Abstract
Background
This study assessed the feasibility of a mentored home-based vegetable gardening intervention and examined changes in health-related outcomes among breast cancer survivors (BCS).
Methods
BCSs were randomized to either a year-long vegetable gardening intervention to begin immediately or a wait-list control. Master Gardeners (MG) mentored participants in planning, planting and maintaining three seasonal gardens over a year. Participant accrual, retention, and satisfaction rates of ≥80% served as feasibility (primary outcome) benchmarks. Secondary outcomes (i.e., vegetable consumption, physical activity, performance and function, anthropometrics, biomarkers, and health-related quality of life) were collected at baseline and post-intervention (one-year follow-up) using subjective and objective measures.
Results
The trial surpassed all feasibility benchmarks: 82% of targeted accrual; 95% retention; and 100% satisfaction (i.e., experience ratings of “Good-to-Excellent” and willingness to “do it again”). Compared to the controls, intervention participants reported significantly greater improvements in moderate physical activity (+14 vs. −17 minutes/week) and demonstrated improvements in the 2-Minute Step Test (+22 vs. +10 steps), and Arm Curl (+2.7 vs +0.1 repetitions) (p-values<0.05). A trend toward improved vegetable consumption was observed (+0.9 vs. +0.2 servings/day; p=0.06). Eighty-six percent continued to garden at two-year follow-up.
Conclusions
Findings suggest that a mentored, home-based vegetable gardening intervention proved feasible and offers an integrative and durable approach to improve health behaviors and outcomes among BCSs. Harvest for Health led to establishing a group of trained MGs and gave rise to local and global community-based programs. Larger studies are needed to confirm results and define applicability across broader populations of survivors.
Keywords: Survivorship, Vegetable Gardening, Nutrition, Physical Function, Breast Cancer
Introduction
More than 3.5 million women in the United States have a history of breast cancer, and are at an increased risk for secondary malignancies, cardiovascular disease, and diabetes, as well as impaired physical functioning and reduced health-related quality of life (HRQOL).1 Evidence indicates that adopting healthier lifestyle behaviors may improve overall health, physical functioning, and HRQOL among breast cancer survivors (BCSs).2–4 The American Cancer Society recommends that cancer survivors eat at least five daily servings of vegetables and fruits and engage in 150 minutes of moderate or 75 min of vigorous physical activity/week.5 However, research indicates that most BCSs do not meet these recommendations.6,7
Vegetable gardening may provide a holistic approach to improving diet quality, physical activity, body weight status, and psychosocial well-being.8–11 Vegetable gardening improves access to fresh produce and has been demonstrated to increase vegetable consumption across several populations.8,9 Healthy senior adults have been shown to meet physical activity recommendations through gardening.10 The Growing a Healthier Older Population project found that 68% of gardeners met physical activity recommendations, compared to only 25% of same-aged non-gardeners.12 Community gardeners are less likely to be overweight or obese as compared to age- and gender-matched non-gardeners living in the same neighborhood.11 Moreover, the therapeutic nature of gardening is associated with improved physical and psychosocial well-being.13,14 Previously, in other populations of cancer survivors, we have found that vegetable gardening interventions have resulted in improvements in diet quality, physical activity and function, and HRQOL.9,15
The current Harvest for Health initiative, the Birmingham Breast Cancer Survivors (BBCS) feasibility trial, delivered and evaluated a one-year home-based vegetable gardening intervention among BCSs residing in the Birmingham, Alabama metropolitan area. Here we report feasibility and changes in measures of health-related outcomes among BCSs.
Methods
This two-arm, feasibility trial randomly assigned BCSs to a one-year mentored vegetable gardening intervention or to a wait-list control. Most study staff were blinded to randomization status, and all study staff who collected follow-up data were blinded with regard to previously collected data. A detailed description of the study protocol was published previously.16
Ethical Considerations
The study protocol received approval from the University of Alabama at Birmingham (UAB) Institutional Review Board. Written informed consent was obtained from all participants before baseline data collection.
Setting and Population
BCSs residing in the Birmingham metropolitan area were recruited via mailed invitation between August 2013 and May 2014. Potential participants were identified via the Alabama State Cancer Registry and individual hospital registries. Self-referrals were sought using support groups and various media (e.g., television, radio). Interested participants were screened to ensure they met study eligibility criteria.
Inclusion/exclusion criteria were delineated to reduce ceiling effects on behavioral or health outcome data, intervention failure, or adverse events (e.g., potential infections arising from contact with fertilizer/soil during immunocompromised states). Inclusion criteria were: (1) completion of cancer treatment (i.e., surgery, chemotherapy, and/or radiation therapy); (2) currently eating <5 servings of vegetables and fruits/day; (3) exercising <150 minutes/week; (4) ≥ 1 physical function limitation; (5) English-speaking and -writing; (6) residing ≤15 miles of a Master Gardener (MG); (7) residence with ≥6 hours of sun/day, running water, and accommodation for one raised bed (4′x 8′) or four Grow Boxes (24″ × 50″); and (8) willingness to be randomized to either study group. Exclusion criteria were: (1) comorbid conditions that would impair ability to complete study assessments or participate in unsupervised physical activity; (2) currently taking pharmacologic doses of warfarin; or (3) tended a successful vegetable garden within the past two years.
Intervention
BCSs were individually paired with Cooperative Extension-certified MGs. The Cooperative Extension is affiliated with land-grant universities nationwide and certifies MGs across North America (http://articles.extension.org/mastergardener). To maintain certification, MGs must volunteer 50 hours/year. A survey conducted among over 2,200 MGs in Alabama suggested that 71% were extremely interested in volunteering for this project. These MGs interfaced bi-monthly (home visits alternating with telephone or email contact) with BCSs to mentor in the planning, planting, and maintaining of three (Spring, Summer, Fall) home-based vegetable gardens over the course of a year. BCSs were provided with: (1) one raised bed or four Grow Boxes; (2) gardening supplies (i.e., soil, seeds, plants, fertilizer, natural pest repellent, gardening hose and tools, watering can, frost cover, and trellis); (3) gardening workbook detailing the planning, planting, tending, and harvesting of the three gardens; (4) a MG contact schedule; (5) contact information for their MG, county Cooperative Extension agent, and the study staff; and (6) a gardening journal to record their observations and notes.16 Additionally, BCSs were encouraged to participate in a private Facebook® group to facilitate interaction with other BCSs and MGs.
Intervention Adherence and Fidelity
Process data were used to evaluate adherence to and fidelity with the intervention. MG monthly home-visits, garden photographs, and bi-monthly emails/phone calls were tracked by study staff.
Data Collection
Feasibility
Feasibility (primary outcome) criteria consisted of participant accrual, retention, and satisfaction rates of ≥80% and intervention safety (no adverse events attributable to the intervention). Targeted accrual was set at 100 and based on building MG capacity in the greater Birmingham metropolitan area (i.e., establishing a critical mass of trained MGs who could sustain the intervention long-term). Data on participant satisfaction, gardening fidelity, future gardening plans, and study suggestions were collected after study completion via a 22-item structured telephone debriefing. To explore gardening sustainability among intervention participants, an extended follow-up was conducted at two-years via a three-item telephone survey.
Health-related outcomes
Health-related outcomes (secondary outcomes of vegetable consumption, physical activity, performance and function, HRQOL, anthropometrics, and biomarkers) were collected in participant’s homes at baseline and post-intervention (one-year later). Study questionnaires and accelerometers were mailed to participants two weeks prior to their scheduled appointment. To minimize attrition, participants were compensated $15 for each completed home visit.
Vegetable Consumption
Vegetable consumption data were collected using the NCI Diet History Questionnaire,17 a food frequency questionnaire consisting of 144 food items. Fifteen items assessing vegetable intake over the previous 12 months were analyzed.
Physical Activity
Self-reported physical activity data were collected using the Godin Leisure-Time Exercise Questionnaire,18 a five-item questionnaire that measures usual leisure-time physical activity frequency over a seven-day period. Objective physical activity data were collected via accelerometers (ActiGraph®, Fort Walton, FL), 19 which were preprogrammed and included instructions for a seven-day data collection.
HRQOL
The Medical Outcomes Survey Short-Form 36 Health Survey (SF-36),20 a 36-item questionnaire, was used to measure HRQOL across both physical and mental domains.
Physical Performance
The physical performance battery included: 1) 30-Second Chair Stand (lower body strength); 2) Arm Curl (upper body strength); 3) Sit-and-Reach (lower body flexibility); 4) Back Scratch (upper body flexibility); 5) 8-Foot Get-Up & Go (agility/dynamic balance); 6) 2-Minute Step Test (endurance); 7) and Hand Grip Strength (via dynamometer [Omron®, Kyoto, Japan]).21,22
Anthropometrics
Anthropometric measures included height, body weight, and waist circumference using a calibrated scale and non-stretch tape measure. Standard measures were taken to the nearest tenth of a kilogram (weight) or centimeter (height and waist circumference) in light clothing and without shoes.23
Biomarkers
Using the methods of Warnock et al.,24 cortisol was assessed in toenail clippings and served as a measure of chronic stress levels.25 Telomerase (biomarker associated with healthful aging) was assessed in peripheral blood mononuclear cells via the methods of Saldanha et al.26 Interleukin (IL)-6 (biomarker of inflammation) was assessed in plasma via electrochemiluminescence.
Statistical Analyses
Feasibility-based outcomes (i.e., accrual, retention, satisfaction, and absence of serious adverse events) were the primary focus of this investigation. Other comparisons were secondary and conducted using SAS® version 9.4 (Cary, NC). Within-group comparisons over time were assessed using paired t-tests (interval data) and McNemars tests (dichotomous data). Baseline to post-intervention change scores between groups were compared using paired t-tests and chi-square tests, controlling for the number of comorbidities. While feasibility was the focus of this investigation, a priori power calculations indicated 80% power to detect a between-group difference of at least five points on the SF-36 physical function subscale with the assumptions of 20% attrition, alpha <0.05, and a proportional between-group difference of 15% vs. 55% using the Fisher’s exact test for proportions.
Results
Feasibility
Accrual, Retention, and Safety
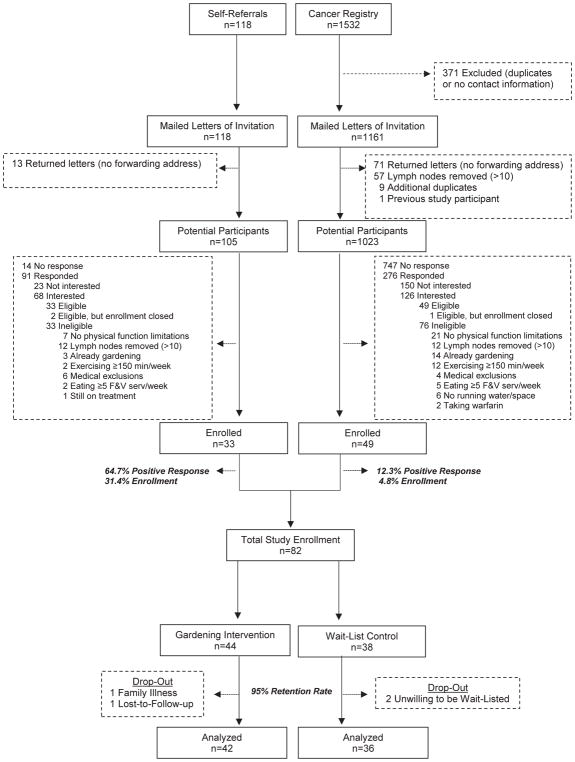
Figure 1 (CONSORT diagram), details case ascertainment, eligibility, and retention. The study enrolled 82 BCS (60% cancer registry; 40% self-referral), thus achieving 82% of the accrual target. Of the 82, four did not complete the study (two refused to be wait-listed due to wanting to garden immediately, one withdrew due to family illness, and one was lost to follow-up), resulting in a 95% retention rate over the one-year study period. During the course of the study, there were no adverse events attributable to the intervention.
Figure 1.
CONSORT diagram
Participant Characteristics
Participants consisted of Caucasian and African-American BCSs with a mean age of 60 years (Table 1). Overall, participants were well-educated (most having attended or graduated from college), employed, married, and living with other family members. Most resided in urban counties, as compared to rural counties. Mean time since diagnosis was five years, with most diagnosed with localized breast cancer. Most participants were overweight or obese, and living with multiple co-morbidities and functional limitations. Nearly 10% were current smokers. Mean daily consumption of vegetables and weekly minutes of physical activity were well below recommendations for cancer survivors.5
Table 1.
Characteristics of breast cancer survivors participating in a vegetable gardening trial
| Variable | Total (N=82) | Intervention (n=44) | Control (n=38) | Between Group Difference | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean(SD) Range |
n(%) | Mean(SD) Range |
n(%) | Mean(SD) Range |
n(%) | p-value | |
| Age (years) | 60.5(9.4) 32–84 |
60(8.4) 39–79 |
61(10.5) 32–84 |
0.66 | |||
| Race | 0.11 | ||||||
| Caucasian | 60(73.2%) | 29(65.9%) | 31(81.6%) | ||||
| African-American | 22(26.8%) | 15(34.1%) | 7(18.4%) | ||||
| Education | 0.18 | ||||||
| <High School | 5(6.1%) | 4(9.1%) | 1(2.6%) | ||||
| High School Grad | 7(8.5%) | 6(13.6%) | 1(2.6%) | ||||
| Some College | 25(30.5%) | 12(27.3%) | 13(34.2%) | ||||
| College Grad | 19(23.2%) | 11(25.0%) | 8(21.1%) | ||||
| Post-Grad | 24(29.3%) | 11(25.0%) | 13(34.2%) | ||||
| Marital Status | 0.24 | ||||||
| Married | 48(58.5%) | 23(52.3%) | 25(65.8%) | ||||
| Widowed | 6(7.3%) | 4(9.1%) | 2(5.3%) | ||||
| Divorced/Separated | 21(25.6%) | 14(31.9%) | 7(18.4%) | ||||
| Single | 5(6.1%) | 3(6.8%) | 2(5.3%) | ||||
| Rural County of Residence | 15(18.3%) | 9(20.5%) | 6(15.8%) | 0.59 | |||
| Currently Employed | 38(46.3%) | 20(45.5%) | 18(47.4%) | 0.69 | |||
| Persons in Household | 0.64 | ||||||
| 1 | 22.0%(18) | 11(25.0%) | 7(18.4%) | ||||
| 2 | 46.3%(38) | 19(43.2%) | 19(50.0%) | ||||
| 3 or more | 29.2%(24) | 14(31.9%) | 10(26.3%) | ||||
| Time Since Diagnosis (years) | 5.4(4.8) 0.5–23 |
4.6(3.3) 0.5–14 |
6.3(6.0) 0.8–23 |
0.12 | |||
| Breast Cancer Stage | 0.33 | ||||||
| Stage 0 | 12(14.6%) | 8(18.1%) | 4(10.5%) | ||||
| Stage 1 | 35(42.7%) | 16(36.4%) | 19(50%) | ||||
| Stage 2 | 18(22%) | 9(20.5%) | 9(23.7%) | ||||
| Stage 3 | 2(2.4%) | 2(4.5%) | 0 | ||||
| Unknown | 15(18.3%) | 9(20.5%) | 6(15.8%) | ||||
| Cancer Treatment | |||||||
| Surgery | 80(97.6%) | 43(100%) | 36(94.7%) | 0.36 | |||
| Radiation | 53(64.6%) | 27(61.4%) | 24(63.2%) | 0.72 | |||
| Chemotherapy | 51(62.2%) | 29(68.2%) | 23(60.5%) | 0.74 | |||
| Hormonal Therapy | 34(41.5%) | 17(36.4%) | 16(42.1%) | 0.66 | |||
| Co-morbidities | 3.5(2.6) 0–12 |
3.0(2.3) 0–9 |
4.2(2.8) 1–12 |
0.04 | |||
| Functional Limitations | 4.6(2.8) 1–10 |
4.4(3.0) 1–10 |
4.8(2.6) 1–9 |
||||
| 1 | 11(13.4%) | 8(18.2%) | 3(7.9%) | 0.54 | |||
| 2 or more | 71(86.6%) | 36(81.8%) | 35(92.2%) | 0.43 | |||
| Body Weight Status | 0.99 | ||||||
| Under weight (BMI<18.5) | 2(2.4%) | 1(2.3%) | 1(2.6%) | ||||
| Normal weight (BMI=18.5–24.9) | 13(15.9%) | 7(15.9%) | 6(15.8%) | ||||
| Overweight (BMI=25.0–29.9) | 23(28.0%) | 13(29.5%) | 10(26.3%) | ||||
| Obese (BMI>30) | 44(53.7%) | 23(52.3%) | 21(55.3%) | ||||
| Current Smoker | 8(9.8%) | 6(13.6%) | 2(5.3%) | 0.23 | |||
p-values in bold text indicate significance at p<0.05
Participant Satisfaction
All participants completing the intervention (n=42) rated their experience as “Good-to-Excellent,” reported that they would “do it again,” and planned to “continue to garden.” Over 88% of participants reported gardening either daily or several times weekly. When asked about the influence of gardening on motivating behavior change, participants reported that gardening strongly motivated them to “eat a healthier diet,” “eat more and try new vegetables,” and “become more physically active,” though gardening was not attributed to increasing fruit consumption.
MG communication occurred less frequently than bi-monthly among 61% of the participants, with 43% of the participants preferring more communication. Overall, MGs were rated strongly (scores exceeding 4-out-of-5) with regard to the design and planting of gardens and answering questions. Participants giving lower MG ratings would have preferred a MG who was “more supportive,” “communicated better,” and was “more hands-on.” When asked about the need for additional information on gardening or healthy eating, most replied “no;” however, some requested more information on pest control, fertilizers, planting schedules, and healthy recipes. All gardening tools, except the watering can, were considered useful. While some valued the intervention below the actual cost, the clear majority valued the intervention at or above $500, with 20% indicating that the intervention was “invaluable.” Many participants voiced positive feelings about Harvest for Health’s impact on their lives. One participant stated, “I learned something new, changed my life, and nourished my body!”
Gardening Sustainability
At two-years, 86% of the intervention participants who completed the study reported that they were still gardening, with 36% of these gardeners reporting a garden expansion. Garden expansions included additional raised beds, containers, planting tables, converting of existing flower gardens, and tilling in-ground gardens.
Heath-Related Outcomes
Changes in vegetable consumption, physical activity, performance and function, anthropometrics, biomarkers, and HRQOL are reported in Table 2. Since this was a feasibility study, directionality of the data was the focus of the secondary outcomes and was assessed via change scores; however, within- and between-group differences were explored. Vegetable consumption increased significantly in the intervention group but not among controls, with the resulting between-group difference approaching significance (p=0.06). A statistically significant between-group difference was observed for self-reported moderate physical activity, with change scores of +14 minutes/week in the intervention and −17 minutes/week among controls. However, accelerometers detected no statistically significant within- or between-group differences over time. Positive change scores in HRQOL were seen in 9-of-10 summary and subscale scores among both groups. Statistically significant improvements in emotional role among the control group were observed, with no other within- or between-group differences. Non-significant trends were observed for body weight and BMI, with decreases in intervention participants and increases in controls. In contrast, significant increases in waist circumference were observed in both groups over the year-long study. Overall, positive change scores were observed among both groups in 7-of-7 physical performance measures; however, the intervention group demonstrated significant improvements in 6-of-7 tests, compared to only 2-of-7 among controls. In the 2-Minute Step Test and the Arm Curl, improvements were significantly greater in the intervention group compared to controls. No significant between-arm differences and few within-arm differences were observed in biomarkers. Telomerase decreased in both groups, though only statistically significant among controls.
Table 2.
Health-Related Outcomes
| Intervention | Control | Between- Group Difference |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=44) |
Post- Intervention (n=42) |
Change Score (n=42) |
Within- Group Difference |
Baseline (n=38) |
Post- Intervention (n=36) |
Change Score (n=36) |
Within- Group Difference |
||
|
| |||||||||
| Mean(SD) | p-value | Mean(SD) | p-value | p-value | |||||
| Lifestyle Behaviors | |||||||||
| Vegetable Consumption (servings/day) | 1.46(1.21) | 2.41(2.13) | +0.86(1.27) | 0.0002 | 1.89(1.06) | 2.20(1.71) | +0.23(1.38) | 0.36 | 0.06 |
| Physical Activity | |||||||||
| - Self-report (Moderate minutes/week) | 37.9(61.0) | 54.2(93.1) | +14.2(94.8) | 0.34 | 34.4(55.2) | 17.3(31.7) | −17.1(54.3) | 0.08 | 0.02 |
| - Accelerometer (MET hours/week) | 47.3(24.5) | 45.1(24.5) | −2.0(24.8) | 0.68 | 39.2(25.9) | 36.8(24.6) | −2.1(25.6) | 0.71 | 0.93 |
| Health Related Quality of Life | |||||||||
| Physical Health Component Score | 69.18(19.70) | 70.35(22.92) | +0.82(12.94) | 0.69 | 62.11(16.92) | 63.21(18.76) | +1.38(11.18) | 0.48 | 0.84 |
| - Physical functioning | 71.31(23.27) | 73.54(27.57) | +1.76(13.16) | 0.40 | 67.18(19.09) | 67.29(22.80) | +1.23(17.98) | 0.69 | 0.88 |
| - Physical role limitations | 75.43(26.29) | 75.30(28.33) | −0.46(22.98) | 0.90 | 64.24(27.17) | 64.11(29.01) | −0.38(22.56) | 0.92 | 0.88 |
| - Pain | 66.88(23.43) | 66.71(25.34) | +0.67(22.72) | 0.85 | 58.40(18.24) | 61.21(22.13) | +3.82(17.89) | 0.22 | 0.35 |
| - General Health | 63.10(21.96) | 65.85(20.46) | +2.65(11.73) | 0.16 | 58.61(20.34) | 60.25(19.69) | +0.11(12.75) | 0.96 | 0.37 |
| Emotional Health Component Score | 72.01(20.65) | 74.07(20.95) | +2.12(14.10) | 0.34 | 68.16(15.32) | 71.40(19.73) | +4.01(14.52) | 0.12 | 0.57 |
| - Emotional well-being | 75.45(20.20) | 76.71(20.93) | +1.83(12.08) | 0.34 | 74.03(14.97) | 75.43(19.30) | +1.03(14.13) | 0.67 | 0.79 |
| - Emotional role limitations | 80.49(24.44) | 80.69(27.54) | +0.81(25.06) | 0.84 | 77.31(19.68) | 83.57(23.7) | +9.07(19.72) | 0.01 | 0.07 |
| - Vitality | 54.55(21.58) | 57.47(22.06) | +3.51(15.88) | 0.17 | 46.99(16.19) | 49.11(23.44) | +2.27(15.55) | 0.40 | 0.74 |
| - Social Functioning | 77.56(26.77) | 81.40(25.33) | +3.96(23.79) | 0.29 | 74.31(21.12) | 77.50(24.59) | +3.68(22.09) | 0.34 | 0.96 |
| Anthropometric Measures | |||||||||
| Weight (kg) | 80.41(16.75) | 78.26(15.25) | −1.00(5.82) | 0.28 | 80.42(17.12) | 80.60(16.71) | +0.15(5.72) | 0.88 | 0.39 |
| BMI (kg/m2) | 30.90(7.25) | 29.87(6.51) | −0.35(2.20) | 0.32 | 30.54(6.43) | 30.65(6.25) | +0.03(2.18) | 0.60 | 0.21 |
| Waist Circumference (cm) | 92.2(12.9) | 95.3(12.6) | +4.2(7.6) | 0.001 | 93.2(11.8) | 97.8(13.92) | +4.5(7.1) | 0.0005 | 0.82 |
| Physical Performance | |||||||||
| 2-Minute Step Test (steps) | 63.9(23.2) | 89.1(23.6) | +21.9(22.0) | <0.0001 | 63.71(16.9) | 71.2(22.1) | +10.0(15.4) | 0.001 | 0.01 |
| 8-Foot Get-Up & Go (seconds) | 7.7(3.4) | 7.0(1.9) | −0.3(1.6) | 0.54 | 8.4(2.8) | 8.1(3.0) | +0.4(1.5) | 0.15 | 0.80 |
| 30-Second Chair Stand (# of rises) | 12.6(3.3) | 14.2(3.7) | +1.5(2.5) | 0.0004 | 11.1(2.8) | 12.6(3.6) | +1.5(2.9) | 0.005 | 0.95 |
| Chair Sit & Reach (inches) | 0.2(3.7) | 1.4(2.8) | +0.9(2.0) | 0.01 | 0.1(4.7) | 1.1(3.9) | +1.21(4.1) | 0.09 | 0.63 |
| Back Scratch (inches) | −3.6(4.6) | −2.1(3.9) | +1.5(4.2) | 0.03 | −4.02(4.0) | −3.4(4.1) | +0.8(3.5) | 0.17 | 0.51 |
| Arm Curl (# of curls) | 15.9(4.0) | 18.7(4.1) | +2.7(3.3) | <0.0001 | 15.9(4.0) | 15.9(3.3) | +0.1(4.0) | 0.93 | 0.002 |
| Hand Grip Strength (kg) | 23.1(4.5) | 24.4(4.8) | +1.4(4.2) | 0.04 | 21.63(4.8) | 23.5(4.4) | +1.9(3.6) | 0.004 | 0.61 |
| Biomarkers | |||||||||
| Toenail Cortisol (ng/g) | 0.02(0.07) | 0.007(0.01) | −0.001(0.01) | 0.67 | 0.03(0.09) | 0.009(0.01) | −0.03(0.09) | 0.24 | 0.18 |
| Telomerase | 0.12(0.13) | 0.07(0.08) | −0.05(0.16) | 0.08 | 0.11(0.13) | 0.06(0.04) | −0.05(0.13) | 0.02 | 0.80 |
| IL-6 (pg/ml) | 1.24(0.77) | 1.44(1.57) | +0.23(1.40) | 0.30 | 2.16(3.44) | 2.02(2.70) | −0.15(1.91) | 0.65 | 0.33 |
p-values in bold text indicate significance at p<0.05
Discussion
Feasibility (Primary Outcome)
The BBCS feasibility trial proved to be safe and surpassed all feasibility benchmarks. Moreover, the vast majority of intervention participants were still gardening at two-year follow-up, demonstrating the potential for this intervention to have long-lasting benefits. However, recruitment was a challenge. Initial contact with cancer survivors using the cancer registry data was difficult, since current address information was often missing. Additionally, survivors residing in rural, farming communities were already gardening and thus ineligible. Also, many cancer survivors screened-out because they were high functioning and adhered to healthy lifestyles. These issues were inherent with the research design and the need to avoid ceiling effects, which are not a concern for community-based programs, as discussed later.
Health-Related (Secondary) Outcomes
Compelling data were seen for vegetable consumption and physical performance. Of clinical relevance were the daily serving increase in vegetable consumption and improvements in seven objective measures of physical performance in the intervention group. This improvement in physical function may translate into the reduction of premature mortality among cancer survivors. Brown and colleagues found that each one-unit increase in the short physical performance battery score predicted a 12% reduction in premature mortality.27 Moreover, a 2014 meta-analysis on a pooled cohort of 833,234 adults found increasing vegetable consumption by one serving/day decreased all-cause mortality by 5%.28
While underpowered, positive trends were seen in body weight status, physical activity, and HRQOL. Among BCSs, weight gain is associated with higher all-cause mortality rates.29 A recent meta-analysis revealed that, among pre- and post-menopausal BCS each 5 kg/m2 increment of BMI increased risks for breast cancer mortality (29%) and all-cause mortality (8%).30 Current cancer survivorship guidelines recommend that survivors achieve and maintain a healthy body weight (18.5 kg/m2 ≤ BMI ≤ 24.9 kg/m2) and engage in ≥150 minutes of moderate physical activity/week.5 Our findings suggest that vegetable gardening may aid BCSs in this endeavor. Given that the majority of participants were either overweight or obese, improvements in body weight status and physical activity (even if small) may be beneficial.4, 29–31
Strengths and Limitations
As with all studies, this feasibility trial had both strengths and limitations. Strengths included representation of African-American BCSs (26.8%), high retention (95%), and use of objective and subjective measures. Limitations included a modest sample size (n=82), no attention control group, and participant representation from only one geographical area (i.e., the Birmingham, Alabama metropolitan area). The design of the intervention was both a strength and a weakness. A notable strength was the reliance on the Cooperative Extension MG program, an extant and sustainable resource available across North America; thus, enhancing potential for dissemination. However, while the home-based garden eliminated survivors’ barriers to travel and was well received, it required more time from MGs than community-based classes or gardens (described in projects that follow). Also, to assure intervention standardization, gardening supplies were provided to all participants. Again, this approach has merit within the context of a controlled clinical trial, but is an obvious barrier to dissemination since external support is required for sustainability. Cost effectiveness studies could be undertaken to assess whether the $500 in gardening supplies is offset by charges in hospital or nursing home admissions, doctor appointments, medical procedures, and/or medications assessed through insurance/Medicare claims. This approach is currently being implemented in a larger, R01-funded clinical trial of Harvest for Health among 426 older cancer survivors across Alabama (NCT02985411).
Further Dissemination
In addition to the larger clinical trial, Harvest for Health has been expanded to other local and global communities. In Alabama, Harvest for Health has validated and supported the creation of Forge Breast Cancer Survivor Center’s community-outreach Gardening Lifestyle Program (www.forgeon.org). In partnership with the Birmingham Botanical Gardens (www.bbgardens.org), the Alabama Cooperative Extension System (www.aces.edu), and the Jefferson County MGs (www.jeffcomg.org), Forge’s monthly gardening lifestyle classes (serving approximately 300 participants annually) link gardening with survivorship concerns and health. Globally, Harvest for Health has informed the development of Healing Gardens (www.healinggardenswur.nl), a six-month supervised community-based vegetable gardening intervention among cancer survivors in the Netherlands led by Wageningen University.
Conclusions
The vegetable gardening intervention proved to be feasible and provided new knowledge about the influence of gardening on motivating behavior change among BCSs. Findings suggest that mentored home-based vegetable gardening may offer an integrative approach to improve vegetable consumption, physical activity and function, body weight status, and HRQOL among BCSs. In addition, Harvest for Health has led to the establishment of a group of trained MGs and given rise to local and global community-based programs. Nevertheless, larger and broader studies are warranted to document the potential benefits of gardening across various groups of cancer survivors.
Acknowledgments
Funding:
Funding was provided by the Women’s Breast Health Fund of the Community Foundation of Greater Birmingham, the National Cancer Institute (R25 CA047888 and 5R25 CA76023), and the Diana Dyer Endowment of the American Institute for Cancer Research.
The authors acknowledge the contributions of Madeline Harris, Dr. Carrie Howell, Dr. Yuko Tsuruta, Teresa Martin, the UAB Cancer Research Experiences for Students (CaRES) interns (Hannah Brown Guthrie, Silvana Janssen, Amber Hardeman, and Justine Goetzman), and work study students (Isabella Mak and Lora Roberson) for their contributions in collecting these data and in recruitment. We also acknowledge Bob Shepard for his contribution in recruiting the study sample. Further, the authors appreciate the efforts of Caroline McClain, Susan Rossman, Nancy Smith, Leonora Roberson, and Jennifer Hicks for their pioneering spirits and effort and we thank Alabama Cooperative Extension agents: Tony Glover, Nelson Wynn, Bethany O’Rear, Dan Porch, Charles Pinkston, and Renee Thompson. We are grateful to the following organizations for providing in kind donations: Territorial Seeds; Johnny’s Selected Seeds; and Hanna’s Garden Shop and the Little Garden Club of Birmingham, AL.
Footnotes
Conflict of Interest Statement:
Drs. Demark-Wahnefried, Frugé, Cases, and Cantor report grants from Diane Dyer Endowment of the American Institute for Cancer Research, National Cancer Institute, and Women’s Breast Health Fund of the Community Foundation of Greater Birmingham during the conduct of the study. Dr. Locher reports grants from National Institutes of Health during the conduct of the study.
Author Contributions:
Jennifer R. Bail: writing - original draft, visualization. Andrew D. Frugé: investigation, data curation, writing - review and editing. Mallory G. Cases: investigation, data curation, writing - review and editing. Jennifer De Los Santos: conceptualization, funding acquisition, writing - review and editing. Julie L. Locher: conceptualization, funding acquisition, writing - review and editing. Kerry P. Smith: methodology, funding acquisition, writing - review and editing. Alan B. Cantor: data curation, formal analysis, writing - review and editing. Harvey J. Cohen: conceptualization, funding acquisition, writing - review and editing. Wendy Demark-Wahnefried: conceptualization, methodology, funding acquisition, project administration, writing - review and editing.
Contributor Information
Jennifer R. Bail, Postdoctoral Fellow, Department of Nutrition Sciences, University of Alabama at Birmingham (UAB), WTI 102C, 1824 6th Ave. S., Birmingham, AL, 35294-3300.
Andrew D. Frugé, Assistant Professor, Department of Nutrition, Dietetics, and Hospitality Management, Auburn University, Auburn, AL.
Mallory G. Cases, Postdoctoral Fellow, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA.
Jennifer De Los Santos, Professor, Department of Radiation Oncology, UAB, Birmingham, AL.
Julie L. Locher, Professor of Medicine, UAB, Birmingham, AL.
Kerry P. Smith, Alabama Cooperative Extension System, Auburn University, Auburn, AL.
Alan B. Cantor, Professor, Department of Preventive Medicine, UAB, Birmingham, AL.
Harvey J. Cohen, Professor of Medicine, Duke University, Durham, NC.
Wendy Demark-Wahnefried, Professor of Nutrition Sciences, Birmingham, AL.
References
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Blanchard CM, Stein KD, Baker F, et al. Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychol Health. 2004;19(1):1–13. [Google Scholar]
- 3.Liese AD, Krebs-Smith SM, Subar AF, et al. The Dietary Patterns Methods Project: Synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145(3):393–402. doi: 10.3945/jn.114.205336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 5.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 6.Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer. 2015;121(23):4212–4221. doi: 10.1002/cncr.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 8.Sommerfeld AJ, McFarland AL, Waliczek TM, Zajicek JM. Growing Minds: Evaluating the Relationship between Gardening and Fruit and Vegetable Consumption in Older Adults. Horttechnology. 2010;20(4):711–717. [Google Scholar]
- 9.Blair CK, Madan-Swain A, Locher JL, et al. Harvest for health gardening intervention feasibility study in cancer survivors. Acta Oncol. 2013;52(6):1110–1118. doi: 10.3109/0284186X.2013.770165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Shoemaker C, Haub M. Can older gardeners meet the physical activity recommendation through gardening? Horttechnology. 2008;18(4):639–643. [Google Scholar]
- 11.Zick CD, Smith KR, Kowaleski-Jones L, Uno C, Merrill BJ. Harvesting more than vegetables: The potential weight control benefits of community gardening. Am J Public Health. 2013;103(6):1110–1115. doi: 10.2105/AJPH.2012.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins J, Mercer J, Clayton D. [Accessed April 5, 2018];Growing a Healthy Older Population in Wales (GHOP): Project summary & key findings. https://www.cardiffmet.ac.uk/health/research/cha/Documents/GHOP%20Report%20Online%20compressed.pdf.
- 13.Annerstedt M, Währborg P. Nature-assisted therapy: Systematic review of controlled and observational studies. Scand J Soc Med. 2011;39(4):371–388. doi: 10.1177/1403494810396400. [DOI] [PubMed] [Google Scholar]
- 14.Cutillo A, Rathore N, Reynolds N, et al. A Literature Review of Nature-Based Therapy and its Application in Cancer Care. Journal of Therapeutic Horticulture. 2015;25(1) [Google Scholar]
- 15.Demark-Wahnefried W, Cases MG, Cantor AB, et al. Pilot Randomized Controlled Trial of a Home Vegetable Gardening Intervention among Older Cancer Survivors Shows Feasibility, Satisfaction, and Promise in Improving Vegetable and Fruit Consumption, Reassurance of Worth, and the Trajectory of Central Adiposity. J Acad Nutr Diet. 2018 doi: 10.1016/j.jand.2017.11.001. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cases MG, Fruge AD, De Los Santos JF, et al. Detailed methods of two home-based vegetable gardening intervention trials to improve diet, physical activity, and quality of life in two different populations of cancer survivors. Contemp Clin Trials. 2016;50:201–212. doi: 10.1016/j.cct.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health Applied Research Program. Diet History Questionnaire, Version 1.0. National Cancer Institute; 2007. [Google Scholar]
- 18.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 19.Welk GJ, Schaben JA, Morrow JR. Reliability of accelerometry-based activity monitors: A generalizability study. Med Sci Sport Exer. 2004;36(9):1637–1645. [PubMed] [Google Scholar]
- 20.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 21.Rikli RE, Chodzko-Zajko W, Rikli RE, Jones CJ. The Development and National Norming of a Functional Fitness Test for Older Adults. Med Sci Sport Exer. 1999;31(Supplement):S399. [Google Scholar]
- 22.Innes E. Handgrip strength testing: A review of the literature. Aust Occup Ther J. 1999;46(3):120–140. [Google Scholar]
- 23.Lohman TJ, Roache AF, Martorell R. Anthropometric Standardization Reference Manual. Med Sci Sport Exer. 1992;24(8):952. [Google Scholar]
- 24.Warnock F, McElwee K, Seo RJ, et al. Measuring cortisol and DHEA in fingernails: A pilot study. Neuropsych Dis Treat. 2010;6:1. [PMC free article] [PubMed] [Google Scholar]
- 25.Frugé AD, Cases MG, Howell CR, et al. Fingernail and toenail clippings as a non-invasive measure of chronic cortisol levels in adult cancer survivors. Cancer Cause Control. 2017 doi: 10.1007/s10552-017-0989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saldanha SN, Andrews LG, Tollefsbol TO. Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Anal Biochem. 2003;315(1):1–21. doi: 10.1016/s0003-2697(02)00663-2. [DOI] [PubMed] [Google Scholar]
- 27.Brown J, Harhay M, Harhay M. Physical function as a prognostic biomarker among cancer survivors. Brit J Cancer. 2015;112(1):194–198. doi: 10.1038/bjc.2014.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight Gain After Breast Cancer Diagnosis and All-Cause Mortality: Systematic Review and Meta-Analysis. J Natl Cancer I. 2015;107(12):djv275–djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer: Systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zomer E, Gurusamy K, Leach R, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: A systematic review and meta-analysis. Obes Rev. 2016;17(10):1001–1011. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]