Abstract
Proton MR spectroscopic imaging (MRSI) has great clinical potential for metabolic mapping of the healthy and pathological human brain. Unfortunately, the promise has not yet been fully achieved due to numerous technical challenges related to insufficient spectral quality caused by magnetic field inhomogeneity, insufficient RF transmit power and incomplete lipid suppression. Here a robust, novel method for lipid suppression in 1H MRSI is presented. The method is based on 2D spatial localization of an elliptical region-of-interest using pulsed second-order spherical harmonics (SH) magnetic fields. A dedicated, high-amplitude second-order SH gradient setup was designed and constructed, containing coils to generate Z2, X2Y2 and XY magnetic fields. Simulations and phantom MRI results are used to demonstrate the principles of the method and illustrate the manifestation of chemical shift displacement. 1H MRSI on human brain in vivo demonstrate high-quality 1H MRSI with robust suppression of extracranial lipids. The method allows a wide range of inner or outer volume selection or suppression and should find application in MRSI, reduced field-of-view MRI and single-volume MRS.
Keywords: proton MRSI, lipid suppression, second-order magnetic fields, human brain
Graphical Abstract
A novel method for lipid suppression in 1H MRSI is presented based on spatial localization of an elliptical region-of-interest using pulsed second-order spherical harmonics (SH) magnetic fields. A high-amplitude second-order SH gradient setup was designed and constructed, containing coils to generate Z2, X2Y2 and XY magnetic fields. Simulations and phantom results demonstrate the principles of the method and illustrate the manifestation of chemical shift displacement. 1H MRSI on human brain in vivo demonstrates high-quality 1H MRSI with robust suppression of extracranial lipids.
INTRODUCTION
Proton MR spectroscopic imaging (MRSI) is a powerful, non-invasive method to map the metabolic profile of the human brain. The intrinsic chemical specificity of MRSI can reveal altered metabolism in regions that would appear normal on standard MRI. MRSI has successfully been used for brain tumor classification and detection of infiltration, planning of radiation therapy and surgery (1–3) and has helped to characterize a range of neurodegenerative diseases such as multiple sclerosis (4–6), Alzheimer’s disease (7,8) and epilepsy (9,10).
Despite its merits and successes, MRSI is not a widespread clinical imaging modality because the method is hampered by a number of technical challenges related to magnetic field B0 inhomogeneity, limited transmit B1+ amplitude and signal contamination from extracranial lipids. Existing lipid suppression methods include inner volume selection (IVS) of a large cuboidal volume based on single-volume MR techniques like STEAM, PRESS or (semi)-LASER (11–14), and outer volume suppression (OVS) of the skull region with up to 12 slice-selective excitation pulses (15,16). The lipid signals can also be reduced with frequency-selective suppression (17), T1-based nulling (18), increased k-space coverage (19), post-processing methods (20), RF-based gradients (21,22) and dedicated crusher coils (23). Each lipid suppression method has its own advantages in terms of ease-of-use and robustness and its own disadvantages regarding level of attainable lipid suppression, peak and average RF power deposition and brain coverage (24).
Here we present a novel method for IVS or OVS based on high-amplitude, second-order spherical harmonic (SH) magnetic fields. The method is referred to as ‘elliptical localization using pulsed second-order fields’ or ECLIPSE. Experimental MR images show the principle of elliptical volume selection using Z2 and X2Y2 shim fields, as well as rotation and translation with XY and linear fields, respectively. Proton MRSI of the human brain in vivo is performed to demonstrate high-quality lipid suppression at low RF power deposition.
METHODS
MR system
MR measurements were performed on a 4 T MR system consisting of an actively shielded whole-body magnet (Ø 940 mm) interfaced to a Bruker Avance III HD spectrometer, running on Paravision 6 (Bruker Biospin Corporation, Billerica, MA, USA). The gradient system (Ø 670 mm) is capable of switching 30 mT/m in 1100 μs and provides up to third order shimming. RF transmission and reception was achieved with a 16-element Tx/Rx volume coil (ID = 230 mm, OD = 334 mm, length = 300 mm), whereby the 16 Tx-elements were used in a fixed-phase configuration.
Second-order gradient coil design and construction
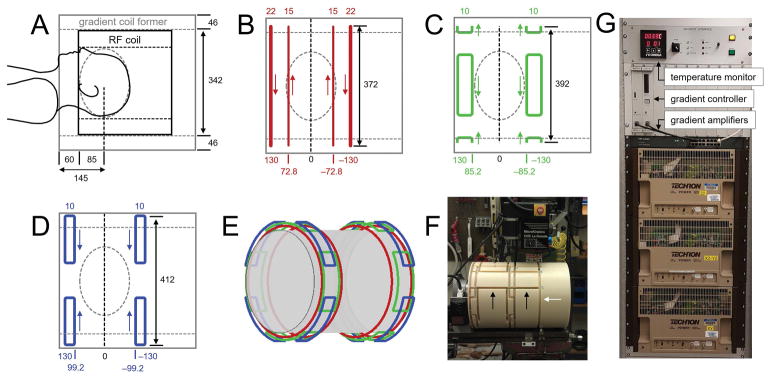
The gradient coil was constructed on a nylon cylinder (The Plastic Factory, Bridgeport, CT, ID 342 mm, OD 434 mm) with a 46 mm wall thickness and 600 mm length, capable of accommodating the 16-element RF coil. Dimensions of the gradient coil were guided by anatomical measurements using standard MRI scans on three subjects (Fig. 1A). This led to using 145 mm as distance between the edge and center positions of the gradient coil, and an ellipsoidal region-of-interest (ROI) with diameters of 200 mm, 220 mm and 160 mm for the X, Y and Z spatial directions, for the anticipated volume selection covering the head. All magnetic field optimizations involved in gradient coil wire placements were performed over this ROI.
Figure 1.
Second-order gradient coil design and construction. (A) RF coil and gradient coil former in relation to the human head. The center of the gradient coil windings is placed at 145 mm from the edge as dictated by the empirically measured distance between shoulders and the middle of the brain. Wire patterns for the (B) Z2, (C) X2Y2 and (D) XY coils as optimized over a 200 × 220 × 160 mm diameter ellipsoid (dotted line). The wire positions relative to the gradient center and the number of turns are indicated below and above the gradient former, respectively. The relative current directions are indicated by small arrows. (E) 3D rendering of the Z2 (red), X2Y2 (green) and XY (blue) coils showing the radial separation. (F) Photograph of the gradient coil former towards the end of the milling process. In addition to the fundamental coil element positions, additional tracks are visible to connect the various coil elements (black arrows) and provide return paths (white arrow). All distances are in mm. (G) Gradient driver and amplifier setup and temperature monitoring.
Wire placements for second-order Z2, X2Y2 and XY coils were based on the basic designs of Romeo and Hoult (25) using current loops and arcs, whereby the short length compared to the diameter of the proposed gradient coils demanded a re-optimization of the relative wire placements and currents. Specifically, the wire center positions for all three coils was limited to [−130.. +130 mm] relative to the coil center in order to ensure a 10 mm wall thickness. Using the spherical harmonics functions A2,0(2Z2 − (X2 + Y2)), A2,2(X2 − Y2) and A2,-2XY the target field amplitudes were set as A2,0 = 2.5 Hz/mm2, A2,2 = 1.25 Hz/mm2 and A2,-2 = 1.25 Hz/mm2 for a 50 A input current. The wire positions and number of loops were optimized through least-squares minimization, while maintaining the inherent symmetry of the individual coils. Magnetic fields were calculated through numerical integration of the Biot-Savart law. All magnetic field calculations and optimizations were performed in Matlab 8.3 (The Mathworks, Natick, MA, USA).
The wire tracks for the coils were milled with a CNC-controlled (MicroKinetics Corporation, Kennesaw, GA, USA) Bridgeport Series I Vertical Milling Machine (Bridgeport Machines, Inc., Bridgeport, CT, USA). Tracks were 12.7 mm wide, except for the inner two Z2 wire tracks which had a width of 9.5 mm.
The three coils were manually wound from AWG 12 (Ø 2.1 mm) polyurethane-coated copper wire (Bulk Wire, Yorba Linda, CA, USA) and placed within the wire tracks milled in the nylon former. Following placement of six PT100 thermal sensor probes (two per coil, Omega Engineering Inc, Norwalk, CT, USA) the wires were permanently fixed with a two-part epoxy adhesive (Loctite, Henkel Coorporation, Rocky Hill, CT, USA). No effort was made to remove air bubbles from the epoxy. The final gradient coils were characterized by inductances of 859, 182, and 154 μH and resistances of 475, 240 and 238 mΩ for the Z2, X2Y2 and XY coils.
Three Techron 7780 gradient amplifiers (AE Techron, Elkhart, IN, USA) drove the coils with 130 V and 100 A, leading to rise times of 661, 140 and 118 μs for the Z2, X2Y2 and XY coils (Fig. 1G). In order to achieve a straightforward temporal alignment with the MR system’s linear gradient pulses, the rise time for all three coils was set to the gradient rise time of 1100 μs. Coil currents were controlled with a home-build, multi-channel gradient controller (26).
MR spectroscopic imaging
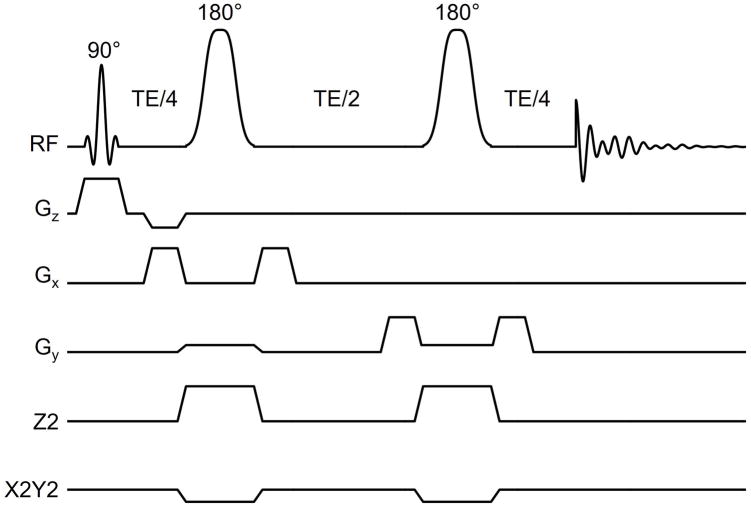
All MR studies were performed with an adiabatic double-spin-echo sequence (Fig. 2). A 2.0 ms Shinnar-Le Roux excitation pulse ((27), 2.8 kHz bandwidth) selected a 10 mm thick slice in the Z direction. Two 6.0 ms adiabatic full passage pulses (HS2 modulation (28), 3.33 kHz bandwidth) were used for refocusing in the presence of a combination of X, Y, Z, Z2, X2Y2 and XY magnetic fields. The refocusing pulses were surrounded by 3.0 ms 25 mT/m magnetic field crusher gradients to ensure destruction of signal excited by the 180° pulses from remote locations (Fig. 2). MR images were acquired with gradient-echo frequency encoding (100 kHz spectral width) and phase encoding following the final 180° pulse (FOV 210 × 210 mm, 128 × 128 matrix). Following a one-time calibration of the gradient coil magnetic field amplitudes, ECLIPSE magnetic fields and offsets were calculated in Matlab 8.3 on a subject-specific MR image. An elliptical ROI was interactively adjusted (center position, two radii and an in-plane rotation angle) to maximize brain inclusion, while simultaneously minimizing extracranial tissues. MR spectroscopic images were acquired with two phase-encoding gradients following the final 180° pulse sampling a circularly restricted k-space (FOV 210 × 210 mm, 21 × 21 matrix) for a nominal resolution of 1 mL. All scans were acquired with TR/TE = 1,500/50 ms leading to an 8.3 min MRSI acquisition time. The minimum allowable TR based on RF power deposition considerations was 850 ms, whereas the minimum possible TE due to RF and gradient pulses was 35 ms. Water suppression was achieved with a seven-pulse VAPOR sequence (29). Magnetic field homogeneity across the MRSI slice was optimized with first-and second order SH shims calculated from a MRI-based B0 map. MRSI data processing used a separately acquired water MRSI for receiver phase correction, amplitude-weighting and correction of temporal magnetic field variations (30).
Figure 2.
ECLIPSE pulse sequence for inner volume selection based on an adiabatic double-spin-echo. The 90° excitation pulse selects an axial slice after which the refocusing pulses provide in-plane ROI selection with second-order SH magnetic fields. Small linear magnetic fields are required to compensate first-order SH imperfections in the second-order SH fields or to achieve translation of the elliptical volume. Linear gradients in the X and Y directions are used as TE crushers surrounding the refocusing pulses. For MRI and MRSI applications the sequence is extended with frequency and phase-encoding gradients following the second refocusing pulse (not shown).
Chemical shift displacement
As a fundamental concept in MRI and localized MRS it is well-known that a frequency-selective RF pulse applied during a linear gradient results in the selection of a 1D spatial slice. Different chemical shifts will result in a displacement of the spatial slice, an effect known as the chemical shift displacement artifact (CSDA). For a given slice thickness the CSDA can only be minimized by increasing the RF bandwidth. The ECLIPSE method uses a frequency selective RF pulse during second-order Z2 and X2Y2 magnetic fields, leading to the selection of a 2D elliptical volume (Fig. 3). The slice boundary r (in mm) for a spin with Larmor frequency ν0 is given by
Figure 3.
Chemical shift displacement artifact (CSDA) during ECLIPSE. (A, B) Frequency distributions during (A) 78.7 Hz/cm2 and (B) 138.9 Hz/cm2 second-order magnetic field gradients. The curves shift up and down relative to an on-resonance condition (green) for positive (red, +500 Hz) and negative (blue, −500 Hz) frequency offsets, respectively. For on-resonance spins a frequency selective RF pulse (bandwidth BW = 3.33 kHz) selects a 2 × 60 = 120 mm wide slice for IVS with an offset νRF = −0.5 kHz (A, green line). OVS with 17.5 mm wide slices centered at +/− 68.8 mm is achieved with an offset of 6.66 kHz (B, green line). Off-resonance spins will select wider or narrower slices depending on the chemical shift and the sign of the second-order gradient field. (C) Selection of an 120 × 170 mm ellipse (green, BW = 3.33 kHz, νRF = 2.83 kHz) for on-resonance spins leads to the selection of 130.2 × 184.4 mm and 108.9 × 154.3 mm ellipses for spins that are off-resonance by −500 Hz and +500 Hz, respectively. (D) Selection of a 120 × 170 mm ID OVS ring (17.5 × 24.8 mm thickness, BW = 3.33 kHz, νRF = 5.0 kHz) for on-resonance spins leads to the selection of 125.9 × 178.3 mm (16.8 × 23.9 mm thickness) and 113.8 × 161.3 mm (18.2 × 25.8 mm thickness) ID OVS rings for spins that are off-resonance by −500 Hz and +500 Hz, respectively. For both IVS and OVS the blue and red ROIs are swapped in the presence of a negative second-order magnetic field.
| (1) |
where νRF is the RF transmit frequency, BW is the RF pulse bandwidth (in Hz) and G is the second-order gradient strength (in Hz/mm2). Depending on the RF transmit frequency, Eq. (1) has between zero and four solutions. The most common situations arise when the RF pulse selects two slice boundaries (Fig. 3A and C) for IVS or four slice boundaries (Fig. 3B and D) for OVS. The green ROIs in Figs. 3C and D depict the spatial region selected for on-resonance spins (i.e. ν0 = 0). The blue and red ROIs depict the spatial region selected for spins that are off-resonance by −500 Hz and +500 Hz, respectively, during a positive second-order field gradient. Note that the CSDA during ECLIPSE manifests itself as an increase or decrease of the selected ROI. For most applications it is preferable to execute ECLIPSE with negative second-order fields such that when the green ROI depicts the selected region for lipids, the blue ROI depicts the region for water and most metabolites resonating at higher Larmor frequencies. Note that the CSDA is substantially smaller for OVS than for IVS due to the steeper magnetic field gradient at the edge of the brain. Similar to conventional OVS using linear magnetic field gradients, the absolute CSDA for ECLIPSE is reduced by selecting the narrowest possible slice that still covers the entire skull region. On most subjects an ROI thickness of 20–30 mm would be sufficient.
RESULTS
The calibration of the second-order gradient coil revealed good agreement with the target design field shapes and amplitudes. Z2, X2Y2 and XY gradient coils were calibrated at 5.48, 2.58 and 2.76 Hz/cm2/A field amplitudes with minor linear gradient field imperfections on the order of 10 Hz/cm/A. The linear imperfections were compensated with the system’s linear field gradients. Despite the absence of active water or air cooling, no gradient coil temperature increases were observed during coil calibration or ECLIPSE. This is likely due to the low 5 A maximum current during calibration (at 30% duty cycle) and the low duty cycle during ECLIPSE (0.4 % maximum duty cycle). RF transmit B1+ and signal-to-noise ratio maps confirmed the absence of RF perturbations arising from the second-order gradient coils. Besides a minor B0 magnetic field modulation (1.3 Hz/A with a decay constant of 260 ms), no eddy currents were observed for the pulsed second-order SH coils. This is likely due to the large physical separation between the second-order ECLIPSE coils and the magnet cryostat.
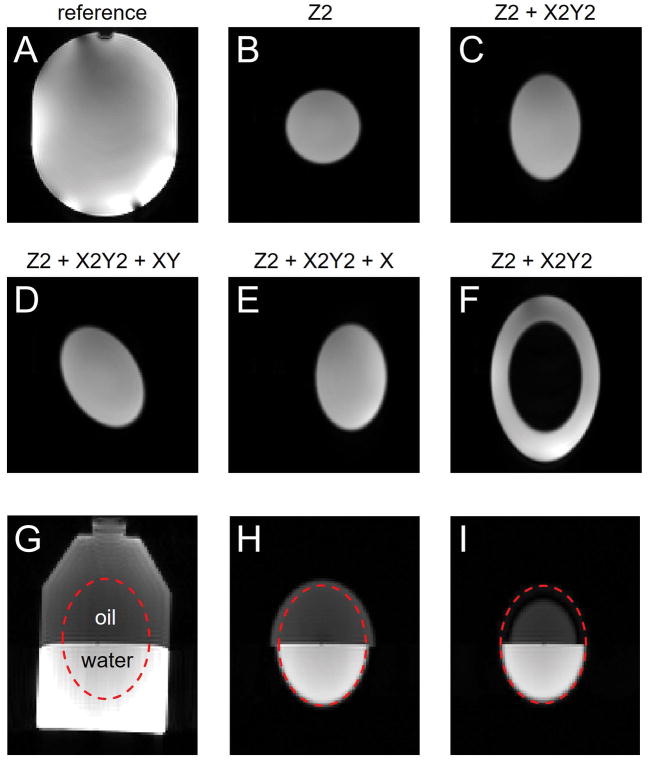
Fig. 4 demonstrates the localization capabilities of ECLIPSE. In the presence of a Z2 magnetic field, a frequency-selective RF pulse selects a 2D circle (Fig. 4B). Adding an X2Y2 magnetic field results in the selection of a 2D ellipse (Fig. 4C). The elliptical volume can be rotated (Fig. 4D) and shifted (Fig. 4E) by adding XY and X magnetic fields, respectively. By adjusting the RF pulse center frequency, the outside of the ellipse shown in Fig. 4C can be selected (Fig. 4F). Fig. 4G–I provide an experimental validation of the CSDA during ECLIPSE for an oil/water phantom. When selecting an elliptical volume during a positive second-order field (Fig. 4H) the lower-frequency oil spins appear as a larger ellipse, in correspondence with Fig. 3C. When the second-order fields are negative the oil ellipse is smaller than the water ellipse (Fig. 4I). A weaker intensity, larger oil ellipse is visible in Fig. 4I due to the presence of multiple oil resonances closer to the water frequency.
Figure 4.
Experimental volume selection using ECLIPSE. (A) Baseline MRI acquired in the absence of magnetic field gradients during the refocusing pulses. (B–F) MR image in the presence of a Z2 magnetic field and additional (C) X2Y2, (D) X2Y2 and XY, (E) X2Y2 and X and (F) X2Y2 magnetic fields. In all cases the gradient strengths were adjusted to select a (B) 80 mm diameter circle or a (C–F) 80 × 120 mm diameter ellipse. The RF frequency offsets in (B–E) were adjusted to provide IVS, whereas the offset in (F) was adjusted for OVS. (G–I) Experimental demonstration of the CSDA in ECLIPSE on an oil-water phantom. (G) MRI acquired without in-plane volume selection and (H, I) with in-plane selection of an elliptical VOI during (H) negative and (I) positive second-order magnetic fields. A faint larger ring is visible in (I) due to the multiple resonances in vegetable oil leading to a range of chemical shift displacements.
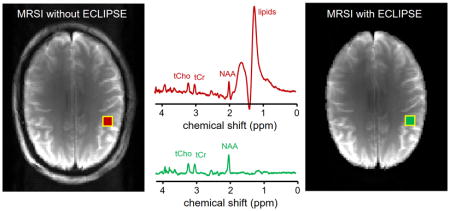
Fig. 5 shows 1H MRSI on the human brain in vivo (A–D) without and (E–H) with 2D in-plane localization using ECLIPSE. Without OVS the extracranial lipid signals dominate each MR spectra in the 21 × 21 MRSI dataset, including spectra well within the brain (Fig. 5D). The MR image acquired with ECLIPSE (Fig. 5E) demonstrates the high-quality elliptical localization similar to that achieved in phantom studies (Fig. 4). The localization performance is confirmed by the 1H MRSI data (Fig. 5F/G) showing the absence of lipid signals from all voxels while retaining the metabolite signals at full intensity.
Figure 5.
ECLIPSE MRI and MRSI on human brain in vivo. (A) MRI and (B) MRSI without in-plane volume selection show strong extracranial lipid signals that contaminate every voxel, including those from within the brain (red box, spectrum shown in (D)). The 3 × 3 grid as indicated in (A) is extracted from the full 21 × 21 MRSI grid. (C) Metabolic image obtained through (absolute-valued) spectral integration of the 1.8 – 2.2 ppm chemical shift range is dominated by strong extracranial lipid signals. (E) MRI and (F) MRSI in the presence of in-plane selection of a 125 × 170 mm elliptical volume. The extracranial lipid signals are suppressed while retaining the metabolite signals at full intensity. (G) Metabolic image from the 1.8 – 2.2 ppm region provides a NAA spatial distribution map without lipid artifacts. Note that the vertical scale in (G) is 50 times higher than in (C). The metabolic images in (C, G) are not intensity corrected for the receiver sensitivity profiles. (H) 1H MR spectrum extracted from the identical voxel as shown in (D).
DISCUSSION
Here we have presented a novel method for 2D volume selection for lipid suppression in proton MRSI based on frequency-selective RF pulses in the presence of a second-order SH magnetic field. The principle of using second-order SH magnetic fields for spatial localization was first described by Cho and co-workers (31–33) using a dedicated Z2 gradient coil. Unfortunately, the method never moved beyond the proof-of-principle stage. More recent reports have used standard second-order SH shim coils to achieve field-of-view restriction in MRI (34–36). The introduction of multi-coil (MC) shimming (37,38) has provided complex magnetic field shaping that is ideally suited for 2D localization (39,40). However, the relatively small magnetic field strengths generated by SH or MC shim setups become a significant limitation for spectroscopic localization, where a small magnetic field gradient is synonymous with a small RF bandwidth and hence a large CSDA. The success of ECLIPSE for spectroscopic applications therefore rests on the availability of high-amplitude magnetic field gradients. In this study a dedicated, home-build second-order gradient coil was designed and constructed to achieve high-quality 1H MRSI without lipid contamination. The constructed gradient coil is characterized by a small length-to-diameter ratio of circa 0.82, but was after optimization of the wire positions, able to achieve high magnetic field efficiencies with good fidelity over the ellipsoidal ROI. The availability of commercial, high-powered shim coil inserts (41) should help with the wider dissemination of the ECLIPSE method. The successful demonstration of high-quality lipid suppression with second-order SH fields certainly provides incentive to pursue a high-powered MC setup for improved ROI shaping.
For any gradient-based localization method the CSDA is minimized by increasing the bandwidth of the RF pulses, which requires an increase of the gradient field in order to attain the same ROI. In previous publications the bandwidth was limited by the small magnetic field strength generated by the SH or MC setups (35–38). The high-amplitude SH fields used here has shifted the limitation to the maximum RF amplitude available with a typical human RF coil and amplifier combination. A robust method to further increase the RF pulse bandwidth without increasing the maximum RF amplitude is to employ gradient modulation. The bandwidth of RF pulses used during regular 1D slice selection can be increased ten-fold by employing the principles of gradient offset-independent adiabaticity (GOIA, (42)) or frequency offset corrected inversion (FOCI, (43)). A similar modulation of the gradient field to achieve an improved RF bandwidth should be suitable for ECLIPSE.
The current ECLIPSE method was implemented with three out of the five second-order SH fields, allowing the selection and rotating of an elliptical ROI within the axial plane. Selection of an elliptical ROI is possible in any sagittal, coronal or oblique orientation by changing the relative contributions of the Z2 and X2Y2 coils. However, the two missing ZX and ZY fields will be required for rotation in a non-axial plane. ECLIPSE with a complete second-order SH coil set will be able to select an elliptical ROI in any single or double-oblique slice orientation.
Applying a frequency-selective RF pulse in the presence of a Z2 magnetic field leads to the selection of a circular ROI for Z = 0. However, since the Z2 field diverges for Z ≠ 0 the selected ROI will increasingly deviate from a circle for larger Z offsets. At an in-plane ROI size suitable for the human head (circa 120 × 170 mm), ECLIPSE can select a 40 mm slab without significant deviation of the in-plane shape, allowing for 3D MRSI across central areas of the human brain. However, as the brain shape and size changes along the superior-inferior direction, whole-brain OVS is not possible with the current ECLIPSE or any other slice-based OVS method. Whole-brain OVS will therefore have to be based on multiple slab selections or the use of non-SH-based magnetic fields.
The MRI and MRSI results presented here were all based on IVS. While providing T1-independent lipid suppression, the slice definition and CSDA are often less optimal than that achieved with OVS (see Fig. 3). Classical OVS based on frequency-selective RF pulses selecting multiple 1D slices is hampered by T1 relaxation and incomplete signal dephasing in regions selected by overlapping slices (16). The fact that ECLIPSE can achieve 2D localization with a single RF pulse avoids slice overlap, such that ECLIPSE-based OVS can be optimized with respect to T1 relaxation and B1+ magnetic field homogeneity, similar to water suppression methods like WET (44) and VAPOR (29). Work is currently in progress to re-evaluate OVS methods based on ECLIPSE.
The ECLIPSE method has been demonstrated for lipid suppression in MRSI. However, ECLIPSE can also find application in single-volume MRS and reduced field-of-view MRI. For MRS the main advantages would be related to lower SAR and shorter echo-times due to the reduced minimum number of RF pulses. In addition, ECLIPSE can be beneficial in pathologies (tumors, multiple sclerosis lesions) where an elliptical VOI provides improved coverage of the tissue under investigation.
Acknowledgments
This research was supported by NIH grant R01-EB014861.
ABBREVIATIONS
- CSDA
Chemical shift displacement artifact
- ECLIPSE
Elliptical localization with pulsed second-order fields
- FOCI
Frequency offset corrected inversion
- GOIA
Gradient offset-independent adiabaticity
- IVS
Inner volume selection
- LASER
Localization by adiabatic spin-echo refocusing
- MC
Multi-coil
- OVS
Outer volume suppression
- PRESS
Point-resolved spectroscopy
- SH
Spherical harmonics
- STEAM
Stimulated-echo acquisition mode
- VAPOR
Variable pulse power and optimized relaxation delays
- WET
Water suppression enhanced through T1 effects
References
- 1.Graves EE, Nelson SJ, Vigneron DB, Chin C, Verhey L, McDermott M, Larson D, Sneed PK, Chang S, Prados MD, Lamborn K, Dillon WP. A preliminary study of the prognostic value of proton magnetic resonance spectroscopic imaging in gamma knife radiosurgery of recurrent malignant gliomas. Neurosurgery. 2000;46:319–326. doi: 10.1097/00006123-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Nelson SJ, Graves E, Pirzkall A, Li X, Antiniw Chan A, Vigneron DB, McKnight TR. In vivo molecular imaging for planning radiation therapy of gliomas: an application of 1H MRSI. J Magn Reson Imaging. 2002;16:464–476. doi: 10.1002/jmri.10183. [DOI] [PubMed] [Google Scholar]
- 3.Pirzkall A, Li X, Oh J, Chang S, Berger MS, Larson DA, Verhey LJ, Dillon WP, Nelson SJ. 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. International journal of radiation oncology, biology, physics. 2004;59:126–137. doi: 10.1016/j.ijrobp.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Suhy J, Rooney WD, Goodkin DE, Capizzano AA, Soher BJ, Maudsley AA, Waubant E, Andersson PB, Weiner MW. 1H MRSI comparison of white matter and lesions in primary progressive and relapsing-remitting MS. Multiple sclerosis (Houndmills, Basingstoke, England) 2000;6:148–155. doi: 10.1177/135245850000600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sijens PE, Irwan R, Potze JH, Mostert JP, De Keyser J, Oudkerk M. Analysis of the human brain in primary progressive multiple sclerosis with mapping of the spatial distributions using 1H MR spectroscopy and diffusion tensor imaging. Eur Radiol. 2005;15:1686–1693. doi: 10.1007/s00330-005-2775-0. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging. 2010;28:163–170. doi: 10.1016/j.mri.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Schuff N, Amend D, Ezekiel F, Steinman SK, Tanabe J, Norman D, Jagust W, Kramer JH, Mastrianni JA, Fein G, Weiner MW. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49:1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Schuff N, Kornak J, Soher B, Yaffe K, Kramer JH, Ezekiel F, Miller BL, Jagust WJ, Weiner MW. Effects of Alzheimer disease on fronto-parietal brain N-acetyl aspartate and myo-inositol using magnetic resonance spectroscopic imaging. Alzheimer disease and associated disorders. 2006;20:77–85. doi: 10.1097/01.wad.0000213809.12553.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maudsley AA, Domenig C, Ramsay RE, Bowen BC. Application of volumetric MR spectroscopic imaging for localization of neocortical epilepsy. Epilepsy research. 2010;88:127–138. doi: 10.1016/j.eplepsyres.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan JW, Duckrow RB, Gerrard J, Ong C, Hirsch LJ, Resor SR, Jr, Zhang Y, Petroff O, Spencer S, Hetherington HP, Spencer DD. 7T MR spectroscopic imaging in the localization of surgical epilepsy. Epilepsia. 2013;54:1668–1678. doi: 10.1111/epi.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frahm J, Merboldt KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987;72:502–508. doi: 10.1002/mrm.1910170113. [DOI] [PubMed] [Google Scholar]
- 12.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 13.Scheenen TW, Klomp DW, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59:1–6. doi: 10.1002/mrm.21302. [DOI] [PubMed] [Google Scholar]
- 14.Bogner W, Gagoski B, Hess AT, Bhat H, Tisdall MD, van der Kouwe AJ, Strasser B, Marjanska M, Trattnig S, Grant E, Rosen B, Andronesi OC. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. Neuroimage. 2014;103:290–302. doi: 10.1016/j.neuroimage.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CT. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993;188:277–282. doi: 10.1148/radiology.188.1.8511313. [DOI] [PubMed] [Google Scholar]
- 16.Henning A, Schar M, Schulte RF, Wilm B, Pruessmann KP, Boesiger P. SELOVS: brain MRSI localization based on highly selective T1- and B1- insensitive outer-volume suppression at 3T. Magn Reson Med. 2008;59:40–51. doi: 10.1002/mrm.21374. [DOI] [PubMed] [Google Scholar]
- 17.Esmaeili M, Bathen TF, Rosen BR, Andronesi OC. Three-dimensional MR spectroscopic imaging using adiabatic spin echo and hypergeometric dual-band suppression for metabolic mapping over the entire brain. Magn Reson Med. 2017;77:490–497. doi: 10.1002/mrm.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebel A, Govindaraju V, Maudsley AA. Comparison of inversion recovery preparation schemes for lipid suppression in 1H MRSI of human brain. Magn Reson Med. 2003;49:903–908. doi: 10.1002/mrm.10444. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Patel M, Ugurbil K. A new strategy for spectroscopic imaging. J Magn Reson B. 1994;103:30–38. doi: 10.1006/jmrb.1994.1004. [DOI] [PubMed] [Google Scholar]
- 20.Haupt CI, Schuff N, Weiner MW, Maudsley AA. Removal of lipid artifacts in 1H spectroscopic imaging by data extrapolation. Magn Reson Med. 1996;35:678–687. doi: 10.1002/mrm.1910350509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med. 2010;63:9–19. doi: 10.1002/mrm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boer VO, Klomp DW, Juchem C, Luijten PR, de Graaf RA. Multi-slice MRSI of the human brain at 7 Tesla using dynamic B0 and B1 shimming. Proc Int Soc Magn Reson Med. 2011;19:142. doi: 10.1002/mrm.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boer VO, van de Lindt T, Luijten PR, Klomp DW. Lipid suppression for brain MRI and MRSI by means of a dedicated crusher coil. Magn Reson Med. 2015;73:2062–2068. doi: 10.1002/mrm.25331. [DOI] [PubMed] [Google Scholar]
- 24.de Graaf RA. Principles and Techniques. Chichester: John Wiley; 2007. In Vivo NMR Spectroscopy. [Google Scholar]
- 25.Romeo F, Hoult DI. Magnet field profiling: analysis and correcting coil design. Magn Reson Med. 1984;1:44–65. doi: 10.1002/mrm.1910010107. [DOI] [PubMed] [Google Scholar]
- 26.Nixon TW, McIntyre S, de Graaf RA. The design and implementation of a 64 channel arbitrary gradient waveform controller. Proc Int Soc Magn Reson Med. 2017;25:969. [Google Scholar]
- 27.Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm. IEEE Trans Med Imaging. 1991;10:53–65. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- 28.Tannus A, Garwood M. Improved performance of frequency-swept pulses using offset-independent adiabaticity. J Magn Reson A. 1996;120:133–137. [Google Scholar]
- 29.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Cho ZH. Localized volume selection technique using an additional radial gradient coil. Magn Reson Med. 1989;12:56–63. doi: 10.1002/mrm.1910120107. [DOI] [PubMed] [Google Scholar]
- 32.Oh CH, Hilal SK, Cho ZH, Mun IK. New spatial localization method using pulsed high-order field gradients (SHOT: Selection with High-Order gradienT) Magn Reson Med. 1991;18:63–70. doi: 10.1002/mrm.1910180108. [DOI] [PubMed] [Google Scholar]
- 33.Wu EX, Johnson G, Hilal SK, Cho ZH. A new 3D localization technique using quadratic field gradients. Magn Reson Med. 1994;32:242–245. doi: 10.1002/mrm.1910320214. [DOI] [PubMed] [Google Scholar]
- 34.de Graaf RA, Rothman DL, Nixon TW. Spatial localization with pulsed second-order shims. Proc Int Soc Magn Reson Med. 2007;15:1350. [Google Scholar]
- 35.Ma C, Xu D, King KF, Liang ZP. Reduced field-of-view excitation using second-order gradients and spatial-spectral radiofrequency pulses. Magn Reson Med. 2013;69:503–508. doi: 10.1002/mrm.24259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam H, Glover GH. Reduced field of view imaging using a static second-order gradient for functional MRI applications. Magn Reson Med. 2016;75:817–822. doi: 10.1002/mrm.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juchem C, Brown PB, Nixon TW, McIntyre S, Rothman DL, de Graaf RA. Multicoil shimming of the mouse brain. Magn Reson Med. 2011;66:893–900. doi: 10.1002/mrm.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juchem C, Nixon TW, McIntyre S, Boer VO, Rothman DL, de Graaf RA. Dynamic multi-coil shimming of the human brain at 7T. J Magn Reson. 2011;212:280–288. doi: 10.1016/j.jmr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juchem C, Nixon TW, Brown PB, McIntyre S, Rothman DL, de Graaf RA. Spatial selection through multi-coil magnetic field shaping. Proc Int Soc Magn Reson Med. 2011;19:385. [Google Scholar]
- 40.Rudrapatna US, de Graaf RA, Nixon TW, Juchem C. Multi-dimensional reduced field-of-view excitation by integrated RF pulse and DYNAMITE B0 field design. Proc Int Soc Magn Reson Med. 2016;24:1010. [Google Scholar]
- 41.Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 Tesla. Magn Reson Med. 2013;68:1007–1017. doi: 10.1002/mrm.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tannus A, Garwood M. Adiabatic pulses. NMR Biomed. 1997;10(8):423–434. doi: 10.1002/(sici)1099-1492(199712)10:8<423::aid-nbm488>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 43.Ordidge RJ, Wylezinska M, Hugg JW, Butterworth E, Franconi F. Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn Reson Med. 1996;36:562–566. doi: 10.1002/mrm.1910360410. [DOI] [PubMed] [Google Scholar]
- 44.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]