Abstract
Background
Aspirin may reduce the risk of several types of cancer, but consistent evidence is lacking for basal cell carcinoma (BCC). Little is known about whether folic acid is associated with BCC risk.
Methods
BCC incidence was evaluated in the Aspirin/Folate Polyp Prevention Study, a randomized, double-blind, placebo-controlled clinical trial of aspirin (81 mg/d or 325 mg/d for approximately 3 years) and/or folic acid (1 mg/d for approximately 6 years) for the prevention of colorectal adenomas among 1121 participants with a previous adenoma. BCC was confirmed by blinded review of pathology reports.
Results
In total, 104 of 958 non-Hispanic white participants were diagnosed with BCC over a median follow-up of 13.5 years. Cumulative incidence of BCC was 12% (95% confidence interval, CI, 7-17%) for placebo, 16% (11-21%) for 81 mg/d aspirin, and 15% (10-20%) for 325 mg/d aspirin (hazard ratio, HR for any aspirin, 1.45; CI 0.93-2.26; HR for 81 mg/d, 1.57; CI, 0.96-2.56; HR for 325 mg/d, 1.33; CI, 0.80-2.20). BCC risk was higher with aspirin use for those without previous skin cancer, but lower with aspirin use for those with previous skin cancer (P-interaction=0.02 for 81 mg/d; P-interaction=0.03 for 325 mg/d). Folic acid supplementation was unrelated to BCC incidence (HR, 0.85; CI, 0.57-1.27).
Conclusions
Neither aspirin nor folic acid treatment had a statistically significant effect on BCC risk. Subgroup analysis by skin cancer history suggested that chemopreventive effects of nonsteroidal anti-inflammatory drugs observed in previous studies may be specific to those at high risk for BCC.
More than 1 million cases of basal cell carcinoma (BCC) are diagnosed annually in the U.S.1 Exposure to ultraviolet (UV) radiation is the primary risk factor for common skin cancers such as BCC.2 Aspirin (acetylsalicylic acid) and other commonly used nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce the risk of certain cancers,3 with consistent evidence across observational and randomized studies for malignancies of the large intestine, stomach, and esophagus.4 For BCC, some studies reported reduced risk among aspirin users, but others found no association.5 The topical NSAID diclofenac effectively treats actinic keratosis (AK), precursors for cutaneous squamous cell carcinomas (SCC),6 and a randomized clinical trial among individuals with numerous AKs found that the oral cyclooxygenase (COX)-2 inhibitor celecoxib reduced the short-term risk of BCC by 60%.7 Folic acid plays an essential role in DNA synthesis and repair, and folate deficiency is a hypothesized risk factor for cancer.8 Few studies have specifically evaluated folic acid exposure and BCC risk. Large prospective cohort studies observed that high dietary folate intake was associated with a modest increase in risk of BCC,9 but not SCC.10 The Aspirin/Folate Polyp Prevention Study provided an opportunity to investigate both aspirin and folic acid use and the incidence of BCC in a randomized, double-blind, placebo-controlled study.
Methods
Study design
Details of the study design and primary findings of the Aspirin/Folate Polyp Prevention Study have been previously published.11,12 From July 1994 to March 1998, patients 21-80 years of age recently diagnosed with colorectal adenomas were enrolled to evaluate the efficacy of aspirin, folic acid, or both to prevent future adenomas. The phase III study had a 3×2 factorial design, comparing 81 mg/d and 325 mg/d of aspirin with placebo and 1 mg/d of folic acid with placebo. Participants were recruited from medical centers affiliated with Dartmouth-Hitchcock Medical Center (Lebanon, NH); University of North Carolina (Chapel Hill, NC); University of Southern California (Los Angeles, CA); University of Colorado (Denver CO); Henry Ford Health System (Detroit, MI); University of Toronto (Toronto, ON); University of Iowa (Iowa City, IA); Cleveland Clinic Foundation (Cleveland, OH); and University of Minnesota (Minneapolis, MN).
Exclusion criteria included a history of invasive colorectal cancer, familial polyposis, inflammatory bowel disease (IBD), and conditions treated or worsened with aspirin or folate, such as anemia, vitamin B12 deficiency, arthritis, and atherosclerotic cardiovascular disease. After a 3-month placebo run-in period, 1121 participants taking ≥80% of allocated pills underwent blocked randomization stratified by center, sex, and age (Supplemental Figure 1). A baseline questionnaire ascertained demographics and medical history including previous diagnosis of melanoma and non-melanoma skin cancer, but did not distinguish subtypes. Institutional review boards at the 9 participating clinical centers approved study protocols; all participants provided written informed consent. The study was registered at clinicaltrials.gov (NCT00272324).
Follow-up and outcome ascertainment
Treatment with aspirin/placebo ended after a surveillance colonoscopy anticipated 3 years after a baseline colonoscopy. To assess longer exposure to folic acid, participants were invited to continue folic acid/placebo for an additional 3- or 5-year colonoscopy interval before October 1, 2004.12 Observational follow-up continued after the end of the active treatment periods. Questionnaires were completed every 4 months during the treatment phase to assess adherence to study pills, use of prescription and over-the-counter medications, nutritional supplements, and the occurrence of medical events. A similar questionnaire was completed annually during observational follow-up until withdrawal or December 31, 2006. A final questionnaire was completed between February 1, 2010 and April 31, 2012 in order to update medication use and medical events since the previous contact. Blinded study physicians reviewed clinical records to verify reported events. BCC or SCC histology was confirmed from pathology reports. Anatomic location of lesions was ascertained from pathology or clinical records.
Statistical analyses
Time to first BCC diagnosis was calculated from date of randomization and censored at date of death or last contact. Cumulative incidence was estimated from the Kaplan-Meier method, and hazard ratios (HR) estimated from proportional hazards regression with adjustment for the randomization stratification variables (center, sex, and age). Given the low prevalence of skin cancer in non-white populations, we excluded all study participants that self-reported a race/ethnicity other than non-Hispanic white. All analyses were performed according to the intention-to-treat approach.
Effect modification of the aspirin effect by folic acid treatment (and vice versa) was assessed by including an interaction term in regression models. A subgroup analysis was conducted to address potential effect modification: by geographic location of study center as a proxy for overall UV exposure (distinguishing study centers in Iowa, Michigan, Minnesota, New Hampshire, Ohio, and Ontario from study centers in California, Colorado, and North Carolina, areas with generally more UV exposure). A similar analysis assessed effect modification by previous history of any skin cancer (including any diagnoses prior to baseline of BCC, SCC, melanoma or other malignancies of the skin). SCC was included as a secondary endpoint, but subgroup analyses were not considered for SCC because of small numbers. The proportional hazards assumption was assessed by testing for the statistical significance of interaction terms with log-transformed time since randomization. Two-sided p-values ≤0.05 were considered statistically significant. All analyses were performed using R 3.3.0.
Results
Randomization, follow-up, and outcomes
The original study included 1121 participants randomized to aspirin or placebo, including a total of 100 participants that had been randomized to aspirin or placebo prior to the time of folic acid randomization. After exclusion of 163 participants who were not non-Hispanic white, and thus at very low risk for skin cancer, a total of 958 participants were included in analyses for aspirin (329 assigned to 81 mg/d aspirin, 322 assigned to 325 mg/d aspirin, and 307 assigned to placebo), and 874 participants were included in analyses for folic acid (443 assigned to 1 mg/d folic acid and 431 assigned to placebo). The distribution of participant characteristics was similar across treatment arms at baseline (Table 1). The average age was 57 years and 64% were men. The majority (74%) were enrolled from study centers in the northern U.S. or Canada. A total of 47 (5%) reported a previous diagnosis of BCC, SCC, melanoma, or other skin cancer.
Table 1.
Baseline characteristics of non-Hispanic white participants in the Aspirin/Folate Polyp Prevention Study.
| Characteristic at baseline | Aspirin Treatment Assignment | Folic Acid Treatment Assignmenta | |||
|---|---|---|---|---|---|
|
|
|
||||
| Aspirin Placebo (N=307) | Aspirin 81 mg/d (N=329) | Aspirin 325 mg/d (N=322) | Folic Acid Placebo (N=431) | Folic Acid 1 mg/d (N=443) | |
| Age, mean (SD), y | 57.5 (10.0) | 57.1 (9.7) | 58.0 (9.3) | 57.7 (9.3) | 57.4 (9.8) |
| Men, n (%) | 190 (62) | 214 (65) | 206 (64) | 154 (64) | 161 (64) |
| Body mass index, mean (SD), kg/m2 | 27.2 (4.3) | 27.3 (4.5) | 27.3 (4.3) | 27.3 (4.3) | 27.4 (4.6) |
| Current cigarette smoker, n (%) | 40 (13) | 43 (13) | 47 (15) | 57 (13) | 59 (13) |
| Aspirin useb, n (%) | 28 (9) | 35 (11) | 27 (8) | 53 (12) | 35 (8) |
| Non-aspirin NSAIDs usea, n (%) | 19 (6) | 22 (7) | 24 (7) | 27 (6) | 35 (8) |
| Plasma folate, mean (SD), ng/mL | 10.5 (8.1) | 10.7 (8.2) | 10.5 (7.1) | 10.8 (7.7) | 10.8 (8.1) |
| High UV study centerc, n (%) | 77 (25) | 89 (27) | 83 (26) | 108 (25) | 117 (26) |
| History of skin cancerd, n (%) | 12 (4) | 18 (5) | 17 (5) | 22 (5) | 21 (5) |
Abbreviations: BCC, basal cell carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation; UV, ultraviolet radiation.
Excludes N=84 participants randomized to aspirin/placebo only.
Defined as use for >4 days/month on average throughout the past year.
High UV study centers are located in California, Colorado, and North Carolina. Low UV study centers located in Iowa, Michigan, Minnesota, New Hampshire, Ohio, and Ontario.
Self-reported personal history of basal cell carcinoma, squamous cell carcinoma, melanoma, or other skin cancer.
Note: 2 participants were missing body mass index, 3 participants were missing smoking status, and 106 participants were missing plasma folate.
During the first year of follow-up, 94% of participants reported taking at least 85% of aspirin tablets, 74% reported no use of non-protocol aspirin or NSAIDs outside of the study (4% using for >4 days/month).11 Adherence to folic acid or placebo was similar.12 Of the 958 randomized participants included in this analysis, a total of 874 (91%) completed a planned surveillance colonoscopy approximately 3 years after the baseline colonoscopy; of these, 637 (73%) continued folic acid/placebo treatment and 237 (27%) discontinued study pills, but agreed to be followed observationally for study endpoints. The median time on aspirin or placebo was 2.6 years and the median time on folic acid or placebo was 6.0 years. During a median follow-up of 13.5 years after randomization, 104 (11%) participants were diagnosed with BCC. The majority of BCC lesions were found on the head or neck (Supplemental Table 1). A total of 31 patients were diagnosed with SCC (4 during the aspirin treatment period and 27 afterward).
Aspirin treatment
The 15-year cumulative incidence of BCC was 12% (CI, 7-17%) for placebo, 16% (11-21%) for 81 mg/d aspirin, and 15% (10-20%) for 325 mg/d aspirin (Figure 1). Relative to placebo, participants assigned any dose of aspirin were more likely to develop BCC (HR, 1.45; 95% confidence interval, CI, 0.93-2.26), with similar magnitude associations according to dose (HR, 1.57, CI, 0.96-2.56 for 81 mg/d aspirin and HR, 1.33; CI, 0.80-2.20 for 325 mg/d aspirin). For SCC, the 15-year cumulative incidence was 4% (CI, 1-7%) for placebo, 4% (2-6%) for 81 mg/d aspirin, and 6% (2-9%) for 325 mg/d aspirin. Hazard ratios for SCC were in the same direction as for BCC, but also were not statistically significant (Supplemental Table 2).
Fig 1.
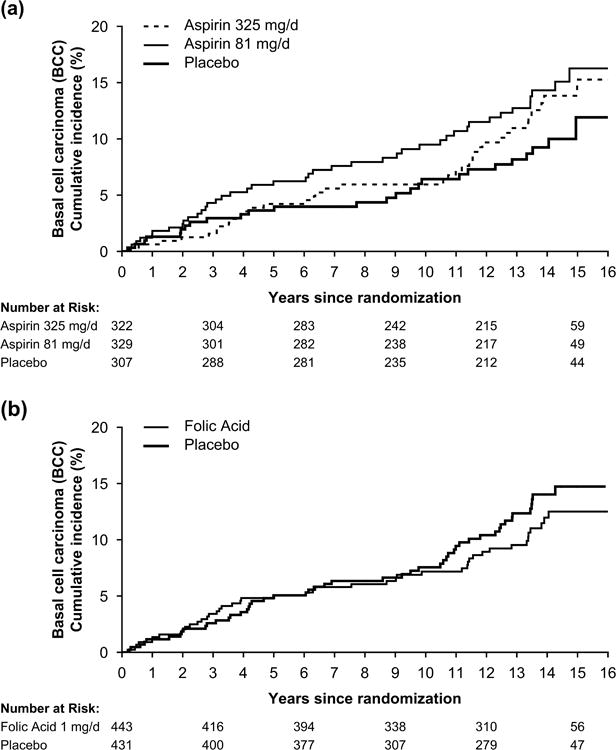
Kaplan-Meier estimates of the cumulative incidence of basal cell carcinoma (BCC) according to randomized aspirin treatment assignment (A) and randomized folic acid treatment assignment (B) in the Aspirin/Folate Polyp Prevention Study.
There was evidence that the aspirin treatment effect on BCC risk depended on whether participants had a history of skin cancer (Table 2; P for interaction = 0.02 for 81 mg/d vs placebo and P for interaction = 0.03 for 325 mg/d vs placebo). For the 911 participants who reported no history of skin cancer at baseline, BCC risk was higher with aspirin treatment over all follow-up (P = 0.04 for 81 mg/d vs placebo and P = 0.11 for 325 mg/d vs placebo). However, for the 47 participants who did report a history of skin cancer, BCC risk was lower with aspirin treatment (P = 0.15 for 80 mg/d vs placebo and P = 0.09 for 325 mg/d vs placebo). Although the cumulative incidence of BCC during the study was higher in participants recruited from medical centers in regions with more daily sunshine exposure, the aspirin treatment effect did not appear to be modified by geography. There was also no evidence of interaction between aspirin and folic acid treatment.
Table 2.
Hazard ratios of basal cell carcinoma (BCC) for aspirin treatment assignment overall and according to subgroup.
| Subgroup at Baseline | BCC Events/At Risk (%) | Aspirin 81 mg/d vs Placebo | Aspirin 325 mg/d vs Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Placebo | Aspirin 81 mg/d | Aspirin 325 mg/d | HRa (95% CI) | P | Pint b | HRa (95% CI) | P | Pint b | |
| Overall | 26/307 (8) | 42/329 (13) | 36/322 (11) | 1.57 (0.96, 2.56) | 0.07 | 1.33 (0.80, 2.20) | 0.27 | ||
| Location of Study Centerc | 0.77 | 0.49 | |||||||
| Low UV | 17/230 (7) | 26/240 (11) | 20/239 (8) | 1.49 (0.81, 2.74) | 0.20 | 1.15 (0.60, 2.19) | 0.67 | ||
| High UV | 9/77 (12) | 16/89 (18) | 16/83 (19) | 1.73 (0.76, 3.92) | 0.19 | 1.66 (0.73, 3.75) | 0.23 | ||
| History of Skin Cancerd | 0.02 | 0.03 | |||||||
| No | 19/295 (6) | 34/311 (11) | 31/305 (10) | 1.80 (1.03, 3.16) | 0.04 | 1.60 (0.90, 2.83) | 0.11 | ||
| Yes | 7/12 (58) | 8/18 (44) | 5/17 (29) | 0.47 (0.17, 1.31) | 0.15 | 0.37 (0.12, 1.19) | 0.09 | ||
| Folic Acid Assignmente | 0.45 | 0.31 | |||||||
| Folic Acid Placebo | 12/135 (9) | 24/149 (16) | 14/147 (10) | 1.82 (0.91, 3.64) | 0.09 | 1.01 (0.46, 2.17) | 0.99 | ||
| Folic Acid 1 mg/d | 12/146 (8) | 14/151 (9) | 19/146 (13) | 1.21 (0.56, 2.63) | 0.62 | 1.73 (0.84, 3.58) | 0.14 | ||
Abbreviations: BCC, basal cell carcinoma; CI, confidence interval; HR, hazard ratio; UV, ultraviolet radiation.
HR adjusted for age at randomization (<60 vs ≥60 years), sex (male vs female), study center (high vs low UV).
P-value for interaction for displayed aspirin dose compared to placebo.
High UV study centers are located in California, Colorado, and North Carolina. Low UV study centers located in Iowa, Michigan, Minnesota, New Hampshire, Ohio, and Ontario.
Self-reported personal history of basal cell carcinoma, squamous cell carcinoma, melanoma, or other skin cancer.
Excludes 84 participants randomized to aspirin/placebo only, of whom 9 were diagnosed with BCC over follow-up.
In total, 24 participants were diagnosed with BCC during the aspirin treatment period and 80 were diagnosed afterward. Participants began using aspirin and other NSAIDs frequently after the intervention period, but this did not vary substantially by treatment assignment (Supplemental Table 3). Estimates for randomized aspirin treatment assignment changed very little when additionally adjusted for self-reported use of non-protocol aspirin and NSAIDs (≥4 days/month) as a time-varying variable over all available follow-up, including after the treatment period (HR for 81 mg/d, 1.57, CI, 0.96-2.56, P=0.07; and HR for 325 mg/d, 1.33, CI, 0.81-2.20, P=0.27). Across all analyses, we concluded that modelling proportional hazards over time was appropriate. Results were also similar using data from the full study of 1121 participants without excluding the 163 individuals who reported a race/ethnicity other than non-Hispanic white (data not shown).
Folic acid treatment
The 15-year cumulative incidence of BCC was 15% (CI, 11-19%) for placebo and 13% (9-16%) for 1 mg/d folic acid. Folic acid supplementation at 1 mg/d was not associated with BCC risk (Figure 1) and was consistent across geography and history of skin cancer (Table 3). In total, 47 participants were diagnosed with BCC during the folic acid treatment period and 57 were diagnosed afterward. The folic acid treatment effect was also not statistically significant for risk of first primary SCC after randomization (Supplementary Table 2).
Table 3.
Hazard ratios of basal cell carcinoma (BCC) for folic acid treatment assignment overall and according to subgroup.
| Subgroup at Baseline | BCC Events/At Risk (%) | Folic Acid 1 mg/d vs Placebo | |||
|---|---|---|---|---|---|
|
|
|
||||
| Placebo | Folic Acid 1 mg/d | HRa (95% CI) | P | Pint b | |
| Overall | 50/431 (12) | 45/443 (10) | 0.85 (0.57, 1.27) | 0.42 | |
| Location of Study Centerc | 0.38 | ||||
| Low UV | 29/323 (9) | 30/326 (9) | 0.97 (0.58, 1.62) | 0.92 | |
| High UV | 21/108 (19) | 15/117 (13) | 0.67 (0.35, 1.30) | 0.24 | |
| History of Skin Cancerd | 0.60 | ||||
| No | 41/409 (10) | 36/422 (9) | 0.82 (0.52, 1.28) | 0.38 | |
| Yes | 9/22 (41) | 9/21 (43) | 1.08 (0.42, 2.76) | 0.87 | |
| Aspirin Assignmente | 0.15 | ||||
| Aspirin Placebo | 12/135 (9) | 12/146 (8) | 0.83 (0.37, 1.84) | 0.64 | |
| Aspirin 81 mg/d | 24/149 (16) | 14/151 (9) | 0.55 (0.29, 1.07) | 0.08 | |
| Aspirin 325 mg/d | 14/147 (10) | 19/146 (13) | 1.43 (0.72, 2.85) | 0.31 | |
Abbreviations: BCC, basal cell carcinoma; CI, confidence interval; HR, hazard ratio; UV, ultraviolet radiation.
HR adjusted for age at randomization (<60 vs ≥60 years), sex (male vs female), study center (high vs low UV).
P-value for interaction.
High UV study centers are located in California, Colorado, and North Carolina. Low UV study centers located in Iowa, Michigan, Minnesota, New Hampshire, Ohio, and Ontario.
Self-reported personal history of basal cell carcinoma, squamous cell carcinoma, melanoma, or other skin cancer.
Excludes 84 participants randomized to aspirin/placebo only, of whom 9 were diagnosed with BCC over follow-up.
Discussion
In the Aspirin/Folate Polyp Prevention Study, daily use of low- or high-dose aspirin did not decrease the risk of BCC overall, and there was an unexpected non-significant suggestion of an increased risk. Only in the small subgroup of participants with a history of skin cancer did aspirin treatment appear to reduce BCC risk, consistent with the magnitude and direction of treatment effects observed in a previous clinical trial of oral celecoxib in individuals with multiple AKs.7 Small numbers of SCCs precluded detailed analysis, but the overall direction and magnitude of the effect for low- and high-dose aspirin did not seem substantially different for SCC than BCC. In addition, we did not observe a statistically significant treatment effect for folic acid supplementation at 1 mg/d for about 6 years.
How aspirin affects skin responses to UV radiation is not fully understood. Observation of a delay in cutaneous erythema from UV light with aspirin treatment was made 50 years ago,13 however, rare phototoxic reactions have also been attributed to certain anti-inflammatory agents.14 Cutaneous adverse reactions to aspirin are rare and often acute; a Type II hypersensitivity in those with chronic idiopathic urticaria is recognized.15 Preclinical studies point to an important role for COX-2 in the development of UV-induced skin cancers.16. COX-2 expression has anti-apoptotic and proliferatory effects on keratinocytes,17,18 and is overexpressed in AKs, BCCs, and SCCs.19,20 Treatment of UV-exposed hairless mice with celecoxib and non-selective indomethacin, both in oral and topical preparations, reduces the occurrence of cutaneous tumors, but whether COX-2 inhibition is effective as primary or secondary preventive remains unclear as many studies have focused on new lesions in mice with at least one previous tumor.17,21,22
Two placebo-controlled randomized clinical trials have evaluated oral celecoxib therapy for prevention of keratinocyte carcinoma in high-risk individuals. Elmets et al. found that 200 mg celecoxib for 9 months reduced the incidence of BCC by 56% in 11 months relative to placebo in a study of 240 individuals with ≥10 AKs.7 Of note, this study was designed to evaluate actinic lesions as primary endpoints, and celecoxib had no effect on the regression of prevalent AKs or on the incidence of new AKs. A trial of 200 mg celecoxib twice daily in 60 basal cell nevus syndrome patients found that BCC counts increased over 2 years in both the treatment and placebo groups, but with a lower annual increase for those taking celecoxib.23 Comparing our results for aspirin to previous studies of celecoxib should be done with recognition that mechanistic studies point to an important role for COX-2, but not COX-1, in the development of UV-induced skin cancers,16 and consequently, many preclinical and clinical studies have focused on celecoxib rather than aspirin, which is not COX-2 selective.24
Across cancers of all types, aspirin appears to have the strongest and most consistent antineoplastic effects for adenocarcinoma, particularly in the luminal gastrointestinal tract.4 Observational studies of the association of aspirin or NSAID use with risk of BCC and SCC have generally reported either no association or a modest decrease in risk.5,25 Our findings of an inverse association only among those with a history of skin cancer is consistent with results from the Women's Health Initiative Observational Study, which found that questionnaire-reported NSAID use was associated with a reduced risk of non-melanoma skin cancer only in the subgroup of study participants with a prior non-melanoma skin cancer.26 At least one previous observational study reported an association in the same direction as our findings. A case-control study among members of Kaiser Permanente Northern California identified a 38% increase in SCC risk for those who used aspirin once a week for >1 year relative to less frequent and never users, but not with statistical significance (adjusted RR, 1.38; 95% CI, 0.96-1.97).27
The factorial design of the Aspirin/Folate Polyp Prevention Study permitted an evaluation of whether aspirin and folic acid treatment interact, and we did not find evidence of this interaction. In addition, there was no suggestion that the treatment effects differed by geography, which may offer some characterization of the participant's cumulative sun exposure. We also considered the potential of interaction with previous history of skin cancer, given that previous clinical trials of NSAIDs have focused on populations with numerous precancerous lesions, that observational studies have reported inverse associations with NSAID use specific to individuals at high risk for skin cancer, and that studies of BCC incidence often do not exclude participants with BCC history because recurrence of the condition is relatively common. Our observation of modification of the aspirin treatment effect by baseline history of any type of skin cancer, if confirmed in other populations, suggests that aspirin may play a role in BCC susceptibility in some individuals, while potentially preventing BCC in others.
Molecular mechanisms whereby aspirin could have opposing effects on keratinocyte proliferation depending on the baseline level of actinic damage remains unclear. Findings from previous clinical studies in humans underscore a complex cutaneous response to NSAID therapy that may depend on skin characteristics. A case-control study found that the odds ratio for SCC increased with increasing levels of arachidonic acid in red blood cell membranes among those who did not report current use NSAIDs. There were noted differences in the dose-response relationship in models that did, and did not, adjust for previous history of AKs.28 A Phase II study involving 90-day treatment of forearm skin with topical difluoromethylornithine (DMFO) in combination with topical diclofenac found that actinic damage to skin cells measured by karyometry unexpectedly increased for DMFO alone, diclofenac alone, and DMFO with diclofenac.29 Interestingly, the motivation for testing the addition of DMFO to NSAIDs for treatment of skin was based primarily on evidence from chemoprevention studies conducted among those with colorectal adenomas.30
In addition to aspirin, our study also investigated daily supplementation with 1 mg of folic acid. Total folate levels in the human dermis and epidermis are known to correlate with circulating concentrations,31 and UV exposure can degrade folic acid present both in blood and skin cells.32-35 Preclinical and clinical evidence of whether folic acid supplementation alters skin cancer risk is limited. A meta-analysis of randomized studies of folic acid supplementation showed no clear effect on cancer risk across multiple cancer types, but did not include non-melanoma skin cancer.36 Our study suggests no association between folic acid supplementation at 1 mg/d for about 6 years and risk of first primary BCC or SCC after randomization.
Strength of the Aspirin/Folate Polyp Prevention Study include its randomized design, which helped control confounding by baseline factors and prevented bias from self-report of medication use. The long follow-up period provided sufficient time to evaluate delayed effects of the study treatments. Adherence was excellent, and drop-out was minimal and unrelated to treatment.11 Patients with IBD, who may be at increased risk of BCC from immunosuppressive therapy with thiopurines,37 were ineligible. The cumulative incidence of BCC over follow-up was consistent with age-specific nationwide estimates.1
The study also has limitations. The Aspirin/Folate Polyp Prevention Study was not originally designed to examine skin cancer, and did not collect information on sun exposure history, skin type, or pre-existing AKs. Based on the randomized design, however, it is expected that these factors would be balanced according to treatment assignment. We believe that it is unlikely that participants in the aspirin treatment arms would have been subject to more intense sun exposure or skin cancer screening after the study treatment period than those in the placebo arm, but cannot directly test this assumption. Although all occurrences of BCC and SCC during follow-up were verified by pathology reports, history of skin cancer at baseline was based on self-report, not validated, and not specified by subtype.
Participants were followed-up for several years beyond the 3-year aspirin treatment period, and over time the balanced distribution of potential confounding variables created by randomization could have been lost. In particular, aspirin use occurring after the end of the study treatment period may have affected our findings, but because self-reported use over several years following the end of the treatment period did not substantially differ by treatment assignment, any influence of uncontrolled use over extended follow-up may be modest. Although we did not observe an aspirin dose-response relationship for BCC incidence, which would have provided additional support for a causal mechanism, it should be noted that there was also no evidence of a dose-response for the chemopreventive effects of aspirin on the primary colorectal adenoma endpoints in this trial.11 The biologic basis of this lack of consistent dose-response relationship warrants further investigation.
Evidence of a potentially increased risk of BCC over long-term follow-up for those without a personal history of skin cancer was unexpected and warrants further investigation. Subgroup analyses should always be interpreted with caution, and we acknowledge that secondary analyses of a completed clinical trial should be considered exploratory. Larger clinical trials designed to evaluate BCC incidence will likely have improved statistical power relative to our study. Our results, however, may inform the planning of future clinical trials of NSAID treatment for skin cancer prevention with broader inclusion criteria than the previous studies that have focused on patients with multiple AKs. As there is limited previous evidence from randomized studies of NSAID use with long-term skin cancer outcomes, further research is needed to determine whether daily use of these drugs can have opposing effects in those at low- and high-risk of developing skin cancer.
Supplementary Material
What's already known about this topic?
Previous clinical trials have found that short-term use of oral celecoxib may reduce the risk of basal cell carcinoma in those with multiple actinic keratoses or basal cell nevus syndrome
Observational studies have reported no association or a modest decrease in the risk of developing basal cell carcinoma or cutaneous squamous cell carcinoma with use of aspirin or other non-steroidal anti-inflammatory drugs.
Dietary consumption of folic acid does not appear to be linked to basal cell carcinoma, but evidence from randomized clinical trials is lacking.
What does this study add?
In the Aspirin/Folate Polyp Prevention Study, a randomized, double-blind, placebo-controlled clinical trial, neither aspirin (81 mg/d or 325 mg/d for approximately 3 years) nor folic acid (1 mg/d for approximately 6 years) resulted in a statistically significant alteration in the risk of first primary basal cell carcinoma after randomization.
Subgroup analysis revealed that the treatment effect for aspirin differed for those with and without a history of skin cancer.
Acknowledgments
On behalf of the Polyp Prevention Study Group, the authors express their appreciation to the study participants, investigators, and staff.
Funding sources: This work was funded by grants from the National Cancer Institute, National Institutes of Health (R01CA59005 and R01CA057494) and the National Institute of General Medical Sciences, National Institutes of Health (P20GM104416).
Footnotes
Conflicts of interest: Together with the Trustees of Dartmouth College, Dr. John A. Baron holds a use patent, not currently licensed, for the chemopreventive use of aspirin for colorectal cancer.
References
- 1.Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081–6. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–85. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 4.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 5.Muranushi C, Olsen CM, Green AC, et al. Can oral nonsteroidal antiinflammatory drugs play a role in the prevention of basal cell carcinoma? A systematic review and metaanalysis. J Am Acad Dermatol. 2016;74:108–19 e1. doi: 10.1016/j.jaad.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600–8. [PubMed] [Google Scholar]
- 7.Elmets CA, Viner JL, Pentland AP, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835–44. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–5. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam RM, Huang Z, Giovannucci E, et al. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am J Clin Nutr. 2000;71:135–41. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 10.Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110–5. doi: 10.1002/ijc.10798. [DOI] [PubMed] [Google Scholar]
- 11.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 12.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 13.Miller WS, Ruderman FR, Smith JG., Jr Aspirin and ultraviolet light-induced erythema in man. Arch Dermatol. 1967;95:357–8. [PubMed] [Google Scholar]
- 14.Przybilla B, Schwab-Przybilla U, Ruzicka T, et al. Phototoxicity of non-steroidal anti-inflammatory drugs demonstrated in vitro by a photo-basophil-histamine-release test. Photodermatol. 1987;4:73–8. [PubMed] [Google Scholar]
- 15.Kowalski ML, Makowska JS, Blanca M, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011;66:818–29. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 16.Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84:322–9. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 17.Tripp CS, Blomme EA, Chinn KS, et al. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J Invest Dermatol. 2003;121:853–61. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- 18.Akunda JK, Chun KS, Sessoms AR, et al. Cyclooxygenase-2 deficiency increases epidermal apoptosis and impairs recovery following acute UVB exposure. Mol Carcinog. 2007;46:354–62. doi: 10.1002/mc.20290. [DOI] [PubMed] [Google Scholar]
- 19.Buckman SY, Gresham A, Hale P, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–9. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 20.An KP, Athar M, Tang X, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Fischer SM, Lo HH, Gordon GB, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–40. [PubMed] [Google Scholar]
- 22.Pentland AP, Schoggins JW, Scott GA, et al. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–44. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 23.Tang JY, Aszterbaum M, Athar M, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/- humans and mice. Cancer Prev Res (Phila) 2010;3:25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–8. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 25.Muranushi C, Olsen CM, Pandeya N, et al. Aspirin and nonsteroidal anti-inflammatory drugs can prevent cutaneous squamous cell carcinoma: a systematic review and meta-analysis. J Invest Dermatol. 2015;135:975–83. doi: 10.1038/jid.2014.531. [DOI] [PubMed] [Google Scholar]
- 26.Wysong A, Ally MS, Gamba CS, et al. Non-melanoma skin cancer and NSAID use in women with a history of skin cancer in the Women's Health Initiative. Prev Med. 2014;69:8–12. doi: 10.1016/j.ypmed.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Asgari MM, Chren MM, Warton EM, et al. Association between nonsteroidal anti-inflammatory drug use and cutaneous squamous cell carcinoma. Arch Dermatol. 2010;146:388–95. doi: 10.1001/archdermatol.2009.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RB, Foote JA, Hakim IA, et al. Fatty acid composition of red blood cell membranes and risk of squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2005;14:906–12. doi: 10.1158/1055-9965.EPI-04-0670. [DOI] [PubMed] [Google Scholar]
- 29.Jeter JM, Curiel-Lewandrowski C, Stratton SP, et al. Phase IIB randomized study of topical difluoromethylornithine and topical diclofenac on sun-damaged skin of the forearm. Cancer Prev Res (Phila) 2016;9:128–34. doi: 10.1158/1940-6207.CAPR-15-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasoun LZ, Bailey SW, Outlaw KK, et al. Effect of serum folate status on total folate and 5-methyltetrahydrofolate in human skin. Am J Clin Nutr. 2013;98:42–8. doi: 10.3945/ajcn.112.057562. [DOI] [PubMed] [Google Scholar]
- 32.Off MK, Steindal AE, Porojnicu AC, et al. Ultraviolet photodegradation of folic acid. J Photochem Photobiol B. 2005;80:47–55. doi: 10.1016/j.jphotobiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Fukuwatari T, Fujita M, Shibata K. Effects of UVA irradiation on the concentration of folate in human blood. Biosci Biotechnol Biochem. 2009;73:322–7. doi: 10.1271/bbb.80530. [DOI] [PubMed] [Google Scholar]
- 34.Hasoun LZ, Bailey SW, Outlaw KK, et al. Rearrangement and depletion of folate in human skin by ultraviolet radiation. Br J Dermatol. 2015;173:1087–90. doi: 10.1111/bjd.13885. [DOI] [PubMed] [Google Scholar]
- 35.Juzeniene A, Grigalavicius M, Ma LW, et al. Folic acid and its photoproducts, 6-formylpterin and pterin-6-carboxylic acid, as generators of reactive oxygen species in skin cells during UVA exposure. J Photochem Photobiol B. 2016;155:116–21. doi: 10.1016/j.jphotobiol.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029–36. doi: 10.1016/S0140-6736(12)62001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–74. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


