Abstract
Piperlongumine (PL), a biologically active compound from the Piper species, has been shown to exert various pharmacological effects in a number of conditions, including tumours, diabetes, pain, psychiatric disorders and neurodegenerative disease. In this study, we evaluated the therapeutic effects of PL on hippocampal function and cognition decline in aged mice. PL (50 mg/kg/day) was intragastrically administrated to 23-month-old female C57BL/6J mice for 8 weeks. Novel object recognition and nest building behaviour tests were used to assess cognitive and social functions. Additionally, immunohistochemistry and western blot analysis were performed to examine the effects of PL on the hippocampus. We found that the oral administration of PL significantly improved novel object recognition and nest building behaviour in aged mice. Although neither the percentage area occupied by astrocytes and microglia nor the level of 4-hydroxynonenal protein, a specific marker of lipid peroxidation, were altered by PL treatment, the phosphorylation levels of N-methyl-D-aspartate receptor subtype 2B (NR2B), calmodulin-dependent protein kinase II alpha (CaMKIIα) and extracellular signal-regulated kinase 1/2 (ERK1/2) were markedly increased in the hippocampus of aged mice following the administration of PL. We also found that PL treatment resulted in a CA3-specific increase in the phosphorylation level of cyclic AMP response element binding protein, which is recognized as a potent marker of neuronal plasticity, learning and memory. Moreover, the number of doublecortin-positive cells, a specific marker of neurogenesis, was significantly increased following PL treatment in the dentate gyrus of the hippocampus. On the whole, these data demonstrate that PL treatment may be a potential novel approach in the treatment of age-related cognitive impairment and hippocampal changes.
Keywords: aging, cognitive impairment, piperlongumine, hippocampus, neurogenesis
Introduction
The aging population is increasing at a rapid rate worldwide, giving rise to a number of age-related diseases that have a significant social and economic burden on the community. With normal aging, the brain undergoes synaptic dysfunction, extensive neuronal death and declined neurogenesis. Learning and memory impairment and cognitive deficits are well-known characteristics of the aging process (1-3). In addition, aging is associated with various debilitating neurodegenerative conditions, including Alzheimer's disease (AD). Thus, the prevention or delay of the onset of age-related diseases and age-related cognitive decline may improve the quality of life.
The hippocampus, located in the medial temporal lobe of the brain, is crucial for normal learning and memory consolidation. This region is particularly vulnerable to the aging process (2,4). The hippocampus has been shown to undergo several structural and functional changes with age (2). Significant aged-related neuronal atrophy and volume decreases of the hippocampus, as well as hippocampal-dependent learning and memory decline have been demonstrated (5). An upregulation in the levels of pro-inflammatory genes and inflammatory parameters has also been observed in the hippocampus during aging (6,7). Additionally, changes in synaptic plasticity have been detected in the hippocampi of aged humans and rodents (8,9). Although the mechanisms underlying age-related synaptic plasticity impairment are still under investigation, dysregulations and alterations in the expression levels of several proteins, that play key roles in synaptogenesis and synaptic stabilization, in the hippocampus have been reported (2,10).
Piperlongumine (PL, 5,6-dihydro-1-[(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propenyl]-2(1H)-pyridinone) is a natural alkaloid that can be isolated from the long pepper (Piper longum L.). PL is found in the fruits and roots of the plant (11). Cumulative evidence has indicated that PL has a number of pharmacological activities, including antidepressant, anxiolytic, anti-fungal, antidiabetic, antinociceptive and antitumour properties (11-16). Moreover, in our previous study, it was demonstrated that administration of PL improves cognitive function in a transgenic mouse model of AD (17). Thus, we hypothesized that PL would enhance cognitive function in aged mice. In the present study, we demonstrate that PL treatment modulates age-related cognitive decline and hippocampal dysfunction in aged mice.
Materials and methods
Preparation of PL
PL was isolated from Piper longum. Preparation was performed as described in previous studies (17-19). Dried fruits (500 g) of Piper longum were extracted with ethyl acetate (EtOH; 1 liter x 3 times) at room temperature for 1 week. The combined EtOH extracts were concentrated to yield a dry residue (32.5 g), which was subsequently suspended in water (H2O; 500 ml) and partitioned with EtOAc (3×500 ml). The partial EtOAc extract (6.0 g), which was subjected to a silica gel column chromatography (CC; 5×40 cm), was eluted with a gradient n-hexane/acetone system (20:1 to 1:1) to yield 5 fractions (F1-F5). Fractions F3 and F4 were combined and further applied to a reversed phase-C18 CC (3×30 cm) with methanol (MeOH)/H2O (1:1 to 9:1). Subfraction F34.3 (60.8 mg) was purified by high-performance liquid chromatography [mobile phase: MeOH in H2O containing (0-40 min: 65% MeOH); flow rate: 2 ml/min; UV detection at 205 and 254 nm] to yield a compound (tR=17.2 min, 14 mg). The chemical structure of the isolated compound was confirmed by comparison with the reported chemical structure of PL using 1D and 2D nuclear magnetic resonance spectroscopy.
Animals
Female C57BL/6J mice, at 3 months (n=7, weighing 19-22 g) and 23 months of age (n=28, 28-34 g), were obtained from the Korea Research Institute of Bioscience and Biotechnology (KRIBB, Daejeon, Korea) and housed in regular polycarbonate plastic cages in an environment with a controlled temperature (21-22°C) and humidity (50-60%) and a 12-h light/dark cycle (lights on at 7 a.m.). The mice were maintained on an ad libitum diet of lab chow (Teklad 2018S, Harlan, WI, USA) with free access to water. The cages were filled to an approximate depth of 1.5 cm with bedding made of chopped wood particles (JSBio, Daejeon, Korea). All materials used were autoclaved and gamma-irradiated. The animal room was maintained in specific-pathogen-free conditions. The C57BL/6J mice at 23 months of age were randomized into the vehicle [0.5% carboxymethyl cellulose (CMC), Aged vehicle, n=14)] and PL (Aged PL, n=14) groups. The PL extract was suspended in 0.5% CMC at a concentration of 5 mg/ml as a stock solution. The 23-month-old female mice were orally administrated 10 μl/g/day of PL stock solution or 0.5% CMC for 8 weeks. The 3-month-old female mice were used as young controls (n=7). Multiple behaviour tests were performed on a single cohort of mice and the following order was obeyed: Open field test → novel object recognition test → nest-building behaviour test (17,20). All the animal experiments were approved by the Institutional Animal Use and Care Committee of the KRIBB (KRIBB-AEC-14074).
Open filed locomotor activity
The mice were individually placed in an open field box (45×45×45 cm3) for 30 min. The horizontal locomotion of the mouse was measured using a computerized video tracking system, SMART (Panlab, Barcelona, Spain).
Novel object recognition test
The novel object recognition test was performed as described in previous studies (21,22). The mice were individually habituated to a testing chamber (40×20×20 cm3) with no objects for 5 min and then placed in a testing chamber for 10 min with two identical objects (familiar, acquisition session). The mice were then returned to the home cages. One day later, the mice were placed back into the testing chamber in the presence of one of the original objects and one novel object (novel, recognition session) for 10 min. The original objects were cylindrical wooden blocks 10 cm high x 2 cm in diameter. The novel object was a 10×2.5×2 cm rectangular wooden block. The acquisition and recognition sessions were video-recorded and an observer, who was blinded to the drug treatment, scored the time spent exploring the objects. The chambers and objects were cleaned with ethanol between trials. Exploration was defined as sniffing and touching the object with the nose and/or forepaws. Sitting on the object was not considered exploratory behaviour. A discrimination index was calculated for each animal and expressed using the following formula: [time (number) of contacts with the novel object-time (number) of contacts with the familiar object]/[time (number) of contacts with the novel object + time (number) of contacts with the familiar object] on day 2.
Nest-building behaviour test
The nest building behaviour test was performed as described in a previous study (23). The mice were housed in single cages containing chopped wood particles for 5 days. On the first day of testing, one piece of cotton (5×5 cm; Nestlets, Ancare, Bellmore, NY, USA) was introduced into the home cage to permit nesting. The presence and quality of nesting was rated 1 day later on a 5-point scale ranging from 1 to 5 as follows: 1, nestlet not noticeably touched (>90% intact); 2, nestlet partially torn up (50-90% remaining intact); 3, mostly shredded, but often no identifiable nest site; 4, an identifiable but flat nest; and 5, a (near) perfect nest. Immediately afterward, the mice were group-housed as before.
Western blot analysis
Western blot analysis was performed as described in a previous study (21). Following 8 weeks of PL treatment, the mice were sacrificed and the hippocampal tissues were rapidly removed and homogenized in a homogenization buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate and 0.1% sodium deoxycholate) containing a cocktail of protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). Protein samples were resolved by performing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The samples were then transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The blots were incubated with primary antibodies followed by secondary antibodies, and specific signals were visualized using an Enhanced Chemi Luminescence kit (Intron Biotechnology, Gyeonggi-do, Korea). Western blot images were quantified using Quantity One 1-D analysis software version 4.6.1 (Bio-Rad Laboratories, Inc.). The primary antibodies used were vesicular glutamate transporter 1 (VGLUT1; 1:1,000, #135 302, SYSY, Göttingen, Germany), vesicular glutamate transporter 2 (VGLUT2; 1:1,000, #75-067 UC Davis/NIH NeuroMab Facility, Davis, CA, USA), glutamate receptor 1 (GluR1; a gift from Dr J.R. Lee, KRIBB, Daejeon, Korea, 1:1,000), N-methyl-D-aspartate receptor subtype 2B [(NR2B, 1:1,000, #4212, Cell Signaling Technology (CST), Danvers, MA, USA)], phosphorylated (p-)NR2B (p-Tyr-1472-NR2B, 1:1,000, #4208, CST), synaptophysin (1:1,000, #S5768, Sigma-Aldrich Co. LLC; Merck KGaA, Darmstadt, Germany), post-synaptic density protein 95 (PSD-95, 1:1,000, #124 014, SYSY), glutamate decarboxylase 65/67 (GAD65/67, 1:1,000, #AB1511, Merck KGaA), gephyrin (1:1,000, #147 011, SYSY), vesicular GABA transporter (VGAT, 1:1,000, #131 002, SYSY), cAMP response element binding protein (CREB, 1:1,000, #06-863, Merck KGaA), p-CREB (p-Ser133-CREB, 1:1,000, #06-519, Merck KGaA), calcium/calmodulin-dependent protein kinase type II α (CaMKIIα, 1:1,000, #sc-13141, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p-CaMKIIα (p-Thr-286-CaMKIIα, 1:1,000, #sc-12886, Santa Cruz Biotechnology, Inc.), extra cellular signal-regulated kinases 1/2 (ERK1/2, 1:1,000, #9102, CST), p-ERK1/2 (p-Thr202/Tyr204-ERK1/2, 1:1,000, #9101, CST) and β-actin (1:1,000, #MAB1501, Merck KGaA). The secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000, #NCI1460KR, Thermo Fisher Scientific, Inc., Waltham, MA, USA) or goat anti-mouse (1:2,000, #sc-2005, Santa Cruz Biotechnology).
Histological analysis
Immunohistochemistry and immunofluorescence staining were performed as previously described (21,24-26). Following 8 weeks of PL treatment, the mice were deeply anesthetized (250 mg/kg Avertin, intraperitoneally) and transcardially perfused with saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). The brains were removed, post-fixed overnight, and then cut into 40-μm-thick coronal sections using a vibratome (Vibratome VT1000A, Leica Microsystems GmbH, Wetzlar, Germany). The free-floating sections were then incubated in PBS containing 3% H2O2 (v/v), rinsed 3 times in PBS, and blocked with serum for 1 h at room temperature. The sections were then incubated with the phospho-CREB (Ser133, 1:1,000, #06-519, Merck KGaA), doublecortin (DCX, 1:1,000, #sc-8666, Santa Cruz Biotechnology), 4-hydroxy-2-nonenal (4-HNE, 1:1,000, #HNE11-S, Alpha Diagnostic, San Antonio, TX, USA), ionized calcium-binding adapter molecule 1 (Iba1, 1:1,000, #019-19741, Wako Chemicals USA, Inc., Richmond, VA, USA) and glial fibrillary acidic protein (GFAP, 1:1,000, #Z-0334, Dako, Glostrup, Denmark) primary antibodies overnight at 4°C. The sections were then washed and incubated with biotinylated secondary anti-rabbit IgG (1:200, #BA-1000, Vector Laboratories, Inc., Burlingame, CA, USA), followed by the avidin-biotinylated peroxidase complex (Vector Laboratories, Inc.) and 3,3′-diaminobenzidine (Sigma-Aldrich Co. LLC; Merck KGaA). Immunofluorescence staining was then performed with an Alexa Fluor 594 goat anti-rabbit IgG antibody (secondary antibody, 1:200, #A11012, Thermo Fisher Scientific, Inc.). Sections containing the hippocampus were selected and the number of doublecortin-positive cells in the dentate gyrus (DG) were counted under a microscope (Olympus Corp., Tokyo, Japan). The intensity of 4-HNE- and p-CREB-stained cells and the percentage area occupied by GFAP- and Iba-1-positive cells in hippocampal CA1, CA3 and DG were assessed using the MetaMorph image analyser (Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
GraphPad PRISM (GraphPad Software, Inc., La Jolla, CA, USA) software was used to perform the statistical analyses. Two-sample comparisons were performed using a Student's t-test, while multiple comparisons were made using a one-way ANOVA followed by the Tukey-Kramer's post hoc test. Associations between distance and discrimination index were examined by Pearson's correlation coefficient. All data are presented as the means ± SEM and statistical differences are accepted at the 5% level (P<0.05), unless otherwise indicated.
Results
PL improves the performance of aged mice in novel object recognition and nest building tasks
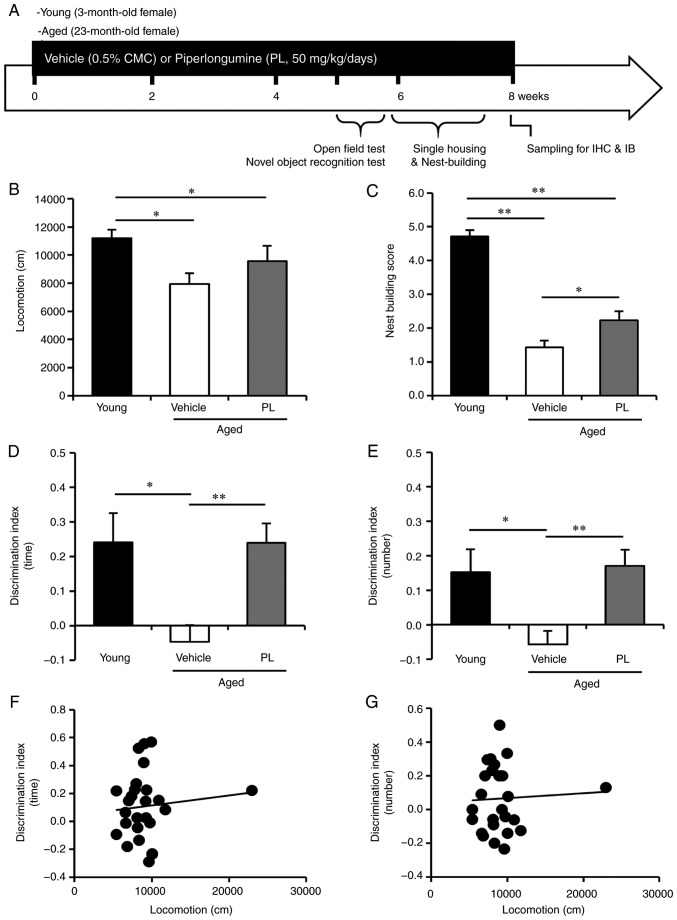
The aged female C57BL/6J mice (23 months old) were randomly separated into the vehicle- and PL-treated groups. PL was administered at a dose of 50 mg/kg/day for 8 weeks, from the ages of 23 to 25 months. The experimental design is presented in Fig. 1A. The aged mice (24 months of age) exhibited a significantly lower locomotor activity in the open field test than the young control mice (Fig. 1B, P<0.05). PL treatment did not markedly affect the exploratory behaviour of the aged mice compared to the aged vehicle group (Fig. 1B, P>0.05). To determine whether PL can improve cognitive function in aged mice, we performed the novel object recognition test. In the recognition session, with two different objects (one novel and the other familiar), the young control mice explored the novel object for a relatively long time period and a made contact with it a relatively high number of times, yielding a discrimination index (DI) of approximately 0.24±0.08 and 0.15±0.07, indicating that they had a memory of the familiar object (Fig. 1D and E). By contrast, the aged mice treated with the vehicle exhibited a DI that was significantly lower than that of the younger controls (−0.05±0.05 and −0.06±0.04, Fig. 1D and E), which is consistent with impaired cognition. PL treatment markedly increased the DI in aged mice to approximately 0.24±0.06 and 0.17±0.05 (Fig. 1D and E), reflecting a therapeutic effect of PL on age-related cognitive impairment. PL treatment did not alter the total exploration time (aged vehicle, 10.14±1.11 sec; aged PL, 8.97±0.48 sec, P=0.466) and total number of contacts (aged vehicle, 16.92±2.22; aged PL, 19.71±1.25, P=0.680) to both objects (familiar + novel) on day 2, indicating no influence on the total exploration activity of PL in the novel object recognition test. Additionally, we could not find any association between the distance in the open field test and the DI in the novel object recognition test in the aged mice (Fig. 1F and G, P>0.05).
Figure 1.
Effect of piperlongumine (PL) on novel object recognition and nest building in aged female mice. (A) Experimental design for PL treatment, behaviour testing and sampling. Open field test, novel object recognition test and nest building behaviour test were performed at 39, 42 and 54 days of PL treatment. (B) Total locomotor activity for a 30-min period in young control mice and aged mice following treatment with the vehicle or PL (young control; n=7, aged vehicle; n=14, aged PL; n=14). (C) The presence and quality of nesting over a 24 h period, rated on a 5-point scale, in young control mice and aged mice following treatment with the vehicle, or PL (young control; n=7, aged vehicle; n=8, aged PL; n=9). (D and E) The discrimination index [(D) the time spent exploring and (E) the number of contacts] of the young mice, and aged mice following treatment with the vehicle or PL in the novel object recognition test (young control; n=6, aged vehicle; n=14, aged PL; n=14). (F and G) The correlation between locomotor activity in open field test and discrimination index in novel object recognition test in aged mice was absent [F, between locomotion (B) and DI (time, D), r=0.09955, P=0.6285; G, between locomotion (B) and DI (number, E), r=0.04872, P=0.8132, n=26]. *P<0.05 and **P<0.01, significant differences from an indicated group, determined by one-way ANOVA, followed by Tukey-Kramer's post-hoc test.
Previous studies have reported that nest building, which is an indicator of well-being and social context in mice, is decreased in aging in rodent models of AD (27,28). Reduced nesting has also been observed in mice with hippocampal lesions (29). In this study, the nesting score in the nest building test was significantly lower in the aged mice than in the young control mice (Fig. 1C, P<0.05). PL significantly increased the nesting score in the aged mice (Fig. 1C, P<0.05). These results indicate that treatment with PL may improve cognitive and social decline without affecting locomotion in aged mice.
PL did not alter the glia activation and lipid peroxidation in the hippocampus of aged mice
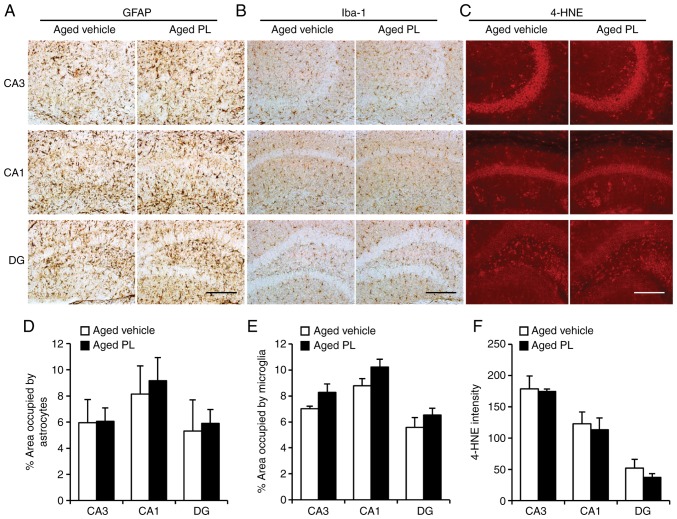
An upregulation of inflammatory responses and oxidative stress have been observed in the hippocampus in aging (30-33). An increase in inflammation in aging implicates the activation of microglia and astrocytes in the brain over this period (34). In aged brains, there is an increase in the number, size and activation of microglia (34). In this study, to investigate the effects of PL on microglia and astrocytes in aging, we measured the percentage area occupied by astrocytes (Fig. 2A and D) and microglia (Fig. 2B and E) in hippocampus through immunohistochemical assay. Additionally, immunofluorescence analysis for oxidative stress (4-HNE, an indicator of lipid peroxidation) in the hippocampus was performed (Fig. 2C and F). PL administration at a dose of 50 mg/kg/day for 8 weeks had no significant effect on glial activation and oxidative stress in the hippocampus at this point in aging.
Figure 2.
Effect of piperlongumine (PL) on neuroinflammation and oxidative stress in the hippocampus. Activation of microglia and astrocytes was analysed by immunohistochemical staining against glial fibrillary acidic protein (GFAP), ionized calcium binding adaptor molecule 1 (Iba1) and 4-hydroxynonenal (4-HNE), respectively. Images showing (A) GFAP, (B) Iba1, and (C) 4-HNE labelling in the hippocampus of aged mice treated with the vehicle or PL. (D) Percentage area of hippocampus [CA3, CA1 and dentate gyrus (DG)] occupied by astrocytes (thus GFAP labelled; aged vehicle; n=6, aged PL; n=9) in aged mice treated with the vehicle or PL. (E) Percentage area of hippocampus (CA3, CA1, and DG) occupied by microglia (thus Iba-1 labelled; aged vehicle; n=6, aged PL; n=9) in aged mice treated with the vehicle or PL. (F) 4-HNE-intensity in the hippocampus (CA3, CA1, DG) in aged mice treated with the vehicle or PL aged vehicle; n=4, aged PL; n=4). Scale bar, 200 μm. Data are presented as the means ± SEM.
PL increases the phosphorylation of NR2B, ERK1/2 and CaMKIIα in the hippocampus of aged mice
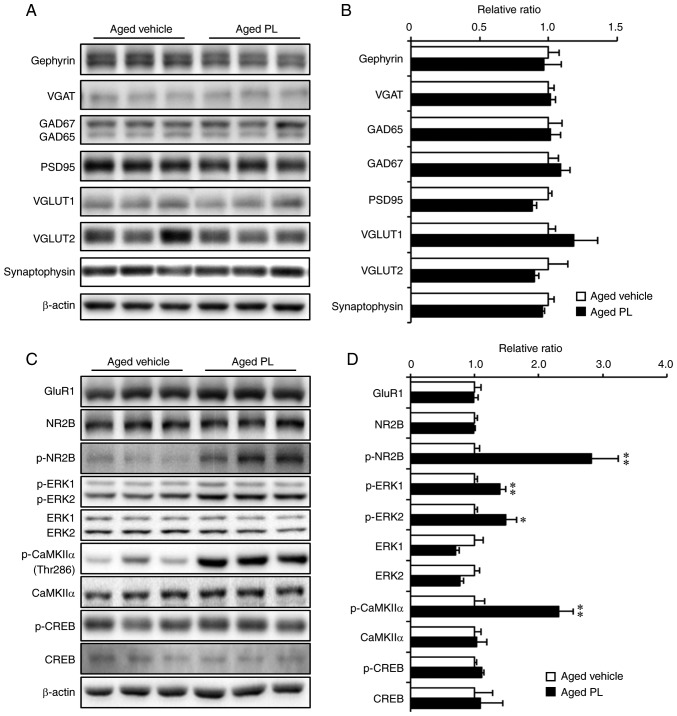
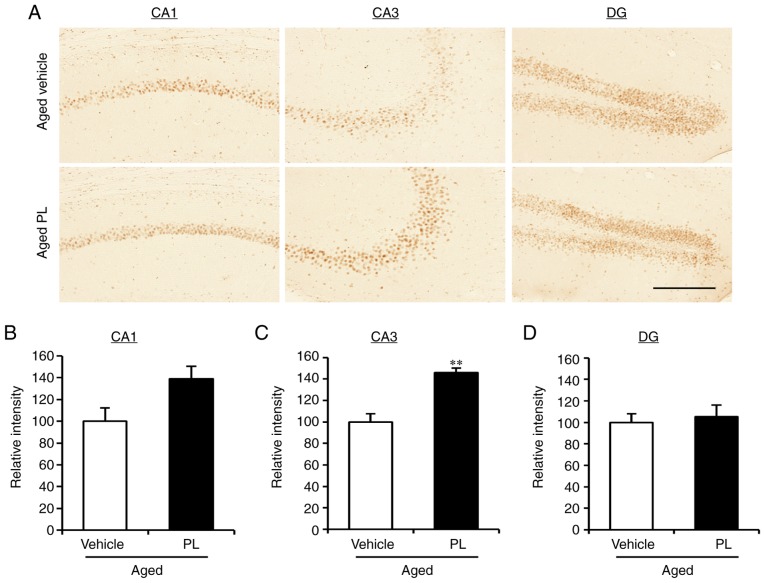
As the results from the behavioural tests pointed to a reduction in age-related cognitive impairment with PL treatment, we examined the level of synaptic markers in the hippocampus of the aged mice treated with the vehicle or PL. As indicated by the results of western blot analysis, the expression levels of gephyrin, VGAT, GAD65/67, PSD95, VGLUT1, VGLUT2 and synaptophysin were similar between the aged vehicle and aged PL groups (Fig. 3A and B). Additionally, PL had no effect on the protein expression of the AMPA (GluR1) or NMDA (NR2B) receptors (Fig. 3C and D). Of note, the levels of phosphorylation of NR2B (Tyr1472), ERK1/2 (Thr202/Tyr204) and (Thr286) were significantly higher in the aged mice treated with PL than in the aged mice treated with the vehicle (Fig. 3C and D). There was a tendency for the phosphorylation of CREB (Ser133) to be slightly higher in the aged PL group than the aged vehicle group, although this difference was not significant. To further investigate the level of p-CREB in the areas of the hippocampus, we measured the integrated optical density (IOD) of p-CREB by immunohistochemical assay in the CA1, CA3, and DG of the aged vehicle- and aged PL-treated mice (Fig. 4). The IOD in the CA3 was markedly higher in the aged mice treated with PL than in the aged mice treated with the vehicle (Fig. 4A and C, P<0.01); however, the level of p-CREB in the CA1 and DG did not differ significantly between the groups (Fig. 4A, B and D). Taken together, these results suggest that the molecular signalling pathways involving NR2B, CaMKIIα, ERK1/2 and CREB are regulated by PL treatment in the hippocampus of the aged mice.
Figure 3.
Effect of piperlongumine (PL) on the expression of synaptic proteins and NMDAR signalling proteins. (A and B) Western blot analysis and quantitative analysis of the expression of synaptic proteins [gephyrin, vesicular GABA transporter (VGAT), glutamate decarboxylase 65/67 (GAD65/67), postsynaptic density protein 95 (PSD95), vesicular glutamate transporter 1 (VGLUT1), vesicular glutamate transporter 2 (VGLUT2) and synaptophysin, aged vehicle; n=5, aged PL; n=7] in hippocampal homogenates of aged mice treated with the vehicle or PL. (C and D) Western blot analysis and quantitative analysis of the expression of NMDAR signalling proteins [glutamate receptor 1 (GluR1), N-methyl-D-aspartate receptor subtype 2B (NR2B), p-NR2B, extracellular signal-regulated kinase (ERK)1/2, p-ERK1/2, calcium/calmodulin-dependent protein kinase type II α (CaMKIIα), p-CaMKIIα, cAMP response element binding protein (CREB) and p-CREB, aged vehicle; n=5, aged PL; n=7] in hippocampal homogenates of aged mice treated with the vehicle or PL. *P<0.05 and **P<0.01, significant differences from the aged vehicle, as shown by the Student's t-test. Data are presented as the means ± SEM.
Figure 4.
Effect of piperlongumine (PL) on the phosphorylation of CREB in the hippocampus of aged mice. (A) Images showing anti-p-CREB antibody-stained CA1, CA3 and dentate gyrus (DG) of the hippocampus in aged mice treated with the vehicle or PL. Results of the quantitative analysis of the relative intensity of p-CREB in the (B) CA1, (C) CA3, and (D) DG of aged mice treated with the vehicle or PL (aged vehicle; n=5, aged PL; n=4). Scale bar, 200 μm. **P<0.01, significant differences from the aged vehicle, as shown by the Student's t-test. Data are presented as the means ± SEM.
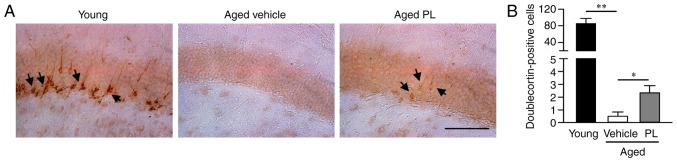
PL increases neurogenesis in the DG of aged mice
Neurogenesis markedly declines with aging and, thus, the maintenance of an adequate level of hippocampal neurogenesis is another important factor to consider in maintaining cognitive function (35). In this study, to investigate whether PL treatment affects hippocampal neurogenesis, we examined neuronal proliferation by immunohistochemistry using the neuroblast marker, DCX, in the DG of aged mice treated with the vehicle or PL (Fig. 5). The number of DCX-positive cells was markedly lower in the aged mice treated with the vehicle than in the young control group (Fig. 5). However, the number of DCX-positive cells was significantly higher in the PL treated aged mice than in the vehicle treated aged mice (Fig. 5, P<0.05). These results suggest that PL increases adult neurogenesis in the DG of aged mice.
Figure 5.
Effect of piperlongumine (PL) on hippocampal neurogenesis. (A) Representative photomicrographs of the DG of the hippocampus of young control, aged vehicle and aged PL groups. Arrows indicate doublecortin (DCX)-positive cells. (B) The number of DCX-positive cells in the DG area of aged mice treated with the vehicle or PL and young mice (young control; n=5, aged vehicle; n=6, aged PL; n=9). Scale bar, 100 μm. *P<0.05 and **P<0.01, significant differences from an indicated group, determined by one-way ANOVA, followed by Tukey-Kramer's post-hoc test. Data are presented as the means ± SEM.
Discussion
Aging is a natural biological process that is associated with physical and cognitive decline. Notably, in both normal aging and under pathological conditions, cognitive decline can diminish the quality of life. In the present study, we found that treatment with piperlongumine (PL), isolated from the long pepper, significantly improved cognitive function in novel object recognition and performance in nest building in 25-month-old female mice. These effects appear to be partly due to the modulation of neuronal activity and neurogenesis in the hippocampus. We found that treatment with PL increased the phosphorylation levels of the NR2B subunit of the NMDA receptor in the hippocampus of aged mice. Furthermore, we observed that PL significantly increased the phosphorylation of ERK1/2 at Thr202/Tyr204, CaMKIIα at Thr286, and CREB at Ser133, and increased the number of doublecortin-positive cells.
PL is a primary constituent of Piper longum, which has been reported to kill multiple types of cancer cells through the targeting of the stress response to reactive oxygen species (ROS) (14,36). Diagnosis with certain tumours, such as age-related degenerative diseases, increases with age and the molecular alterations that occur in aging can favour carcinogenesis (37). Senescent cells can drive hyperplastic pathology and promote age-related neurodegeneration (38,39). Recently, PL has been reported to be a potential novel lead for the development of senolytic agents (40) and the selective depletion of senescence cells as an anti-aging strategy may prevent cancer and aging-related degenerative diseases. Although in this study, we did not investigate the anti-tumour activities of PL in aged mice, PL treatment may be beneficial through the apoptosis of age-related senescence cells. Cellular senescence is associated with oxidative stress and inflammation (39). An increase in the expression of GFAP has been the most common change to be observed in astrocytes with aging (41). The results of this study demonstrated that PL did not affect the size of area occupied by glia, such as microglia and astrocytes, in the hippocampus of the aged mice (Fig. 2). We also observed that lipid peroxidation in the hippocampus was not altered in the aged mice (Fig. 2). However, previously, we have demonstrated that PL effectively decreases astrogliosis and microglia activation in the parietal cortex in animal models of AD (17). The results indicated that the inflammation and microglia activation that was triggered by pathological conditions were effectively suppressed by PL treatment.
The precise mechanism of action through which PL improves cognitive function remains unclear. The results of this study demonstrated that PL modulates the NR2B subunit of the NMDA receptor and CaMKII in the hippocampus (Fig. 3). The phosphorylation of NR2B at Tyr-1472 in hippocampus was increased by treatment with PL (Fig. 3C and D). The level of Tyr-1472 phosphorylation is increased after the induction of long-term potentiation (LTP) in the hippocampus, indicating that the phosphorylation of Tyr-1472 is involved in synaptic plasticity (42). Additionally, CaMKII is the main protein of post-synaptic density and is an essential protein for the induction of NMDAR-dependent LTP (43). CaMKIIα promotes synaptic formation, strengthening, and integration into existing neural circuits (44). Autophosphorylation at Thr286 of CaMKIIα is also required for NMDAR-dependent LTP and hippocampus-dependent learning (45). However, CaMKIIα activation is impaired in an age-dependent manner in the hippocampus and amygdala (46). The loss of CaMKIIα activity results in severe electrophysiological abnormalities that are associated with impaired synaptic plasticity and memory formation, while the overexpression of CaMKIIα improves cognitive performance, as assessed by Morris water maze testing (45,47). NR2B-containing NMDARs is coupled to ERK activation (48). The present study demonstrates that the oral administration of PL also significantly increased ERK1/2 and CREB phosphorylation in the hippocampus (Figs. 3 and 4). One of the key signalling proteins activated downstream of CaMKII and ERK is CREB (49,50). It has been well-documented that CREB plays a role in LTP and memory formation (51). A reduction and deficit in CREB signalling has been observed in aged animals (52). The phosphorylation of Ser133 seems to be a critical step in CREB activation (51,53). Total CREB levels do not appear to change; however, the level of p-CREB is decreased in aged rats (53,54). Additionally, the level of p-CREB expression has been found to be associated with performance in emotional memory tests, where a higher level of p-CREB is indicative of a better emotional memory performance (56,57). In the current study, PL significantly increased the phosphorylation of CREB in the CA3 region of the hippocampus (Fig. 4). Therefore, considering the functional role of these molecules in the regulation of cognitive function, the modulation of CaMKII/ERK/CREB signalling transduction could account for the therapeutic effect of PL.
The age-related decline in adult neurogenesis is a well-documented process (58). In mice, aging is associated with a decreased number of neural stem cells in the hippocampus (59). New-born neurons in aged mice are highly associated with neurogenesis-dependent cognition (60). Moreover, hippocampal neurogenesis in response to exercise and enriched environment contributes to hippocampal plasticity (58,61). Previously, we reported that PL markedly increases sirtuin 1 deacetylase activity in in vitro assays (17). Sirtuin 1 is one of seven mammalian sirtuins and has been shown to modulate aging and memory (62,63). Although the regulation of neurogenesis by sirtuin 1 has not been investigated in this study, it has been reported that the activation of sirtuin 1 restores cognitive performance and neurogenesis in mice exhibiting reduced adult neurogenesis and lowered hippocampal cognitive abilities (64). In the present study, there were few DCX-positive neuroblasts in the DG of 25-month-old female mice (Fig. 5). Moreover, the aged mice treated with PL exhibited significantly higher number of DCX-positive cells in the DG than in the aged mice treated with the vehicle (Fig. 5). These results suggest that PL may have an effect on neurogenesis by preventing or reversing age-related decline. However, the precise mechanisms responsible for the effect of PL on neurogenesis in aged mice are not yet clear. Further studies, therefore, are warranted to investigate the effects of PL on neurogenesis, including in in vitro models. Additionally, studies on target mediators of signalling pathways involved in the formation of new neurons can be utilized to determine the effect of PL on neurogenesis in the adult brain.
In conclusion, our in vivo analysis of aged female mice demonstrates that PL improves some properties of aging, such as age-associated cognitive impairments, synaptic dysfunction and the decline in neurogenesis. Although additional studies are required to elucidate the underlying molecular mechanisms and validate the anti-aging effects of PL in male mice, the results of the present study suggest that the activation of NR2B, CaMKIIα, ERK1/2 and CREB, and the increase in neurogenesis following PL treatment may contribute to hippocampal neuronal activity in the aged brain.
Acknowledgments
The authors would like to thank Dr Jae-Ran Lee (KRIBB, Republic of Korea) for the gift of GluR1 antiserum and Mr. In-Bok Lee, Ms. Jung-Hyun Choi, Mr. Young-Keun Choi and Ms. Yun-Jeong Seo for their technical assistance.
Abbreviations
- PL
piperlongumine
- AD
Alzheimer's disease
- EtOH
ethanol
- EtOAc
ethyl acetate
- MeOH
methanol
- CMC
carboxymethyl cellulose
- VGLUT1
vesicular glutamate transporter 1
- VGLUT2
vesicular glutamate transporter 2
- NR2B
N-methyl-D-aspartate receptor subtype 2B
- PSD-95
postsynaptic density protein 95
- GAD65/67
glutamate decarboxylase 65/67
- VGAT
vesicular GABA transporter
- CREB
cAMP response element binding protein
- CaMKIIα
calcium/calmodulin-dependent protein kinase type II α
- ERK1/2
extracellular signal-regulated kinases 1/2
- PBS
phosphate-buffered saline
- Iba1
ionized calcium-binding adapter molecule 1
- GFAP
glial fibrillary acidic protein
- 4-HNE
4-hydroxy-2-nonenal
- LTP
long-term potentiation
Funding
This study was supported by the KRIBB Research Initiative Program of the Republic of Korea, and the Development of Platform Technology for Innovative Medical Measurements funded by Korea Research Institute of Standards and Science (KRISS-2017-GP2017-0020).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JG, TSP, HYP, GHH, and YKR carried out the experiment and analysed the data. CHL, SK, WKO, and KSK conceived and planned the experiments. JG, CHL, and KSK wrote the manuscript. YHK, JHH, DHC, DYH and JRN contributed to sample preparation and analysed the data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Use and Care Committee of the KRIBB (KRIBB-AEC-14074).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 2.Bettio LEB, Rajendran L, Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Aaboe K, Knop FK, Vilsboll T, Vølund A, Simonsen U, Deacon CF, Madsbad S, Holst JJ, Krarup T. KATP channel closure ameliorates the impaired insulinotropic effect of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:603–608. doi: 10.1210/jc.2008-1731. [DOI] [PubMed] [Google Scholar]
- 4.Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: Behavioral, electrophysiological and morphological perspectives. Prog Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-L. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: A multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 7.Ojo JO, Rezaie P, Gabbott PL, Stewart MG. Impact of age-related neuroglial cell responses on hippocampal deterioration. Front Aging Neurosci. 2015;7:57. doi: 10.3389/fnagi.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gureviciene I, Gurevicius K, Tanila H. Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus. J Neural Transm (Vienna) 2009;116:13–22. doi: 10.1007/s00702-008-0149-x. [DOI] [PubMed] [Google Scholar]
- 9.Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Arch Neurol. 2009;66:829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- 10.Nyffeler M, Zhang WN, Feldon J, Knuesel I. Differential expression of PSD proteins in age-related spatial learning impairments. Neurobiol Aging. 2007;28:143–155. doi: 10.1016/j.neurobiolaging.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Bezerra DP, Pessoa C, de Moraes MO, Saker-Neto N, Silveira ER, Costa-Lotufo LV. Overview of the therapeutic potential of piplartine (piperlongumine) Eur J Pharm Sci. 2013;48:453–463. doi: 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Cícero Bezerra Felipe F, Trajano Sousa Filho J, de Oliveira Souza LE, Alexandre Silveira J, Esdras de Andrade Uchoa D, Rocha Silveira E, Deusdênia Loiola Pessoa O, de Barros Viana GS. Piplartine, an amide alkaloid from Piper tuberculatum, presents anxiolytic and antidepressant effects in mice. Phytomedicine. 2007;14:605–612. doi: 10.1016/j.phymed.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues RV, Lanznaster D, Longhi Balbinot DT, de Gadotti VM, Facundo VA, Santos AR. Antinociceptive effect of crude extract, fractions and three alkaloids obtained from fruits of Piper tuberculatum. Biol Pharm Bull. 2009;32:1809–1812. doi: 10.1248/bpb.32.1809. [DOI] [PubMed] [Google Scholar]
- 14.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Rao VR, Muthenna P, Shankaraiah G, Akileshwari C, Babu KH, Suresh G, Babu KS, Chandra Kumar RS, Prasad KR, Yadav PA, et al. Synthesis and biological evaluation of new piplartine analogues as potent aldose reductase inhibitors (ARIs) Eur J Med Chem. 2012;57:344–361. doi: 10.1016/j.ejmech.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navickiene HM, Alécio AC, Kato MJ, Bolzani VD, Young MC, Cavalheiro AJ, Furlan M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry. 2000;55:621–626. doi: 10.1016/S0031-9422(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 17.Go J, Ha TKQ, Seo JY, Park TS, Ryu YK, Park HY, Noh JR, Kim YH, Hwang JH, Choi DH, et al. Piperlongumine activates Sirtuin1 and improves cognitive function in a murine model of Alzheimer's disease. J Funct Foods. 2018;43:103–111. doi: 10.1016/j.jff.2018.02.002. [DOI] [Google Scholar]
- 18.Peng S, Zhang B, Meng X, Yao J, Fang J. Synthesis of piper-longumine analogues and discovery of nuclear factor erythroid 2-related factor 2 (Nrf2) activators as potential neuroprotective agents. J Med Chem. 2015;58:5242–5255. doi: 10.1021/acs.jmedchem.5b00410. [DOI] [PubMed] [Google Scholar]
- 19.Tabuneng W, Bando H, Amiya T. Studies on the constituents of the crude drug 'piperis longi fructus'. On the alkaloids of fruits of piper longum L. Chem Pharm Bull. 1983;31:3562–3565. doi: 10.1248/cpb.31.3562. [DOI] [Google Scholar]
- 20.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HY, Ryu YK, Kim YH, Park TS, Go J, Hwang JH, Choi DH, Rhee M, Lee CH, Kim KS. Gadd45β ameliorates L-DOPA-induced dyskinesia in a Parkinson's disease mouse model. Neurobiol Dis. 2016;89:169–179. doi: 10.1016/j.nbd.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Park TS, Ryu YK, Park HY, Kim JY, Go J, Noh JR, Kim YH, Hwang JH, Choi DH, Oh WK, et al. Humulus japonicus inhibits the progression of Alzheimer's disease in a APP/PS1 transgenic mouse model. Int J Mol Med. 2017;39:21–30. doi: 10.3892/ijmm.2016.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deacon RM, Cholerton LL, Talbot K, Nair-Roberts RG, Sanderson DJ, Romberg C, Koros E, Bornemann KD, Rawlins JN. Age-dependent and -independent behavioral deficits in Tg2576 mice. Behav Brain Res. 2008;189:126–138. doi: 10.1016/j.bbr.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim YJ, Kang Y, Park HY, Lee JR, Yu DY, Murata T, Gondo Y, Hwang JH, Kim YH, Lee CH, et al. STEP signaling pathway mediates psychomotor stimulation and morphine withdrawal symptoms, but not for reward, analgesia and tolerance. Exp Mol Med. 2016;48:e212. doi: 10.1038/emm.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu YK, Kang Y, Go J, Park HY, Noh JR, Kim YH, Hwang JH, Choi DH, Han SS, Oh WK, et al. Humulus japonicus prevents dopaminergic neuron death in 6-hydroxydopamine-induced models of Parkinson's disease. J Med Food. 2017;20:116–123. doi: 10.1089/jmf.2016.3851. [DOI] [PubMed] [Google Scholar]
- 26.Ryu YK, Park HY, Go J, Choi DH, Kim YH, Hwang JH, Noh JR, Lee TG, Lee CH, Kim KS. Metformin inhibits the development of L-DOPA-induced dyskinesia in a murine model of Parkinson's disease. Mol Neurobiol. 2018;55:5715–5726. doi: 10.1007/s12035-017-0752-7. [DOI] [PubMed] [Google Scholar]
- 27.Wesson DW, Wilson DA. Age and gene overexpression interact to abolish nesting behavior in Tg2576 amyloid precursor protein (APP) mice. Behav Brain Res. 2011;216:408–413. doi: 10.1016/j.bbr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filali M, Lalonde R, Rivest S. Subchronic memantine administration on spatial learning, exploratory activity, and nest-building in an APP/PS1 mouse model of Alzheimer's disease. Neuropharmacology. 2011;60:930–936. doi: 10.1016/j.neuropharm.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Deacon RM, Croucher A, Rawlins JN. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res. 2002;132:203–213. doi: 10.1016/S0166-4328(01)00401-6. [DOI] [PubMed] [Google Scholar]
- 30.Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J Neuroinflamm. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 32.Stebbings KA, Choi HW, Ravindra A, Llano DA. The impact of aging, hearing loss, and body weight on mouse hippocampal redox state, measured in brain slices using fluorescence imaging. Neurobiol Aging. 2016;42:101–109. doi: 10.1016/j.neurobiolaging.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-E. [DOI] [PubMed] [Google Scholar]
- 34.von Bernhardi R, Eugenín-von Bernhardi L, Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 2015;7:124. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav Brain Res. 2012;227:497–507. doi: 10.1016/j.bbr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharadwaj U, Eckols TK, Kolosov M, Kasembeli MM, Adam A, Torres D, Zhang X, Dobrolecki LE, Wei W, Lewis MT, et al. Drug-repositioning screening identified piperlongumine as a direct STAT3 inhibitor with potent activity against breast cancer. Oncogene. 2015;34:1341–1353. doi: 10.1038/onc.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balducci L, Ershler WB. Cancer and ageing: A nexus at several levels. Nat Rev Cancer. 2005;5:655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- 38.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 39.Campisi J. Aging, cellular senescence, and cancer. Ann Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, Zhou D, Zheng G. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 2016;8:2915–2926. doi: 10.18632/aging.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-P. [DOI] [PubMed] [Google Scholar]
- 42.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 43.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 44.Asrican B, Lisman J, Otmakhov N. Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II. J Neurosci. 2007;27:14007–14011. doi: 10.1523/JNEUROSCI.3587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 46.Fang T, Kasbi K, Rothe S, Aziz W, Giese KP. Age-dependent changes in autophosphorylation of alpha calcium/calmodulin dependent kinase II in hippocampus and amygdala after contextual fear conditioning. Brain Res Bull. 2017;134:18–23. doi: 10.1016/j.brainresbull.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/S0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 49.Kreusser MM, Backs J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front Pharmacol. 2014;5:36. doi: 10.3389/fphar.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 51.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Ann Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 52.Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippo-campal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 54.Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15:1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- 55.Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Cowansage KK, Bush DE, Josselyn SA, Klann E, Ledoux JE. Basal variability in CREB phosphorylation predicts trait-like differences in amygdala-dependent memory. Proc Natl Acad Sci USA. 2013;110:16645–16650. doi: 10.1073/pnas.1304665110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu XW, Oh MM, Disterhoft JF. CREB, cellular excitability, and cognition: Implications for aging. Behav Brain Res. 2017;322:206–211. doi: 10.1016/j.bbr.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan X, Wheatley EG, Villeda SA. Mechanisms of Hippocampal Aging and the Potential for Rejuvenation. Ann Rev Neurosci. 2017;40:251–272. doi: 10.1146/annurev-neuro-072116-031357. [DOI] [PubMed] [Google Scholar]
- 59.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgenstern NA, Lombardi G, Schinder AF. Newborn granule cells in the ageing dentate gyrus. J Physiol. 2008;586:3751–3757. doi: 10.1113/jphysiol.2008.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 62.Herskovits AZ, Guarente L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013;23:746–758. doi: 10.1038/cr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sellner S, Paricio-Montesinos R, Spieß A, Masuch A, Erny D, Harsan LA, Elverfeldt DV, Schwabenland M, Biber K, Staszewski O, et al. Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun. 2016;4:102. doi: 10.1186/s40478-016-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.