Highlights
-
•
Moderate or high titer donor-specific antibodies were present in 12 of 41 adults with SCD.
-
•
G-CSF can be safely administered post-transplantation in adults with SCD with HbS < 30%.
-
•
Modified Hopkins haplo-protocol led to long-term engraftment in 7 of 8 adults with SCD.
Key Words: Sickle cell disease, Transplantation, Haploidentical, Donor specific antibody, G-CSF
Abstract
We report on the screening and development of haploidentical hematopoietic stem cell transplantation (HSCT) for adult patients with clinically aggressive sickle cell disease (SCD) at our institution. Of 50 adult SCD patients referred for HSCT between January 2014 and March 2017, 20% were denied by insurance. Of 41 patients initially screened, 10% lacked an available haploidentical donor, 29% had elevated donor-specific antibodies (DSAs), and 34% declined to proceed to HSCT. All 10 patients who were transplanted received peripheral blood stem cells. The initial 2 were conditioned with alemtuzumab/total body irradiation (TBI) 3 Gy followed by post-transplant cyclophosphamide and failed to engraft. The next 8 patients received the regimen developed at Johns Hopkins University with TBI 3 Gy. Granulocyte colony-stimulating factor was administered from day +12 in those with HbS < 30%. All 8 patients engrafted with a median time to neutrophil >.5 × 109/L of 22 days (range, 18 to 23). One patient subsequently lost the graft, and 7 (87.5%) maintained >95% donor cell chimerism at 1-year post-HSCT. Two patients developed acute graft-versus-host disease (GVHD) of at least grade II. One had chronic GVHD and died >1 year after HSCT of unknown causes. With a median follow-up of 16 months (range, 11 to 29), 7 patients (87.5%) are alive. Our findings suggest that limited insurance coverage, high rate of DSAs, and patient declining HSCT may limit the availability of haploidentical HSCT in adult SCD patients. The modified Hopkins regimen used here demonstrates high engraftment and low morbidity rates and should be tested in larger, multicenter, prospective clinical trials.
Introduction
Allogeneic nonmyeloablative hematopoietic stem cell transplantation (HSCT) from HLA-matched related donors results in event-free survival rates of 87% to 92%, overall survival rates of 97% to 100%, and 0% acute or chronic graft-versus host disease (GVHD) in adults with sickle cell disease (SCD) 1, 2. There is an unmet need for alternative donors because only 18% of SCD patients have an HLA-matched sibling [3]. HSCT using HLA-matched unrelated donors is limited by the difficulty in finding HLA-compatible donors for this largely nonwhite patient population 4, 5. Additionally, a study reported high rates of chronic GVHD (62%) and transplant-related mortality (21%) in SCD patients receiving transplants from HLA-matched unrelated donors [6].
The discovery that post-transplant cyclophosphamide (PTCy) allows patients to engraft stem cells from haploidentical donors without an increased risk of GVHD has led to the rapid expansion of haploidentical HSCT in patients with hematologic malignancies [7]. This strategy was used in 14 SCD patients transplanted with bone marrow cells after conditioning with fludarabine, cyclophosphamide, 2 Gy total body irradiation (TBI), antithymocyte globulin, and GVHD prophylaxis with PTCy on days +3 and +4; mycophenolate mofetil for 30 days; and tacrolimus or sirolimus for at least 1 year (the Hopkins protocol) [8]. No severe transplant-related complications were reported, and 57% of patients achieved a stable donor cell engraftment. In a subsequent study the same regimen resulted in the engraftment of only 2 of 5 SCD patients [9]. Modifications to this regimen, including the addition of azathioprine and hydroxyurea for 3 months pre-HSCT, hypertransfusion, and thiotepa on day –7, improved engraftment to 91%, with 18% acute GVHD and 14% mortality in a pediatric series of SCD patients [9].
Our institution has the largest adult sickle cell program in the Chicago area. Because only 20% of our SCD patients eligible for HSCT had a matched related donor, we initiated a haploidentical HSCT program [1]. Here we report our center's real-life experience of screening and treating adult SCD patients with haploidentical HSCT.
Methods
Patients
Transplant eligibility requirements were similar to those for match related donor transplants [1] and in accordance with the international expert panel for alternative donor transplantation in SCD [10], with the additional requirement that the recipient be negative for donor-specific HLA antibodies (DSAs). Positivity for DSAs was considered moderate with a mean fluorescent intensity between 2000 to 5000 and high with a mean fluorescent intensity > 5000 [11]. Institutional Review Board approval was obtained before collecting and analyzing the clinical data.
Donors
Haploidentical donors were either HbAA or HbAS. The donors received granulocyte colony-stimulating factor (G-CSF) subcutaneously at a dose of 5 µg/kg/twice daily for 5 days followed by peripheral blood stem cell (PBSC) collection.
Conditioning Regimens
The conditioning protocol in the first 2 patients was as follows: alemtuzumab (.03 mg/kg on day –7, .1 mg/kg on day –6, .3 mg/kg on days –5 to –3), single-dose TBI 3 Gy on day –2, and cyclophosphamide (50 mg/kg on days +3 and +4). Because both patients failed to engraft donor cells, we adopted the Hopkins protocol [8] for the next 8 patients with 2 modifications aimed at improving engraftment: (1) increasing the dose of TBI from 2 Gy to 3 Gy and (2) infusing growth factor–mobilized PBSCs instead of bone marrow cells (Figure 1A ). The conditioning was as follows: rabbit antithymocyte globulin (Sanofi Genzyme, Cambridge, MA) (.5 mg/kg on day –9, 2 mg/kg on days –8 and –7), cyclophosphamide (14.5 mg/kg on days –6 and –5), fludarabine (30 mg/m2 on days –6 to –2), and single-dose TBI 3 Gy on day –1. GVHD prophylaxis consisted of cyclophosphamide (50 mg/kg i.v. on days +3 and +4), oral mycophenolate mofetil (15 mg/kg 3 times daily from days +5 to +35), and sirolimus from day +5 dosed for a target trough of 5 to 15 ng/mL. In patients with T cell chimerism > 50% at 1 year post-HSCT and without signs of GVHD, treatment with sirolimus was tapered off over 3 months.
Figure 1.
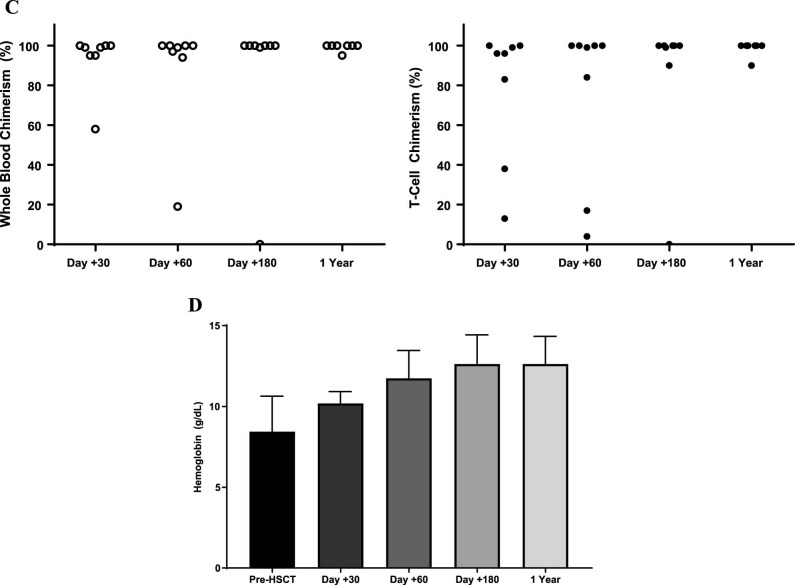
(A) Regimen for haploidentical transplantation in adult patients with SCD. (B) Screening process for haploidentical transplantation in adult patients with SCD. Of 50 patients referred to the SCD transplant clinic, 41 proceeded with HLA typing. Twenty-five (61%) had a suitable HLA-haploidentical donor with 4 (10%) lacking an available HLA-haploidentical relative and 12 (29%) having DSAs. In addition, 10 (20%) were denied by insurance and 14 (34%) declined or deferred transplantation. (C) Stable whole blood and CD3+ T cell engraftment after haploidentical transplantation in adult patients with SCD. (D) Improvements in hemoglobin concentration after haploidentical transplantation in adult patients with SCD.
Recipients underwent RBC exchange transfusion on day –10 (goal HbS < 30%), and hydroxyurea was permanently discontinued on day –9. Platelet transfusions were administered to maintain platelet counts >50 × 109 cells/L and penicillin V 250 mg p.o. was administered twice daily, in addition to standard antimicrobial prophylaxis. Donor cell engraftment was assessed by chimerism analysis on circulating mononuclear cells and CD3+ T cells on days +30, +60, +180, and +365.
Graft-versus-Host Disease
Patients were monitored in the University of Illinois at Chicago bone barrow transplant clinic at least weekly until day +60, monthly until day +180, and bimonthly until 1 year post-HSCT. Acute and chronic GVHD were graded according to standard consensus diagnostic criteria 12, 13
Results
Between January 2014 and March 2017, 50 adult SCD patients meeting haploidentical HSCT eligibility requirements [10] were referred to our program (Figure 1B). Nine patients were initially denied by insurance, and therefore only 41 could be screened for donor availability. Of these, 4 (10%) lacked an available haploidentical donor and 12 (29%) had moderate (n = 7) or high (n = 5) levels of DSAs, a strong predictor of graft rejection in haploidentical HSCT [11]. Of the 25 patients (61%) with an identified haploidentical donor, 1 additional patient was denied by insurance before transplant and 14 (34%) ultimately declined or deferred HSCT, resulting in a total of 10 patients being transplanted. All recipients received PBSCs from their haploidentical donors. In 7 HbAS donors, mobilization with G-CSF did not lead to increased side effects and produced similar stem cell collections as in HbAA donors.
The first 2 patients (Table 1, top ) were conditioned with alemtuzumab/3 Gy TBI + PTCy but failed to engraft donor cells and recovered autologous neutrophils on day +39 and day +34, respectively. The following 8 patients (Table 1, bottom) were conditioned with the modified Hopkins regimen (TBI 3 Gy and PBSCs as the graft source) (Figure 1A). All 8 patients engrafted > .5 × 109 neutrophils/L at a median of 22 days (range, 18 to 23). One patient had low donor T cell chimerism levels at days +30 and +60 that spontaneously improved by day +180 without any changes in immunosuppression. Another patient had a progressive decline of donor whole blood and T cell chimerisms and experienced secondary graft failure on day +90 with autologous hematopoietic recovery. At 1 year post-HSCT, 7 patients maintained an average donor mononuclear chimerism > 95% and T cell chimerism ≥ 90% (Figure 1C).
Table 1.
Characteristics and Outcomes of 2 Patients with SCD Who Underwent HSCT from a Haploidentical, Related Donor Treated with Alemtuzumab, TBI, and PTCy (top) and of 8 Patients with SCD Who Underwent HSCT from a Haploidentical, Related Donor Treated with the Modified Hopkins Regimen(bottom)
| Transplantation Characteristics |
Transplantation Outcomes |
Transplant-Related Toxicity |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age at HSCT (yr) |
Sex | Hemoglobin Genotype |
Indications* | Prior Therapy | Donor | CD34+ Dose (×106/kg) |
Duration of Follow-up (mo)/Current Donor T Cell Chimerism |
Living Status |
Neutrophil Engraftment | Infectious Complications |
CMV Reactivation |
Acute GVHD | Chronic GVHD |
| Two patients treated with alemtuzumab, TBI, and PTCy | ||||||||||||||
| A | 24 | F | SS | 20 VOC/year 3 ACS/lifetime |
Hydroxyurea | Mother (Hb AS) |
5.9 | 37 | Alive | Autologous, day +39 | None | None | None | None |
| B | 52 | M | SC | 10 VOC/year | Hydroxyurea | Sister (Hb AA) |
5.4 | 36 | Alive | Autologous, day +34 | None | Day +11 | None | None |
| Eight patients treated with modified Hopkins regimen | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | M | SS | 8 VOC/year 3 ACS/lifetime |
Hydroxyurea | Daughter (Hb AS) |
14.2 | 30 / 100% | Alive | Day +23 | Escherichia coli UTI | Day +19 | None | None |
| 2 | 20 | M | Sβ+-thal | 10 VOC/year | Hydroxyurea | Sister (Hb AA) |
6.0 | 23 / 90% | Alive | Day +20 | None | None | None | None |
| 3 | 21 | M | SS | 10 VOC/year TRJV = 2.7 |
Hydroxyurea | Mother (Hb AS) |
5.3 | 15 | Deceased | Day +18 | Oral HSV1 coronavirus influenza |
Day +20 | Grade II skin Grade IV liver |
Moderate - eye - liver |
| 4 | 27 | M | SS | 4 VOC/year 2 ACS/lifetime |
Hydroxyurea | Father (Hb AS) |
8.2 | 17 / 0% | Alive | Day +23 | None | None | None | None |
| 5 | 31 | M | SS | Stroke 11 VOC/year 2 ACS/last 2 years |
Hydroxyurea | Brother (Hb AA) |
4.2 | 17 / 100% | Alive | Day +22 | None | None | None | None |
| 6 | 27 | F | SS | 19 VOC/year 2 ACS/last 2 years |
Hydroxyurea | Mother (Hb AS) |
10.8 | 13 / 100% | Alive | Day +22 | Enterococcus UTI | None | None | None |
| 7 | 37 | M | SS | Stroke 3 VOC/year 2 ACS/last 2 years |
Chronic transfusion | Mother (Hb AS) |
12.2 | 13 / 100% | Alive | Day +19 | None | None | None | None |
| 8 | 29 | F | SS | 7 VOC/year 2 ACS/lifetime |
Hydroxyurea | Mother (Hb AS) |
6.1 | 12 / 100% | Alive | Day +18 | None | None | Grade II GI | None |
CMV indicates cytomegalovirus; VOC, vaso-occlusive crisis; ACS, acute chest syndrome; UTI, urinary tract infection; TRJV, tricuscpid regurgitant jet velocity; GI, gastrointestinal.
The VOC rate is an average of the rate over the 2 years preceding the date of consultation for transplantation.
Transplant-related toxicities included at least grade 2 mucositis in 3 patients and cytomegalovirus reactivation in 2 patients without occurrence of cytomegalovirus infection. Seven neutropenic patients with HbS < 30% received a median of 7 doses (range, 3 to 14) of G-CSF at 5 µg/kg starting at day +12 post-HSCT. Only 1 patient experienced mild bone pain in the lower extremities. Small subarachnoid hemorrhages occurred in 2 patients. The first patient had a history of multiple RBC antibodies, became refractory to platelet transfusions, and developed multifocal small subarachnoid hemorrhages in the left parietal lobe on day +10. Symptoms and brain imaging improved 4 days later after platelet counts were maintained at >50 × 109 cells/L with cross-matched platelets. The second patient, who had a prior stroke history, developed a seizure when the platelet count was 68 × 109 cells/L. Magnetic resonance imaging of the brain demonstrated a right frontal subarachnoid hemorrhage on day +12. Symptoms and imaging results improved 2 days later, after initiating levetiracetam and maintaining platelets >100 × 109 cells/L. Acute GVHD was observed in 2 patients and chronic GVHD in 1 patient. One patient developed acute on chronic GVHD involving the skin, liver, and eyes on day +83. Treatment with steroids and strict compliance with sirolimus improved eye symptoms and bilirubin levels, but the patient died unexpectedly at home on day +407. Another patient developed grade II acute gut GVHD that completely resolved after a short course of steroid therapy.
With a median follow-up of 17 months (range, 12 to 30), 7 patients are alive and 6 maintain >95% stable donor engraftment (Figure 1C) with improvements in their hemoglobin concentrations (Figure 1D). Three patients have stopped immunosuppression, and the other 3 are being tapered off immunosuppression.
Discussion
In this single-center experience of a haploidentical HSCT program for adults with SCD, we demonstrated several real-life barriers for access to haploidentical transplantation, the safety of G-CSF post-HSCT, and a high rate of long-term engraftment using PBSCs with a modified Hopkins regimen. After our positive results in match related donor HSCT [1], we initially attempted to apply the same alemtuzumab-based regimen with the addition of PTCy in 2 patients undergoing haploidentical HSCT. Both experienced graft failure, consistent with the experience reported using the same approach at the National Institutes of Health [14].
Because the risk of transplant-related mortality is higher in adults with SCD using standard myeloablative regimens [15], we transplanted the next 8 patients with a nonmyeloablative regimen developed at Johns Hopkins University [8]. To decrease the high rate of rejection reported in the original study (43%), we modified the Hopkins protocol by increasing the dose of TBI from 2 Gy to 3 Gy and using PBSCs instead of marrow cells. This led to improvements in stable donor cell engraftment from 40% to 57% previously reported 8, 9 to 87.5%, while maintaining manageable toxicities. The findings we report here should be considered in the context of the few small series of haploidentical HSCT in SCD patients that have been published to date (Table 2 ).
Table 2.
Current Published Experience for Haploidentical Transplantation in SCD
| Study | No. of Patients | Age Range (yr) |
Conditioning Regimen | Stem Cell Source | Acute GVHD | Chronic GVHD | Stable Engraftment |
Overall Survival |
|---|---|---|---|---|---|---|---|---|
| Bolanos-Meade et al., 2012 [8] | 14 | 15-42 | Flu 30 mg/m2/day, Cy 14.5 mg/kg/day, ATG, TBI 2 Gy, PTCy 50 mg/kg/day | Bone marrow | 0 | 0 | 8 | 14 |
| Dallas et al., 2013 [16] | 8 | 4-17 | 1) Flu 150-200 mg/m2, thiotepa 10 mg/kg, Bu (target 900 ng/mL), ATG (10 mg/kg), muromonab-CD3 (.1 mg/kg) 2) HU/azathioprine 3 months pretransplant; Bu (target 900 ng/mL), thiotepa, Cy (200 mg/kg), muomonab-CD3 (.1 mg/kg) |
Bone marrow | 4 | 3 | 5 | 6 |
| Dhedin et al., 2016 [9] | 5 | 12-50 | 1) Flu 30 mg/m2/day, Cy 14.5 mg/kg/day, ATG, TBI 2 Gy, PTCy 50 mg/kg/day | Bone marrow | 0 | 0 | 2 | 5 |
| 8 | 7-26 | 2) Thiotepa 10 mg/kg/day, Flu 30 mg/m2/day, Cy 14.5 mg/kg/day, ATG, TBI 2 Gy, PTCy 50 mg/kg/day | 1 | 0 | 7 | 8 | ||
| 23 | 3-18 | 3) Preconditioning for 3 months withazathioprine 3 mg/kg/day and HU 30 mg/kg/day; thiotepa 10 mg/kg/day, Flu 30 mg/m2/day, Cy 14.5 mg/kg/day, ATG, TBI 2 Gy, PTCy 50 mg/kg/day | 4 | 0 | 21 | 18 | ||
| Fitzhugh et al., 2017 [14] | 12 | 20-56 | Alemtuzumab 1 mg/kg, TBI 4 Gy, PTCy 50 mg/kg/day | PBSCs | 1 | 1 | 6 | 11 |
| Pawlowska et al., 2018 [17] | 4 | 13-23 | Flu 40 mg/m2/day, dexamethasone 25 mg/m2/day × 2 cycles pre-HSCT Rabbit ATG 1.5 mg/kg/day, Flu 35 mg/m2/day, Bu 130 mg/m2/day, PTCy 50 mg/kg/day |
3 Bone marrow, 1 PBSCs |
1 | 3 | 4 | 4 |
| Current Study | 8 | 20-38 | Flu 30 mg/m2/day, Cy 14.5 mg/kg/day, ATG, TBI 3 Gy, PTCy 50 mg/kg/day | PBSCs | 2 | 1 | 7 | 7 |
| Summary | 82 | 3-51 | — | — | 13 (16%) | 8 (10%) | 60 (73%) | 73 (89%) |
Flu indicates fludarabine, ATG, antithymocyte globulin; Bu, busulfan; HU, hydroxyurea.
Our engraftment results are comparable with those obtained in a pediatric series where azathioprine, hydroxyurea, and thiotepa were added to the preparative regimen [8]. Use of PBSCs has been associated with a greater risk for acute and chronic GVHD compared with unstimulated bone marrow in patients with hematologic malignancies undergoing a T cell–replete haploidentical HSCT [18]. Although we observed chronic GVHD in only 1 of 8 patients, this risk should be carefully considered in future studies testing the benefits of PBSCs for reducing rejection in patients with nonmalignant diseases conditioned with low intensity regimens.
In our experience of 50 adult SCD patients referred for HSCT, only 20% ended up receiving a haploidentical HSCT. Medical insurance denial accounted for 20% of the lack of access for HSCT. Other factors, such as high rates of DSAs in frequently transfused SCD patients and personal decisions to decline HSCT, also played significant roles. Our rate of available haploidentical donors is lower than was previously observed at Johns Hopkins University, where 90% of SCD patients referred had haploidentical donors [8]. This difference may be because many of the SCD patients reported on here were already being followed in our clinics and had not been prescreened or selected by referring physicians. The presence of DSAs is a major barrier to haploidentical HSCT in SCD that should be addressed when discussing treatment options with patients. A possible strategy to increase the donor pool could be to select patients with low DSA titers and a negative cross-match result. Desensitization protocols used in hematologic malignancies [11] should also be tested in clinical trials for SCD patients with clinically aggressive disease. Our findings are consistent with a previous report showing that a substantial proportion of eligible SCD patients do not proceed to HSCT because of fear of toxicity and satisfaction with the current quality of life [19]. The 2 main reasons that patients reported for declining HSCT were the risk of GVHD in an HLA-mismatched HSCT and the toxicity associated with chemotherapy in the conditioning regimen. Interestingly, in previous studies the severity of SCD was not associated with the degree of risk that a parent or a patient is willing to accept for cure 20, 21, 22, 23. Only 35% of adolescents and 46% of parents would accept HSCT if recommended by their hematologist [24]. Thirty-two percent of adolescents believe that SCD will shorten their life span, and only 26% believe that SCD will prevent the achievement of life goals, which is in contrast to 86% of adults who perceived that employment opportunities are affected by SCD 21, 24. There is a need for better education of patients about the course of SCD and for large, prospective haploidentical HSCT studies to guide the decisions of patients and their families for the risk-to-benefit assessment of HSCT. The risks of curative treatment with alternative donors will need to be carefully considered as new therapies, such as gene therapy [25] and nontransplant therapies [26], are being developed for SCD.
We also observed that G-CSF could be safely administered to SCD adults to shorten the duration of post-HSCT neutropenia. Concern for the safety of G-CSF was highlighted in a previous case series of 11 SCD patients receiving G-CSF to reduce the duration of neutropenia after chemotherapy or to mobilize autologous stem cells [27]. In that case series 7 of 11 patients had serious adverse events with G-CSF use, and a lower HbS level did not reduce the rate of adverse events. In contrast, in a pediatric cohort of children with SCD undergoing matched related donor HSCT, G-CSF 5 µg/kg/day was safely given starting on day +7 until full neutrophil recovery [28]. Only 1 patient in our study had mild lower extremity pain while receiving G-CSF consistent with tolerability of G-CSF post-HSCT in SCD. Although sickle cell trait donors did not experience any severe side effects with G-CSF mobilization, an alternative strategy to mobilize stem cells in sickle cell trait donors and to mobilize autologous stem cells from SCD patients may be the use of plerixafor, as safely demonstrated in gene therapy studies [29].
In conclusion, our findings suggest that a nonmyeloablative haploidentical PBSC transplant using TBI 3 Gy and PTCy could cure many adult patients with advanced SCD, and larger clinical studies are warranted. Based on our findings, barriers limiting the access to haploidentical HSCT for SCD patients should also be addressed by the transplant community with multilevel interventions.
Acknowledgments
Financial disclosure: S.L.S. receives research support from the National Institutes of Health through grant K23HL125984. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study has been partially supported by a Michael Reese Research & Education Foundation endowment to D.R.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: S.L.S and D.R. designed the study and wrote the manuscript. S.L.S, A.L.O., P.R.P., K.S., M.K., S.C.-L., J.G.Q., R.E.M., N.M., V.R.G., and D.R. performed the research and collected, analyzed, and interpreted the data. M.G., S.J., D.P., and I.K. performed the research and contributed to the data analysis. All the authors critically reviewed the manuscript.
Footnotes
Financial disclosure: See Acknowledgments on page 1764.
References
- 1.Saraf S.L., Oh A.L., Patel P.R. Nonmyeloablative stem cell transplantation with alemtuzumab/low-dose irradiation to cure and improve the quality of life of adults with sickle cell disease. Biol Blood Marrow Transplant. 2016;22:441–448. doi: 10.1016/j.bbmt.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh M.M., Fitzhugh C.D., Weitzel R.P. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 2014;312:48–56. doi: 10.1001/jama.2014.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mentzer W.C., Heller S., Pearle P.R., Hackney E., Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16:27–29. [PubMed] [Google Scholar]
- 4.Eapen M., Horowitz M.M. Alternative donor transplantation for aplastic anemia. Hematolo Am Soc Hematol Educ Progr. 2010;30:43–46. doi: 10.1182/asheducation-2010.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Gragert L., Eapen M., Williams E. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy S., Eapen M., Panepinto J.A. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. 2016;128:2561–2567. doi: 10.1182/blood-2016-05-715870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luznik L., O'Donnell P.V., Symons H.J. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolanos-Meade J., Fuchs E.J., Luznik L. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhedin N., Fuente J., Bernaudin F. Haploidentical bone marrow transplant with post-transplant cytoxan plus thiotepa improves donor engraftment in patients with sickle cell anemia: results of an international multicenter learning collaborative. Blood. 2016;128:1233. doi: 10.1016/j.bbmt.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Angelucci E., Matthes-Martin S., Baronciani D. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99:811–820. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciurea S.O., de Lima M., Cano P. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovich A.H., Weisdorf D., Pavletic S. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D., Weisdorf D., Martin P. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Fitzhugh C.D., Hsieh M., Taylor T. Cyclophosphamide improves engraftment in patients with SCD and severe organ damage who undergo haploidentical PBSCT. Blood Adv. 2017;1:652–661. doi: 10.1182/bloodadvances.2016002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluckman E., Cappelli B., Bernaudin F. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2016;29:1548–1556. doi: 10.1182/blood-2016-10-745711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallas M.H., Triplett B., Shook D.R. Long-term outcome and evaluation of organ function in pediatric patients undergoing haploidentical and matched related hematopoietic cell transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2013;19:820–830. doi: 10.1016/j.bbmt.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlowska A.B., Cheng J.C., Karras N.A. HLA haploidentical stem cell transplant with pretransplant immunosuppression for patients with sickle cell disease. Biol Blood Marrow Transplant. 2018;24:185–189. doi: 10.1016/j.bbmt.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Bashey A., Zhang M.J., McCurdy S.R. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansbury E.N., Schultz W.H., Ware R.E., Aygun B. Bone marrow transplant options and preferences in a sickle cell anemia cohort on chronic transfusions. Pediatr Blood Cancer. 2012;58:611–615. doi: 10.1002/pbc.23304. [DOI] [PubMed] [Google Scholar]
- 20.Meier E.R., Dioguardi J.V., Kamani N. Current attitudes of parents and patients toward hematopoietic stem cell transplantation for sickle cell anemia. Pediatr Blood Cancer. 2015;62:1277–1284. doi: 10.1002/pbc.25446. [DOI] [PubMed] [Google Scholar]
- 21.Chakrabarti S., Bareford D. A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone Marrow Transplant. 2007;39:447–451. doi: 10.1038/sj.bmt.1705622. [DOI] [PubMed] [Google Scholar]
- 22.van Besien K., Koshy M., Anderson-Shaw L. Allogeneic stem cell transplantation for sickle cell disease. A study of patients' decisions. Bone Marrow Transplant. 2001;28:545–549. doi: 10.1038/sj.bmt.1703208. [DOI] [PubMed] [Google Scholar]
- 23.Kodish E., Lantos J., Stocking C., Singer P.A., Siegler M., Johnson F.L. Bone marrow transplantation for sickle cell disease. A study of parents' decisions. N Engl J Med. 1991;325:1349–1353. doi: 10.1056/NEJM199111073251905. [DOI] [PubMed] [Google Scholar]
- 24.Roth M., Krystal J., Manwani D., Driscoll C., Ricafort R. Stem cell transplant for children with sickle cell anemia: parent and patient interest. Biol Blood Marrow Transplant. 2012;18:1709–1715. doi: 10.1016/j.bbmt.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Ribeil J.A., Hacein-Bey-Abina S., Payen E. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 26.de Montalembert M., Brousse V., Chakravorty S. Are the risks of treatment to cure a child with severe sickle cell disease too high? BMJ. 2017;359:j5250. doi: 10.1136/bmj.j5250. [DOI] [PubMed] [Google Scholar]
- 27.Fitzhugh C.D., Hsieh M.M., Bolan C.D., Saenz C., Tisdale J.F. Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy. 2009;11:464–471. doi: 10.1080/14653240902849788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King A.A., Kamani N., Bunin N. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am J Hematol. 2015;90:1093–1098. doi: 10.1002/ajh.24183. [DOI] [PubMed] [Google Scholar]
- 29.Lagresle-Peyrou C., Lefrere F., Magrin E. Plerixafor enables the safe, rapid, efficient mobilization of haematopoietic stem cells in sickle cell disease patients after exchange transfusion. Haematologica. 2018;103:778–786. doi: 10.3324/haematol.2017.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]