA and Rap were not only used to examine the expression of autophagy-related proteins, but also to investigate the effects of autophagy on withdrawal threshold, withdrawal latency and astrocyte activation. Neuropathic pain led to a decrease in the withdrawal threshold, withdrawal latency and astrocyte activation, and increased GFAP expression; 3-MA caused a further decrease in the withdrawal threshold, withdrawal latency and astrocyte activation, whereas Rap significantly reversed the effects of the autophagy inhibitors on withdrawal threshold, withdrawal latency and astrocyte activation. These results indicate that there is a negative association between LC3II, Beclin 1 and p62, and after the nerve is injured by ligation, tissues and cells react to stress and activate autophagy-mediated physiological and pathological changes in the rat body. However, after the nerve damage reaches a certain extent, autophagy impairment leads to tissue and cell dysfunction.
The autophagosome formation pathway consists of several stages, including initiation (formation of a PAS, leading to an isolation membrane, or phagophore), vesicle elongation, autophagosome maturation and cargo sequestration, and autophagosome-lysosome fusion. In the final phase, autophagosomal contents are degraded by lysosomal acid hydrolases and released for metabolic recycling (14). An increase in autophagy can promote LC3II and Beclin 1 protein accumulation; however, whether LC3II and Beclin 1 accumulation fully explain the results of autophagy increase or autophagosome-lysosome fusion malfunction remains unclear. In lysosomal storage diseases, defects in specific lysosomal hydrolases have been shown to result in lysosomal dysfunction and autophagy impairment (65,66). It has been reported that lysosomal dysfunction contributes to the pathological accumulation of autophagosomes, neuronal dysfunction and death (67). Therefore, the entire process of autophagy, including autophagosome formation and the fusion of autophagosomes and lysosomes, should be taken into consideration to accurately evaluate autophagy. In an attempt to elucidate this matter, it was found that the lysosomal inhibitors, Baf and CQ, inhibited the late-stage fusion of autophagosomes and lysosomes. LAMP2A, apart from acting as a receptor of autophagy, is also indispensable for efficient fusion of autophagosomes and lysosomes (68,69). RAB7 is necessary for the maturation of late autophagosomes and the fusion of autophagosomes with lysosomes (70). In the present study, we found that the expression of LAMP2 and RAB7 increased from day 1 to 14 after CCI; these expression leveks peaked at day 3 and gradually declined thereafter. The lysosomal inhibitors, Baf and CQ, markedly increased the accumulation of LC3II and Beclin 1 and inhibited the expression of LAMP2 and RAB7. Rap increased the expression of LAMP2 and RAB7 in rats with neuropathic pain when compared with the CCI group. LC3 accumulation persisted due to the increased autophagy following the fusion of autophagosomes with lysosomes (15,71). Once fusion is inhibited, LC3II accumulates, even if autophagy is no longer occurring. In the present study, compared with the CCI group, Rap + CQ or Rap + Baf treatment led to the excessive accumulation of LC3II and Beclin 1 and a decrease in LAMP2 and RAB7 levels. It was also observed that the lysosomal inhibitors, Baf and CQ, exacerbated mechanical and thermal hyperalgesia via a reduction in the withdrawal threshold and withdrawal latency. These results indicated that neuropathic pain induced autophagy, thereby increasing LC3II, Beclin 1, LAMP2 and RAB7 levels; the autophagy inducer, Rap, further increased the expression of LC3II, Beclin 1, LAMP2 and RAB7. The inhibition of the late stage of autophagy (the fusion stage of autophagosomes with lysosomes) increase LC3II and Beclin 1 levels, and decreased those of the lysosome proteins, LAMP2 and RAB7.
In conclusion, this study demonstrates that neuropathic pain induces the expression of the autophagy-related proteins, LC3II and Beclin 1, and that of the lysosome-related proteins, LAMP2 and RAB7. Increased autophagy alleviates mechanical and thermal hyperalgesia and astrocyte activation. The autophagy-lysosomal pathway was found to be responsible for the neuropathic pain induced by CCI of the sciatic nerve.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YY conceived and designed the study. HC, YH, KX, YC and HW conducted the experimental protocols in the present study. HC, YC and HW acquired analytical reagents and tools. YH, YB, YW and AD performed data analysis. YY and HC prepared the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University and were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 2.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Wang W, Wang Y, Huang J, Wu S, Li YQ. Temporal changes of astrocyte activation and glutamate transporter-1 expression in the spinal cord after spinal nerve ligation-induced neuropathic pain. Anat Rec (Hoboken) 2008;291:513–318. doi: 10.1002/ar.20673. [DOI] [PubMed] [Google Scholar]
- 5.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: New players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- 9.Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, Luo ZD. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors (corrected) Pain. 2009;143:114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/A:1007677003387. [DOI] [PubMed] [Google Scholar]
- 11.Mccall MA, Gregg RG, Behringer RR, Brenner M, Delaney CL, Galbreath EJ, Zhang CL, Pearce RA, Chiu SY, Messing A. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci USA. 1996;93:6361–6366. doi: 10.1073/pnas.93.13.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, et al. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16:587–599. doi: 10.1016/S0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Katoh A, Oguro K, Shimazaki K, Gomi H, Itohara S, Masuzawa T, Kawai N. Disturbance of hippocampal long-term potentiation after transient ischemia in GFAP deficient mice. J Neurosci Res. 2002;67:11–20. doi: 10.1002/jnr.10004. [DOI] [PubMed] [Google Scholar]
- 14.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh Ch, Pai PY, Hsueh HW, Yuan SS, Hsieh YC. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann Surg. 2011;253:1190–1200. doi: 10.1097/SLA.0b013e318214b67e. [DOI] [PubMed] [Google Scholar]
- 16.Barth S, Glick D, Macleod KF. Autophagy: Assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeger PA, Wyss-Coray T. All-you-can-eat: Autophagy in neurodegeneration and neuroprotection. Mol Neurodegener. 2009;4:16. doi: 10.1186/1750-1326-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YD, Wang ZB, Han G, Zhao P. Hyperbaric oxygen treatment attenuates neuropathic pain by elevating autophagy flux via inhibiting mTOR pathway. Am J Transl Res. 2017;9:2629–2638. [PMC free article] [PubMed] [Google Scholar]
- 19.Guo JS, Jing PB, Wang JA, Zhang R, Jiang BC, Gao YJ, Zhang ZJ. Increased autophagic activity in dorsal root ganglion attenuates neuropathic pain following peripheral nerve injury. Neurosci Lett. 2015;599:158–163. doi: 10.1016/j.neulet.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Tateda S, Kanno H, Ozawa H, Sekiguchi A, Yahata K, Yamaya S, Itoi E. Rapamycin suppresses microglial activation and reduces the development of neuropathic pain after spinal cord injury. J Orthop Res. 2017;35:93–103. doi: 10.1002/jor.23328. [DOI] [PubMed] [Google Scholar]
- 21.Piao Y, Gwon DH, Kang DW, Hwang TW, Shin N, Kwon HH, Shin HJ, Yin Y, Kim JJ, Hong J, et al. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol Brain. 2018;11:11. doi: 10.1186/s13041-018-0354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2017;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Tang G, Yue Z, Talloczy Z, Hagemann T, Cho W, Messing A, Sulzer DL, Goldman JE. Autophagy induced by Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK and mTOR signaling pathways. Hum Mol Genet. 2008;17:1540–1555. doi: 10.1093/hmg/ddn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, Yang L, Liu J, Qin ZH, Zhang HL. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6:738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 26.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 27.Lo S, Yuan SS, Hsu C, Cheng YJ, Chang YF, Hsueh HW, Lee PH, Hsieh YC. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann Surg. 2013;257:352–363. doi: 10.1097/SLA.0b013e318269d0e2. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi W, Watanabe E, Fujimura L, Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S, Hatano M. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit Care. 2013;17:R160. doi: 10.1186/cc12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yalcin I, Choucair-Jaafar N, Benbouzid M, Tessier LH, Muller A, Hein L, Freund-Mercier MJ, Barrot M. beta(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann Neurol. 2009;65:218–225. doi: 10.1002/ana.21542. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi M, Sacerdote P, Ricciardi-Castagnoli P, Mantegazza P, Panerai AE. Central effects of tumor necrosis factor alpha and interleukin-1 alpha on nociceptive thresholds and spontaneous locomotor activity. Neurosci Lett. 1992;148:76–80. doi: 10.1016/0304-3940(92)90808-K. [DOI] [PubMed] [Google Scholar]
- 31.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizushima N, Hara T. Intracellular quality control by autophagy: How does autophagy prevent neurodegeneration? Autophagy. 2006;2:302–304. doi: 10.4161/auto.2945. [DOI] [PubMed] [Google Scholar]
- 33.Svensson CI, Brodin E. Spinal astrocytes in pain processing: Non-neuronal cells as therapeutic targets. Mol Interv. 2010;10:25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- 34.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berliocchi L, Maiarù M, Varano GP, Russo R, Corasaniti MT, Bagetta G, Tassorelli C. Spinal autophagy is differently modulated in distinct mouse models of neuropathic pain. Mol Pain. 2015;11:3. doi: 10.1186/1744-8069-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolf CJ, Mannion RJ. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 37.Hamidi GA, Jafari-Sabet M, Abed A, Mesdaghinia A, Mahlooji M, Banafshe HR. Gabapentin enhances anti-nociceptive effects of morphine on heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Iran J Basic Med Sci. 2014;17:753–759. [PMC free article] [PubMed] [Google Scholar]
- 38.Lattanzi R, Maftei D, Marconi V, Florenzano F, Franchi S, Borsani E, Rodella LF, Balboni G, Salvadori S, Sacerdote P, Negri L. Prokineticin 2 upregulation in the peripheral nervous system has a major role in triggering and maintaining neuropathic pain in the chronic constriction injury model. Biomed Res Int. 2015;2015:301292. doi: 10.1155/2015/301292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/S0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 40.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 41.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Booth LA, Tavallai S, Hamed HA, Cruickshanks N, Dent P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal. 2014;26:549–555. doi: 10.1016/j.cellsig.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 47.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 48.Kegel KB, Kim M, Sapp E, McIntyre C, Castaño JG, Aronin N, DiFiglia M. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 2000;20:7268–7278. doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: Disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J Neurosci. 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 51.Den YH, He HY, Yang LQ, Zhang PY. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural Regen Res. 2016;11:1108–1114. doi: 10.4103/1673-5374.187045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boellaard JW, Kao M, Schlote W, Diringer H. Neuronal autophagy in experimental scrapie. Acta Neuropathol. 1991;82:225–228. doi: 10.1007/BF00294449. [DOI] [PubMed] [Google Scholar]
- 53.Berliocchi L, Russo R, Maiarù M, Levato A, Bagetta G, Corasaniti MT. Autophagy impairment in a mouse model of neuropathic pain. Mol Pain. 2011;7:83. doi: 10.1186/1744-8069-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10:2208–2222. doi: 10.4161/15548627.2014.981787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JP, Yang YX, Liu QL, Zhou ZW, Pan ST, He ZX, Zhang X, Yang T, Pan SY, Duan W, et al. The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells. Drug Des Devel Ther. 2015;9:1027–1062. doi: 10.2147/DDDT.S74412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Fan H, Zhou Y, Duan P, Zhao G, Wu G. Knockdown of autophagy-related gene BECLIN1 promotes cell growth and inhibits apoptosis in the A549 human lung cancer cell line. Mol Med Rep. 2013;7:1501–1505. doi: 10.3892/mmr.2013.1379. [DOI] [PubMed] [Google Scholar]
- 57.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ling H, Chen H, Wei M, Meng X, Yu Y, Xie K. The effect of autophagy on inflammation cytokines in renal ischemia/reperfusion injury. Infammation. 2016;39:347–356. doi: 10.1007/s10753-015-0255-5. [DOI] [PubMed] [Google Scholar]
- 60.Dong AL, Chen HG, Xie KL, Yu YH. Role of autophagy in lung injury in septic mice. Chin J Anesthesiol. 2015;35:1124–1127. [Google Scholar]
- 61.Wang HX, Huo XD, Chen HG, Li B, Liu J, Ma WT, Wang XJ, Xie KL, Yu YH, Shi KM. Hydrogen-rich saline activated autophagy via HIF-1α pathways in neuropathic pain model. BioMed Res Int. 2018;2018 doi: 10.1155/2018/4670834. Article ID 4670834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong A, Wang L, Wang YY, Bian YX, Yu YH, Xie KL. Role of autophagy in hydrogen-induced reduction of lung injury in septic mice. Chin J Anesthesiol. 2017;37:632–634. In Chinese. [Google Scholar]
- 63.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 64.Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget's disease of bone. J Biol Chem. 2003;278:37409–37412. doi: 10.1074/jbc.M307416200. [DOI] [PubMed] [Google Scholar]
- 65.Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- 66.Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy. 2008;4:113–114. doi: 10.4161/auto.5227. [DOI] [PubMed] [Google Scholar]
- 67.Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, Klein C, Vila M, Bezard E. Lysosomal impairment in Parkinson's disease. Mov Disord. 2013;28:725–732. doi: 10.1002/mds.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saftig P, Eskelinen EL. Live longer with LAMP-2. Nat Med. 2008;14:909–910. doi: 10.1038/nm0908-909. [DOI] [PubMed] [Google Scholar]
- 69.Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T, Xiao GD, Yang YP, Liu CF. The autophagy-lysosome pathway: A novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun. 2010;394:377–382. doi: 10.1016/j.bbrc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Gutierrez MG, Munafó DB, Berón W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: Deciding cell fate after trauma and sepsis. Trends Mol Med. 2009;15:129–138. doi: 10.1016/j.molmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.