Abstract
The present study investigated the role of bidirectional ephrin-B2/erythropoietin-producing human hepatocellular receptor 4 (ephB4) signaling in the regulation of wear particle-mediated osteoclastogenesis in vitro. Mouse bone marrow macrophages (BMMs) were induced into osteoclasts by receptor activator of nuclear factor-κB ligand (RANKL, 50 ng/ml). EphB4-Fc, an osteoblast membrane surface receptor (4 µg/ml), was used to stimulate the ephrin-B2 ligand of osteoclasts in the presence and absence of titanium (Ti). Tartrate-resistant acid phosphatase (TRAP) staining was used to detect the number of osteoclasts, and phalloidin staining was used to examine the cytoskeletons of the osteoclasts. A bone pit absorption experiment was used to measure osteoclast function. Reverse transcription quantitative polymerase chain reaction and western blot analysis were used to examine osteoclastogenesis. ELISAs were used to detect the production of inflammatory factors. The data demonstrated that Ti significantly promoted the differentiation of BMMs into mature osteoclasts in the presence of RANKL and significantly promoted expression of the ephrin-B2, nuclear factor of activated T-cells 1 (NFATc1), TRAP, Fos proto-oncogene, AP-1 transcription factor subunit (C-FOS), and matrix metalloproteinase 9 (MMP9) genes. Phalloidin and TRAP staining revealed that following the addition of ephB4-Fc, the number, size and cytoskeletal elements of Key words: osteoclasts, osteoblasts, remodeling, ephrin-B2, osteoclastogenesis osteoclasts were significantly decreased compared with those in the titanium particle group without ephB4-Fc. Compared with the titanium particle group, the bone pit absorption experiment revealed significantly decreased absorption pit areas in the titanium particle+ephB4-Fc group. The expression of the NFATc1, TRAP, C-FOS and MMP9 genes was markedly decreased in the ephB4-Fc group; however, the expression of the ephrin-B2 gene was increased compared with the Ti particle group without ephB4-Fc after 5 days. Production of inflammatory cytokines was inhibited by Ti particles through bidirectional signals. Addition of ephB4-Fc inhibited the osteoclast-mediated formation of Ti particles via bidirectional ephrin-B2/ephB4 signaling. Activation of this bidirectional signaling pathway may be a potential clinical treatment for osteolysis surrounding prostheses.
Keywords: osteoclasts, osteoblasts, remodeling, ephrin-B2, osteoclastogenesis
Introduction
In the previous few decades, a large number of joint replacement surgeries have been conducted worldwide, and arthroplasty has become the most important treatment for severe joint diseases, benefitting millions of patients each year (1–3). However, aseptic loosening remains an issue with total joint replacement procedures (4–6). Inflammation and subsequent periprosthetic osteolysis induced by wear particles, including titanium (Ti), ceramic and polymethylmethacrylate, has been demonstrated to be the leading pathological mechanism contributing to aseptic loosening (7,8). Various studies on the effects of wear particles on bone remodeling have been conducted at the molecular level; for example, wear particles may activate a number of osteoclast signaling pathways, including calcineurin/nuclear factor of activated T-cells (NFAT), nuclear factor-κB (NF-κB), extracellular signal-regulated kinase-mitogen-activated protein kinase and protein kinase B (1,9–12), and may accelerate osteoclast differentiation and bone resorption functions. These signaling pathways should be the focus of future studies into the prevention and treatment of aseptic loosening.
Bone reconstruction is based on achieving a dynamic equilibrium between osteoblast-mediated bone formation and osteoclast-mediated bone resorption (13). The communication and interaction between osteoblasts and osteoclasts is the key mechanism maintaining homeostasis in bone remodeling (14). In previous decades, it was widely acknowledged that osteoblasts were the leading factor in this communication, affecting the biological behavior of osteoclasts through the receptor activator of nuclear factor κB (RANK)/RANK ligand (RANKL) signaling pathway (15). However, accumulating evidence suggests that osteoclasts may similarly affect differentiation, osteogenesis and apoptosis of osteoblasts; consequently, the communication is now thought to be bidirectional (16).
The erythropoietin-producing human hepatocellular (eph) receptors, the largest family of tyrosine kinase receptors, are located on the cell membrane and are divided into two classes. Ephrin proteins are ligands of eph receptors and are divided into 2 types, A and B (16). Previous studies investigating eph/ephrin signaling have primarily concentrated on neurology, hematology and gastroenterology (17); few studies have focused on orthopedics. This role of bidirectional ephrin-B2/EPH receptor 4 (ephB4) signaling was previously investigated in the field of bone formation (18). Preliminary data suggested that osteoblasts primarily express ephB4, osteoclasts primarily express ephrin-B2 and that the cells communicate through ephrin-B2/ephB4 signaling (19). When the intracellular Fos proto-oncogene, AP-1 transcription factor subunit (C-FOS)/NFATc1 signal is activated in osteoclasts, the ephrin-B2 gene located downstream of the signal is translated into ephrin-B2 and expressed on the cell membrane. Activation of the ephrin-B2 signal may reverse inhibition of osteoclast differentiation into mature osteoclasts. A number of signaling pathways are involved in the imbalance of bone remodeling induced by wear debris, and ascertaining which of these is the most important is imperative (20).
Based on previous data, we hypothesized that ephrin-B2/ephB4 signaling may be a vital pathway for the imbalance of bone remodeling surrounding prostheses. The aim of the present study was to determine whether Ti particle-mediated osteolysis may be inhibited by activation of ephrin-B2, which may provide a potential therapeutic target for the treatment of aseptic loosening.
Materials and methods
Reagents
α-minimum essential medium (α-MEM), fetal bovine serum (FBS) and a penicillin-streptomycin solution were obtained from Gibco; Thermo Fisher Scientific, Inc., (Waltham, MA, USA). Soluble recombinant mouse macrophage-colony stimulating factor (M-CSF), recombinant mouse RANKL, recombinant mouse ephB4-Fc and Fc fragments were obtained from R&D Systems, Inc., (Minneapolis, MN, USA). Tartrate-resistant acid phosphatase (TRAP) was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). High purity Ti particles (average diameter. 3–5 µM) were obtained from Johnson Matthey (cat. no. 00681; London, UK). Dexamethasone (Sigma-Aldrich; Merck KGaA), ascorbic acid (Sigma-Aldrich; Merck KGaA), and β-glycerol phosphate sodium (Sigma-Aldrich; Merck KGaA). The common antibodies, GAPDH, C-FOS, NFATc1, TRAP and cathepsin-K (CK) were purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA). The ephrin-B2 antibodies were purchased from R&D Systems. ELISA kits for detecting mouse interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α and IL-10 were purchased from R&D Systems, Inc.
Cell culture
A total of 24 healthy female C57BL/6 mice (16–18 g/per) were obtained from the Animal Center Research Committee of the Shanghai Ninth People's Hospital (Shanghai, China). All animal procedures were approved by the Animal's Hospital affiliated with Shanghai Jiao Tong University. All mice were housed in a temperature range of 20–26°C, relative humidity of 70%, light intensity of ≥200 Lux (12 h light/dark) and free access to water and feeding. MC-3T3-E1 cells were purchased from the Chinese Academy of Science (Shanghai, China). Bone marrow macrophages (BMMs) were collected from the tibias and femurs of 4–6-week-old C57BL/6 mice, then incubated in α-MEM containing 30 ng/ml M-CSF, 10% FBS and 1% penicillin-streptomycin at 37°C for 3 days; the medium was changed on the third day. Non-adherent cells were removed from the medium after 7 days. At ~80% confluence, the cells were dissociated with 0.25% (v/v) trypsin, then plated onto culture plates for subsequent experiments. For co-cultures, BMMs were seeded at a density of 5×105 cells in 12-well plates containing 5×104 MC-3T3-E1 cells in 1 ml α-MEM supplemented with 10% FBS, 10−8 M dexamethasone, 50 mg/l ascorbic acid and 10 mM β-glycerol phosphate sodium. To stimulate reverse signaling, soluble recombinant mouse ephB4-Fc and Fc fragments were added to the medium at a concentration of 4 µg/ml.
Preparation of Ti particles
Ti particles were prepared as described previously (1,11). Briefly, the particles were soaked in anhydrous 75% (v/v) ethanol to remove endotoxins for 48 h, following which endotoxin concentration was <0.1 EU/ml. A chromogenic end-point TAL with Diazo coupling kit (Xiamen Houshiji, Fujian, China) was used to detect endotoxins. A 20 mg/ml Ti stock solution was prepared with PBS and co-cultured with the osteoclasts at a concentration of 0.1 mg/ml to simulate the concentration of Ti particles surrounding the prostheses in clinical settings (21,22).
Alkaline phosphatase (ALP) staining
To detect the level of osteogenic function, 3T3-E1 cells (1×105 cells/well) were seeded on 24-well plates in triplicate. When the medium was changed the next day, medium containing 10–8 M dexa-methasone, 50 mg/l ascorbic acid and 10 mM β-glycerol phosphate sodium was added to the plate. A total of four cell groups: 3T3-E1; 3T3-E1 ephrinB2-Fc (4 µg/ml); 3T3-E1+Ti (0.1 mg/ml); and 3T3-E1+ephrinB2-Fc (4 µg/ml)+Ti (0.1 mg/ml) groups were formed. The medium was changed every 2 days. Paraformaldehyde (4%; PFA) was added to the plates for 20 min at 37°C on day 7. According to the protocol of the manufacturer, the cells were stained with an ALP kit (Renbao, Shanghai, China), and observed by light microscopy (magnification, ×10). ALP activity was determined at 405 nm using and the P-nitrophenyl phosphate (pNPP) (Sigma-Aldrich; Merck KGaA) served as the substrate. The bicinchoninic acid assay method was used to determined the protein contents. Quantitative and statistical analysis of ALP data was also performed as described below.
TRAP staining
BMMs (1×104 cells/well) were seeded onto 96-well plates in triplicate. After 24 h, the medium containing the cell suspension was replaced with a medium containing 30 ng/ml M-CSF and 50 ng/ml RANKL. After 7 days of culture, 4% PFA was used to fix osteoclasts for 20 min at 37°C then the cells were rinsed three times with PBS. The TRAP staining kit (Sigma-Aldrich; Merck KGaA) was used to detect osteoclast activity according to the manufacturer's protocol. Cells were considered TRAP positive if the number of nuclei was >3. Mature osteoclasts were counted using a light microscope. The total area of mature osteoclasts was quantified using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
F-actin ring formation assay
A total of four groups of samples were fixed with 4% PFA for 20 min at 37°C then permeabilized with 0.1% (v/v) Triton-100 (Sigma-Aldrich; Merck KGaA). Cells were stained with rhodamine-conjugated phalloidin (Cytoskeleton, Inc., Denver, CO, USA) at 37°C for 1 h. The cells were then washed with PBS three times, each time for 10 min. An LSM5 confocal microscope (magnification, ×10; Carl Zeiss AG, Oberkochen, Germany) was used to visualize the cytoskeleton (F-actin ring). Images were analyzed using Image-Pro Plus 6.0. Experiments were performed in triplicate.
Bone pits resorption assay
Sterile bone pieces from the four groups were placed into 96-well plates, then washed with PBS three times. BMMs (1×104 cells) were seeded into plates with 30 ng/ml M-CSF and 50 ng/ml RANKL, and the medium was changed every two days. After 12 days, the cells on the bone pieces were digested with 0.25% (v/v) trypsin at 37°C for 5 min, and then washed 3–5 times with PBS. Bone absorption images were obtained with a Quanta 250 scanning electron microscope (SEM; FEI; Thermo Fisher Scientific, Inc.) with a magnification of 10 kV. The resorption area was measured using Image-Pro Plus 6.0. Experiments were performed in triplicate
Immunofluorescence staining of ephrin-B2 localization
BMMs were seeded at a density of 1×105 cells into the plates and divided into two groups; a BMMs+ephB4-Fc; and a clustered BMMs+ephB4-Fc groups. When mature osteoclasts formed, they were fixed with 4% PFA for 20 min at 37°C and washed 2–3 times with PBS for 10 min, then permeabilized in 0.1% Triton X-100. The cells were incubated (37°C) with 5% bovine serum albumin (cat. no. A602440; Sangon Biotech Co., Ltd., Shanghai, China) for 60 min, and then a mouse anti-ephrin-b2 monoclonal antibody (cat. no. ab150411; 1:250; Abcam, Cambridge, UK) as added to the plates at 4°C for 12 h. Goat Anti-Rabbit IgG (Alexa Fluor®488; cat. no. 111-547-008; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) conjugated with flFluor®488 (1:250) at room temperature for 1 h in darkness. An LSM5 confocal microscope (magnification, x40) was used to visualize ephrin-b2 localization.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
BMMs were seeded at a density of 4×106 cells into 6-well plates with Ti particles (30 ng/ml M-CSF, 50 ng/ml RANKL) and treated with or without 4 µg/ml ephB4-Fc. The 3T3-E1 cells (5×105 cells/well) were seeded on 6-well plates. The cell culture conditions were as aforementioned. We set up four groups as described previously. Total RNA was collected using an RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) at 1, 3 and 5 days and reverse transcribed into cDNA (Takara Bio, Inc., Otsu, Japan). The SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.) and an ABI 7500 Sequencing Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to perform qPCR. The following thermocycling conditions were used: 40 cycles of denaturation at 95°C for 5 sec and amplification at 60°C for 24 sec. GAPDH was used as the reference gene, and all the reactions were run in triplicate. The PCR primers were designed as follows: Ephrin-B2 forward (F), 5′-AAT CTC CTG GGT TCC GAA GT-3′; reverse (R), 5′-GTC TCC TGC GGT ACT TGA GC-3; NFATc1 F, 5′-CAA CGC CCT GAC CAC CGA TAG-3′; NFATc1 R, 5′-GGC TGC CTT CCG TCT CAT AGT-3′; TRAP F, 5′-AAA TCA CTC TTT AAG ACC AG-3′; TRAP R, 5′-TTA TTG AAT AGC AGT GAC AG-3′; CK F, 5′-CCT CTC TTG GTG TCC ATA CA-3′; CK R, 5′-ATC TCT CTG TAC CCT CTG CA-3′; matrix metalloproteinase 9 (MMP9) F, 5′-AGA CGA CAT AGA CGG CAT CC-3′; MMP9 R, 5′-TGG GAC ACA TAG TGG GAG GT-3′; GAPDH F, 5′-CAC CAC CAT GGA GAA GGC CG-3′; GAPDH R, 5′-ATG ATG TTC TGG GCA GCC CC-3′; RANKl F, 5′-CCC ATC GGG TTC CCA TAA AGT-3′; RANKl R, 5′-CGA CCA GTT TTT CGT GCT CC-3′; osteoclastogenesis inhibitory factor (OPG) F, 5′-CGA GCG CAG ATG GAT CCT AA-3′; OPG R, 5′-CCA CAT CCA ACC ATG AGC CT-3′; EphB4 F, 5′-TGA GGT TGC TGC TTT GAA TG-3′; EphB4 R, 5′-AGG CAC TGC AGG AGA AAG AA-3′; Collagen 1 F, 5′-GAG AGG TGA ACA AGG TCC CG-3′; Collagen 1 R, 5′-AAA CCT CTC TCG CCT CTT GC-3′; runt related transcription factor 2 (RUNX2) F, 5′-TCG GAG AGG TAC CAG ATG GG-3′; RUNX2 R, 5′-TGA AAC TCT TGC CTC GTC CG-3′.
Western blot analysis
The protein expressions levels of ephrin-b2, NFATc1, TRAP, CK, MPP9 and GAPDH were measured with and without ephB4-Fc treatment. After 5 days, radioimmunoprecipitation assay (RIPA) lysis buffer (cat. no. C500005; Sangon Biotech Co., Ltd.) containing 1 µM protease inhibitor was added to 2×107 BMMs in 6-well plates for 15 min. Samples were centrifuged (4°C) at 12,000 × g for 10 min and the supernatant was collected. Total protein concentration was measured by the bicinchoninic acid assay (BCA). Equal amounts of the protein lysates were separated via SDS-PAGE (10% gel) and gels were transferred to polyvinylidene difluoride membranes, blocked for 1 h with 5% (w/v) milk, and incubated at 37°C with primary antibodies against GAPDH (cat. no. #8884; 1:1,000; Cell Signaling Technology, Inc.), C-FOS (cat. no. #2250; 1:1,000; Cell Signaling Technology, Inc.), NFATc1 (cat. no. #8032; 1:1,000; Cell Signaling Technology, Inc.) and TRAP (cat. no. ab133238; 1:1,000; Abcam) overnight. The Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA) was used to detect horseradish peroxidase-conjugated secondary antibodies (cat. no. #7074; 1:5,000; Cell Signaling Technology, Inc.) reactivity.
Statistical analysis
Data are expressed as means ± standard deviation. Differences among groups were analyzed by one-way analysis of variance and the post hoc tests with the Student-Newman-Keuls post-hoc test with SPSS software (version 11.0; SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Direct co-culture inhibits osteoclast differentiation
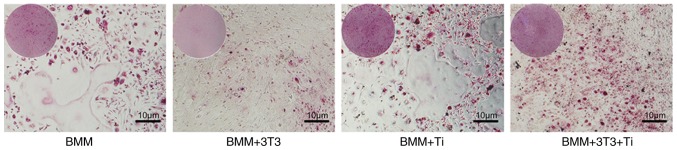
BMMs were seeded at a density of 5×105 cells in 12-well plates containing 1×105 3T3 cells and osteogenic induction media with 30 ng/ml M-CSF and 50 ng/ml RANKL. Cells were divided into four groups: BMMs; BMMs+EphB4-Fc; BMMs+Ti; and BMMs+EphB4-Fc+Ti groups. TRAP staining indicated that the number of TRAP-positive multinucleated cells in the BMMs+EphB4-Fc+Ti group was significantly decreased compared with the BMMs+Ti group. This suggested that direct co-culture inhibited osteoclast differentiation into mature osteoclasts (Fig. 1).
Figure 1.
TRAP staining of 3T3 and BMMs co-cultures. The results indicated that the number of TRAP-positive staining was significantly decreased compared with that of BMMs with Ti particles. BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; Ti, titanium.
ALP
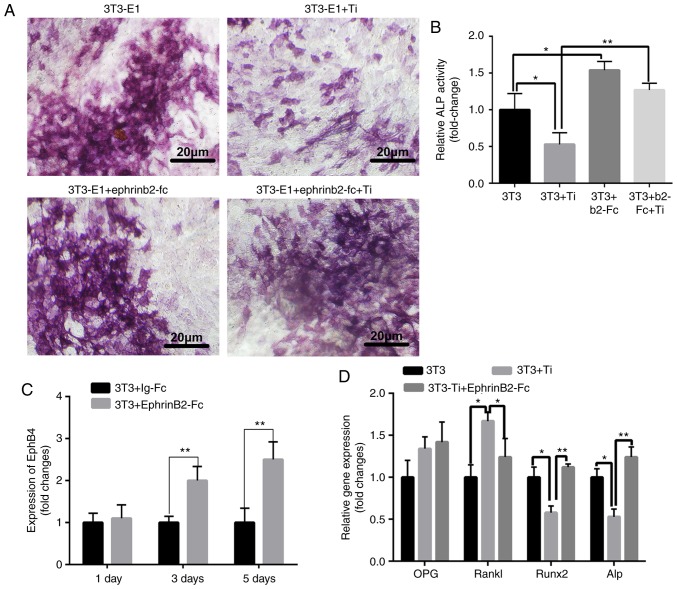
The osteogenic effects of the different groups (3T3-E1, 3T3-E1+ephrinB2-Fc, 3T3-E1+Ti and 3T3-E1+ephrinb2-Fc+Ti) were investigated according to ALP staining. Fig. 2A demonstrates ALP staining images obtained of 3T3-E1 cells following culture with or without ephrinB2-Fc and Ti. Quantification of the ALP staining images identified that ALP protein expression was significantly decreased in the Ti group compared with the control group, but the addition ephrinB2-Fc abrogated the effect of titanium particles on osteoblast differentiation (Fig. 2B).
Figure 2.
Changes in osteoclasts with different treatments. (A) Alkaline phosphatase staining. (B) Quantitative analysis of alkaline phosphatase staining. (C) The expression of ephb4 on osteoblast membranes at different times. (D) The change in osteoclasts-associated genes. *P<0.05 and **P<0.01. Ti, titanium; ephb4, erythropoietin-producing human hepatocellular receptor 4; OPG, osteoclastogenesis inhibitory factor; RANKL, receptor activator of nuclear factor κB ligand; RUNX2, runt related transcription factor associated 2; ALP, alkaline phosphatase.
Detection of changes in osteogenic genes in bidirectional signaling
To detect osteoblast membrane ephB4, ephrinB2-Fc was added to 3T3 cells. RT-qPCR demonstrated that ephrinB2-Fc increased the expression of ephB4 receptors on 3T3 cells (Fig. 2C). This demonstrated that the bidirectional signaling pathway may be modulated to favor bone formation. When Ti wear particles were added, expression levels of osteogenic genes (ALP and RUNX2) were significantly inhibited compared with those in the control group. However, compared with 3T3 group, 3T3+Ti+EphrinB2-Fc group, RANKL gene expression was significantly increased in the 3T3+Ti group. OPG gene expression was not significantly altered. The addition of ephrinB2-Fc significantly suppressed RANKL gene expression compared with that in the 3T3+Ti group (Fig. 2D).
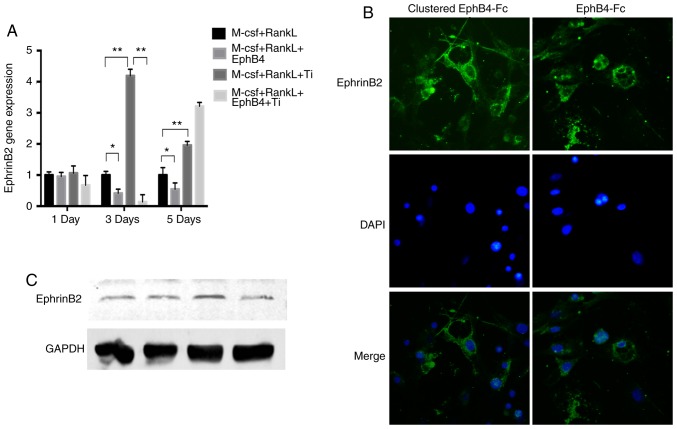
Effects of ephB4-Fc on osteoclast-associated ephrin-B2 gene expression and ephrin-B2 protein localization to the cell membrane
RT-qPCR indicated that the ephrin-B2 gene expression level was increased in the Ti particle group compared with in the BMMs group (Fig. 3A). However, 3 days after the addition of ephB4-Fc, ephrin-B2 gene expression was significantly decreased compared with in the Ti particle group. After 5 days, ephrin-B2 gene expression was abnormally increased in the EphB4-Fc+Ti group compared with that in the Ti group. Immunofluorescence staining demonstrated that ephrin-B2 was localized at the surface of the BMM membrane, exhibiting a circular pattern of staining. The clustering of proteins with or without anti-Fc was compared, and it was identified that the staining in the group with ephrin-B2 protein without anti-Fc was weaker compared with that with the anti-Fc group. However, there were also marked ephrin-B2 formations (Fig. 3B). The same result was also demonstrated in the western blot analysis results (Fig. 3C).
Figure 3.
Localization of EphrinB2 and the effect of EphrinB2 expression without Ti and EphB4-Fc. (A) Detection of ephrin B2 gene expression by reverse transcription quantitative polymerase chain reaction in different treatment groups on days 1, 3 and 5. (B) EphrinB2 expression on the cell membrane surface visualized using immunofluorescence microscopy. (C) The protein expression level of the cell membrane ligand was detected by western blot analysis on day 3. Data are presented as mean ± standard deviation. *P<0.05 and **P<0.01. M-CSF, macrophage-colony stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; ephB4, erythropoietin-producing human hepatocellular receptor 4; Ti, titanium.
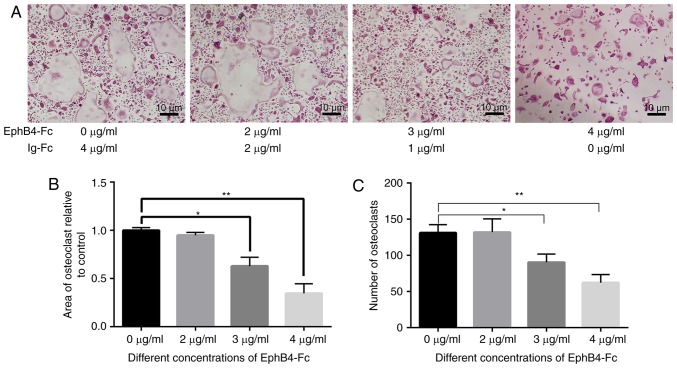
Establishment of Ti model with simulated co-culture
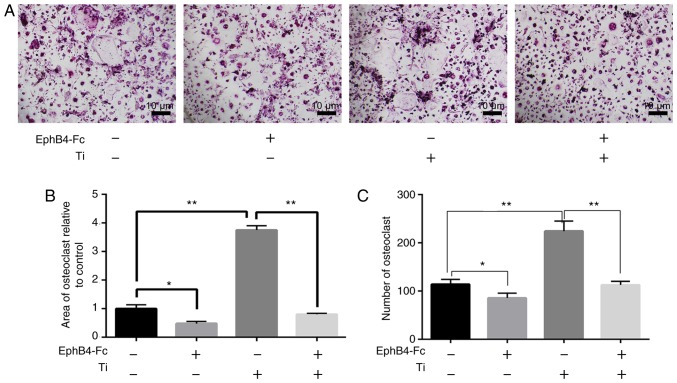
The size of the area and number of osteoclasts demonstrated that different concentrations of ephB4-Fc inhibited osteoclast differentiation. The concentration of 4 µg/ml ephB4-Fc exhibited the most significant inhibitory effect (Fig. 4). As the concentration increased, the inhibitory effect on osteoclasts was similar to that of 4 µg/ml. Therefore, 4 µg/ml was added to the BMMs in the simulated co-culture environment.
Figure 4.
Effects of different concentrations of ephB4-Fc on osteoclast differentiation. (A) BMMs cells were treated with different concentrations of ephB4-Fc in the presence of RANKL (50 ng/ml) and M-CSF (30 ng/ml) stimulation. (B) The area of osteoclasts relative to control. (C) The number of tartrate-resistant acid phosphatase-positive BMMs. Data are presented as mean ± standard deviation. *P<0.05 and **P<0.01. ephB4, erythropoietin-producing human hepatocellular receptor 4; BMMs, bone marrow macrophages; RANKL, receptor activator of nuclear factor κB ligand; M-CSF, macrophage-colony stimulating factor.
Suppression of Ti sample TRAP-positive multinucleated cells with ephB4-Fc and F-actin rings
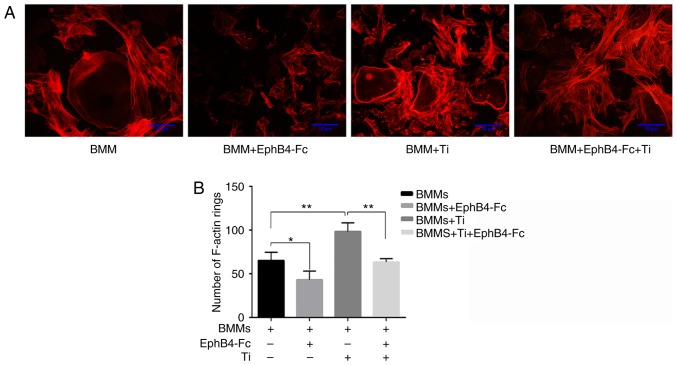
In the BMMs+Ti group, the size of the area and the number of osteoclasts were significantly increased compared with the BMMs group. This suggested that the addition of Ti particles promoted the differentiation of BMMs into osteoclasts and promoted the overexpression of the ephrin-B2 gene (Fig. 3A). However, the addition of ephB4-Fc to the BMMs+Ti group significantly decreased the number and area of osteoclasts (Fig. 5A). EphB4-Fc affected F-actin ring formation and morphology, decreasing the number of F-actin rings, which are essential prerequisites for bone resorption (Fig. 6).
Figure 5.
Effects of different treatments on bone differentiation. (A) The number of tartrate-resistant acid phosphatase-positive cells in BMMs, BMMs+EphB4, BMMs+Ti and BMMs+EphB4+Ti groups, in the presence of macrophage-colony stimulating factor and receptor activator of nuclear factor κB after 7 days of staining. (B) The area of osteoclasts relative to control (C) The number of osteoclasts. Data are presented as mean ± standard deviation. *P<0.05 and **P<0.01. ehpB4, erythropoietin-producing human hepatocellular receptor 4; Ti, titanium; BMMs, bone marrow macrophages.
Figure 6.
Effect on the F-actin ring by different treatments. (A) Osteoclasts were stained with phalloidin during the induction of differentiation by Ti particles and Ephb4-Fc after day 7. (B) The number of F-actin rings in the 5 randomized fields. *P<0.05 and **P<0.01. Ti, titanium; BMMs, bone marrow macrophages; ephB4, erythropoietin-producing human hepatocellular receptor 4.
Suppression of bone resorption function by addition of ephB4-Fc
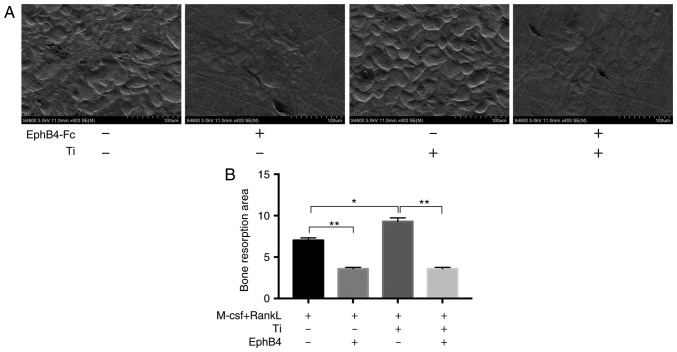
Next, it was verified that the addition of ephB4-Fc inhibited bone dissolution mediated by Ti particles. The number and depth of the lacunae in the area of the entire bone was observed using SEM (Fig. 7A). There were an increased number of lacunae in the TI group compared with the BMMs group, and the depth of bone resorption was also increased. However, when ephB4-Fc was added, it was identified that the level of bone resorption was significantly decreased (Fig. 7).
Figure 7.
Bone absorption experiment under different treatments. (A) A total of four groups of bone pieces were placed on a 96-well plate with 4 bone pieces per group. BMMs were seeded on the surface bone pieces at a density of 1×105 cells/well without EphB4-Fc or Ti in the presence of M-CSF and RANKL for 12 days. (B) Histogram of the total area of bone resorption was based on the size of the above pits. *P<0.05 and **P<0.01. M-CSF, macrophage-colony stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; ephB4, erythropoietin-producing human hepatocellular receptor 4; Ti, titanium.
Effects of ephB4-Fc on osteoclastogenesis-associated proteins and gene expression and suppressive effect of ephB4-Fc on NFATc1 signaling
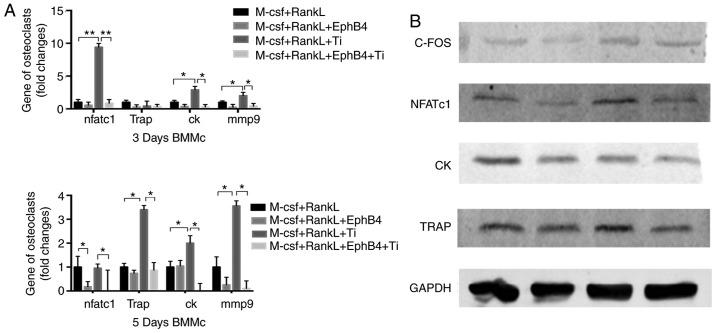
NFATc1 is considered a key player in the transformation of bone marrow macrophages to mature osteoclasts (1), and the present study confirmed that the addition of Ti particles significantly increased NFTAc1 expression. The inhibition of differentiation by the NFATc1 signal was not been confirmed in the Ti group following the addition of EphB4-fc in a previous study (23). However, the results of the present study were as expected. When ephB4-Fc was added to the Ti group, NFATc1 protein and gene expression decreased. The expression levels of osteoclast differentiation- and maturation-associated genes and proteins were significantly decreased by the additions of ephB4 and Ti to BMMs compared with levels subsequent to the addition of titanium particles alone (Fig. 8).
Figure 8.
(A and B) EphB4-Fc inhibited the NFATc1 signaling pathway caused by Ti and inhibited osteoclast-associated genes and protein expression. The mRNA expression of NFATc1, TRAP, MMP9 and CK was detected by reverse transcription quantitative polymerase chain reaction on days 3 and 5, and the protein expression of NFATc1, C-FOS, CK and TRAP was detected by western blot analysis on day 3. Data are presented as mean ± standard deviation. *P<0.05 and **P<0.01. C-FOS, Fos proto-oncogene, AP-1 transcription factor subunit; NFATc1, nuclear factor of activated T-cells 1; CK, cathepsin-K; TRAP, tartrate-resistant acid phosphatase; M-CSF; RANKL, receptor activator of nuclear factor κB; ephB4, erythropoietin-producing human hepatocellular receptor 4; BMMs, bone marrow macrophages; Ti, titanium.
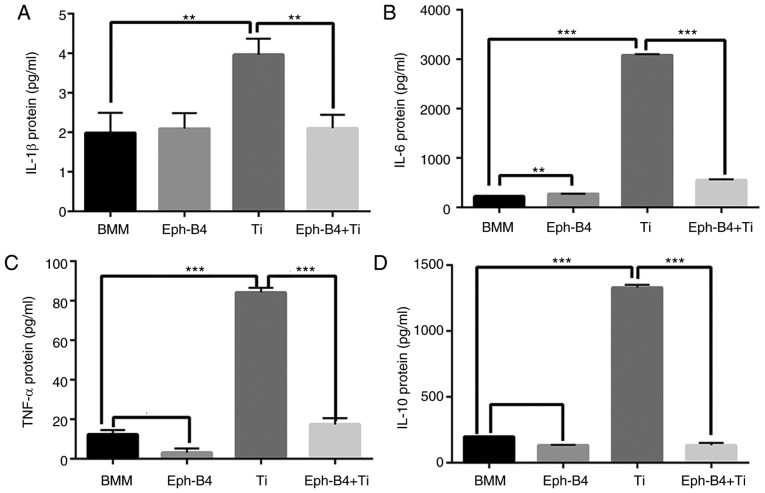
Effects of ephB4-Fc on cytokine production
To additionally investigate the effect of bidirectional signaling on the cytokine production in the process of osteoclast differentiation induced by Ti wear particles, ELISA assays were performed. The IL-6, IL-1β, TNF-α and IL-10 expression levels in the Ti group were significantly increased compared with those in the control group. However, when ephB4-Fc was added, the expression levels of these cytokines were significantly decreased (Fig. 9).
Figure 9.
Levels of cytokines produced following different treatments. A total of four groups were formed. BMMs, BMMs+ephB4-Fc, BMMs+ephB4-Fc+Ti. ELISA conducted to evaluated to (A) IL-1β, (B) IL-6, (C) TNF-α and (D) IL-10. Data are presented as mean ± standard deviation. *P<0.05 and **P<0.01. IL, interleukin; BMMs, bone marrow macrophages; ephB4, erythropoietin-producing human hepatocellular receptor 4; Ti, titanium; TNF-α, tumor necrosis factor α.
Discussion
Aseptic loosening is currently the most common complication of total hip arthroplasty, and previous studies have examined the differentiation of osteoclasts in its pathogenesis (5,24–26). At present, inhibitors of osteoclast differentiation have been a starting point for a number of studies (1,11). Drugs have also been used to inhibit the formation and differentiation of osteoclasts during in vitro and in vivo experiments (26,27), and magnesium metal ions also purportedly inhibit osteoclast formation (28). The exact mechanism of osteolysis surrounding prostheses has not been confirmed. However, the majority of studies on osteoclast formation and differentiation have been specific to osteoclasts themselves and have not investigated direct contact between cells to explain osteolysis surrounding prostheses. Previous studies have indicated that osteoblasts engage in bidirectional communication with osteoclasts (29), and that this communication is essential. Therefore, osteoclasts and osteoblasts should be explored as a unit in studies examining bone remodeling. The present study investigated the role of wear particle-induced osteolysis in this bidirectional signaling pathway.
Previous studies have indicated that the eph/ephrin signaling pathway regulates bone regeneration by direct contact (19,30–35). The most common subfamily of the tyrosine protein kinase receptor is the eph receptor family, and its ligands are proteins of the ephrin family (36,37). A previous study identified that a co-culture group of 3T3 and BMM cells (in a 1:5 ratio) with Ti particles exhibited decreased formation of osteoclasts compared with that of a BMM group with Ti particles (13). EphB4-Fc was selected to simulate ephB4 on the surface of pre-osteoblasts. The activation of the ephrin-b2 gene with ephB4-Fc inhibited the differentiation of pre-osteoclasts by inhibiting the NFATc1 signal; however, the ephrin-B2 gene was not highly expressed in the absence of RANKL in BMMs (13). Addition of Ti particles and RANKL increased the expression of the ephrin-B2 gene mediated by RANKL. It was then concluded that Ti and RANKL synergistically activated the NFATc1 signal, thereby continuing to activate the ephrin-B2 gene. How ephrin-B2 activation inhibits Ti particle-induced osteoclast differentiation had not yet been confirmed. The in vitro experiments of the present study demonstrated that Ti particles significantly promoted osteoclast-associated gene expression, including that of NFATc1, TRAP, MMP9 and CK. In addition, ephrin-B2 gene expression was significantly increased in the Ti group. By adding ephB4-Fc to the Ti particle group, it was identified that osteoclast differentiation was inhibited. Staining with phalloidin indicated that ephB4-Fc inhibited the cytoskeleton formation of osteoclasts, and bone resorption was significantly decreased. In addition, the NFATc1 gene in the NF-κB signaling pathway and its downstream genes, MMP9, CK and TRAP, were also inhibited. The ephrin-B2 gene is downstream of NFATc1, and activation of the ephrin-B2 membrane protein may reverse the inhibition of C-FOS and NFATc1 gene expression (38). Corresponding examination on osteoblasts was also conducted. The addition of Ti particles to the osteoblast and osteoclast system promoted a high expression of the RANKL gene and inhibited the differentiation and maturation of osteoblasts. However, RANKL cytokines are the specific cytokines required for osteoclast differentiation. It was then identified that following the addition of the recombinant protein ephrinB2-Fc, Ti particle-mediated inhibition of osteoblasts was abrogated, and also RANKL-mediated secretion of cytokines by osteoblasts was inhibited. Compared with that in the Ti group, the OPG/RANKL ratio was increased subsequent to the addition of ephrinB2-Fc; therefore, ephrinB2-Fc indirectly inhibited the differentiation of osteoclasts. In addition, Ti particles increased the release of proinflammatory cytokines (IL-6, IL-1β, TNF-α and IL-10), which promoted the differentiation of bone marrow macrophages into osteoclasts and their bone resorption. The production of inflammatory factors was additionally examined by ELISA. Through morphological examination, it was identified that bone marrow macrophages engulfed a large number of wear particles. The supernatants of BMM, BMM+Ti, BMM+ephB4-Fc, and BMM+Ti+ephB4-Fc cell suspensions were examined, and it was demonstrated that the activation of ephrinB2 inhibited the release of inflammatory cytokines mediated by Ti particles, thereby additionally inhibiting the differentiation and maturation of osteoclasts.
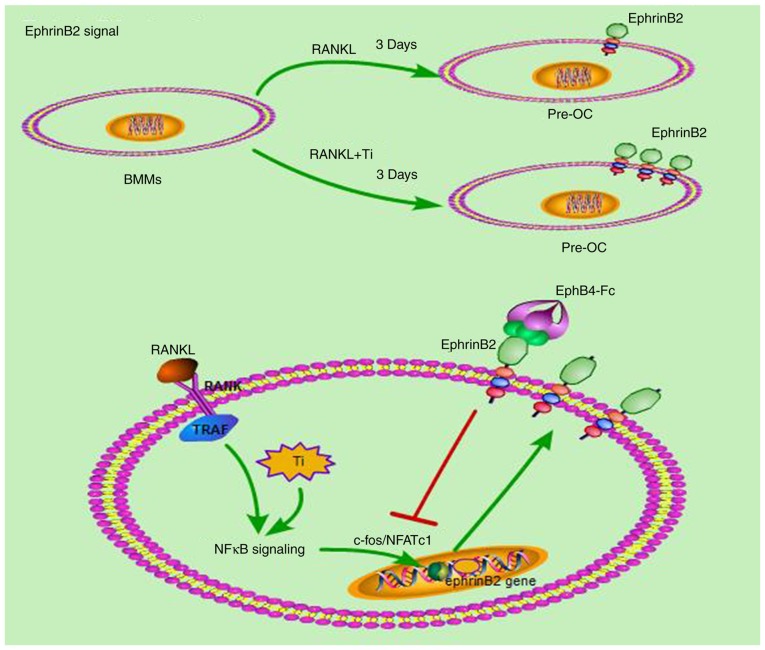
In the present study, bidirectional ephrin- B2/ephb4 signaling-based intercellular communication in particle-induced bone remodeling imbalance was investigated. The results indicated that the ephrin-B2 gene was highly expressed in the Ti particle group and that RANKL co-stimulated ephrin-B2 gene expression. Finally, ephB44-Fc inhibited the NFATc1 signal by activating the ephrin-B2 gene. As observed in the Ti particle group, activation of NFATc1 is crucial to the osteoclastogenesis pathway induced by Ti particles, and the ephrin-B2 gene is located downstream of the NFATc1 gene (Fig. 10). By activating this bidirectional signal, the release of inflammatory factors was inhibited, and consequently the differentiation of bone marrow macrophages into osteoclasts was inhibited. Activation of this bidirectional pathway with ephB4-Fc may be a prospective therapy for treating wear particle-induced periprosthetic osteolysis.
Figure 10.
Schematic diagram demonstrating that ephB4-Fc suppresses osteoclast formation and bone resorption induced by Ti particles. Ti particles activated the NFκB pathway in the presence of RANKL. A total of 3 days later, ephrinB2 expression increased significantly. By adding ephB4-Fc, ephrinB2 was activated, which inhibited the C-FOS/NFATc1 signaling pathway and inhibited the gene expression of cathepsin-K, tartrate-resistant acid phosphatase and matrix metalloproteinase 9, by RANKL and Ti particles. Finally, ephB4-Fc inhibits osteoclasts differentiation and bone resorption by Ti particles. Ti, titanium; ephB4, erythropoietin-producing hepatocellular receptor 4; OC, osteoclast; NF-κB, nuclear factor κB; RANK, receptor activator of NF-κB; RANKL, RANK ligand; TRAP, tartrate-resistant acid phosphatase; C-FOS, Fos proto-oncogene, AP-1 transcription factor subunit; NFATc1, nuclear factor of activated T-cells 1.
Acknowledgments
The present study was supported by the Department of Orthopedic Surgery, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81572158, 81772361 and 81572116).
Availability of data and materials
All datasets generated or analyzed are included in this published article.
Authors' contributions
YM designed the research. The experiments were performed by YG, ZL, ZS, DY and KF. YG and ZL conducted the data analysis and wrote the manuscript. ZZ contributed to the study design and revised the manuscript.
Ethics approval and consent to participate
All animal procedures were approved by the Animal Hospital affiliated with Shanghai Jiao Tong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu F, Zhu Z, Mao Y, Liu M, Tang T, Qiu S. Inhibition of titanium particle-induced osteoclastogenesis through inactivation of NFATc1 by VIVIT peptide. Biomaterials. 2009;30:1756–1762. doi: 10.1016/j.biomaterials.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Zhu S, Cui J, Shao H, Zhang W, Yang H, Xu Y, Geng D, Yu L. Strontium ranelate inhibits titanium-particle-induced osteolysis by restraining inflammatory osteoclastogenesis in vivo. Acta Biomater. 2014;10:4912–4918. doi: 10.1016/j.actbio.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Geng D, Wu J, Shao H, Zhu S, Wang Y, Zhang W, Ping Z, Hu X, Zhu X, Xu Y, Yang H. Pharmaceutical inhibition of glycogen synthetase kinase 3 beta suppresses wear debris-induced osteolysis. Biomaterials. 2015;69:12–21. doi: 10.1016/j.biomaterials.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 4.Gallo J, Goodman SB, Konttinen YT, Wimmer MA, Holinka M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013;9:8046–8058. doi: 10.1016/j.actbio.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H, Ouyang Z, Xu X, Wang W, Fan Q, et al. The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials. 2014;35:5721–5730. doi: 10.1016/j.biomaterials.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Vallés G, Pérez C, Boré A, Martín-Saavedra F, Saldaña L, Vilaboa N. Simvastatin prevents the induction of interleukin-6 gene expression by titanium particles in human osteoblastic cells. Acta Biomater. 2013;9:4916–4925. doi: 10.1016/j.actbio.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, et al. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101:3033–3045. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pajarinen J, Kouri VP, Jämsen E, Li TF, Mandelin J, Konttinen YT. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013;9:9229–9240. doi: 10.1016/j.actbio.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Lee HG, Minematsu H, Kim KO, Celil Aydemir AB, Shin MJ, Nizami SA, Chung KJ, Hsu AC, Jacobs CR, Lee FY. Actin and ERK1/2-CEBPβ signaling mediates phagocytosis-induced innate immune response of osteoprogenitor cells. Biomaterials. 2011;32:9197–9206. doi: 10.1016/j.biomaterials.2011.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JB, Ding Y, Huang DS, Zeng WK, Guan ZP, Zhang ML. rna interference targeting p110β reduces tumor necrosis factor-alpha production in cellular response to wear particles in vitro and osteolysis in vivo. Inflammation. 2013;36:1041–1054. doi: 10.1007/s10753-013-9636-9. [DOI] [PubMed] [Google Scholar]
- 11.Liu FX, Wu CL, Zhu ZA, Li MQ, Mao YQ, Liu M, Wang XQ, Yu DG, Tang TT. Calcineurin/NFAT pathway mediates wear particle-induced TNF-alpha release and osteoclastogenesis from mice bone marrow macrophages in vitro. Acta Pharmacol Sin. 2013;34:1457–1466. doi: 10.1038/aps.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JA, Ihn HJ, Park JY, Lim J, Hong JM, Kim SH, Kim SY, Shin HI, Park EK. Inhibitory effects of triptolide on titanium particle-induced osteolysis and receptor activator of nuclear factor-κB ligand-mediated osteoclast differentiation. Int Orthop. 2015;39:173–182. doi: 10.1007/s00264-014-2596-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 15.Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, Shen Z, Fu Q. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42. doi: 10.1016/j.bone.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo K. Eph and ephrin interactions in bone. Adv Exp Med Biol. 2010;658:95–103. doi: 10.1007/978-1-4419-1050-9_10. [DOI] [PubMed] [Google Scholar]
- 18.Luiz de Freitas PH, Li M, Ninomiya T, Nakamura M, Ubaidus S, Oda K, Udagawa N, Maeda T, Takagi R, Amizuka N. Intermittent PTH administration stimulates pre-osteoblastic proliferation without leading to enhanced bone formation in osteoclast-less c-fos(−/−) mice. J Bone Miner Res. 2009;24:1586–1597. doi: 10.1359/jbmr.090413. [DOI] [PubMed] [Google Scholar]
- 19.Fan WB, Zhao JN, Bao NR. Effects of bidirectional EphB4-EphrinB2 signaling on bone remodeling. Zhongguo Gu Shang. 2013;26:705–708. In Chinese. [PubMed] [Google Scholar]
- 20.Zuo C, Huang Y, Bajis R, Sahih M, Li YP, Dai K, Zhang X. Osteoblastogenesis regulation signals in bone remodeling. Osteoporos Int. 2012;23:1653–1663. doi: 10.1007/s00198-012-1909-x. [DOI] [PubMed] [Google Scholar]
- 21.von Knoch M, Jewison DE, Sibonga JD, Sprecher C, Morrey BF, Loer F, Berry DJ, Scully SP. The effectiveness of polyethylene versus titanium particles in inducing osteolysis in vivo. J Orthop Res. 2004;22:237–243. doi: 10.1016/j.orthres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Lee SS, Woo CH, Chang JD, Kim JH. Roles of Rac and cytosolic phospholipase A2 in the intracellular signalling in response to titanium particles. Cell Signal. 2003;15:339–345. doi: 10.1016/S0898-6568(02)00118-3. [DOI] [PubMed] [Google Scholar]
- 23.Takyar FM, Tonna S, Ho PW, Crimeen-Irwin B, Baker EK, Martin TJ, Sims NA. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28:912–925. doi: 10.1002/jbmr.1820. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang Z, Zhai Z, Li H, Liu X, Qu X, Li X, Fan Q, Tang T, Qin A, Dai K. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem Pharmacol. 2014;90:276–287. doi: 10.1016/j.bcp.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang XC, Bao XF, Hu M, Yu WX. Effects of Porphyromonas gingivalis lipopolysaccharide on osteoblast-osteoclast bidirectional EphB4-EphrinB2 signaling. Exp Ther Med. 2014;7:80–84. doi: 10.3892/etm.2013.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Wang W, Tian B, Liu X, Qu X, Zhai Z, Li H, Liu F, Fan Q, Tang T, et al. Myricetin prevents titanium particle-induced osteolysis in vivo and inhibits RANKL-induced osteoclastogenesis in vitro. Biochem Pharmacol. 2015;93:59–71. doi: 10.1016/j.bcp.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Shao H, Shen J, Wang M, Cui J, Wang Y, Zhu S, Zhang W, Yang H, Xu Y, Geng D. Icariin protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials. 2015;60:92–99. doi: 10.1016/j.biomaterials.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Zhai Z, Qu X, Li H, Yang K, Wan P, Tan L, Ouyang Z, Liu X, Tian B, Xiao F, et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials. 2014;35:6299–6310. doi: 10.1016/j.biomaterials.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59:99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT, Krasnoperov V, Sims NA. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. doi: 10.1007/978-1-4419-1050-9_6. [DOI] [PubMed] [Google Scholar]
- 32.Gooi JH, Pompolo S, Karsdal MA, Kulkarni NH, Kalajzic I, McAhren SH, Han B, Onyia JE, Ho PW, Gillespie MT, et al. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone. 2010;46:1486–1497. doi: 10.1016/j.bone.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Benson MD, Opperman LA, Westerlund J, Fernandez CR, San Miguel S, Henkemeyer M, Chenaux G. Ephrin-B stimulation of calvarial bone formation. Dev Dyn. 2012;241:1901–1910. doi: 10.1002/dvdy.23874. [DOI] [PubMed] [Google Scholar]
- 34.Arthur A, Panagopoulos RA, Cooper L, Menicanin D, Parkinson IH, Codrington JD, Vandyke K, Zannettino AC, Koblar SA, Sims NA, et al. EphB4 enhances the process of endochondral ossification and inhibits remodeling during bone fracture repair. J Bone Miner Res. 2013;28:926–935. doi: 10.1002/jbmr.1821. [DOI] [PubMed] [Google Scholar]
- 35.Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE, Martin TJ. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 36.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Zhang J, Wang C, Qi Y, Du M, Liu W, Yang C, Yang P. Low concentrations of TNF-alpha promote osteogenic differentiation via activation of the ephrinB2-EphB4 signalling pathway. Cell Prolif. 2017;50 doi: 10.1111/cpr.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Shi C, Kim J, Chen Y, Ni S, Jiang L, Zheng C, Li D, Hou J, Taichman RS, Sun H. Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J Dent Res. 2015;94:455–463. doi: 10.1177/0022034514566431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated or analyzed are included in this published article.