Abstract
This multicenter study evaluated a treosulfan-based regimen in children and young adults with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) undergoing allogeneic hematopoietic cell transplant (HCT). Forty patients with median age 11 years (1–19) underwent allogeneic HCT for AML in first (n=18), second (n=11), third or greater remission (n=3); or MDS (n=8) using bone marrow (n=25), peripheral blood stem cells (n=5) or cord blood (n=9). The regimen consisted of body surface area (BSA)-based treosulfan 10 g/m2/day (BSA ≤ 0.5 m2), 12 g/m2/day (BSA > 0.5 – 1.0 m2), or 14 g/m2/day (BSA >1.0 m2) on days −6 to −4; fludarabine 30 mg/m2/day on days −6 to −2; and a single fraction of 200 centigray total body irradiation on day - 1. Graft-versus-host disease (GVHD) prophylaxis included tacrolimus and methotrexate for marrow and peripheral blood stem cell and cyclosporine/mycophenolate mofetil for cord blood. One-year overall survival, disease-free survival, and non-relapse mortality were 80%, 73% and 3%, respectively. One-year relapse was 38% for AML and 13% for MDS. No serious organ toxicities were observed. All 37 evaluable patients engrafted. Cumulative incidences of grade II-IV acute and chronic GVHD were 22% and 40%. BSA-based treosulfan dosing resulted in predictable area under the curve and maximum concentration, which is required for dosing without measuring individual pharmacokinetic parameters. Observed differences in pharmacokinetics did not impact disease control or regimen toxicity. This BSA-based treosulfan regimen resulted in excellent engraftment and disease-free survival, minimal toxicity and transplant-related mortality (3%) in children and young adults with AML and MDS.
Keywords: Stem Cell Transplantation, Conditioning Regimen, Myelodysplastic Syndromes, Acute Myeloid Leukemia
INTRODUCTION
Allogeneic hematopoietic cell transplant (HCT) is potentially curative for children and young adults with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Conventional myeloablative conditioning regimens (MAC) containing busulfan are the most widely used for these indications in this younger age group. The success of these regimens is limited by toxicity and transplant-related mortality (TRM).1–4 Reduced intensity conditioning (RIC) regimens may be associated with lower rates of TRM, but at the expense of more relapse.5–8 Thus, children with AML and MDS are still in need of a better conditioning regimen that results in sustained remission without the substantial acute toxicity and NRM observed with conventional regimens.
Treosulfan (Ovastat®, L-threitol-1,4-dimethasulfonate, (2S,3S)-1,4-dimesyloxy-2,3-butanediol, dihydroxybusulfan) is a water-soluble prodrug of a bifunctional alkylating agent approved for therapy of advanced ovarian carcinoma in Europe.9, 10 This drug has several characteristics that make it attractive for use in HCT compared to busulfan. Treosulfan metabolism bypasses the liver, has a highly predictable pharmacokinetic (PK) profile in adults, is sufficiently immunosuppressive for engraftment of donor cells across histocompatibility barriers, and is highly anti-leukemic. 11–17 In adults, treosulfan-based conditioning for allogeneic HCT for hematologic malignancies have shown safety and efficacy.18–22 However, there is little data on the use of treosulfan regimens for childhood hematologic malignancies.21-24 This phase II prospective multicenter study evaluated the safety and efficacy of a treosulfan combined with fludarabine and 200 cGy total body irradiation (TBI) in children and young adults with AML or MDS undergoing allogeneic HCT. Our hypothesis was that this regimen would yield lower toxicity without compromise of disease control when compared to standard MAC regimens.
METHODS
Patient and donor characteristics
Forty patients with median age 11 years (1–19) were enrolled in a multicenter prospective, open label, non-randomized clinical trial at 13 U.S. transplant centers between September 2013 and April 2014. Patient characteristics are summarized in Table 1. Patients included were children and young adults with a diagnosis of AML in first or greater complete remission (CR), or any MDS subtype undergoing marrow (BM) or peripheral blood stem cells (PBSC) from a related or unrelated donor matched at ≥ 7/8 human leukocyte antigens (HLA) or unrelated cord blood matched at ≥ 4/6 HLA antigens.
Table 1.
Patient and graft characteristics
| Variable | N(%) |
|---|---|
| Number of patients | 40 |
| Age at HCT (years), median (range) | 11 (1–19) |
| Sex | |
| Male | 16(40) |
| Female | 24 (60) |
| BSA, m2 | |
| ≤0.5 | 5(13) |
| >0.5–1 | 10(25) |
| > 1 | 25 (62) |
| AMLa | 32 (80) |
| 1st CR | 18(56) |
| 2nd or greater CR | 14(44) |
| MDS | 8(20) |
| Treated prior to HCT | 1 (13) |
| Not treated prior to HCT | 7(87) |
| Days from diagnosis to HCT, median (range) | |
| AML | 229(62–1574) |
| MDS | 83 (29–853) |
| Second HCT | 5(13) |
| Previous autologous | 1(3) |
| Previous allogeneic | 4(10) |
| Cytogenetics at diagnosis for AML patientsb | |
| Good | 2(6) |
| Intermediate | 18(58) |
| Poor | 11 (35) |
| Unknown | 1 |
| CIBMTR disease risk groupc | |
| Standard | 21(52) |
| Intermediate | 15(38) |
| Poor | 4(10) |
| Graft type and HLA-matching | |
| BM/PBSC | 25/6 (62)/(15) |
| 8/8 related | 10 |
| 8/8 unrelated | 15 |
| 7/8 unrelated | 6 |
| Unrelated CBU | 9(23) |
| 6/6 | 2 |
| 5/6 | 3 |
| 4/6d | 4 |
6 AML patients had residual detectable disease by flow cytometry at HCT
Cytogenetic risk classification
Disease risk groups: Standard = AML 1st CR; MDS = refractory cytopenia or refractory anemia; Intermediate = AML 2nd or greater CR; Poor = MDS RAEB/RAEB-T
2 patients received double CBUs; the lower of the 2 HLA matches was reported
The diagnosis of AML or MDS was made according to World Health Organization (WHO) criteria and confirmed by review of clinical pathology reports at each institution. Disease status prior to HOT was determined by a bone marrow aspirate obtained within 28 days prior to the start of conditioning. AML in morphologic remission was defined as < 5% blasts in a bone marrow aspirate of adequate cellularity. Minimal residual disease (MRD) was defined as the presence of detectable disease by flow cytometry, cytogenetic analysis, or fluorescent in-situ hybridization (FISH) in patients with less than 5% BM blasts by morphology. Patients with refractory anemia with excess blast-2 could proceed directly to transplant, but also be considered for induction chemotherapy before transplant. Patients with ≥ 20% morphologic marrow blasts required induction therapy to reduce morphologic marrow blasts below 5% before transplant.
Patients with low general performance scores (i.e., Karnofsky or Lansky Play-Performance Scale score < 70% on pre-HCT evaluation), Fanconi anemia, human immunodeficiency virus (HIV) or uncontrolled systemic infections, active central nervous system leukemia or extramedullary disease at the time of HCT, or significant cardiopulmonary, renal or hepatic dysfunction were excluded. Those undergoing chemotherapy using other investigational agents within four weeks prior to start of conditioning, as well as pregnant and lactating females were also excluded. Patients who had undergone a single previous HCT were eligible for inclusion if time from the first transplant was ≥ 6 months.
Related donors were matched for human leukocyte antigen (HLA)-A, -B, and -C at intermediate resolution and -DRB1 at high resolution by molecular typing. Unrelated donors were matched for HLA-A, -B, -C and -DRB1 defined by high resolution molecular typing. A single HLA antigen or allele mismatch (7/8 matched) was permitted. BM or granulocyte-colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC) were permitted. Unrelated cord blood unit (CBU) donors were matched at a minimum of 4 of 6 loci at HLA-A and -B by intermediate resolution, and -DRB1 by high resolution, with a minimum total cell dose of 3 × 107 total nucleated cells (TNC) per kg of recipient weight. Double CBUs were allowed for patients lacking a single CBU with sufficient cell dose, following the same HLA-matching and cell dose criteria for single CBUs.
This study was a cooperative effort between the Pediatric Blood and Marrow Transplant Consortium (PBMTC) study and the Center for International Blood and Marrow Transplant Research® (CIBMTR). The study protocol underwent scientific and ethics reviews, and obtained approval from the National Marrow Donor Program (NMDP) institutional review board (IRB). Patients, and legal guardians for patients younger than 18 years, were informed of the investigational nature of the study and signed consent and assent forms approved by the IRB at each institution, in accordance with the Declaration of Helsinki.
Transplant regimen
Treosulfan was given intravenously (IV) on days −6 to −4 at a daily dose determined by BSA of 10 g/m2 (≤ 0.5 m2), 12 g/m2 (> 0.5 −1 m2) or 14 g/m2 (> 1 m2). The latter was the maximum tolerated dose based on previous studies, where limiting toxicities were observed with doses above 42 g/m2.5 BSA dosing was based on population PK modeling [medac GmbH, data not published]. Fludarabine 30 mg/m2/day IV was given on days −6 to −2 (total dose 150 mg/m2). Ideal body weight was used in patients whose actual body weight exceeded 125% of ideal body weight. A single fraction of 200 cGy TBI was administered on day −1. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus to keep serum trough levels of 8–12 ng/mL starting on day −1 and methotrexate 10 mg/m2/dose on days +1, +3, +6 and +11 for those receiving BM/PBSC. In the absence of GVHD, tacrolimus was tapered starting on day +56 and discontinued by day +180. For CBU recipients, GVHD prophylaxis consisted of cyclosporine A (CSA) starting on day −3 to keep trough levels of 250–500 ng/mL by immunoassay and mycophenolate mofetil (MMF) 15 mg/kg every 8 hours starting on day 0. In the absence of GVHD, CSA was tapered starting on day +100 and discontinued after day +180. MMF was tapered starting on day +42 or 7 days after engraftment, whichever occurred later, through day +96. Supportive care was provided as detailed in the supplemental on-line information.
Pharmacokinetic (PK) studies
Blood samples were collected for treosulfan PK analysis from patients weighing less than 40 kg. Samples were batched and analyzed at the University of Essen, Germany, using refractometric detection methods previously described.25 Blood samples of 1 mL each were collected at ten time points after the first and third dose of treosulfan (hours 0, 2, 2:20, 2:40, 3, 4, 5, 6, 7 and 24) and 2 hours after the second dose. To avoid artificial ex vivo degradation, the samples were adjusted to a final pH of 5.5 by adding them to prefilled tubes with citrate buffer. Samples were centrifuged at 4°C and 1,000 g for 10 minutes to separate plasma. The cell-free supernatant was further microfiltrated and analyzed by reversed phase high-performance liquid chromatography (RP-HPLC). Individual PK parameters were evaluated by two-compartment disposition modeling using TopFit software, version 2.0.26,27 PK parameters analysed included area under the curve (AUC), maximum concentration (Cmax), half-life (t 1/2), volume of distribution (Vss) and total clearance (CL).
Study design and statistical methods
This primary objective of this study was to determine the safety and preliminary efficacy of a regimen of treosulfan-based regimen for children and young adults with AML or MDS undergoing allogeneic HCT. The primary endpoint was OS at one-year post-HCT. Secondary endpoints included graft failure, relapse, time to neutrophil and platelet engraftment, disease-free survival (DFS), GVHD/relapse-free survival (GRFS), NRM, incidence of acute and chronic GVHD, transplant-related toxicities and PK parameters of treosulfan in patients weighing less than 40 kg. Transplant-related toxicities were defined as organ toxicities not attributable to primary disease, infection or GVHD. Grading of organ toxicities was done using National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0.
Estimates of OS and DFS were calculated using Kaplan-Meier estimates. Relapse or death from any cause were considered failure for the endpoint of DFS. Relapse, death, presence of acute GVHD grades III-IV or chronic GVHD were considered failure for the endpoint of GRFS. Cumulative incidence curves were used to estimate the probabilities of acute and chronic GVHD, relapse, and NRM. Death was treated as a competing risk for neutrophil and platelet engraftment, GVHD and relapse. Relapse was considered a competing risk for NRM. Statistical significance was evaluated using the Cox regression model. Independent variables examined for the regression models included type of disease, disease status at HCT, donor and HCT source. All reported two-sided p-values from regression models were derived from the Wald test. The statistical analysis was performed using SAS® Enterprise Guide (Cary, NC, USA).
Time to neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) of 0.5 × 109/L or greater. Platelet engraftment was defined as the first of three consecutive days with a platelet count greater than 20 × 109/L without the need for platelet transfusions. Primary graft failure was evaluated separately for BM/PBSC and CBU, and defined as lack of donor-derived neutrophil engraftment by day +56. Relapse and death from other causes prior to engraftment were considered as competing risks for the endpoint of graft failure. Presence of donor engraftment was also assessed by chimerism testing in whole blood fractions sorted for T cell lymphoid (CD3) and myeloid (CD33) subset markers collected on days +42 (± 14), +100 (± 20), +180 (± 20) and +365 (± 30). Acute and chronic GVHD were diagnosed and graded using CIBMTR criteria.28,29
RESULTS
Treosulfan PK
BSA-based treosulfan dosing resulted in reliably predictable AUC and Cmax across BSA groups (Table 2). Half-life did not differ across groups. Significant differences were observed in CL and Vss across treosulfan dose groups, as expected for patients of different size. The number of patients sampled for PK and the number of events are too small to reach any conclusions regarding the impact of treosulfan dosing in the risk of relapse, engraftment or toxicity across groups of differently sized patients.
Table 2.
Treosulfan PK profile by BSA-based dosing groups
| Treosulfan dose (g/m2) | |||
|---|---|---|---|
| 10 | 12 | 14 | |
| N | 5 | 10 | 4 |
| PK Parameter | |||
| Age (years) *,+,#,‡ | 1 (0.9–1) | 6 (4–8) | 9 (9–11) |
| BSA (m2) *,+,#,‡ | 0.43 (0.38–0.50) | 0.86 (0.52–0.99) | 1.11 (1.05–1.40) |
| Weight (kg) *,+,#,‡ | 9.2 (8.1–14.6) | 22.7 (19.4–25.1) | 30.7 (30.1–37.5) |
| AUC (mcg/mL*h)† | 2762 ± 1058 | 2240 ± 538 | 2235 ± 96 |
| Cmax (mcg/mL)† | 977 ± 412 | 799 ± 201 | 788 ± 28 |
| t½ (h)† | 1.39 ± 0.25 | 1.49 ± 0.14 | 1.38 ± 0.11 |
| Vss (L) *,+,† | 6.7± 2.3 | 9.5 ± 2.5 | 9.9 ± 0.6 |
| CL (mL/min) *,+,† | 69.4 ± 22.5 | 94.9 ± 18.2 | 104.5 ± 4.5 |
AUC: area under the curve; Cmax: maximum concentration; t½: half-life; Vss: volume of distribution; CL: total clearance
AUC, Cmax, t½Vss and CL expressed in mean + standard deviation (SD)
Age, BSA and weight expressed in median (range)
*,+,#Significant differences between BSA-based treosulfan doses (p < 0.05 (using t-test)):
10 vs.12 g/m2;
10 vs.14 g/m2;
12 vs.14g/m2
Engraftment and donor cell chimerism
Three patients were not evaluable for neutrophil engraftment due to never becoming neutropenic (n=1) or early relapse (n=2). Neutrophil engraftment occurred in 93% (90%CI, 87–100%) of the 37 evaluable patients. Median times to neutrophil and platelet engraftment were 19 days (12–28) and 25 days (12–66), respectively. More than 95% of patients achieved full donor T-cell and myeloid cell chimerisms by day +100.
Transplant-related toxicity, graft-versus-host disease, and non-relapse mortality
As expected, the conditioning regimen was minimally toxic. There were no instances of veno- occlusive disease of the liver or hemorrhagic cystitis. No severe conditioning regimen-related toxicities occurred on this study including the five patients who had undergone HCT previously. In addition, the cumulative incidence of severe grade III-IV acute GVHD by day +100 was 14% (90%CI, 6–25). Chronic GVHD developed in 40% (90%CI, 27–54%) of patients by one-year. The single non-relapse death was due to GVHD complicated by invasive fungal infection on day +288. As a result, the cumulative incidence of NRM at day +100 was 0% and at one year reached 3% (90%CI, 0–8%).
Relapse and survival
The one-year cumulative incidence of relapse was 33% (90%CI, 20–47%) All seven patients who relapsed died. Figure 1 shows the one-year OS was 80% (90%CI, 69–89%), and DFS 73% (90%CI, 60–83%). One-year DFS was 63% (90%CI, 46–77%) for the 32 patients with AML, and 87% (90% CI, 53–100%) for the 8 patients with MDS.
Figure 1.
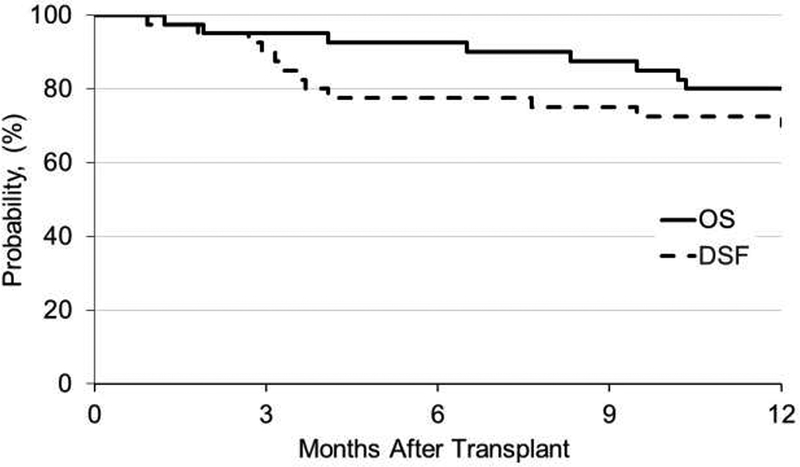
Overall and disease-free survival outcomes at 1-year post-HCT
OS: overall survival; DFS: disease-free survival
DISCUSSION
This is the first prospective study using a BSA-based treosulfan regimen in pediatric patients with AML or MDS. BSA-based treosulfan regimen was safe and provided excellent disease control. Although this regimen was myeloablative, toxicity was minimal. Engraftment was robust and sustained, and full donor chimerism was persistent, even in cord blood recipients.
This results in this prospective study are similar to a recent retrospective study of children and adolescents transplanted with treosulfan. Boztug et al reported TRM of 14% with three-year OS of 46% for AML, 64% for MDS23 The novel conditioning regimen used in this study resulted in exceptionally low NRM, especially when compared to busulfan-based regimens in a similar patient population (3% vs 10.5–20%).30–32 As hypothesized, relapse rates were not higher than those observed in busulfan-based regimens, which suggests that treosulfan-based regimen may improve survival. Futhermore, other than GVHD, non-fatal severe toxicities, including VOD, were essentially absent in this study, which may prove to be another benefit from this approach. Treosulfan-based conditioning did not eliminate severe GVHD, but the observed GVHD rate in this study was comparable to previously reports in similar patient populations.30–33
The BSA-based treosulfan dosing schema used in this study yielded predictable pharmacokinetics across groups, confirming that individualized PK adjustment for treosulfan is not necessary, a great advantage of this drug compared to busulfan. Ongoing prospective pediatric studies in Europe are collecting additional treosulfan PK data in children with malignant and nonmalignant disorders undergoing treatment with the same BSA-based treosulfan dosing used in our study. This information will be particularly useful for determining dosing in infants for whom data remains very limited. If treosulfan is confirmed to have a predictable PK profile in these studies, it could become the drug of choice for MAC regimens in children and young adults, given its low toxicity profile without the need for individual PK parameter testing.
One current limitation to the use of treosulfan is its lack of availability for commercial use in several countries including the United States. Studies in progress in adults with hematologic malignancies and children with malignant and nonmalignant disorders are addressing this gap, with the goal of making this drug more available in the future, if it proves to be effective in larger clinical trials. Our treosulfan-based regimen appears to be at least as effective as busulfan-based regimens, with the potential to yield better disease-free survival, by virtue of lower NRM with similar relapse rates. Clinical studies comparing busulfan versus treosulfan-based regimens in children may provide valuable information towards the efficacy of this drug.
Table 3.
Survival and GVHD outcomes by stem cell source and donor group
| Stem cell source | Donor* | ||||
|---|---|---|---|---|---|
| Outcome‡ | BM (n=25) | PBSC (n=6) | CBU (n=9) | Related (n=10) | Unrelated (n=21) |
| Acute GVHD at day +100 | |||||
| Grades II-IV | 5 (0–14) | 50 (19–81) | 50 (23–77) | 0 | 22 (9–40) |
| Grades III-IV | 0 | 50 (19–81) | 25 (5–53) | 0 | 18 (5–35) |
| Chronic GVHD | |||||
| at day +180 | 5 (0–14) | 67 (34–92) | 38 (13–66) | 0 | 28 (12–46) |
| at 1 year | 25 (11–42) | 67 (34–92) | 63 (34–87) | 11 (0–34) | 46 (27–66) |
| Off IST at 1 year | 60 | 33 | 100 | 60 | 52 |
| OS at 1 year | 72 (56–85) | 83 (53–99) | 100 | 70 (45–90) | 76 (80–89) |
| DFS at 1 year | 64 (48–79) | 83 (53–99) | 89 (67–100) | 60 (34–83) | 71 (54–86) |
| GRFS at 1 year | 44 (28–60) | 17 (1–47) | 22 (5–48) | 50 (25–75) | 33 (18–51) |
| Relapse at 1 year | 36 (21–52) | 0 | 11 (0–33) | 40 (17–66) | 24 (11–40) |
| NRM at 1 year (n) | 0 | 1 | 0 | 0 | 1 |
| Deaths at 1 year (n) | 7 | 1 | 0 | 3 | 5 |
Results presented as probability (%) and 90%CI, unless noted otherwise.
GVHD: graft-versus-host disease; IST: immunosuppressive therapy; OS: overall survival; DFS: disease-free survival; GRFS GVHD/relapse-free survival; NRM: non-relapse mortality
Includes non-cord allogeneic donor sources only.
ACKNOWLEDGMENTS
The authors would like to thank the patients, study personnel, and care providers who participated in this study.
The study was supported in part by grants from the Johnny Crisstopher Children’s Charitable Foundation St. Baldrick’s Consortium Grant and medac GmbH.
Footnotes
*Corporate Members
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014–17-1–2388 and N0014–17-1–2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *lncyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; Medlmmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. - Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota.
DISCLAIMERS
The views expressed in the submitted article are his or her own and not an official position of the institutions or sources of support (including the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government).
DISCLOSURE OF CONFLICTS OF INTEREST
Ralf A. Hilger reports receipt of payment/services/products from medac GmbH during the conduct of the study.
Lauri Burroughs reports a financial relationship with medac GmbH outside the submitted work. Michael A. Pulsipher reports a financial relationship with medac GmbH outside the submitted work.
Colleen Delaney reports a study drug supply and regulatory oversight agreement with medac GmbH during the conduct of the study; a drug transfer agreement and IND management agreement outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lucchini G, Labopin M, Beohou E, Dalissier A, Dalle JH, Cornish J, et al. Impact of Conditioning Regimen on Outcomes for Children with Acute Myeloid Leukemia Undergoing Transplantation in First Complete Remission. An Analysis on Behalf of the Pediatric Disease Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23(3):467–74. [DOI] [PubMed] [Google Scholar]
- 2.de Berranger E, Cousien A, Petit A, Peffault de Latour R, Galambrun C, Bertrand Y, et al. Impact on long-term OS of conditioning regimen in allogeneic BMT for children with AML in first CR: TBI+CY versus BU+CY: a report from the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Bone Marrow Transplant. 2014;49(3):382–8. [DOI] [PubMed] [Google Scholar]
- 3.Strahm B, Nöllke P, Zecca M, Korthof ET, Bierings M, Furlan I, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25(3):455–62. [DOI] [PubMed] [Google Scholar]
- 4.Sisler IY, Koehler E, Koyama T, Domm JA, Ryan R, Levine JE, et al. Impact of conditioning regimen in allogeneic hematopoetic stem cell transplantation for children with acute myelogenous leukemia beyond first complete remission: a pediatric blood and marrow transplant consortium (PBMTC) study. Biol Blood Marrow Transplant. 2009;15(12):1620–7. [DOI] [PubMed] [Google Scholar]
- 5.Paillard C, Rochette E, Lutz P, Bertrand Y, Michel G, Bordigoni P, et al. Reduced-intensity conditioning followed by allogeneic transplantation in pediatric malignancies: a report from the Société Française des Cancers de I’Enfant and the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Bone Marrow Transplant. 2013;48(11):1401–8. [DOI] [PubMed] [Google Scholar]
- 6.Rio B, Chevret S, Vigouroux S, Chevallier P, Fürst S, Sirvent A, et al. Decreased nonrelapse mortality after unrelated cord blood transplantation for acute myeloid leukemia using reduced-intensity conditioning: a prospective phase II multicenter trial. Biol Blood Marrow Transplant. 2015;21(3):445–53. [DOI] [PubMed] [Google Scholar]
- 7.Pulsipher MA, Boucher KM, Wall D, Frangoul H, Duval M, Goyal RK, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114(7):1429–36. [DOI] [PubMed] [Google Scholar]
- 8.Lawitschka A, Faraci M, Yaniv I, Veys P, Bader P, Wachowiak J, et al. Paediatric reduced intensity conditioning: analysis of centre strategies on regimens and definitions by the EBMT Paediatric Diseases and Complications and Quality of Life WP. Bone Marrow Transplant. 2015;50(4):592–7. [DOI] [PubMed] [Google Scholar]
- 9.Feit PW, Rastrup-Andersen N, Matagne R. Epoxide formation from (2S,3S)-threitol 1,4-bismethanesulfonate. The preparation and biological activity of (2S,3S)-1,2-epoxy-3,4-butanediol 4-methanesulfonate. J Med Chem. 1970;13(6):1173–75. [DOI] [PubMed] [Google Scholar]
- 10.Aabo K, Hald I, Hørbov S, Dombernowsky P, Hansen HH, Sørensen HM, et al. A randomized study of single agent vs combination chemotherapy in FIGO stages IIB, III and IV ovarian adenocarcinoma. Eur J Cancer Clin Oncol. 1985;21(4):475–81. [DOI] [PubMed] [Google Scholar]
- 11.Ploemacher RE, Johnson KW, Rombouts EJ, Etienne K, Westerhof GR, Baumgart J, et al. Addition of treosulfan to a nonmyeloablative conditioning regimen results in enhanced chimerism and immunologic tolerance in an experimental allogeneic bone marrow transplant model. Biol Blood Marrow Transplant. 2004;10(4):236–45. [DOI] [PubMed] [Google Scholar]
- 12.Hilger RA, Baumgart J, Scheulen ME, Trenschel R, Strumberg D, Seeber S, et al. Pharmacokinetics of treosulfan in a myeloablative combination with cyclophosphamide prior to allogeneic hematopoietic stem cell transplantation. Int J Clin Pharmacol Ther. 2004;42(11):654–55. [DOI] [PubMed] [Google Scholar]
- 13.Hilger RA, Harstrick A, Eberhardt W, Oberhoff C, Skorzec M, Baumgart J, et al. Clinical pharmacokinetics of intravenous treosulfan in patients with advanced solid tumors. Cancer Chemother Pharmacol. 1998;42(2):99–104. [DOI] [PubMed] [Google Scholar]
- 14.Lindley C, Shea T, McCune J, Shord S, Decker J, Harvey D, et al. Intraindividual variability in busulfan pharmacokinetics in patients undergoing a bone marrow transplant: assessment of a test dose and first dose strategy. Anticancer Drugs. 2004; 15(5):453–59. [DOI] [PubMed] [Google Scholar]
- 15.Schuler US, Renner UD, Kroschinsky F, Johne C, Jenke A, Naumann R, et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. British J Haematol. 2001;114(4):944–50. [DOI] [PubMed] [Google Scholar]
- 16.Schmidmaier R, Oellerich M, Baumgart J, Emmerich B, Meinhardt G. Treosulfan-induced apoptosis in acute myeloid leukemia cells is accompanied by translocation of protein kinase C delta and enhanced by bryostatin-1. Exp Hematol. 2004;32(1):76–86. [DOI] [PubMed] [Google Scholar]
- 17.Fichtner I, Becker M, Baumgart J. Antileukaemic activity of treosulfan in xenografted human acute lymphoblastic leukaemias (ALL). Eur J Cancer. 2003;39(6):801–7. [DOI] [PubMed] [Google Scholar]
- 18.Beelen DW, Trenschel R, Casper J, Freund M, Hilger RA, Scheulen ME, et al. Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications. Bone Marrow Transplant. 2005;35(3):233–41. [DOI] [PubMed] [Google Scholar]
- 19.Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol. 2010;28(20):3344–51. [DOI] [PubMed] [Google Scholar]
- 20.Ruutu T, Volin L, Beelen DW, Trenschel R, Finke J, Schnitzler M, et al. Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica. 2011;96(9):1344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(3):341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyurkocza B, Gutman J, Nemecek ER, Bar M, Milano F, Ramakrishnan A, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(4):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boztug H, Sykora KW, Slatter M, Zecca M, Veys P, Lankester A, et al. European Society for Blood and Marrow Transplantation Analysis of Treosulfan Conditioning Before Hematopoietic Stem Cell Transplantation in Children and Adolescents With Hematological Malignancies. Pediatr Blood Cancer. 2016;63(1):139–48. [DOI] [PubMed] [Google Scholar]
- 24.Beier R, Schulz A, Hönig M, Eyrich M, Schlegel PG, Holter W, et al. Long-term follow-up of children conditioned with Treosulfan: German and Austrian experience. Bone Marrow Transplant. 2013;48(4):491–501. [DOI] [PubMed] [Google Scholar]
- 25.Scheulen ME, Hilger RA, Oberhoff C, Casper J, Freund M, Josten KM, et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res. 2000;6(11):4209–16. [PubMed] [Google Scholar]
- 26.Tanswell P1, Koup J. TopFit: a PC-based pharmacokinetic/pharmacodynamic data analysis program. Int J Clin Pharmacol Ther Toxicol. 1993;31(10):514–20. [PubMed] [Google Scholar]
- 27.Heinzel G, Woloszak R, Thomann P. Topfit v.2.0 Pharmacokinetic and Pharmacodynamic Data Analysis System for PC. Stuttgart, Jena, New York: Gustav Fisher; 1993. [Google Scholar]
- 28.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–14. [DOI] [PubMed] [Google Scholar]
- 30.Lucchini G, Labopin M, Beohou E, Dalissier A, Dalle JH, Cornish J, et al. Impact of Conditioning Regimen on Outcomes for Children with Acute Myeloid Leukemia Undergoing Transplantation in First Complete Remission. An Analysis on Behalf of the Pediatric Disease Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23(3):467–474. [DOI] [PubMed] [Google Scholar]
- 31.de Berranger E, Cousien A, Petit A, Peffault de Latour R, Galambrun C, Bertrand Y, et al. Impact on long-term OS of conditioning regimen in allogeneic BMT for children with AML in first CR: TBI+CY versus BU+CY: a report from the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Bone Marrow Transplant. 2014;49(3):382–8. [DOI] [PubMed] [Google Scholar]
- 32.Sisler IY, Koehler E, Koyama T, Domm JA, Ryan R, Levine JE, et al. Impact of conditioning regimen in allogeneic hematopoetic stem cell transplantation for children with acute myelogenous leukemia beyond first complete remission: a pediatric blood and marrow transplant consortium (PBMTC) study. Biol Blood Marrow Transplant. 2009; 15(12): 1620–7. [DOI] [PubMed] [Google Scholar]
- 33.Davies SM, Wang D, Wang T, Arora M, Ringden O, Anasetti C, et al. Recent decrease in acute graft-versus-host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2009;15(3):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


