Summary
Pseudomonas aeruginosa uses its type III secretion system to inject the effector proteins ExoS and ExoU into eukaryotic cells, which subverts these cells to the bacterium’s advantage and contributes to severe infections. We studied the environmental reservoirs of exoS+ and exoU+ strains of P. aeruginosa by collecting water, soil, moist substrates, and plant samples from environments in the Chicago region and neighboring states. Whole-genome sequencing was used to determine the phylogeny and type III secretion system genotypes of 120 environmental isolates. No correlation existed between geographic separation of isolates and their genetic relatedness, which confirmed previous findings of both high genetic diversity within a single site and the widespread distribution of P. aeruginosa clonal complexes. After excluding clonal isolates cultured from the same samples, 74 exoS+ isolates and 16 exoU+ isolates remained. Of the exoS+ isolates, 41 (55%) were from natural environmental sites and 33 (45%) were from man-made sites. Of the exoU+ isolates, only 3 (19%) were from natural environmental sites and 13 (81%) were from man-made sites (p < 0.05). These findings suggest that man-made water systems may be a reservoir from which patients acquire exoU+ P. aeruginosa strains.
Introduction
Pseudomonas aeruginosa is an important cause of severe infections in hospitalized patients and those with cystic fibrosis (Driscoll et al., 2007). This Gram-negative bacterium is primarily found as a free-living microbe in soil, water, and plants (Green et al., 1974; Hoadley, 1977; Kominos et al., 1977; Pellett et al., 1983), and the predominant selective pressures governing its evolution appear to be in the natural environment and not in human hosts (Selezska et al., 2012). The P. aeruginosa type III secretion system (T3SS) (Hauser, 2009) plays a critical role in the pathogenesis of infections in people (Hauser, 2009) but likely evolved to protect P. aeruginosa from predation in the environment (Ferguson et al., 2001; Matz et al., 2008). Many of the proteins encoded by this system (e.g. PscF) form a needle-like apparatus on the surface of P. aeruginosa. Effector proteins such as ExoS and ExoU are injected by this apparatus into the cytosol of eukaryotic cells to promote infection. Interestingly, the exoS and exoU genes are nearly always mutually exclusive; very few P. aeruginosa isolates contain both genes or neither gene (Feltman et al., 2001). One explanation for this exclusivity is that ExoS and ExoU provide enhanced fitness in distinct ecological niches.
Although the major reservoir of P. aeruginosa is the natural environment, the majority of epidemiological studies have focused on clinical rather than environmental isolates, and research into the environmental distribution of strains with specific type III secretion effector genes is limited. We cultured 120 P. aeruginosa isolates from largely non-healthcare sources and found that exoU+ and exoS+ isolates have different distributions in the environment. Whereas exoS+ isolates were cultured from a variety of sample types, including lakes, streams, faucets, drains, soil, and plant material, exoU+ isolates were more commonly associated with man-made water systems.
Results and Discussion
Identification and population structure of P. aeruginosa isolates from environmental samples
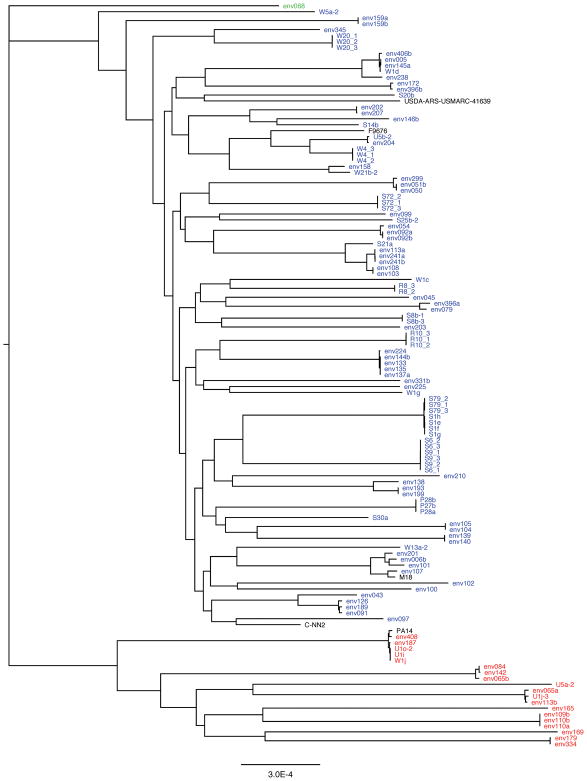
A total of 700 environmental samples of soil, water, plants and moist substrates were collected from sites in the Chicago region and neighboring states (Supporting Information Fig. S1). Samples were enriched for P. aeruginosa (Supporting Information Fig. S2), and the resulting bacteria were sequenced, which identified 97 P. aeruginosa isolates. This collection was supplemented with 23 isolates previously collected from environmental sites in central Minnesota (Supporting Information Fig. S1), yielding a total of 120 P. aeruginosa isolates from environmental sites (Supporting Information Table S1). We next generated a maximum likelihood phylogenetic tree based upon the core genomes of the 120 isolates (Fig. 1). As previously reported (Wiehlmann et al., 2007; Freschi et al., 2015; Thrane et al., 2015; Klockgether and Tummler, 2017), nearly all the isolates fell into one large clade and one smaller clade. Five isolates were closely related to the reference strain PA14, which is a member of a widespread clonal complex of P. aeruginosa isolates (Wiehlmann et al., 2007). Interestingly, none of the isolates were highly similar to the reference strain C-NN2, which is a member of a clonal complex of clone C-like strains commonly cultured in Europe from aquatic environments and individuals with cystic fibrosis (Romling et al., 1994; Romling et al., 2005). Closer inspection of the tree indicated that nearly identical isolates and distantly related isolates were often found at sites within close proximity of each other. Also, some nearly identical isolates were found at sites quite distant from each other. For example, isolates from Wisconsin (env091), Ohio (env189), and Iowa (env126) had core genomes that were nearly identical (Fig. 1, Supporting Information Table S1). These results agree with prior studies demonstrating that local populations of P. aeruginosa are nearly as diverse as global populations (Kiewitz and Tummler, 2000; Pirnay et al., 2005; Kidd et al., 2012; Selezska et al., 2012) and that clonal groups of P. aeruginosa can be found across broad geographic distances (Romling et al., 1994; Pirnay et al., 2002; Dinesh et al., 2003; Romling et al., 2005). Taken together, these findings suggested the absence of a correlation between P. aeruginosa genotype and geographical location. Indeed, the pairwise genetic distance between isolates was not appreciably correlated with the geographic distance between the collection sites (Supporting Information Fig. S3). These results confirm that P. aeruginosa has a nonclonal epidemic population structure consisting of widely distributed epidemic clones dispersed among a background of non-clonal strains (Romling et al., 1994; Johnson et al., 2007; Wiehlmann et al., 2007; Pirnay et al., 2009; Maatallah et al., 2011).
Figure 1.
The phylogeny of environmental P. aeruginosa isolates. A maximum likelihood tree was generated from core genome alignments following removal of sequences under the influence of recombination. Isolates in red are exoU+, those in blue are exoS+, and the isolate in green is exoS−/exoU−. Reference strains PA14 (exoU+) and C-NN2 (clone c, exoS+) and three previously sequenced environmental isolates, M18 (Wu et al., 2011b), F9676 (Shi et al., 2015), and USDA-ARS-USMARC-41639 (Winsor et al., 2016), are shown in black.
We next compared the genotypes of our isolates to those previously reported by Wiehlmann and colleagues from a large global collection of 2921 P. aeruginosa isolates, of which 278 were environmental isolates (Wiehlmann et al., 2015). Each isolate had been genotyped using a set of 16-genetic markers (Wiehlmann et al., 2007). We searched the genomes of our isolates for these markers to similarly genotype them. We found that our 120 isolates comprised 53 genotypes, of which 31 were also in the Wiehlmann collection (Supplemental Information Table S2). Of the four most common genotypes in the Wiehlmann collection, two were found in our collection, indicating that some isolates from the Midwestern United States had a global distribution. A total of 51 (43%) of the 120 environmental isolates in our collection had genotypes that were not present in the Wiehlmann collection (Supplemental Information Table S3). These results indicate that some P. aeruginosa clones are not geographically isolated but rather widely dispersed, while most environmental isolates represent distinct clones.
The ecological niche of P. aeruginosa
We next examined how frequently P. aeruginosa was cultured from the different types of environmental samples. Data on sample collection were not available for the 23 central Minnesota isolates, so they were excluded from this analysis. The yield of P. aeruginosa was highest in soil and moist substrates, somewhat lower in water samples, and lowest in plant samples (Table 1). Our results are in agreement with previous reports that P. aeruginosa is found ubiquitously throughout moist environments (Hoadley, 1977; Pellett et al., 1983; Pirnay et al., 2005).
Table 1.
Yield of P. aeruginosa from different sample types.
| earth/soil | water | moist substrates | plant | |
|---|---|---|---|---|
| total # samples* | 113 | 164 | 293 | 113 |
| # samples from which P. aeruginosa grew (%) | 18 (15.9) | 16 (9.8) | 47 (16.0) | 8 (7.1) |
samples from central Minnesota (site 44) are excluded
Type III secretion genotypes and environmental sites
Next we examined the type III secretion genotypes of the 120 environmental isolates. Each isolate contained the pscF gene, which is located in a large chromosomal cluster of genes that encode the structural and regulatory components of the type III secretion system. All contained the exoT gene, 115 (96%) contained the exoY gene, 100 (83%) contained the exoS gene, and 19 (16%) contained the exoU gene (Supporting Information Table S1). These values are within the ranges observed by others of 100%, 95%, 61–80%, and 18–33% for exoT, exoY, exoS, and exoU, respectively, in P. aeruginosa isolates from the natural environment (Feltman et al., 2001; Pirnay et al., 2009; Selezska et al., 2012). Interestingly, these values differ somewhat from those reported for isolates from acute infections, in which the exoT gene was found in 92–100% of isolates, the exoY gene in 89%, the exoS gene in 56–75%, and the exoU gene in 21–44% (Fleiszig et al., 1997; Feltman et al., 2001; Lomholt et al., 2001; Berthelot et al., 2003; Wareham and Curtis, 2007; Garey et al., 2008; Agnello and Wong-Beringer, 2012; Pena et al., 2014). Together, these data suggest two points. First, nearly all environmental isolates contain the exoT and exoY genes, perhaps because these genes play an important role in the survival of P. aeruginosa in diverse natural environments. Second, the exoU gene is more commonly present in P. aeruginosa isolates causing acute infections than isolates found in the environment.
As previously noted, the larger clade of the phylogenetic tree was comprised of the exoS+ isolates and the smaller clade of the exoU+ isolates (Fig. 1) (Ozer et al.; Wiehlmann et al., 2007; Selezska et al., 2012; Freschi et al., 2015; Klockgether and Tummler, 2017). Such cladogenesis may have resulted from ecological divergence, in which exoU+ and exoS+ strains thrived in distinct niches (Cohan and Koeppel, 2008). Selezska and colleagues noted similar findings with regard to a largely exoU+ “extended clonal complex” that they identified and named eccB. These authors state that eccB “is quite a diverse but clonal group, with an apparently elevated sexual isolation to other lineages” (Selezska et al., 2012). Only one of our 120 isolates lacked both exoS and exoU (env068); it was distantly related to the exoS+ and exoU+ clades (Fig. 1). Closer examination of env068 indicated that it is distinct from PA7-like strains (which also lack exoU and exoS) in that it contains several type III secretion genes (e.g. exoT, exsA, pscF, and pscJ) but lacks the type V secretion gene exlA (Roy et al., 2010; Huber et al., 2016).
To validate our findings, we compared the type III secretion genotypes of our isolates to those of other environmental P. aeruginosa isolates with publically available sequences. We included all P. aeruginosa strains with whole-genome sequences deposited in the NCBI FTP site (ftp.ncbi.nlm.nih.gov accessed on January 7, 2018) with a source label of “water,” “plant,” “soil,” or “environment” (excluding isolates from hospital or industrial settings). We identified 56 isolates, of which 37 (66%) were exoS+, 16 (29%) were exoU+, 3 (5%) were exoS-/exoU−, and 0 were exoS+/exoU+. We expect that these results differ somewhat from ours because the proportion of each environmental sample type also differed from ours. Nonetheless, these results confirm that the majority of environmental isolates are exoS+.
Environmental niches of exoS+ and exoU+ isolates
We next examined the types of sites from which the exoS+ and exoU+ isolates were cultured. We categorized sites as either “natural” or “man-made,” based upon whether they were naturally occurring (e.g. lakes, streams, soil, plants) or fabricated (e.g. sinks, drains, toilets, fountains, hoses, pipes) (Supporting Information Table S1). To prevent bias from repeat sampling of the same bacterial clone at the same site, we included only one clonal isolate per sample type from each collection site in these analyses. This left a total of 90 P. aeruginosa isolates, 74 of which contained the exoS gene and 16 of which contained the exoU gene. While exoS+ isolates were frequently cultured from soil and plant matter, no exoU+ isolates were grown from these sources. Of the exoS+ isolates, 41 (55%) were from natural environments and 33 (45%) were from man-made environments (Table 2). Of the 16 exoU+ isolates, only 3 (19%) were from natural environments and 13 (81%) were from man-made environments. Thus relative to exoS+ isolates, exoU+ isolates of P. aeruginosa were more frequently found in man-made environments (p < 0.01). Our results are consistent with those of Vincent and colleagues (Vincent et al., 2017), who noted that all 16 P. aeruginosa isolates from dental water lines fell into a phylogenetic clade corresponding to our exoU+ group. These findings suggest that exoS provides a fitness advantage in many types of ecological niches, whereas fitness attributable to exoU may be limited predominantly to man-made water systems. One explanation is that ExoS targets and confers protection against a broad variety of predatory eukaryotic organisms, whereas ExoU is active against a more narrow range of organisms that inhabit man-made water systems. Free-living amoebae are known to colonize and form biofilms in domestic plumbing systems (Shoff et al., 2008; Thomas et al., 2010), and P. aeruginosa bacteria can survive and multiply within these amoebae (Michel et al., 1995) or even kill them to prevent predation (Matz et al., 2008). Furthermore, the effector complement of P. aeruginosa strains influences their ability to kill Acanthamoeba castellaniii amoebae (Matz et al., 2008). At first glance, the presence of both exoS+ and exoU+ isolates in man-made water systems argues against the ecological isolation of these two types of P. aeruginosa strains. Indeed, exoS+ and exoU+ isolates were cultured from the same drinking fountain (env113a and env113b) and from a shower drain and sink drain within the same bathroom (env189 and env187). It is possible, however, that within these water systems the exoS+ and exoU+ strains inhabit distinct micro-niches, as has been described for Vibrio cyclitrophicus, which is associated with different zoo- and phytoplankton or organic particle types in the same samples of seawater (Shapiro et al., 2012).
Table 2.
Number of exoS+ and exoU+ P. aeruginosa isolates from man-made and natural environments, using only one clonal isolate per collection site and sample type.
| exoS+ | exoU+ | Total | |
|---|---|---|---|
| Natural environment | 41 (55%) | 3 (19%) | 44 |
| water | 14 | 2 | |
| moist substrates | 4 | 1 | |
| earth/soil | 16 | ||
| plant matter | 7 | ||
| Man-made environment | 33 (45%) | 13 (81%) | 46 |
| water | 4 | 1 | |
| moist substrates | 28 | 12 | |
| earth/soil | 1 | ||
| plant matter | |||
| Total | 74 | 16 | 90 |
% values reflect percentage of all exoS+ isolates or of all exoU+ isolates
Our results are consistent with several published reports that have found exoS+ isolates in soil (Ferguson et al., 2001) and a paucity of exoU+ eccB isolates in a Belgian river (Pirnay et al., 2005; Selezska et al., 2012). However, Selezska and colleagues found eccB to be common in a river system in Northern Germany (Selezska et al., 2012) and Wiehlmann and colleagues reported that 18% of soil, plant, and freshwater isolates were exoU+, compared to 7% in our study (Wiehlmann et al., 2015). These findings may reflect differing levels of pollution or contamination of natural water systems with human-manipulated water such as sewage. In support of the latter hypothesis, two of the three exoU+ isolates we identified in natural water systems came from the Chicago River, which is highly affected by industrial and residential development and has even been engineered to reverse its direction of flow in several segments. These river isolates had nearly identical core genomes to isolates cultured from sink and shower drains and a drinking fountain (Fig. 1 and Supporting Information Table S1). Interestingly, exoU+ eccB strains had a survival advantage relative to exoS+ eccS42 strains when incubated in autoclaved highly polluted river water (Selezska et al., 2012), suggesting that high levels of pollution may favor exoU+ strains. Additional studies over wider geographical areas will be necessary to more definitively define the factors that are associated with the presence of exoU+ strains.
These findings shed light on potential reservoirs from which patients acquire their P. aeruginosa infections. exoU+ strains are over-represented in hospital-associated infections relative to the natural environment (21–44% vs. 16%, respectively), suggesting that many infected patients acquire their strains from man-made water systems. Indeed, hospital-acquired infections have been linked to the presence of P. aeruginosa in hospital water supplies (Trautmann et al., 2005; Trautmann et al., 2006; Kizny Gordon et al., 2017; Lefebvre et al., 2017), and isolates collected from the sinks and washtubs of intensive care units were more likely to be exoU+ (Bradbury et al., 2010). We would predict that community-acquired outbreaks of P. aeruginosa dermatitis-folliculitis, which are associated with man-made water systems such as hot tubs and swimming pools (Fiorillo et al., 2001; Wu et al., 2011a; Michl et al., 2012), would be caused largely by exoU+ strains. Although to our knowledge such strains have not been analyzed for T3SS genotypes, they are commonly serogroup O-11 (Ayi, 2015), which tend to be exoU+ (Berthelot et al., 2003; Zhu et al., 2006). Perhaps the most intriguing disease manifestation in this regard is keratitis, in which exoU+ strains are associated with contact lens use and exoS+ strains are more common in patients who do not wear contact lenses (Lomholt et al., 2001; Winstanley et al., 2005; Zhu et al., 2006; Choy et al., 2008; Shankar et al., 2012; Yamaguchi et al., 2014). It is conceivable that keratitis in contact-lens wearers originates from P. aeruginosa strains in tap water that contaminates contact lenses through the washing of the lenses and cases (Stapleton et al., 2007), whereas keratitis in the absence of contact lenses is caused by P. aeruginosa acquired from the natural environment. Additional studies are necessary to more clearly define the roles of environmental reservoirs in dictating the prevalence of exoU+ vs. exoS+ strains in different types of infections.
Our study has several limitations. First, it examined only a localized region within the Midwestern United States, and it is not clear whether our results can be generalized to other areas. Second, our isolation protocols may not have detected the full diversity of P. aeruginosa strains present. Third, our sample size was limited to 120 P. aeruginosa isolates. Finally, we largely excluded hospital and other medical sites from the study. This was done intentionally to ensure that the results represented the distribution of P. aeruginosa outside of the healthcare setting. In future studies, we plan to collect P. aeruginosa isolates from healthcare settings within the same region and to compare these isolates to those of the current study.
Supplementary Material
Originality-Significance Statement.
ExoU and ExoS are important virulence factors of Pseudomonas aeruginosa, and isolates of this bacterium tend to contain either the exoU or the exoS gene. Our results confirm previous findings that exoU+ and exoS+ strains of P. aeruginosa comprise distinct phylogenetic clades. To our knowledge, this is the first study to associate exoU+ strains with man-made water systems. This finding has implications regarding how patients acquire the P. aeruginosa strains that subsequently cause severe infections.
Acknowledgments
We thank Drs. Larry K. Kociolek, Maulin Soneji, Maureen Bolon, and Fiorella Krapp Lopez for their assistance with collecting environmental isolates from within hospitals. This work was supported by the National Institutes of Health grants R01 AI053674, R01 AI118257, and K24 AI04831 (ARH) and by the Northwestern University Bioscientist Program.
References
- Agnello M, Wong-Beringer A. Differentiation in quinolone resistance by virulence genotype in Pseudomonas aeruginosa. PLoS One. 2012;7:e42973. doi: 10.1371/journal.pone.0042973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayi B. Infections acquired via fresh water: from lakes to hot tubs. Microbiol Spectr. 2015:3. doi: 10.1128/microbiolspec.IOL5-0019-2015. [DOI] [PubMed] [Google Scholar]
- Berthelot P, Attree I, Plesiat P, Chabert J, de Bentzmann S, Pozzetto B, Grattard F. Genotypic and phenotypic analysis of type III secretion system in a cohort of Pseudomonas aeruginosa bacteremia isolates: Evidence for a possible association between O serotypes and exo genes. J Infect Dis. 2003;188:512–518. doi: 10.1086/377000. [DOI] [PubMed] [Google Scholar]
- Bradbury RS, Roddam LF, Merritt A, Reid DW, Champion AC. Virulence gene distribution in clinical, nosocomial and environmental isolates of Pseudomonas aeruginosa. J Med Microbiol. 2010;59:881–890. doi: 10.1099/jmm.0.018283-0. [DOI] [PubMed] [Google Scholar]
- Choy MH, Stapleton F, Willcox MD, Zhu H. Comparison of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens- and non-contact lens-related keratitis. J Med Microbiol. 2008;57:1539–15346. doi: 10.1099/jmm.0.2008/003723-0. [DOI] [PubMed] [Google Scholar]
- Cohan FM, Koeppel AF. The origins of ecological diversity in prokaryotes. Curr Biol. 2008;18:R1024–R1034. doi: 10.1016/j.cub.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Dinesh SD, Grundmann H, Pitt TL, Romling U. European-wide distribution of Pseudomonas aeruginosa clone C. Clin Microbiol Infect. 2003;9:1228–1233. doi: 10.1111/j.1469-0691.2003.00793.x. [DOI] [PubMed] [Google Scholar]
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, Maxwell JA, Vincent TS, da Silva J, Olson JC. Comparison of the exoS gene and protein expression in soil and clinical isolates of Pseudomonas aeruginosa. Infect Immun. 2001;69:2198–2210. doi: 10.1128/IAI.69.4.2198-2210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo L, Zucker M, Sawyer D, Lin AN. The Pseudomonas hot-foot syndrome. N Engl J Med. 2001;345:335–338. doi: 10.1056/NEJM200108023450504. [DOI] [PubMed] [Google Scholar]
- Fleiszig SMJ, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov K, Kanada D, et al. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi L, Jeukens J, Kukavica-Ibrulj I, Boyle B, Dupont MJ, Laroche J, et al. Clinical utilization of genomics data produced by the international Pseudomonas aeruginosa consortium. Front Microbiol. 2015;6:1036. doi: 10.3389/fmicb.2015.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey KW, Vo QP, Larocco MT, Gentry LO, Tam VH. Prevalence of type III secretion protein exoenzymes and antimicrobial susceptibility patterns from bloodstream isolates of patients with Pseudomonas aeruginosa bacteremia. J Chemother. 2008;20:714–720. doi: 10.1179/joc.2008.20.6.714. [DOI] [PubMed] [Google Scholar]
- Green SK, Schroth MN, Cho JJ, Kominos SK, Vitanza-jack VB. Agricultural plants and soil as a reservoir for Pseudomonas aeruginosa. Appl Microbiol. 1974;28:987–991. doi: 10.1128/am.28.6.987-991.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley AW. Pseudomonas aeruginosa in surface waters. In: Young VM, editor. Pseudomonas aeruginosa: Ecological aspects and patient colonization. New York: Raven Press; 1977. pp. 31–57. [Google Scholar]
- Huber P, Basso P, Reboud E, Attree I. Pseudomonas aeruginosa renews its virulence factors. Environ Microbiol Rep. 2016 doi: 10.1111/1758-2229.12443. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Arduino SM, Stine OC, Johnson JA, Harris AD. Multilocus sequence typing compared to pulsed-field gel electrophoresis for molecular typing of Pseudomonas aeruginosa. J Clin Microbiol. 2007;45:3707–3712. doi: 10.1128/JCM.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd TJ, Ritchie SR, Ramsay KA, Grimwood K, Bell SC, Rainey PB. Pseudomonas aeruginosa exhibits frequent recombination, but only a limited association between genotype and ecological setting. PLoS One. 2012;7:e44199. doi: 10.1371/journal.pone.0044199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiewitz C, Tummler B. Sequence diversity of Pseudomonas aeruginosa: impact on population structure and genome evolution. J Bacteriol. 2000;182:3125–3135. doi: 10.1128/jb.182.11.3125-3135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections-A systematic review of the literature. Clin Infect Dis. 2017;64:1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- Klockgether J, Tummler B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Res. 2017;6:1261. doi: 10.12688/f1000research.10506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominos SD, Copeland CE, Delenko CA. Pseudomonas aeruginosa from vegetables, salads, and other foods served to patients with burns. In: Young VM, editor. Pseudomonas aeruginosa: Ecological aspects and patient colonization. New York: Raven Press; 1977. pp. 59–75. [Google Scholar]
- Lefebvre A, Bertrand X, Quantin C, Vanhems P, Lucet JC, Nuemi G, et al. Association between Pseudomonas aeruginosa positive water samples and healthcare-associated cases: nine-year study at one university hospital. J Hosp Infect. 2017;96:238–243. doi: 10.1016/j.jhin.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Lomholt JA, Poulsen K, Kilian M. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect Immun. 2001;69:6284–6295. doi: 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatallah M, Cheriaa J, Backhrouf A, Iversen A, Grundmann H, Do T, et al. Population structure of Pseudomonas aeruginosa from five Mediterranean countries: evidence for frequent recombination and epidemic occurrence of CC235. PLoS One. 2011;6:e25617. doi: 10.1371/journal.pone.0025617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz C, Moreno AM, Alhede M, Manefield M, Hauser AR, Givskov M, Kjelleberg S. Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae. ISME J. 2008;2:843–852. doi: 10.1038/ismej.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R, Burghardt H, Bergmann H. Acanthamoeba, naturally intracellularly infected with Pseudomonas aeruginosa, after their isolation from a microbiologically contaminated drinking water system in a hospital. Zentralbl Hyg Umweltmed. 1995;196:532–544. [PubMed] [Google Scholar]
- Michl RK, Rusche T, Grimm S, Limpert E, Beck JF, Dost A. Outbreak of hot-foot syndrome - caused by Pseudomonas aeruginosa. Klin Padiatr. 2012;224:252–255. doi: 10.1055/s-0031-1297949. [DOI] [PubMed] [Google Scholar]
- Ozer EA, Nnah E, Didelot X, Hauser AR. exoU+ and exoS+ isolates of Pseudomonas aeruginosa comprise distinct populations with characteristics of adaptation to distinct ecological niches. Submitted. [Google Scholar]
- Pellett S, Bigley DV, Grimes DJ. Distribution of Pseudomonas aeruginosa in a riverine ecosystem. Appl Environ Microbiol. 1983;45:328–332. doi: 10.1128/aem.45.1.328-332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena C, Cabot G, Gomez-Zorrilla S, Zamorano L, Ocampo-Sosa A, Murillas J, et al. Influence of virulence genotype and resistance profile in the mortality of Pseudomonas aeruginosa bloodstream infections. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu866. [DOI] [PubMed] [Google Scholar]
- Pirnay JP, De Vos D, Cochez C, Bilocq F, Vanderkelen A, Zizi M, et al. Pseudomonas aeruginosa displays an epidemic population structure. Environ Microbiol. 2002;4:898–911. doi: 10.1046/j.1462-2920.2002.00321.x. [DOI] [PubMed] [Google Scholar]
- Pirnay JP, Matthijs S, Colak H, Chablain P, Bilocq F, Van Eldere J, et al. Global Pseudomonas aeruginosa biodiversity as reflected in a Belgian river. Environ Microbiol. 2005;7:969–980. doi: 10.1111/j.1462-2920.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- Pirnay JP, Bilocq F, Pot B, Cornelis P, Zizi M, Van Eldere J, et al. Pseudomonas aeruginosa population structure revisited. PLoS One. 2009;4:e7740. doi: 10.1371/journal.pone.0007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Wingender J, Muller H, Tummler B. A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl Environ Microbiol. 1994;60:1734–1738. doi: 10.1128/aem.60.6.1734-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Kader A, Sriramulu DD, Simm R, Kronvall G. Worldwide distribution of Pseudomonas aeruginosa clone C strains in the aquatic environment and cystic fibrosis patients. Environ Microbiol. 2005;7:1029–1038. doi: 10.1111/j.1462-2920.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Roy PH, Tetu SG, Larouche A, Elbourne L, Tremblay S, Ren Q, et al. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS One. 2010;5:e8842. doi: 10.1371/journal.pone.0008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selezska K, Kazmierczak M, Musken M, Garbe J, Schobert M, Haussler S, et al. Pseudomonas aeruginosa population structure revisited under environmental focus: impact of water quality and phage pressure. Environ Microbiol. 2012;14:1952–1967. doi: 10.1111/j.1462-2920.2012.02719.x. [DOI] [PubMed] [Google Scholar]
- Shankar J, Sueke H, Wiehlmann L, Horsburgh MJ, Tuft S, Neal TJ, et al. Genotypic analysis of UK keratitis-associated Pseudomonas aeruginosa suggests adaptation to environmental water as a key component in the development of eye infections. FEMS Microbiol Lett. 2012;334:79–86. doi: 10.1111/j.1574-6968.2012.02621.x. [DOI] [PubMed] [Google Scholar]
- Shapiro BJ, Friedman J, Cordero OX, Preheim SP, Timberlake SC, Szabo G, et al. Population genomics of early events in the ecological differentiation of bacteria. Science. 2012;336:48–51. doi: 10.1126/science.1218198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Ren D, Hu S, Hu X, Wu L, Lin H, et al. Whole genome sequence of Pseudomonas aeruginosa F9676, an antagonistic bacterium isolated from rice seed. J Biotechnol. 2015;211:77–78. doi: 10.1016/j.jbiotec.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Shoff ME, Rogerson A, Kessler K, Schatz S, Seal DV. Prevalence of Acanthamoeba and other naked amoebae in South Florida domestic water. J Water Health. 2008;6:99–104. doi: 10.2166/wh.2007.014. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84:257–272. doi: 10.1097/OPX.0b013e3180485d5f. [DOI] [PubMed] [Google Scholar]
- Thomas V, McDonnell G, Denyer SP, Maillard JY. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol Rev. 2010;34:231–259. doi: 10.1111/j.1574-6976.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- Thrane SW, Taylor VL, Freschi L, Kukavica-Ibrulj I, Boyle B, Laroche J, et al. The widespread multidrug-resistant Serotype O12 Pseudomonas aeruginosa clone emerged through concomitant horizontal transfer of serotype antigen and antibiotic resistance gene clusters. MBio. 2015;6:e01396–01315. doi: 10.1128/mBio.01396-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann M, Lepper PM, Haller M. Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am J Infect Control. 2005;33:S41–S49. doi: 10.1016/j.ajic.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Trautmann M, Bauer C, Schumann C, Hahn P, Hoher M, Haller M, Lepper PM. Common RAPD pattern of Pseudomonas aeruginosa from patients and tap water in a medical intensive care unit. Int J Hyg Environ Health. 2006;209:325–331. doi: 10.1016/j.ijheh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Vincent AT, Freschi L, Jeukens J, Kukavica-Ibrulj I, Emond-Rheault JG, Leduc A, et al. Genomic characterisation of environmental Pseudomonas aeruginosa isolated from dental unit waterlines revealed the insertion sequence ISPa11 as a chaotropic element. FEMS Microbiol Ecol. 2017:93. doi: 10.1093/femsec/fix106. [DOI] [PubMed] [Google Scholar]
- Wareham DW, Curtis MA. A genotypic and phenotypic comparison of type III secretion profiles of Pseudomonas aeruginosa cystic fibrosis and bacteremia isolates. Int J Med Microbiol. 2007;297:227–234. doi: 10.1016/j.ijmm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Wiehlmann L, Cramer N, Tummler B. Habitat-associated skew of clone abundance in the Pseudomonas aeruginosa population. Environ Microbiol Rep. 2015;7:955–960. doi: 10.1111/1758-2229.12340. [DOI] [PubMed] [Google Scholar]
- Wiehlmann L, Wagner G, Cramer N, Siebert B, Gudowius P, Morales G, et al. Population structure of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FS. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016;44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C, Kaye SB, Neal TJ, Chilton HJ, Miksch S, Hart CA. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J Med Microbiol. 2005;54:519–526. doi: 10.1099/jmm.0.46005-0. [DOI] [PubMed] [Google Scholar]
- Wu DC, Chan WW, Metelitsa AI, Fiorillo L, Lin AN. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011a;12:157–169. doi: 10.2165/11539770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wu DQ, Ye J, Ou HY, Wei X, Huang X, He YW, Xu Y. Genomic analysis and temperature-dependent transcriptome profiles of the rhizosphere originating strain Pseudomonas aeruginosa M18. BMC Genomics. 2011b;12:438. doi: 10.1186/1471-2164-12-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Suzuki T, Kobayashi T, Oka N, Ishikawa E, Shinomiya H, Ohashi Y. Genotypic analysis of Pseudomonas aeruginosa isolated from ocular infection. J Infect Chemother. 2014;20:407–411. doi: 10.1016/j.jiac.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Zhu H, Conibear TC, Bandara R, Aliwarga Y, Stapleton F, Willcox MD. Type III secretion system-associated toxins, proteases, serotypes, and antibiotic resistance of Pseudomonas aeruginosa isolates associated with keratitis. Curr Eye Res. 2006;31:297–306. doi: 10.1080/02713680500536746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.